Abstract
Background
Osteosarcoma of the scapula is extremely rare. Hence, there is no consensus regarding its optimal management. In this study, we report the demographics, characteristic features, and outcomes of scapulectomy with or without scapular allograft reconstruction in a series of patients with primary scapular osteosarcoma.
Materials and Methods
Twelve patients with primary scapular osteosarcoma who were treated by scapulectomy with or without scapular allograft reconstruction (five and seven patients, respectively) were included. The allograft was fixed in place using a dacron tape without a plate or screw. The function of the shoulder was evaluated using the Musculoskeletal Tumor Society (MSTS) score for the upper extremity and Toronto Extremity Salvage Score (TESS).
Results
The study population included seven (58.3%) males and five (41.7%) females with a mean age of 30 ± 8.2 years. The histologic type of the tumor was osteoblastic in the majority of patients (n = 8, 66.7%). At an average follow-up of 6.5 ± 2.3 years, only one local recurrence (8.3%) occurred in our patients that coincided with lung metastasis. The mean MSTS score was 78.7 ± 3.8% and 66.2 ± 4% in patients with and without scapular allograft, respectively (P = 0.006). The mean TESS was 78.6 ± 5.6 and 68.4 ± 2.4 in patients with and without scapular allograft, respectively (P = 0.005). The overall 5-year survival of the patients was 85.7%.
Conclusion
Osteosarcoma of scapula more frequently occurs in the fourth decade of life, mainly presented with a sclerotic radiologic appearance. Patients with scapular allograft impaction after scapulectomy have better functional outcomes compared to those without boney reconstruction.
Keywords: Scapula, Osteosarcoma, Scapulectomy, Scapular allograft
Introduction
The scapula is a wide flat bone with several muscle attachments. It is an important bone in shoulder movements, and its dysfunction is associated with significant morbidity [1]. Bone and soft-tissue tumors of the scapula are uncommon. In a series of 566 bone tumors reported by Baena-Ocampo Ldel et al., the scapula was involved in 1.6% of cases only [2]. In a large series of 1853 bone cancers reported by Dahlin et al., the scapula was involved in 3.6% of them [3].
Chondrosarcoma is the most common malignant bone tumor of the scapula, accounting for about 32% of all malignant lesions in this location [4]. Ewing sarcoma is the second most common malignant bone tumor of the scapula accounting for nearly 16.2% of all malignant lesions of this bone [4]. Although osteosarcoma is the most common primary malignant bone tumor, it less frequently involves the scapula, so it comprises about 14.3% [4] of the malignant tumors of the scapula. Osteosarcoma of the scapula has only been reported in a small number of case reports, and no case series study is available in the literature.
Due to the scarcity of published literature describing scapular osteosarcoma, the characteristic feature and outcomes of treatment are not well-understood. In this study, we aimed to describe demographic, clinical, histologic, and radiologic characteristics in 12 patients with primary scapular osteosarcoma. We also evaluated the outcomes of scapulectomy with or without scapular allograft reconstruction for this cohort of patients.
Patients and Methods
This study was approved by the review board of our institute. Using the electronic database of our hospital, patients who were treated at our orthopedic oncology center between 1978 and 2019 with a malignant tumor of scapula were identified. Of a total of 118 patients, 21 patients had osteosarcoma. Inclusion criteria were histologic diagnosis of osteosarcoma, absence of metastasis at presentation, treatment by total scapulectomy, and a minimum follow-up of 2 years. Patients who underwent partial scapulectomy (n = 2), patients with inadequate biopsy (n = 1), patients who died before 2 years (n = 2), patients who lost to follow-up (n = 1), patients with extra-articular resection (n = 2), and patients with secondary osteosarcoma (n = 1) were excluded. The remaining 12 patients were included in the analysis.
Staging studies were done by radiographs, Tc 99 bone scan, CT scan of the chest, and CT scan of the scapula. MRI of the scapula was also implemented after 1992. Eight patients had guided core needle biopsy, and four patients had open incisional biopsy. After neoadjuvant chemotherapy, the restaging of the tumor was performed by obtaining new radiographs and an MRI of the involved area. Joint involvement was evaluated by observing effusion in the restaging MRI.
Demographic and clinical characteristics of the patients were extracted from their medical records. Histologic and radiologic characteristics of the tumor were also extracted that included histologic type, tumor size at its largest diameter, tumor location, and radiologic appearance. Tumor size was regarded as the largest diameter of the tumor on CT scanning. The location of the tumor was categorized into three zones according to the Jamshidi et al. classification: zone 1 (body of scapula), zone 2 (neck and coracoid), and zone 3 (acromion) [5]. Neo-adjuvant and adjuvant chemotherapies were implemented for all patients.
Surgical Procedure
Under general anesthesia, the patient was positioned in a lateral position. A posterior longitudinal incision encompassing the elliptical biopsy tract was made, which started with a deltopectoral approach crossing the lateral third of the acromion, extending distally and medially to the inferior angle of the scapula. Gross inspection and frozen section analysis of synovium were employed to inspect joint involvement. Total scapulectomy was performed for large tumors or tumors centered on the glenoid neck or nearby [6]. In this respect, the entire scapula, biopsy tract, and a cuff of normal surrounding muscle were resected. In all patients, zones 1 and 2 were involved. For these patients, attached muscles, including the infraspinatus, subscapularis, and supraspinatus, were resected to obtain an adequate clear margin. Intra-articular resection was performed, as well. When the acromion (zone 3) was involved, we resected the acromioclavicular joint medial to trapezoid ligament. Before 1998, patients underwent scapulectomy without scapular allograft reconstruction. After that, osteoarticular allograft reconstruction was performed following the scapulectomy. In patients without scapular allograft reconstruction, we created a hole in the distal end of the clavicle, and static stabilization was performed by attaching the remnants of the capsule and rotator cuff to the clavicle using non-absorbable material. Dynamic stabilization was achieved by suturing the remnant of deltoid and trapezius to the pectoralis major muscle. For the scapular allograft reconstruction group, first, a matched scapular fresh frozen osteo-articular allograft was selected and placed in the correct position. Then the remnant of the capsule and rotator cuff of the proximal humerus was sutured to the corresponding capsule of allograft with non-absorbable materials. A 3 mm wide dacron tape was looped around the coracoid of the allograft and the host clavicle. The remaining muscles of the levator scapula, rhomboid, trapezius, serratus anterior, and teres major and minor were anchored to allograft using an interosseous suture. For the restoration of the supraspinatus function, the clavicular portion of the pectoralis major was transferred to greater tuberosity [7].
Postoperative Protocol and Follow-Up
The shoulder was immobilized in a sling and swathe for 4 weeks. Passive range of motion was allowed afterward. Active exercises started after 6 weeks. Follow-up visits were done every 3 months in the first 2 years, every 6 months for the next 3 years, and annually thereafter. In the last follow-up session, the function of the shoulder was evaluated using the Musculoskeletal Tumor Society (MSTS) score for the upper extremity [8] and The Toronto Extremity Salvage Score questionnaire [9]. MSTS questionnaire consisted of 30 questions. The TESS questionnaire consisted of 29 questions. Both scores were presented as percentages, and in both of them, a higher score was indicative of better function. The shoulder range of motion was also evaluated in the last follow-up session using a standard goniometer.
Statistical Analysis
SPSS for Windows, version 16 (SPSS Inc., Chicago, Ill., USA) was used for the statistical analysis of the data. Descriptive data were demonstrated by mean ± standard deviation (SD) or number and percentage. Mann–Whitney U test was used to compare the mean values between the patients with and without a scapular allograft augmentation. Kaplan–Meier method was used for the survival analysis. A P-value < 0.05 was considered significant.
Results
The study population included seven (58.3%) males and five (41.7%) females with a mean age of 30 ± 8.2 years (range 20–46). Pain was the main symptom at the presentation that was observed in all patients. Mass and muscle atrophy was detected in six (50%) and five (41.7%) patients, respectively. The mean symptom duration was 9.6 ± 5.5 months (range 4–24). The mean follow-up of the patients was 6.5 ± 2.3 years (range 4–11). The mean tumor size was 8.6 ± 1.8 cm (range 6–11). Characteristic features of the patients are summarized in Table 1.
Table 1.
Demographic, clinical, histologic, and radiologic characteristics of patients with osteosarcoma of the scapula
| No | Age/sex | Symptom | Symptom duration (months) | Locationa | Follow-up (year) | Tumor size | Histologic type | Imaging appearance | Surgery | Shoulder flexion/abduction (°) | MSTS (%) | TESS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 42/F | Pain and muscle atrophy | 12 | Zone 1, 2 | 5 | 6 cm | Osteoblastic | Sclerotic | Scapulectomy | 15/10 | 70 | 68.9 |
| 2 | 40/F | Pain and mass | 9 | Zone 1, 2, 3 | 8 | 10 cm | Chondroblastic |
Expansile Lytic Sclerotic Soft-tissue component |
Scapulectomy | 10/0 | 60 | 65.5 |
| 3 | 30/F | Pain and mass | 24 | Zone 1, 2 | 4 | 8 cm | Osteoblastic |
Expansile Sclerotic Soft-tissue component |
Scapulectomy + Scapular allograft |
25/15 | 80 | 75.9 |
| 4 | 20/M | Pain and mass | 6 | Zone 1, 2, 3 | 4 | 11 cm | Chondroblastic |
Expansile Lytic, sclerotic Soft-tissue component |
Scapulectomy | 10/5 | 63.3 | 68.9 |
| 5 | 25/M | Pain and muscle atrophy | 12 | Zone 1, 2 | 5 | 7 cm | Osteoblastic |
Expansile Sclerotic Soft-tissue component |
Scapulectomy | 10/10 | 63.3 | 65.5 |
| 6 | 31/M | Pain and muscle atrophy | 6 | Zone 1, 2, 3 | 7 | 10 cm | Osteoblastic |
Expansile Sclerotic Soft-tissue component |
Scapulectomy + scapular allograft | 20/25 | 73.3 | 72.4 |
| 7 | 30/M | Pain | 12 | Zone 1, 2 | 6 | 7 cm | Osteoblastic |
Expansile Sclerotic |
Scapulectomy | 15/10 | 66.7 | 68.9 |
| 8 | 29/F | Pain and mass | 9 | Zone 1, 2 | 6 | 9 cm | Chondroblastic |
Sclerotic Soft-tissue component |
Scapulectomy + scapular allograft | 45/30 | 80 | 82.7 |
| 9 | 39/M | Pain and muscle atrophy | 6 | Zone 1, 2, 3 | 7 | 9 cm | Osteoblastic |
Expansile Lytic, sclerotic Soft-tissue component |
Scapulectomy + scapular allograft | 15/20 | 76.7 | 75.9 |
| 10 | 24/F | Pain and mass | 6 | Zone 1, 2, 3 | 9 | 8 cm | Chondroblastic |
Expansile Sclerotic Soft-tissue component |
Scapulectomy | 15/15 | 70 | 68.9 |
| 11 | 20/M | Pain and muscle atrophy | 9 | Zone 1, 2 | 6 | 7 cm | Osteoblastic |
Expansile Sclerotic Soft-tissue component |
Scapulectomy | 30/20 | 70 | 72.4 |
| 12 | 30/ M | Pain and mass | 4 | Zone 1, 2, 3 | 11 | 11 cm | Osteoblastic |
Expansile Lytic Sclerotic soft-tissue component |
Scapulectomy + scapular allograft | 60/45 | 83.3 | 86.2 |
aJamshidi et al. classification: zone 1 (body of scapula), zone 2 (neck and coracoid), and zone 3 (acromion)
The histologic type of the tumor was osteoblastic in eight (66.7%) patients and chondroblasts in four (33.3%). Imaging appearance was sclerotic in one (8.3%), expansile-sclerotic in seven (58.3%) patients, and expansile-mixed in four (33.3%). Ten (83.3%) lesions also had a soft-tissue component.
Functional Outcomes
The mean MSTS score of the patients was 71.8 ± 7.4% (range 60–83.3). The mean TESS of the patients was 72.6 ± 6.5 (range 65–82.6). The mean active forward flexion was 22.5° ± 15.5° (range 10–60). The mean active shoulder abduction was 17.1° ± 12.1° (range 0–45).
Scapulectomy was performed without scapular allograft in seven (58.3%) patients (Fig. 1) and with scapular allograft reconstruction in five (41.7%) (Fig. 2). The mean active forward flexion was 37.5 ± 18 in patients with scapular allograft and 15 ± 7 in patients without scapular allograft (P = 0.01). The mean active abduction was 28.7 ± 12.5 in patients with scapular allograft and 11.3 ± 6.9 in patients without scapular allograft (P = 0.02) (Fig. 3). The mean MSTS score was 80 ± 2.7 and 67 ± 4.5 for patients with and without a scapular allograft, respectively (P = 0.006). The mean TESS was 80.2 ± 5.1 and 68.9 ± 2.6 for patients with and without a scapular allograft, respectively (P = 0.005).
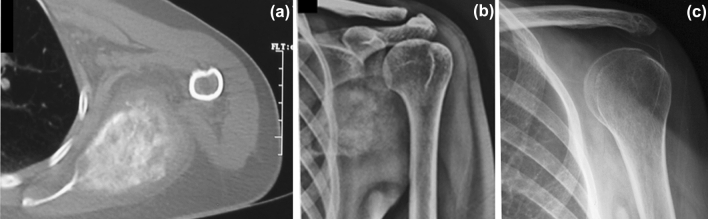
Fig. 1.
a Preoperative CT scan of a scapular osteosarcoma in a 20-year-old male with sclerotic appearance and soft-tissue component; b anteroposterior radiograph of the patients before the surgery; c anteroposterior radiograph of the patients 6 years after the scapulectomy without bony reconstruction
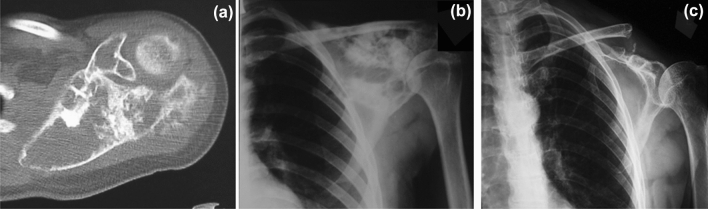
Fig. 2.
a Preoperative CT scan of a scapular osteosarcoma in a 30-year-old male with sclerotic-lytic appearance and soft-tissue component; b anteroposterior radiograph of the patients before the surgery; c anteroposterior radiograph of the patients 4 years after the scapulectomy with osteoarticular allograft reconstruction
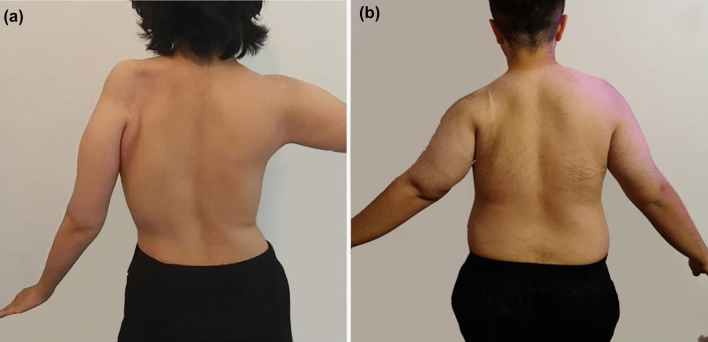
Fig. 3.
a Postoperative shoulder active abduction of 15° in a 24-year-old female with scapular osteosarcoma of the left shoulder treated by scapulectomy without reconstruction; b postoperative shoulder active abduction of 45° in a 30-year-old male with scapular osteosarcoma of the left shoulder treated by scapulectomy and osteoarticular allograft reconstruction
Oncologic Outcome
Two patients who died before 2 years owing to lung metastasis and one patient who was lost to follow-up were also included in the oncologic outcome analysis, making a total number of 15 patients for this section. Accordingly, the overall 5-year survival of the patient was 85.7%. In total, one local recurrence (6.7%) occurred in this series 30 months after the index surgery. The recurrence occurred in the soft tissue and coincided with lung metastasis. Re-excision of the lesion was performed for the management of local recurrence. Treatment of lung metastasis was done by mastectomy and re-chemotherapy. No relapse was observed until the last follow-up of the patient (18 months after the excision of the recurrent lesion).
Postoperative Complications
Two postoperative complications were recorded in this series that included one case of wound dehiscence in the group without allograft and one case of infection in the allograft-reconstructed group. Wound dehiscence was managed with debridement and intravenous antibiotic administration. Infection was managed with the removal of the allograft, debridement, and intravenous antibiotic administration. No allograft resorption occurred in this series.
Discussion
Characterization of tumors in rare locations is of critical importance as the scarce amount of available information makes the optimal management of these tumors unclear [10–12]. In this case series, we described the characteristic features and outcome of the scapular osteosarcoma that was treated with scapulectomy, with or without scapular allograft reconstruction. Only one local recurrence was observed in patients of this series. This observation reveals that in patients with massive osteosarcoma of the scapula involving the neck and glenoid, total scapulectomy could be regarded as an efficient treatment option to prevent the local recurrence of the lesion. In addition, the functional outcomes were superior in patients who underwent scapular allograft reconstruction.
Osteosarcoma of the scapula has been reported in a small number of case reports, and no case series study is available in this regard. Herget et al. reported a case of scapular epithelioid osteosarcoma in a 53-year-old female who presented with a history of chronic shoulder pain for 3 years. Owing to the rapidly progressive disease, lung, and vertebral metastases, and bilateral malignant pleural effusion, no surgical intervention was performed [13]. Saberi and Mortazavi reported a case of scapular osteosarcoma in a 34-year-old man that was managed with total scapulectomy without reconstruction [14]. Bedi et al. reported a case of scapular osteosarcoma presenting in a 37-year-old woman with severe congenital osteogenesis imperfect [15]. Logan et al. reported two cases of secondary osteosarcoma of the scapula attributed to radiation therapy [16].
Osteosarcoma contains a bimodal age distribution, with the first peak in adolescence (age of 10–14 years) and the second peak in the older population (> 65 years) [17]. Based on our observations, the age distribution of scapular osteosarcoma is different from that reported in the other locations. In this respect, the mean age of patients with scapular osteosarcoma was 30 years in the present study. Consistent with our results, the age of involvement was 53 years in the study of Herget et al. [13] and 34 years in the study of Saberi and Mortazavi [14]. Osteosarcoma generally shows a combination of lytic and sclerotic imaging appearance, and a soft-tissue component is usually present [18]. The same imaging appearance was observed in the present series. The overall 5-year survival of osteosarcoma is reported to be around 71% [19]. The overall 5-year survival was 85.7% in the present study. Accordingly, the overall survival of osteosarcoma in scapula seems to be higher compared to other locations. The rate of local recurrence in our patients was (6.7%), which is also lower than overall local recurrences of other sites (8–14.8%) [20]. This lower rate can be attributed to the muscles and fascia covering the scapula, making it an intra-compartmental lesion. In addition, the histologic type of the tumor was osteoblastic in the majority of patients, which is reported to be associated with better survival [21].
The outcome of scapulectomy for malignant tumors of the scapula has been reported in earlier studies. Vahanan et al. reported the outcome of scapulectomy without reconstruction in 23. Only one patient had osteosarcoma. Total scapulectomy was performed for 15 patients, while the rest retained their glenoids. They concluded that subtotal resection (retention of the glenohumeral articulation) provides superior functional results [22]. Since the glenoid and neck region were involved in all patients of the present series, we selected total scapulectomy to reduce the rate of local recurrence, which turned out to be successful (only one local recurrence).
Mnaymneh et al. reported the outcome of allograft reconstruction following the resection of malignant tumors of the scapula in six patients. None of the tumors was osteosarcoma. Total and subtotal scapulectomy were performed for five and one patient, respectively. Scapular allografts were fixed with plates and screws. At an average follow-up of 3.8 years, cosmetic and functional outcomes were good in all patients. The mean MSTS score was nearly 24.6/30. One allograft fracture was the only complication of this series that occurred following a falling down [23]. We did a total scapulectomy in all patients. Allograft was fixed with a dacron tape and not a plate and screw. The mean MSTS of the patients in the reconstruction group was 23.6, which was comparable to the study of Mnaymneh et al.
Capanna et al. reported the outcome of allograft reconstruction following the total scapulectomy in six patients with malignant tumors of the scapula. Preservation of at least one of the rotator cuff muscles was regarded as the prerequisite for the surgery. None of the tumors was osteosarcoma. The allograft was fixed to host residual acromion using plates and screws. At an average follow-up was 5.5 years, the average functional scores were 20 points according to MSTS score. Two plate fractures and two allograft fractures occurred in their series, which led to revision surgery in two patients (33.3%) [6]. We did not fix allografts with plates and screws. In addition, to achieve a wide margin, we transferred pectoralis major instead of maintaining one rotator cuff muscle. While our procedure omits the complications of plate fracture, the functional outcome remains comparable with that of plate fixation.
Other reconstruction techniques are also available for scapular tumors. Hayashi et al. compared the outcomes of prosthesis reconstruction, humeral suspension, and no bony reconstruction following the scapulectomy. According to their results, reconstruction with scapular prosthesis provided superior function and shoulder ROM compared to the other groups [24]. Although we used scapular allograft for reconstruction, similar results were observed, further supporting the use of bony reconstruction after scapulectomy.
Dislocations and wound infections are considered the most common complications of the scapulectomy with bony reconstruction, particularly if reconstructed with a scapular prosthesis (10–20%) [25]. Zhang et al. reported one infection in seven patients (14%) who underwent scapulectomy and allograft reconstruction of shoulder girdle after tumor resection [26]. The rate of infection was 20% (one in five) in the allograft-reconstructed group of the present study, which seems a little higher compared to the earlier studies using prosthesis or allograft reconstruction.
Altogether, the results of the present study suggest that total scapulectomy is a safe and effective method in the treatment of scapular osteosarcoma and, if combined with reconstruction, provides even better functional outcomes. However, this study was not without limitations. The main limitation of the study was the small number of patients who were dictated by the rare incidence of osteosarcoma in the scapula. The retrospective design was another limitation of the study, with its own potential biases.
Conclusion
Osteosarcoma of scapula more frequently occurs in the fourth decade of life and mainly presents with a sclerotic radiologic appearance. Total scapulectomy could be regarded as an adequate treatment for lesions involving the neck and glenoid regions. Patients who underwent allograft reconstruction of the scapula after scapulectomy tended to have better functional outcomes than non-reconstructed patients. While plate fixation of allograft has provided satisfying outcomes in the earlier investigations, the results of the present study revealed that reconstruction without plate fixation could also provide satisfying outcomes and lacks the complications of plate fixation, such as plate fracture.
Author contributions
KJ: supervisor. MHAB: data collection. EM: data collection. AB: reviewing manuscript critically. AM: drafting the manuscript.
Funding
None. Manuscript has been read and approved by all the authors, represents honest work, and not been submitted elsewhere.
Data availability
The data that support the findings of this article are not shared online, but are available from the corresponding author, [AM], upon reasonable request.
Declarations
Conflict of interest
The authors of this article declare no conflict of interest to disclose.
Ethical standard statement
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed consent
For this type of study, informed consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Voleti PB, Namdari S, Mehta S. Fractures of the scapula. Advances in Orthopedics. 2012 doi: 10.1155/2012/903850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baena-Ocampo Ldel C, Ramirez-Perez E, Linares-Gonzalez LM, Delgado-Chavez R. Epidemiology of bone tumors in Mexico City: Retrospective clinicopathologic study of 566 patients at a referral institution. Annals of Diagnostic Pathology. 2009;13(1):16–21. doi: 10.1016/j.anndiagpath.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Huvos AG. Bone tumors: Diagnosis, treatment and prognosis. New York: W.B. Saunders CBS Educ. and Professional Publ; 1987. [Google Scholar]
- 4.Priemel MH, Erler JM, Zustin J, Luebke AM, Stiel N, Spiro AS. Histological, epidemiological and anatomical analysis of 193 bone tumours of the scapula. Journal of Bone Oncology. 2019;18:100258. doi: 10.1016/j.jbo.2019.100258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jamshidi K, Bozorgi MHA, Hajializade M, Bagherifard A, Mirzaei A. Tailored treatment of aneurysmal bone cyst of the scapula: En bloc resection for the body and extended curettage for the neck and acromion. Journal of Shoulder and Elbow Surgery. 2020;29(5):961–967. doi: 10.1016/j.jse.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Capanna R, Totti F, Van der Geest IC, Müller DA. Scapular allograft reconstruction after total scapulectomy: Surgical technique and functional results. Journal of Shoulder and Elbow Surgery. 2015;24(8):e203–e211. doi: 10.1016/j.jse.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Resch H, Povacz P, Ritter E, Matschi W. Transfer of the pectoralis major muscle for the treatment of irreparable rupture of the subscapularis tendon. Journal of Bone and Joint Surgery. American Volume. 2000;82(3):372–382. doi: 10.2106/00004623-200003000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clinical Orthopaedics and Related Research. 1993;286:241–246. doi: 10.1097/00003086-199301000-00035. [DOI] [PubMed] [Google Scholar]
- 9.Davis AM, Wright JG, Williams JI, Bombardier C, Griffin A, Bell RS. Development of a measure of physical function for patients with bone and soft tissue sarcoma. Quality of Life Research. 1996;5(5):508–516. doi: 10.1007/BF00540024. [DOI] [PubMed] [Google Scholar]
- 10.Jamshidi K, Bagherifard A, Mirzaei A, Bahrabadi M. Giant cell tumor of the sacrum: Series of 19 patients and review of the literature. Archives of Bone and Joint Surgery. 2017;5(6):443. [PMC free article] [PubMed] [Google Scholar]
- 11.Jamshidi K, Karimi A, Bagherifard A, Mirzaei A. Aneurysmal bone cysts of the clavicle: A comparison of extended curettage and segmental resection with bone reconstruction. Journal of Shoulder and Elbow Surgery. 2019;28(9):1654–1657. doi: 10.1016/j.jse.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Jamshidi K, Zandrahimi F, Bagherifard A, Mohammadi F, Mirzaei A. An unusual presentation of osteosarcoma in the proximal femur with peculiar diagnostic characteristics: A retrospective series. Current Orthopaedic Practice. 2021;32(4):377–382. doi: 10.1097/BCO.0000000000001016. [DOI] [Google Scholar]
- 13.Herget GW, Otto C, Kurz P, Uhl M, Adler CP, Südkamp NP, et al. Epithelioid osteosarcoma of the scapula. Acta Chirurgiae Orthopaedicae et Traumatologiae Cechoslovaca. 2014;81(4):288–291. [PubMed] [Google Scholar]
- 14.Saberi M, Mortazavi N. Scapula osteosarcoma. Biomedical Journal of Scientific & Technical Research. 2017 doi: 10.26717/BJSTR.2017.01.000291. [DOI] [Google Scholar]
- 15.Bedi HS, Kaufman DV, Choong PF, Slavin JL. Osteosarcoma of the scapula arising in osteogenesis imperfecta. Pathology. 1999;31(1):52–54. doi: 10.1080/003130299105557. [DOI] [PubMed] [Google Scholar]
- 16.Logan PM, Munk PL, O'Connell JX, Connell DG, Janzen DL. Post-radiation osteosarcoma of the scapula. Skeletal Radiology. 1996;25(6):596–601. doi: 10.1007/s002560050144. [DOI] [PubMed] [Google Scholar]
- 17.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treatment and Research. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 18.Logan PM, Mitchell MJ, Munk PL. Imaging of variant osteosarcomas with an emphasis on CT and MR imaging. American Journal of Roentgenology. 1998;171(6):1531–1537. doi: 10.2214/ajr.171.6.9843284. [DOI] [PubMed] [Google Scholar]
- 19.Friebele JC, Peck J, Pan X, Abdel-Rasoul M, Mayerson JL. Osteosarcoma: A meta-analysis and review of the literature. American Journal of Orthopedics (Belle Mead, N.J.) 2015;44(12):547–553. [PubMed] [Google Scholar]
- 20.Salunke AA, Chen Y, Tan JH, Chen X, Khin LW, Puhaindran ME. Does a pathological fracture affect the prognosis in patients with osteosarcoma of the extremities? A systematic review and meta-analysis. The Bone and Joint Journal. 2014;96-B(10):1396–1403. doi: 10.1302/0301-620X.96B10.34370. [DOI] [PubMed] [Google Scholar]
- 21.Sathiyamoorthy S, Ali SZ. Osteoblastic osteosarcoma: Cytomorphologic characteristics and differential diagnosis on fine-needle aspiration. Acta Cytologica. 2012;56(5):481–486. doi: 10.1159/000339196. [DOI] [PubMed] [Google Scholar]
- 22.Mayil Vahanan N, Mohanlal P, Bose JC, Gangadharan R, Karthisundar V. The functional and oncological results after scapulectomy for scapular tumours: 2–16-year results. International Orthopaedics. 2007;31(6):831–836. doi: 10.1007/s00264-006-0261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mnaymneh WA, Temple HT, Malinin TI. Allograft reconstruction after resection of malignant tumors of the scapula. Clinical Orthopaedics and Related Research. 2002;405:223–229. doi: 10.1097/00003086-200212000-00029. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi K, Niu X, Tang X, Singh VA, Asavamongkolkul A, Kawai A, et al. Experience of total scapular excision for musculoskeletal tumor and reconstruction in eastern Asian countries. Journal of Bone Oncology. 2017;9:55–58. doi: 10.1016/j.jbo.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biazzo A, De Paolis M, Donati DM. Scapular reconstructions after resection for bone tumors: A single-institution experience and review of the literature. Acta Bio-Medica. 2018;89(3):415–422. doi: 10.23750/abm.v89i3.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang K, Duan H, Xiang Z, Tu C. Surgical technique and clinical results for scapular allograft reconstruction following resection of scapular tumors. Journal of Experimental & Clinical Cancer Research. 2009;28(1):45. doi: 10.1186/1756-9966-28-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this article are not shared online, but are available from the corresponding author, [AM], upon reasonable request.