Highlights
-
•
Effect of three cooking methods on taste, flavor and chemical profiles of yellow-fleshed sweetpotato was investigated.
-
•
More soluble sugar, more furans and terpenes were enriched, making the baked YFSP the sweetest and most flavorful.
-
•
72 VOCs and 706 metabolites were identified in cooked YFSP and broad insight was provided in cooked table-stock sweetpotatoes.
-
•
Bioactive substances were preserved maximally in cooked YFSP.
Keywords: Yellow-fleshed sweetpotato, Sweetness, Carotenoids, Volatile compounds, Metabolites
Abstract
This study investigated the impact of baking, boiling, and steaming on the taste, flavor, and chemical profile of yellow-fleshed sweetpotatoes (YFSP). Baked YFSP were sweeter, more palatable, and more flavorful than both steamed and boiled YFSP. Baking increased the YFSP soluble sugar content from 9.12% to 36.65%. Specifically, maltose increased by 200-fold and this possibly accounted for the sweetness of baked YFSP. From the Gas Chromatography-Mass Spectrometry analysis, the contents of furans and terpenes increased with baking, endowing baked YFSP with an aroma. On the contrary, boiling retained more carotenoids than the other cooking methods. Although cooking clearly altered YFSP, bioactive substances were predominantly preserved as only 72 out of 706 metabolites were identified as differentially accumulated metabolites between cooked and raw samples. Taken together, baked YFSP had high levels of sugars and volatile compounds, and the three cooking methods had little effect on chemical compounds. This comprehensive evaluation of cooked YFSP is a basis for sweetpotato processing and consumer choice.
1. Introduction
Sweetpotato is the third most important crop — in terms of production value — in the world and historically served as a famine-relief crop (CIP, 2022). In Pacific communities, it is cultivated as part of food security and climate change adaptation projects (Iese, Holland, Wairiu, Havea, et al., 2018). As a food crop, it not only has a high yield, but also diverse nutrients like carbohydrates, proteins, carotenoids, flavonoids, anthocyanins, phenolic acids, and various vitamins and minerals (Alam, 2021). Traditionally, sweetpotato was cultivated as a source of starch, table-stock, processed food, and animal feed. In recent years, consumers prefer whole sweetpotatoes due to their useful bioactive compounds and health benefits. Thus, the proportion of table-stock has gradually increased, accounting for 40–50 % in China and Japan (Katayama, Kobayashi, Sakai, Kuranouchi, et al., 2017). Among them, yellow and orange-fleshed sweetpotatoes are predominant. Thus, the flavor and nutritional properties of table-stock sweetpotatoes with pigmented flesh are of much interest to the public, especially the health-conscious food market. Relatedly, flavor and nutrient retention during processing are important evaluation indicators for table-stock sweetpotato breeding.
Harvested table-stock sweetpotatoes are domestically consumed after steaming, boiling, or baking. Different kinds of cooking methods improve the sensory and textural features of the sweetpotatoes. Moreover, thermal processes during cooking may also alter chemical compounds in the sweetpotato. Most studies on sweetpotato processing focused on the content and structure of starch, total sugars, total carotenoid, and antioxidant activities (Franková et al., 2022, Kourouma et al., 2019). For example, the starch content and final amylase activity reduced during cooking whereas the content of reducing sugars increased (Ogliari, Soares, Teixeira, Schwarz, et al., 2020). The total carotenoid content in sweetpotato decreased after cooking and compared to microwaving and frying, boiling and steaming maintained the carotenoid content and antioxidant activities of orange-fleshed sweetpotatoes (Islam et al., 2016, Kourouma et al., 2019). Thermal processing, especially deep-frying, significantly increased not only the total phenolic content but also individual phenolic acids and antioxidant activities of sweetpotatoes. This could be explained by the fact that heat processing possibly damaged root cellular structures, resulting in easier extraction of antioxidant components either from the root itself or from its peel (Bellail, Shaltout, Youssef, & Gamal, 2012). However, a systemic assessment of the dynamic changes in the flavor and chemical profile of sweetpotatoes when cooked by different methods remains to be undertaken. Understanding the chemical profile in relation to cooking methods may provide new dietary health insights into the nutritional and bioactive substances of sweetpotatoes, and therefore promote technological improvements in the sweetpotato processing industry. Currently, metabolomics based on gas and liquid chromatography-mass spectrometry (GC/LC-MS) for the determination of volatile and non-volatile metabolites is a basis for comprehensive evaluation of the volatile compounds and metabolites in sweetpotatoes. However, there is a paucity of studies on changes in metabolites of table-stock sweetpotato with yellow flesh treated by different cooking methods, via the use of metabolomics assays.
Yellow-fleshed sweetpotatoes (YFSP) are the traditional predominant edible sweetpotatoes in Africa and Asia (Tomlins et al., 2007, Xie et al., 2018). Compared to orange and purple flesh sweetpotatoes, YFSP are more palatable and flavorful. In this study, Guangshu11-139, a new YSFP with a sweet, starchy, moisty and pasty taste and typical sweetpotato aroma, was selected to systemically assess the dynamic changes of taste, flavor and chemical profile when cooked with different methods, by combining traditional analytical techniques and high throughput detection methods. These findings are both a baseline for understanding the preservation of nutritional and functional ingredients in domestically cooked table-stock sweetpotato and a basis for the consumption of sweetpotatoes within a healthy diet. At the same time, these findings provide insights that are valuable for table-stock sweetpotato breeding.
2. Materials and methods
2.1. Plant materials, cooking methods, and sample preparation
The YFSP (cv. Guangshu11-139) was bred by the Crops Research Institute of Guangdong Academy of Agricultural Sciences (Guangzhou, China) and used as a core breeding parent for starchy, sweet, and flavorful sweetpotatoes, which were descendants of Jieshu95-16 and Guangshu79. It inherited excellent crop yield, quality, and sensory taste traits from its parents. It was cultivated randomly in a field plot according to standard agricultural practices at the Baiyun Experimental Station (23°23́N, 113°26́E; 20 m above sea level) of Guangdong Academy of Agricultural Sciences, Guangzhou, China. They were planted in May and transplanted in July. The roots were harvested 130 days after transplantation.
Twelve medium-sized (100–150 g) sweetpotatoes were selected, cleaned with distilled water, air-dried, weighed, and randomly divided into four groups. One group was kept as a raw sample and each of the other three groups was processed by a specific cooking method. Detailed information on the cooking methods is shown in Table S1. All samples were unpeeled before the heat treatment. For steaming, an entire root was put in a plate and steamed for 40 min. For baking, a whole root was baked in an oven for 90 min at 200 °C. For boiling, whole roots were cooked in boiling water for 30 min.
The raw and cooked samples were cut into small pieces and frozen in liquid nitrogen. Then, half of each sample was ground to powder in a liquid nitrogen grinder (A10 basic, IKA, Staufen, Germany) and the remaining half was freeze-dried for 72 h and then ground into powder. Sample powders were then stored at −80 °C until further experimentation.
2.2. Determination of the color of sweetpotato flesh
The color parameters for different sweetpotato samples were detected using a spectrophotometer (CM-700d/600d, Konicaminolta, Japan) according to the manufacturer’s instructions. The spectrophotometer was calibrated using standard white before sample measurement. Measurements for each sample were done in triplicates based on three parameters: L (lightness), a (redness and greenness), and b (yellowness and blueness).
2.3. Sensory analysis
Sensory analysis was performed as described (Zhang, Tang, Jiang, Mo, et al., 2021) by ten well-trained panelists. Eight sensory descriptors, including firmness, aroma, sweetness, starchiness, viscosity, coarse texture, fibrous texture, and overall taste were selected to assess the sweetpotato samples. The aroma intensity was described from zero (not aromatic) to three (intensely aromatic), and the overall taste was evaluated out of a maximum score of 100. The intensity of other descriptors was evaluated on a five-point scale from zero (not perceivable) to five (strongly perceivable).
2.4. Determination of total starch and amylose contents
The total starch and amylose contents were respectively measured using a Total Starch Assay Kit (Megazyme, Ireland) and Amylose/Amylopecin Assay Kit (Megazyme, Ireland) according to the manufacturer’s instructions.
2.5. Determination of the soluble sugar content
2.5.1. Detection of the total soluble sugar content
The total soluble sugar was determined using anthrone-H2SO4 method (Guo, Liu, Xu, Zhang, et al., 2019). Briefly, 0.1 g of freeze-dried sample powder was put into a 15 ml tube containing 10 ml 80 % ethanol (v/v). The sample was incubated at 80 °C for 30 min, then at room temperature for 5 min before centrifugation at 4000 rpm for 10 min. The supernatant was decanted and mixed with 10 ml 80 % ethanol (v/v) for a second extraction before dilution to a final volume of 25 ml with 80 % ethanol. Then, 100 μl of the extract solution was mixed with an equal amount of distilled water and 3 ml anthrone solution, before incubation at 100 °C for 10 min. The absorbance was determined by a spectrophotometer at 620 nm with a calibration curve of d-glucose as the standard.
2.5.2. Determination of soluble sugars concentration by HPAEC
About 10 mg of freeze-dried sample powder was placed in a 2 ml tube containing 0.7 ml 80 % ethanol (v/v). This was mixed by vortexing before incubation at 50 °C for 2 h. To this, 0.7 ml ultra-pure water (Millipore, Bedford, MA, USA) was added before centrifugation at 12000 rpm for 5 min. The extracts were then injected into a high-performance anion-exchange chromatography (HPAEC) system equipped with a CarboPac PA-1 anion-exchange column (4.0 * 250 mm; Dionex) and a pulsed amperometric detector (PAD; Dionex ICS 5000 system). The flow rate was 1.0 ml/min and injection volume was 10 μl. The column temperature was 30 °C. The gradient programs (H2O:0.1 M NaOH) were: 95:5V/V from 0 min, 95:5 V/V at 9 min, 0:100 V/V at 20 min, 0:100 V/V at 40 min, 95:5 V/V at 40.1 min, 95:5 V/V at 60 min. Data were acquired with an ICS5000 system (Thermo Scientific) and processed using Chromeleon 7.2 CDS (Thermo Scientific). Identification of soluble sugars was based on the retention times of pure standards and quantification was done by external calibration (Table S2).
To ascertain and compare sweetness levels in the samples, sweetness (sucrose equivalent) was calculated using this equation: Sucrose Equivalent (SE) = 1.2 fructose + 1 sucrose + 0.64 glucose + 0.43 maltose (Kays, Wang, & McLaurin, 2005).
2.6. Determination of volatile compounds by GC–MS
A gram of frozen sample powder was stored in 2 ml of saturated NaCl solution in a 20 ml headspace vial. To this, 5 μl of 1-heptanol (0.137 μg ml−1, CAS: 111-70-6) was added as an internal standard and the mixture was gently homogenized. The raw sample was incubated at 40 °C for 30 min and the cooked samples were incubated at 70 °C for 30 min. Volatile compounds were then extracted by an SPME device with a DVB/CAR/PDMS fiber for 30 min at the same temperature.
The extracted volatile components trapped by the SPME fiber were desorbed in the GC injection port for 3 min at 250 °C in splitless mode. GC–MS analysis was performed using an Aglient 7890B gas chromatography system coupled to a 5977B mass spectrometer (Agilent Technologies, CA, USA) and equipped with an HP-5 ms column (30 m, 250 μm, 0.25 μm) (Agilent Technologies, CA, USA). A constant helium flow of 1 ml min−1 was used for chromatographic separation. The oven temperature program was: an initial 35 °C for 2 min, 5 °C min−1 ramp up to 190 °C, hold for 1 min, 20 °C min−1 ramp up to 250 °C, hold for 2 min, and solvent delay 1 min. The detector was operated in electron impact (EI) ionization mode at 70 eV in a full scan mode with the mass/charge ratio (m z−1) range set from 35 to 450.
Chromatograms and mass spectra were analyzed using the Enhanced ChemStation software (Agilent Technologies, CA, USA). Identification of putative volatile compounds was achieved by querying mass spectra against the data system library (NIST 2017) and linear retention index (RI) of the NIST Standard Reference Database. The relative content of each compound was calculated from the peak area of the internal standard. All analyses were done in triplicates.
2.7. Detection of metabolites by LC-MS
2.7.1. Sample preparation
The frozen fresh sample powders (100 mg) were homogenized in 100 μl of ice-cold H2O for 60 s using a homogenizer. To this, 400 μl of a methanol/acetonitrile solution was added for metabolite extraction. This solution was then sonicated for 30 min at 4 °C. Following centrifugation at 12,000 rpm at 4 °C for 20 min, the supernatant was freeze-dried and dissolved in 200 μl of 30 % ACN (v/v) (Ghirardo et al., 2020, Lee et al., 2019).
2.7.2. Identification and quantification of metabolites
The sample extracts were injected into a UPLC–Orbitrap-MS system (UPLC, Vanquish; MS, QE), equipped with a Waters HSS T3 column (50*2.1 mm, 1.8 μm) (Yang, Mei, Wang, Chen, et al., 2021). The mobile phase consisted of pure water with 0.1 % acetic acid (solvent A) and acetonitrile with 0.1 % acetic acid (solvent B). Samples were assessed via an isocratic elution (solvent A:solvent B): 90:10 (v/v) and an elution duration of 9.0 min. The column temperature was set to 40 °C. The flow rate was 0.3 ml/min and the injection volume was 2 μl. HRMS data were recorded on a Q Exactive hybrid Q–Orbitrap mass spectrometer equipped with a heated electrospray ionization (ESI) source (Thermo Fisher Scientific) utilizing SIM MS acquisition methods. The raw MS data were acquired on the Q-Exactive using Xcalibur 4.1 (Thermo Scientific) and processed using Progenesis QI (Waters Corporation, Milford, USA). A mixture of all samples was used for quality control to evaluate the technical reproducibility. An OPLS-DA model was made from comparisons between each cooked and raw sample, which presented the differences. The variable influence on projection (VIP) value was then calculated by this model. Metabolites with VIP value >1 were further assessed using a univariate Student’s t-test to determine the significance of the differences in concentration of the same metabolite from cooked and the raw samples —p values less than 0.05 were considered statistically significant.
2.8. HPLC determination of carotenoid components
2.8.1. Carotenoid extraction
Carotenoids were extracted by the method of Ma et al (Ma, Zhang, Iida, Madono, et al., 2017) with slight modifications. Fifty-milligrams of freeze-dried powder was extracted with a solvent containing n-hexane:acetone:ethanol (1:1:1, V/V/V) and 0.01 % BHT. To this, an internal standard — β-apo-8′-carotenal (Sigma, MO, USA) — was added. The extract was vortexed for 20 min at room temperature (22 °C) then centrifuged, before collection of the supernatant. The residue was re-extracted as aforementioned. The solvent extract was evaporated to dryness under a nitrogen gas stream and resuspended in a solution of methanol:methyltert-butyl ether (1:1, V/V), which was then filtered through a 0.22 μm pore-size nylon membrane syringe filter (ANPLE, Shanghai, China) for further HPLC analysis.
2.8.2. Quantification of carotenoid by HPLC
The carotenoid contents were detected according to a previously reported method (Liu, Lv, Liu, Wang, et al., 2020). Sample extracts were injected into an HPLC system (Agilent 1260) equipped with YMC C30 column and i.d. 2 × 100 mm columns. Mobile phases were MeCN-MeOH (3:1, V/V) with 0.01 % BHT and 0.1 % formic acid (eluent A) and 100 % MTBE with 0.01 % BHT (eluent B). Methanol was used to wash the probes. The flow rate was set to 0.8 ml/min, the column temperature to 28 °C, and the injection volume was 2 μl. The gradient program (eluent A: eluent B) was as follows: 100:0 V/V from 0 min, 30:70 V/V at 5.0 min, 5:95 V/V at 9.0 min, 100:0 V/V at 9.1 min, 100:0 V/V at 11.0 min. Identification of the carotenoids was by the combined analyses of the retention times and co-chromatography with pure standards while quantification was done by external calibration (Table S3). The following are the carotenoid standards used in this study: Phytoene (CAS: 540-04-5), ε-Carotene (CAS: 472-89-9), α-Carotene (CAS: 7488-99-5), β-Carotene (CAS: 7235-40-7), Lycopene (CAS: 502-65-8), Violaxanthin (CAS: 126-29-4), Neoxanthin (CAS: 14660-91-4), Antheraxanthin (CAS: 640-03-9), Lutein (CAS: 127-40-2), Zeaxanthin (CAS: 144-68-3), α-Cryptoxanthin (CAS: 24480-38-4), β-Cryptoxanthin (CAS: 472-70-8), γ-Carotene (CAS: 472-93-5) and δ-Carotene (CAS: 31063-33-9).
2.9. Statistical analysis
Each group was assessed with either triplicates or sextuplicates to ensure reliability of the experimental results. All data were analyzed using SPSS statistics 24 and expressed as mean ± SD. One-way analysis of variance (ANOVA) was implemented and followed by Tukey’s significant difference test at an alpha level of 0.05. The unsupervised principal component analysis (PCA) score plot and heatmap were drawn using the prcomp, ggplot2 (v3.3.5) and pheatmap (v1.0.12) packages of R (x64 4.1.2). The data used to draw the heatmap was normalized and scaled by raw values.
3. Results and discussion
3.1. Phenotypic investigation
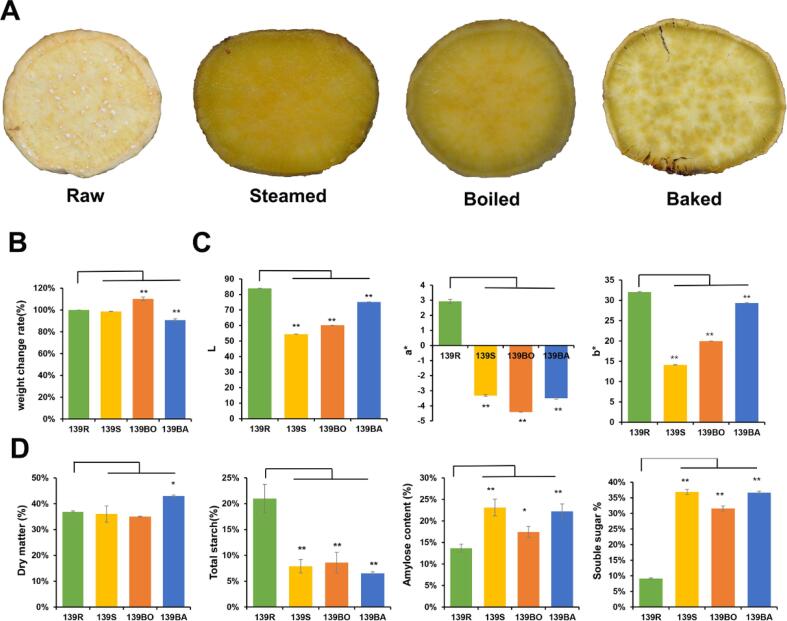
To investigate the effect of different cooking methods on the appearance of YFSP, we assessed the weight and color of the flesh (Fig. 1A). The weight of the boiled sweetpotato was significantly heavier than that of the raw sample whereas that of the baked sweetpotato was significantly lighter (Fig. 1B). The effect of steaming on the weight of the sweetpotato was negligible. We hypothesize that changes in the weight of cooked YFSP are related to changes in their moisture content. The boiled samples absorbed the most water whereas baked samples lost the most.
Fig. 1.
Variation of yellow-fleshed sweetpotato with different cooking methods. (A) Morphological observation of different cooking methods of processed sweetpotatoes. (B) The investigation of sweetpotato weight change rate. The average weight of treated sweetpotato was divided by the original weight. (C) Spectrophotometer detection of 3 color parameters values (L, a*, b*) of sweetpotato. (D) Investigation of dry matter, starch content and soluble sugar content in different cooking methods of yellow-fleshed sweetpotato. Statistically significant differences based on t-test (**P < 0.01, *P < 0.05).
The color parameters (L*, a*, b*) of the raw and cooked sweetpotato samples are shown in Fig. 1C. L* significantly decreased in cooked YFSP and was the least in the steamed sample. This illustrated that all three cooking methods reduced the brightness of sweetpotato flesh, with steaming reducing it the most. Similarly, both a* and b* were significantly lower in cooked than raw samples. The least values for a* and b* were for the boiled and steamed samples respectively. Overall, the three cooking methods significantly impacted the appearance of sweetpotatoes. The appearance of steamed and boiled YFSP was more alluring and palatable due to the golden color and moist textured flesh respectively, whereas baked YFSP flesh had a relatively light color and solid texture. During cooking, the starch absorbs water and swells, causing drastic damage to the cell walls. However, the water in baked samples dries out due to high amounts of dry heat whereas the steamed and boiled samples retain water resulting in their moist texture (Valetudie, Gallant, Bouchet, Colonna, et al., 1999).
3.2. Sensory evaluation
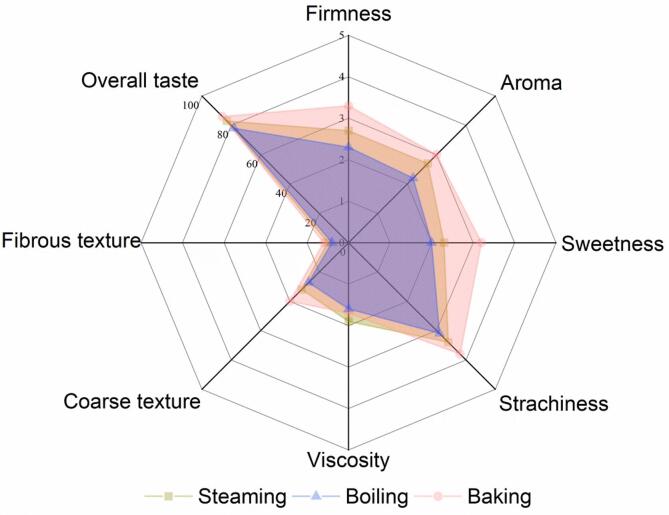
Sensory evaluation directly reflects the differences between sweetpotatoes that are cooked differently. The overall taste score of Guangshu11-139 cooked by various methods was around 80 (Fig. 2), which presented a high acceptability of cooked sweetpotato, such a score promotes sweetpotato breeding. Baked YFSP were assessed as more sweet, flavorful, and starchy yet firmer than the rest (Fig. 2). Based on its excellent taste, it had the highest overall acceptability. Boiled YFSP were softer and moister than the rest but had the worst overall taste since they had less sweetness and aroma. For steamed YFSP, their score for each descriptor was between those of baked and boiled samples and its taste is indeed between the two. The sensory evaluation results corroborated those of the appearance analysis — the boiled and steamed samples were moister whereas the baked sample was firmer.
Fig. 2.
Comprehensive sensory evaluation of yellow fleshed sweetpotato with different cooking method.
3.3. Starch content
Tomlins et al. showed that the sensory attributes of sweet taste, starchy and watery textures were correlated with the dry matter content (Tomlins, Owori, Bechoff, Menya, et al., 2012). Therefore, the dry matter content was determined to evaluate the effect of different cooking methods on YFSP. The dry matter of the raw sample was 37 %, whereas that of the baked sample was significantly higher at 47 %. Those of steamed and boiled samples (36 % and 35 % respectively, Fig. 1D) did not significantly differ from that of the raw sample. The high dry matter of baked YFSP means it had the least moisture content, implying that the highest water dehydration occurred during baking, hence the firm texture of baked YFSP.
The total starch and amylose content of the YFSP samples were measured since starch is the main component of the sweetpotato dry matter (Fig. 1D). The total starch content in the raw samples was 20.99 % of the fresh weight (56.72 % of the dry weight (DW)) and reduced to 7.89 % (21.92 % DW), 8.58 % (24.51 % DW) and 6.50 % (15.11 % DW) in the steamed, boiled and baked samples, respectively. The total starch content significantly decreased due to cooking, yet no significant difference was observed between cooked groups. This demonstrated that starch content reduced after cooking yet the cooking method did not significantly influence this reduction. Moreover, the percentage of amylose in the total starch was significantly higher in cooked than raw samples — the amylose contents in raw, steamed, boiled, and baked samples were 13.67 %, 23.12 %, 17.42 %, and 22.22 %, respectively. This higher measured amylose content of cooked samples is probably related to starch dextrins generated by alpha amylase during heating.
3.4. Soluble sugar content
The soluble sugar content is directly related to the sweetness of a cooked sweetpotato. Thus, it was measured to investigate how it varies with the cooking method. It significantly differed between raw (9.12 %) and cooked samples —36.87 %, 31.56 % and 36.65 % for steamed, boiled and baked samples respectively. There was significantly less total soluble sugar content in boiled than steamed and baked samples. Generally, cooking significantly increased the total soluble sugar content corroborating findings that cooking decreased starch and increased reducing sugar contents, respectively (Wei, Lu, & Cao, 2017).
To determine which kinds of sugars were altered by cooking, we assessed the samples for 13 different kinds of soluble sugars. In total, five sugars (galactose, glucose, fructose, sucrose, and maltose) were detected in all samples (Table 1). Sucrose was the main sugar in raw samples (44.55 ± 0.21 μg/mg) and significantly decreased after steaming and boiling, but remained the same after baking. Maltose was the dominant soluble sugar in cooked samples, and its content increased with cooking from 0.66 ± 0.01 μg/mg in raw samples to 107.64 ± 1.92 μg/mg, 74.64 ± 1.66 μg/mg, and 130.55 ± 2.14 μg/mg in steamed, boiled, and baked samples respectively. Likewise, the fructose content increased significantly after cooking and was the highest in the baked samples (6.44 ± 0.09 μg/mg). This increase in fructose content is probably due to sucrose hydrolysis during cooking. The galactose content was the least among the soluble sugars and increased significantly after cooking. Salvador et al. reported high amounts of galactose in the cell wall of sweetpotato tubers (Salvador, Suganuma, Kitahara, Tanoue, et al., 2000). Thus, we hypothesize that the increased galactose content is due to homogalacturonan, important component of pectin in cell wall, was depolymerized during thermal treatments at temperatures higher than 80 °C by a beta-eliminative reaction (Moens, Plas, Van Ceunebroeck, Van Loey, et al., 2021). The glucose content increased in steamed and baked samples but decreased in the boiled samples. In general, sucrose was the main soluble sugar in raw YFSP and its content reduced slightly after cooking since it was broken down to maltose by cooking, which thus became the main soluble sugar in cooked YFSP.
Table 1.
The soluble sugars content (µg/mg on dry weight basis) in YFSP with different processing methods.
| Item | Galactose | Glucose | Fructose | Sucrose | Maltose | SE* |
|---|---|---|---|---|---|---|
| 139R | 0.06 ± 0.00c | 4.58 ± 0.02c | 3.41 ± 0.06d | 44.55 ± 0.21a | 0.66 ± 0.01d | 51.87 |
| 139S | 0.14 ± 0.00a | 4.98 ± 0.10b | 4.59 ± 0.00b | 39.81 ± 0.29b | 107.64 ± 1.92b | 94.80 |
| 139BO | 0.15 ± 0.00a | 3.08 ± 0.08d | 3.61 ± 0.04c | 39.05 ± 0.60b | 74.64 ± 1.66c | 77.44 |
| 139BA | 0.11 ± 0.00b | 7.76 ± 0.06a | 6.44 ± 0.09a | 44.47 ± 0.41a | 130.55 ± 2.14a | 113.30 |
*SE: Sucrose Equivalent, calculated to evaluate the sweetness level.
Different letters represent significant (p < 0.05) differences between means according to ANOVA combined with Duncan’s multiple range test. Each value represents the mean ± standard deviation (n = 3).
These changes in soluble sugar contents after cooking corroborate sucrose and maltose as the dominant soluble sugars in raw and cooked sweetpotatoes respectively (Adu-Kwarteng, Sakyi-Dawson, Ayernor, Truong, et al., 2014). During cooking, gelatinized starch is hydrolyzed by three enzymes: α-amylase, β-amylase, and starch phosphorylase. At the onset of gelatinization, α-amylase rapidly hydrolyzes starch into small-chain dextrins. β-Amlyase is the most abundant among the three and accounts for up to 5 % of the total protein content of sweetpotato roots (Banda, Kyallo, Domelevo Entfellner, Moyo, et al., 2021). It is thermostable, and during cooking it hydrolyzes the second α-1,4-glycosidic bond from the non-reducing end of starch, releasing maltose and maltodextrins (Chalse, Suryawanshi, Kharat, Mundhe, et al., 2016). The variations in soluble sugar content with the cooking method are probably due to differences in β-amylase activity, which is influenced by the cooking temperature.
To study the effect of the cooking methods on the sweetness of YFSP, the sucrose equivalent (SE) was calculated from the individual sugars in the YFSP samples based on the sweetness factors. The contribution of galactose to sweetness has been overlooked due to its low content and sweetness factor. All cooked sweetpotatoes were sweeter than the raw ones —the sweetest were baked, then steamed then boiled sweetpotatoes, which corroborated the sensory evaluation.
3.5. Volatile metabolite profiles
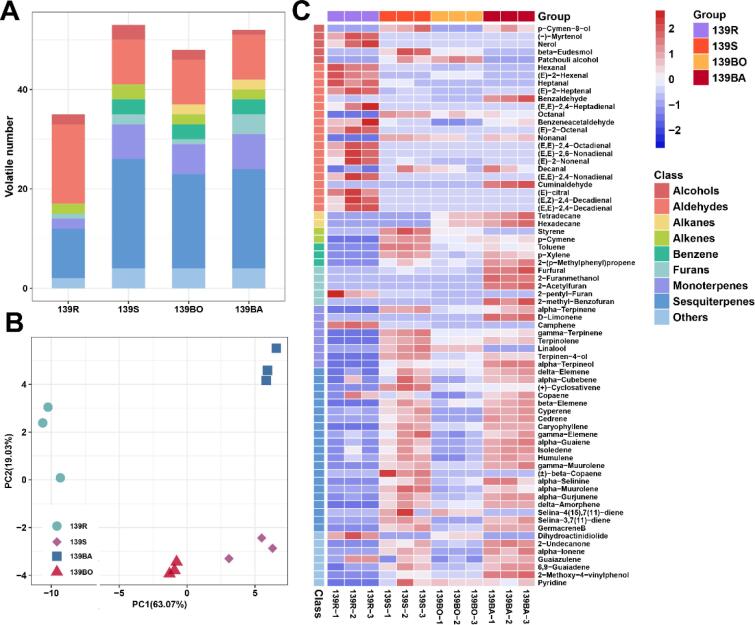
In addition to texture and sweetness, different cooking methods clearly affected the aroma of sweetpotatoes. To comprehensively evaluate the change in putative volatile organic compounds (VOCs) before and after steaming, boiling, and baking of sweetpotatoes, volatile metabolomic analyses were done using GC–MS. Consequently, 72 volatile compounds were identified from all experimental groups. Detailed information on volatile compounds and their relative content is given in Table S5. In total, 35, 53, 48, and 52 VOCs were identified in the raw (139R), steamed (139S), boiled (139BO) and baked samples (139BA), respectively (Fig. 3A). Based on their chemical structures, the VOCs were grouped into nine distinct chemical classes: alcohols, aldehydes, alkanes, alkenes, benzene, furans, monoterpenes, sesquiterpenes, and others. Aldehydes and terpenes (monoterpenes and sesquiterpenes) accounted for a large proportion of VOCs in all experimental groups (Fig. 3A). Fifteen aldehydes were identified in raw samples and nine from each of the cooked groups. Thirty terpenes, including nine monoterpenes and 21 sesquiterpenes were identified, among which three monoterpenes and 10 sesquiterpenes were detected in raw samples whereas approximately double the number of these two clusters were identified in the three cooked groups (Fig. 3A). Three benzene VOCs were uniquely detected in cooked samples. Likewise, two alkanes were uniquely detected in boiled and baked samples (Table S5).
Fig. 3.
Analysis of volatile compounds in the yellow-flesh sweetpotato treated by different cooking methods. (A) Category statistics for volatile compounds in each experimental group. (B) The PCA score plots, each dot represents an independent experimental repeat. (C) Heat map visualization of all the metabolites. The red blocks indicate the up-regulated metabolites, blue blocks indicate down-regulated metabolites, white blocks represent the average relative expressed intensity of all volatile compounds.
Based on the accumulation level of different VOCs, a principal component analysis (PCA) was conducted. The four experimental groups were clearly distinct. The first (PC1) and second components (PC2) accounted for 63.07 % and 19.03 % variance, respectively, which summed up to 82.1 % (Fig. 3B). From the PCA analysis, the VOCs of sweetpotatoes that were cooked differently were largely distinct.
To better understand the accumulation level differences of VOCs from each experimental group, a heatmap was drawn (Fig. 3C) and differences in their relative contents were assessed by one-way ANOVA (Table S5). The raw samples were clearly distinguishable from cooked samples and most VOCs except aldehydes had higher levels of accumulations in cooked than raw samples (Fig. 3C). The VOCs in steamed and baked samples had similar levels of accumulation, with many of their VOCs having higher levels than those in raw samples.
The highest relative VOC content in the raw samples was aldehydes, accounting for 71.85 % of the total VOCs. Yet its content was lower than the 20 % seen in all three cooked groups (Table S5). As shown in Fig. 2C, a large proportion of the aldehydes in the raw sample had higher levels of accumulation level than in cooked sweetpotatoes. Among them, hexanal, (E)-2-heptenal, benzaldehyde, benzeneacetaldehyde, (E)-2-nonenal and decanal were common in all four groups whereas seven aldehydes, including (E,E)-2,4-Heptadienal, (E)-2-Octenal, (E,E)-2,4-Octadienal, (E,E)-2,6-Nonadienal, (E,E)-2,4-Nonadienal, (E,Z)-2,4-Decadienal and (E,E)-2,4-Decadienal were specific to the raw samples. The relative contents of hexanal, (E)-2-heptenal, (E)-2-nonenal and benzeneacetaldehyde were significantly higher in the raw than cooked samples, and (E)-2-Octenal and hexanal were the most and second most abundant VOCs respectively in the raw samples (Table S5). Hexanal and (E)-2-Octenal have strong antifungal activity and are natural metabolizable fungicides (Liarzi, Benichis, Gamliel, & Ezra, 2020). The effectiveness of hexanal in preventing the growth of postharvest pathogens of fruits, vegetables, and crops is well established (Utto et al., 2008, Zhang et al., 2021, Zhang et al., 2018) and (E)-2-Octenal inhibited and killed the fungus Sclerotium rolfsii both in vitro and in the soil (Liarzi, Benichis, Gamliel, & Ezra, 2020). This large amount of hexanal and (E)-2-Octenal may thus be related to the antifungal activity of raw sweetpotatoes. Except for benzaldehyde and benzeneacetaldehyde, all aforementioned aldehydes arose from the enzymatic oxidation of linoleic acid, which contributes to fat, tallow, or green flavors, resulting in inferior flavors (Dudareva, Klempien, Muhlemann, & Kaplan, 2013) that may be important contributors to the flavor of raw sweetpotatoes. Overall, we hypothesize two reasons for the decreased total aldehyde content in the cooked samples. First, a few volatile low molecular weight aldehydes were released by heating. Second, for volatile compounds extracted by HS-SPME the relative content was reported. For the cooked samples, volatile compounds were added to the headspace, which caused the relative content of extracted aldehydes in the fiber to be lower than in the raw samples. This resulted in low detection rates depending on the use of HS-SPME/GC–MS; a few volatile compounds that had very low levels were not detected.
Terpenes are an important aromatic class of VOCs since they are the largest and most diverse class of secondary metabolites. Monoterpenes and sesquiterpenes are common VOCs in sweetpotatoes. In this study, terpenes accounted for ca. 60 % and 21 % of all VOCs in cooked and raw samples, respectively. All terpenes except camphene, a monoterpene with a typical camphoraceous flavor, had higher levels in cooked than in raw samples and were more abundant in steamed and baked samples (Fig. 3C, Table S5). Most of these terpenes were possibly present in the raw samples, but low extraction temperatures hampered their volatilization. Cooking promoted the volatilization of terpenes in sweetpotatoes and resulted in their retention at high rates. Moreover, some terpenes arose from the degradation of polyterpenes during heating. In cooked YFSP, the amount and content of sesquiterpenes were much higher than those reported for orange and purple flesh sweetpotatoes (Zhang, Tang, Jiang, Mo, et al., 2021), and we suggest this as the reason why YFSP are more flavorful than other colored sweetpotatoes.
Furans are important aromatic compounds in cooked sweetpotatoes that often arise from Maillard reactions. In the present study, five furans (Furfural, 2-Furanmethanol, 2-Acetylfuran, 2-pentyl-Furan, 2-methyl-Benzofuran) were identified. In the raw, steamed, and boiled samples, only a few furans were identified, whereas four were identified in baked sample. Except for 2-pentyl-furan with green bean and butter note, which was abundant in raw samples, all furans had the highest levels of accumulation in the baked samples (Fig. 3C, Table S5). Specifically, furfural, a source of bread and sweetness odors (Abeynayake, Panter, Chapman, Webster, et al., 2011), conferred either a burnt or roast flavor to the baked sweetpotatoes. The presence of most furans in baked sweetpotatoes was probably due to the Maillard reaction that occurs at high baking temperatures. This corroborates the identification of furfural, 2-furanmethanol and 2-acetylfuran in the baked samples and their contribution as important caramel flavors for baked samples, which causes a better aromatic flavor of baking sweetpotatoes than those cooked by other methods (Wang & Kays, 2000). In general, cooked YFSP had a rich aromatic flavor and baked ones were the most flavorful.
3.6. Non-volatile metabolite profiles
Different cooking methods not only make a difference in the sensory-related characteristics of YFSP, but also their nutrients. Untargeted metabolomics was used to analyze metabolites in sweetpotato samples that were cooked differently, via UHPLC-QE. A total of 706 non-volatile metabolites were detected in all experimental materials (Table S6). According to their chemical properties, they were divided into 24 classes (Table S6). Lipids (125) had the highest amount and included five subclasses: fatty acids (66), glycerophospholipids (26), prenol lipids (14), steroids (11), and glycerolipids (8). Other compound classes were carbohydrate derivatives (60), peptides (57), terpenoids (49), amino acids and their derivatives (46), flavonoids (44), carboxylic acids (44), phenylpropanoids (40), phenols (25), and vitamins (10). This demonstrates that sweetpotatoes contain many functional compounds and this is an important factor for their selection by consumers.
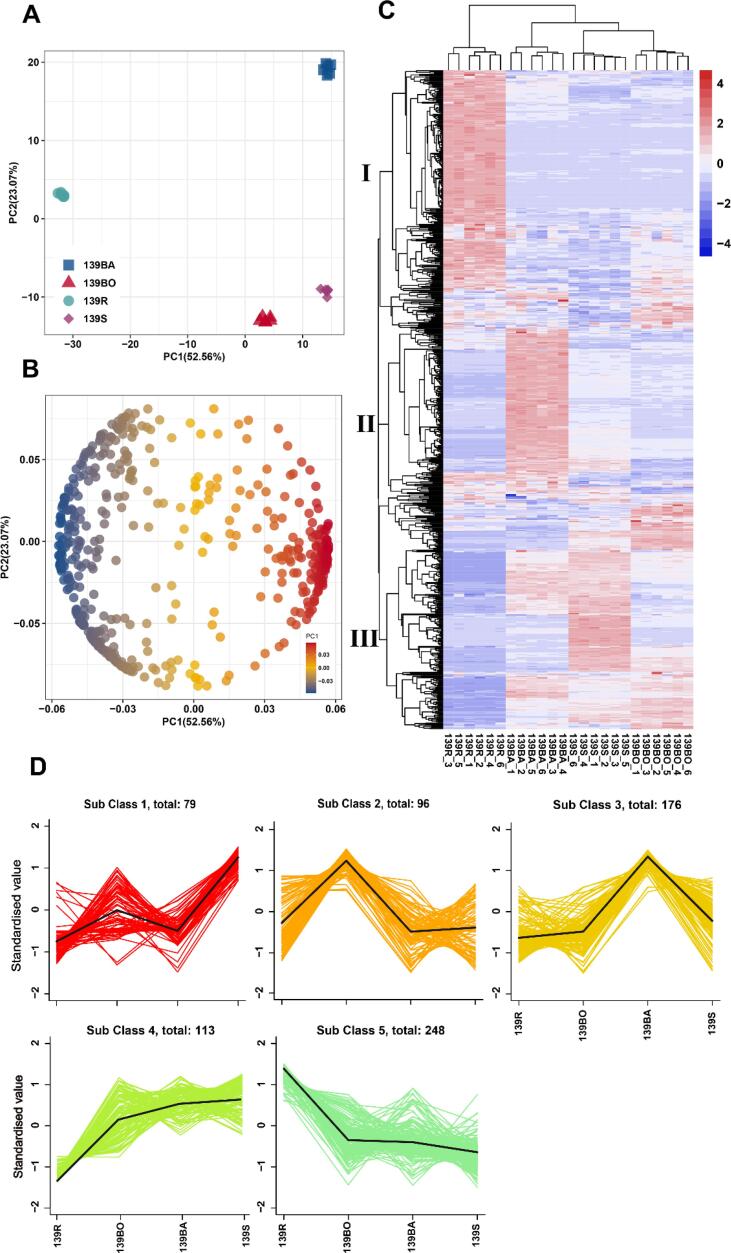
The principal component analysis (PCA) for all samples is shown in Fig. 4A. The six replicates of each group had little variation, indicating the reliability and reproducibility of the results. From the PCA, the four experimental groups were clearly separated. The first principal component (PC1) separated the raw and cooked samples well with a 52.56 % variance contribution value. The baked group was separated from the other cooked groups by the second principal component (PC2), with a value of 23.07 %. The loading plot showed that the metabolites were responsible for the separation (Fig. 4B).
Fig. 4.
Analysis of the metabolites in the yellow-flesh sweetpotato treated by different cooking methods. (A) The PCA score plots, each dot represents an independent experimental repeat. (B) The PCA loading plots, each dot represents an identified metabolite. (C) Heatmap visualization of all the metabolites. The red blocks indicate the up-regulated metabolites, blue blocks indicate down-regulated metabolites, white blocks represent the average relative expressed intensity of all metabolites. (D) K-means analysis for all metabolites.
Results of hierarchal clustering analysis (HCA) of the non-volatile metabolite profiles are shown in the heatmap (Fig. 4C). There were three main clusters in the pattern of metabolite accumulation. The cluster I metabolites were significantly less in the three cooked groups than in the raw samples, and in cluster III metabolites were less in raw samples than in the cooked groups. Thus, the cooked groups had metabolites that were distinguishable from those in raw samples. However, the cluster II metabolites had higher levels in the baked group. Overall, the HCA suggested that the metabolite profile of sweetpotatoes changed with the cooking method.
To investigate the variation tendency of sweetpotato after treatment with different cooking methods, K-means cluster analysis was performed (Fig. 4D). All the metabolites were divided into five subclasses with similar tendencies. Subclass five contained the greatest number of metabolites and their median values were significantly lower in cooked groups. Conversely, the metabolites in subclass four had a contrasting tendency to those of subclass five as they were more abundant in cooked groups. Furthermore, in subclasses one, two, and three, metabolites had the highest median values in steamed, boiled, and baked samples, respectively.
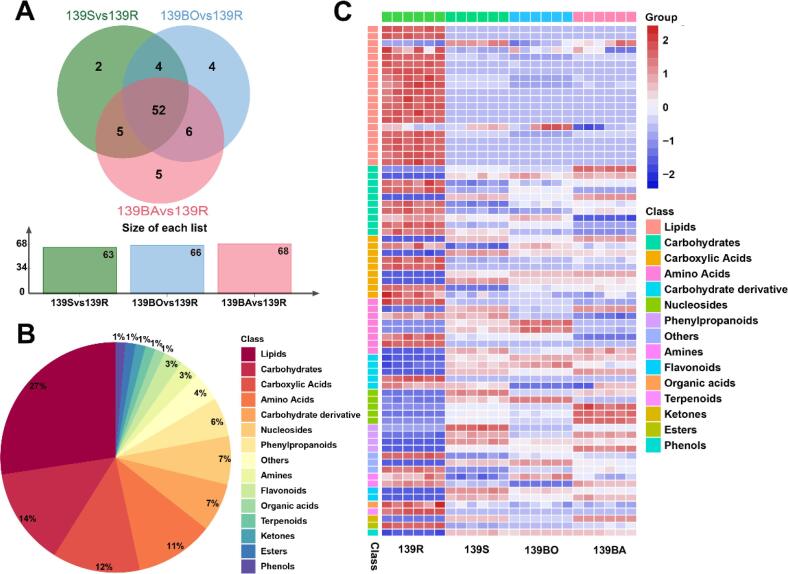
To reflect the influence of different cooking methods on sweetpotatoes more accurately, an OPLS-DA model was created between each cooked and the raw sample. As per these models, the R2X and Q2 were both > 0.9, indicating that they had a high separating capacity and could clearly distinguish the sweetpotato samples based on how they were cooked (Fig. S1). Based on the variable influence on projection (VIP) of the OPLS-DA model, significant variances were found for metabolites of sweetpotatoes from different cooking methods. We then screened the variances using the following criteria: Log2(fold change) ≥ 1 or Log2(fold change) ≤ -1, VIP ≥ 1, and p < 0.05. The results shown in Table S7–9 indicated that there were 63, 66, and 68 differentially accumulated metabolites (DAMs) between the raw samples and steamed, boiled, and baked samples, respectively. A total of 52 DAMs were identified in both the raw samples and all cooked groups (Fig. 5A). All DAMs were divided into 15 categories depending on their chemical structures (Fig. 5B). Among them, the lipid group was the most abundant, and almost all lipids —mostly glycerophospholipids— were downregulated in the cooked groups (Fig. 5C). The carbohydrate group was similarly predominantly downregulated in cooked groups —a few were upregulated. Carboxylic acids constituted nine DAMs, four of which were upregulated during cooking whereas the remaining five were downregulated after cooking. In eight amino acids DAMs, half were downregulated in the cooked groups, especially in baked samples, and the remainder were upregulated in either the steamed or boiled samples. Furthermore, nucleosides, phenylpropanoids, and flavonoids DAMs were all upregulated after cooking (Fig. 5C).
Fig. 5.
Analysis of the differential metabolites in yellow-flesh sweetpotato treated by different cooking methods. (A) Venn diagram showing the distribution of differential metabolites (B) Category statistics for differential metabolites (C) Heatmap visualization of differential metabolites classified in distinct categories in cooked sweetpotato.
This is the first analysis of the metabolites differences between raw and cooked sweetpotatoes. Many metabolites were identified in cooked sweetpotatoes. DAMs analysis showed that the levels of most of the metabolites, including bioactive compounds that were classified as terpenoids, amino acids, flavonoids, phenylpropanoids, phenols and vitamins —these are all positively associated with human health— did not differ significantly before and after cooking. This result indicated that steaming, boiling, and baking of sweetpotatoes largely retained its nutritional and functional components. This demonstrated that steamed, boiled, and baked sweetpotatoes aid people to obtain the full nutrient value of sweetpotatoes. Some phenylpropanoids and flavonoids increased after cooking corroborating a previous study where thermal processing significantly increased the total phenolic content and antioxidant capacities (Kourouma, Mu, Zhang, & Sun, 2019). In cooked YFSP, the most altered metabolites were lipids and carbohydrates. Lipids play essential roles in the structure and organization, signaling, protein regulation, metabolic transformation, and trafficking of all plant cells. Five to 10 % (dry weight) of the vegetative plant cell is composed of lipids, which are almost entirely found in the membranes (Della Corte, Chitarrini, Di Gangi, Masuero, et al., 2015). In this study, glycerophospholipids were the type of lipid most altered by cooking. Glycerophospholipids are the main constituent of the cellular membrane. We hypothesized that the swelling of starch caused cells to rupture, which made easy the hydrolyzation of cellular membrane glycerophospholipids by phospholipases. Thus, levels of glycerophospholipids were lower in the cooked than raw sweetpotatoes. The reduced carbohydrate content of cooked sweetpotatoes is likely related to the starch hydrolysis, whereas for baked samples the carbohydrate-based Maillard and caramelization reactions may have contributed to their reduced carbohydrate content.
Moreover, alterations in amino acid and peptide contents were probably related to Maillard reactions during cooking. This is a non-enzymatic reaction between a carbonyl group of a reducing sugar and a free amino group of protein, peptide, or amino acid, whose products contribute to the pleasing aroma, color, and taste of various food products (Yang, Wang, & Song, 2012). In this study, some VOCs such as benzaldehyde, benzeneacetaldehyde, and furans were typical products of Maillard reactions.
3.7. Carotenoid content
Carotenoid is an important nutrient and pigment that endows sweetpotato flesh with its distinct colors and fragrance. As previously shown, the flesh colors of YFSP changed with the cooking method. Nevertheless, due to the limited extraction methods and databases, carotenoids were not detected in the untargeted metabolome. Accordingly, the carotenoid content and composition in differently cooked sweetpotatoes were estimated by HPLC analysis (Table 2A) to calculate the total carotenoid content and the remaining rates in cooked samples. This was an unprecedented evaluation of the impact of cooking methods on the composition of carotenoids in YFSP.
Table 2.
A. Carotenoid content (µg/g on dry weight basis) in YFSP with different cooking methods.
| Item | Violaxanthin | Neoxanthin | Antheraxanthin | Lutein | Zeaxanthin | α-Cryptoxanthin | ε-Carotene | β-Cryptoxanthin | β-Carotene | γ-Carotene | Total | Remaining rate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 139R | 0.96 ± 0.00a | 2.77 ± 0.25a | 7.82 ± 0.10ab | 13.01 ± 1.11a | 3.64 ± 0.77a | 0.12 ± 0.03a | – | – | 6.17 ± 0.52a | 1.30 ± 0.18a | 35.78 ± 3.67a | |
| 139S | 0.57 ± 0.03b | 0.28 ± 0.05c | 7.02 ± 0.50ab | – | 2.73 ± 0.33b | – | 1.81 ± 0.31a | 0.09 ± 0.07a | 3.19 ± 0.49b | 0.46 ± 0.18b | 16.14 ± 0.81b | 45.11 % |
| 139BO | 0.61 ± 0.10b | 0.70 ± 0.05b | 8.33 ± 0.03a | 1.18 ± 0.10b | 3.12 ± 0.20ab | – | 0.29 ± 0.01c | – | 2.23 ± 0.07b | 1.03 ± 0.13a | 17.49 ± 0.91b | 48.88 % |
| 139BA | 0.48 ± 0.01b | – | 6.23 ± 0.21b | – | 2.25 ± 0.01b | – | 0.91 ± 0.06b | 0.02 ± 0a | 3.25 ± 0.09b | 0.11 ± 0.01b | 13.28 ± 0.22b | 37.12 % |
|
B. The Pearson’s correlation coefficient between carotenoids and color parameters. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Violaxanthin | Neoxanthin | Antheraxanthin | Lutein | Zeaxanthin | α-Cryptoxanthin | ε-Carotene | β-Cryptoxanthin | β-Carotene | γ-Carotene | |
| L | 0.54 | 0.64 | −0.24 | 0.74* | 0.05 | 0.73* | −0.71* | −0.02 | 0.73* | 0.13 |
| a | 0.87** | 0.93** | 0.17 | 0.97** | 0.49 | 0.96** | −0.41 | −0.26 | 0.98** | 0.53 |
| b | 0.44 | 0.54 | −0.32 | 0.64 | −0.06 | 0.62 | −0.77* | 0 | 0.61 | 0.06 |
Different letters represent significant (p < 0.05) differences between means according to ANOVA combined with Duncan’s multiple range test. Each value represents the mean ± standard deviation (n = 3).
*and**mean significant differences at p < 0.05 and p < 0.01, respectively.
In total, 10 carotenoids were detected in the four experimental groups. Among them, five carotenoids were identified in all materials: violaxanthin, antheraxanthin, zeaxanthin, β-carotene and γ-carotene. Antheraxanthin was the most abundant carotenoid in cooked samples, followed by β-carotene, zeaxanthin, γ-carotene, and violaxanthin. Antheraxanthin accounted for 46.89 % (7.02 ± 0.50 μg/g), 47.64 % (6.23 ± 0.21 μg/g), and 43.49 % (8.33 ± 0.03 μg/g) of the total carotenoid in the steamed, baked and boiled samples, respectively. However, its content did not significantly differ between the raw and cooked samples. The other four common carotenoids showed varying degrees of reduction after cooking. The β-carotene content in raw samples was almost double that of cooked samples. Kim et al. observed that loss of β-carotene content occurred for all home cooking methods assessed and steaming, boiling and baking had a similar loss ratio of β-carotene (Kim et al., 2015, Kourouma et al., 2019). Zeaxanthin and γ-carotene contents significantly decreased after steaming and baking, but their level in boiled samples was like that in raw samples. The level of violaxanthin was significantly reduced to low amounts by cooking. Lutein was the most abundant carotenoid in raw samples (13.01 ± 1.11 μg/g) and constituted 36.36 % of its total carotenoid content. For cooked samples, lutein was only detected in trace amounts in boiled samples (1.18 ± 0.10 μg/g), illustrating its drastic reduction due to cooking. This is inconsistent with the finding that lutein concentrations in potatoes were not affected by cooking (Blessington, Nzaramba, Scheuring, Hale, et al., 2010), suggesting that the degradation of lutein during cooking is probably species-specific. Neoxanthinn concentrations were predominantly reduced by cooking and it was undetectable in the baked samples. α-Cryptoxanthin was only detected in the raw samples and had low concentrations (0.12 ± 0.03 μg/g), and ε-Carotene was uniquely detected in cooked samples with significant variations that were probably produced by the choice of cooking method. The correlation between color parameters and individual carotenoid content was analyzed (Table 2B). The contents of violaxanthin, neoxanthin, lutein, and β-carotene were positively correlated with values of a*, and β-carotene was also positively correlated with values of b*. The a* value represents greenness (−a) to gray (0) to redness (+a) and the b* value represents blueness (−b) to gray (0) to yellowness (+b). Therefore, alterations in the β-carotene content probably affected the color change of cooked sweetpotato flesh.
Moreover, the total carotenoid content was assessed. It was highest (35.78 μg/g) in raw samples and at a similar level in cooked groups (16.14 ± 0.81, 17.49 ± 0.91 and 13.28 ± 0.22 μg/g in steamed, boiled, and baked samples, respectively), which was significantly lower than that of raw samples. Compared to the raw sample, the carotenoids in YFSP had degraded after the various cooking methods. The remaining rates were 45.11 %, 48.88 % and 37.12 % in steamed, boiled, and baked samples, respectively. Boiling retained the highest content of carotenoids and baking lost the most corroborating findings of (Kidmose, Christensen, Agili, & Thilsted, 2007) that boiling had a greater retention than roasting for different varieties of sweetpotatoes.
4. Conclusion
In this study, the taste, flavor, and chemical composition of YFSP were assessed before and after steaming, boiling, and baking. Baked YFSP were sweetest and had the highest levels of sugars and volatile compounds. This sweetness, which was higher than that of steamed and boiled YFSP, was probably due to the increased degradation of starch into soluble sugars, especially maltose. Seventy-two volatile compounds were identified in cooked YFSP. The most abundant compounds in raw and cooked YFSP were aldehydes and terpenoids, respectively. Most furans and terpenoids were detected at high relative contents, which might be the cause of the distinctive flavor of the baked YFSP. From the metabolomics analysis, 702 compounds were identified and classified into 24 classes. Only 72 compounds were identified as DAMs between cooked and raw samples with the majority common to all cooked YFSPs. Therefore, compared to raw YFSP, even the three cooking methods affected the composition of many chemical compounds, the nutrients and bioactivities of YFSP were retained despite cooking. Overall, baked YFSP were sweetest and most flavorful and boiling retained carotenoids better than other cooking methods. However, the nutrients were largely retained in all three cooked YFSPs. This study provides a reference not only for the commercial processing of sweetpotatoes but also for the selection of a suitable sweetpotato cooking method by various consumers.
Funding
This work was supported by grants from the construction and operation of the Food Nutrition and Health Research Center of Guangdong Academy of Agricultural Sciences (XTXM 202205), the China Agriculture Research System of MOF and MARA, the Guangdong Modern Agro-industry Technology Research System (2021KJ111, 2022KJ111), Presidential foundation of Guangdong Academy of Agricultural Sciences (BZ202009).
CRediT authorship contribution statement
Rong Zhang: Conceptualization, Methodology, Formal analysis, Data curation, Writing – original draft, Visualization. Haocheng Chen: Formal analysis, Data curation, Writing – original draft, Visualization. Yihang Chen: Formal analysis. Chaochen Tang: Writing – review & editing. Bingzhi Jiang: Writing – review & editing. Zhangying Wang: Conceptualization, Methodology, Writing – review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100542.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- Abeynayake S.W., Panter S., Chapman R., Webster T., Rochfort S., Mouradov A., Spangenberg G. Biosynthesis of proanthocyanidins in white clover flowers: Cross talk within the flavonoid pathway. Plant Physiology. 2011;158(2):666–678. doi: 10.1104/pp.111.189258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adu-Kwarteng E., Sakyi-Dawson E.O., Ayernor G.S., Truong V.-D., Shih F.F., Daigle K. Variability of sugars in staple-type sweet potato (Ipomoea batatas) cultivars: The effects of harvest time and storage. International Journal of Food Properties. 2014;17(2):410–420. doi: 10.1080/10942912.2011.642439. [DOI] [Google Scholar]
- Alam M.K. A comprehensive review of sweet potato (Ipomoea batatas [L.] Lam): Revisiting the associated health benefits. Trends in Food Science & Technology. 2021;115:512–529. doi: 10.1016/j.tifs.2021.07.001. [DOI] [Google Scholar]
- Banda L., Kyallo M., Domelevo Entfellner J.-B., Moyo M., Swanckaert J., Mwanga R.O.M.…Muzhingi T. Analysis of β-amylase gene (Amyβ) variation reveals allele association with low enzyme activity and increased firmness in cooked sweetpotato (Ipomoea batatas) from East Africa. Journal of Agriculture and Food Research. 2021;4 doi: 10.1016/j.jafr.2021.100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellail A.A., Shaltout O.E., Youssef M.M., Gamal A.M.A.E. Effect of home-cooking methods on phenolic composition and antioxidant activity of sweetpotato (Ipomoea batatas (L.) Lam.) cultivars grown in Egypt. Food and Nutrition Sciences. 2012;03(04):10. doi: 10.4236/fns.2012.34069. [DOI] [Google Scholar]
- Blessington T., Nzaramba M.N., Scheuring D.C., Hale A.L., Reddivari L., Miller J.C. Cooking methods and storage treatments of potato: Effects on carotenoids, antioxidant activity, and phenolics. American Journal of Potato Research. 2010;87(6):479–491. doi: 10.1007/s12230-010-9150-7. [DOI] [Google Scholar]
- Chalse M.N., Suryawanshi D.K., Kharat R.B., Mundhe T.S., Shinde P.P., Jogdand K.B., Rathod R.V. Extraction and partial purification and kinetic study of beta-amylase from sweet potato. J. Glob. Biosci. 2016;5(4):3892–3901. [Google Scholar]
- CIP. (2022). Sweetpotato facts and figures - International Potato Center. In, vol. 2022): International Potato Center.
- Della Corte A., Chitarrini G., Di Gangi I.M., Masuero D., Soini E., Mattivi F., Vrhovsek U. A rapid LC–MS/MS method for quantitative profiling of fatty acids, sterols, glycerolipids, glycerophospholipids and sphingolipids in grapes. Talanta. 2015;140:52–61. doi: 10.1016/j.talanta.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Dudareva N., Klempien A., Muhlemann J.K., Kaplan I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytologist. 2013;198(1):16–32. doi: 10.1111/nph.12145. [DOI] [PubMed] [Google Scholar]
- Franková H., Musilová J., Árvay J., Šnirc M., Jančo I., Lidiková J., Vollmannová A. Changes in antioxidant properties and phenolics in sweet potatoes (Ipomoea batatas L.) due to heat treatments. Molecules. 2022;27(6):1884. doi: 10.3390/molecules27061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirardo A., Fochi V., Lange B., Witting M., Schnitzler J.-P., Perotto S., Balestrini R. Metabolomic adjustments in the orchid mycorrhizal fungus Tulasnella calospora during symbiosis with Serapias vomeracea. New Phytologist. 2020;228(6):1939–1952. doi: 10.1111/nph.16812. [DOI] [PubMed] [Google Scholar]
- Guo K., Liu T., Xu A., Zhang L., Bian X., Wei C. Structural and functional properties of starches from root tubers of white, yellow, and purple sweet potatoes. Food Hydrocolloids. 2019;89:829–836. doi: 10.1016/j.foodhyd.2018.11.058. [DOI] [Google Scholar]
- Iese V., Holland E., Wairiu M., Havea R., Patolo S., Nishi M.…Waqainabete L. Facing food security risks: The rise and rise of the sweet potato in the Pacific Islands. Global Food Security. 2018;18:48–56. doi: 10.1016/j.gfs.2018.07.004. [DOI] [Google Scholar]
- Islam S.N., Nusrat T., Begum P., Ahsan M. Carotenoids and β-carotene in orange fleshed sweet potato: A possible solution to vitamin A deficiency. Food Chemistry. 2016;199:628–631. doi: 10.1016/j.foodchem.2015.12.057. [DOI] [PubMed] [Google Scholar]
- Katayama K., Kobayashi A., Sakai T., Kuranouchi T., Kai Y. Recent progress in sweetpotato breeding and cultivars for diverse applications in Japan. Breed Science. 2017;67(1):3–14. doi: 10.1270/jsbbs.16129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kays S.J., Wang Y., McLaurin W.J. Chemical and geographical assessment of the sweetness of the cultivated sweetpotato clones of the world. Journal of the American Society for Horticultural Science. 2005;130(4):591–597. doi: 10.21273/JASHS.130.4.591. [DOI] [Google Scholar]
- Kidmose U., Christensen L.P., Agili S.M., Thilsted S.H. Effect of home preparation practices on the content of provitamin A carotenoids in coloured sweet potato varieties (Ipomoea batatas Lam.) from Kenya. Innovative Food Science & Emerging Technologies. 2007;8(3):399–406. doi: 10.1016/j.ifset.2007.03.025. [DOI] [Google Scholar]
- Kim H.J., Park W.S., Bae J.-Y., Kang S.Y., Yang M.H., Lee S.…Ahn M.-J. Variations in the carotenoid and anthocyanin contents of Korean cultural varieties and home-processed sweet potatoes. Journal of Food Composition and Analysis. 2015;41:188–193. doi: 10.1016/j.jfca.2015.01.012. [DOI] [Google Scholar]
- Kourouma V., Mu T.-H., Zhang M., Sun H.-N. Effects of cooking process on carotenoids and antioxidant activity of orange-fleshed sweet potato. LWT - Food Science and Technology. 2019;104:134–141. doi: 10.1016/j.lwt.2019.01.011. [DOI] [Google Scholar]
- Lee E.M., Park S.J., Lee J.-E., Lee B.M., Shin B.K., Kang D.J.…Lee D.Y. Highly geographical specificity of metabolomic traits among Korean domestic soybeans (Glycine max) Food Research International. 2019;120:12–18. doi: 10.1016/j.foodres.2019.02.021. [DOI] [PubMed] [Google Scholar]
- Liarzi O., Benichis M., Gamliel A., Ezra D. Trans-2-Octenal, a single compound of a fungal origin, controls Sclerotium rolfsii, both in vitro and in soil. Pest Management Science. 2020;76(6):2068–2071. doi: 10.1002/ps.5744. [DOI] [PubMed] [Google Scholar]
- Liu Y., Lv J., Liu Z., Wang J., Yang B., Chen W.…Zou X. Integrative analysis of metabolome and transcriptome reveals the mechanism of color formation in pepper fruit (Capsicum annuum L.) Food Chemistry. 2020;306 doi: 10.1016/j.foodchem.2019.125629. [DOI] [PubMed] [Google Scholar]
- Ma G., Zhang L., Iida K., Madono Y., Yungyuen W., Yahata M.…Kato M. Identification and quantitative analysis of β-cryptoxanthin and β-citraurin esters in Satsuma mandarin fruit during the ripening process. Food Chemistry. 2017;234:356–364. doi: 10.1016/j.foodchem.2017.05.015. [DOI] [PubMed] [Google Scholar]
- Moens L.G., Plas K., Van Ceunebroeck J.-C., Van Loey A.M., Hendrickx M.E.G. Effect of pulsed electric field, mild thermal pretreatment and calcium on texture changes of potato (Solanum tuberosum L.) during subsequent cooking. Innovative Food Science & Emerging Technologies. 2021;74 doi: 10.1016/j.ifset.2021.102830. [DOI] [Google Scholar]
- Ogliari R., Soares J.M., Teixeira F., Schwarz K., da Silva K.A., Schiessel D.L., Novello D. Chemical, nutritional and sensory characterization of sweet potato submitted to different cooking methods. International Journal of Research. Granthaalayah. 2020;8:147–156. doi: 10.29121/granthaalayah.v8.i10.2020.1881. [DOI] [Google Scholar]
- Salvador L.D., Suganuma T., Kitahara K., Tanoue H., Ichiki M. Monosaccharide composition of sweetpotato fiber and cell wall polysaccharides from sweetpotato, cassava, and potato analyzed by the high-performance anion exchange chromatography with pulsed amperometric detection method. Journal of Agricultural and Food Chemistry. 2000;48(8):3448–3454. doi: 10.1021/jf991089z. [DOI] [PubMed] [Google Scholar]
- Tomlins K., Ndunguru G., Stambul K., Joshua N., Ngendello T., Rwiza E.…Westby A. Sensory evaluation and consumer acceptability of pale-fleshed and orange-fleshed sweetpotato by school children and mothers with preschool children. Journal of the Science of Food and Agriculture. 2007;87(13):2436–2446. doi: 10.1002/jsfa.2931. [DOI] [Google Scholar]
- Tomlins K., Owori C., Bechoff A., Menya G., Westby A. Relationship among the carotenoid content, dry matter content and sensory attributes of sweet potato. Food Chemistry. 2012;131(1):14–21. doi: 10.1016/j.foodchem.2011.07.072. [DOI] [Google Scholar]
- Utto W., Mawson A.J., Bronlund J.E. Hexanal reduces infection of tomatoes by Botrytis cinerea whilst maintaining quality. Postharvest Biology and Technology. 2008;47(3):434–437. doi: 10.1016/j.postharvbio.2007.08.008. [DOI] [Google Scholar]
- Valetudie J.-C., Gallant D.J., Bouchet B., Colonna P., Champ M. Influcence of cooking procedures on structure and biochemical changes in sweet potato. Starch - Stärke. 1999;51(11–12):389–397. doi: 10.1002/(SICI)1521-379X(199912)51:11/12<389::AID-STAR389>3.0.CO;2-H. [DOI] [Google Scholar]
- Wang Y., Kays S.J. Effect of cooking method on the aroma consistuents of sweet potato [Ipomoea Batatas (L.) Lam.] Journal of Food Quality. 2000;24(1):67–78. doi: 10.1111/j.1745-4557.2001.tb00591.x. [DOI] [Google Scholar]
- Wei S., Lu G., Cao H. Effects of cooking methods on starch and sugar composition of sweetpotato storage roots. PLoS One. 2017;12(8):e0182604. doi: 10.1371/journal.pone.0182604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Y., Guo, X., Jia, Z., Ma, P., Bian, X., & Yu, Y. (2018). Progresses and prospects on edible sweetpotato breeding in China. Jiangsu Agricultural Sciences, 34(06), 1419-1424. https://kns.cnki.net/kcms/detail/32.1213.S.20181229.1023.060.html.
- Yang C., Wang R., Song H. The mechanism of peptide bonds cleavage and volatile compounds generated from pentapeptide to heptapeptide via Maillard reaction. Food Chemistry. 2012;133(2):373–382. doi: 10.1016/j.foodchem.2012.01.044. [DOI] [PubMed] [Google Scholar]
- Yang Q., Mei X., Wang Z., Chen X., Zhang R., Chen Q., Kan J. Comprehensive identification of non-volatile bitter-tasting compounds in Zanthoxylum bungeanum Maxim. by untargeted metabolomics combined with sensory-guided fractionation technique. Food Chemistry. 2021;347 doi: 10.1016/j.foodchem.2021.129085. [DOI] [PubMed] [Google Scholar]
- Zhang R., Tang C., Jiang B., Mo X., Wang Z. Optimization of HS-SPME for GC-MS analysis and its application in characterization of volatile compounds in sweet potato. Molecules. 2021;26(19):5808. doi: 10.3390/molecules26195808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Zheng M., Zhai H., Ma P.A., Lyu Y., Hu Y., Cai J. Effects of hexanal fumigation on fungal spoilage and grain quality of stored wheat. Grain & Oil Science and Technology. 2021;4(1):10–17. doi: 10.1016/j.gaost.2020.12.002. [DOI] [Google Scholar]
- Zhang Y., Kong J., Huang F., Xie Y., Guo Y., Cheng Y.…Yao W. Hexanal as a QS inhibitor of extracellular enzyme activity of Erwinia carotovora and Pseudomonas fluorescens and its application in vegetables. Food Chemistry. 2018;255:1–7. doi: 10.1016/j.foodchem.2018.02.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.