Abstract
Objective: To determine the feasibility, safety, and efficacy on PaO2/FIO2 ratio of prone positioning (PP) for acute respiratory distress syndrome (ARDS) after cardiac surgery.
Design: Retrospective review of information entered prospectively in the authors' database.
Setting: A private community nonteaching hospital.
Participants: Sixteen patients who developed ARDS after cardiac surgery from January 2004 through June 2005.
Interventions: PP to improve oxygenation.
Measurements and Main Results: After a median duration of 18 (range, 14-27) hours in PP, PaO2/FIO2 improved in 14 (87.5%) patients. For the entire population, median PaO2/FIO2 rose from 87 (range, 56-161) before PP to 194 (range, 94-460; p < 0.05) after it. After supine repositioning (SR), PaO2/FIO2 declined to 146 (range, 72-320; not significant). PaO2/FIO2 at the end of PP and 1 day after SR were comparable, respectively, 194 (range, 94-460) and 184 (range, 105-342). No severe complication was associated with PP, but 5 patients developed pressure sores and 2 others had superficial sternal wound infections. Intensive care unit mortality of 37.5% reflected the number of organ failure(s); there were no deaths with 2 failures, and 60% with ≥3 organ failures died (p = 0.03). Mortality rates were comparable regardless of whether patients were PaO2/FIO2 responders or their PaCO2 decreased by ≥1 mmHg.
Conclusion: PP to treat ARDS after cardiac surgery is feasible, safe, and can efficiently improve oxygenation. Measures to prevent pressure sores are mandatory.
Key words: acute respiratory distress syndrome, cardiac surgery, prone positioning
ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) sometimes complicates the postoperative course of cardiac surgery with cardiopulmonary bypass (CPB).1, 2, 3 Its prognosis is associated with high mortality.2, 4, 5 Oxygenation can be improved by using different techniques.6 Although prone positioning (PP) of the patients seems to improve oxygenation effectively, this method is associated with potentially severe complications.7, 8 In addition, some physicians might be reluctant to use this technique for unstable postoperative patients because it also raises concern about the quality of sternal wound healing and the risk of surgical wound infection. Guérin et al8 included sternotomy during the preceding 15 days as an exclusion criterion. This study was designed to retrospectively evaluate the feasibility, safety, and impact on PaO2/FIO2 of the first PP session for patients with ARDS after cardiac surgery with CPB.
Methods
All patients treated with PP for ARDS after cardiac surgery between January 1, 2004, and June 31, 2005, were retrospectively identified. Inclusion criteria were (1) presence of ARDS as defined by the North American–European consensus conference,9 (2) persistence of severely impaired oxygenation (PaO2/FIO2 ≤200) after a positive end-expiratory pressure (PEEP) test and/or a pressure plateau >30 cmH2O despite low tidal volume, and (3) informed consent of the family member(s) to put the patient in a prone position. Because of the type of information collected and the retrospective design of the study with normal management that included sequential arterial blood gas measurements, institutional review board approval was not required at this institution.
All the patients included in this study were sedated with midazolam (2-5 mg/h) and sufentanil (5-10 μg/h). All were ventilated in a volume-controlled mode, with a plateau pressure <30 cmH2O. Heart rate, arterial systolic pressure (with an indwelling radial artery catheter), and oxygen saturation (with a pulse oximeter) were monitored continuously. Arterial blood gas analyses and arterial lactate concentrations were determined using a commercial blood-gas analyzer (Rapidlab 864; Bayer, Leverkusen, Germany).
All patients' charts were reviewed. For each patient, the following information was collected: age, sex, body mass index, cardiovascular risk factors, simplified acute physiology score II10 at intensive care unit (ICU) admission and the day of PP, number and type of organ failure(s) using the organ dysfunction and/or infection score11 (Appendix 1) on the day of PP, and the Murray lung injury score.12 The following mechanical ventilation parameters were recorded before PP, at the end of PP, and 1 day after supine repositioning (SR): tidal volume/weight, respiratory rate, FIO2, application, and the level of PEEP. The number of PPs for each patient was noted. The effect of PP on arterial blood gases was evaluated during the first session. PaO2/FIO2, pH, PaCO2, and lactate values were recorded at just before (baseline) PP, at the end of PP, within 1 hour, and 1 day after SR.
The following PP-associated complications were recorded: accidental extubation, selective intubation, endotracheal tube obstruction, accidental removal of arterial or venous central catheters, surgical wound infection,13 pressure sores, ocular complications, and hemodynamic instability. The duration of mechanical ventilation, ICU length of stay, and ICU mortality were also noted.
The algorithm proposed by Messerole et al14 was used to place patients in PP. Briefly, 5 to 7 staff members, 1 or 2 intensivists, and 2 to 4 nurses turned the patient with the help of a respiratory physical therapist. Before turning, the patient's weight-bearing sites (ie, face, shoulders, knees, and so on) were protected by using Biatin (Coloplast, Humlebaek, Denmark). To avoid ocular lesions, the face and the shoulders were appropriately cushioned. During the PP session, the patient's bed was inclined with the head 30° higher than the foot, and the patient's face was turned toward the ventilator.
A patient was considered to be a PaO2 responder when his/her PaO2/FIO2 increased 20 mmHg,15 to have sustained benefit when his/her PaO2/FIO2 was stable after SR compared with the value at the end of PP, and to be a PaCO2 responder when his/her PaCO2 decreased ≥1 mmHg during PP.15
Data are expressed as medians (range) or number of patients (percentage). A Fisher exact test was used to compare percentages, and mean ± standard deviation PaO2/FIO2, PaCO2, and lactate values were compared by using a 2-way analysis of variance for repeated measurements followed by Bonferroni's multiple comparison procedure; p ≤ 0.05 was considered statistically significant. Statistical analyses were performed by using StatView version 5.0 (SAS Institute Inc, Cary, NC).
Results
Between January 2004 and June 2005, 1,200 patients were admitted to the ICU after cardiac surgery; 16 were placed in a prone position to treat ARDS with persistent and severe hypoxemia despite a PEEP trial. Their main clinical characteristics, including risk factors for ARDS, are reported in Table 1. PP was performed a median of 3 (1-10) days after cardiac surgery and after a median of 2 (1-10) days of mechanical ventilation. At baseline PP, median PaO2/FIO2 was 87 (56-161) and the median Murray score was 4 (2-4). All patients were given inotropic agents for hemodynamic instability.
Table 1.
Clinical Characteristics of the 16 Patients Treated With PP
| Characteristic | Value⁎ |
|---|---|
| Age (y) | 74 [44-83] |
| Male sex | 10 (62.5) |
| Body mass index (kg/m2) | 27 [20-37] |
| Nonelective surgery | 3 (18.8) |
| Redo | 4 (25) |
| Chronic obstructive pulmonary disease | 4 (25) |
| Left ventricular ejection fraction (%) | 60 (25-80) |
| Type of surgery | |
| CABG | 5 (31.3) |
| Valve replacement | 5 (31.3) |
| CABG and valve replacement | 3 (18.8) |
| Miscellaneous | 3 (18.8) |
| CPB duration (min) | 109 [68-240] |
| SAPS II at ICU admission (points) | 42 [18-54] |
| SAPS II day of PP (points) | 41 [33-64] |
| Nonpulmonary organ failure(s) the day of PP (n) | 3 [2-5] |
| Type of nonpulmonary organ failure the day of PP† | |
| Cardiac | 16 (100) |
| Renal | 7 (43.8) |
| Hematologic | 2 (12.5) |
| Neurologic | 2 (12.5) |
| Infection | 10 (62.5) |
| Hepatic | 0 (0) |
| ARDS risk factors‡ | |
| Cardiopulmonary bypass | 16 (100) |
| Pneumonia | 4 (25) |
| Shock | 3 (18.8) |
| Sepsis | 2 (12.5) |
| Massive transfusion | 1 (6.3) |
Abbreviations: CABG, coronary artery bypass graft; SAPS, simplified acute physiology score.
Values are expressed as median [range] or number (%).
Some patients experienced >1 organ failure the day of PP.
Some patients had >1 risk factor for ARDS.
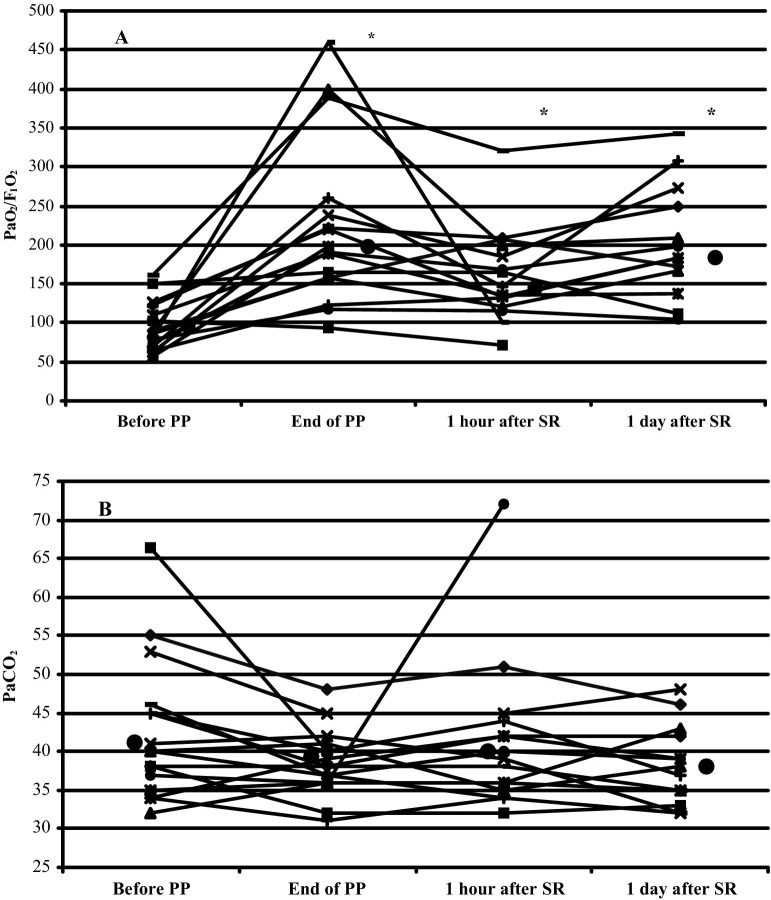
The median and individual changes in PaO2/FIO2 are shown in Figure 1A. After a median PP duration of 18 (14-27) hours, PaO2/FIO2 had dramatically risen from 87 (56-161) to 194 (94-460) (95 ± 31 v 223 ± 106, respectively; p < 0.0001). Fourteen (87.5%) patients were PaO2 responders. Within 1 hour of SR, PaO2/FIO2 declined from 194 (94-460) to 146 (72-320, not significant) but remained significantly higher than baseline values (95 ± 31 v 160 ± 60, respectively; p < 0.05). PaO2/FIO2 had increased slightly 24 hours after SR compared with 1 hour after SR (not significant). PaO2/FIO2 at the end of PP and 1 day after SR were comparable, respectively (194 [94-460] and 184 [105-342]).
Fig 1.
Median (filled circle) and individual evolutions of (A) PaO2/FIO2 ratios and (B) PaCO2 from just before to during PP and 1 hour and 1 day after SR; *p < 0.05 versus baseline PaO2/FIO2.
Median and individual changes in PaCO2 are shown in Figure 1B. The median PaCO2 change from before to the end of PP was −3 (−26 to 5) mmHg (41.1 ± 5.1 v 38.5 ± 0.5, respectively; p = 0.11); PaCO2 declined in 10 patients (PaCO2 responders, median PaCO2 decrease −7 [−26 to −1] mmHg) but remained constant or rose in 6 patients (PaCO2 nonresponders, median PaCO2 increase 1 [0-5] mmHg) with comparable ventilator settings (Table 2). Between baseline PP and 1 day after SR, median lactate values decreased, respectively, from 1.7 (1-6.7) to 1.3 (range, 0.8-2.1) mmol/L (2.3 ± 1.6 v 1.4 ± 0.5, respectively; p = 0.11).
Table 2.
Ventilator Settings
| Before PP | During PP | 1 Day After SR | |
|---|---|---|---|
| TV/weight (mL/kg) | 8 [5-13] | 8 [6-13] | 8 [6-12] |
| Respiratory rate | 15 [12-40] | 16 [12-42] | 16 [12-32] |
| FIO2 | 0.8 [0.6-1] | 0.6 [0.4-0.9] | 0.6 [0.4-1] |
| PEEP (cmH2O) | 5 [0-20] | 5 [0-15] | 10 [0-15] |
NOTE. Data are expressed as median [range].
Abbreviation: TV, tidal volume.
None of the reported severe complications (accidental extubation and accidental removal of central catheter) occurred during or after 34 PP sessions. Neither sedation nor inotropic agent doses were modified during PP. Despite inclination of the patient's bed, facial edema was constant, but it regressed rapidly after SR. Survivors experienced no ocular complications, but 5 patients developed pressure sores on the chin (n = 2), cheekbone (n = 2), or knee (n = 1) without persistent esthetic facial sequelae. Two superficial sternal wound infections were successfully treated with surgical debridement and antibiotic therapy. No sternal instability was observed.
Two patients were successfully treated with cardioversion for atrial fibrillation during PP. Continuous venovenous hemofiltration without technical difficulties was also performed during PP for 1 patient in acute renal failure.
Thirty-four PP sessions were performed during the study period. Thirteen patients underwent a single session: 1 died, PP lacked efficacy for 3, and improved oxygenation for the remaining 9. ICU mortality was 37.5%. Death was related to cardiogenic shock (n = 2), multiorgan failure (n = 2), refractory hypoxemia (n = 1), and malignant ventricular arrhythmia (n = 1). Death in the ICU was significantly associated with the number of organ failure(s); no patients with 2 organ failures died, whereas those with ≥3 organ dysfunctions suffered 60% mortality (p = 0.03). The respective mortality rates for PaO2 responders and nonresponders (28.6% and 100%) and PaCO2 responders and nonresponders (40% and 33%) did not differ significantly.
Discussion
ARDS is a rare complication after cardiac surgery with CPB, and the results of this study showed that, although PP safely improved oxygenation for most patients, it did not seem to modify the natural history of ARDS and its poor prognosis. Among this institution's 1,200 postcardiac surgery patients whose files were reviewed for this study, fewer than 1% developed ARDS that required PP. Reported ARDS frequency after cardiac surgery with CPB ranged from 0.4%3 to 2.5%2, depending on the definitions used and the population studied.1, 2, 3, 4, 5 The rate does not seem to be influenced by the time period considered, despite technical improvements in the 1990s, especially concerning CPB.1, 2, 3, 4, 5
Studies on PP after cardiac surgery are very scarce16, 17, 18, 19 including case reports from India18 and Spain19 and an article on 36 patients published in Russian.17 Brussel et al16 observed significantly increased PaO2/FIO2 after prolonged PP ventilation in 9 of their 10 patients with postoperative acute respiratory failure after coronary artery bypass graft surgery. Most of the present patients (87.5%) responded to PP with substantial increases of their PaO2/FIO2 ratios. These results are in accordance with those of most recent multicenter studies, with PaO2 responder rates exceeding 70%.7, 8, 20
The authors arbitrarily chose to use prolonged PP sessions (median duration, 18 hours) because the optimal PP duration is unknown; it ranges from 414, 21 to >18 hours,16, 20, 22, 23 with comparable PaO2/FIO2 improvement and percentages of responders. It could be hypothesized that prolonged PP would achieve better consolidation of the physiologic modifications associated with the improved oxygenation obtained with this technique including increased end-expiratory lung volume,24 better ventilation-perfusion matching,25 and regional changes of ventilation26 associated with a return toward normal chest-wall mechanics.27
Despite the positive PP effect on oxygenation, the most recent studies failed to obtain significantly lower mortality with repeated PP sessions.7, 8, 20 In contrast, decreased PaCO2 with PP is predictive of a better outcome.15 Indeed, improved efficiency of alveolar ventilation is an important marker of patients who will survive. Elevated deadspace fraction was also significantly associated with an increased risk of death from ARDS.28 Finally, in ARDS, low tidal volume (6 mL/kg of predicted body weight) is the sole therapy associated with a significantly better prognosis.29
ARDS-associated mortality exceeded 38% in a community population of patients admitted for ARDS.30 After cardiac surgery, the death rate from ARDS ranged from 15% to >90%.1, 2, 3, 4, 5 These widely differing mortality rates might be explained by the study period, the ARDS definitions applied, and/or the number of nonpulmonary organ failure(s) associated with ARDS.1, 2, 3, 4, 5
A potential limitation of PP therapy is the rate of associated severe complications.7, 8 No complications resulted directly from PP in the present study. Recently, Mancebo et al20 reported a very low complication rate in their multicenter study (68 events for 718 turnings). Routine use of this technique, with strict respect for the procedure and a large team, might explain the low rate of PP-induced complications. Sternal wound infection is another potential limitation that could explain why Guérin et al8 considered it an exclusion criterion. In the present study, 2 patients developed superficial sternal wound infections that did not influence their outcomes. This “fear” should not lead to hesitation to use PP, even early after cardiac surgery.
This study has some limitations, primarily that it was retrospective without a control group. All postoperative patients with ARDS were not systematically treated with PP, regardless of whether the PEEP test was successful or not. Also, the sample size was small so gas exchange measurements were the only parameters studied and not 28-day mortality, 1-year survival rate, or health-related quality of life. However, in light of the scarcity of data on this therapy after cardiac surgery16, 17, 18, 19 and its potential severe complications, the authors believe it was important to initially assess the feasibility, safety, and efficacy of PP use specifically in this population.
Indeed, these observations clearly showed that after cardiac surgery PP can be applied to patients who develop ARDS and multiorgan dysfunctions, and it can efficiently improve oxygenation, but the prognosis remains poor. Complications associated with PP can be limited or avoided by using a strict protocol and paying special attention to preventing pressure sores.
Appendix
Appendix 1.
Definitions of Organ Dysfunctions and/or Infection Applied in the Organ Dysfunction and/or Infection Model
| Cardiac dysfunction | Infusion of dopamine and/or epinephrine and/or norepinephrine; or systemic arterial pressure <90 mmHg; or peripheral signs of shock |
| Pulmonary dysfunction | Mechanical ventilation or PaO2 (21% FIO2) <60 mmHg |
| Renal dysfunction | Creatinine level >300 μmol/L or diuresis <500 mL/d |
| Neurologic dysfunction | Glasgow coma score <6 or confusion, coma |
| Hepatic dysfunction | Bilirubin level >100 IU/mL or alkaline phosphatase >600 IU/mL |
| Hematologic dysfunction | White blood cell <2,000/mm3 or platelet level <40,000/mm3 or hematocrit <20% |
| Infection | Clinically documented infection or temperature <36.5°C or >38.3°C |
NOTE. Each element was scored as follows: absence = 0, presence = 1.
References
- 1.Asimakopoulos G., Taylor K.M., Smith P.L., et al. Prevalence of acute respiratory distress syndrome after cardiac surgery. J Thorac Cardiovasc Surg. 1999;117:620–621. doi: 10.1016/s0022-5223(99)70348-x. [DOI] [PubMed] [Google Scholar]
- 2.Kaul T.K., Fields B.L., Riggins L.S., et al. Adult respiratory distress syndrome following cardiopulmonary bypass: Incidence, prophylaxis and management. J Cardiovasc Surg. 1998;39:777–781. [PubMed] [Google Scholar]
- 3.Milot J., Perron J., Lacasse Y., et al. Incidence and predictors of ARDS after cardiac surgery. Chest. 2001;119:884–888. doi: 10.1378/chest.119.3.884. [DOI] [PubMed] [Google Scholar]
- 4.Messent M., Sullivan K., Keogh B.F., et al. Adult respiratory distress syndrome following cardiopulmonary bypass: Incidence and prediction. Anaesthesia. 1992;47:267–268. doi: 10.1111/j.1365-2044.1992.tb02134.x. [DOI] [PubMed] [Google Scholar]
- 5.Christenson J.T., Aeberhard J.M., Badel P., et al. Adult respiratory distress syndrome after cardiac surgery. Cardiovasc Surg. 1996;4:15–21. doi: 10.1016/0967-2109(96)83778-1. [DOI] [PubMed] [Google Scholar]
- 6.Marini J., Gattinoni L. Ventilatory management of acute respiratory distress syndrome: A consensus of two. Crit Care Med. 2004;32:250–255. doi: 10.1097/01.CCM.0000104946.66723.A8. [DOI] [PubMed] [Google Scholar]
- 7.Gattinoni L., Tognoni G., Pesenti A., et al. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med. 2001;345:568–573. doi: 10.1056/NEJMoa010043. [DOI] [PubMed] [Google Scholar]
- 8.Guérin C., Gaillard S., Lemasson S., et al. Effects of systematic prone positioning in hypoxemic acute respiratory failure: A randomized controlled trial. JAMA. 2004;292:2379–2387. doi: 10.1001/jama.292.19.2379. [DOI] [PubMed] [Google Scholar]
- 9.Bernard G.R., Artigas A., Brigham K.L., et al. The American-European Consensus Conference on ARDS: Definitions, mechanisms, relevant outcomes and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 10.Le Gall J.R., Lemeshow S., Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 11.Fagon J.Y., Chastre J.Y., Novara A., et al. Characterization of intensive care unit patients using a model based on the presence or absence of organ dysfunctions and/or infection: The ODIN model. Intensive Care Med. 1993;19:137–144. doi: 10.1007/BF01720528. [DOI] [PubMed] [Google Scholar]
- 12.Murray J.F., Matthay M.A., Luce J.M., et al. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 13.CDC definitions of nosocomial surgical site infections, 1992: A modification of CDC definitions of surgical wound infections. Am J Infect Control. 1992;20:271–274. doi: 10.1016/s0196-6553(05)80201-9. [DOI] [PubMed] [Google Scholar]
- 14.Messerole E., Peine P., Wittkopp S., et al. The pragmatics of prone position. Am J Respir Crit Care Med. 2002;165:1359–1363. doi: 10.1164/rccm.2107005. [DOI] [PubMed] [Google Scholar]
- 15.Gattinoni L., Vagginelli F., Carlesso E., et al. Decrease in PaCO2 with prone position is predictive of improved outcome in acute respiratory distress syndrome. Crit Care Med. 2003;31:2727–2733. doi: 10.1097/01.CCM.0000098032.34052.F9. [DOI] [PubMed] [Google Scholar]
- 16.Brüssel T., Hachenberg T., Roos N., et al. Mechanical ventilation in the prone position for acute respiratory failure after cardiac surgery. J Cardiothorac Vasc Anesth. 1993;7:541–546. doi: 10.1016/1053-0770(93)90311-8. [DOI] [PubMed] [Google Scholar]
- 17.Eremenko A.A., Egorov V.M., Levikov D.I. Results of the treatment of cardiac surgery patients with postoperative acute respiratory distress syndrome by prone-position pulmonary ventilation. Anesteziol Reanimatol. 2000;5:42–45. [PubMed] [Google Scholar]
- 18.Firodiya M., Mehta Y., Juneja R., et al. Mechanical ventilation in the prone position: A strategy for acute respiratory failure after cardiac surgery. Indian Heart J. 2001;53:83–86. [PubMed] [Google Scholar]
- 19.Rama-Maceiras P., Duro J., Figueira-Moure A., et al. Ventilation in prone decubitus in a patient with respiratory distress during heart surgery. Rev Esp Anestesiol Reanim. 1999;46:81–84. [PubMed] [Google Scholar]
- 20.Mancebo J., Fernandez R., Blanch L., et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;173:1233–1239. doi: 10.1164/rccm.200503-353OC. [DOI] [PubMed] [Google Scholar]
- 21.Langer M., Mascheroni D., Marcolin R., et al. The prone position in ARDS patients: A clinical study. Chest. 1988;94:103–107. doi: 10.1378/chest.94.1.103. [DOI] [PubMed] [Google Scholar]
- 22.McAuley D.F., Giles S., Fichter H., et al. What is the optimal duration of ventilation in the prone position in acute lung injury and acute respiratory distress syndrome? Intensive Care Med. 2002;28:414–418. doi: 10.1007/s00134-002-1248-z. [DOI] [PubMed] [Google Scholar]
- 23.Fridrich P., Krafft P., Hochleuthner H., et al. The effects of long-term positioning in patients with trauma-induced adult respiratory distress syndrome. Anesth Analg. 1996;83:1206–1211. doi: 10.1097/00000539-199612000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Douglas W.W., Rehder K., Beynen F.M., et al. Improved oxygenation in patients with acute respiratory failure: The prone position. Am Rev Respir Dis. 1977;115:559–566. doi: 10.1164/arrd.1977.115.4.559. [DOI] [PubMed] [Google Scholar]
- 25.Pappert D., Rossaint R., Slama K., et al. Influence of the positioning on ventilation-perfusion relationships in severe adult respiratory distress syndrome. Chest. 1994;106:1511–1516. doi: 10.1378/chest.106.5.1511. [DOI] [PubMed] [Google Scholar]
- 26.Albert R.K., Leasa D., Sanderson M., et al. The prone position improves arterial oxygenation and reduces shunt in oleic-acid-induced acute lung injury. Am Rev Respir Dis. 1987;135:628–633. doi: 10.1164/arrd.1987.135.3.628. [DOI] [PubMed] [Google Scholar]
- 27.Pelosi P., Tubiolo D., Mascheroni D., et al. Effects of the prone position on respiratory mechanics and gas exchange during acute lung injury. Am J Respir Crit Care Med. 1998;157:387–393. doi: 10.1164/ajrccm.157.2.97-04023. [DOI] [PubMed] [Google Scholar]
- 28.Nuckton T.J., Alonso J.A., Kallet R.H., et al. Pulmonary deadspace fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002;346:1281–1286. doi: 10.1056/NEJMoa012835. [DOI] [PubMed] [Google Scholar]
- 29.The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 30.Rubenfeld G.D., Caldwell E., Peabody E., et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]