Abstract
Introduction
Informed consent documentation is often the first area of interest for lawyers and insurers when a medico-legal malpractice suit is concerned. However, there is a lack of uniformity and standard procedure for obtaining informed consent for total hip arthroplasty (THA). We aimed to develop a solution for this need for a pre-designed, evidence-based informed consent form for THA cases.
Materials and Methods
We extensively reviewed the literature on the medico-legal aspects of THA, medico-legal aspects of informed consent, and medico-legal aspects of informed consent in THA. We then conducted semi-structured interviews with orthopaedic surgeons and patients who had previously undergone THA in the previous year. Based on all of the above, we developed an informed consent form that was evidence-based. We then had the form reviewed by a legal expert. The final form was utilised for THA cases at our institution for 1 year.
Results
Legally sound, evidence-based Informed Consent Form for Total Hip Arthroplasty is given in Form A.
Conclusion
The use of legally sound, evidence-based informed consent for total hip arthroplasty cases would be beneficial to orthopaedic surgeons and patients alike. It would uphold the rights of the patient, and promote open discussion and transparency. In the event of a lawsuit, it would be a vital document in the defence of the surgeon and withstand the scrutiny of lawyers and the judiciary.
Keywords: Informed consent, Total hip arthroplasty, Hip replacement, Medico-legal, Lawsuit, Consent
Introduction
Informed consent is an exercise designed to protect the rights of the patients and provide them with adequate information before undergoing a medical procedure. Its importance has risen significantly in the past few decades with it being the crux of several lawsuits. It is often the first area of interest for lawyers and insurers when a medico-legal malpractice suit is concerned. Total Hip Arthroplasty (THA) is one of the most commonly performed orthopaedic surgeries worldwide. However, there is a lack of uniformity and standard procedure for obtaining informed consent for the procedure. This leads to informed consent forms which often lack several key aspects and would jeopardise their legal standing in a court of law. This substantiates the need for a pre-designed, evidence-based informed consent form for total hip replacement cases specifically [1].
Materials and Methods
After obtaining the institute ethical clearance, we extensively reviewed the literature on informed consent in total hip arthroplasty. We also explored literature on the medico-legal aspects of total hip arthroplasty, informed consent, and informed consent in THA. We additionally reviewed the literature on complications occurring after THA. The electronic databases of PubMed and Cochrane Library were explored using the following search terms and Boolean operators: ‘medico-legal’ OR ‘lawsuit’ OR ‘malpractice’ OR ‘litigation’ AND ‘total hip arthroplasty’ OR ‘hip arthroplasty’ OR ‘hip replacement’ OR ‘total hip replacement’ OR ‘THA’. The databases were also searched using the terms and Boolean operators: ‘Informed consent’ OR ‘consent’ OR ‘patient consent’ AND ‘total hip replacement’ OR ‘hip replacement’ OR ‘hip arthroplasty. Further searches included the terms and Boolean operators: ‘total hip replacement’ AND ‘complications’ OR ‘adverse events. No restriction on publication date was applied. The manuscript language was restricted to English. In addition, a comprehensive search of reference lists of all identified articles was conducted to identify additional studies. Information about specific medico-legal proceedings involving THA cases in legal courts, state and national consumer dispute redressal forums, and state medical councils were obtained from different books having a compendium of medico-legal judgements. The results of this literature review were curated and documented.
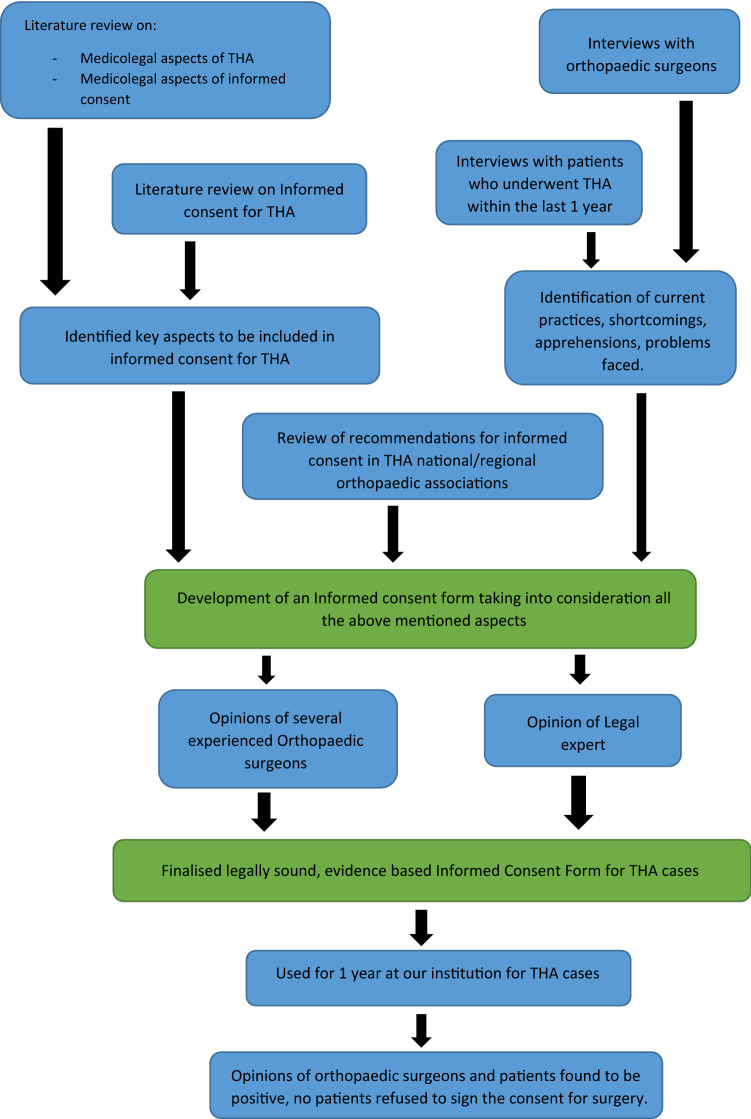
We then conducted semi-structured interviews with orthopaedic surgeons from different institutes to understand the common practices about informed consent in THA, the difficulties they faced, their experiences with contentious informed consent, disputes/concerns patients had raised regarding consent forms, and personal experiences in any lawsuits involving THA cases. We then held semi-structured interviews with patients who had previously undergone THA in the previous year from the date of the interview. We asked them about their personal experience in the process of giving their informed consent, the usefulness of the process, and any doubts which were not satisfactorily addressed in the informed consent. Based on all of the above, we developed an evidence-based informed consent form. This consent form was presented to several experienced orthopaedic surgeons for their personal opinion and suggestions for further improvement. It was also run by a legal expert. Minor modifications were made based on their suggestions and the final consent form was prepared. It was used at our institution for 1 year and no concerns were raised regarding the consent form. The consent form was used for a total of 346 patients, with none of the patients refusing to sign the consent form. No additional modification of the consent form was found necessary in 97% of the cases. In 11 cases (3.1% cases), an additional “High Risk Consent” was obtained owing severe co-morbidities of the patient and an increased risk to life. The overall response of orthopaedic surgeons and patients was positive, with no patient refusing to consent to the procedure (Fig. 1).
Fig. 1.
Flow diagram depicting the steps involved in the formulation of the consent form
Validation of Instrument
The prepared tools along with the objectives, blueprints, and criteria rating scale were given to six internal [3 from the Department of Orthopaedics, Chennai; 1 from the Department of Forensic Medicine, Chennai; and 2 from the Department of Community Medicine, Chennai] and external experts [1 from the Department of Orthopaedics, Delhi; 1 from the Department of Orthopaedics, Bhubaneswar; 2 from the Department of Forensic Medicine, Patna, and 2 from the Department of Community Medicine, Mumbai]. All the tools were returned after validation of the content.
Baseline Proforma of the Study Participants
There was 100% agreement on most of the items in the informed consent proforma. The informed consent proforma for study participants had thus 27 items. The modifications were performed as per the validators’ suggestions. There was less than 60% agreement on three items of the informed consent proforma and so those items were deleted after the consultation with the expert. Thus, in the present study, the informed consent form had 24 items.
To examine the content validity rate (CVR), the questionnaire was given to six experts in the specialities related to the field of the study; the answers were designed based on a three-point Likert scale consisting of necessary, helpful but not necessary, and not necessary. Then, the questionnaire’s CVR was assessed; according to the Lawsche table, if the item score was over 0.95, the item was considered an appropriate and necessary one. Regarding the obtained scores at this stage, those comments, and views of the respondents and the rethought on the items with lower scores, those that seemed unable to measure the desired concept, or those that had little connection with the issue were excluded.
The indexes of “relevance”, “clarity”, “simplicity”, and “ambiguity” were examined. The experts were asked to respond to two questions: (1) the viewpoints they believed should be imposed and (2) suggestions for the items that should be entered into the questionnaire. A separate content validity index (CVI) was calculated for each item and scale [2]. Thus, we calculated scale-content validity index S-CVI/Average for the overall six constructs (1.00 + 1.00 + 0.83 + 1.00 + 1.00 + 0.83)/6 = 0.94.
Results
A Legally sound, Evidence-based Informed Consent Form for Total Hip Arthroplasty is given in Form A as Annexure 1. The original form (Form B—Annexure 2) was used at authors' institution, and it was modified at some places during the journal review process.
Discussion
Informed consent is often the most vital document in the defence of a malpractice lawsuit. However, almost all available literature suggests that informed consent taken for THA is incomplete and requires improvement. These shortcomings in informed consent leave the orthopaedic surgeon liable for malpractice claims and can be used by the patients in a court of law. The use of generic forms which can be utilised for multiple different types of procedures has been found to lead to insufficient documentation of clinically significant complications, and hence, the use of standardised, procedure-specific consent forms has been recommended for THA [3]. It is important to document the diagnosis and discuss alternative treatment options with patients before surgery. Alternative treatment options are one of the most commonly missed parts of informed consent forms [4]. Patients should be explained the procedure in brief in a simplified way that all patients can understand. It is also vital that the surgeon discusses the goal of the surgery with the patient, so that the patient has realistic expectations from the surgery. The expected outcome and activities they will be able to do/will not be able to do after THA were a very important part of the informed consent in the view of patients [5]. Clarification that the surgery does not guarantee complete resolution of pain is paramount. This discussion with the patient on the expected outcome of arthroplasty has been found to decrease medical malpractice claims [6]. A discussion on the prosthesis that is planned to be used is another aspect which is often wrongly omitted. The orthopaedic surgeon must discuss with the patient the implant options available, the implant the surgeon feels most appropriate for the patient and the reasons for the same, the scientific evidence present regarding the implant, and also disclose any financial relationships or conflicts of interest for the surgeon [7]. This is extremely important, as, in the event of an implant failure/breakage, patients very often allege a poor-quality implant was used [8]. Patients should also be informed about the longevity of the prosthesis and the possibility of the need for a revision procedure [5].
Patients should be explained about the common complications occurring after THA, as well as some of the rarer grave complications. While most consent forms list non-specific complications like infection, bleeding, and neurovascular injury, most consent forms are lacking in addressing complications specific to THA like limb length discrepancy, prosthesis wear/loosening, or risk of dislocation [3]. There remains confusion among orthopaedic surgeons regarding which complications to list in the informed consent, and hence, it is advisable to follow the guidelines/recommendation of a national/regional association of orthopaedic surgeons for the same. Such a recommendation is, however, absent in most countries and is the reason for a lack of uniformity and absence of standard practice. Our consent form lists all the relevant complications based on the recommendation by the British Orthopaedic Association [9], and the Hip Society (USA) [10], as well as taking into account the common causes of litigation in THA cases [11, 12]. Another aspect often neglected by orthopaedic surgeons is counselling regarding the need and course of post-operative physiotherapy and rehabilitation. Though some may contend it is not essential as it is not a part of the surgical procedure, it should not be omitted, as its significance is realised only in the event of a fall during the post-operative period or an adverse outcome occurring as a result of the patient not following the surgeon’s advice with regards to rehabilitation [8, 11]. Obtaining prior consent for photography/recording of the surgery for education purposes/publication in scientific journals is always recommended as a part of research ethics.
In the event of an unexpected situation occurring in the operation theatre, warranting a modification/abandonment of the procedure, the patient being unable to consent given being under the effect of general anaesthesia/sedation, we recommended taking in writing the preference of the patient to either plan the procedure at a later date or have a designated representative to consent for any modification/additional procedure if the need arises. This would not only safeguard the surgeon who takes actions in the best interest of the patient in the event of an unforeseeable complication but also uphold the interests of the patient to avoid an additional surgery on account of being unable to give consent at the time. The language of the consent form poses a challenge when dealing with individuals who cannot read the English language, especially in countries with diverse dialects. For the consent to be valid even for such patients in a court of law, we recommend documenting the language it was translated to, the details of the translator, and the signature of the translator as well. The translator can be any individual who can read English and translate it to a language the patient understands. Obtaining the signature of the patient and the doctor obtaining the consent is paramount. The signature of a witness is not required as per law but is recommended as an additional safeguard measure. This form is currently available only in English, and cross-cultural validations should be undertaken, so that it can be used in the other languages [13, 14].
Despite our efforts to provide an all encompassing consent form for THR, we understand that the consent form may need to be tailored according to the particular patient. While this consent form can be used is most routine cases of THR, it is a possibility that in some special cases, additional points may need to be handwritten and consent obtained for it separately. This consent form relies on medical evidence and a thorough review of the legal aspects; however, the absolute objective assessment of a consent form in terms of law is seldom possible. Only if it were to be put through a legal trial whereby the consent form was analysed and discussed, can it be thought to be full-proof, and even then would only be circumstantial for that particular case. In our opinion, it is prudent to be prepared, aware, and always in line with the law of land, and this consent form we believe will help in that regard.
Conclusion
The use of legally sound, evidence-based informed consent for total hip arthroplasty cases would be beneficial to orthopaedic surgeons and patients alike. It would uphold the rights of the patient, and promote open discussion and transparency. In the event of a lawsuit, it would be a vital document in the defence of the surgeon and withstand the scrutiny of lawyers and the judiciary.
Appendix
Annexure 1—Form A—Informed Consent
I, _________________________ hereby authorise the performance of the operation TOTAL HIP ARTHROPLASTY of the ____________ hip joint(s) of ________________________ (Myself/Name of the patient), ________ years of age, with hospital ID _________________.
I have been explained that I/the patient have been diagnosed to have _____________________________________________ since __________years.
I have been informed regarding the other treatment options for the condition including conservative management with analgesics and physiotherapy. I have also discussed other surgical options. After discussion with my doctor, we have decided to proceed with total hip arthroplasty.
I have been explained briefly regarding the steps of the procedure, including the replacement of the head and proximal part of femur and acetabulum with a prosthesis.
I understand that the procedure is being performed to attain a stable, pain-free joint and allow for easier mobilisation than the current condition. However, I also understand that the procedure does not guarantee complete resolution of pain or achieve complete range of movements of the hip joint.
I authorise Dr. _________________________ and such associates and assistants as may be selected by him/her to perform any part of the surgery upon myself/the patient.
I have discussed with the doctor regarding the prosthesis _____________________________________________________________________which is/are planned to be used for the surgery. I am satisfied with the nature and characteristics of the specific prosthesis and approve for the same to be used in the surgery. I have been explained of the possibility of the prosthesis type and design being changed during the surgery after considering the hip condition and anatomy intra-operatively. The fixation of the prosthesis may be cemented, uncemented, or hybrid. The decision on use of cement may be changed intra-operatively if found appropriate. I have been informed that in the event of the prosthesis or cement being found to be faulty, the liability would be with the manufacturer and not the operating surgeon.
I have been explained about the possible requirement for additional procedures like plate/screw/wire fixation for bony reconstruction/fixation of periprosthetic fracture, bone grafting from iliac crest or fibula. I give my consent for such procedures to be carried out in the same surgery if deemed to be required.
I have been explained about the possible complications that can occur during the surgery including, but not restricted to, excessive bleeding, infection, nerve damage, periprosthetic fracture, cement implantation syndrome, damage to femoral or external iliac blood vessels, deep vein thrombosis, pulmonary embolism, etc.
I have been explained about the need for anaesthesia and its potential risks. I have been counselled regarding the benefits and drawbacks of different methods of anaesthesia including general and regional anaesthesia. I consent for the method of anaesthesia to be used as deemed appropriate by the anaesthetist.
I also understand that the surgery may be abandoned at any point during surgery in my own interest, due to anaesthetic complications, implant issues, suspected infection, or other intra-operative complications.
I have also been explained that following the surgery the patient may have limb length discrepancy, incomplete range of movements of the hip, heterotopic ossification, dislocation, periprosthetic fracture, and implant loosening/breakage. The patient may be unable to squat or sit cross legged.
I understand the prosthesis will undergo wear with time. I understand the degree of wear and longevity of the implant will depend on my/the patient’s weight, activities, strain and, therefore, cannot be accurately predicted. Therefore, a revision surgery may be required after a couple of years. I have been told that revision surgery is often complicated and decreases the benefits with each revision.
I understand the prosthesis may be incompatible with different modes of magnetic resonance imaging. This is as per the manufacturer of the prosthesis and is beyond the scope of the surgeon.
I have been counselled regarding the need for physiotherapy and monitored rehabilitation following surgery. I have been made aware of the risk of falls, fractures, and stiffness following the surgery. I have also been informed of the increased risk of complications if I were to not follow the recommendations of the doctors in terms of weight bearing, mobilisation, follow-up visits, post-operative wound care, and physiotherapy.
I give my consent for care in an Intensive Care Unit (ICU) and ventilator support, if the need arises.
I have been informed regarding the possible need for transfusion of blood/blood products preceding/during/following the surgery and give my consent for the same. I have been informed that despite careful screening in accordance with regulations, there are rare instances of infections such as HIV and Hepatitis, or possibility of unpredictable reactions to the transfusion.
I consent to the photography/recording/viewing of the surgery for the purpose of advancing medical education, or its publication in scientific journals/presentations, provided my/the patient’s identity is not revealed in any of the texts/images/videos. I also consent for my data to be entered into national, international registries/databases for knee arthroplasty.
If you wish to specifically opt out of any of the above clauses 16, 17, or 18, kindly indicate it by listing the clause number(s) here ___________.
In the event of an unforeseeable/very rare complication occurring during the surgery, that may warrant a modification of the procedure/additional procedure/abandonment of the procedure, I prefer to (tick one):
Plan it later after my due consent is obtained when I am in a state to make an informed decision (OR)
Authorise for consent to be obtained form ________________________ with Mobile number ___________________ on my behalf.
I have fully read this consent form and comprehend all the above-mentioned points.
OR
This consent has been translated to me to the language of _______________ that I can understand by _____________________ with designation/address _____________________________________________
Signed _________________
I hereby give my consent for the surgery after having fully understood all the above aspects.
Name: ___________________________
Signature: _________________________
Date and Time: ___________________________
If consent is being obtained from someone on behalf of the patient, the reason for inability of patient to sign the consent form _____________________, and relation of individual to the patient___________________________.
Declaration by doctor:
All the above points have been explained in detail to the patient/patient representative prior to surgery.
Name: ________________________
Designation: ______________________
Signature: _______________________
Date and Time: ______________________
Declaration by witness (Optional):
I have been present and witnessed the above said doctor/translator explain the above-mentioned points to the patient/patient representative.
Name: _____________________
Designation/Address: ___________________________
Signature: _____________________
Date and Time: ________________________
Annexure 2—Form B—Informed Consent
I, _________________________ hereby authorise the performance of the operation TOTAL HIP ARTHROPLASTY of the ____________ hip joint(s) of ________________________ (Myself/Name of the patient), ________ years of age, with hospital ID _________________.
I have been explained that I/the patient have been diagnosed to have _____________________________________________ since __________years.
I have been informed regarding the other treatment options for the condition including conservative management with analgesics and physiotherapy. I have also discussed. After discussion with my doctor, we have decided to proceed with total hip arthroplasty.
I have been explained briefly regarding the steps of the procedure, including the replacement of the head and proximal part of femur and acetabulum with a prosthesis.
I understand that the procedure is being performed to attain a stable, pain-free joint and allow for easier mobilisation than the current condition. However, I also understand that the procedure does not guarantee complete resolution of pain or achieve complete range of movements of the hip joint.
I authorise Dr. _________________________ and such associates and assistants as may be selected by him/her to perform any part of the surgery upon myself/the patient.
I have discussed with the doctor regarding the prosthesis _____________________________________________________________________which is/are planned to be used for the surgery. I am satisfied with the nature and characteristics of the specific prosthesis and approve for the same to be used in the surgery. I have been explained of the possibility of the prosthesis type and design being changed during the surgery after considering the hip condition and anatomy intra-operatively. The fixation of the prosthesis may be cemented, uncemented, or hybrid. The decision on use of cement may be changed intra-operatively if found appropriate.
I have been explained about the possible requirement for additional procedures like plate/screw/wire fixation for bony reconstruction/fixation of periprosthetic fracture, bone grafting from iliac crest or fibula. I give my consent for such procedures to be carried out in the same surgery if deemed to be required.
I have been explained about the possible complications that can occur during the surgery including, but not restricted to, excessive bleeding, infection, nerve damage, periprosthetic fracture, cement implantation syndrome, damage to femoral or external iliac blood vessels, deep vein thrombosis, pulmonary embolism, etc.
I have been explained about the need for anaesthesia and its potential risks. I have been counselled regarding the benefits and drawbacks of different methods of anaesthesia including general and regional anaesthesia. I consent for the method of anaesthesia to be used as deemed appropriate by the anaesthetist.
I also understand that the surgery may be abandoned at any point during surgery in my own interest, due to anaesthetic complications, implant issues, suspected infection, or other intra-operative complications.
I have also been explained that following the surgery the patient may have limb length discrepancy, incomplete range of movements of the hip, heterotopic ossification, dislocation, periprosthetic fracture, and implant loosening/breakage. The patient may be unable to squat or sit cross legged.
I understand the prosthesis will undergo wear with time. I understand the degree of wear and longevity of the implant will depend on my/the patient’s weight, activities, strain and therefore cannot be accurately predicted. Therefore, a revision surgery may be required after a couple of years. I have been told that revision surgery is often complicated and decrease the benefits with each revision.
I understand the prosthesis may be incompatible with different modes of magnetic resonance imaging. This is as per the manufacturer of the prosthesis and is beyond the scope of the surgeon.
I have been counselled regarding the need for physiotherapy and monitored rehabilitation following surgery. I have been made aware of the risk of falls, fractures, and stiffness following the surgery.
I give my consent for care in an Intensive Care Unit (ICU) and ventilator support, if the need arises.
I have been informed regarding the possible need for transfusion of blood/blood products preceding/during/following the surgery and give my consent for the same. I have been informed that despite careful screening in accordance with regulations, there are rare instances of infections such as HIV and Hepatitis, or possibility of unpredictable reactions to the transfusion.
I consent to the photography/recording/viewing of the surgery for the purpose of advancing medical education, or its publication in scientific journals/presentations, provided my/the patient’s identity is not revealed in any of the texts/images/videos. I also consent for my data to be entered into national, international registries/databases for knee arthroplasty.
If you wish to specifically opt out of any of the above clauses 16, 17 or 18, kindly indicate it by listing the clause number(s) here ___________.
In the event of an unforeseeable/very rare complication occurring during the surgery, that may warrant a modification of the procedure/additional procedure/abandonment of the procedure, I prefer to (tick one):
Plan it later after my due consent is obtained when I am in a state to make an informed decision (OR)
Authorise for consent to be obtained form ________________________ with Mobile number ___________________ on my behalf.
I have fully read this consent form and comprehend all the above-mentioned points.
OR
This consent has been translated to me to the language of _______________ that I can understand by _____________________ with designation/address _____________________________________________
Signed _________________
I hereby give my consent for the surgery after having fully understood all the above aspects.
Name: ___________________________
Signature: _________________________
Date and Time: ___________________________
If consent is being obtained from someone on behalf of the patient, the reason for inability of patient to sign the consent form _____________________, and relation of individual to the patient___________________________.
Declaration by doctor:
All the above points have been explained in detail to the patient/patient representative prior to surgery.
Name: ________________________
Designation: ______________________
Signature: _______________________
Date and Time: ______________________
Declaration by witness (Optional):
I have been present and witnessed the above said doctor/translator explain the above-mentioned points to the patient/patient representative.
Name: _____________________
Designation/Address: ___________________________
Signature: _____________________
Date and Time: ________________________
Funding
None.
Declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
HOSMAT Hospital, Bengaluru.
Informed Consent
For this type of study, informed consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Beresford-Cleary N, Halliday J, Biant L, Breusch S. Consent process for elective total hip and knee arthroplasty. Journal of Orthopaedic Surgery. 2011 doi: 10.1177/230949901101900302. [DOI] [PubMed] [Google Scholar]
- 2.Polit DF, Beck CT. The content validity index: Are you sure you know what's being reported? Critique and recommendations. Research in Nursing & Health. 2006;29(5):489–497. doi: 10.1002/nur.20147. [DOI] [PubMed] [Google Scholar]
- 3.Mussa MA, Sweed TA, Khan A. Informed consent documentation for total hip and knee replacement using generic forms with blank spaces. Journal of Orthopaedic Surgery (Hong Kong) 2014;22(2):214–217. doi: 10.1177/230949901402200220. [DOI] [PubMed] [Google Scholar]
- 4.Tonge XN, Crouch-Smith H, Bhalaik V, Harrison WD. Do we achieve the Montgomery standard for consent in orthopaedic surgery? British Journal of Hospital Medicine (London, England) 2021;82(4):1–7. doi: 10.12968/hmed.2020.0504. [DOI] [PubMed] [Google Scholar]
- 5.Sandiford NA, Mahendra M, Wickramarachchi L, Back D, Bansal M. Informed consent in patients undergoing primary hip and knee arthroplasty: What do patients want to know? Cureus. 2020;12(6):e8457. doi: 10.7759/cureus.8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark CR, Huddleston HD, Schoch EP, 3rd, Thomas BJ. Leg-length discrepancy after total hip arthroplasty. Journal of American Academy of Orthopaedic Surgeons. 2006;14(1):38–45. doi: 10.5435/00124635-200601000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Prokopetz JJ, Katz JN, Losina E, Thornhill TS, Wright J, Lehmann LS. Preceding the procedure: Medical devices and shared decision making. Arthritis Care & Research (Hoboken). 2013;65(1):148–151. doi: 10.1002/acr.21826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiwari S, Baldwa M, Tiwari M, Kuthe A. Textbook on medico legal issues related to various medical specialities. 1. New Delhi: Jaypee Brothers; 2019. pp. 301–306p. [Google Scholar]
- 9.British Orthopaedic Association. Informed consent fortotal hip replacement. Retrieved Oct 17, 2021 from https://www.orthoconsent.com/documents/THR(4)(2).doc
- 10.Healy WL, Iorio R, Clair AJ, Pellegrini VD, Della Valle CJ, Berend KR. Complications of total hip arthroplasty: standardized list, definitions, and stratification developed by the hip society. Clinical Orthopaedics and Related Research. 2016;474(2):357–364. doi: 10.1007/s11999-015-4341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pai S. Medico-legal issues related to hip and knee arthroplasty: A literature review including the indian scenario [published online ahead of print, 2021 Mar 30] Indian Journal of Orthopaedics. 2021 doi: 10.1007/s43465-021-00398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samuel LT, Sultan AA, Rabin JM, et al. Medical malpractice litigation following primary total joint arthroplasty: A comprehensive, nationwide analysis of the past decade. Journal of Arthroplasty. 2019;34(7S):S102–S107. doi: 10.1016/j.arth.2019.02.066. [DOI] [PubMed] [Google Scholar]
- 13.Monticone M, Capone A, Frigau L, et al. Development of the Italian version of the High-Activity Arthroplasty Score (HAAS-I) following hip and knee total arthroplasty: cross-cultural adaptation, reliability, validity and sensitivity to change. Journal of Orthopaedic Surgery and Research. 2018;13(1):81. doi: 10.1186/s13018-018-0782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerciello S, Corona K, Morris BJ, et al. Cross-cultural adaptation and validation of the Italian versions of the Kujala, Larsen, Lysholm and Fulkerson scores in patients with patellofemoral disorders. Journal of Orthopaedics and Traumatology. 2018;19(1):18. doi: 10.1186/s10195-018-0508-9. [DOI] [PMC free article] [PubMed] [Google Scholar]