Abstract
General access to highly valuable seven-membered rings via Büchner-type reaction remains a formidable challenge. Here we report a Cu-catalyzed intermolecular oxidation of alkynes using N-oxides as oxidants, which enables expedient preparation of valuable benzo[6,7]azepino[2,3-b]quinolines and pyridine-based diones. Importantly, in contrast to the well-established gold-catalyzed intermolecular alkyne oxidation, the dissociated pyridine or quinoline partner could be further utilized to construct N-heterocycles in this system and the reaction most likely proceeds by a Büchner-type ring expansion pathway. A mechanistic rationale for this cascade cyclization is supported by DFT calculations.
Subject terms: Synthetic chemistry methodology, Reaction mechanisms, Organocatalysis, Homogeneous catalysis
Organic molecules containing seven-membered rings are important structural motifs for drug candidates, but the construction of seven-membered rings can be challenging through traditional cyclization pathways. Here, the authors report a copper-catalyzed alkyne oxidation and Büchner-type ring-expansion to generate benzo[6,7]azepino[2,3-b]quinolines and pyridine-based diones.
Introduction
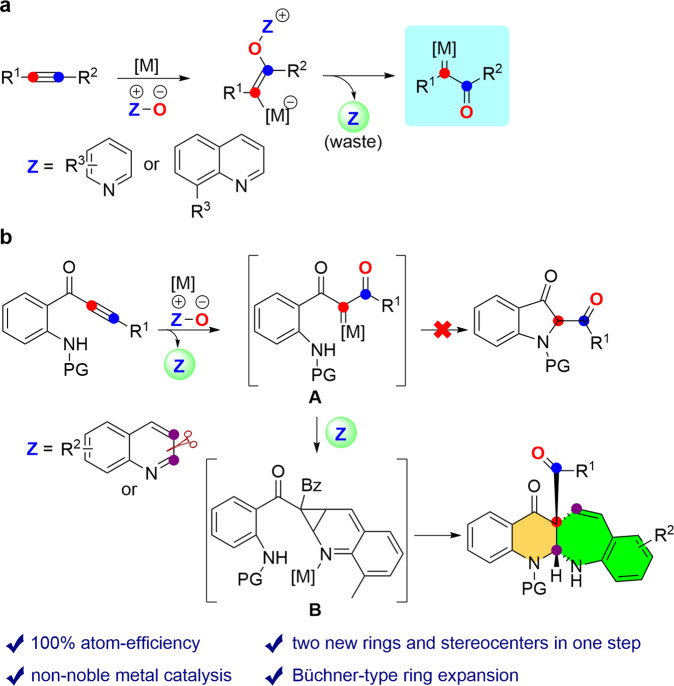
Medium-sized ring-containing organic molecules, especially the seven-membered rings, are important structural motifs that are found in drug candidates as well as in bioactive molecules1–7. However, due to entropic effects and transannular interactions, such frameworks are regarded as difficult structures to access8,9. Compared to the synthesis of five- and six-membered rings, the construction of seven-membered rings can be more challenging through traditional cyclization pathways. Thus, the development of an efficient method to build these seven-membered rings has attracted a significant amount of research attention. Among the numerous methods developed so far, the Büchner-type ring-expansion reaction which has become an effective method for the preparation of seven-membered rings has attracted much attention during the last decade10–14. Traditionally, the Büchner reaction is triggered by the cyclopropanation of the benzene ring to give a norcaradiene, and then the electrocyclic ring opening provides a cycloheptatriene. However, this strategy is hindered by the nature of these α-diazo ketone precursors, which are hazardous, not easily accessible, and potentially explosive. Consequently, the development of catalytic approaches is highly desirable, especially from readily and generally available precursors.
Recently, gold-catalyzed intermolecular alkyne oxidation presumably involving α-oxo gold carbenes has burgeoned, as it avoids the use of difficult and hazardous diazo compounds15–26.
In 2010, Zhang et al.27 disclosed an elegant protocol for the gold-catalyzed intermolecular oxidation of alkynes via a reactive α-oxo gold carbene intermediate (Fig. 1). In addition, the Tang group described that rhodium could also catalyze such an intermolecular alkyne oxidation28. Following this notion, numerous efficient synthetic methods have also been disclosed by Hashmi29,30, Liu31–34, Ye35,36 and others based on this strategy, affording functionalized heterocycles37–49. Despite these great successes, this intermolecular pathway is evidently not atom-economical due to the fact that the procedure generates a stoichiometric amount of pyridines or quinolines, as waste, which may coordinate and poison metal catalysts. Furthermore, a noble-metal catalyst usually is required, and may severely hamper the practical application of this strategy owing to the high cost and toxicity of the catalyst. In our ongoing program of expanding copper catalysis into alkyne transformation50–56, we develop a copper-catalyzed alkyne oxidation/Büchner-type ring-expansion sequence, leading to the benzo[6,7]azepino[2,3-b]quinolines and pyridine-based diones. In particular, the pyridine or quinoline partner could be further utilized to construct N-heterocycles in such an oxidative copper catalysis. Most importantly, mechanistic studies and theoretical calculations demonstrate that the reaction presumably proceeds by a Büchner-type pathway, which is distinctively different from the related gold-catalyzed oxidative cyclization. General access to highly valuable seven-membered rings via Büchner-type reaction remains a formidable challenge. Herein, we describe a copper-catalyzed alkyne oxidation/Büchner-type ring-expansion sequence, thus providing practical access to synthetically useful fused seven-membered ring cyclic compounds. Cyclopropanations of heteroarenes are shown in an intermolecular Büchner-type reaction, while circumventing the use of hazardous diazo carbonyl substrates.
Fig. 1. Typical ways for the generation of α-oxo metal carbenes.
a Previous work. b Our initial design. M metal.
Results and discussion
Optimization of the reaction conditions
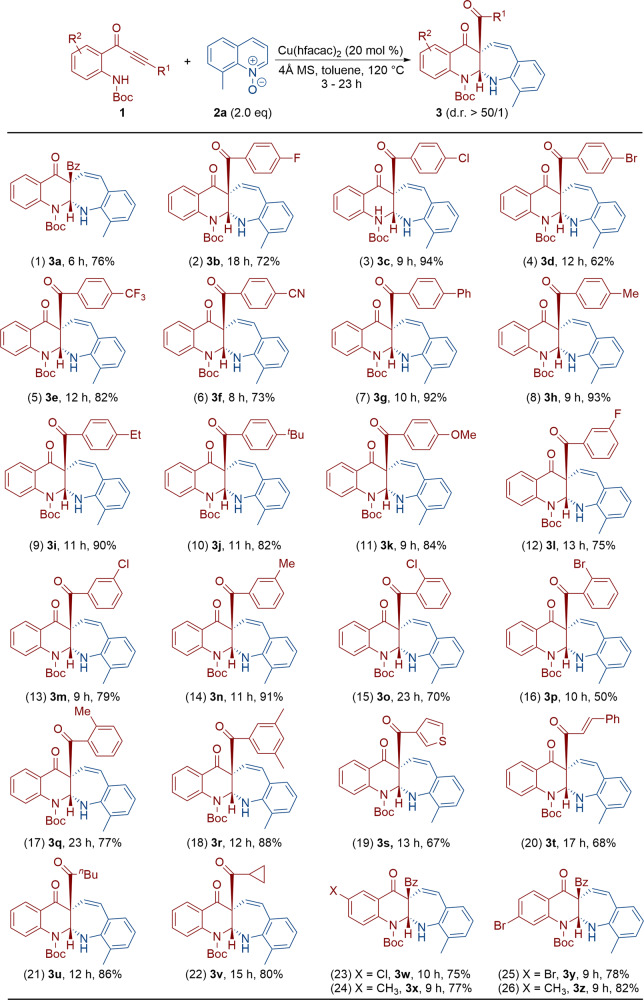
At the outset, alkynone 1a and 8-methylquinoline N-oxide 2a were chosen as model substrates and a range of experiments were executed in order to authenticate our opinion. As documented in Table 1, our initial examination focused on the reaction of the alkynone 1a with 8-methylquinoline N-oxide 2a in DCE at 80 °C in the presence of a copper catalyst. To our pleasure, the expected benzo[6,7]azepino[2,3-b]quinoline 3a was certainly formed in 23% yield, albeit with a lower yield (Table 1, entry 1). The molecular structure of 3a was further confirmed by X-ray diffraction57. Subsequent other copper catalyst screenings indicated that the Cu(hfacac)2 performed obviously better (entries 2–6). Other Lewis acids, including Zn(OTf)2, Y(OTf)3 and Sc(OTf)3, failed to further improve the reaction efficiency (entries 7–9). In addition, the desired 3a was detected in 43% yield when the solvent was changed from DCE to toluene (entry 10). Raising the reaction temperature to 120 °C improved the product yield considerably to 58% (entries 11–12). Doubling of catalyst loading led to an even better yield, affording 3a in 69% yield (entry 13). It should be mentioned that the reaction failed to give even a trace of 3a in the absence of the catalyst (entry 14). Finally, the addition of 4 Å MS led to a slight increase in the yield, forming 3a in 76% yield (entry 15).
Table 1.
Optimization of reaction conditionsa.
| Entry | Cat. (x mol %) | Conditions | Yield (%)b |
|---|---|---|---|
| 1 | Cu(CH3CN)4PF6 (10) | DCE, 80 °C, 55 h | 23 |
| 2 | Cu(CH3CN)4BF4 (10) | DCE, 80 °C, 60 h | 19 |
| 3 | CuOTf (10) | DCE, 80 °C, 60 h | 26 |
| 4 | Cu(PPh3)3Br (10) | DCE, 80 °C, 72 h | <1 |
| 5 | Cu(OTf)2 (10) | DCE, 80 °C, 62 h | 25 |
| 6 | Cu(hfacac)2 (10) | DCE, 80 °C, 48 h | 37 |
| 7 | Zn(OTf)2 (10) | DCE, 80 °C, 29 h | <1 |
| 8 | Y(OTf)3 (10) | DCE, 80 °C, 75 h | 27 |
| 9 | Sc(OTf)3 (10) | DCE, 80 °C, 80 h | 18 |
| 10 | Cu(hfacac)2 (10) | Toluene, 80 °C, 46 h | 43 |
| 11 | Cu(hfacac)2 (10) | Toluene, 100 °C, 20 h | 51 |
| 12 | Cu(hfacac)2 (10) | Toluene, 120 °C, 9 h | 58 |
| 13 | Cu(hfacac)2 (20) | Toluene, 120 °C, 8 h | 69 |
| 14 | None | Toluene, 120 °C, 8 h | <1 |
| 15c | Cu(hfacac)2 (20) | Toluene, 120 °C, 6 h | 76 |
aReaction conditions: 1a (0.1 mmol), 2a (0.2 mmol), catalyst (5–20 mol %), 0.05 M, 80–120 °C, in vials.
bMeasured by 1H NMR using diethyl phthalate as the internal standard.
cUsing 4 Å molecular sieves (20 mg/0.1 mmol) as an additive.
Bold text highlights the optimal reaction condition.
Substrate scope with different alkynones
With the optimized reaction conditions in hand, we then set out to assess the scope of the reaction by varying alkynones 1. The results are presented in Fig. 2. Alkynones with varied aryl groups (Ar = 4-XC6H4, X = F, Cl, Br, CF3, CN, Ph, Me, Et, tBu and OMe) at the C-4 position were first examined, delivering the corresponding benzo[6,7]azepino[2,3-b]quinolines 3a–k in 62–94% yields (entries 1–11). In addition, aryl-substituted alkynones bearing both electron-withdrawing and electron-donating substituents, such as F, Cl and Me, on the phenyl ring were also compatible with this tandem reaction, thus leading to the resulting benzo[6,7]azepino[2,3-b]quinolines 3l–r in 50–91% yields (entries 12–18). In particular, the reaction proceeded smoothly with alkynones bearing sterically hindered ortho substituents. The molecular structure of 3r was further confirmed by X-ray diffraction57. To our satisfaction, thiophene, styryl, nBu and cyclopropyl-substituted alkynones were also suitable substrates for this transformation, affording the expected benzo[6,7]azepino[2,3-b]quinolines 3s–v in 67–86% yields (entries 19–22). For alkynones bearing different R2 substituents, their desired products 3w–z were obtained in 75–82% yields (entries 23–26).
Fig. 2. Reaction scope with different alkynones 1.
Reaction conditions: [1] = 0.05 M; yields are those for the isolated products.
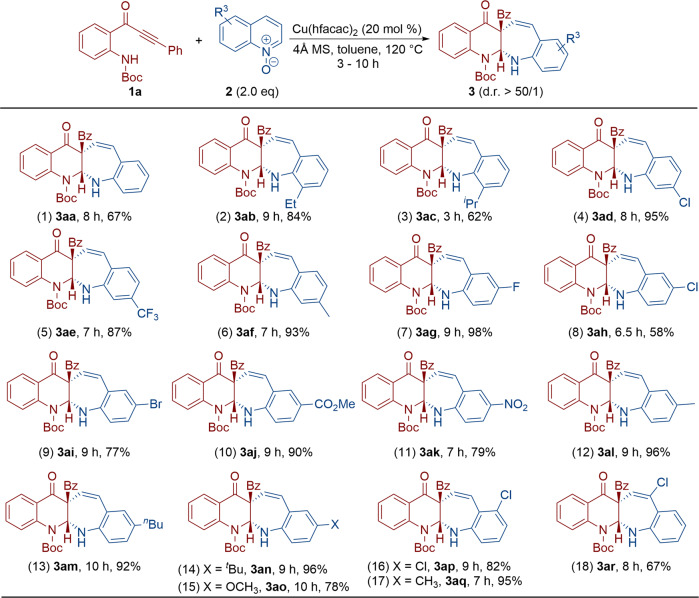
Substrate scope with different quinoline N-oxides
We next extended the reaction to a variety of quinoline N-oxides 2 (Fig. 3). Unsubstituted quinoline N-oxide was first performed, giving the corresponding benzo[6,7]azepino[2,3-b]quinoline 3aa in 67% yield (entry 1). The products 3ab and 3ac were also formed in 84 and 62% yields, respectively, when 8-methylquinoline N-oxide was replaced by 8-ethylquinoline N-oxide or 8-isopropylquinoline N-oxide (entries 2–3). Compounds 2e–g (N-oxide = 7-chloroquinoline N-oxide, 7-trifluoromethylquinoline N-oxide and 7-methylquinoline N-oxide) were converted smoothly into benzo[6,7]azepino[2,3-b]quinolines 3ad–af in 87–95% yields (entries 4–6). This tandem reaction also proceeded for 6-substituted quinoline N-oxides, including substrates with fluoro, chloro, bromo, methyl formate, nitro, methyl, n-butyl, t-butyl and methoxy substituents, and the resulting 3ag–ao were obtained in 58–98% yields (entries 7–15). The related reactions of quinoline N-oxides with additional substitutions at the 5-position and 4-position were either equally or more efficient, affording the expected benzo[6,7]azepino[2,3-b]quinolines 3ap–ar in 67–95% yields (entries 16–18). Accordingly, this approach provided a general and highly efficient strategy for the construction of polycyclic N-heterocycles in organic synthesis. Notable is that the reaction substrates were not only limited to 8-alkylquinoline N-oxides as the oxidants58.
Fig. 3. Reaction scope with different quinoline N-oxides 2.
Reaction conditions: [1] = 0.05 M; yields are those for the isolated products.
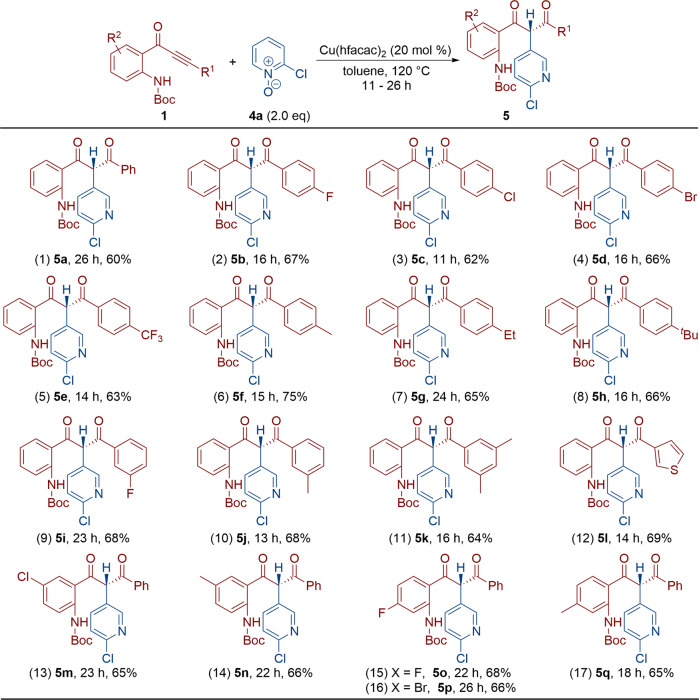
Reaction scope for the formation of pyridine-based diones
Besides quinoline N-oxides, the reaction also proceeded well with pyridine N-oxides to furnish unexpected pyridine-based diones. Thus, the treatment of alkynones 1 with pyridine N-oxide 4a under copper catalysis furnished the resulting pyridine-based diones 5a–q in 60–75% yield (Fig. 4). The molecular structure of 5p was further confirmed by X-ray diffraction57. The reaction presumably involved a copper-catalyzed oxidation-initiated tandem alkyne oxidation/Büchner-type/[1,2]-H shift, and the formation of pyridine-based diones instead of the previous benzo[6,7]azepino[2,3-b]quinolines was attributed to the relatively lower activity (mechanism for the formation of 5a is depicted in Supplementary Information).
Fig. 4. Reaction scope for the formation of pyridine-based diones 5.
Reaction conditions: [1] = 0.05 M; yields are those for the isolated products.
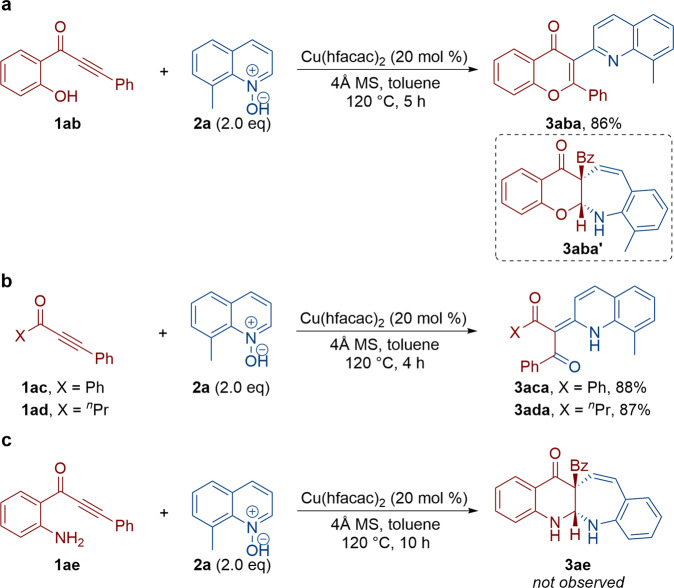
To understand the mechanism of these cyclizations, several control experiments were first conducted (Fig. 5). Our attempts to extend the reaction to hydroxyl-substituted alkynone resulted in the formation of 3-(8-methylquinolin-2-yl)-2-phenyl-4H-chromen-4-one 3aba in 86% yield, and no corresponding azepine compound 3aba’ formation was detected. The molecular structure of 3aba was further confirmed by X-ray diffraction12. Furthermore, when unsubstituted alkynone 1ac and 1ad were subjected to this copper-catalyzed oxidative cyclization, 1,3-diones 3aca and 3ada were generated in 88 and 87% yields, respectively. These results showed that the amino group was crucial for the formation of benzo[6,7]azepino[2,3-b]quinolines. Alkynone without Boc at the amino group was then examined, and no corresponding azepine compound 3ae was obtained, likely due to coordinate and poison copper catalysts.
Fig. 5. Control experiments.
a Hydroxyl-substituted alkynone was used. b Unsubstituted alkynone was used. c Alkynone without Boc at the amino group was used.
Synthetic application
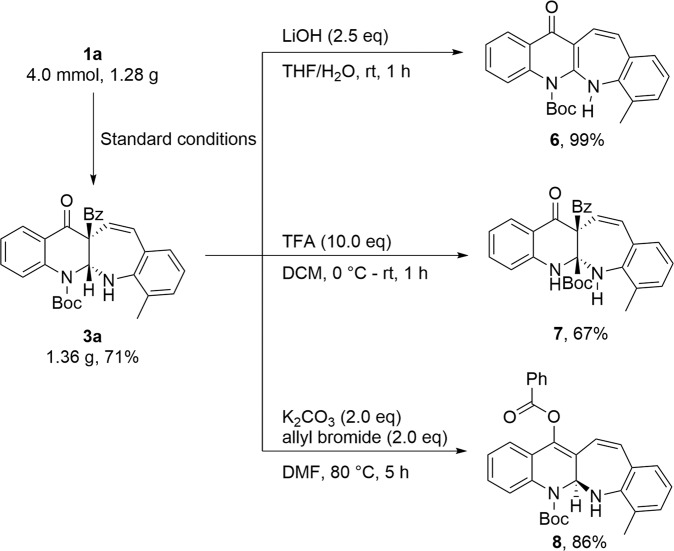
The synthetic utility of the benzo[6,7]azepino[2,3-b]quinolines was examined (Fig. 6). Firstly, 3a was prepared on a gram scale in 71% yield under the optimized reaction conditions. Subsequently, a selective elimination of benzaldehyde of 3a was achieved with LiOH to furnish benzo[6,7]azepino[2,3-b]quinoline derivatives 6 in almost quantitative yield. In addition, 3a could be transformed into compound 7 bearing two contiguous quaternary carbon stereocenters in 67% yield via a 1,2-Boc shift. Furthermore, 3a could be readily converted into compound 8 in 86% yield by a 1,3-benzoyl-migration. The molecular structure of 8 was further confirmed by X-ray diffraction57.
Fig. 6. Synthetic applications.
The synthetic utility of the 3a was examined.
Mechanistic studies
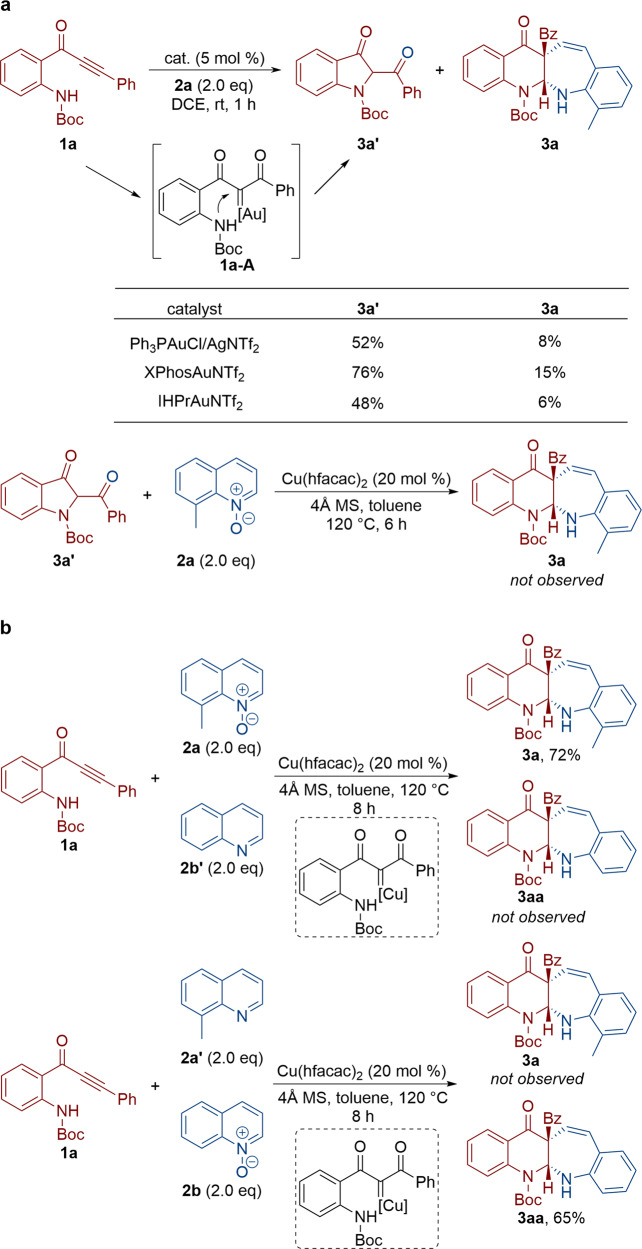
Although a detailed description of mechanistic rationale at present is not possible and deserves further detailed exploration, several control experiments were conducted to gain some further information on potential pathways (Fig. 7). Typical noble-metal catalysts were tested. The direct N-H insertion by the gold carbene in 1a-A was obtained as the main product under gold catalysis conditions. As we considered 3a’ to be possible intermediates in such a tandem sequence, we then subjected 3a’ to the optimal reaction conditions and the formation of 3a was not observed, thus ruling out 3a’ as a potential intermediate for the formation of 3a. Besides, we performed further studies using quinoline as the external nucleophiles. A 1:1 mixture of 8-methylquinoline N-oxide 2a and quinoline 2b’ under the optimized reaction conditions only led to the formation of the corresponding 3a in 72% yield, and no desired 3aa was obtained. Similarly, when quinoline N-oxide 2b and 8-methylquinoline 2a’ was treated under the optimized reaction conditions, only 3aa was obtained in 65% yield. These results indicate that α-oxo copper carbene is not presumably involved in such a copper catalysis.
Fig. 7. Control experiments.
a Typical noble-metal catalysts were tested. b A 1:1 mixture of quinoline N-oxide and quinoline was treated.
Proposed mechanism
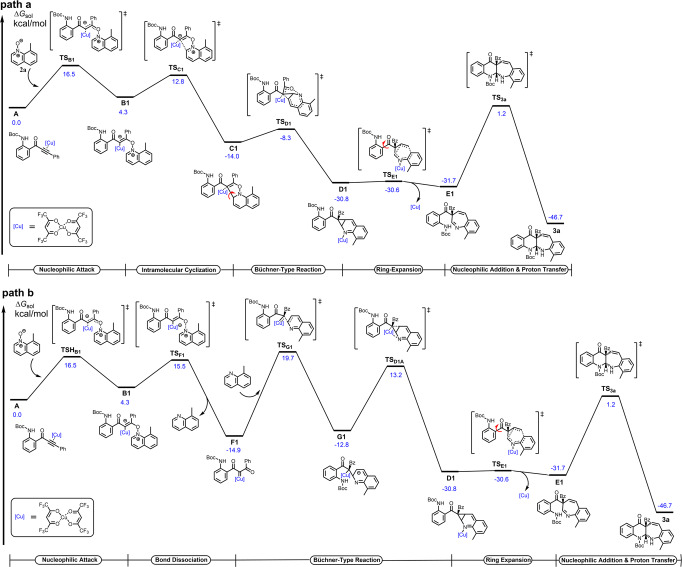
Based on the above experimental observations and density functional theory (DFT) computations (for details, see the Supplementary Information), a possible mechanism to clarify the formation of 3a is documented. As depicted in Fig. 8, there are two plausible mechanisms to rationalize the formation of 3a. It entails an initial copper activation of alkynone 1a in the form of complex A, followed by a nucleophilic attack by 8-methylquinoline N-oxide 2a via the transition state TS-B1 to furnish the vinyl copper intermediate B1 by overcoming a barrier of 16.5 kcal/mol. In path a, the intramolecular cyclization occurs efficiently to form the five-membered-ring intermediate C1, via transition state TS-C1 with an activation energy of 8.5 kcal/mol, which undergoes Büchner-type reaction to deliver the norcaradiene intermediate D1. It should be mentioned that the stabilization of intermediate C1 can be attributed to the coordination of the carbonyl oxygen to the copper atom according to the calculations. Subsequently, an electrocyclic step opens the cyclopropane ring to provide the seven-membered ring intermediate E1 via TS–E1 with a lower activation energy of 0.2 kcal/mol. Going a step further, intramolecular nucleophilic addition of N-Boc to the imine moiety produces the eventual polycyclic N-heterocycle 3a. The whole process is exothermal by 46.7 kcal mol−1 in free energy. In path b, upon N-O bond cleavage, B1 transforms into α-oxo copper carbene intermediate F1 via transition state TS-F1 with a higher activation energy of 11.2 kcal/mol, and thus the formation of the α-oxo copper carbene F1 is unfavorable. Meanwhile, considering the subsequent Büchner-type reaction, via TS-G1 and via TS-D1A, the activation barriers are 34.6 and 26.0 kcal/mol, respectively. Obviously, path a is much favored kinetically over path b. Besides, path a can rationalize our control experiments in Fig. 7 in which α-oxo copper carbene is not presumably involved in such a copper catalysis.
Fig. 8. Plausible reaction mechanism for the formation of 3a.
a α-oxo copper carbene intermediate was not involved. b α-oxo copper carbene intermediate was involved.
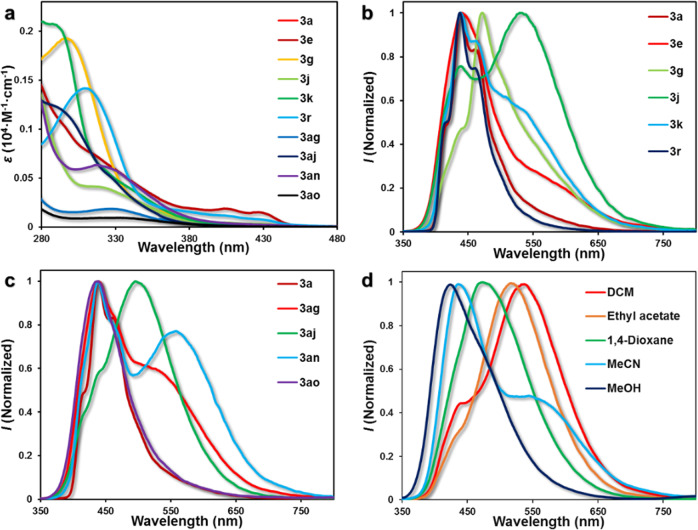
Optical properties
Our next efforts concentrated on the exploration of the optical properties of the obtained benzo [6,7]azepino[2,3-b]quinolines (Fig. 9). According to the impact of substituent on the benzene ring, the absorption and emission maxima of these compounds varied from 287 to 432 nm and from 440 to 557 nm, respectively. The absorption was red-shifted by the presence of an electron-withdrawing substituent as in 3e, and the λmax of 3e was bathochromically shifted by 83 nm compared to 3k. A similar effect was detected for the emission spectra of the 3ag, which showed a longer-wavelength emission band at 536 nm and extended the emission to 750 nm. Furthermore, the fluorescence of 3g displayed a considerable red-shift from a fluorescence maximum wavelength of 440–474 nm by conjugation with an increased benzene ring. In addition, the emission wavelengths were further red-shifted to 536 and 498 nm by the introduction of tBu substituent as in 3j and 3an. These results confirmed that the red-shifting absorption and emission band might be achieved by utilizing the strategy of combining the push–pull design, allowing the facile synthesis of near-infrared polycyclic N-heterocycles.
Fig. 9. Optical properties of 3.
a Absorption spectra of benzo[6,7]azepino[2,3-b]quinolines in DCM (10 μM). b, c Emission spectra of benzo[6,7]azepino[2,3-b]quinolines in DCM at room temperature (10 μM). d Emission spectra of 3r in solvents of different polarities.
Besides, the effect of the solvent environment on their emission properties was also explored with 3r (Fig. 9d). Interestingly, the 3r displayed negative solvatochromism59–61, and the emissions of 3r were blue-shifted from 537 to 423 nm by increasing solvent polarity (the structures of the 3r were optimized and the dipole moment of 3r in the excited and ground states were calculated by Gaussian 09 at the B3LYP/6-31G(d) level). The ground state of 3r was calculated to be slightly more polar (Dipole Moment = 7.28 Debye) than the excited state (Dipole Moment = 6.45 Debye). These results supported the negative solvatochromism of 3r. The solution fluorescence quantum yield (ΦF) was estimated for polycyclic N-heterocycles, as shown in Table 2. The ΦF mainly concentrated between 0.01 and 0.05 in DCM. However, the ΦF of 3r (0.32) was much higher relative to the other compounds, presumably due to the steric effect. The two meta-position methyl groups limited the rotation of the benzene ring, which reduced the intramolecular vibration relaxation and improved the stability of the excited state, thus delivering the higher ΦF62,63.
Table 2.
Photophysical data of benzo [6,7]azepino[2,3-b]quinolinesa.
| Compd | Absorption | Emission | |
|---|---|---|---|
| λmax (nm), ε (104 cm−1M−1) | λmax (nm) | ΦF | |
| 3a | 309 (1.51), 329 (1.02), 403 (0.23), 428 (0.16) | 440, 463 | 0.05 |
| 3e | 314 (0.75), 405 (0.19), 428 (0.14) | 442 | 0.02 |
| 3g | 296 (1.93) | 474 | 0.01 |
| 3j | 318 (0.41), 404 (0.04), 431 (0.03) | 441, 536 | 0.01 |
| 3k | 287 (2.04), 345 (0.35) | 440, 465, 537 | 0.04 |
| 3r | 309 (1.15), 403 (0.12), 432 (0.07) | 438, 463 | 0.32 |
| 3ag | 330 (0.18) | 441, 536 | 0.03 |
| 3aj | 290 (1.20), 328 (0.51) | 441, 498 | 0.03 |
| 3an | 319 (0.63) | 440, 465, 557 | 0.03 |
| 3ao | 332 (0.10) | 441 | 0.02 |
aMeasured in DCM at 1.0 × 10−5 M, RT.
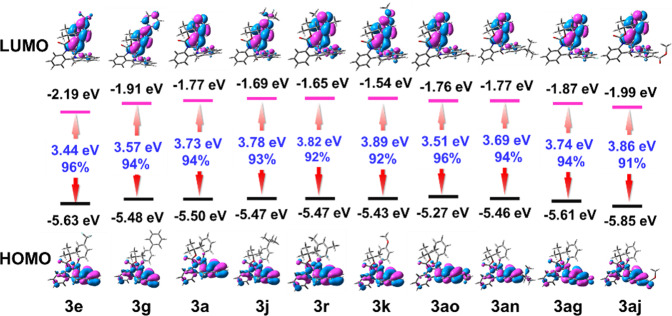
To further explore the structure–property relationship of benzo [6,7]azepino[2,3-b]quinoline derivatives, DFT calculations were performed to obtain the optimized geometries and the frontier orbitals distribution of benzo [6,7]azepino[2,3-b]quinolines64,65. As shown in Fig. 10, due to the non-conjugated and non-planar heterocycle skeleton of benzo [6,7]azepino[2,3-b]quinolines, the Boc and benzoyl groups increased the intramolecular steric hindrance. The HOMO and LUMO levels of these compounds were well separated without significant overlap. The HOMOs of the benzo [6,7]azepino[2,3-b]quinolines mainly distributed on the backbone of the molecule, and the HOMO level and electron cloud distribution could be regulated by introducing different substituents into the backbone. The introduction of electron-donating substituents significantly improved the HOMO levels, such as 3ao (−5.27 eV) to 3aj (−5.85 eV). The LUMOs of benzo [6,7]azepino[2,3-b]quinolines were mainly distributed in the benzoyl groups, and the LUMO levels were regularly decreased by introducing the electron-withdrawing and conjugate groups. These results suggested that the luminescence color and band gap of the compounds could be effectively adjusted, which could have great potential applications in biological imaging and optoelectronic devices.
Fig. 10. Density functional theory calculations.
Molecular orbital and energy levels of benzo [6,7]azepino[2,3-b]quinolines, calculated at the B3LYP/6-31G(d) level.
Conclusions
In conclusion, we have described a copper-catalyzed dearomative annulation between alkynones and quinoline N-oxides, delivering the practical and efficient synthesis of fused polycyclic N-heterocycles. This strategy provides the first example of non-noble-metal catalyzed intermolecular alkyne oxidation via an atom-economical and environmentally friendly pathway where the quinoline partner could be further utilized to construct N-heterocycles instead of the previously reported the departure of a stoichiometric amount of quinolines as waste. Cyclopropanations of heteroarenes are shown in an intermolecular Büchner-type reaction, while circumventing the use of hazardous diazo carbonyl substrates. Of note, mechanistic studies revealed that this copper-catalyzed alkyne oxidation presumably proceeds by a Büchner-type ring-expansion pathway, which is distinctively different from the related gold catalysis. Moreover, such oxidation of alkynes can afford the divergent synthesis of pyridine-based diones with pyridine N-oxides as oxidants. Work to exploit enantioselective variants and acquire a deeper mechanistic understanding is underway.
Methods
Materials
Unless otherwise noted, materials were obtained commercially and used without further purification. All the solvents were treated according to general methods. Flash column chromatography was performed over silica gel (300–400 mesh). See Supplementary methods for experimental details.
General methods
1H NMR spectra were recorded on a Bruker AV-400 spectrometer in chloroform-d3. Chemical shifts are reported in ppm with the internal TMS signal at 0.0 ppm as a standard. The data is being reported as (s = singlet, d = doublet, t = triplet, m = multiplet or unresolved, brs = broad singlet, coupling constant(s) in Hz, integration). 13C NMR spectra were recorded on a Bruker AV-400 spectrometer in chloroform-d3. Chemical shifts are reported in ppm with the internal chloroform signal at 77.0 ppm as a standard. Infrared spectra were recorded on a Nicolet iS 10 spectrometer as thin film and are reported in reciprocal centimeter (cm−1). Mass spectra were recorded with Micromass Q-Exactive Focus mass spectrometer using electron spray ionization. 1H NMR, and 13C NMR are supplied for all compounds: see Supplementary Figs. 1–68. More mechanism studies are supplied: see Supplementary Figs. 69–74. Representative synthetic procedures for the preparation of alkynones are supplied: see Supplementary Fig. 75. General procedure for the synthesis of benzo[6,7]azepino[2,3-b]quinolines 3 is supplied: see Supplementary Fig. 76. General procedure for the synthesis of pyridine-based diones 5 are supplied: see Supplementary Fig. 77. Synthetic applications are supplied: see Supplementary Figs. 78–80. Crystal data are supplied: see Supplementary Tables 1–5. TD-DFT computational data are supplied: see Supplementary Tables 6–15. See Supplementary methods for the characterization data of compounds not listed in this part.
General procedure for the synthesis of benzo[6,7]azepino[2,3-b]quinolines 3
Quinoline N-oxide 2 (0.4 mmol), 4 Å molecular sieves (40 mg) and Cu(hfacac)2 (0.04 mmol, 19.1 mg) were added in this order to the alkynones 1 (0.2 mmol) in toluene (4.0 mL) at room temperature. The reaction mixture was stirred at 120 °C (120 °C, heating mantle temperature) and the progress of the reaction was monitored by TLC. Upon completion, the mixture was then concentrated and the residue was purified by chromatography on silica gel (eluent: petroleum ether/ethyl acetate) to afford the desired product 3.
General procedure for the synthesis of pyridine-based diones 5
2-Chloropyridine N-oxide 4a (0.4 mmol, 51.8 mg) and Cu(hfacac)2 (0.04 mmol, 19.1 mg) were added in this order to the alkynones 1 (0.2 mmol) in toluene (4.0 mL) at room temperature. The reaction mixture was stirred at 120 °C (120 °C, heating mantle temperature) and the progress of the reaction was monitored by TLC. Upon completion, the mixture was then concentrated and the residue was purified by chromatography on silica gel (eluent: petroleum ether/ethyl acetate) to afford the desired product 5.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We are grateful for the financial support from the NNSFC (22001059), the Top-Notch Talents Program of Henan Agricultural University (30500739 and 30501032).
Author contributions
X.-L.J., Q.L., K.-F.W., T.-T.Z., G.M. and X.-H.Z. performed experiments. G.-X.R. revised the manuscript. L.-J.L. and L.-R.H. performed DFT calculations. W.-B.S. conceived and directed the project and wrote the paper. All authors discussed the results and commented on the manuscript.
Peer review
Peer review information
Communications Chemistry thanks Jiaxi Xu and the other, anonymous, reviewers for their contribution to the peer review of this work.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and the Supplementary Information as well as from the authors upon reasonable request. DFT calculations are available in Supplementary Data 1. The compound characterizations are available in Supplementary Data 2. The X-ray crystallographic coordinates for structures 3a, 3r, 3aba, 5p, and 8, reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under CCDC 2122556 (3a, Supplementary Data 3), 2126067 (3r, Supplementary Data 4), 2122557 (3aba, Supplementary Data 5), 2144833 (5p, Supplementary Data 6) and 2122558 (8, Supplementary Data 7), respectively. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Guang-Xin Ru, Email: ruguangxin@126.com.
Lijie Liu, Email: lijieliu@henau.edu.cn.
Lian-Rui Hu, Email: lrhu@chem.ecnu.edu.cn.
Wen-Bo Shen, Email: wenboshen@henau.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s42004-023-00840-6.
References
- 1.Schultz C, et al. Paullones, a series of cyclin-dependent kinase inhibitors: synthesis, evaluation of CDK1/Cyclin B inhibition, and in vitro antitumor activity. J. Med. Chem. 1999;42:2909–2919. doi: 10.1021/jm9900570. [DOI] [PubMed] [Google Scholar]
- 2.Sharma SK, Sharma S, Agarwal PK, Kundu B. Application of 7-endo-trig Pictet–Spengler cyclization to the formation of the benzazepine ring: synthesis of benzazepinoindoles. Eur. J. Org. Chem. 2009;2009:1309–1312. doi: 10.1002/ejoc.200801201. [DOI] [Google Scholar]
- 3.Magolan J, Kerr MA. Expanding the scope of Mn(OAc)3-mediated cyclizations: synthesis of the tetracyclic core of tronocarpine. Org. Lett. 2006;8:4561–4564. doi: 10.1021/ol061698+. [DOI] [PubMed] [Google Scholar]
- 4.Wang L-L, et al. Chalcone-based pyridinium salts and their diastereoselective dearomatization to access bibridged benzoazepines. Org. Lett. 2020;22:873–878. doi: 10.1021/acs.orglett.9b04398. [DOI] [PubMed] [Google Scholar]
- 5.Watson MD, Fechtenkötter A, Müllen K. Big is beautiful−“aromaticity” revisited from the viewpoint of macromolecular and supramolecular benzene chemistry. Chem. Rev. 2001;101:1267–1300. doi: 10.1021/cr990322p. [DOI] [PubMed] [Google Scholar]
- 6.Anthony JE. Functionalized acenes and heteroacenes for organic electronics. Chem. Rev. 2006;106:5028–5048. doi: 10.1021/cr050966z. [DOI] [PubMed] [Google Scholar]
- 7.Georgakilas V, Perman JA, Tucek J, Zboril R. Broad family of carbon nanoallotropes: classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem. Rev. 2015;115:4744–4822. doi: 10.1021/cr500304f. [DOI] [PubMed] [Google Scholar]
- 8.Yet L. Metal-mediated synthesis of medium-sized rings. Chem. Rev. 2000;100:2963–3008. doi: 10.1021/cr990407q. [DOI] [PubMed] [Google Scholar]
- 9.Nubbemeyer U. Synthesis of medium-sized ring lactams. Top. Curr. Chem. 2001;216:125–196. doi: 10.1007/3-540-44726-1_4. [DOI] [Google Scholar]
- 10.Nakayama H, Harada S, Kono M, Nemoto T. Chemoselective asymmetric intramolecular dearomatization of phenols with α-diazoacetamides catalyzed by silver phosphate. J. Am. Chem. Soc. 2017;139:10188–10191. doi: 10.1021/jacs.7b04813. [DOI] [PubMed] [Google Scholar]
- 11.Ji K, Zhang L. Cyclopropanation of benzene rings by oxidatively generated α-oxo gold carbene: one-pot access to tetrahydropyranone-fused cycloheptatrienes from propargyl benzyl ethers. Adv. Synth. Catal. 2018;360:647–651. doi: 10.1002/adsc.201701322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu D, Cao T, Chen K, Zhu S. Rh2(II)-catalyzed enantioselective intramolecular Büchner reaction and aromatic substitution of donor–donor carbenes. Chem. Sci. 2022;13:1992–2000. doi: 10.1039/D1SC05374D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan D-F, et al. Hypervalent iodine promoted the synthesis of cycloheptatrienes and cyclopropanes. Chem. Sci. 2022;13:478–485. doi: 10.1039/D1SC05429E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng Q, et al. Divergent construction of macrocyclic alkynes via catalytic metal carbene C(sp2)–H insertion and the buchner reaction. ACS Catal. 2019;9:10773–10779. doi: 10.1021/acscatal.9b04199. [DOI] [Google Scholar]
- 15.Zheng Z, et al. Homogeneous gold-catalyzed oxidation reactions. Chem. Rev. 2021;121:8979–9038. doi: 10.1021/acs.chemrev.0c00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye L-W, et al. Nitrene transfer and carbene transfer in gold catalysis. Chem. Rev. 2021;121:9039–9112. doi: 10.1021/acs.chemrev.0c00348. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L. A non-diazo approach to α-oxo gold carbenes via gold-catalyzed alkyne oxidation. Acc. Chem. Res. 2014;47:877–888. doi: 10.1021/ar400181x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeom H-S, Shin S. Catalytic access to α-oxo gold carbenes by N-O bond oxidants. Acc. Chem. Res. 2014;47:966–977. doi: 10.1021/ar4001839. [DOI] [PubMed] [Google Scholar]
- 19.Dorel R, Echavarren AM. Gold(I)-catalyzed activation of alkynes for the construction of molecular complexity. Chem. Rev. 2015;115:9028–9072. doi: 10.1021/cr500691k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qian D, Zhang J. Gold-catalyzed cyclopropanation reactions using a carbenoid precursor toolbox. Chem. Soc. Rev. 2015;44:677–698. doi: 10.1039/C4CS00304G. [DOI] [PubMed] [Google Scholar]
- 21.Shen W-B, Tang X-T. Recent advances in catalytic asymmetric intermolecular oxidation of alkynes. Org. Biomol. Chem. 2019;17:7106–7113. doi: 10.1039/C9OB01096C. [DOI] [PubMed] [Google Scholar]
- 22.Ru G-X, et al. Recent progress towards the transition-metal-catalyzed Nazarov cyclization of alkynes via metal carbenes. Org. Biomol. Chem. 2021;19:5274–5283. doi: 10.1039/D1OB00744K. [DOI] [PubMed] [Google Scholar]
- 23.Tang X-T, et al. Recent progress in N-heterocyclic carbene gold-catalyzed reactions of alkynes involving oxidation/amination/cycloaddition. Catalysts. 2020;10:350–359. doi: 10.3390/catal10030350. [DOI] [Google Scholar]
- 24.Huple DB, Ghorpade S, Liu R-S. Recent advances in gold-catalyzed N- and O-functionalizations of alkynes with nitrones, nitroso, nitro and nitroxy species. Adv. Synth. Catal. 2016;358:1348–1367. doi: 10.1002/adsc.201600018. [DOI] [Google Scholar]
- 25.Xiao J, Li X. Gold α-oxo carbenoids in catalysis: catalytic oxygen-atom transfer to alkynes. Angew. Chem. Int. Ed. 2011;50:7226–7236. doi: 10.1002/anie.201100148. [DOI] [PubMed] [Google Scholar]
- 26.Bhunia S, Ghosh P, Patra SR. Gold-catalyzed oxidative alkyne functionalization by N-O/S-O/C-O bond oxidants. Adv. Synth. Catal. 2020;362:3664–3708. doi: 10.1002/adsc.202000274. [DOI] [Google Scholar]
- 27.Ye L, Cui L, Zhang G, Zhang L. Alkynes as equivalents of α-diazo ketones in generating oxo metal carbenes: a gold-catalyzed expedient synthesis of dihydrofuran-3-ones. J. Am. Chem. Soc. 2010;132:3258–3259. doi: 10.1021/ja100041e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu R, et al. Generation of rhodium(I) carbenes from ynamides and their reactions with alkynes and alkenes. J. Am. Chem. Soc. 2013;135:8201–8204. doi: 10.1021/ja4047069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nösel P, et al. 1,6-Carbene transfer: gold-catalyzed oxidative diyne cyclizations. J. Am. Chem. Soc. 2013;135:15662–15666. doi: 10.1021/ja4085385. [DOI] [PubMed] [Google Scholar]
- 30.Wang T, et al. Synthesis of highly substituted 3-formylfurans by a gold(I)-catalyzed oxidation/1,2-alkynyl migration/cyclization cascade. Angew. Chem. Int. Ed. 2014;53:3715–3719. doi: 10.1002/anie.201310146. [DOI] [PubMed] [Google Scholar]
- 31.Vasu D, et al. Gold-catalyzed oxidative cyclization of 1,5-enynes using external oxidants. Angew. Chem. Int. Ed. 2011;50:6911–6914. doi: 10.1002/anie.201102581. [DOI] [PubMed] [Google Scholar]
- 32.Bhunia S, Ghorpade S, Huple DB, Liu R-S. Gold-catalyzed oxidative cyclizations of cis-3-en-1-ynes to form cyclopentenone derivatives. Angew. Chem. Int. Ed. 2012;51:2939–2942. doi: 10.1002/anie.201108027. [DOI] [PubMed] [Google Scholar]
- 33.Ghorpade S, Su M-D, Liu R-S. Gold-catalyzed oxidative cyclizations on 1,4-enynes: evidence for a γ-substituent effect on Wagner-Meerwein rearrangements. Angew. Chem. Int. Ed. 2013;52:4229–4234. doi: 10.1002/anie.201210313. [DOI] [PubMed] [Google Scholar]
- 34.Karad SN, Liu R-S. Gold-catalyzed 1,2-oxoarylations of nitriles with pyridine-derived oxides. Angew. Chem. Int. Ed. 2014;53:5444–5448. doi: 10.1002/anie.201403015. [DOI] [PubMed] [Google Scholar]
- 35.Li L, et al. Generation of gold carbenes in water: efficient intermolecular trapping of the α-oxo gold carbenoids by indoles and anilines. Chem. Sci. 2014;5:4057–4064. doi: 10.1039/C4SC00983E. [DOI] [Google Scholar]
- 36.Shu C, et al. Generation of endocyclic vinyl carbene complexes via gold-catalyzed oxidative cyclization of terminal diynes: toward naphthoquinones and carbazolequinones. ACS Catal. 2019;9:1019–1025. doi: 10.1021/acscatal.8b04455. [DOI] [Google Scholar]
- 37.Hamada N, Yamaguchi A, Inuki S, Oishi S, Ohno H. Gold(I)-catalyzed oxidative cascade cyclization of 1,4-diyn-3-ones for the construction of tropone-fused furan scaffolds. Org. Lett. 2018;20:4401–4405. doi: 10.1021/acs.orglett.8b01524. [DOI] [PubMed] [Google Scholar]
- 38.Qian D, et al. Gold(I)-catalyzed highly diastereo-and enantioselective alkyne oxidation/cyclopropanation of 1,6-enynes. Angew. Chem. Int. Ed. 2014;53:13751–13755. doi: 10.1002/anie.201407717. [DOI] [PubMed] [Google Scholar]
- 39.Wang A, Lu M, Liu Y. Gold-catalyzed oxidative cyclization involving nucleophilic attack to the keto group of α,α′-dioxo gold carbene and 1,2-alkynyl migration: synthesis of furan-3-carboxylates. Org. Lett. 2021;23:6813–6818. doi: 10.1021/acs.orglett.1c02389. [DOI] [PubMed] [Google Scholar]
- 40.Zhao J, et al. Gold-catalyzed oxidative cyclizations of {o-(alkynyl)phenyl propargyl} silyl ether derivatives involving 1,2-enynyl migration: synthesis of functionalized 1H-isochromenes and 2H-pyrans. Org. Lett. 2018;20:5461–5465. doi: 10.1021/acs.orglett.8b02380. [DOI] [PubMed] [Google Scholar]
- 41.Ji K, Liu X, Du B, Yang F, Gao J. Gold-catalyzed selective oxidation of 4-oxahepta-1,6-diynes to 2H-pyran-3(6H)-ones and chromen-3(4H)-ones via β-gold vinyl cation intermediates. Chem. Commun. 2015;51:10318–10321. doi: 10.1039/C5CC02952J. [DOI] [PubMed] [Google Scholar]
- 42.Li J, Xing H-W, Yang F, Chen Z-S, Ji K. Gold(III)-catalyzed regioselective oxidation/cycloisomerization of diynes: an approach to fused Furan derivatives. Org. Lett. 2018;20:4622–4626. doi: 10.1021/acs.orglett.8b01915. [DOI] [PubMed] [Google Scholar]
- 43.Wagh SB, Singh RR, Sahani RL, Liu R-S. Gold-catalyzed oxidative hydrative alkenylations of propargyl aryl thioethers with quinoline N‑oxides involving a 1,3-sulfur migration. Org. Lett. 2019;21:2755–2758. doi: 10.1021/acs.orglett.9b00705. [DOI] [PubMed] [Google Scholar]
- 44.Nan J, et al. CuII-catalyzed coupling with two ynone units by selective triple and sigma C−C and C−H bond cleavages. Org. Lett. 2021;23:1928–1933. doi: 10.1021/acs.orglett.1c00371. [DOI] [PubMed] [Google Scholar]
- 45.Chen Z-S, et al. Metal-free, site-selective addition to ynones: an approach to synthesize substituted quinoline derivatives. Org. Lett. 2016;18:5828–5831. doi: 10.1021/acs.orglett.6b02813. [DOI] [PubMed] [Google Scholar]
- 46.Zhang S, Wu C, Zhang Z, Wang T. Metal-free synthesis of 3‑(iso)quinolinyl 4‑chromenones and 3‑(iso)quinolinyl 4-quinolones from (iso)quinoline N‑oxides and ynones. Org. Lett. 2019;21:9995–9998. doi: 10.1021/acs.orglett.9b03921. [DOI] [PubMed] [Google Scholar]
- 47.Liu J, Ba D, Chen Y, Wen S, Cheng G. Synthesis of 3-(2-quinolyl) chromones from ynones and quinoline N-oxides via tandem reactions under transition metal- and additive-free conditions. Chem. Commun. 2020;56:4078–4081. doi: 10.1039/C9CC09460A. [DOI] [PubMed] [Google Scholar]
- 48.Park CP, Nagle A, Yoon CH, Chen C, Jung KW. Formal aromatic C−H insertion for stereoselective isoquinolinone synthesis and studies on mechanistic insights into the C−C bond formation. J. Org. Chem. 2009;74:6231–6236. doi: 10.1021/jo9011255. [DOI] [PubMed] [Google Scholar]
- 49.Mo S, Xu J. Chemospecific intramolecular Buchner reaction catalyzed by copper(II) acetylacetonate. ChemCatChem. 2014;6:1679–1683. doi: 10.1002/cctc.201400014. [DOI] [Google Scholar]
- 50.Ru G-X, et al. Copper catalyzed dearomatization by Michael-type addition of indolyl ynones: divergent synthesis of functionalized spiroindoles and cyclopenta[c]quinolin-3-ones. Org. Chem. Front. 2022;9:2621–2626. doi: 10.1039/D2QO00275B. [DOI] [Google Scholar]
- 51.Shen W-B, et al. Copper(I)-catalyzed enyne oxidation/cyclopropanation: divergent and enantioselective synthesis of cyclopropanes. Org. Lett. 2021;23:1285–1290. doi: 10.1021/acs.orglett.0c04268. [DOI] [PubMed] [Google Scholar]
- 52.Shen W-B, et al. Cu(I)- and Au(I)-catalyzed regioselective oxidation of diynes: divergent synthesis of N-heterocycles. Org. Chem. Front. 2021;8:4960–4966. doi: 10.1039/D1QO00912E. [DOI] [Google Scholar]
- 53.Shen W-B, et al. Cu(I)-catalyzed oxidative cyclization of enynamides: regioselective access to cyclopentadiene frameworks and 2-aminofurans. Org. Lett. 2020;22:6799–6804. doi: 10.1021/acs.orglett.0c02317. [DOI] [PubMed] [Google Scholar]
- 54.Zheng Y, Zhang T-T, Shen W-B. Gold-catalyzed oxidative cyclization of amide-alkynes: access to functionalized γ-lactams. Org. Biomol. Chem. 2021;19:9688–9691. doi: 10.1039/D1OB01846A. [DOI] [PubMed] [Google Scholar]
- 55.Shen W-B, et al. Highly site selective formal [5+2] and [4+2] annulations of isoxazoles with heterosubstituted alkynes by platinum catalysis: rapid access to functionalized 1,3-oxazepines and 2,5-dihydropyridines. Angew. Chem. Int. Ed. 2017;56:605–609. doi: 10.1002/anie.201610042. [DOI] [PubMed] [Google Scholar]
- 56.Shen W-B, et al. Divergent synthesis of N-heterocycles via controllable cyclization of azido-diynes catalyzed by copper and gold. Nat. Commun. 2017;8:1748. doi: 10.1038/s41467-017-01853-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.CCDC 2122556 (3a), CCDC 2126067 (3r), CCDC 2122557 (3aba), CCDC 2144833 (5p), and CCDC 212255 (7) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre.
- 58.Lu B, Li C, Zhang L. Gold-catalyzed highly regioselective oxidation of C-C triple bonds without acid additives: propargyl moieties as masked α,β-unsaturated carbonyls. J. Am. Chem. Soc. 2010;132:14070–14072. doi: 10.1021/ja1072614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim, J. J. et al. Negative solovatochromism of azo dyes derived from (dialkylamino)thiazole dimers. Chem. Commun. 753–754 (2000).
- 60.Iuliano V, et al. Negative solvatochromism in a N-linked p-pyridiniumcalix[4]arene derivative. Org. Lett. 2019;21:2704–2707. doi: 10.1021/acs.orglett.9b00683. [DOI] [PubMed] [Google Scholar]
- 61.Sharma R, et al. High singlet oxygen production and negative solvatochromism of octabrominated 3-pyrrolyl boron dipyrromethenes. RSC Adv. 2016;6:24111–24114. doi: 10.1039/C6RA01294A. [DOI] [Google Scholar]
- 62.Zhao Z, Zhang H, Lam JWY, Tang BZ. Synergistic N-heterocyclic carbene/palladium-catalyzed umpolung 1,4-addition of aryl iodides to enals. Angew. Chem. Int. Ed. 2020;59:2. doi: 10.1002/anie.201914768. [DOI] [PubMed] [Google Scholar]
- 63.Tu Y, Zhao Z, Lam JWY, Tang BZ. Mechanistic connotations of restriction of intramolecular motions (RIM) Nat. Sci. Rev. 2021;8:nwaa260. doi: 10.1093/nsr/nwaa260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cai X-M, et al. BioAIEgens derived from rosin: how does molecular motion affect their photophysical processes in solid state? Nat. Commun. 2021;12:1773. doi: 10.1038/s41467-021-22061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu, D., et al. Molecular core–shell structure design: facilitating delayed fluorescence in aggregates toward highly efficient solution-processed OLEDs. Aggregate3, e164 (2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and the Supplementary Information as well as from the authors upon reasonable request. DFT calculations are available in Supplementary Data 1. The compound characterizations are available in Supplementary Data 2. The X-ray crystallographic coordinates for structures 3a, 3r, 3aba, 5p, and 8, reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under CCDC 2122556 (3a, Supplementary Data 3), 2126067 (3r, Supplementary Data 4), 2122557 (3aba, Supplementary Data 5), 2144833 (5p, Supplementary Data 6) and 2122558 (8, Supplementary Data 7), respectively. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.