Dear editor,
We read with interest the article by Yang et al. suggesting no significant association between dyslipidemia and COVID-19 mortality1. However, whereas most studies have focused on dyslipidemia during the acute phase, more recent studies suggest that COVID-19 is associated with future cardiovascular events,2 but the mechanisms for this are still unclear3. Moreover, while most studies have measured traditional lipid parameters such as low-density (LDL) and high-density lipoprotein (HDL) cholesterol, recent studies suggest that LDL aggregation and particle characteristics can serve as markers and mediators of progressive atherosclerosis,4, 5 but data on these mechanisms in COVID-19 are lacking.
This study analyzed plasma samples from 66 adult hospitalized COVID-19 patients (≥18 years old) from the NOR-SOLIDARITY trial6 (35 standard of care [SoC], 18 hydroxychloroquine [HCQ] and 13 remdesivir) who attended a 3-month follow-up following hospitalization for confirmed SARS-CoV-2 infection and 42 matched healthy controls (HC) ( Fig. 1A). The study was approved by the South-Eastern Norway Regional Health Authority (ref 118684) and registered on ClinicalTrials.gov (NCT04321616).
Fig. 1.
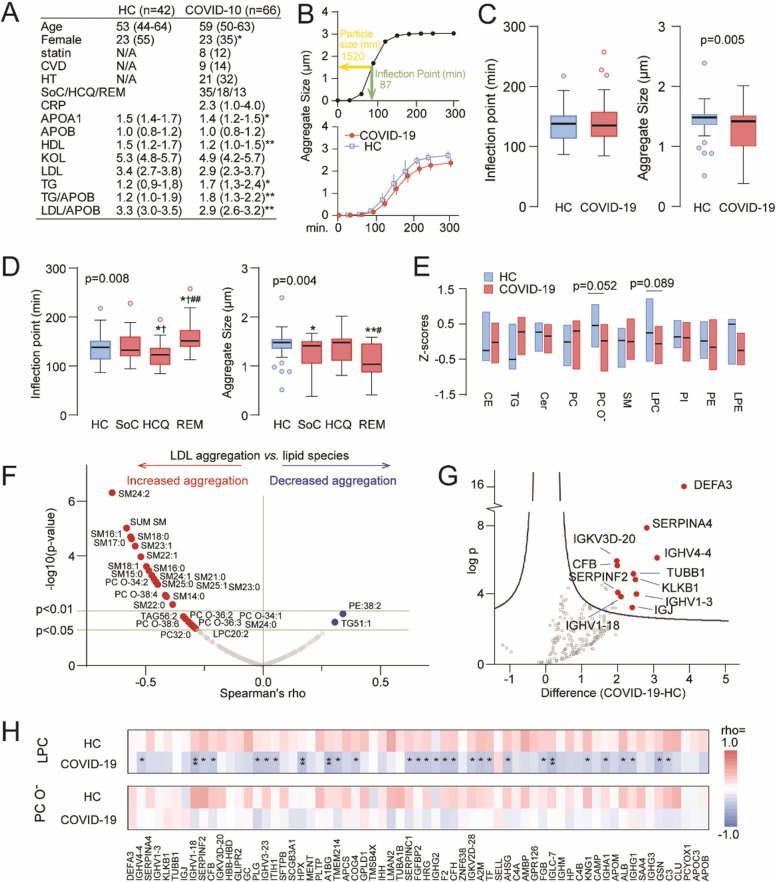
LDL aggregation following severe COVID-19 disease. (A) Demographics and lipoprotein levels in the study population. (B) Representative aggregation curves from a study subject (top panel) and in HC and COVID-19 patients (lower panel). (C) Tukey plots showing the inflection point (left panel) and LDL aggregate size (right panel) in HC and COVID-19 patients. (D) Tukey plots showing the inflection point (left panel) and LDL aggregate size (right panel) according to treatment modalities. *p < 0.05, **p < 0.01 vs. HC; †p < 0.05 vs. Standard of Care (SoC); #p < 0.05, ##p < 0.01 vs. hydroxychloroquine (HCQ), REM=remdesivir. (E) Electrospray ionization mass spectrometry (ESI-MS/MS) analyses of LDL lipid composition (CE, cholesterol esters; TG, triglycerides; Cer, ceramides; PC, phosphatidylcholines; PC O- l, ether ether-linked PC; SM, sphingomyelins; LPC, lyso-phosphatidylcholines; PI, phosphatidylinositols; PE, phosphatidylethanolamines; LPE, Lysophosphatidylethanolamine). (F) Volcano plot showing sphingomyelins from ESI-MS/MS analysis and association with LDL aggregation. (G) Volcano plot from LC/MS analyses of the protein cargo of plasma LDL. (H) Heatmap of spearman rhos from correlating LPC and PC O- with increased proteins from the MS analysis of LDL particles in HC and COVID-19 patients. Significant correlation is rho>0.34. *p < 0.05, **p < 0.01.
LDL was isolated and LDL aggregation susceptibility defined as the inflection point of the aggregate size vs. time curves obtained during incubation of LDL with sphingomyelinase as previously described (n = 108).4, 5 LDL lipid (n = 51) and protein (n = 49) compositions were determined from LDL samples as described4, 7. Plasma levels of lipoproteins were analyzed at an accredited clinical chemistry laboratory at Oslo University Hospital Rikshospitalet, Oslo, Norway.
Patient characteristics were compared using student's t-test or Χ2 for continuous and categorical variables, respectively (Fig. 1A). Comparison of lipoproteins, LDL aggregation, and LDL aggregate size between HC and COVID-19 patients or in relation to comorbid disease or treatment modalities was performed using MANCOVA with sex as a covariate. Correlation was assessed with Spearman's rank correlation. T-test with permutation-based FDR < 0.05 (250 randomizations) as the criteria for statistical significance was used to identify dysregulated proteins in the liquid chromatography–mass spectrometry (LC–MS) analysis of plasma LDL.
Fig. 1A shows that COVID-19 patients had lower plasma levels of apolipoprotein A-I (APOA-I), a major component of the “anti-inflammatory” HDL, lower levels the LDL/APOB ratio, a proxy for LDL particle size and low levels are associated with adverse event in patients with atherosclerosis8, and higher levels of triglycerides (TG) and TG/APOB ratio. Fig. 1B shows a representative LDL-aggregation curve highlighting the inflection point (green bar) and aggregate size at the inflection point (yellow bar). Average aggregate size vs. time curves in HC and COVID-19 patients are shown in the lower panel.
We observed no difference in LDL aggregation (Fig. 1C, left panel), but aggregate size was significantly smaller in COVID-19 patients (right panel) and correlated with the LDL/APOB ratio, an index of LDL-particle size, in patients (r = 0.68, p < 0.001), but not in HC (r = 0.15, p = 0.46). Evaluation of treatment modalities, stopped after a maximum of 10 days after hospital admission, and about 80 days before plasma collection, revealed higher inflection point indicating slower LDL aggregation and smaller aggregate size in remdesivir treated patients, whereas patients receiving HCQ had normal aggregate size and enhanced aggregation (Fig. 1D). ESI-MS/MS analysis of LDL lipid composition (Fig. 1E) showed trend level decreases in phosphatidylcholines (PC, p = 0.052) and lysophosphatidylcholines (LPC, p = 0.089). All but one sphingomyelin (SM) and the sum of the SM species correlated with increased aggregation (Fig. 1F). MS analyses of the protein cargo of plasma LDL particles identified increased levels of 69 proteins in COVID-19 patients with alpha defensin 3 (DEFA3), a protein involved in adaptive and innate immunity and kallistatin (SERPINA4), a kallikrein inhibitor, as well as several immunoglobulin components as the most regulated, as compared with HC (volcano plot in Fig. 1G). Furthermore, 29 proteins correlated negatively with LPC with a similar tendency for most remaining proteins (Fig. 1H). Conversely, these associations were mostly positive in HC. Interestingly, Prenylcysteine oxidase 1, an LDL-oxidizing enzyme linked with atherosclerosis9 was associated with both rapid LDL aggregation (r = −0.36, p = 0.011) and large aggregate size (r = 0.33, p = 0.021), and this oxidase was upregulated in HCQ treated patients. In contrast, DEFA3 correlated with decreased aggregate size (r = −0.39, p = 0.005).
No HC used statins, and in COVID-19 patients, there was no association between statin use and LDL aggregation, particle size or lipid composition within the aggregates.
Our data show that COVID-19 patients 3 months after hospital admission had decreased LDL particle size, accompanied by an inflammatory protein cargo, without overall differences in LDL aggregation in vitro. Furthermore, remdesivir- and HCQ-treated patients had differences in LDL functionality and composition potentially reflecting differences in proatherogenic properties, although the potential impact of these exploratory data should be interpreted with caution.
We suggest that the composition of the LDL particles from COVID-19 patients characterized by inflammatory protein content may have larger potential for docking, entry, retention, and accumulation in the arterial wall. Low LPC levels associate with enhanced atherosclerotic burden and reliably predict future cardiovascular events10. The consistent inverse correlations of LPC with inflammatory protein components in the same LDL particles in COVID-19 patients, but not in HC, further suggest a proatherogenic potential of sustained alterations in LDL particle composition in COVID-19. Importantly, the differences in LDL composition and function were observed 3 months after hospitalization. Since LDL has a half-life in plasma of only a few days, the observed differences in the proteome and the lipidome of LDL indicate that COVID-19 can induce long-term disturbances in lipoprotein metabolism.
Whereas our data suggest novel mechanisms for enhanced cardiovascular risk in COVID-19 patients, further and larger studies, that examine the association of these LDL characteristics with clinical events, are needed to support our novel findings further.
Funding
This study was supported by the South-Eastern Norway Regional Health Authority (2021071), the Academy of Finland (332564), Novo Nordisk Fonden (NNF19OC0057411), Emil Aaltonen Foundation (220244K), Ida Montin Foundation (20220113), Finnish Foundation for Cardiovascular Research, and Sigrid Jusélius Foundation. Mass spectrometry-based proteomic analyses were performed by the Proteomics Core Facility, Department of Immunology, University of Oslo/Oslo University Hospital, which is supported by the Core Facilities program of the South-Eastern Norway Regional Health Authority. This core facility is also a member of the National Network of Advanced Proteomics Infrastructure (NAPI), which is funded by the Research Council of Norway INFRASTRUKTUR-program (project number: 295910).
Study group members
The NOR-SOLIDARITY consortium members are: Jan Terje Andersen, Anette Kolderup, Trine Kåsine, Fridtjof Lund-Johansen, Inge Christoffer Olsen, Karoline Hansen Skåra, Trung Tran, Cathrine Fladeby, Liv Hesstvedt, Mona Holberg-Petersen, Synne Jenum, Simreen Kaur Johal, Dag Henrik Reikvam, Kjerstin Røstad, Anne Katrine Steffensen, Birgitte Stiksrud, Eline Brenno Vaage, Erik Egeland Christensen, Marthe Jøntvedt Jørgensen, Fridtjof Lund-Johansen, Sarah Nur, Vidar Ormaasen, Frank Olav Pettersen (Oslo University Hospital); Saad Aballi, Jorunn Brynhildsen, Waleed Ghanima, Anne Marie Halstensen (Østfold Hospital Trust); Åse Berg (Stavanger University Hospital); Bjørn Blomberg, Reidar Kvåle, Nina Langeland, Kristin Greve Isdahl Mohn (Haukeland University Hospital); Olav Dalgard (Akershus University Hospital); Ragnhild Eiken, Richard Alexander Molvik, Carl Magnus Ystrøm (Innlandet Hospital Trust); Gernot Ernst, Lars Thoresen (Vestre Viken Hospital Trust); Lise Tuset Gustad, Lars Mølgaard Saxhaug, Nina Vibeche Skei (Nord-Trøndelag Hospital Trust); Raisa Hannula (Trondheim University Hospital); Mette Haugli, Roy Bjørkholt Olsen (Sørlandet Hospital Trust); Dag Arne Lihaug Hoff (Ålesund Hospital); Asgeir Johannessen, Bjørn Åsheim-Hansen (Vestfold Hospital Trust); Bård Reikvam Kittang (Haraldsplass Deaconess Hospital); Lan Ai Kieu Le (Haugesund Hospital); Ravinea Manotheepan, Hans Schmidt Rasmussen, Grethe-Elisabeth Stenvik, Ruth Foseide Thorkildsen, Leif Erik Vinge (Diakonhjemmet Hospital); Pawel Mielnik (Førde Hospital); Vegard Skogen (University Hospital of North Norway); Hilde Skudal (Telemark Hospital Trust); Birgitte Tholin (Molde Hospital).
Conflict of interest
LH has stock ownership in Algipharma AS; AMDR has received funding from Vivaldi Invest A/S owned by Jon Stephenson von Tetzchner; KÖ has a patent regarding the LDL aggregation method (P4629US01).
Footnotes
Thor Ueland and Lauri A.O. Äikäs shared first authorship.
References
- 1.Yang H., Hou H., Liang X., Xu J., Wang Y. Lack of significant association between dyslipidemia and COVID-19 mortality. J Infect. 2021;82(6):276–316. doi: 10.1016/j.jinf.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie Y., Xu E., Bowe B., Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28(3):583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liuzzo G., Volpe M. SARS-CoV-2 infection markedly increases long-term cardiovascular risk. Eur Heart J. 2022;43(20):1899–1900. doi: 10.1093/eurheartj/ehac168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruuth M., Nguyen S.D., Vihervaara T., et al. Susceptibility of low-density lipoprotein particles to aggregate depends on particle lipidome, is modifiable, and associates with future cardiovascular deaths. Eur Heart J. 2018;39(27):2562–2573. doi: 10.1093/eurheartj/ehy319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heffron S.P., Ruuth M.K., Xia Y., et al. Low-density lipoprotein aggregation predicts adverse cardiovascular events in peripheral artery disease. Atherosclerosis. 2021;316:53–57. doi: 10.1016/j.atherosclerosis.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barratt-Due A., Olsen I.C., Nezvalova-Henriksen K., et al. Evaluation of the effects of remdesivir and hydroxychloroquine on viral clearance in COVID-19: a randomized trial. Ann Intern Med. 2021;174(9):1261–1269. doi: 10.7326/m21-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holm S., Kared H., Michelsen A.E., et al. Immune complexes, innate immunity, and NETosis in ChAdOx1 vaccine-induced thrombocytopenia. Eur Heart J. 2021;42(39):4064–4072. doi: 10.1093/eurheartj/ehab506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drexel H., Larcher B., Mader A., et al. The LDL-C/ApoB ratio predicts major cardiovascular events in patients with established atherosclerotic cardiovascular disease. Atherosclerosis. 2021;329:44–49. doi: 10.1016/j.atherosclerosis.2021.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Banfi C., Baetta R., Barbieri S.S., et al. Prenylcysteine oxidase 1, an emerging player in atherosclerosis. Commun Biol. 2021;4(1):1109. doi: 10.1038/s42003-021-02630-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganna A., Salihovic S., Sundström J., et al. Large-scale metabolomic profiling identifies novel biomarkers for incident coronary heart disease. PLoS Genet. 2014;10(12) doi: 10.1371/journal.pgen.1004801. [DOI] [PMC free article] [PubMed] [Google Scholar]