Abstract
Inositol 1,4,5-trisphosphate (IP3) plays a key role in calcium signaling. After stimulation, it diffuses from the plasma membrane where it is produced to the endoplasmic reticulum where its receptors are localized. Based on in vitro measurements, IP3 was long thought to be a global messenger characterized by a diffusion coefficient of ~ 280 μm2s−1. However, in vivo observations revealed that this value does not match with the timing of localized Ca2+ increases induced by the confined release of a non-metabolizable IP3 analog. A theoretical analysis of these data concluded that in intact cells diffusion of IP3 is strongly hindered, leading to a 30-fold reduction of the diffusion coefficient. Here, we performed a new computational analysis of the same observations using a stochastic model of Ca2+ puffs. Our simulations concluded that the value of the effective IP3 diffusion coefficient is close to 100 μm2s−1. Such moderate reduction with respect to in vitro estimations quantitatively agrees with a buffering effect by non-fully bound inactive IP3 receptors. The model also reveals that IP3 spreading is not much affected by the endoplasmic reticulum, which represents an obstacle to the free displacement of molecules, but can be significantly increased in cells displaying elongated, 1-dimensional like geometries.
Subject terms: Cell signalling, Computational biology and bioinformatics
Introduction
Within all cell types, Ca2+ signaling is controlled by a variety of channels and pumps that allow for rapid and highly regulated Ca2+ fluxes at specific locations of the cell1. In many instances, Ca2+ increases are initiated by the formation of inositol 1,4,5-trisphosphate (IP3) at the plasma membrane2. IP3 diffuses in the cytoplasm and binds its receptors located on the endoplasmic reticulum that contains a large quantity3 of rapidly mobilizable Ca2+. Activity of these receptors is also regulated by cytosolic Ca2+, both positively and negatively4. At the spatial level, IP3 receptors (IP3R) are not homogeneously scattered on the ER membrane, but rather grouped in clusters of ~ 10–20 channels5,6. Because the number of clusters in cells is limited, coupling between clusters has been much investigated to understand how Ca2+ signaling can be coordinated at the cellular level, giving rise to Ca2+ oscillations and waves7–9. Most studies have focused on Ca2+-mediated communication between clusters, since it was assumed that all clusters rapidly experience the increase of IP3 that results from the stimulation of the cell. In cytosolic extracts of Xenopus oocytes, apparent diffusion coefficients of Ca2+ and IP3 indeed equal 38 ± 11 μm2s−1 and 283 ± 53 μm2s−1, respectively10. Considering in addition the respective rates of Ca2+ removal from the cytoplasm and IP3 metabolism, these values led Allbritton et al.10 to conclude that Ca2+ mostly acts in restricted domains and that IP3 is a global messenger.
The notion that IP3 acts as a global messenger was however contradicted by indirect in vivo observations11. To evaluate the rate at which IP3 diffuses in an intact cell, the group of Ian Parker used the IP3-evoked liberation of Ca2+ from a cluster of IP3Rs as a detector of the presence of IP3 at the cluster location. Such Ca2+ increases evoked by clusters of IP3Rs are well-known as Ca2+ puffs12. If a non-metabolizable IP3 analog is released at one extremity of an elongated SH-SY5Y cell, the time lapse between the localized IP3 increase and the occurrence of the first Ca2+ puff significantly increases with the distance between the spot of IP3 release and the location of Ca2+ rise. This time lapse, called latency, reflects the time taken by IP3 to diffuse on this distance. Accordingly, when the IP3 analog is uniformly released across the entire cell, latency does not show any systematic variation along the cell length but decreases with increasing IP3 concentration. The fact that latency increases with the distance from the IP3 release spot clearly indicates that IP3 does not act as a global messenger in these conditions. From these observations, Dickinson et al.11 inferred the value of the IP3 diffusion coefficient by resorting to a simplified mathematical expression for the probability of Ca2+ puff occurrence coupled to 1-dimensional (1D) simulations of IP3 diffusion. These calculations predicted an effective IP3 diffusion coefficient lower than 10 μm2s−1, i.e. about 30 times slower than in vitro estimations.
This slowing down of IP3 diffusion within cells as compared to cytosolic extracts was ascribed to the presence of IP3Rs that are not fully bound to IP311,13. IP3Rs are indeed tetramers that release Ca2+ only when each IP3R monomer is occupied by IP314. Given that IP3 binding on IP3Rs is not cooperative, most of the receptors are partially bound as long as [IP3] remains lower than the KD of IP3 binding, i.e. ~ 100 nM (KD = 119 nM reported by Taylor and Konieczny13) and thus act as a buffer of IP3. Assuming fast binding and unbinding of IP3 to and from its receptor, the resulting effective diffusion coefficient15 is given by:
| 1 |
where D represents the IP3 diffusion coefficient in the absence of buffers, ST the concentration of IP3R monomers, and KD the equilibrium dissociation constant of IP3 from its receptor. ST is cell dependent, in the range of 80 nM to 2 μM13,16–18 with an estimated value of 542 nM in SH-SY5Y cells18. Thus, the buffering effect described by Eq. 1 is expected to induce a reduction of the effective diffusion coefficient of ~ 2.25 at [IP3] = KD. In Dickinson et al.11 experiments, [IP3] was in the Ca2+ oscillatory range. Because IP3 concentrations are typically of the order or lower than the KD of the IP3R in the oscillatory range19, one can estimate that the effective diffusion coefficient of IP3 considering IP3 buffering by not fully bound receptors (DI) should be ~ 100 μm2s–1 (Fig. 1). Values around 5–10 μm2s–1 would correspond to situations in which IP3 concentrations are lower than KD/2 and IP3R concentrations at least higher than 1 μM, in which IP3 binding to its receptors exceeds what occurs in SH-SY5Y cells. Such discrepancy led Dickinson et al.11 to hypothesize the existence of two distinct populations of IP3Rs, with the most active exhibiting different binding and/or gating properties. These different populations were proposed to be the molecular bases of the observed two modes of Ca2+ release: one early pulsatile Ca2+ activity at puff sites and one later, spatially more diffuse Ca2+ liberation20.
Figure 1.
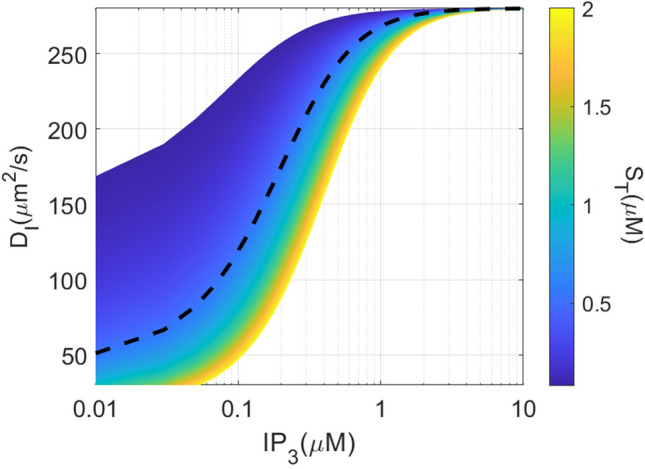
Theoretical values of the effective IP3 diffusion coefficient depending on the concentration of IP3 and of the monomers of IP3 receptors (ST). The dashed line shows this diffusion coefficient as a function of [IP3] for ST = 542 nM, which is the value estimated for SH-S5Y5 neuroblastoma cells. Values have been calculated using Eq. 1. See text for details.
Besides, a value of DI lower than 10 μm2s−1 raises questions about some observations related to Ca2+ waves. For example, in ascidian eggs that have a radius between 25 and 38 μm, Ca2+ waves propagate across the entire fertilized egg in less than 10 s while IP3 is only synthesized at the plasma membrane21. The question is even more critical for intercellular Ca2+ waves, which in many cases rely on the propagation of IP3 through gap junctions22. Intracellular diffusion of IP3 with a coefficient ≤ 10 μm2s−1 could not account for waves propagating at rates ≥ 10 μms−1 among a cell population23,24. More generally, an accurate quantitative description of IP3 diffusion is necessary for the numerous modelling studies devoted to the physiological responses induced by IP3-controlled Ca2+ signaling, such as, for example, the Ca2+- and IP3-regulated nitric oxide production in neurons25, the metabolism of β-amyloids during the development of Alzheimer's disease26 or salivary secretion by acinar cells27. IP3 spreading from its location of synthesis to its receptors also plays a key role in cardiac cells28,29.
The aim of the present study is to re-investigate the characteristics of IP3 diffusion using a stochastic model that explicitly simulates Ca2+ puff dynamics and allows a realistic computational description of the experiments performed by Dickinson et al.11. Based on the observations of puff latencies performed by these authors, we propose a new computational treatment to infer the value of the effective diffusion coefficient of IP3, leading to different conclusions. The reasons for the different outcomes between the two studies are analyzed in the discussion. We also investigate how cell geometry and the presence of the ER membranes, which provide an obstacle to the free displacement of molecules, affect the rates at which IP3 diffuses in a cell-like environment.
Results
Methodology
We performed explicit stochastic simulations of Ca2+14 releasing activities of puff sites located at different distances from an IP3 source, in order to directly simulate the experimental protocol that was used to estimate the IP3 diffusion coefficient in vivo11. The mathematical model is based on a previously proposed fully stochastic description of the Ca2+ exchanges between the ER and the cytosol via IP3Rs, SERCA pumps and a leak from the ER9. In this work, SERCA pumps, Ca2+ leakage and Ca2+ diffusion are described deterministically. The effective value of the Ca2+ diffusion coefficient taking Ca2+ buffering into account10 is considered, i.e. 40 μm2s−1. To simulate Ca2+ puffs, the release of Ca2+ via the IP3Rs is described stochastically, using the Gillespie’s algorithm30. Each cluster of IP3Rs (Supplemental Figure S1) is described as a whole and can be in four states: one open (O), one closed (C) and two different inhibited ones (I1 and I2). These states describe the global behavior of a cluster composed of close-by IP3Rs. The phenomenological model was shown to reproduce experimentally observed statistical properties of Ca2+ puffs31 and to describe the passage from localized puffs to global Ca2+ spikes when clusters are effectively coupled by Ca2+ diffusion9.
To evaluate the effective diffusion coefficient of IP3, this model is extended to take the dependence of puff occurrence on IP3 concentration into account. Thus, in the Gillespie's simulations, the propensity of transition of the cluster from the closed (C) to the open (O) state now writes:
| 2 |
where kCO stands for the rate constant that characterizes the passage of the cluster from the closed to the open state, NCa is the number of cytosolic Ca2+ ions and Ω is the extensivity parameter. NIP and KIP represent the number of IP3 molecules and the IP3 dissociation constant of the IP3R (multiplied by Ω), respectively. Although the model does not simulate individual IP3 receptors, we assumed that puff firing probability is related to the probability of one tetrameric IP3R to be fully bound to IP313. As shown below, Eq. 2 indeed allows to reproduce the exponential dependence of mean first puff latency on IP3 concentration reported experimentally11,32.
The evolution of IP3 concentration is described deterministically and two different protocols of photorelease of caged IP3 are simulated. The first one corresponds to a spot photorelease of caged IP3 in a small region of the cell:
| 3 |
| 4 |
In the second one, called “distributed photorelease”, IP3 is liberated at different spots to get an increase in [IP3] that is nearly spatially homogeneous in the whole cell. In this case:
| 5 |
| 6 |
In Eqs. (3–6), DI stands for the effective IP3 diffusion coefficient, for the rate of IP3 release upon laser flash and H for Heaviside function. This function equals 1 if t ≤ tph and 0 otherwise. Considering that is determined by the intensity of the flash in the spot photorelease case (Eq. 3), a ten times smaller value is used to simulate distributed photorelease (Eq. 5) because the same total amount of IP3 is distributed among the ten IP3 releasing sites. Basal IP3 concentration is set to 50 nM33.
A detailed description of the algorithm is provided in the Supplementary Information (Sect. 1), together with a Table listing the propensities used for the stochastic part of the algorithm and the evolution equations used in the deterministic part (Table Supplement 1). Code are available at https://github.com/RobertoOrnelasGuevara/ca2-puffs/tree/main. Except for the process describing IP3 dynamics listed here above, the values of parameters are taken from Voorsluijs et al.9 and are listed in Table Supplement 2.
Validation of the model
Low concentrations of IP3 typically evoke local Ca2+ signals known as Ca2+ puffs12,34. As the concentration of IP3 increases, Ca2+ puffs become more frequent and transform into Ca2+ waves spreading regeneratively across the cell. These repetitive waves, also known as Ca2+ spikes, are more regular than puffs and are often referred to as Ca2+ oscillations. Their stochastic origin is visible by the linear relation between the variance on the interspike interval and the mean interspike interval itself8,35. In experiments, Ca2+ waves propagation can be hindered by loading the cells with the slow Ca2+ buffer EGTA, which reduces communication between puff sites36. At high EGTA concentrations, each cluster practically behaves as an independent entity and thus generates Ca2+ puffs on a large range of [IP3].
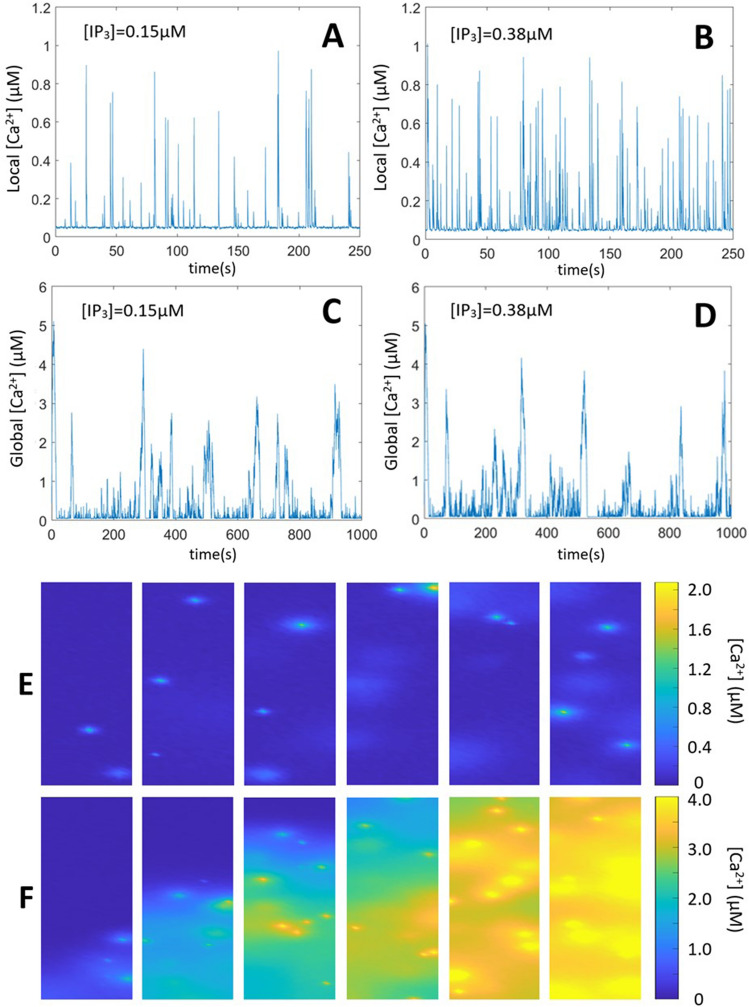
Such behavior is well reproduced by the stochastic model, as seen by simulations in a simplified 2D geometry (Fig. 2A–D). Four different conditions were used: low and intermediate IP3 concentration, with and without coupling between clusters by Ca2+ diffusion. In all cases, [IP3] is constant in time and space. To model puff dynamics when clusters are uncoupled (corresponding to the presence of EGTA), a single cluster was simulated and the local Ca2+ concentration averaged in 1fL around the cluster was monitored. The resulting dynamics of [Ca2+] (Fig. 2A,B) corresponds to Ca2+ puffs, with statistical properties in good agreement with observations and an average inter-puffs interval that decreases with [IP3]9. The mean interpuff intervals are 4.60 ± 4.25 s and 1.86 ± 1.79 at 0.15 μM and 0.38 μM [IP3], respectively. Thus, the model recovers the characteristic stochastic activity of an isolated cluster of IP3Rs. To model spikes dynamics when clusters are coupled by diffusion, 10 clusters of IP3Rs were randomly distributed in a 5 μm × 5 μm system and the global [Ca2+] averaged over the whole system was monitored (Fig. 2C,D). The mean interspike interval and the coefficient of variation (CV) decrease when increasing [IP3], as reported previously35: the mean interspike intervals are 129.6 ± 64.00 s and 108.26 ± 49.22 s at 0.15 μM and 0.38 μM [IP3], respectively. This exemplifies that global, more regular Ca2+ increases occur when clusters communicate via Ca2+ diffusion. To simulate the spatio-temporal profiles of puffs and spikes, simulations were performed in a 50 μm × 10 μm system, while keeping the same average density of clusters (Fig. 2E,F). While a low, homogeneous concentration of IP3 induces random puff activity, a large bolus of IP3 released at one extremity of the cell initiates a global Ca2+ spike that propagates as a Ca2+ wave.
Figure 2.
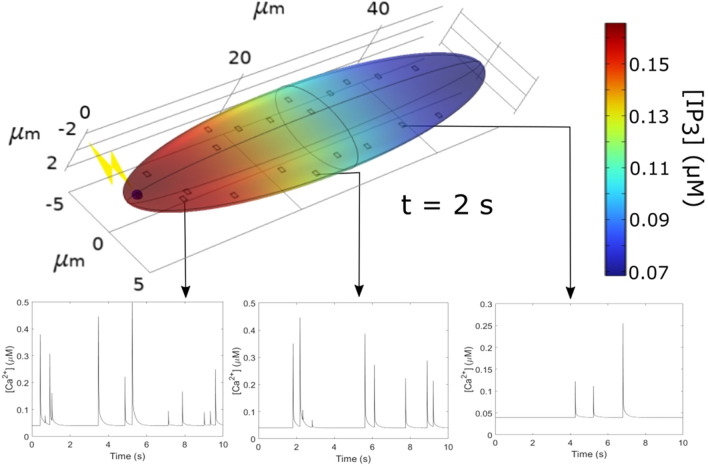
Stochastic simulations of Ca2+ puffs and spikes. (A and B) show the evolution of Ca2+ concentration in a 1 fL volume (1.5 × 1.5 μm2 in the 2D simulations, see Voorsluijs et al.9) centered around the cluster at two [IP3]. For the 2 panels, one cluster, located at the centre of a 5 × 5 μm2 2D system is considered. For panels (C and D), a larger 5 × 10 μm2 system containing 10 clusters is simulated. All values of parameters are the same as in A and B and listed in Table S2. Time series show the evolution of Ca2+ concentration averaged on the whole system. Panels (E and F) show the spatio-temporal evolutions of Ca2+ puffs and spikes, respectively. In the two cases, the system is 50 × 10 μm2 large and 10% of the total surface is occupied by clusters (200 clusters). For panel E, [IP3] is constant and equal to 0.075 μM everywhere in the cell. For panel F, IP3 was released in one spot (with dimensions 0.5 × 0.5 μm2) located in the bottom center of the simulated cell. For panels E and F, times corresponding to each subpanels are: 0.1, 0.6, 2.5, 3.4 and 5.2 s after the increase in [IP3] from 10 nM. In panels A-E, [IP3] is increased at time 0, and Eqs. (3) and (5) are not considered. In panel F, the release of IP3 is simulated using Eq. (3), with = 1000µMs−1, DI = 100 μm2s−1 and tph = 500 ms.
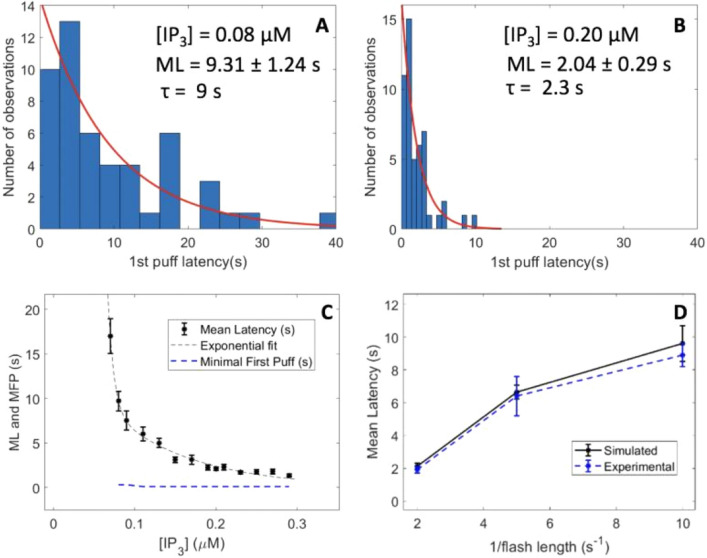
Upon a spatially uniform increase in IP3 concentration, latencies of first puff occurrence are exponentially distributed12,32. Such distributions, obtained by model simulations, are shown in Fig. 3A–C, together with their means and associated standard error of the mean (SEM) values. First puff latencies are exponentially distributed with an average that decreases with the IP3 concentration, consistent with observations in SH-SY5Y cells11. The characteristic decrease times of the exponential distributions (τ) also decrease with [IP3]. These results were used to infer the values of the rate of release of caged IP3 in response to the flash, i.e. the value of parameter θ in Eqs. 3 and 5. Starting from the relation between mean first puff latency and flash duration reported by Dickinson et al.11 for the distributed photorelease of IP3 -and replotted in Fig. 3D, we seek the values of IP3 concentrations that, in the simulations, gave the same mean latencies as those reported experimentally using Fig. 3C. Next, we numerically evaluated the value of θ allowing to reach these spatially uniform concentrations of IP3 when assuming 10 photorelease spots of durations equal to 0.1, 0.2 and 0.5 s respectively, as in the experiments. Good agreement between mean latencies and flash durations was found for θ = 600 μM/s (Fig. 3D).
Figure 3.
Statistics of the latencies of first puffs simulated with the model. Panels (A and B) show histograms of first puff latencies resulting from simulations of one cluster site in a square 5 × 5μm2 2D geometry. A puff is defined as an increase in the cytosolic Ca2+ concentration in a 1 fL volume (1.5 × 1.5 μm2 in the 2D simulations, see Voorsluijs et al.9) centered around the cluster that exceeds 0.1 μM. First puff latencies show an exponential distribution with characteristic decrease times matching experimental observations. Time t = 0 corresponds to the moment of [IP3] increase from 50 nM to indicated values. The black stars in panel (C) show the mean first puff latencies (ML) as a function of [IP3]. The blue dashed line shows the minimal first puff latencies (MFP), i.e. the time at which the first puff occurred. Minimal first puffs are practically independent of [IP3] and are always close to 300 ms. For each [IP3], 50 independent simulations were run. In panels A, B and C, [IP3] is increased at time 0, and Eqs. (3) and (5) are not considered. In panel D, photorelease of caged IP3 is also simulated, using Eq. (5). Best fit with the observations of Dickinson et al.11 was found when considering θ = 600 μMs−1. This value was found by looking for the value of θ that allows to obtain the steady states [IP3] leading to the ML’s corresponding to the flash durations (tph) used in Dickinson et al.’s experiments, i.e. 8.9 ± 0.5 s, 6.4 ± 1.2 s and 1.9 ± 0.2 s for the 0.1, 0.2 and 0.5 s flash durations, respectively. Error bars indicate ± SEM.
When caged IP3 is photoreleased at one extremity of the cell, puffs begin on average after longer latencies at greater distance from the spot11. This reflects the time taken for IP3 to diffuse up to the cluster, and IP3 dilution. Dickinson et al.11 used mean first puff latencies at different distances from the photorelease spot to estimate the effective diffusion coefficient of IP3. In addition to mean first puff latencies, the time interval between the flash and the observation of the first puff at a given distance from the release site is also a relevant quantity. We called this latency “minimal first puff latency”. It can be regarded as the time lapse during which all clusters located at a given distance from the flash site remain silent, indicating that IP3 diffusion up to this point has been negligible. As visible in the distribution of puff latencies upon a global IP3 increase, the minimal first puff latency is shorter than 0.5 s as soon as [IP3] exceeds 80 nM (Fig. 3C, blue dashed line). In agreement with this, the minimal first puff latency is not affected by the duration of the flash in the case of distributed photorelease of IP311. If the source of IP3 is spatially restricted, minimal first puff latency provides a reliable indication of the time at which IP3 has started to increase at a given location since IP3 diffusion can be viewed as a deterministic process and since the number of puff sites analyzed is large enough. Because puff activity is a stochastic process that also depends on the Ca2+ concentration around the cluster, minimal first puff latencies in principle overestimate the time required for IP3 to spread up to a given location. However, given that minimal first puffs are shorter than 0.5 s at any IP3 concentration, this delay is anyway small compared to the time needed for IP3 to spread over distances larger than several microns. Moreover, as discussed below, the minimal first puff latency is not significantly influenced by the possible incomplete Ca2+ buffering by EGTA, which could accelerate puff triggering. Indeed, first puffs arise in conditions where most, if not all, nearby clusters are inactive.
Effective diffusion coefficient of IP3
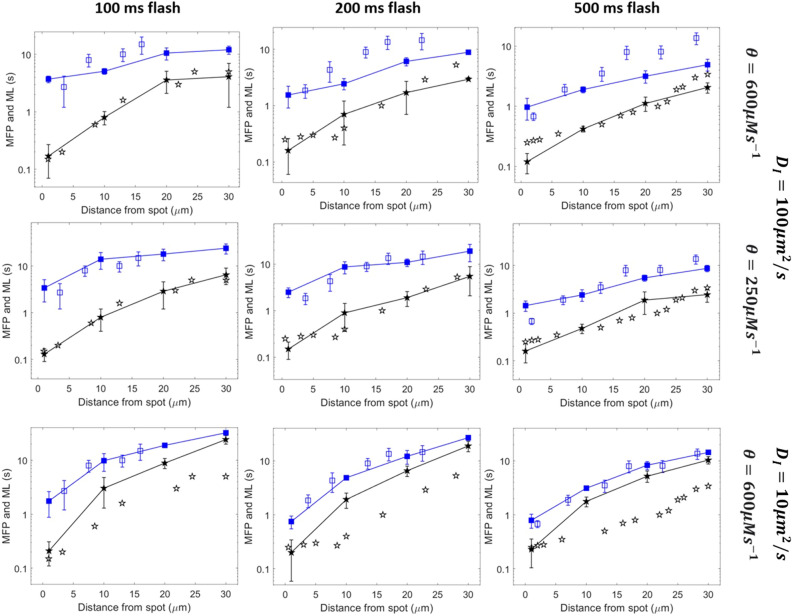
We investigated the relation between first puff latency and distance from spot photorelease by performing independent simulations for twelve clusters, each one located at a different distance from the photorelease spot in an ellipse-shaped 2D cell. Such a configuration allows to reproduce the absence of communication between the cluster sites that results from the addition of EGTA. Binding of IP3 to IP3Rs is taken into account in the value of the effective IP3 diffusion coefficient, DI (see Eq. 1). The ellipse shape was chosen instead of the rectangle used in the previous figures to avoid artefactual effects in the corners. Each simulation was run up to the opening of the cluster and this time was then monitored. The same simulation was performed 50 times for each distance between the spot and the cluster. The averages of the latencies of the first puffs correspond to the mean latencies. For the minimal first puff latencies, we divided the 50 simulations in five groups of ten. In each group, the minimal value was spotted. The average of these minimal values is defined as the minimal first puff latency. As shown in Fig. 4 (upper row), minimal first puffs latencies (black stars) simulated with an effective IP3 diffusion coefficient of 100 μm2s−1 –which corresponds to the expected value given the IP3 buffering capacity of the cytosol of SH-SY5Y cells, as discussed in the Introduction– are in agreement with experimental observations of Dickinson et al.11. Thus, the time taken by IP3 to diffuse from the photorelease spot to the locations of the clusters is such that the relation between the distance from the flash spot and the duration of the period of inactivity are well reproduced. Agreement between simulated and observed mean first puff latencies is limited to the case of the 0.1 s flash, i.e. the lowest IP3 concentration. For larger IP3 concentrations (0.2 and 0.5 s flash), we reasoned that the discrepancy between the idealized simulations of cells containing one single cluster and experiments may be due to a slight stimulation of puff activity by Ca2+ in the experiments, which is not totally buffered by EGTA. At 5 μM EGTA, the distance on which free Ca2+ ions diffuse before being captured by EGTA is indeed between 2.1 and 3.8 μm (see Sect. 2 of Supplementary Information). As these distances are of the order of the mean distance between clusters in the SH-SY5Y cells, there is some Ca2+ stimulation of puff activity in the distributed photorelease protocol. Because we only model one cluster at a time, this effect does not occur in our simulations. Thus, the value θ = 600 μMs−1 deduced in the previous section overestimates the IP3 release rate and must be seen as an upper limit.
Figure 4.
Mean latencies (ML) and minimal first puff latencies (MFP) as simulated with an effective IP3 diffusion coefficient equal to 100 μm2s−1 (first and second rows) or 10 μm2s−1 (third row) and a rate of IP3 increase at the photorelease spot of 600 μMs−1 (first and third rows) or 250 μMs−1 (second row). In all panels, squares represent mean latencies and stars, minimal first puff latencies. Plain symbols are theoretical predictions while empty symbols are the experimental observations of Dickinson et al.11, estimated from their figures. Lines are drawn between simulation results. Simulations are performed in a 2D ellipse-shaped geometry with the spot of IP3 release occurring in a 0.25 μm radius circle at one extremity of the simulated cell. For each theoretical point, 50 independent simulations were run, considering one cluster at a time. Error bars indicate ± SEM. For minimal first puffs, the 50 simulations were divided in 10 groups of 5 simulations among which the minimal first puff was considered, except for the case θ = 250 μMs−1 and the 500 ms flash for which 100 simulations were run for the shortest distance.
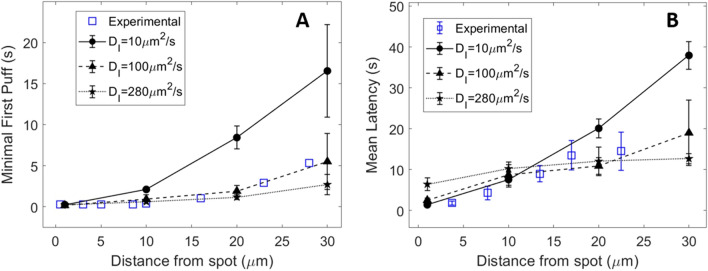
In line with this assumption, a lower value of parameter θ (250 μMs−1) representing the rate of IP3 release due to the flash (Eqs. 3 and 5) allows to get a good agreement both for the mean and the minimal first puff latencies (Fig. 4, medium row). This value of θ also allows us to reproduce the experimentally observed dependency of the mean latency on the flash duration when considering, in the simulations, that the Ca2+ level is increased at cluster location during puff activity upon distributed IP3 photorelease (Supplemental Figure S2). It should be stressed that the 250 μMs−1 value for θ was found to provide a good fit with observations but remains arbitrary since it cannot be computed on the basis of a detailed quantitative knowledge of the level of communication between the clusters via Ca2+. However, the third row of Fig. 4 shows that agreement with observations cannot be obtained when keeping the large value of θ (600 μMs−1) and decreasing the value of DI. In this case, despite the agreement for mean latencies, there is a systematic and important overestimation of the minimal first puff latencies. Such disagreement cannot be ascribed to a reduced number of experimental observations because a larger number of data is only expected to further decrease the shortest possible latency at a given distance. In contrast, it reveals that the low value of DI is unable to simulate the quite rapid, moderate IP3 increase away from the spot. The larger concentration of IP3 due to the overestimated θ explains why mean latencies agree with observations, as they compensate in average for the slow diffusion. Simulations also predict that with θ = 250 μMs−1 best agreement is obtained with DI = 100 μm2s−1, as compared to smaller or larger values (Fig. 5). Altogether, simulations are in good agreement with experimental observations concerning mean and minimal first puff latencies with the value of the IP3 diffusion coefficient taking IP3 binding to the receptors into account, i.e. 100 μm2s−1. The latter value also allowed to reproduce the relation between puff latencies and distance from the flash measured in COS-7 cells (Supplemental Figure S3).
Figure 5.
Comparison between puff latencies theoretically predicted with the rate of IP3 release θ = 250 μm2s−1 and observed by Dickinson et al.11. Panels (A and B) show minimal first puff latencies and mean latencies simulated with different values of the effective diffusion coefficient for IP3, DI. All points correspond to a 200 ms flash. Plain symbols are theoretical predictions while empty symbols are the experimental observations of Dickinson et al.11. Lines are drawn between simulation results. Simulations are performed in a 2D ellipse-shaped system with the spot of IP3 release being a 0.25 μm radius disk at one extremity of the simulated cell. For each theoretical point, 50 independent simulations were run. Error bars indicate ± SEM. For minimal first puffs, the 50 simulations were divided in 10 groups of 5 simulations among which the minimal first puff was considered.
Influence of cell shape
Up to this point, simulations have been performed in 2D, rectangular or ellipse-shaped systems. However, the 3D character and the specific geometry of the cells are expected to influence the rate at which IP3 propagates into the cytoplasm through diffusion. To address this question using computational simulations (Fig. 6), we first considered an ellipsoid (50 × 10 × 5 µm) in which 20 cluster sites are located randomly at a distance shorter than 0.1 μm from the plasma membrane37. IP3 is assumed to be released in a 0.25 μm radius sphere located at the left extremity of the cell. The rate of IP3 increase (parameter θ in Eqs. 3 and 5) was adapted according to the change in the cytoplasmic volume. Two seconds after the simulated flash, a gradient of IP3 is established (Fig. 6). Consequently, the dynamics of puff activity is rather different depending on the distance from the flash (compare the Ca2+ time series in Fig. 6). Agreement between minimal and mean first puff latencies computed in simulations with an effective diffusion coefficient of IP3 equal to 100 μm2s−1 and observations of Dickinson et al.11 is slightly improved in this 3D configuration as compared to the 2D situation (Supplemental Figure S4).
Figure 6.
Computational simulations of IP3 diffusion and Ca2+ puff occurrence in response to the localized photorelease of a non-metabolizable IP3 analogue in an ellipsoidal 3D geometry, assuming an effective diffusion coefficient of IP3 (DI) of 100 μm2s−1. The upper panel shows IP3 distribution 2 s after the flash. Lower panels show time series of local Ca2+ concentrations at cluster sites (in a 1 fL volume) located at increasing distances from the flash. Simulation procedures are the same as for Fig. 4. The rate of localized IP3 photorelease, θ, is taken equal to 2500 μMs−1, which allows to obtain the same spatiotemporal profile of IP3 increase as the 250 μMs−1 value for the 2D case, because of changes in volume.
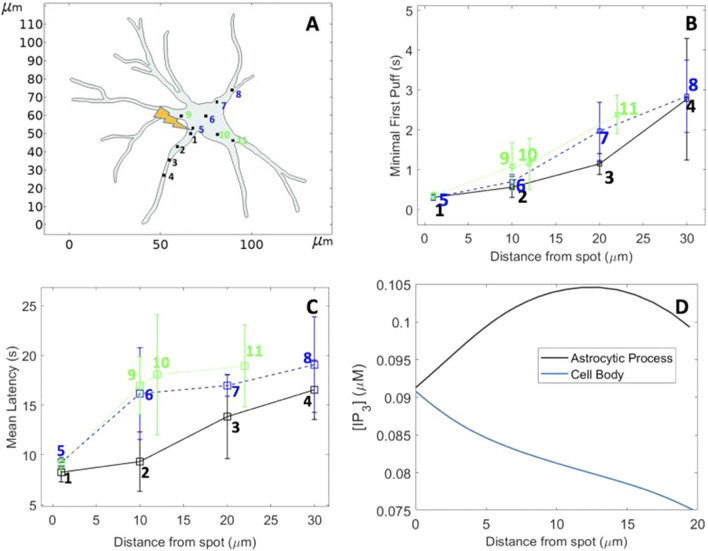
The shape of the cell is expected to influence the rate of IP3 spreading. We investigated this possible influence by simulating IP3 diffusion in a two-dimensional representation of an astrocyte (Fig. 7). In response to a release of IP3 at the intersection between the cell body and one process, Ca2+ puffs occur sooner in the cell process than in the body. This is related to a larger rate of IP3 spreading in elongated structures where dilution is much reduced. For example, 2 s after the simulated flash, more elevated IP3 concentrations are seen in the process than in the cell body (Fig. 7D). Thus, at the same time and the same distance from the site of IP3 release, IP3 reaches higher concentrations in the process and is able to propagate on longer distances (Supplemental Video S5).
Figure 7.
Computational simulations of IP3 diffusion and Ca2+ puff occurrence in response to the localized photorelease of a non-metabolizable IP3 analogue in a 2D geometry resembling an astrocyte, assuming an effective diffusion coefficient of IP3 (DI) of 100 μm2s−1. (A) Shape of the astrocyte redrawn in COMSOL Multiphysics from images of Gonçalves-Pimentel et al.38. Locations of the clusters considered in the simulations are indicated and labelled in black in the astrocytic process and in blue and green in the cell body. IP3 photorelease was assumed to occur between locations 1 and 5. Simulation procedures are the same as for Fig. 4 (main text), with θ = 250 μms−1 and flash duration = 500 ms. Panels (B and C) show the minimal first puffs and the mean first puff latencies at the different locations, respectively. At equal distance from the IP3 release point, puffs occur sooner in the process than in the body. Panel (D) shows the distribution of IP3 2 s after the flash: higher local IP3 concentrations are reached in the process in which there is no dilution effect. The spatio-temporal evolution of [IP3] can be seen in the Supplement Video S5.
In conclusion, the value inferred for the effective IP3 diffusion coefficient is not significantly affected by considering a 3D system instead of a 2D one. On the other hand, cell shape can considerably affect spreading, with elongated geometries increasing concentration gradients and thereby favoring fast diffusion.
Realistic ER geometry
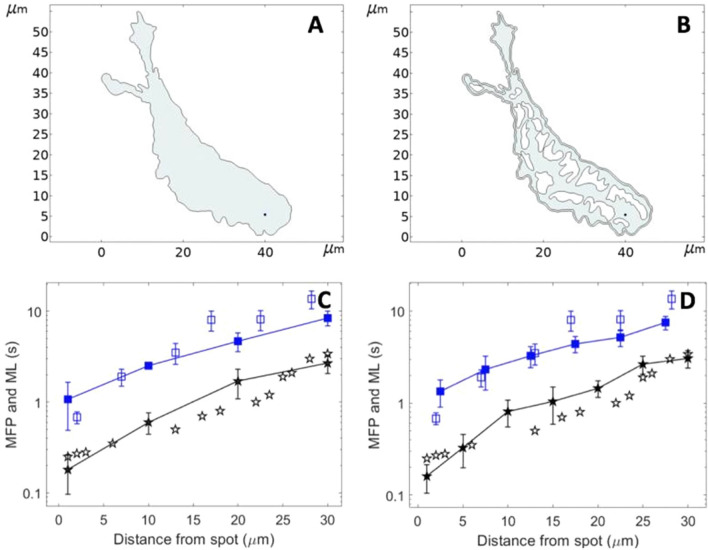
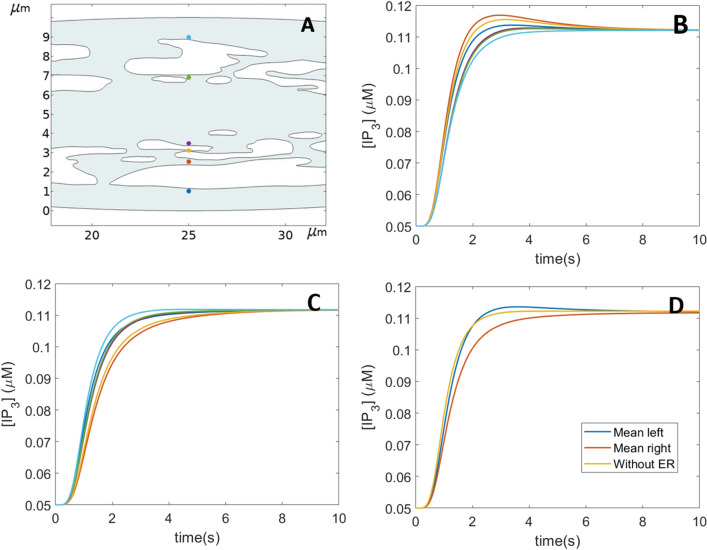
The ER consists in a network of tubules and flattened sacs, resulting in a complex shape. Since the IP3 receptors are located within its membrane, spreading of IP3 to the receptors may be affected by the ER structure. Taking advantage of the great flexibility of Comsol Multiphysics, we performed simulations in which the ER structure was explicitly considered. Thus, we simulated the geometry of a SH-SY5Y cell, in which we inserted an ER whose shape was largely inspired from 2D images of ER in DC-3F cells39. We first compared the puff latencies simulated in the absence and in the presence of ER. The values of the rates of IP3 release were kept the same as in the simulations above (Fig. 4), using a scaling factor to take into account the changes in the cytosolic volume. Puff latencies were in average not much affected by the presence of the ER (Fig. 8). However, the time evolution of the corresponding IP3 profiles indicates that spreading is locally affected by the extent and the shape of the accessible portions of cytosol between the IP3 release point and a given puff location. This is most easily visualized in an idealized, ellipse-shaped geometry. In this case, the IP3 profiles along a fictive line located at half the cell length and perpendicular to the gradient are indeed slightly different when IP3 is released at the right or the left extremity of the cell (Fig. 9A–C). Thus, IP3R clusters locally experience somewhat different IP3 increases depending on the ER shape, which represents an additional source of randomness in puff activity. When compared to simulations where the ER structure is not considered, average IP3 increases are in average either a bit slower or similar (Fig. 9D). However, locally, the presence of the ER can also allow for a faster IP3 increase (Fig. 9B).
Figure 8.
Theoretical investigation of the influence of the ER membranes on the diffusion of IP3 and Ca2+ puff occurrence. Panels (A and B) show the 2D geometry considered in the computational simulations of IP3 diffusion and Ca2+ puff occurrence in response to the localized photorelease of a non-metabolizable IP3 analogue. The shape of the cell was redrawn from Dickinson et al. (2016) and that of the ER was largely inspired from De Angelis et al.39. Panels (C and D) show the simulated (plain symbols) and experimental (empty symbols) mean latencies (blue) and minimal first puff latencies (black). Flash duration is 500 ms. Simulation procedures are the same as for Fig. 4. The rates of localized IP3 photorelease, θ, is taken equal to 400.6 μMs−1 and 284.65 μMs−1, respectively which corresponds to the 250 μMs−1 used in the other simulations with the volumetric adjustments.
Figure 9.
Theoretical investigation of the influence of the ER membranes on the diffusion of IP3 in a 2D ellipse-shaped cell (50 × 10 μm2). Panel (A) shows the central part of the simulated cell together with the locations of the clusters considered. Panels (B and C) show the IP3 temporal profiles at the different cluster locations considering a spot of photorelease of IP3 located at the left (B) or at the right (C) extremity of the cell. Line colors correspond to the points in panel A. In panel (D), the profiles of the IP3 concentrations averaged over the six cluster sites are shown for the two situations corresponding to B and C. Simulation procedures are the same as for Fig. 4. The rate of localized IP3 photorelease, θ, is taken equal to 179.42 μMs−1, which corresponds to the 250 μMs−1 used in the other simulations with the volumetric adjustments.
In conclusion, simulations indicate that the ER does not much affect average spreading of IP3 in the cytosol. Although it constitutes a physical barrier that can locally slow down spreading, it also creates cytosolic channel-like structures in which diffusion is accelerated because ions cannot spread in all directions.
Discussion
IP3 plays a major role in Ca2+ signaling by mobilizing Ca2+ from the ER, which is the main intracellular Ca2+ store. After stimulation, IP3 must diffuse from the plasma membrane where it is produced across the cytoplasm to trigger Ca2+ release from the ER. Thus, IP3 diffusion plays an essential role in Ca2+ signaling. Following measurements in a medium devoid of IP3R, IP3 diffusion was assumed to be fast, with a diffusion coefficient10 of 283 ± 53 μm2s−1. This estimation is in line with those of compounds of similar molecular weight such as ATP for example40. However, this value cannot account for the observation that in response to a localized release of a non-metabolizable analog of IP3, there is a ~ 10 s delay in the occurrence of Ca2+ puffs 30 μm away from the site of IP3 release11. In the same manner, such a rapid diffusion cannot account for the drastic influence of the localization of the IP3-metabolizing 5-phosphatase enzyme on Ca2+ signaling observed in CHO cells by De Smedt et al.41. The latter authors developed a mutant InsP3 5-phosphatase in which the C-terminal cysteine cannot be farnesylated, which hinders its binding to the plasma membrane. While the Ca2+ oscillations detected in the presence of 1 μM ATP were totally lost in 87.5% of intact (farnesylated) InsP3 5-phosphatase-transfected cells, a loss of Ca2+ signal occurred in only 1.1% of the mutant InsP3 5-phosphatase-transfected cells41. Such a sensitivity to the location of an IP3 metabolizing enzyme could not be observed if IP3 was indeed such a fast-diffusing molecule that rapidly becomes homogeneously distributed in the whole cell.
Accordingly, a much lower value of the effective diffusion coefficient of IP3 was predicted by Dickinson et al.11 on the basis of their observations of puff latencies in SH-SY5Y cells in response to the photorelease of a caged non metabolisable IP3 analog. Although this indirect evaluation of the rate of IP3 spreading is submitted to uncertainties due to the stochastic nature of Ca2+ puffs and to their dependence on Ca2+ concentration that is not fully controlled by EGTA, it has the unique advantage of corresponding to realistic cellular conditions. Based on a simplified description of Ca2+ puff dynamics, these authors proposed that observations can be accounted for if the effective diffusion coefficient of IP3 in these cells is in the 3–10 μm2s−1 range. This conclusion was re-examined in the present study, based on a detailed description of Ca2+ puff dynamics. Our results also point to a lower value of the effective diffusion coefficient of IP3 in intact cells than in cytosolic extracts of Xenopus oocytes but concluded to a decrease by a factor ~ 3 instead of ~ 30. On the basis of their in vitro measurement, Allbritton et al.10 predicted the range of action of IP3 to be ~ 24 μm. This is an estimation of the range on which IP3 can diffuse before being metabolized into another inositol phosphate. Given that this range is proportional to , we would predict a range of action of 14 μm. This value is estimated at 4.5 μm with the DI value inferred in Dickinson et al.11. Since most mammalian cells have a diameter of ~ 12 μm42, the two values lead to significantly different physiological conclusions. With an effective diffusion coefficient of 100 μm2s−1, IP3 acts as a global messenger in many cases, although this would not be the case in very large cells such as oocytes or some adipocytes.
Besides the different computational framework, our approach differs from that developed by Dickinson et al. (2016) in three ways. First, only one spatial dimension was considered in the latter study, while we performed 2D or 3D simulations. As illustrated for simulations of IP3 diffusion in the 1D-like astrocytic process (Fig. 7 and Video S5), diffusion is faster in 1D. The 1D approach of the previous study led to an underestimation of the diffusion coefficient because the value was fitted to reproduce the effective rate of IP3 diffusion that was observed in 3D. A second important difference relates to the relation between IP3 concentration and the probability of Ca2+ puff occurrence. At low IP3 concentration, the linear relation considered in the previous study predicts a larger probability of puff occurrence than the nonlinear function used in the present study (Eq. 2). Again, this higher probability of puff occurrence was compensated by a lower value of the IP3 diffusion coefficient. Thirdly, because we here considered both the mean and the minimal first puff latencies, we inferred the rate of IP3 release by the flash (parameter in Eqs. 3 and 5) in a more accurate way. We reasoned that the mean first puff latency is affected by an increase in Ca2+ because the latter is not fully prevented by the EGTA injected in the cell at a final concentration of 5 μM. In line with this hypothesis, agreement for both mean and first puff latencies was only obtained in our simulations when considering that locally the level of Ca2+ was in average 120 nM above basal level when puff activity was monitored after distributed photorelease of IP3. Because this Ca2+ increase was not taken into account in the previous study of Dickinson et al.11, the value of was overestimated, leading again to an underestimation of the IP3 diffusion coefficient.
To confirm that the IP3 diffusion coefficient is indeed larger than 10 μm2s−1, we simulated the experimental protocol of distributed photorelease of IP3 used in Dickinson et al.11 to induce a spatially uniform rise in IP3 concentration and described in the methodology section here above. Simulations performed with DI = 10 μm2s−1 and shown in Figure S6B&D show that IP3 concentration remains spatially inhomogeneous up to at least 10 s after the flash. This is not in agreement with the observation that first puff latencies are independent from the distance from cell end under this protocol. In contrast, when DI = 100 μm2s−1, IP3 rapidly equilibrates in the whole cell (Figure S6C).
Simulations predict that IP3 spreading is not much affected by the proximity of the plasma membrane. Indeed, times of puffs occurrence are mainly determined by the distance of the clusters from the spot of IP3 photorelease and not by their positions in the bulk of the cytoplasm (as in Fig. 4) or close to the plasma membrane (as in Fig. 6). This is relevant because clusters of IP3 receptors are most of the time located very close to the plasma membrane37. Similarly, IP3 spreading is not much affected by the precise shape of the cell. Simulations of puff activity indeed lead to similar results in a 2D ellipse-shaped cell (Fig. 4), in a 3D ellipsoid (Fig. 6) or in a 2D configuration resembling a SH-SY5Y cell (Fig. 8). However, elongated geometries favor fast diffusion and allow for higher local [IP3] than in extended systems. This was shown here by simulations in a situation corresponding to an astrocyte. Accordingly, rates of Ca2+ waves propagation in astrocytic processes are larger than in most cell types (33–100 μms−1, see Cornell-Bell and Finkbeiner43 versus 10–20 μms−1, see Dupont et al.23). Surprisingly, the presence of the ER, which acts as an obstacle to free diffusion, does not much affect mean effective diffusion. This lack of global effect can be ascribed to the fact that while some paths are slower because of obstruction, others are faster because of the presence of channel-like structures which favor fast diffusion thanks to the absence of dilution effect. It should be noted that we did not consider the tubular-shaped of this network, nor its dynamical evolution into a more fragmented structure44.
The value of 100 μm2s−1 for the effective diffusion coefficient of IP3 that emerges from our simulations is in line with the buffering effect exerted by the non-fully bound IP3R tetramers, as proposed previously11,13. Thus, it is expected to be smaller in cell types with higher expression levels of IP3R. In the same line, effective diffusion is much accelerated upon increasing [IP3] because buffers become saturated. Thus, upon cell stimulation by an agonist that generally leads to a surge in [IP3] followed by a decrease, the properties of diffusion are expected to vary. Even more significant in this respect are the IP3 oscillations that have been observed in several cell types and that arise from the activation by Ca2+ of IP3 synthesis by PLC45 and/or of IP3 metabolism by a 3-kinase46,47. Further studies are required to assess the consequences of the interplay between the temporal changes in IP3 concentration and its diffusional properties.
Supplementary Information
Acknowledgements
This work was supported by a PDR FRS-FNRS project (T.0073.21). GD is Research Director at the Belgian “Fonds National pour la Recherche Scientifique” (FRS-FNRS). We thank Benjamin Wacquier and Laurent Combettes for scientific help and fruitful discussions, as well as Silvina Ponce Dawson for constructive comments.
Author contributions
Conceptualization by ROG and GD. Code development by ROG, VV and DG. Analysis of the simulations by all authors. Writing of original draft by GD and ROG. Editing by all others.
Data availability
Codes are available at https://github.com/RobertoOrnelasGuevara/ca2-puffs/tree/main.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-29876-3.
References
- 1.Bootman M, Collins T, Peppiatt C, Prothero L, MacKenzie L, De Smet P, Travers M, Tovey S, Deo J, Berridge M, Ciccolini F, Lipp P. Calcium signalling – an overview. Seminar Cell Dev. Biol. 2001;12:3–10. doi: 10.1006/scdb.2000.0211. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ. Elementary and global aspects of calcium signalling. J. Physiol. 1997;499:291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sammels E, Parys J, Missiaen L, De Smedt H, Bultynck G. Intracellular Ca2+ storage in health and disease: a dynamic equilibrium. Cell Calcium. 2010;47:297–314. doi: 10.1016/j.ceca.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Bezprozvanny I, Watras J, Ehrlich B. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- 5.Smith I, Parker I. Imaging the quantal substructure of single IP3R channel activity during Ca2+ puffs in intact mammalian cells. Proc. Natl. Acad. Sci. USA. 2009;106:6404–6409. doi: 10.1073/pnas.0810799106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swillens S, Dupont G, Combettes L, Champeil P. From calcium blips to calcium puffs: theoretical analysis of the requirements for interchannel communication. Proc. Natl. Acad. Sci. USA. 1999;96:13750–13755. doi: 10.1073/pnas.96.24.13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bootman M, Berridge M, Lipp P. Cooking with calcium: The recipes for composing global signals from elementary events. Cell. 1997;91:367–373. doi: 10.1016/S0092-8674(00)80420-1. [DOI] [PubMed] [Google Scholar]
- 8.Skupin A, Kettenman H, Winkler U, Wartenberg M, Sauer H, Tovey S, Taylor C, Falcke M. How does intracellular Ca2+ oscillate: By chance or by the clock? Biophys. J. 2008;94:2404–2411. doi: 10.1529/biophysj.107.119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voorsluijs V, Ponce Dawson S, De Decker Y, Dupont G. Deterministic limit of intracellular calcium spikes. Phys. Rev. Lett. 2019;122:088101. doi: 10.1103/PhysRevLett.122.088101. [DOI] [PubMed] [Google Scholar]
- 10.Allbritton N, Meyer T, Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992;258:1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- 11.Dickinson G, Ellefsen K, Ponce Dawson S, Pearson J, Parker I. Hindered cytoplasmic diffusion of inositol trisphosphate restricts its cellular range of action. Sci. Signal. 2016;9:ra108. doi: 10.1126/scisignal.aag1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao Y, Choi I, Parker I. Quantal puffs of intracellular Ca2+ evoked by inositol trisphosphate in Xenopus oocytes. J. Physiol. 1995;482:533–553. doi: 10.1113/jphysiol.1995.sp020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor, C. & Konieczny, V. IP3 receptors: Take four IP3 to open. Science Signaling9(422):pe1 (2016) [DOI] [PMC free article] [PubMed]
- 14.Alzayady K, Wang L, Chandrasekhar R, Wagner L, Van Petegem F, Yule D. Defining the stoichiometry of inositol 1,4,5-trisphosphate binding required to initiate Ca2+ release. Science Signaling. 2016;9(422):ra35. doi: 10.1126/scisignal.aad6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dupont, G., Falcke, M., Kirk, V. & Sneyd, J. Models of calcium signalling. Interdisciplinary Applied Mathematics (Springer International Publishing, Switzerland), Vol 43. (2016)
- 16.Dupont G, Abou-Lovergne A, Combettes L. Stochastic aspects of oscillatory Ca2+ dynamics in hepatocytes. Biophys. J. 2008;95:2193–2202. doi: 10.1529/biophysj.108.133777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spät A, Bradford P, McKinney J, Rubin R, Putney J. A saturable receptor for 32P-inositol-1,4,5-trisphosphate in hepatocytes and neutrophils. Nature. 1986;319:514–516. doi: 10.1038/319514a0. [DOI] [PubMed] [Google Scholar]
- 18.Wojcikiewicz R. Type I, II, and III inositol 1,4,5-trisphosphate receptors are unequally susceptible to down-regulation and are expressed in markedly different proportions in different cell types. J. Biol. Chem. 1995;270:11678–11683. doi: 10.1074/jbc.270.19.11678. [DOI] [PubMed] [Google Scholar]
- 19.Tanimura A, Morita T, Nezu A, Shitara A, Hashimoto N, Tojyo Y. Use of fluorescence resonance energy tansfer-based biosensors for the quantitative analysis of inositol 1,4,5-trisphosphate dynamics in calcium oscillations. J. Biol. Chem. 2009;284:8910–8917. doi: 10.1074/jbc.M805865200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lock, J. & Parker, I. IP3 mediated global Ca2+ signals arise through two temporally and spatially distinct modes of Ca2+ release. eLife9: e55008 (2020) [DOI] [PMC free article] [PubMed]
- 21.Dupont G, Dumollard R. Simulation of calcium waves in ascidian eggs: insights into the origin of the pacemaker sites and the possible nature of the sperm factor. J. Cell Sci. 2004;117:4313–4323. doi: 10.1242/jcs.01278. [DOI] [PubMed] [Google Scholar]
- 22.Decrock E, De Bock M, Wang N, Gadicherla A, Bol M, Delvaye T, Vandenabeele P, Vinken M, Bultynck G, Krysko D, Leybaert L. IP3, a small molecule with a powerful message. Biochim. Biophys. Acta. 2013;1833:1772–1786. doi: 10.1016/j.bbamcr.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 23.Dupont G, Combettes L, Leybaert L. Calcium dynamics: spatio-temporal organization from the subcellular to the organ level. Int. Rev. Cytol. 2007;261:193–245. doi: 10.1016/S0074-7696(07)61005-5. [DOI] [PubMed] [Google Scholar]
- 24.Leybaert L. IP3, still on the move but now in the slow lane. Sci. Signal. 2016;9:fs17. doi: 10.1126/scisignal.aal1929. [DOI] [PubMed] [Google Scholar]
- 25.Pawar A, Pardasani K. Effects of disorders in interdependent calcium and IP3 dynamics on nitric oxide production in a neuron cell. Eur. Phys. Journ. Plus. 2022;137:543. doi: 10.1140/epjp/s13360-022-02743-2. [DOI] [Google Scholar]
- 26.Pawar, A., Pardasani, K. Effects of disturbances in neuronal calcium and IP3 dynamics on b-amyloid production and degradation. Cognitive Neurodyn.10.1007/s11571-022-09815-0 (2022) [DOI] [PMC free article] [PubMed]
- 27.Sneyd J, Means S, Zhu D, Rugis J, Won JH, Yule D. Modeling calcium waves in an anatomically accurate three-dimensional parotid acinar cells. J. Theor. Biol. 2017;419:383–393. doi: 10.1016/j.jtbi.2016.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hohendanner F, McCulloch A, Blatter L, Michailova A. Calcium and IP3 dynamics in cardiac myocytes: experimental and computational perspectives and approaches. Front. Pharmacol. 2014;10:3389. doi: 10.3389/fphar.2014.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh N, Adlakha N. A mathematical model for interdependent calcium and inositol 1,4,5-trisphosphate in cardiac myocyte. Netw. Model. Anal. Health Informat. Bioinformat. 2019;8:18. doi: 10.1007/s13721-019-0198-0. [DOI] [Google Scholar]
- 30.Gillespie D. A general method for numerically simulating the stochastic time evolution of coupled chemical reactions. J. Comput. Phys. 1976;22:403–434. doi: 10.1016/0021-9991(76)90041-3. [DOI] [Google Scholar]
- 31.Calabrese A, Fraiman D, Zysman D, Ponce Dawson S. Stochastic fire-diffuse-fire model with realistic cluster dynamics. Phys. Rev. E. 2010;82:031910. doi: 10.1103/PhysRevE.82.031910. [DOI] [PubMed] [Google Scholar]
- 32.Dickinson G, Swaminathan D, Parker I. The probability of triggering calcium puffs is linearly related to the number of inositol trisphosphate receptors in a cluster. Biophys. J. 2012;102:1826–1836. doi: 10.1016/j.bpj.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luzzi V, Sims C, Soughayer J, Allbritton N. The physiological concentration of inositol 1,4,5-trisphosphate in the oocytes of Xenopus laevis. J. Biol. Chem. 1998;273:28657–28662. doi: 10.1074/jbc.273.44.28657. [DOI] [PubMed] [Google Scholar]
- 34.Tovey S, de Smet P, Lipp P, Thomas D, Young K, Missiaen L, De Smedt H, Parys J, Berridge M, Thuring J, Holmes A, Bootman D. Calcium puffs are generic InsP3-activated elementary calcium signals and are downregulated by prolonged hormonal stimulation to inhibit cellular calcium responses. J. Cell Sci. 2001;114:3979–3989. doi: 10.1242/jcs.114.22.3979. [DOI] [PubMed] [Google Scholar]
- 35.Thurley, K., Tovey, S., Moenke, G., Prince, V., Meena, A., Thomas, A., Skupin, A., Taylor, C. & Falcke, M. Reliable encoding of stimulus intensities with random sequences of intracellular Ca2+ spikes. Sci. Signal.7(331): ra59 (2014) [DOI] [PMC free article] [PubMed]
- 36.Dargan S, Parker I. Buffer kinetics shape the spatiotemporal patterns of IP3-evoked Ca2+ signals. J. Physiol. 2003;553:775–788. doi: 10.1113/jphysiol.2003.054247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith I, Wiltgen S, Parker I. Localization of puff sites adjacent to the plasma membrane: functional and spatial characterization of Ca2+ signaling in SH-SY5Y cells utilizing membrane-permeant caged IP3. Cell Calcium. 2009;45:65–76. doi: 10.1016/j.ceca.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonçalves-Pimentel C, Moreno G, Trindade B, Isaac A, Rodrigues C, Savarira M, de Albuquerque A, de Andrade Aguiar J, Andrade-da-Costa B. Cellulose exopolysaccharide from sugarcane molasses as a suitable substrate for 2D and 3D neuron and astrocyte primary cultures. J. Mater. Sci. Mater. Med. 2018;29:139. doi: 10.1007/s10856-018-6147-0. [DOI] [PubMed] [Google Scholar]
- 39.De Angelis A, Denzi A, Merla C, Andre F, Garcia-Sanchez T, Mir L, Apollonio F, Liberti M. Microdosimetric realistic model of a cell with endoplasmic reticulum. Ann. Int. Conf. IEEE Eng. Med. Biol. Soc. 2019;2019:134–137. doi: 10.1109/EMBC.2019.8857540. [DOI] [PubMed] [Google Scholar]
- 40.Hubley M, Moerland T, Rosanke R. Diffusion coefficient of ATP and creatine phosphate in isolated muscle: pulsed gradient 31P NMR of small biological sample. NMR Biomed. 1995;8:72–78. doi: 10.1002/nbm.1940080205. [DOI] [PubMed] [Google Scholar]
- 41.De Smedt F, Missiaen L, Parys J, Vanweyenberg V, De Smedt H, Erneux C. Isoprenylated human brain type I inositol 1,4,5-trisphosphate 5-phosphatase controls Ca2+ oscillations induced by ATP in Chinese hamster ovary cells. J. Biol. Chem. 1997;272:17367–17375. doi: 10.1074/jbc.272.28.17367. [DOI] [PubMed] [Google Scholar]
- 42.Milo R, Phillips R. Cell biology by the numbers. CRC Press; 2015. [Google Scholar]
- 43.Cornell-Bell A, Finkbeiner S. Ca2+ waves in astrocytes. Cell Calcium. 1991;12:185–204. doi: 10.1016/0143-4160(91)90020-F. [DOI] [PubMed] [Google Scholar]
- 44.Subramanian K, Meyer T. Calcium-induced restructuring of nuclear envelope and endoplasmic reticulum calcium stores. Cell. 1997;89:963–971. doi: 10.1016/S0092-8674(00)80281-0. [DOI] [PubMed] [Google Scholar]
- 45.Politi A, Gaspers L, Thomas A, Höfer T. Models of IP3 and Ca2+ oscillations: frequency encoding and identification of underlying feedbacks. Biophys. J. 2006;90:3120–3133. doi: 10.1529/biophysj.105.072249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dupont G, Erneux C. Simulations of the effects of inositol 1,4,5-trisphosphate 3-kinase and 5-phosphatase activities on Ca2+ oscillations. Cell Calcium. 1997;22:321–331. doi: 10.1016/S0143-4160(97)90017-8. [DOI] [PubMed] [Google Scholar]
- 47.Sneyd J, Tsaneva-Atanasova K, Reznikov V, Bai Y, Sanderson M, Yule D. (2006) A method for determining the dependence of calcium oscillations on inositol trisphosphate oscillations. Proc. Natl. Acad. Sci. USA. 2006;103:1675–1680. doi: 10.1073/pnas.0506135103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Codes are available at https://github.com/RobertoOrnelasGuevara/ca2-puffs/tree/main.