Abstract
Low back pain (LBP) is a common and important clinical problem. In addition to pain, patients are also affected by personal, social, and economic burdens. Intervertebral disc (IVD) degeneration is a common cause of LBP, further increasing the patient’s morbidity and medical costs. The limitations of current treatment strategies for long-term pain relief mean that increasing attention has been paid to regenerative medicine. We carried out a narrative review to explore the roles of four types of regenerative medicine for treating LBP: marrow-derived stem cells, growth factors, platelet-rich plasma, and prolotherapy. Marrow-derived stem cells are regarded as an ideal cell source for IVD regeneration. Growth factors may stimulate the synthesis of extracellular matrix and attenuate or reverse the degenerative process in IVD, while platelet-rich plasma, which contains multiple growth factors, is thought to be a promising alternative therapy for IVD degeneration. Prolotherapy can initiate the body’s inflammatory healing response to repair injured joints and connective tissues. This review summarizes the mechanisms, in vitro and in vivo studies, and clinical applications of these four types of regenerative medicine in patients with LBP.
Keywords: Low back pain, intervertebral disc degeneration, regenerative medicine, marrow-derived stem cell, growth factor, platelet-rich plasma, prolotherapy
Introduction
Low back pain (LBP) is a common clinical condition with negative impacts on the patient’s psychosocial well-being, function, and quality of life, as well as posing a major socioeconomic burden.1 LBP is the primary cause of disability in individuals below 50 years of age, with an annual incidence of 15% and a prevalence rate of nearly 30%.2,3 Women have higher prevalence of LBP compared with men,1 and it is especially prevalent during adolescence, with 37.0% of individuals reporting LBP at least monthly.4 Discogenic pain arising from intervertebral disc (IVD) degeneration accounts for around 42% of all cases of non-specific LBP,5–8 and younger patients are relatively more likely to have discogenic LBP.9–11 Neurotrophic factors participate in the pathogenesis of discogenic LBP, contributing to the transmission of physiological and pathological pain.12,13
Steroid injections into the epidural space have been used to treat LBP since the early 1950s.14 Transforaminal epidural steroids have demonstrated a modest analgesic effect for up to 3 months in patients with lumbosacral radicular pain from herniated IVDs.15 Compared with a placebo, epidural prolotherapy was shown to reduce pain for up to 48 hours, but not for 2 weeks, in patients with chronic LBP.16 However, lumbar epidural steroid injections can result in transient complications due to various side effects, including insomnia, impaired glucose control, and hypertension.17 As a result, the benefit of epidural steroid injections remains controversial, and they are also limited by a short duration of analgesia.18,19 The first-line pharmacological treatments for LBP are non-steroidal anti-inflammatory drugs (NSAIDS) and acetaminophen.20 Although NSAIDs are more effective than acetaminophen, they are associated with more side effects such as gastric ulceration, cardiovascular events, and renal impairment.20,21
There has been a growing interest in the use of regenerative medicine for treating chronic pain. Unlike steroids, which suppress inflammation, the rationale for regenerative medicine is to stimulate the repair of damaged structures.22 Various regenerative medicine strategies have been studied, including tissue engineering, cell therapy, gene therapy, and growth factors.23 Given that IVD degeneration is a major cause of LBP, this review aims to summarize four current common regenerative therapies for LBP, especially in relation to IVD degeneration: marrow-derived stem cells (MSCs), growth factors, platelet-rich plasma (PRP), and prolotherapy.
Article search methodology
We searched PubMed, Google Scholar, and ClinicalTrials.gov for original papers published from the start of the respective databases until June 2022, including any one of the following keywords in the title or abstract: “regenerative medicine” and either “pain” or “low back pain” or “intervertebral disc” or “IVD”. MSC studies must contain the words “marrow-derived stem cell”, “marrow derived stem cells”, “mesenchymal stem cell”, “MSC”, or “stem cell”; growth factor studies must contain the words “growth factor”; PRP studies must contain the words “platelet rich plasma”, “platelet-rich plasma”, or “PRP”; and prolotherapy studies must contain the words “prolotherapy”, “sclerosant therapy”, or “sclerotherapy”. Moreover, we also assessed the reference lists of the screened articles and previously published reviews and meta-analyses in the related subject areas to identify any relevant information.
Intervertebral disc degeneration
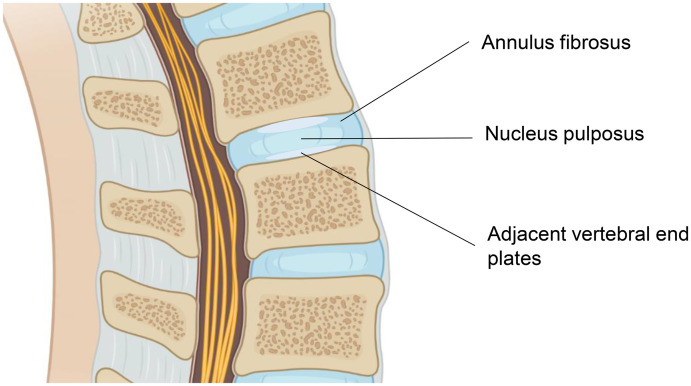
The causes of chronic LBP involve the discs in about 42% of cases, the sacro-iliac joint in 31%, and the zygapophyseal joint in 18%.5 IVD degeneration is one of the major causes of LBP. IVDs comprise three key components that work together: the inner nucleus pulposus (NP), the outer annulus fibrosus (AF) supporting the NP, and the adjacent vertebral end plates (Figure 1).22,24 The NP is surrounded by the AF circumferentially, and the vertebral end plates bound the IVD superiorly and inferiorly.24 This interconnected unit of tissues contains different ratios of proteoglycans, collagen, and water. The NP acts like a cushion because of its dramatic water content, while the AF and vertebral end plates join together to decrease the compression forces and protect the vertebral bodies from bony compromise.24 All three parts can be potentially targeted by regenerative medicine.22
Figure 1.
Structural overview of intervertebral disc (IVD). The IVD is composed of three key components: the inner nucleus pulposus, the outer annulus fibrosus, and the adjacent vertebral end plates.
IVD degeneration is a cell-mediated process responding to the functional and structural failure of the disc.25 IVD degeneration has various causes, including nutritional, genetic, and mechanical influences, and is characterized by a progressive decline in NP hydration as the result of a decrease in extracellular matrix (ECM) molecules, e.g. collagen and aggrecan.26–28 A high ECM content is maintained within IVD cells, and the disc matrix has an elaborate structure to attract and hold water.29 The integrity of the IVD partially depends on the balance between matrix synthesis and degradation, and failure of this balance may lead to disc degeneration.30
Dysregulated disc hydration could affect the mechanical tension in the collagen fibers of the AF, leading to abnormal axial spinal loading forces and segmental instability.31 Finally, other pathologies can develop as a result of IVD, including IVD herniation, spinal canal stenosis, spondylolisthesis, and facet joint pain.32,33 Various regenerative medicine therapies are available depending on the degree of IVD degeneration. Early-stage degeneration is usually treated by biomolecular therapies, such as PRP, prolotherapy, and hyaluronic acid.22 Cell-based therapies such as stem cells may be more useful for intermediate degeneration,22 while tissue engineering may be considered for advanced disc degeneration involving severe structural damage, when few viable cells are available.22 Extensive research has been carried out to explore the use of biological therapies and regenerative approaches for IVD degeneration by inhibiting abnormal cytokine production and stimulating matrix anabolism.34
Marrow-derived stem cells
Stem cell-based strategies, consisting of tissue-engineering and biomaterials science, are the basic science behind regenerative medicine.34 Adult stem cells, including hematopoietic and mesenchymal stem cells, can be found in most tissues in the body and are responsible for tissue maintenance and response to injury.35 MSCs are emerging as a frontier in the areas of regenerative medicine and musculoskeletal disorders.36 MSCs are undifferentiated multipotent stem cells derived from adult bone marrow, which can differentiate into various cell types, including bone, tendon, adipose, cartilage, and muscle cells.37 MSCs also have anti-inflammatory and immunosuppressive properties and can be injected or surgically implanted into the target tissues.38
The degeneration of ECM components can lead to apoptosis of NP cells. The proteoglycan content is reduced in IVD degeneration, partially due to apoptosis or necrosis of NP cells,39,40 but MSCs can replenish these NP cells and core ECM to regenerate the IVD. More specifically, MSCs can differentiate into NP-like cells to secrete ECM components, leading to the supplementation of NP cells and stimulation of NP reconstruction.29 MSCs have three kinds of regenerative properties: i) differentiation into target cell types; ii) activation of proliferation of resident cells; and iii) improving nutrient supply by paracrine effects.29
Disc degeneration may be caused by increased cell senescence and dysregulated cellular activities. Their limited nutrient and oxygen supplies, as well as constant high mechanical stress, mean that IVDs may find it difficult to regenerate themselves following degeneration and injury.29 It has been proposed that MSCs may be used to treat diseased or injured musculoskeletal tissue by selecting stem cells that are characteristically similar to the differentiated target tissue.41 MSCs play a vital role in IVD regeneration, due to their immunomodulatory functions and capacity to differentiate into chondrocytes in suitable microenvironments, and have thus been regarded as an ideal cell source for IVD regeneration.6
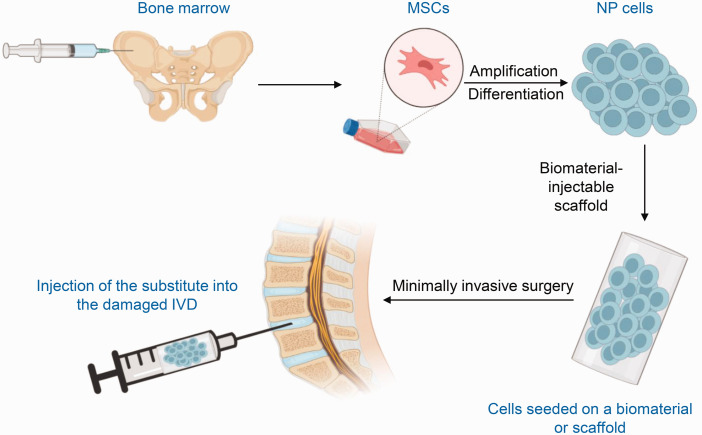
The main procedures of IVD tissue engineering are summarized in Figure 2.42 MSCs are generally collected from the bone marrow and then amplified in vitro. The cells are further cultured under various conditions, including growth factors and culture in hypoxic conditions or in a three-dimensional environment, to produce the NP cell phenotype. The differentiated cells are then seeded on a biomaterial or scaffold and the substitute is injected into the damaged IVD.
Figure 2.
Graphical representation of marrow-derived stem cell (MSC) therapy for intervertebral disc (IVD) degeneration. MSCs are collected from bone marrow, amplified in vitro, and then cultured under various conditions to differentiate into nucleus pulposus (NP) cells. The differentiated cells are then seeded on a biomaterial or scaffold and the substitute is injected into the target IVD.
Researchers have focused on differentiating MSCs into an NP cell-like phenotype. MSCs cultured with growth/differentiation factor 5 in a hypoxic environment were shown to upregulate ECM-related genes in the IVD and NP markers.43 The resulting MSCs acquired an NP cell-like phenotype and may be used for IVD regenerative therapy. Other growth factors, including transforming growth factor-β3 and growth/differentiation factor 6, significantly increased the expression of NP marker genes and the production of glycosaminoglycans in MSCs.44 MSCs seeded in scaffolds have been regarded as a popular option for tissue engineering. Scaffolds can mimic the tissue and provide structural supports for cell attachment, proliferation, and ECM accumulation.45 MSCs were shown to differentiate into an NP-like phenotype when seeded in genetically engineered silk fleeces with growth factors.46
Cell culture and animal model studies have demonstrated the potential application of MSC implantation to regenerate the IVD. Both in vitro and in vivo studies have been performed to explore the feasibility of stem cell therapy for IVD degeneration.47 Co-culture of adult human NP cells and MSCs in vitro resulted in increased ECM production.47 In an in vivo study, allogenic adult rabbit MSCs were injected into healthy adult rabbit discs to investigate the survival and engraftment of MSCs in living disc tissue. MSCs were identified in histological sections of the discs, indicating that the stem cells had the potential for migration and engraftment into the inner AF layer of the IVD.47 These results provide useful information supporting the therapeutic effects of stem cell therapy in IVD degeneration.
MSC injection is a potentially attractive treatment option in clinical practice, due to the safe isolation of MSCs from patient tissues and negligible immunogenicity.48 A prospective controlled trial to evaluate the effectiveness of autologous MSCs in patients with chronic LBP with severe lumbar spinal degeneration compared the effects of a one-time bone marrow concentrate injection into the spine with conventional treatment with NSAIDs at 1, 3, 6, and 12 months after treatment.49 The results demonstrated significant improvements in pain relief and function in patients who received the bone marrow concentrate injection compared with the control group.49 MSC implantation in IVDs has been reported to lead to osteophyte formation by cell leakage,28 and the combined injection of MSCs and hyaluronic acid (HA) has been proposed to reduce the risk of osteophyte formation.50,51 In a clinical phase I study, patients with chronic discogenic LBP who received a single intradiscal injection of combined HA derivative and adipose tissue-derived MSCs were compared with patients who received conventional treatments, with a 12-month follow-up.28 The results demonstrated good safety and tolerability of the combination of MSCs and HA derivative, as well as significant improvements in pain scores compared with conventional treatments.28
Growth factors
Growth factors are polypeptides originally discovered in body fluids and tissue extracts, which can modulate cell growth and differentiation.52 Growth factors activate intercellular signaling cascades by binding to cell membranes via specific transmembrane receptors, leading to the stimulation of cell differentiation, proliferation, migration, and apoptosis.53 Growth factors can perform biological functions via endocrine, paracrine, and autocrine mechanisms.53 In the IVD, growth factors are delivered by tissue fluids through endocrine mechanisms, while the autocrine and paracrine systems are regarded as the major regulatory mechanisms in IVD tissues.54 The use of growth factors as a potential therapeutic method for the regeneration of IVD tissues is currently under investigation.
Various growth factors have demonstrated the potential to modulate anabolic and anti-catabolic effects in IVD tissue engineering both in vitro and in animal studies.24 Increasing attention has been paid to investigating the molecular degeneration process and to identifying factors that could stop or reverse the degenerative process. Growth factors administered into the IVD can stimulate the synthesis of ECM, reduce inflammation, and slow down the degenerative process.55 Growth differentiation factor family members have reportedly shown the potential for disc regeneration in IVD degeneration models both in vitro and in vivo, to be vital for IVD homeostatic processes, and to upregulate marker genes of healthy NP cells in degenerative cells.56 The safety and efficacy of growth factor bone morphogenetic proteins (BMPs) for the regeneration of mildly degenerated IVD have been explored by conjugating BMPs to a fibrin/HA hydrogel in an animal model.57 The results demonstrated that BMPs could increase the expression and synthesis of ECM in human IVD cells, and thus have regenerative potential in IVD applications.
Injection of a single growth factor may have limited use because of its short half-life and instability, meaning that it is insufficient to reverse the degenerative process.58,59 As a result, combined treatment with multiple growth factors may be encouraged to enhance the gradual release at target sites to optimize the therapeutic effects for IVD degeneration.58 PRP includes multiple growth factors, and much attention has thus been paid to its use as a promising therapeutic alternative for IVD regeneration.59
Platelet-rich plasma
PRP therapy is based on the theory that platelets and plasma in the blood can heal and repair injured tissues.60 PRP was developed in the 1970s.61 PRP is generated by centrifugation of whole blood to increase the platelet concentration by three- to five-fold compared with the physiological baseline, and is widely applied for tissue regeneration and repair.1,62,63 Activated PRP is composed of activated platelets and high levels of growth factors that are responsible for tissue healing and regeneration.35 Platelets can regulate hemostasis via the processes of adhesion, activation, and aggregation.1 Growth factor release modulates inflammation and enhances revascularization, which accelerates epithelial regeneration in the wound-healing process.62 The growth factors present in PRP include transforming growth factor-β, basic fibroblast growth factor, and epidermal growth factor, which can promote cell proliferation, migration, differentiation, and angiogenesis.35,64,65
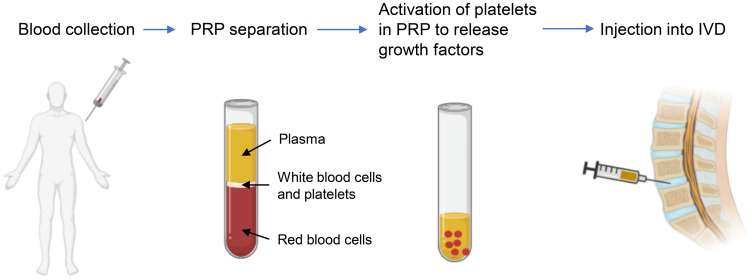
The process of PRP therapy is shown in Figure 3.66 Blood is first drawn from the patient and centrifuged to concentrate the platelets by removing most of the plasma and red blood cells. The platelets are then activated to release growth factors and the activated PRP is then reintroduced into the target site.
Figure 3.
Platelet-rich plasma (PRP) preparation and injection into the intervertebral disc (IVD). A small blood sample is harvested from the patient and then centrifuged. The platelets in PRP are activated to release growth factors and the activated plasma is then reintroduced into the IVD via injection.
PRP can be injected epidurally or intradiscally for the management of LBP from IVD pathology.67 The safety and efficacy of intradiscal injection of PRP for IVD degeneration have been studied in a clinical trial.68 In this study, PRP was injected into the center of the NP in patients with chronic LBP, and pain scores were significantly decreased after 10 months of follow-up compared with pre-treatment, with no adverse effects.68 In a case report, a 42-year-old male patient with IVD degeneration showed considerable improvements in pain and range of motion 6 weeks after a single intradiscal PRP injection, and improvement in LBP after 1 year.69 In a prospective randomized controlled study, patients with chronic nonspecific LBP were treated with PRP injection or control lidocaine injection at the lumbopelvic ligaments for more than 3 years.70 The results showed decreased pain intensity in the PRP group compared with the lidocaine group at 6 months after the intervention, and disability indices were decreased in the PRP group at both 3 and 6 months after treatment.70 In a prospective randomized, open, blinded, endpoint study, patients with chronic LBP were injected with PRP or saline control for 3 months, and the pain score was significantly decreased in the PRP group compared with the saline group at 6 weeks and 3 months.71 In another prospective trial, patients with discogenic LBP received a single intradiscal injection of PRP,72 and 47% of patients had at least a 50% pain improvement and a 30% improvement in the Oswestry Disability Index score at 6 months of follow-up.72 A randomized controlled trial compared lumbar facet steroid injection versus PRP73 and showed that although both treatments reduced pain, PRP provided a longer duration of analgesic efficacy.73 In a case series, PRP was injected into the facet joints in two patients with worsening chronic LBP.74 One patient had at least a 30% improvement in pain after the 1st injection, 60% after the 2nd injection, and a numerical rating scale pain score of 1/10 at 9 months of follow-up, while another patient showed a 70% improvement in symptoms and increased functional status after the 3rd injection.74 In a prospective clinical evaluation, intra-articular injections of autologous PRP into the lumbar facet joints was reported to be effective and safe in patients with axial LBP.75
Prolotherapy
Prolotherapy, also known as sclerotherapy, involves the injection of biological substances (e.g. saline and dextrose) that trigger the body’s inflammatory healing response and thereby help to repair the injured tissues. Prolotherapy has been applied to treat chronic LBP for more than 50 years.76 The injection of prolotherapy agents into ligamentous tissue is believed to trigger a series of activities, from the influx of granulocytes, macrophages, and fibroblasts to the release of growth factors, finally leading to collagen deposition. The consequences of these activities include the strengthening of ligaments and reduced pain and disability.76
Some clinical trials found that prolotherapy injection was no more effective than the control treatment for chronic LBP (Table 1). A randomized, double-blind, placebo-controlled trial of prolotherapy for facet joint-mediated pain found no significant difference in pain scores at 6 months between the prolotherapy and control (saline) groups in patients with chronic LBP.77 Another randomized double-blind trial examined the efficacy of dextrose–glycerine–phenol injections into the posterior ligaments, fascia, and joint capsules for treating chronic LBP, compared with saline control.78 Patients received six injections of either proliferant or saline into the posterior sacroiliac and interspinous ligaments, fascia, and joint capsules of the low back from L4 to the sacrum. Pain and disability scores were significantly decreased in both groups at 6 months after injection, with no significant difference between the two groups.78 In a randomized trial, patients with nonspecific LBP were injected with 20% glucose/0.2% lignocaine or normal saline into tender lumbo-pelvic ligaments, followed by either flexion/extension exercises or normal activity for more than 6 months,79 with no significant difference between the treatment and control groups.79 In contrast, other clinical trials found that prolotherapy injections were more effective than control treatments for patients with LBP (Table 1). In a randomized clinical trial, patients with chronic LBP were treated with an empirically devised regimen of forceful spinal manipulation combined with injections of a dextrose–glycerine–phenol solution into soft-tissue structures, compared with the same treatment and saline injection in the control group.80 There was a significantly greater reduction in mean visual analogue scale pain scores at 6 months and a >50% improvement in disability scores from baseline to 6 months in the treatment group compared with the control group.80 In a three-patient case report, ultrasound-guided combined prolotherapy and PRP injection to the sacroiliac ligaments, facet joint capsule, and epidural space was performed successfully and safely in patients with chronic LBP,81 resulting in decreased pain level, reduced or eliminated oral analgesic use, and improved function at 1-year follow-up.81
Table 1.
Clinical studies of prolotherapy.
| Author (year) | Study details | Results |
|---|---|---|
| Dechow et al. (1999)77 | Design: randomized, double-blind, placebo-controlled trialIntervention: prolotherapy vs saline injections into the facet jointsFollow-up: 6 months | Treatment of facet joint-mediated pain using prolotherapy demonstrated no significant difference in pain scores compared with normal saline with 1% lignocaine (control group) |
| Klein et al. (1993)78 | Design: randomized double-blind trialIntervention: prolotherapy vs saline injection into the posterior ligaments, fascia, and joint capsulesFollow-up: 6 months | No significant difference in pain and disability scores between the two groups |
| Yelland et al. (2004)79 | Design: randomized controlled trialIntervention: prolotherapy vs saline injection into tender lumbo-pelvic ligamentsFollow-up: 6 months | No significant difference between treatment and control groups |
| Ongley et al. (1987)80 | Design: randomized clinical trialIntervention: prolotherapy vs saline injection into soft-tissue structuresFollow-up: 6 months | Significantly greater reduction in mean VAS pain score in treatment compared with control group. Treatment group had >50% improvement in disability score from baseline to 6 months, compared with control group |
| Tolbert et al. (2013)81 | Design: case reportIntervention: prolotherapy and PRP injection into sacroiliac ligaments, facet joint capsule and epidural spaceFollow-up: 1–11 months | Decreased pain level, reduced or eliminated oral analgesic use, and improved function |
VAS, visual analogue scale.
Discussion
Chronic LBP is responsible for an enormous burden on both the affected individuals and wider society. IVD degeneration is an important cause of chronic LBP. In this review, we described the mechanisms, in vitro and in vivo studies, and clinical evidence regarding four major regenerative medicine treatment modalities for managing chronic LBP, especially in relation to IVD degeneration. MSCs can replenish NP cells and the core ECM to regenerate IVDs, and have been regarded as an ideal cell source for IVD regeneration due to their immunomodulatory functions and capacity to differentiate into chondrocytes under suitable microenvironments. Growth factors could stimulate the synthesis of ECM, relieve inflammation, and attenuate or reverse the degenerative process in IVD. Injection of a single growth factor may have limited use due to its short half-life and unstable status; however, much attention has been paid to PRP, which includes multiple growth factors, as a promising alternative therapeutic method for IVD regeneration. Prolotherapy could initiate an inflammatory healing cascade by mimicking the body’s own response to treat LBP.
Although these different regenerative medicine approaches seem to provide a promising future for the treatment of LBP, some issues remain. Although some clinical studies have demonstrated the effects of these treatments for reducing chronic LBP and disability scores, clinical evidence is still limited. More randomized controlled trials comparing the various regenerative treatment modalities with standard treatments for LBP are therefore needed to determine their efficacy and side effect profiles.
MSC treatment has some potential problems. Most studies have used exogenous stem cells, but these may be limited by their potential immunogenicity. In addition, some studies used animal models and the results may therefore not be applicable to humans. Although MSCs have been shown to arrest the degenerative processes, they can only partially regenerate the degenerative disc, given that the degeneration process often continues after a certain period of time.82 Moreover, needle injection into the disc or epidural space may result in complications, including infection and discitis.29 Furthermore, the short monitoring times of the experiments may be not sufficient to generate conclusions about treatment efficacy, and further studies are needed to demonstrate the safety of MSC treatment in human patients. MSC infusion may also have tumorigenic effects, given that MSCs can transform into malignant cells or promote tumor formation.83 In addition, MSCs can enhance tumor growth and metastasis by inhibiting the immune system and promoting the formation of new blood vessels, and because MSCs can differentiate into fibroblasts, fibrotic reactions may also occur during the repair process.84 Given the limited availability of stem cells derived from the bone marrow, bone dust has emerged as a potential alternative option. Bone dust is usually regarded as surgical waste from transforaminal lumbar interbody fusion procedures, but has been shown to produce cells with the characteristics of MSCs in vitro.85 The harvested cells were positive for MSC markers and differentiated into osteogenic and adipogenic cells, which grew robustly and proliferated well in cell culture. Bone dust is thus a potential source of MSCs, as an alternative to bone marrow.85 However, further clinical studies are needed to evaluate the application of MSCs from bone dust in the treatment of LBP.
Some clinical studies support the use of PRP for chronic LBP. However, its duration of effect may be limited due to the short half-life of growth factors from PRP.66 The functional role of PRP depends on the amount of residual viable cells, which may not be sufficient in patients with advanced IVD degeneration. PRP may thus be less effective in patients with severe disease, which could be an important limitation. In addition, the optimal amount, best timing of administration, and potential adverse effects of PRP injections all require further investigation.67
Researchers found that prolotherapy injections alone were no more effective than control treatments in patients with chronic LBP, but may be more effective when combined with spinal manipulation, exercise, or other therapies.76 The main side effects of prolotherapy are pain and mild bleeding from needle trauma. Although prolotherapy injections carried out by an experienced person should be safe, the injection of irritant solutions into ligaments, tendons and joints is theoretically associated with safety concerns, including light-headedness, allergic reaction, infection, and nerve damage. Prolotherapy agents should thus be injected with caution, with the patient in a prone position.86
In conclusion, we have reviewed the roles and applications of MSCs, growth factors, PRP, and prolotherapy in patients with LBP. These treatments can regenerate the IVD by stimulating the synthesis of ECM and replenishing NP cells, or by initiating an inflammatory healing cascade to treat LBP. However, despite their promising therapeutic applications, clinical evidence in this area is still limited, and further clinical trials are required to evaluate the efficacy and safety of these methods compared with current standard treatments.
Acknowledgement
We would like to acknowledge BioRender.com for figure creation.
Footnotes
Author contributions: FW and SSCW determined the objective of this review and established the research questions. FW performed the literature search, reviewed the literature, and selected the relevant articles. FW was responsible for writing the manuscript. CWC and SSCW critically revised the manuscript. All the authors approved the submitted version.
The authors have no conflicts of interests to declare.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Department of Anaesthesiology, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong.
ORCID iD: Stanley Sau Ching Wong https://orcid.org/0000-0002-8763-5687
References
- 1.Akeda K, Yamada J, Linn ET, et al. Platelet-rich plasma in the management of chronic low back pain: a critical review. J Pain Res 2019; 12: 753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biyani A, Andersson GB. Low back pain: pathophysiology and management. J Am Acad Orthop Surg 2004; 12: 106–115. [DOI] [PubMed] [Google Scholar]
- 3.Andersson GB. Epidemiological features of chronic low-back pain. Lancet 1999; 354: 581–585. [DOI] [PubMed] [Google Scholar]
- 4.Swain MS, Henschke N, Kamper SJ, et al. An international survey of pain in adolescents. BMC Public Health 2014; 14: 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piuzzi NS, Chahla J, Jiandong H, et al. Analysis of cell therapies used in clinical trials for the treatment of osteonecrosis of the femoral head: a systematic review of the literature. J Arthroplasty 2017; 32: 2612–2618. [DOI] [PubMed] [Google Scholar]
- 6.Esquijarosa Hechavarria M, Richard SA. Edifying the focal factors influencing mesenchymal stem cells by the microenvironment of intervertebral disc degeneration in low back pain. Pain Res Manag 2022; 2022: 6235400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, Zhu L, Wu G, et al. A comparison between nucleus pulposus-derived stem cell transplantation and nucleus pulposus cell transplantation for the treatment of intervertebral disc degeneration in a rabbit model. Int J Surg 2016; 28: 77–82. [DOI] [PubMed] [Google Scholar]
- 8.Maher C, Underwood M, Buchbinder R. Non-specific low back pain. Lancet 2017; 389: 736–747. [DOI] [PubMed] [Google Scholar]
- 9.Teraguchi M, Yoshimura N, Hashizume H, et al. The association of combination of disc degeneration, end plate signal change, and Schmorl node with low back pain in a large population study: the Wakayama Spine Study. Spine J 2015; 15: 622–628. [DOI] [PubMed] [Google Scholar]
- 10.Teraguchi M, Yoshimura N, Hashizume H, et al. Prevalence and distribution of intervertebral disc degeneration over the entire spine in a population-based cohort: the Wakayama Spine Study. Osteoarthritis Cartilage 2014; 22: 104–110. [DOI] [PubMed] [Google Scholar]
- 11.Cheung KM, Karppinen J, Chan D, et al. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine (Phila Pa 1976) 2009; 34: 934–940. [DOI] [PubMed] [Google Scholar]
- 12.Ohtori S, Inoue G, Miyagi M, et al. Pathomechanisms of discogenic low back pain in humans and animal models. Spine J 2015; 15: 1347–1355. [DOI] [PubMed] [Google Scholar]
- 13.García‐Cosamalón J, Del Valle ME, Calavia MG, et al. Intervertebral disc, sensory nerves and neurotrophins: who is who in discogenic pain? J Anat 2010; 217: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lievre J, Bloch-Michel H, Attali P. Epidural hydrocortisone in the treatment of sciatica. Rev Rhum Mal Osteoartic 1955; 22: 696–697. [PubMed] [Google Scholar]
- 15.Bhatia A, Flamer D, Shah PS, et al. Transforaminal epidural steroid injections for treating lumbosacral radicular pain from herniated intervertebral discs: a systematic review and meta-analysis. Anesth Analg 2016; 122: 857–870. [DOI] [PubMed] [Google Scholar]
- 16.Maniquis-Smigel L, Reeves KD, Rosen HJ, et al. Short term analgesic effects of 5% dextrose epidural injections for chronic low back pain: a randomized controlled trial. Anesth Pain Med 2017; 7: e42550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stout A. Epidural steroid injections for low back pain. Phys Med Rehabil Clin N Am 2010; 21: 825–834. [DOI] [PubMed] [Google Scholar]
- 18.Staal JB, Nelemans PJ, De Bie RA. Spinal injection therapy for low back pain. Jama 2013; 309: 2439–2440. [DOI] [PubMed] [Google Scholar]
- 19.Pinto RZ, Maher CG, Ferreira ML, et al. Epidural corticosteroid injections in the management of sciatica: a systematic review and meta-analysis. Ann Intern Med 2012; 157: 865–877. [DOI] [PubMed] [Google Scholar]
- 20.Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med 2007; 147: 478–491. [DOI] [PubMed] [Google Scholar]
- 21.Gutthann SP, Rodríguez LAG, Raiford DS. Individual nonsteroidal antiinflammatory drugs and other risk factors for upper gastrointestinal bleeding and perforation. Epidemiology 1997: 18–24. [DOI] [PubMed] [Google Scholar]
- 22.Cooper G, Herrera J, Kirkbride J, et al. Regenerative Medicine for Spine and Joint Pain. Springer, 2020. [Google Scholar]
- 23.Yalcinkaya TM, Sittadjody S, Opara EC. Scientific principles of regenerative medicine and their application in the female reproductive system. Maturitas 2014; 77: 12–19. [DOI] [PubMed] [Google Scholar]
- 24.Kennon JC, Awad ME, Chutkan N, et al. Current insights on use of growth factors as therapy for intervertebral disc degeneration. Biomol Concepts 2018; 9: 43–52. [DOI] [PubMed] [Google Scholar]
- 25.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976) 2006; 31: 2151–2161. [DOI] [PubMed] [Google Scholar]
- 26.Oehme D, Goldschlager T, Ghosh P, et al. Cell-based therapies used to treat lumbar degenerative disc disease: a systematic review of animal studies and human clinical trials. Stem Cells Int 2015; 2015: 946031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han I, Ropper AE, Konya D, et al. Biological approaches to treating intervertebral disk degeneration: devising stem cell therapies. Cell Transplant 2015; 24: 2197–2208. [DOI] [PubMed] [Google Scholar]
- 28.Kumar H, Ha DH, Lee EJ, et al. Safety and tolerability of intradiscal implantation of combined autologous adipose-derived mesenchymal stem cells and hyaluronic acid in patients with chronic discogenic low back pain: 1-year follow-up of a phase I study. Stem Cell Res Ther 2017; 8: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long D, Liang S, Liu H, et al. Mesenchymal stem cell in the intervertebral disc. Mesenchymal Stem Cells-Isolation, Characterization and Applications. IntechOpen, 2017.
- 30.Mwale F, Roughley P, Antoniou J. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: a requisite for tissue engineering of intervertebral disc. Eur Cell Mater 2004; 8: 63–64. [DOI] [PubMed] [Google Scholar]
- 31.Pennicooke B, Moriguchi Y, Hussain I, et al. Biological treatment approaches for degenerative disc disease: a review of clinical trials and future directions. Cureus 2016; 8: e892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antoniou J, Steffen T, Nelson F, et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest 1996; 98: 996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin MD, Boxell CM, Malone DG. Pathophysiology of lumbar disc degeneration: a review of the literature. Neurosurg Focus 2002; 13: E1. [DOI] [PubMed] [Google Scholar]
- 34.Richardson SM, Kalamegam G, Pushparaj PN, et al. Mesenchymal stem cells in regenerative medicine: focus on articular cartilage and intervertebral disc regeneration. Methods 2016; 99: 69–80. [DOI] [PubMed] [Google Scholar]
- 35.Charchian B, Tribuzio B, Zappaterra M, et al. Regenerative spinal therapies for low back pain. Curr Phys Med Rehabil Rep 2014; 2: 41–47. [Google Scholar]
- 36.Smith M, Segal NA. State of regenerative medicine in musculoskeletal rehabilitation practice. Curr Phys Med Rehabil Rep 2016; 4: 19–27. [Google Scholar]
- 37.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143–147. [DOI] [PubMed] [Google Scholar]
- 38.Steinert AF, Rackwitz L, Gilbert F, et al. Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem Cells Transl Med 2012; 1: 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park JY, Kuh SU, Park HS, et al. Comparative expression of matrix-associated genes and inflammatory cytokines-associated genes according to disc degeneration: analysis of living human nucleus pulposus. Clinical Spine Surgery 2011; 24: 352–357. [DOI] [PubMed] [Google Scholar]
- 40.Huang S, Tam V, MC Cheung K, et al. Stem cell-based approaches for intervertebral disc regeneration. Curr Stem Cell Res Ther 2011; 6: 317–326. [DOI] [PubMed] [Google Scholar]
- 41.Centeno CJ. Clinical challenges and opportunities of mesenchymal stem cells in musculoskeletal medicine. PM R 2014; 6: 70–77. [DOI] [PubMed] [Google Scholar]
- 42.Clouet J, Vinatier C, Merceron C, et al. The intervertebral disc: from pathophysiology to tissue engineering. Joint Bone Spine 2009; 76: 614–618. [DOI] [PubMed] [Google Scholar]
- 43.Stoyanov J, Gantenbein-Ritter B, Bertolo A, et al. Role of hypoxia and growth and differentiation factor-5 on differentiation of human mesenchymal stem cells towards intervertebral nucleus pulposus-like cells. Eur Cell Mater 2011; 21: 533–547. [DOI] [PubMed] [Google Scholar]
- 44.Clarke LE, McConnell JC, Sherratt MJ, et al. Growth differentiation factor 6 and transforming growth factor-beta differentially mediate mesenchymal stem cell differentiation, composition, and micromechanical properties of nucleus pulposus constructs. Arthritis Res Ther 2014; 16: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stergar J, Gradisnik L, Velnar T, et al. Intervertebral disc tissue engineering: A brief review. Bosn J Basic Med Sci 2019; 19: 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frauchiger DA, Heeb SR, May RD, et al. Differentiation of MSC and annulus fibrosus cells on genetically engineered silk fleece‐membrane‐composites enriched for GDF‐6 or TGF‐β3. J Orthop Res 2018; 36: 1324–1333. [DOI] [PubMed] [Google Scholar]
- 47.Sobajima S, Vadala G, Shimer A, et al. Feasibility of a stem cell therapy for intervertebral disc degeneration. Spine J 2008; 8: 888–896. [DOI] [PubMed] [Google Scholar]
- 48.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell stem cell 2009; 4: 206–216. [DOI] [PubMed] [Google Scholar]
- 49.Atluri S, Muy MB, Dragella R, et al. Evaluation of the effectiveness of autologous bone marrow mesenchymal stem cells in the treatment of chronic low back pain due to severe lumbar spinal degeneration: A 12-month, open-label, prospective controlled trial. Pain Physician 2022; 25: 193–207. [PubMed] [Google Scholar]
- 50.Vadalà G, Sowa G, Hubert M, et al. Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation. J Tissue Eng Regen Med 2012; 6: 348–355. [DOI] [PubMed] [Google Scholar]
- 51.Li YY, Diao HJ, Chik TK, et al. Delivering mesenchymal stem cells in collagen microsphere carriers to rabbit degenerative disc: reduced risk of osteophyte formation. Tissue Eng Part A 2014; 20: 1379–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Favoni RE, De Cupis A. The role of polypeptide growth factors in human carcinomas: new targets for a novel pharmacological approach. Pharmacol Rev 2000; 52: 179–206. [PubMed] [Google Scholar]
- 53.Masuda K, Oegema TR, Jr, An HS. Growth factors and treatment of intervertebral disc degeneration. Spine (Phila Pa 1976) 2004; 29: 2757–2769. [DOI] [PubMed] [Google Scholar]
- 54.Osada R, Ohshima H, Ishihara H, et al. Autocrine/paracrine mechanism of insulin‐like growth factor‐1 secretion, and the effect of insulin‐like growth factor‐1 on proteoglycan synthesis in bovine intervertebral discs. J Orthop Res 1996; 14: 690–699. [DOI] [PubMed] [Google Scholar]
- 55.Dowdell J, Erwin M, Choma T, et al. Intervertebral disk degeneration and repair. Neurosurgery 2017; 80: S46–S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hodgkinson T, Shen B, Diwan A, et al. Therapeutic potential of growth differentiation factors in the treatment of degenerative disc diseases. JOR spine 2019; 2: e1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peeters M, Detiger SE, Karfeld-Sulzer LS, et al. BMP-2 and BMP-2/7 heterodimers conjugated to a fibrin/hyaluronic acid hydrogel in a large animal model of mild intervertebral disc degeneration. Biores Open Access 2015; 4: 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ekram S, Khalid S, Salim A, et al. Regulating the fate of stem cells for regenerating the intervertebral disc degeneration. World J Stem Cells 2021; 13: 1881–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mahyudin F, Prakoeswa CRS, Notobroto HB, et al. An update of current therapeutic approach for Intervertebral Disc Degeneration: A review article. Ann Med Surg (Lond) 2022: 103619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malhotra A, Pelletier MH, Yu Y, et al. Can platelet-rich plasma (PRP) improve bone healing? A comparison between the theory and experimental outcomes. Arch Orthop Trauma Surg 2013; 133: 153–165. [DOI] [PubMed] [Google Scholar]
- 61.Boyan BD, Schwartz Z, Patterson TE, et al. Clinical use of platelet-rich plasma in orthopaedics. American Academy of Orthopaedic Surgeons Now 2007; 1: 17. [Google Scholar]
- 62.Anitua E, Andia I, Ardanza B, et al. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost 2004; 91: 4–15. [DOI] [PubMed] [Google Scholar]
- 63.Alsousou J, Ali A, Willett K, et al. The role of platelet-rich plasma in tissue regeneration. Platelets 2013; 24: 173–182. [DOI] [PubMed] [Google Scholar]
- 64.Foster TE, Puskas BL, Mandelbaum BR, et al. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med 2009; 37: 2259–2272. [DOI] [PubMed] [Google Scholar]
- 65.Lee JW, Kwon OH, Kim TK, et al. Platelet-rich plasma: quantitative assessment of growth factor levels and comparative analysis of activated and inactivated groups. Arch Plast Surg 2013; 40: 530–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vo TKC, Tanaka Y, Kawamura K. Ovarian rejuvenation using autologous platelet-rich plasma. Endocrines 2021; 2: 15–27. [Google Scholar]
- 67.Chang Y, Yang M, Ke S, et al. Effect of platelet-rich plasma on intervertebral disc degeneration in vivo and in vitro: a critical review. Oxid Med Cell Longev 2020; 2020: 8893819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Akeda K, Imanishi T, Ohishi K, et al. Intradiscal injection of autologous serum isolated from platelet‐rich‐plasma for the treatment of discogenic low back pain: preliminary prospective clinical trial: gp141. In: Spine Journal Meeting Abstracts 2011, LWW.
- 69.Lutz GE. Increased nuclear T2 signal intensity and improved function and pain in a patient one year after an intradiscal platelet–rich plasma injection. Pain Med 2017; 18: 1197–1199. [DOI] [PubMed] [Google Scholar]
- 70.Won SJ, Kim DY, Kim JM. Effect of platelet-rich plasma injections for chronic nonspecific low back pain: A randomized controlled study. Medicine (Baltimore) 2022; 101: e28935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singla V, Batra YK, Bharti N, et al. Steroid vs. platelet‐rich plasma in ultrasound‐guided sacroiliac joint injection for chronic low back pain. Pain Pract 2017; 17: 782–791. [DOI] [PubMed] [Google Scholar]
- 72.Levi D, Horn S, Tyszko S, et al. Intradiscal platelet-rich plasma injection for chronic discogenic low back pain: preliminary results from a prospective trial. Pain Med 2016; 17: 1010–1022. [DOI] [PubMed] [Google Scholar]
- 73.Wu J, Zhou J, Liu C, et al. A prospective study comparing platelet‐rich plasma and local anesthetic (LA)/corticosteroid in intra‐articular injection for the treatment of lumbar facet joint syndrome. Pain Pract 2017; 17: 914–924. [DOI] [PubMed] [Google Scholar]
- 74.Aufiero D, Vincent H, Sampson S, et al. Regenerative injection treatment in the spine: review and case series with platelet rich plasma. J Stem Cells Res Rev & Rep 2015; 2: 1019. [Google Scholar]
- 75.Wu J, Du Z, Yang L, et al. A new technique for the treatment of lumbar facet joint syndrome using intra-articular injection with autologous platelet rich plasma. Pain Physician 2016; 19: 617–625. [PubMed] [Google Scholar]
- 76.Dagenais S, Yelland MJ, Del Mar C, et al. Prolotherapy injections for chronic low‐back pain. Cochrane Database Syst Rev 2007; 2007: CD004059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dechow E, Davies R, Carr A, et al. A randomized, double-blind, placebo-controlled trial of sclerosing injections in patients with chronic low back pain. Rheumatology 1999; 38: 1255–1259. [DOI] [PubMed] [Google Scholar]
- 78.Klein RG, Eek BC, DeLong WB, et al. A randomized double-blind trial of dextrose-glycerine-phenol injections for chronic, low back pain. J Spinal Disord 1993; 6: 23–33. [PubMed] [Google Scholar]
- 79.Yelland MJ, Glasziou PP, Bogduk N, et al. Prolotherapy injections, saline injections, and exercises for chronic low-back pain: a randomized trial. Spine (Phila Pa 1976) 2004; 29: 9–16; discussion 16. [DOI] [PubMed] [Google Scholar]
- 80.Ongley M, Dorman T, Klein R, et al. A new approach to the treatment of chronic low back pain. Lancet 1987; 330: 143–146. [DOI] [PubMed] [Google Scholar]
- 81.Tolbert G, Roy D, Walker V. Ultrasound guided dextrose prolotherapy and platelet rich plasma therapy in chronic low back pain: three case reports. Int J Phys Med Rehabil 2013; 1: 2. [Google Scholar]
- 82.Hiyama A, Skubutyte R, Markova D, et al. Hypoxia activates the notch signaling pathway in cells of the intervertebral disc: implications in degenerative disc disease. Arthritis Rheum 2011; 63: 1355–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fitzsimmons RE, Mazurek MS, Soos A, et al. Mesenchymal stromal/stem cells in regenerative medicine and tissue engineering. Stem Cells Int 2018; 2018: 031718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Using mesenchymal stem cells in regenerative medicine. Blog, Cells in Action, https://promocell.com/blog/using-mesenchymal-stem-cells-in-regenerative-medicine/ (2019, accessed 25 November 2019).
- 85.Ichiyanagi T, Anabuki K, Nishijima Y, et al. Isolation of mesenchymal stem cells from bone marrow wastes of spinal fusion procedure (TLIF) for low back pain patients and preparation of bone dusts for transplantable autologous bone graft with a serum glue. Biosci Trends 2010; 4: 110–118. [PubMed] [Google Scholar]
- 86.Rabago D, Slattengren A, Zgierska A. Prolotherapy in primary care practice. Prim Care 2010; 37: 65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]