Abstract
Objective
The aim of this study was to evaluate the use and effectiveness of non-pharmacological therapies as part of the treatment of COVID-19 and its complications, either combined or not with the usual treatment.
Methods
A systematic review was conducted between August and October 2021 using PubMed, Scopus, CINAHL and Web of Science databases. From a total of 204 articles identified, 33 were included in the final sample (15 clinical trials and 18 quasi-experimental studies). The methodological evaluation was carried out using STROBE and CONSORT guidelines.
Results
There is a growing literature on the use of CAM for COVID-19. Most studies have shown positive findings, particularly for the use of TCM, other herbal therapies and acupuncture. Nevertheless, most studies were carried out in Asia and relied on quasi-experimental designs. The current evidence is available for physical outcomes (mortality rate, pneumonia resolution and other symptoms, negative PCR test, and hospitalization and ICU admissions) and for mental health outcomes.
Conclusion
Despite a positive role of CAM on COVID-19 outcomes, the evidence is still mostly based on quasi-experimental studies. More robust clinical trials are needed in order to generate better evidence in this area.
Keywords: Complementary therapies, Coronavirus, COVID-19, Nonpharmacological interventions, Traditional medicine
Introduction
In December 2019, the Severe Acute Respiratory Syndrome (SARS)-CoV-2 was identified in Wuhan (China).1 The rapid spread of the virus all over the world made the Emergency Commission of the International Health Regulations to determine this outbreak as a “Public Health Emergency of International Importance” (World Health Organization.2 Later, on March 11th, 2020, COVID-19 was declared a pandemic,2 representing the largest global public health crisis since the 1918 influenza pandemic.1
The high mortality associated with this infection has prompted rapid responses from scientists and healthcare professionals, resulting in the research of several pharmacological treatments, such as remdesivir, hydroxychloroquine and interferon,3, 4, 5, 6, 7 and in the development of different vaccines. Current data has shown that the use of immunomodulators such as anakinra and tocilizumab reduced the need for orotracheal intubation in patients by improving pulmonary hyperinflammation.8, 9, 10, 11 Likewise, corticosteroids were also used to reduce the inflammatory response and the progression of respiratory failure, reducing the mortality rate of patients by 8%.4
Despite this promising evidence, results are still conflicting. A study that involved 405 hospitals in 30 countries, with a sample of 11,330 patients, determined that the use of Remdesivir, Hydroxychloroquine, Interferon, and Lopinavir did not show any clinical improvement (i.e., reducing mortality, hospitalization time and mechanical ventilation) in patients hospitalized with COVID-19.12 Likewise, although COVID-19 vaccines showed an effectiveness of 90 to 97% and have proven to reduce contagion and mortality,13 , 14 not all countries are currently at the same rate of vaccination due to social and economic inequalities.15 Another common problem is the misconceptions concerning the use of vaccines by individuals, based on non-scientific beliefs, fear and political reasons.16 These aforementioned reasons and the uncertainty in the initial phase of the outbreak have led to the use of a series of nonpharmacological therapies, including those with and without the appropriate evidence.
In this context, alternative and complementary medicine (CAM) emerge as potential options to be combined or not with the current pharmacological treatment. These therapies are defined as the “set of health practices, approaches, knowledge and beliefs that incorporate medicines based on plants, animals and/or minerals, spiritual therapies, manual techniques and exercises applied individually or in groups, to maintain well-being, in addition to treating, diagnosing and preventing diseases”,17 which are based on a comprehensive and holistic care.18
Background
There are several nonpharmacological therapies being used since the outbreak of the COVID-19 pandemic and the results are diverse. A synthesis study of the literature determined that the herbs Liquorice Root and Baical Skullcap Root seemed to relieve clinical symptoms such as pulmonary congestion, due to their antitussive, expectorant, antipyretic, and anti-inflammatory properties.19 Another review found that various Traditional Chinese Medicine (TCM) formulations were promising to prevent SARS-CoV-2 infection among hospital care workers.20 In the same direction, acupuncture and electroacupuncture have been evaluated showing possible benefits on the appearance of septic symptoms21 and the TCM moxibustion (burning of the herb “àicǎo” or “mugwort” on acupuncture points), seemed to strength the immune system during COVID-19 infection.22
Despite these previous studies, the literature is still conflicting and very controversial on the use of CAM for COVID-19. A systematic compilation of the current evidence is needed to minimize bias and enhance the comprehension of such therapies for COVID-19 patients. Therefore, this systematic review could add to the current scientific literature providing further evidence of the use and effectiveness of non-pharmacological therapies as part of the treatment of COVID-19 and its complications, either combined or not with the usual treatment.23
Methods
Design
This systematic review was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines24 (Supplementary material-Appendix A). The main research question was: What non-pharmacological therapies are offered as a complementary or alternative treatment for COVID-19 and what is their current evidence of their effectiveness?
Search strategy and selection criteria
The search was carried out between August and October 2021 by two researchers independently in four electronic databases (PubMed, Scopus, CINHAL, and Web of Science). The following search strategy was used for all databases: (“traditional medicine” OR “traditional Chinese medicine” OR “alternative medicine” OR “complementary medicine” OR “herbal medicine” OR “cultural practice”) AND (covid OR covid-19 OR SARS-CoV-2).
The eligibility criteria were defined according to the PICOTS strategy (Population, intervention, comparison, outcome, time, and study design) as presented in Table 1 .
Table 1.
PICOTS (population, intervention/exposure, comparator, outcome, time and study design) criteria.
| PICOTS criteria | |
|---|---|
| Population | Patients diagnosed with COVID-19, who were hospitalized or under home confinement. |
| Intervention/Exposure | Non-pharmacological therapies/Traditional interventions |
| Comparator | Standard practice, usual care or no comparator |
| Outcome | Clinical Physical symptoms related to COVID-19 (clinical data, test) |
| Time | Any follow up |
| Study design | Controlled trials and Quasi-experimental studies |
Articles were included if they were quantitative studies published in peer-reviewed journals. They should focus on the use of non-pharmacological complementary and alternative therapies as a method of prevention or of treatment against COVID-19. Patients included in these studies should be in the acute phase of COVID-19 or in the recovery phase after infection. Regarding exclusion criteria, “in vitro” trials and those studies related to the consequences of the pandemic, epidemiological characteristics or risk factors for COVID-19 were excluded. In addition, publications consisting of editorials, letters to the editor, comments, or essays, as well as other syntheses of the literature, were also excluded.
We limited our search to original research studies published in English language during the COVID-19 pandemic, and no geographic restrictions were applied.
Study selection and data extraction
After searching the literature, all references were imported into Mendeley Software 1.19.18, a reference manager software, and two authors reviewed independently all titles and abstracts for assessing the eligibility of articles on the basis of our research question. Duplicate publications were also excluded in this phase. A third author evaluated all disagreements and made final decisions for inclusion or exclusion.
All authors participated in data extraction, and all extracted data were cross-checked by other authors. Any disagreements concerning statistics analyses or results of these included articles were discussed until the team which reached a consensus. The main characteristics of the selected articles were presented in a table (Table 2 ), taking into account the author and geographic area of the study, the type of therapy used, the methodology (participants, measures and instruments), the number of sessions/duration, and the main findings, as well as the methodological quality of each study.
Table 2.
Description of the articles included as Results.
| Reference | Therapy* | Design* | Participants* | Measures* | Instrument* | N° sessions/ duration (days) | Main findings | Methodological quality |
|---|---|---|---|---|---|---|---|---|
| An et al. (2021)/ China | TCM (Shen Ling Bai Zhu San + Xiao Chai Hu Tang Formula) | Retrospective Cohort Study | Total N = 568 discharged COVID-19 patients (IG = 143; CG = 425) | Laboratory parameters (liver - kidney function and cardiac enzymes, inflamatory meadiators) | Blood tests | - / 60 | There was a minor incidence of pro-inflammatory and immunological mediators in blood specimens, and a higher cardiovascular, liver and nutritional protector effect for the IG. There were no significant differences to kidney functions indicators. |
12.72 / 22 (STROBE) |
| Chen et al., (2021)/ China | WM (Umifenovir or Arbidol) + TCM (Shufeng Jiedu Capsules) | Retrospective Cohort Study | Total N = 200 inpatients (IG = 100; CG = 100-only Arbidol) Gender: 65% males Age: 51–75 years old (M = 60.2) |
Clinical symptoms and treatment effectiveness, laboratory indicators, pneumonia resolution and adverse reactions. | Clinical data, blood tests, chest Computed Tomography (CT) | 168 / 14 | IG achieved a better pneumonia resolution (87% on day 7 versus 70% on day 7 on CG). Laboratory indicators showed an increase on inmunity and anti-inflamatory mediators in IG. Only defervescence was significally faster on IG with no markedly findings in other general symptoms resolution. Treatment effectiveness was higher at the end of 14 days in the IG (92% versus 80% on CG). No significant differences on adverse effects were found between the groups. |
18.22 / 22 (STROBE) |
| Chu & Zang (2021)/ Japan | Integrated management mode (TCM and WM): WeChat + TCM (herbal decotions) + Emotional Care + Music Therapy + Vital Qi Strenghtening (30 min) + Observation Tongue and Pulse. | Retrospective Cohort Study | Total N = 142 hospitalized patients. (IG =111; CG = 31) Gender: 51.5% males Age: 59 - 62 years old (M = 60.2) |
Clinical symptoms, pneumonia evolution, days of hospital stay, and survival without ICU admission. | Clinical data, Chest CT | -/- | Patients from IG needed less time to fully recover from COVID-19, obtaining a faster chest CT recovery than the CG (13 days vs. 16.7 days) and shorter days oh hospital stay (16.4 days vs. 24.4 days). There was also a significant increase of survival index within IG (97.3%). General symptoms (fever, cought, shortness of breath, muscle pain) were also solved sooner in the IG. | 17.92 / 22 (STROBE) |
| Doaei et al., (2021)/ United Kingdom | Omega-3 suplementation | RCT | Total N = 101 ICU COVID-19 patients (IG = 28; CG = 73) Gender: 59.4% males Age: 64 - 66 years old (M = 65) |
Inflammatory and biochemical markers) and mental state. | Clinical data, blood tests and Glasgow Coma Scale (GCS) | 14 / 14 | Omega-3 improved 1-month survival rates in IG (21% vs. 3%). Kidney function improved and arterial blood acidosis reduced, with no significant findings on blood oxygen levels within two groups. GSC kept being low on both groups after 14 days. Regarding serum electrolytes, blood clotting function, blood glucose, albumin, O2 sat., no significant differences were found between the groups. |
22 / 25 (CONSORT) |
| Feng et al., (2021)/ South Korea | TCM Shenhuang granules (SHG) | Retrospective Observational Study | N total = 118 ICU patients (IG = 33; CG = 85) Gender: 67.1% males Age: 57 - 75 years old (M = 68) |
Organ dysfunction, laboratory parameters, mortality rate, lenght of ICU stay and need of mechanical ventilation (IVM). | Clinical data, blood test, chest radiographs or CT. | 18 / 9 (median duration) | SHG treatment decreased mortality rates in ICU (45.4% in IG and 80% in CG) and the need of IMV (66.7% in IG vs 84.7% in CG). Lenght of ICU stay was shorter in IG (32 days) than the CG (76 days). Kidney, liver and cardiac complications and also pro-inflamatory mediators were reduced within the IG. |
18.75 / 22 (STROBE) |
| Guo et al., (2020) / China | TCM (Xuebijing Inyection) | Retrospective Case-Control Study | Total N = 58 hospitalized patients [IG = 42 patients (Mild cases = 8 patients + Severe cases = 34 patients); CG = 16 patients (Mild cases = 8 patients + Severe cases = 8 patients)] Gender: 50% females Age: 25–87 years old (M = 52.75) |
Clinical symptoms, hospitalary stay, conversion time of SARS-COV-2 nucleic viral assay and laboratory parameters. | Clinical data, Chest CT, SARS-COV-2 PCR and blood tests. | 24–44 (twice a day) / 12–22 (hospital stay) | IG showed better CT scan results (81.25% vs 62.5%) than in CG, and body temperature decreased. IG got a lower count of pro-inflammatory parameters on their blood specimens compared to patients in the CG. There were no significant differences in cough, sputum, fatigue, or diarrhea before and after treatment between the two groups. After treatment, total length of hospital stay, or the time taken to produce a negative nucleic acid test. |
18.06 / 22 (STROBE) |
| Hu, Guan, Bi et al., (2021)/ China | TCM: Lianhuaqing-wen capsules (LHQW) | RCT | Total N = 284 inpatients (IG = 142; CG = 142) Gender: 52.8% males Age: ≥ 45 years old (M = 51) |
Symptoms recovery, pneumonia evolution and conversion time of SARS-COV-2 viral assay. | Chest CT, clinical data and SARS-COV-2 PCR. | 168 / 14 | LH treatment was associated with a faster recovery of chest CT (IG 83.8% vs CG 64.1%) and symptoms resolution (i.e. fever, fatigue, and coughing): 7 days in the IG vs 10 days in the CG. There was no difference in viral assay conversion nor conversion to severe cases in both groups. | 17 / 25 (CONSORT) |
| Hu-Wang et al., (2021)/ China | WM and TCM: He-Jie-Shen-Shi Decoction (HJSS) | Retrospective Cohort Study | Total N = 81 severe patients with COVID-19 (IG = 47; CG = 34) Gender: 54.3% females Age: 18 - 75 years old (M = 64) |
General symptoms, laboratory parameters, conversion time of SARS-COV-2 viral assay. | Chest CT, blood test, blood oxygen saturation, SARS-COV-2 PCR. | Twice daily (HJSS) + Three times daily (Arbidol-part of the WM) / - | IG obtained better outcomes on the severe infection, with shorter duration of the negative conversion time of nucleic acid (23 days CG vs 20 days IG) and better result on clinical indicators such as leukocyte count and fever. No significant difference was found on blood saturation improvement between the groups. | 18.38 / 22 (STROBE) |
| Koshak et al., (2021)/ Saudi Arabia | Nigella Sativa Oil (NSO, herbal medicine) | RCT | Total N = 183 patients with COVID-19 (mild infection) (IG = 91; CG = 92) Gender: 53% males Age: 22 - 58 years old (M = 36) |
Clinical recovery, duration of symptoms, adverse reactions, and hospital admission due to disease complications. | Clinical data | 20 / 10 | Recovery in the NSO group was significantly higher (63%) than that in the CG (35%), and the days of hospital stay to recover was shorter (10.7 days in IG vs 12.4 days in CG). Patients in the IG had a significantly shorter mean duration of chills, anosmia, runny nose, and loss of appetite as compared to the CG. |
16.5 / 25 (CONSORT) |
| Li et al., (2021) / China | TCM (herbal decoction) Formula 1 for the pathogen residue síndrome and Formula 2 for both qi and yin defi ciency síndrome. |
Prospective case-control Study | Total N = 96 convalescent patients (IG = 64; CG = 32). Gender: 63.5% females Age: >16 years old (M = 49) |
General symptom evolution, and lung inflammation absorption. | Clinical data, chest CT, blood test. | 56 /28 | IG achieved a faster recovery from pneumonia only within the first 14 days (35% complete lung inflamation absortion rate vs. 15%). On the other time points, there was no statistically significant difference between the two groups (28, 56, and 84 of follow-up). Furthermore, by the 28th day after discharge, there was no significant difference in the improvement rates of symptoms. | 17.55 / 22 (STROBE) |
| Liu, Du, Shao et al., (2021) / China | TCM: Qingfei Paidu Decoction (QFPD) | Retrospective Observational Study | Total N = 761 hospitalized patients (IG= 239; CG= 522). Gender: 51.2% males. Age: ≥14 years old (M = 60). |
Death | Clinical data | 6 / 3 | Patients in the QPD group had a significantly lower risk of death than those in the NoQPD group, even after adjusting for baseline age, sex, and diabetes (3.2% of patients in the IG died vs 13.0% in the CG). | 19.31 / 22 (STROBE) |
| Liu, Jiang, Liu et al., (2021a) / China | TCM: Yindan Jiedu Herb (YDJDG) | Prospective Cohort Study | Total N = 131 hospitalized patients (IG = 60; CG= 71) Gender: 51% males Age: 6–86 years old (M = 41) |
Hospital stay for recovery, general symptoms, pneumonia evolution, conversion time of SARS-COV-2 viral assay. | Clinical data, chest CT SARS-COV-2 PCR | 42 / 14 | TCM reduced IG patients hospital stay (21 days vs 27 days in CG). Pneumonia evolution was also more favorable in IG (11.6 days) than in the CG (17.2 days). No significant difference was found on general symptoms resolution nor medium time for negative PCR, except for fever resolution (4.2 days in IG versus 6.4 days in the CG). | 18.22 / 22 (STROBE) |
|
Liu, Shi, Tu et al., (2021b)/ China |
WM (Arbidol) + TCM: LHQW | Retrospective Cohort Study | Total N = 108 inpatients with COVID-19 (IG = 68; CG = 40) Gender: 65% females Age: ≥ 18 years old (M = 54.8) |
Laboratory parameters, renal and liver functions, pneumonia evolution and conversion time of SARS-COV-2 viral assay. |
Chest CT, blood tests and SARS-COV-2 PCR | 15–63 (three times a day) / 5 - 21 | IG showed a higher decrease in pro-inflammatory mediators than in the CG. After 7 days of therapy, the chest CT scans did not find any difference between the 2 groups, but after 14 days of admission (better improvement in CT scans in the IG). In addition, the first negative PCR was sooner obtained in this group, resulting in a more effective reduction of viral load (27.9% of IG patients remained positive by 14th day, vs. 47.5% in the CG). There were no differences between groups for renal and liver function parameters. | 17.39 / 22 (STROBE) |
| Liu, Yang, Liu et al., (2021c)/ China | WM and TCM: Huashi Baidu Granules (Q-14) | RCT | Total N = 204 COVID-19 infected patients (IG = 102; CG = 102) Gender: 62.6% females Age: 18 - 75 years old (M = 56) |
Symptoms recovery, pneumonia evolution, and conversion time SARS-COV-2 viral assay. | Clinical data, 7-point scale (about hospitalization, requiring oxygen and ventilation, and death), chest CT, SARS-COV-2 PCR, blood test. | 28 / 14 | TCM combined with standard care resulted on general symptoms improvement (i.e. fever resolution, cough, fatigue, chest discomfort) except for headache and dry throat. Pneumonia evolution was faster in IG than in the CG, with faster decrease of inflamation (80% vs 51.5%). However, no significant difference was found for the conversion time of SARS-CoV-2 viral assay. | 18.5 / 25 (CONSORT) |
| Ma et al., (2021)/ China | TCM: Reduning intravenous inyection (RDN) | RCT | Total N = 50 patients (IG = 27; CG = 23) Gender: 56% males Age: 21–88 years old (M = 50) |
General symptoms, conversion time of SARS-COV-2 viral assay, duration of hospitalization, pneumonia evolution. | SARS-COV-2 PCR, chest CT clinical data | 14 / 14 | IG patients had a shorter recovery from symptoms within the first 7 days of treatment (96.30% vs 39.13%). The lenght of hospital stay was also shorter in IG (14.8 days) than in the CG (18.5 days). Negative nucleic acid assay and better images on chest CT were obtained sooner in the IG. | 16 / 25 (CONSORT) |
| Natarajan et al., (2021)/ India | Poly-herbal Siddha medicine: Kabasura Kudiner (KSK) | RCT | Total N = 60 confirmed asymptomatic patients with COVID-19 (IG = 30; CG = 30) Gender: 75% males Age: 18 - 55 years old (M = 35.4) |
Reduction in viral load, immunological markers, renal and liver function, and general symptoms. | Blood test, SARS-COV-2 PCR, and clinical data. | 14 / 7 | There was no significant difference in the biochemical parameters between the groups. Although declined viral load was more pronounced in the IG, there was no significant difference as compared with the CG. None of the participants in both group progressed to symptomatic. | 18 / 25 (CONSORT) |
| Ni, Wen, Hu et al., (2021)/ China | TCM: Shuanghua- glian Oral Liquids (SHL) | RCT | Total N = 235 patients with COVID-19 (IG = 176; CG = 59) Gender: 54% females Age: > 18 years old (M = 54) |
Conversion time of SARS-COV-2 viral assay, laboratory parameters and pneumonia evolution. | SARS-COV-2 PCR, blood test, and chest CT. |
IG 1 Low dose: 42 / 14 IG 2 Medium dose: 42 / 14 IG 3 High dose: 42 / 14 |
TCM group obtained a faster negative nucleic acid test result (93.4% IG vs 73.9% CG) and better pulmonary images on CT. Higher dosis of SHL were related to better pulmonary inflammation absorption. There were no differences between blood specimens and time to disease recovery between the groups. |
16.5 / 25 (CONSORT) |
| Parizad et al., (2021)/ Iran | Guided Imagery Method | RCT | Total N = 110 patients (IG = 55; CG = 55). Gender: 56.4% males Age: 18–69 years old (M = 40.2) |
Anxiety levels, pain, vital signs. |
STAI, SF-MPQ, VAS, and the Vital Signs Flow Sheet. | 10 / 5 | Guided imagery reduced anxiety and the intensity and quality of pain among COVID-19 patients. Heart rate, systolic blood pressure and oxygen saturation improved after intervention in IG patients. There was no effect over body temperature, respiratory rate and diastolic blood pressure. | 20/25 (CONSORT) |
| Pawar et al., (2021)/ India | Oral Curcumine and Piperine | RCT | Total N = 140 patients with severe, moderate, and mild COVID-19 infection (IG = 70; CG = 70) Gender: 70.7% males Age: 18–85 years old (M = 33.8) |
Duration of hospitalization, laboratory parameters, remission of symptoms, mechanical ventilation assistance. | Clinical data, blood test. | 28 / 14 | Patients of the IG showed early symptomatic recovery (of fever, cough, sore throat, breathlessness) and better oxygen saturation. These patients also required less mechanical ventilator support and suffered less thromboembolic episodes than in the CG. The duration of hospitalization was significantly lower in the moderate and severe patients from the IG. |
21.5/25 (CONSORT) |
| Shen et al., (2021) / China | TCM: LHQW | Retrospective Cohort Study | Total N = 248 patients with moderate COVID-19 infection (IG = 90, CG = 158). Gender: 52.8% males Age: >18 years old (M = 58.95) |
Blood cell counts, biochemical parameters, inflammation, and coagulation function. | Blood and urine tests, Chest CT | 15–21 / 5–7 |
Regarding blood cell counts, hemoglobin levels were significantly increased in the LHQW treatment group. The differences in terms of general inflammation parameters were not statistically significant, but erythrocyte sedimentation rate. D-dimer in the CG was significantly higher than in the IG, but no significant differences were found between the groups for general coagulation function. |
17.72/ 22 (STROBE) |
| Shi, Guo, Liu et al., (2021)/ China | TCM: Huashi Baidu formula + WM: Lopinavir/Ritonavir. | NRCT | Total N = 60 patients; IG1 (WM+TCM) = 20, IG2 (TCM) = 20, CG (WM) = 20). Gender: 66.7% males Age: 18 - 85 years old (M = 54.5) |
Symptoms remission, quarantine release and biochemical parameters, kidney and liver functions, and myocardial damage. |
Clinical data, chest CT, and blood test. | Twice daily / - | TCM combined with Western medicine improved immunology results on blood specimens. The clinical remission time was 10.8 days in CG, 9.7 days in IG1, and 5.9 days in IG2, being statistically significant. There was no statistical difference among the three groups regarding quarantine times, liver and kidney function and harm to cardiac function. |
19.74/22 (STROBE) |
| Srivastava et al., (2021)/ India | Nilavembu Kudineer (NVK) and Kaba Sura Kudineer (KSK) | RCT | Total N = 120 patients diagnosed with mild to moderate COVID-19; IG1 (NVK) = 40, IG2 (KSK) = 40, CG = 40. Gender: 50% females Age: 18 - 65 years old (M = 42.2) |
SARS-CoV-2 viral load, time taken by the patient to become asymptomatic, hospital stay, biological markers, and general symptoms (fever, dyspnea). | PCR test, blood test, clinical data. | 20 / 10 | In NVK and KSK groups, patients recovered faster in comparison to placebo, so they discharged earlier (65% of males and 55% of females were discharged on day 3). NVK and KSK groups had better results on blood specimens, and also a faster negative result on RT-PCR (within first 6 days) against placebo group (10 days). Time taken by patients to get asymptomatic was 2.5 mean days in the NVK group; 1.7 in the KSK group and 4.2 days in the CG. Within NKV and KSK groups there was a noticeable difference in recovery times, symptoms evolution and RT-PCR results, being more favorable and shorter in KSK group. |
17.5/25 (CONSORT) |
| Thakar et al., (2021)/ India | Ayurveda | Retrospective Cohort study | Total N = 762 early stage COVID-19 patients (IG = 541; CG = 221). Gender: 74% males. Mean age: 34.5 years old. |
Development of symptoms, duration of symptomatic phase in those progressing to symptomatic stage and mortality. | Clinical data through telephone interviews | - / 5 | Risk of progressing to symptomatic COVID-19 disease was not significantly different between groups. However, it was found significantly different for the total duration of the symptomatic period (3.66 days in IG vs. 5.34 days in CG). No mortality was observed in either of the groups. |
17.21/22 (STROBE) |
| Tian et al., (2020)/ China | TCM: Hanshiyi Formula (HSYF) + WM | Retrospective Cohort study | Total N = 721 patients with mild or moderate infection (IG = 430; CG = 291) Gender: 51.9% females. Mean age: 48 years old. |
Progression to a severe disease status | Clinical Data | Unspecified (more than 2 days). | There were no cases that progressed to severe COVID-19 in the IG. In contrast, there were 19 severe cases in the CG (p<0.001). | 17.88/22 (STROBE) |
| Wang, Liu, Lv et al., (2021)/ China | TCM: Chai-hu-jie-du and Fu-zheng-jiu-fei granules. | Retrospective Cohort study | Total N = 130 patients with severe infection (IG=66; CG=64). Gender: 55% females. Age: 57–70 years old (M = 65.6). |
Clinical improvement, mortality rate, and lung lesion. | Chest CT, blood test, and 7-point scale. | Twice Daily / 14 - 28 | No significant difference was observed in clinical improvement (i.e. cough, dyspnea) or deterioration between the groups, bur for the duration of fever. The comparison of lung injury ratio before and after the treatment also did not show differences between groups. However, the 28-day mortality rate was lower in the IG group than in the CG. In addition, the duration of fever was shorter in the IG than in the CG (4 days vs. 7 days). Abnormal liver function and gastrointestinal adverse events were more common in the IG, but no differences were observed when compared with the CG. |
16.88/22 (STROBE) |
| Wang, Lu, Li et al., (2021a) / China | TCM: Huashi Baidu granule combined with the Xiyanping injections, Xuebijing and Shenmai |
Retrospective case series study | Total N = 55 severe COVID-19 patients (IG = 23; CG = 32) Gender: 52.7% males Age: 26–77 years old (M = 58). |
Laboratory parameters, conversion time of SARS-COV-2 viral assay, discharge from hospital. | Blood test, Chest CT, SARS-COV-2 PCR | - / 16 | TCM showed faster CT Scan improvements, so the inflammation absorption was better in the IG. SARS-COV-2 viral assay took longer to become negative in CG treated with WM (15.5 days) than in the IG (12 days). Inflammatory parameters and tisular damage decreased in the IG. The discharge rates of the two groups had no significant difference. | 18.55 / 22 (STROBE) |
| Xu et al., (2021)/ China | TCM: Reduning Inyection | RCT | Total N = 157 patients (IG = 77; CG = 80). Gender: 55.4% males. Age: ≥18 years old (M = 49.8). |
Symptoms recovery, viral load, days of hospital stay, survival rate. |
Clinical data and PCR test. | 14 / 14 |
IG patients improved their health after 7 and 14 days of treatment, which involved a shorter time to symptom resolution, shorter time to negative nucleic acid test, faster resolution of fever, and a shorter hospitalization. The difference in the 28-day survival of the participants was not statistically significant between the groups. |
18.5/25 (CONSORT) |
| Zeng et al., (2021)/ China | TCM: Maxingshigan - Weijing Decoction (MWD) | RCT | Total N = 59 patients with mild or moderate symptoms caused by SARS-CoV-2 infection (IG = 30; CG = 29). Gender: 68% males. Age: 18–85 years old (M = 52). |
Symptoms recovery, time of conversion of SARS-CoV-2 RNA assay, days of hospital stay, conversion to severe cases, liver and kidney functions. | TCM Syndrome Scoring System, blood and PCR test, and clinical data. | 28 / 14 |
The IG patients exhibited a significantly shorter time to the recovery of fever (3 days vs. 7 days), fatigue (9 days vs. 12 days), coughing (9 days vs. 14 days) and difficulty breathing (4.5 days vs. 9.5 days). Treatment with MWD was not associated with a shorter conversion time of SARS-CoV-2 viral assay (14 days vs. 10 days) or a shorter hospitalization period (21 days vs. 18 days). After treatment, hemoglobin levels improved significantly in the IG, but no differences were observed on other blood parameters (leukocytes, neutrophil, lymphocyte, red blood cells and platelets). Kidney and liver functions remained normal throughout the treatment period. |
19/25 (CONSORT) |
| Zhang, Huang, Liu, et al., (2020a)/ China | TCM (natural herbal medicine) + WM | Retrospective case-control study | Total N = 22 patients with mild or moderate symptoms (IG = 11; CG = 11) Gender: 63.6% females. Age: 18–70 years old (M = 42). |
General symptoms (fever, cough, diarrea), COVID RNA persistance, and pneumonia evolution. | Clinical data, PCR test (respiratory tract and fecal specimens), and Chest CT. | 24 / 12 | The duration of fever was markedly shorter in the IG (3.4 days) compared with the CG (5.6 days). However, there were no significant differences between groups in terms of conversion time of SARS-COV-2 viral assay or chest CT scores. | 18.69/22 (STROBE) |
| Zhang, Lv, Zhou et al., (2021a)/ China | TCM: Xiyanping Inyection (XYP) | RCT | Total N = 130 hospitalized adult patients with mild or moderate symptoms (IG = 65; CG = 65). Gender: 53.8% females. Age: ≥18 years old (M = 46.28). |
Clinical symptoms (fever, cough, time to virus clearance). | Body temperature, blood test and PCR test. | 7–14 / 7–14 | The meantime to complete resolution of both fever and cough was significantly shorter for the IG (8.33 days vs. 11.86 days). XYP treatment significantly reduced the time to cough relief (6.89 days 12.25 days). The IG had a significantly shorter time to achieve negative SARS-CoV-2 RNA tests (7.97 days vs. 12.23 days). Six patients in the CG developed severe symptoms during the study, while no patients in the IG showed disease progression. |
17/25 (CONSORT) |
| Zhang, Xue et al., (2020b) / China | TCM: Tanreqing Capsule (TRQC) + WM | Retrospective Cohort study | Total N = 82 hospitalized patients with mild and moderate infection (IG = 25; CG = 57) Gender: 58.5% females Age: 23–58 years old (M = 35.5). |
Conversion time of SARS-COV-2 viral assay and immunological indicators. | PCR (pharyngeal and fecal) and blood test. | Unspecified (three times a day, three pills each time) | The negative conversion time of both fecal and pharyngeal nucleic acid was significantly shorter in the IG, compared to the CG. There was no significant difference between the groups in terms of lymphocyte levels in blood specimens. | 19.21/22 (STROBE) |
| Zhang, Zheng, Bai et al., (2021b)/ China | TCM: Qingfei Paidu Tang (QPT) | Retrospective Cohort study | Total N = 8939 patients with COVID-19 (GI = 2568; CG = 6371). Gender: 53.4% females. Age: ≥ 18 years old (M = 55.9) |
Mortality, acute kidney and liver injury. | Clinical data | ≥ 3 / ≥ 3 | Mortality was significantly lower among IG (1.2%) than in CG patients (4.8%) during hospitalization. Patients in both groups had a comparable incidence of acute kidney injury (1.6% vs. 3%) and acute liver injury (8.9% vs. 9.9%). | 18.08/22 (STROBE) |
| Zhou et al., (2021)/ China | TCM: (SHG) | RCT | Total N = 111 patients with severe/ critical COVID-19 (IG = 57; CG = 54). Gendre: 63.9% males. Age: ≥18 years old (M = 66). |
Clinical improvement, blood cell counts, mortality rate. | Clinical data, and blood test. | 28 / 14 | The IG patients had a higher improvement rate (61.4%) compared to the CG group (24.1%). In the IG, an increased lymphocyte count and a decreased total white blood cell and neutrophil count were observed. Mortality rate of the IG was 5.3% compared to 58.8% for the CG (p < 0.01); 88.2% in the CG advanced to critical status, vs. 47.4% of the IG patients (p < 0.01). No IG patients received an invasive ventilator compared to 58.8% of CG patients. | 18/25 (CONSORT) |
Therapy: (TCM) Traditional Chinese Medicine; (WM) Western Medicine.
Design: (NRCT) Non–Randomized Clinical Trial; (RCT) Randomized Clinical Trial.
Participants: (CG) Control Group; (IG) Intervention Group.
Measures (ICU) Intensive Critical Unit; (IMV) Invasive Mechanical Ventilation; (GCS) Glasgow Coma Scale.
Instrument: (CT) Computed Tomography; (PCR) Polymerase Chain Reaction; (STAI) Spielberger State-Trait Anxiety Inventory; (SF-MPQ) Short-Form McGill Pain Questionnaire; (VAS) Visual Analogue Scale; (VSFS) Vital Signs Flow Sheet.
Development of themes
To develop the themes for this systematic review, a thematic analysis approach was taken.25 The reviewers participating in searches, selection, article evaluation, and data extraction organized descriptive labels, focusing on emerging or persistent therapies and similarities or differences in using them and their outcomes. The coded data from each paper were examined and compared with the data from all the other studies. Finally, the different categories were grouped into different themes.
Assessment of methodological quality
The studies that met the inclusion criteria were assessed by two reviewers independently for methodological validity. Any disagreements that arose between the reviewers were resolved through discussion or by a third reviewer. The methodological quality was assessed using tools that ensure high quality presentation of observational studies (i.e., STROBE),26 and of clinical trials (i.e., CONSORT)27 to determinate a sound methodology within the retrieved studies.
Results
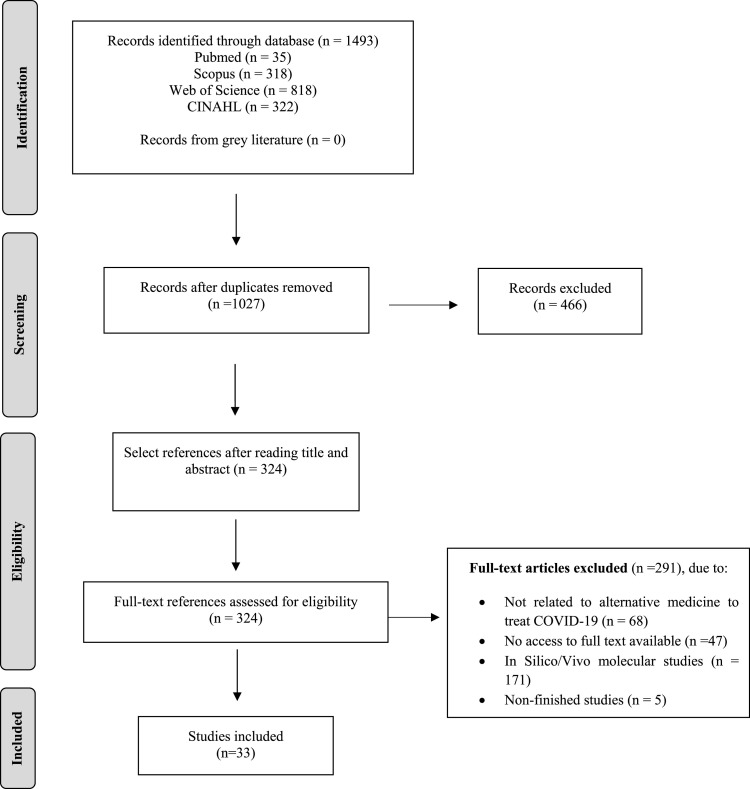
A total of 1493 publications were identified using our search strategy (Fig. 1 ). After removing duplicates, 1027 articles remained. Then, all titles and abstracts were evaluated, and this resulted in another 703 articles excluded, totaling 324 articles that underwent full-text analysis. After reading the full text of the articles, the final sample consisted of 33 studies: 15 clinical trials and 18 quasi-experimental studies.
Fig. 1.
Flowchart for the selection of articles for the systematic review.
From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. For more information, visit www.prisma-statement.org.
Characteristics of the included studies
All the studies included were published during the COVID-19 pandemic, between April 2020 and October 2021. Of a total of 33 studies, 97% were conducted in Asian countries, predominantly in China (75,75%; n = 25), and only one study was carried out in Europe.
Most included studies explored the use of herbal medicine and formulas from the TCM (81,8%; n = 27), and 9 of these combined TCM with usual Western Medicine care (WM). The treatments applied were very diverse, so the doses and duration were heterogeneous. Regarding the sample of the studies, most of them included more males than females (57.6%, n = 19 of the articles had a sample composed of more than 50% males). Among all participants, the mean age was 49.7 years old, and only 5 studies (15%) involved people with a mean age lower than 40 years old.
Concerning outcomes, most studies investigated the effectiveness of CAM on the resolution of pneumonia assessed by chest Computed Tomography (54.5%; n = 18), followed by greater negativation of tests for COVID-19 (48.5%; n = 16), and shorter hospital stays (30.3%; n = 10). Other parameters, such as clinical symptoms recorded in the medical history, laboratory tests and kidney or liver function were also investigated.
In relation to the methodological quality of the studies evaluated by STROBE and CONSORT guidelines, most articles showed high scores, ensuring a good development and content structure of the articles involved (Supplementary material-Appendix B). Only two studies28 , 29 got lower scores in comparison to the others (12.72/22 and 11.16/22).
Below, a brief description of the main results will be presented.
Herbal traditional Chinese medicine in the approach of COVID-19
As reported above, most of the evidence on CAM for the treatment of COVID-19 infection relies on the use of herbal TCM.
In this context, several articles have assessed different outcomes and the use of herbal TCM has promoted an earlier negativation of COVID-19 PCR tests, ,30, 31, 32 , 34, 35, 36 and a reduction of common symptoms associated with coronavirus,37, 38, 39, 40 such as fever,29 , 41, 42, 43 fatigue, cough, and shortness of breath.33 , 35 , 44 , 45
The pneumonia resolution was another outcome measured by these studies, which showed a decrease in lung inflammation visualized by CT. For instance, the administration of Shufeng Jiedu capsules produced a reabsorption of pneumonia after 7 days of treatment in 87% of the patients.37 Other authors evidenced this improvement in reabsorption and pulmonary exudate shortly after starting treatment.29 , 31, 32, 33, 34 , 38 , 41 , 44 , 46
The administration of herbal preparations also had positive effects on inflammatory and biochemical markers in patients with COVID-19. In many cases, inflammatory factors decreased28 , 31 , 34 , 37 and there was an improvement on immunological parameters such as white blood cells, lymphocytes, and platelets.30 , 39 , 47 Although other two studies45 , 48 did not find significant improvements in inflammatory and immunological parameters, they showed increases in the hemoglobin level in patients for the experimental groups.
Other authors have explored the role of TCM on survival. Using Shenhuang granules - a preparation with anti-inflammatory and antiviral properties - in critical patients with COVID-19, several authors47 , 49, 50, 51 showed a decrease in the mortality rate for the intervention group, after using formulas with detoxifying, pulmonary moisturizing, reabsorbing, antiemetic, antitussive, antithermal, and Qi-moving activity.
TCM not only showed promising effects on the treatment of symptoms in patients with COVID-19, but also appeared to influence the evolution of the disease as well. Two studies indicated that none of the participants with mild/moderate infection progressed to severe/critical COVID-19 after the application of herbal TCM.35 , 52 Regarding critically ill patients, the efficacy of TCM capsules in reducing ICU admissions together with the need to apply invasive mechanical ventilation.47 , 49
In general, the TCM studies have shown a reduction in the length of hospital stay.38 , 40 , 41 , 49 However, other studies did not show differences in terms of hospital discharge29 , 45 or time to became negative the PCR29 , 33 , 44 , 45 after the application of both isolated compounds of TCM and a combination of Western medicine with TCM. Despite these results, there was a slight tendency to get a faster viral assay conversion related to the strong affinity main compounds from TCM has over SARS-CoV-2 and its specific proteins and infectious pathways.29 , 44
Our review has also found several clinical trials assessing the use of herbal TCM for COVID-19, which provides more solid evidence to this field of knowledge. Below, a more detailed description of the findings of such clinical trials will be presented.
Hu et al.33 investigated the use of Lianhuaqingwen (LH) capsule in 284 confirmed cases of COVID-19. Recovery was higher for the intervention group (91.5%) as compared to the control group (82.4%). Other findings of this study were faster symptom recover, better rate of improvement in chest tomography and more resolution. However, no differences in the rate of conversion to severe cases or viral assay findings were found.
Another randomized clinical trial carried out by Liu et al.44 included 204 confirmed cases of COVID-19 and found that those treated with Hua Shi Bai Du Granule (Q-14) had shorter recovery, better symptom remission and better chest CT resolution, without statistical significance in the conversion time (COVID-19 negativation).
Ma et al.38 also carried out a randomized clinical trial with 50 COVID-19 patients comparing the use of ReDuNing injection (TCM herbs) as compared to the control group. Intervention group had their symptoms more efficiently reduced after 7 and 10 days, had a shorter median time of resolution, less time to negativation, better imaging and a shorter period of hospitalization (14.8 vs. 18.5 days). These results were in the same direction of another randomized clinical trial, in which the use of ReDuNing injection in a larger sample of 157 COVID-19 patients found that the symptom resolution rate at 14 days was higher in the intervention group [84.4% vs. 60.0%]. Likewise, the resolution of the clinical symptoms, nucleic acid test turning negative, the hospital stay and the time to defervescence were all shorter in the intervention groups as compared to the control group.
Shuanghuanglian oral liquids were also tested among 176 patients with COVID-19 in a randomized clinical trial.32 Results were significantly favorable for the intervention group as compared to the control group regarding negative conversion of SARS-CoV-2 in nucleic acid swab tests (93.4% vs 73.9%) and inflammation resolution in the chest CT. No serious adverse events were detected for both groups.
In other randomized clinical trial,45 59 inpatients with mild or moderate symptoms were enrolled in two groups: Maxingshigan-Weijing Decoction (Intervention group) and a control group. The number of days hospitalized (18 vs 21 days), rate of symptom recovery and the time to recovery of fever, fatigue, coughing and difficulty breathing were significantly shorter in the treatment group as compared to the control group.
The efficacy of Xiyanping herbal injection was also investigated in a clinical trial including 130 COVID-19 patients with mild to moderate symptoms.35 Those receiving Xiyanping injection had a significantly shorter meantime to complete resolution (8.3 vs 11.8 days), as well as reduced time to cough relief, fever resolution and virus clearance.
Finally, Zhou et al. carried out a RCT assessing the efficacy of shenhuang granule in 11 patients with severe/critical COVID-19. Mortality was significantly lower in the intervention group (75.9%) as compared to the control group (38.6%) and no significant increase in adverse events.
Herbal therapies from India and the Middle East
Although most studies included in our review were from TCM, other herbal therapies available in India and in the Middle East were also used and tested for the treatment of COVID-19 in the reviewed articles. Concerning Indian Herbal Medicine, Pawar et al.53 carried out a randomized clinical trial including 140 patients and showed a reduction in days of hospital stay among patients who used oral curcumine and piperine and an improvement in dyspnea, cough, fever, sore throat, and oxygen saturation. Another randomized clinical trial55 including 120 patients, investigated the use of Indian polyherbal formulations like Nilavembu Kudineer (NVK) and Kaba Sura Kudineer (KSK). The intervention group experienced significant reduction in hospital stay time, reduction in viral load of SARS-CoV-2, and the time taken to become symptomatic from asymptomatic as compared to the control group.
Thakar et al.54 found that the application of Ayurveda significantly reduced the duration of general COVID symptoms. A randomized clinical trial56 compared the effect of Kabasura Kudineer (KSK), a poly-herbal Siddha medicine for COVID-19 patients. Authors found a more pronounced reduction in viral load on the seventh day for the intervention group as compared to a control group, without adverse events. Other biochemical parameters had no differences between groups.
Nevertheless, other authors did not find significant differences in biochemical parameters (i.e., kidney function, inflammatory markers, blood cell count, electrolytes) or in the decrease of viral load between both groups.
There was also another study assessing herbal therapies in the Middle East (i.e., Saudi Arabia). Koshak et al.57 conducted a randomized clinical trial and found that, among 173 COVID-19 patients, those using Nigella sativa (an herbal oil) showed a significantly faster recovery (62% in the intervention versus 36% in the control group) and a greater reduction of symptoms such as chills, runny nose, and loss of appetite, as well as a reduction in hospitalization stay.
Other non-pharmacological non-herbal therapies
Certain studies explored the usefulness of other non-herbal therapies that could be combined to the usual treatment against COVID-19, such as: comprehensive nursing interventions58; Omega-3 nutritional supplementation59; and guided imagery techniques.60
Chu & Zang et al.58 combined prescribed drugs with personalized care to address the psychological, physiological, spiritual, and cultural domains of each patient. The application of this comprehensive therapy reduced the hospital stay of patients in the intervention (16.4 days) as compared to the control group (24.4 days) and reduced the number of admissions to the ICU. In addition, the negative results in chest CT occurred earlier in the intervention patients than in the control group (13.0 vs. 16.7), and survival was also higher (97.3% vs 80.6%).
Concerning oral supplements, a clinical trial by Doaei et al.59 carried out in 128 ICU patients showed that those treated with Omega-3 acid supplementation for two weeks had a higher survival rate (21%) as compared to the control group (3%). Furthermore, these patients improved their blood parameters in terms of pH and bicarbonate, thus resolving the metabolic acidosis caused by COVID-19 pneumonia, along with an improvement in renal parameters of creatinine and urea.
Lastly, Parizad et al.60 used the so-called guided visualization technique focused on the imagination of pleasant events and mental images instead of stressful situations. In their randomized clinical trial, among 110 patients affected by coronavirus, those receiving the intervention experienced a significantly reduction in anxiety (38.2 vs 47.2) and pain intensity (37.4 vs 43.4) as compared to the control group. Other parameters such as heart rate, systolic blood pressure and oxygen saturation were also different between groups. In contrast, they have not found evidence of positive effects on the body temperature, respiratory rate, and diastolic blood pressure.
More details of the results are shown as Table 2.
Discussion
This review shows that the use of CAM for COVID-19 was mainly directed towards the treatment of general symptoms of coronavirus, pneumonia resolution, PCR negativation and reduction of mortality rates. However, other outcomes were also assessed such as reduction of mechanical ventilation, hospitalization days, ICU admissions and mental health problems of patients who suffered COVID-19 infection.
Several CAM therapies have been used in the studies included in our review, which is supported by the previous pre-COVID literature, where a great number of CAM were applied.61 As an example, prior to COVID-19, during the Ebola health emergency that took place in 2014–2016, patients made a great use of CAM, including self-medication and traditional healers. The most common CAM used at that time was biological-based therapies (mainly herbal medicine), followed by mind and body therapies (prayer/spirituality, massage).62 The same pattern was also observed during COVID-19 pandemic, where patients reported using herbal products and acupuncture to alleviate their symptoms.63 , 64 Another parallel between Ebola and COVID-19 is the fact that there was a lack of solid evidence-based treatments at the initial phase of the epidemic,62 which resulted in the search for non-pharmacological therapies.
CAM use is widespread all over the world and the reasons for choosing them are several. In the particular case of the COVID-19 pandemic, the use of CAM was also motivated by the distrust to get accurate information, beliefs in conspiracy theories, and endorsement substances that are not part of conventional medicine.65
Nevertheless, there are other reasons that make patients search for CAM. For instance, dissatisfaction with medical therapies tends to intensify the use of CAM. Salamonsen carried out a study in Norway and reported that patients with shorter relationship with their doctors tend to use more CAM.66 Likewise, patients reporting unmet medical needs tend to use more CAM as well.67 Another key element in the use of CAMs is the economic cost since CAM therapies tend to be cheaper than the pharmacological treatments.67 , 68
Another important finding of the present review is regarding the effectiveness of these interventions. It is important to highlight that the studies are very diverse and preliminary. In general, most studies have shown promising results for both physical and psychological outcomes of COVID-19. Nevertheless, these findings were based mostly on quasi-experimental studies and a few clinical trials, and this should be considered when interpreting the possible role of CAM on different outcomes. Future high evidence studies are needed in this area.
Finally, another important issue is that most included studies were from Asian contexts. This could be justified by the fact that the beginning of the pandemic started in Asia and the rapid response of these countries and their openness to CAM may have interfered in these findings. Future studies should be conducted in other continents such as Europe and America in order to improve generalizability of data.
This systematic review has some limitations that should be considered. First, his is a limited example of CAM therapies over all existing and practiced throughout the world. Second, the heterogeneity of interventions and outcomes makes it difficult to generalize the overall effectiveness of CAMs, warranting further studies on this topic or the focus on specific therapies to perform meta-analysis. Third, this study cannot specify all the information on CAMs for the treatment of coronavirus, given that the health problems associated with covid have been changing during the waves of the pandemic and new treatments have been tested to improve health care.
Conclusions
In conclusion, there is a growing literature on the use of CAM for COVID-19. Most studies have shown positive findings, particularly for the use of TCM, other herbal therapies and acupuncture. Nevertheless, most studies were carried out in Asia and relied on quasi-experimental designs. More robust clinical trials are needed in order to generate better evidence in this area.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
All relevant data that supports the findings of this study are within the manuscript.
Authors' contributions
All authors contributed equally to the development of this study.
Ethics approval and consent to participate and consent for publication
Not applicable.
Author agreement
All authors have seen and approved the final version of the manuscript being submitted. They warrant that the article is the authors' original work, hasn't received prior publication and isn't under consideration for publication elsewhere.
References
- 1.Berekaa M.M. Elite edition. Vol. 13. NLM (Medline); 2021. Insights into the COVID-19 pandemic: origin, pathogenesis, diagnosis, and therapeutic interventions; pp. 117–139. (Frontiers in Bioscience). [DOI] [PubMed] [Google Scholar]
- 2.World Health Organisation (WHO). COVID-19 situation report as of 11th march 2020. (n.d.) Retrieved from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10.
- 3.Chakraborty C., Sharma A.R., Sharma G., Bhattacharya M., Lee S.S. SARS-CoV-2 causing pneumonia-associated respiratory disorder (COVID-19): diagnostic and proposed therapeutic options. Eur Rev Med Pharmacol Sci. 2020;24(7):4016–4026. doi: 10.26355/eurrev_202004_20871. [DOI] [PubMed] [Google Scholar]
- 4.Díaz E., Amézaga Menéndez R., Vidal Cortés P., et al. Pharmacological treatment of COVID-19: narrative review of the working group in infectious diseases and sepsis (GTEIS) and the working groups in transfusions and blood products (GTTH) Med Intens. 2020 doi: 10.1016/j.medin.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dragojevic Simic V., Miljkovic M., Stamenkovic D., et al. An Overview of antiviral strategies for coronavirus 2 (SARS-CoV-2) infection with special reference to antimalarial drugs chloroquine and hydroxychloroquine. Int J Clin Pract. 2020 doi: 10.1111/ijcp.13825. [DOI] [PubMed] [Google Scholar]
- 6.Gavriatopoulou M., Ntanasis-Stathopoulos I., Korompoki E., et al. Emerging treatment strategies for COVID-19 infection. Clin Exp Med. 2021;21:167–179. doi: 10.1007/s10238-020-00671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X., Liu C., Liu G., Luo W., Xia N. COVID-19: progress in diagnostics, therapy and vaccination. Theranostics. 2020;10(17):7821–7835. doi: 10.7150/thno.47987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asselah T., Durantel D., Pasmant E., Lau G., Schinazi R.F. COVID-19: discovery, diagnostics and drug development. J Hepatol. 2021;74(1):168–184. doi: 10.1016/j.jhep.2020.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balkhair A., Al-Zakwani I., Al Busaidi M., et al. Anakinra in hospitalized patients with severe COVID-19 pneumonia requiring oxygen therapy: results of a prospective, open-label, interventional study. Int J Infect Dis. 2021;103:288–296. doi: 10.1016/j.ijid.2020.11.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franzetti M., Forastieri A., Borsa N., et al. IL-1 receptor antagonist anakinra in the treatment of COVID-19 acute respiratory distress syndrome: a retrospective, observational study. J Immunol. 2021 doi: 10.4049/jimmunol.2001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasin L., Cavalli G., Navalesi P., et al. Anakinra For Patients With COVID-19: a meta-analysis of non-randomized cohort studies. Eur J Intern Med. 2021 doi: 10.1016/j.ejim.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO Solidarity Trial Consortium. Pan H., Peto R., Henao-Restrepo A.M., et al. Repurposed antiviral drugs for Covid-19 - interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2mRNA Covid- 19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO (2021). COVID vaccines: widening inequality and millions vulnerable. https://news.un.org/en/story/2021/09/1100192.
- 16.Kreps S.E., Goldfarb J.L., Brownstein J.S., Kriner D.L. The Relationship between US Adults’ Misconceptions about COVID-19 Vaccines and Vaccination Preferences. Vaccines (Basel) 2021;9(8):901. doi: 10.3390/vaccines9080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fokunang C.N., Ndikum V., Tabi O.Y., et al. Traditional medicine: past, present and future research and development prospects and integration in the National Health System of Cameroon. African journal of traditional, complementary, and alternative medicines. AJTCAM. 2011;8(3):284–295. doi: 10.4314/ajtcam.v8i3.65276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Sousa I.M.C., Hortale V.A., Bodstein R.C.de A. Traditional complementary and integrative medicine: challenges in constructing an evaluation model of care. Cienc Saude Colet. 2018;23(10):3403–3412. doi: 10.1590/1413-812320182310.23792016. [DOI] [PubMed] [Google Scholar]
- 19.Xiong X., Wang P., Su K., Cho W.C., Xing Y. Vol. 160. 2020. Chinese herbal medicine for coronavirus disease 2019: a systematic review and meta-analysis. (Pharmacological Research). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo H., Tang Q., Ling Shang Y., et al. Can chinese medicine be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chin J Integr Med. 2020;26(4):243–250. doi: 10.1007/s11655-020-3192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He W., Shi X.S., Zhang Z.Y., et al. Discussion on the effect pathways of preventing and treating coronavirus disease 2019 by acupuncture and moxibustion from the regulation of immune inflammatory response. Zhong guo Zhen Jiu. 2020;40(8):799–802. doi: 10.13703/j.0255-2930.20200305-0001. [DOI] [PubMed] [Google Scholar]
- 22.Góngora Gómez O., Riverón Carralero W.J. La Medicina Tradicional China en el tratamiento de la COVID-19. Rev Int Acupunt. 2020;14(3):123–124. doi: 10.1016/j.acu.2020.07.001. [DOI] [Google Scholar]
- 23.Yang Y., Islam M.S., Wang J., Li Y., Chen Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int J Biol Sci. 2020;16(10):1708–1717. doi: 10.7150/ijbs.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021:372. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braun V., Clarke V., Hayfield N., Terry G. In: Handbook of Research Methods in Health Social Sciences. Liamputtong P, editor. Springer; Singapore: 2019. Thematic analysis; pp. 843–860. editor. [Google Scholar]
- 26.Von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P., STROBE Initiative The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 27.Boutron I., Moher D., Altman D.G., Schulz K.F., Ravaud P., CONSORT Group Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148(4):295–309. doi: 10.7326/0003-4819-148-4-200802190-00008. [DOI] [PubMed] [Google Scholar]
- 28.An B. Yuan Y.-.W., Wang J.-.C., Wang C., Liu T.-.T., Song S., Liu H.-.Q. Clinical characteristics and impacts of traditional Chinese medicine treatment on the convalescents of COVID-19. Int J Med Sci. 2021;18(3):646–651. doi: 10.7150/ijms.52664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo H., Zheng J., Huang G., et al. Xuebijing injection in the treatment of COVID-19: a retrospective case-control study. Ann Palliat Med. 2020;9(5):3235–3248. doi: 10.21037/apm-20-1478. [DOI] [PubMed] [Google Scholar]
- 30.Hu H., K.Wang L.Wang, Du Y., et al. He-Jie-Shen-Shi decoction as an adjuvant therapy on severe coronavirus disease 2019: a retrospective cohort and potential mechanistic study. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.700498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu L., Shi F., Tu P., C.Chen M.Zhang, Li X., Li C. Arbidol combined with the Chinese medicine Lianhuaqingwen capsule versus arbidol alone in the treatment of COVID-19. Medicine (Baltimore) 2021;100(4) doi: 10.1097/MD.0000000000024475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ni L., Wen Z., Hu X., et al. Effects of Shuanghuanglian oral liquids on patients with COVID-19: a randomized, open-label, parallel-controlled, multicenter clinical trial. Front Med. 2021;15(5):704–717. doi: 10.1007/s11684-021-0853-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu K., Guan W.J., Bi Y., et al. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomed: Int J Phytother Phytopharmacol. 2021;85 doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y., Lu C., Li H., et al. Efficacy and safety assessment of severe COVID-19 patients with Chinese medicine: a retrospective case series study at early stage of the COVID-19 epidemic in Wuhan, China. J Ethnopharmacol. 2021;277 doi: 10.1016/j.jep.2021.113888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X., Lv L., Zhou Y., et al. Efficacy and safety of Xiyanping injection in the treatment of COVID-19: a multicenter, prospective, open-label and randomized controlled trial. Phytother Res. 2021;35(8):4401–4410. doi: 10.1002/ptr.7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X., Xue Y., Chen X., et al. Effects of Tanreqing Capsule on the negative conversion time of nucleic acid in patients with COVID-19: a retrospective cohort study. J Integr Med. 2021;19(1):36–41. doi: 10.1016/j.joim.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J., Lin S., Niu C., Xiao Q. Clinical evaluation of Shufeng Jiedu capsules combined with umifenovir (Arbidol) in the treatment of common-type COVID-19: a retrospective study. Expert Rev Respir Med. 2021;15(2):257–265. doi: 10.1080/17476348.2020.1822741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma Q., Xie Y., Z.Wang B.Lei, et al. Efficacy and safety of ReDuNing injection as a treatment for COVID-19 and its inhibitory effect against SARS-CoV-2. J Ethnopharmacol. 2021;279 doi: 10.1016/j.jep.2021.114367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi N., L.Guo B.Liu, Bian Y., et al. Efficacy and safety of Chinese herbal medicine versus Lopinavir-Ritonavir in adult patients with coronavirus disease 2019: a non-randomized controlled trial. Phytomedicine. 2021;81 doi: 10.1016/j.phymed.2020.153367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu X., Zhang J., Zheng W., et al. Efficacy and safety of Reduning injection in the treatment of COVID-19: a randomized, multicenter clinical study. Ann Palliat Med. 2021;10(5):5146. doi: 10.21037/apm-20-2121. [DOI] [PubMed] [Google Scholar]
- 41.Liu J., Jiang Y., Liu Y., et al. Yindan Jiedu Granules, a traditional Chinese medicinal formulation, as a potential treatment for coronavirus disease 2019. Front Pharmacol. 2021;11 doi: 10.3389/fphar.2020.634266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y., Liu Y., Lv Q., et al. Effect and safety of Chinese herbal medicine granules in patients with severe coronavirus disease 2019 in Wuhan, China: a retrospective, single-center study with propensity score matching. Phytomedicine. 2021;85 doi: 10.1016/j.phymed.2020.153404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang H.-.T., Huang M.-.X., Liu X., et al. Evaluation of the adjuvant efficacy of natural herbal medicine on COVID-19: a retrospective matched case-control study. Am J Chin Med (Gard City N Y) 2020;48(4):779–792. doi: 10.1142/S0192415X20500391. [DOI] [PubMed] [Google Scholar]
- 44.Liu J., Yang W., Liu Y., et al. Combination of Hua Shi Bai Du granule (Q-14) and standard care in the treatment of patients with coronavirus disease 2019 (COVID-19): a single-center, open-label, randomized controlled trial. Phytomedicine. 2021;91 doi: 10.1016/j.phymed.2021.153671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeng C., Yuan Z., Zhu J., et al. Therapeutic effects of traditional Chinese medicine (Maxingshigan-Weijing Decoction) on COVID-19: an open-label randomized controlled trial. Integrative Medicine Research., 2021;10 doi: 10.1016/j.imr.2021.100782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li L., Gou C.-.Y., Li X.-.M., et al. Effects of Chinese medicine on symptoms, syndrome evolution, and lung inflammation absorption in COVID-19 convalescent patients during 84-day follow-up after hospital discharge: a prospective cohort and nested case-control study. Chin J Integr Med. 2021;27(4):245–251. doi: 10.1007/s11655-021-3328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou S., Feng J., Xie Q., et al. Traditional Chinese medicine shenhuang granule in patients with severe/critical COVID-19: a randomized controlled multicenter trial. Phytomedicine. 2021;89 doi: 10.1016/j.phymed.2021.153612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen P., Li J., Tu S., et al. Positive effects of Lianhuaqingwen granules in COVID-19 patients: a retrospective study of 248 cases. Journal of Ethnopharmacology., 2021;278 doi: 10.1016/j.jep.2021.114220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feng J., Fang B., Zhou D., et al. Clinical effect of traditional Chinese medicine Shenhuang granule in critically ill patients with COVID-19: a single-centered, retrospective, observational study. J. Microbiol. Biotechnol. 2021;31(3):380–386. doi: 10.4014/jmb.2009.09029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Z., Du S., Shao F., et al. Efficacy of Qingfei Paidu Decoction on Patients with COVID-19 Pneumonia in Wuhan, China: a Propensity Score Matching Study. Evid-Based Complement Altern Med. 2021 doi: 10.1155/2021/4303380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang L., Zheng X., Bai X., et al. Association between use of Qingfei Paidu Tang and mortality in hospitalized patients with COVID-19: a national retrospective registry study. Phytomedicine. 2021;85 doi: 10.1016/j.phymed.2021.153531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tian J., Yan S., Wang H., et al. Hanshiyi Formula, a medicine for Sars-CoV2 infection in China, reduced the proportion of mild and moderate COVID-19 patients turning to severe status: a cohort study. Pharmacol Res. 2020;161 doi: 10.1016/j.phrs.2020.105127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pawar K.S., Mastud R.N., Pawar S.K., et al. Oral curcumin with piperine as adjuvant therapy for the treatment of COVID-19: a randomized clinical trial. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.669362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thakar A., Panara K., Patel F., et al. Add-on ayurveda treatment for early stage COVID-19: a single center retrospective cohort study from Gujarat, India. J Evid-Based Integr Med. 2021;26 doi: 10.1177/2515690X211020685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Srivastava A., Rengaraju M., Srivastava S., et al. Efficacy of two siddha polyherbal decoctions, Nilavembu Kudineer and Kaba Sura Kudineer, along with standard allopathy treatment in the management of mild to moderate symptomatic COVID-19 patients—A double-blind, placebo-controlled, clinical trial. Trials. 2021;22(1) doi: 10.1186/s13063-021-05478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Natarajan S., Anbarasi C., Sathiyarajeswaran P., et al. Kabasura Kudineer (KSK), a poly-herbal Siddha medicine, reduced SARS-CoV-2 viral load in asymptomatic COVID-19 individuals as compared to vitamin C and zinc supplementation: findings from a prospective, exploratory, open-labeled, comparative, randomized controlled trial, Tamil Nadu, India. Trials. 2021;22(1):623. doi: 10.1186/s13063-021-05583-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koshak A.E., Koshak E.A., Mobeireek A.F., et al. Nigella sativa for the treatment of COVID-19: an open-label randomized controlled clinical trial. Complement Ther Med. 2021;61 doi: 10.1016/j.ctim.2021.102769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chu L., Zhang &.Y. A study on nursing effect of integrated traditional Chinese and Western medicine management mode on COVID-19. Japan Journal of Nursing Science: JJNS. 2021 doi: 10.1111/jjns.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doaei S., Gholami S., Rastgoo S., et al. The effect of omega-3 fatty acid supplementation on clinical and biochemical parameters of critically ill patients with COVID-19: a randomized clinical trial. J Transl Med. 2021;19(1):128. doi: 10.1186/s12967-021-02795-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parizad N., Goli R., Faraji N., et al. Effect of guided imagery on anxiety, muscle pain, and vital signs in patients with COVID-19: a randomized controlled trial. Complement Ther Clin Pract. 2021;43 doi: 10.1016/j.ctcp.2021.101335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berretta M., Rinaldi L., Taibi R., et al. Physician attitudes and perceptions of complementary and alternative medicine (CAM): a multicentre Italian study. Front Oncol. 2020;10:594. doi: 10.3389/fonc.2020.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bai James P., Wardle J., Steel A., Adams J. American Public Health Association 2019 Annual Meeting and Expo (Philadelphia, United States, 02/11/2019 - 06/11/2019) 2019. Modes of healthcare seeking behaviour and pattern of traditional, complementary and alternative medicine use among Ebola survivors in Sierra Leone (Conference Presentation) [Google Scholar]
- 63.Aljawadi M.H., Khoja A.T., AlOtaibi A.D., et al. The utilization of complementary and alternative medicine among saudi older adults: a population-based study. Evid-Based Complement Altern Med. 2020 doi: 10.1155/2020/4357194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoffman J.L. Everything old is new again: a review of current complementary and alternative medicine trends. Holist Nurs Pract. 2021;35(6):300–305. doi: 10.1097/HNP.0000000000000481. [DOI] [PubMed] [Google Scholar]
- 65.Soveri A., Karlsson L.C., Antfolk J., et al. Unwillingness to engage in behaviors that protect against COVID-19: the role of conspiracy beliefs, trust, and endorsement of complementary and alternative medicine. BMC Public Health. 2021;21:684. doi: 10.1186/s12889-021-10643-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salamonsen A. Doctor-patient communication and cancer patients' choice of alternative therapies as supplement or alternative to conventional care. Scand J Caring Sci. 2013;27:70–76. doi: 10.1111/j.1471-6712.2012.01002.x. [DOI] [PubMed] [Google Scholar]
- 67.Fjær E.L., Landet E.R., McNamara C.L., et al. The use of complementary and alternative medicine (CAM) in Europe. BMC Complement Med Ther. 2020;20:108. doi: 10.1186/s12906-020-02903-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kemppainen L.M., Kemppainen T.T., Reippainen J.A., Salmenniemi S.T., Vuolanto P.H. Use of complementary and alternative medicine in Europe: health-related and sociodemographic determinants. Scand J Public Health. 2018;46(4):448–455. doi: 10.1177/1403494817733869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data that supports the findings of this study are within the manuscript.