Abstract
The regulatory landscape for device-based heart failure (HF) therapies has seen a major shift in the last 7 years. In 2013, the Food and Drug Administration (FDA) released guidance for early feasibility and first-in-human studies, encouraging device innovation and in 2016 the U.S. Congress authorized the Breakthrough Device Program to expedite access for Americans to innovative devices indicated for diagnosis and treatment of serious illnesses, such as HF. Since December 2016 there has been an increase in the number of HF devices seeking approval through the breakthrough designation pathway. This has led to a rapid uptake in the development and evaluation of device-based HF therapies. Here, we review the current and future landscape of device therapies for chronic HF and associated comorbid diseases and the regulatory environment that is driving current and future innovation.
Keywords: Device therapy, Heart failure, Food and Drug Administration
Condensed Abstract
Over the past years, there has been significant changes in the regulatory process for device-based heart failure (HF) therapies in the United States. This coupled with the authorization of the Breakthrough Device Program to expedite access to innovative devices indicated for diagnosis and treatment of serious illnesses, such as HF, has resulted in significant uptake in the development and evaluation of device-based HF therapies. We review the current and future landscape of device therapies for chronic HF and the regulatory environment that is driving current and future innovation.
Despite recent positive trials with drug therapies for heart failure with reduced ejection fraction (HFrEF), the residual risk for these patients remains high. Also, there are no proven treatments for patients with heart failure and preserved ejection fraction (HFpEF). This underscores the need for continued development of innovative treatments for heart failure (HF), including devices. To accelerate innovation, allow earlier access to new therapies, and address unmet clinical needs, in 2013 the Food and Drug Administration (FDA) released guidance for early feasibility and first-in-human studies, encouraging device innovation to “address clinical needs and improve patient care, particularly when alternative treatments or assessments are unavailable, ineffective, or associated with a substantial risk” (1). In 2015, the FDA established the Expedited Access Program in an FDA Guidance Document, which in 2018 evolved to the Breakthrough Devices Program in an FDA Guidance Document to expedite Americans access to innovative devices indicated for diagnosis and treatment of serious illnesses, such as HF. To increase access to breakthrough technologies, the Centers for Medicare and Medicaid Services increased hospital reimbursement for these technologies (2).
The breakthrough device designation program (which replaced the earlier expedited access pathway) has changed the landscape of the approval process for devices targeting life-threatening or irreversibly debilitating diseases or conditions. Novel clinical trial designs may be considered that allow for early market access by accelerating the development, assessment, and review processes, and linking reimbursement from the Centers for Medicare and Medicaid Services (CMS) to FDA marketing approval. The breakthrough device designation program aims to create a pre- and post-market balance in evidence generation while still meeting the statutory requirements for safety and effectiveness at the time of approval. As demonstrating improvements in cardiovascular mortality and HF hospitalization outcomes require more time and larger studies, the breakthrough device designation program may permit assessing effectiveness via patient-centered outcomes such as functional capacity, quality of life, and biomarkers in the initial expedited phase of premarket approval (as long as safety is demonstrated). BeAT-HF was the first approved device-based HF program under this pathway (3). The Phase I (expedited phase) trial used 6-minute walk distance, Minnesota Living With Heart Failure (MLWHF), and NT-proBNP as endpoints for premarket approval. Phase II (extended phase) used HF morbidity and cardiovascular mortality as endpoints. The Phase II trial was used as supplementary for the premarket approval submission and continued after obtaining the marketing approval. By the conclusion of these two phases, data on the composite of patient-centric outcomes, surrogate endpoints, and traditional cardiovascular outcomes will be available. To expedite and facilitate the access to these therapies, CMS waived the requirement that devices enrolled in the breakthrough device designation program show significant clinical improvement in 2019 and increased reimbursement for such devices from 50% to 65% of device costs (4). Since 2019 a new Medicare coverage pathway through the Medicare Coverage of Innovative Technology (MCIT) provides national Medicare coverage for breakthrough device technology (5). Starting the day devices receive FDA market authorization the new pathway guarantees 4 years of coverage for the device to Medicare beneficiaries. Although this new ruling does not require clinical evidence generation during the 4 years, it is expected that manufacturers will be encouraged to voluntarily continue to generate evidence to support and expand on known health outcomes of their technology. Additional evidence generation under the umbrella of Medicare coverage could then strengthen clinical evidence and support future national coverage after the 4-year term.
REGULATORY CONSIDERATIONS
As we enter in an expanding era of device therapies for HF, a number of critical issues must be addressed:
Endpoints: The design, conduct and execution of device-based trials tend to be more complex, expensive, and difficult to conduct than either drug trials for HF or device-based trials for non-HF indications. Thus, many device-based HF trials tend to be smaller and not powered for “hard” clinical outcomes. A concerted effort to define appropriate endpoints is needed to meet the demands by patients, regulatory agencies, academia, companies and payors. Clarification of the relative utility of surrogate endpoints (e.g., change in LVEF and biomarkers), measures of functional capacity or quality of life (e.g., 6-minute walk distance, Kansas City Cardiomyopathy Questionnaire (KCCQ)/MLWHF) and clinical outcomes (e.g., death and HF hospitalizations), as well as the meaningful thresholds for improvement of these endpoints and optimal analytic methods are needed (6).
Novel statistical approaches: The design of device-based trials might benefit from the adoption of novel statistical approaches. For example, the Finkelstein-Schoenfeld is a hierarchical test that combines a time to event measure (like death) with longitudinal measures (nonfatal events like hospitalization for HF) (7). In extension of the Finkelstein-Schoenfeld method, the win ratio approach allows for prioritization of clinical outcomes, testing of hierarchical outcomes, and incorporation of patient-centered endpoints (8). Finally, Bayesian analysis can integrate new study data with existing data, thus reducing uncertainty (9).
Sham design: Sham-controlled device-based trials are the closest counterpart to placebo-controlled drug studies as they minimize bias during the follow-up phase by blinding all but the proceduralists (who should not be involved in the later care of the patient). Compared to placebo-controlled trials, however, sham-controlled trials are more complex; the appropriate sham is often debated; there are ethical issues if procedural risks cannot be minimized; and there is a greater risk of inadvertent unblinding (10). The FDA has advocated for alternative trial designs when blinding is not feasible. A focus on more objective endpoints with blinded evaluation may reduce the need for sham designs, although placebo, nocebo and Hawthorne effects, and ascertainment and other biases are not easily dismissed.
Safety evaluation: The evaluation of device-related safety plays an important role in the approval process. Since device trials tend to be smaller in size, regulatory agencies have to rely not only on the pre-approval safety evidence but also on post approval surveillance of device therapies. A continued effort to strike a balance between accelerated access to the clinical market and continued safety surveillance is needed. Innovative efforts for safety evaluation, including the use of alternative data sources such as post approval registries are underway.
Since December 2016 there has been an increase in the number of devices seeking FDA approval through the breakthrough designation pathway, including a total of 330 device therapies as of August 2020, 88 of which were cardiovascular (Supplemental Figure 1). Herein we review the current and future landscape of device therapies for chronic HF and associated comorbid diseases and describe the regulatory environment that is driving current and future innovation (Central Illustration).
Central Illustration:
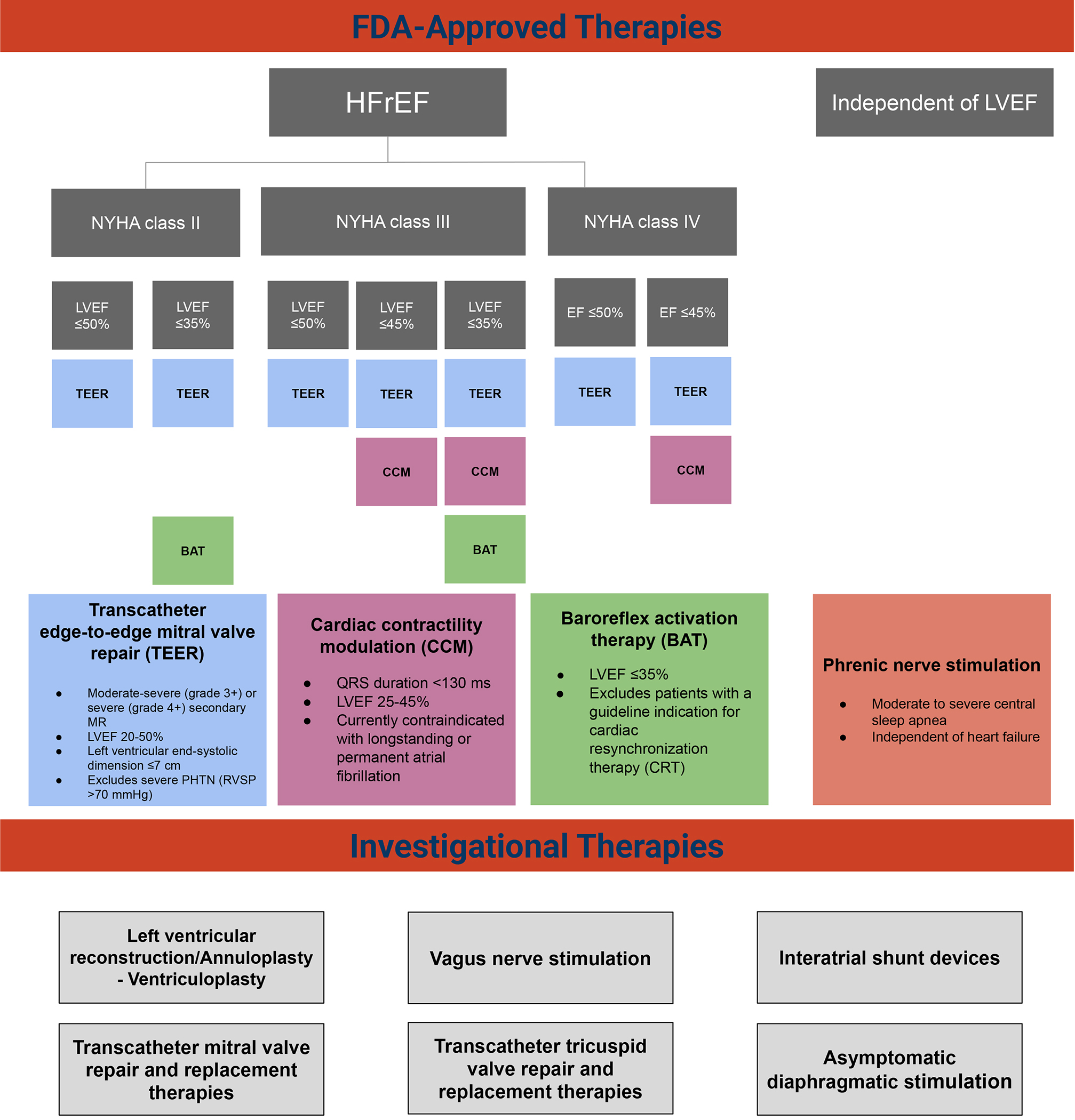
FDA-approved and breakthrough designated device therapies. FDA-approved and breakthrough designated devices in the heart failure space split out by heart failure subtype (HFrEF, HFpEF and LVEF independent)
Abbreviations: HFrEF= heart failure with reduced ejection fraction, HFpEF= heart failure with preserved ejection fraction. FDA= food and drug administration, LVEF= left ventricular ejection fraction. TR= tricuspid regurgitation
VALVE THERAPIES
I. Devices to treat mitral regurgitation
Primary or degenerative mitral regurgitation (MR) is due to pathology of the mitral valve itself, usually due to fibroelastic deficiency or Barlow’s disease, in patients typically with normal left ventricular (LV) systolic function (11). If untreated, primary MR can result in LV dysfunction and HF. In contrast, secondary or functional MR commonly develops due to LV dysfunction and HF that lead to mitral leaflet tethering from dislocated papillary muscles, papillary muscle dyssynchrony with reduced closing forces, and valve tenting due to increased left atrial pressure. In a small proportion of patients pure annular dilatation, usually due to chronic atrial fibrillation, can also result in lack of leaflet coaptation and secondary MR (12). The prognosis in HF patients who develop secondary MR is clearly worse, with a direct quantitively relationship between the severity of secondary MR and both death and hospitalizations for HF (13). Until recently, though, it was uncertain whether reducing secondary MR would improve long-term prognosis as it does nothing to directly address the underlying LV dysfunction. More than 60 transcatheter mitral valve repair or replacement devices have been developed to treat HF patients with primary or secondary MR. Herein we review those that have or are being investigated in pivotal safety and effectiveness randomized trials.
MitraClip (Abbott).
The MitraClip device, a cobalt-chrome implant, allows for an edge-to-edge repair of the mitral valve, restoring coaptation of the anterior and posterior mitral leaflets, thereby reducing MR (14,15). The procedure utilizes a transfemoral venous access and transseptal puncture (16). In patients with primary MR, percutaneous repair of MR with MitraClip compared to surgical interventions is safe and improves quality of life, functional class, and left ventricular size, although in an early randomized trial was found to not be as effective as mitral valve surgical repair (15). The MitraClip device received FDA approval for primary MR in 2013 for patients at prohibitive risk for surgical mitral valve repair.
In contrast to primary MR, surgery has not been convincingly shown to be effective in secondary MR; rather, guideline-directed medical therapy (GDMT) for HF is standard of care, in addition to cardiac resynchronization therapy (CRT) for eligible patients. The Cardiovascular Outcomes Assessment of the MitraClip in Patients with Heart Failure and Secondary Mitral Regurgitation (COAPT) trial has provided a new option for the management of selected patients with primary or secondary MR in HF (17). COAPT was an open-label, randomized, controlled trial in 614 patients with moderate-severe or severe secondary MR, with left ventricular ejection fraction (LVEF) of 20–50%, and New York Heart Association (NYHA) class II-IV symptoms despite maximally-tolerated GDMT and CRT if appropriate. MitraClip reduced HF hospitalizations at 24 months by 47%, lowered all-cause mortality, and improved quality of life and 6-minute walk distance (Figure 1). The COAPT trial led to the approval of the MitraClip in 2019 in the US for secondary MR. A smaller (N=307) randomized controlled trial of MitraClip (Multicentre Study of Percutaneous Mitral Valve Repair MitraClip Device in Patients With Severe Secondary Mitral Regurgitation [MITRA-FR]) (18) found no difference in HF outcomes at 12 months (18). The COAPT trial had more stringent criteria for GDMT prior to enrollment and included a population with more severe MR and less left ventricular dysfunction and excluded severe TR and right heart failure, perhaps underlying the discordant results between these trials (19). The ongoing Reshape-HF2 (A Clinical Evaluation of the Safety and Effectiveness of the MitraClip System in the Treatment of Clinically Significant Functional Mitral Regurgitation; NCT02444338) and the EVOLVE-MR (MitraClip for the Treatment of Moderate Functional Mitral Regurgitation; NCT03705312) aim to provide additional evidence regarding the effectiveness and the appropriate use of MitraClip in HF. Additionally, a number of other device solutions are under investigation (14).
Figure 1.
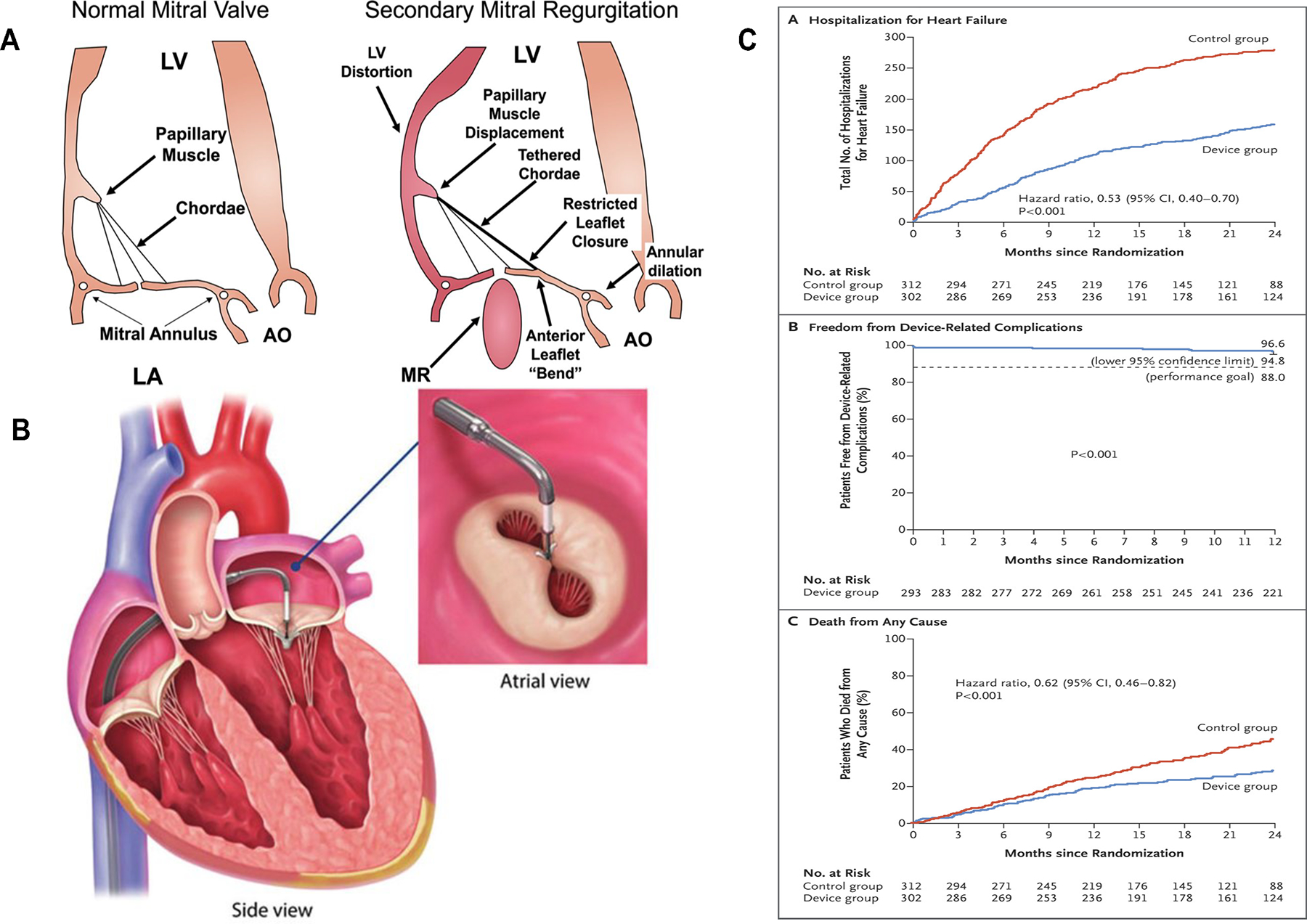
Device therapy for mitral valve regurgitation
Mitral regurgitation development and progression in heart failure is mostly secondary to annular dilatation and leaflet tethering, papillary muscle desynchrony, and valve tenting due to increased left atrial pressure (A). MitraClip is a cobalt-chrome implant that allows for an edge-to-edge repair of the mitral valve (B). (C) Results of the COAPT trial (17). Abbreviations: AO= Aorta, LA= left atrium, LV= left ventricle.
Other edge-to-edge devices have been developed, the most notable being the PASCAL device (Edwards Life Sciences). After a series of promising pilot studies (20–22) the device received the CE mark and is currently being investigated in pivotal randomized approval trials in primary MR (CLASP IID; NCT03706833) and secondary MR (CLASP IIF; NCT03706833).
Carillon Mitral Contour System (CARILLON®).
The Carillon mitral contour system (CMCS) is an indirect percutaneous device placed into the coronary sinus, which when cinched reduces the annular anterior-posterior diameter thereby making it a mitral annuloplasty system (23). The procedure utilizes a transjugular venous access (24). The REDUCE FMR (CARILLON Mitral Contour System® for Reducing Functional Mitral Regurgitation) trial randomized 120 patients with NYHA class II-IV, LVEF of <50%, and functional MR grade 2+ or more on medical therapy (23). At 12-month follow-up there was a median 22.4% decrease in mitral regurgitant volume in the CMCS group compared to a 1.5% increase in the control group. The CMCS resulted in a significant decrease in LV end-diastolic and end-systolic volume, and improvement in 6-min walk distances and NYHA functional class (23). The CMCS is CE marked. The ongoing sham-controlled CARILLON trial (NCT03142152) is enrolling 352 heart failure patients with similar criteria as in REDUCE FMR to evaluate the clinical safety and effectiveness of this device in HF patients.
Transcatheter mitral valve replacement.
Medtronic’s Intrepid transcatheter mitral valve replacement (TMVR) system is a nitinol, self-expanding, dual frame valve with a circular outer fixation frame and an inner stent frame that houses a tri-leaflet bovine pericardium valve and is delivered via a 35-French transapical sheath (25,26). In the Intrepid Global Pilot Study, implantation of the Intrepid TMVR system in patients with symptomatic and severe MR, no more than minimal MV calcification, and LVEF ≥20% resulted in reduction in MR severity to mild or none in all patients, and improved symptoms and quality of life (27). The APOLLO (Transcatheter Mitral Valve Replacement With the Medtronic Intrepid™ TMVR System in Patients With Severe Symptomatic Mitral Regurgitation; NCT03242642) study is an ongoing, non-randomized, open-label, pre-market trial that aims to establish efficacy of the Medtronic Intrepid TMVR system in patients with moderate to severe or severe symptomatic MR and LVEF >30% who are not candidates for approved transcatheter repair or conventional MV surgery. Similarly, the Abbott’s Tendyne is bioprosthetic, repositionable, and retrievable TMVR system that is delivered via a 34-French transapical sheath (28,29) (CE marked). In the Tendyne Global Feasibility Trial, implantation of the Tendyne TMVR system in patients with grade 3 or 4 MR resulted in reduction in MR severity to mild or none in all patients (28). The SUMMIT (Clinical Trial to Evaluate the Safety and Effectiveness of Using the Tendyne Mitral Valve System for the Treatment of Symptomatic Mitral Regurgitation; NCT03433274) study is another ongoing trial with a randomized, parallel assignment design that aims to investigate safety and efficacy of the Tendyne TMVR in symptomatic patients with moderate to severe or severe MR or severe mitral annular calcification and LVEF ≥25%.
Transcatheter mitral valve replacement has been a rapidly growing field (30,31) with several other devices currently under development and investigation, such as Edwards SAPIEN M3 System (NCT04153292), Edwards SAPIEN 3 (NCT01808287), Edwards EVOQUE Eos (NCT02718001), Neovasc Tiara™ (NCT02276547), Abbott’s Cephea, (32) and HighLife™ (NCT02974881).
II. Devices to treat tricuspid regurgitation
Severe tricuspid regurgitation (TR) is associated with right ventricular dysfunction and has been strongly associated with mortality, HF hospitalizations and poor quality of life (33). However, surgery for isolated TR is rarely performed (34), and due to the frequent co-existence of LV dysfunction and other comorbidities, it is uncertain the extent to which correcting TR might improve prognosis in HF. Numerous transcatheter devices have been developed for TR repair or replacement (35), to date the most widely studied of which is the TriClip.
TriClip (Abbott).
TriClip is a minimally invasive, device-based, transcatheter edge-to-edge repair system that is similar to MitraClip (CE marked). The TRILUMINATE (Trial to Evaluate Treatment With Abbott Transcatheter Clip Repair System in Patients With Moderate or Greater Tricuspid Regurgitation; NCT03227757) is a single-arm, multi-center study of 85 patients with moderate or greater TR who underwent TriClip implantation (36). At 6-month follow-up TR severity was reduced by one grade or more in 86% of the patients and only 6% of patients experienced a major adverse event. The TRILUMINATE Pivotal Trial (NCT03904147) is an ongoing randomized trial of 700 subjects that aims to investigate the effectiveness and safety of the TriClip device in symptomatic patients with severe TR and NYHA class II or more despite optimal medical therapy.
Transcatheter tricuspid valve replacement has been a rapidly growing field with several other devices currently under development and investigation, such as Edwards EVOQUE (NCT02718001), Neovasc Tiara™ (NCT02276547), Abbott’s Cephea, (32) and HighLife™ (NCT02974881).
AUTONOMIC MODULATION
I. Baroreflex Activation Therapy
Baroreflex dysfunction results in autonomic dysregulation (i.e., parasympathetic system withdrawal and sympathetic system overactivation) that has been related to the development and progression of HF (37). Afferent input to the baroreflex originates from the carotid sinus and aortic arch receptors, which are stimulated by arterial distension. The baroreflex regulates the efferent sympathetic and parasympathetic output via the rostral ventrolateral medulla and nucleus ambiguous (38). Baroreflex activation therapy (BAT) stimulates the carotid baroreceptor via electrical impulses from an implanted pulse generator (in the pectoral region) resulting in a centrally mediated decrease in sympathetic activity and an increase in the parasympathetic outflow (Figure 2, Panel A-B) (39,40). HOPE4HF was a randomized phase II trial of 146 patients with NYHA class III HF with LVEF ≤35%, where BAT with the Barostim™ neo™ system resulted greater improvements in NYHA class (55% vs. 24%, p=0.002), MLWHF score (−19.5±4.2, p<0.001), and 6-minute walk distance (58.1±19.8 meters, p=0.004) at 6 months (41). Freedom from major adverse neurological and cardiovascular events (MANCE) was 97.2%. There was also a reduction in N-terminal pro-brain natriuretic peptide (NT-proBNP) levels in the treatment group compared with an increase in the control group (−69.0 pg/ml vs. 129.5 pg/ml; p=0.02). LVEF was also improved. The subsequent confirmatory pivotal Baroreflex Activation Therapy for Heart Failure (BeAT-HF) trial was a randomized, parallel-group trial of 408 patients with NYHA class II-III with LVEF ≤35% comparing BAT plus medical therapy to medical therapy alone (two phases: expedited and extended) (42). Compared to the control group, patients treated with BAT were shown to have an improvement in quality of life, 6-minute walk distance, and a reduced level of NT-proBNP at 6 months (42). Neither HOPE4HF or BeAT-HF (expedited) were adequately powered or had follow-up long enough to determine whether BAT reduced death or HF hospitalizations. Based on the results of expedited phase of the BeAT-HF trial, the FDA approved the Barostim™ neo™ system in 2019 for the improvement of HF symptoms of HF in patients NYHA Class III (or Class II with a recent history of Class III) patients, LVEF ≤35% and NT-proBNP <1600 pg/mL who are not CRT candidates. The extended phase of BeAT-HF is ongoing, and the presentation of the morbidity and mortality results is expected in 2022.
Figure 2.
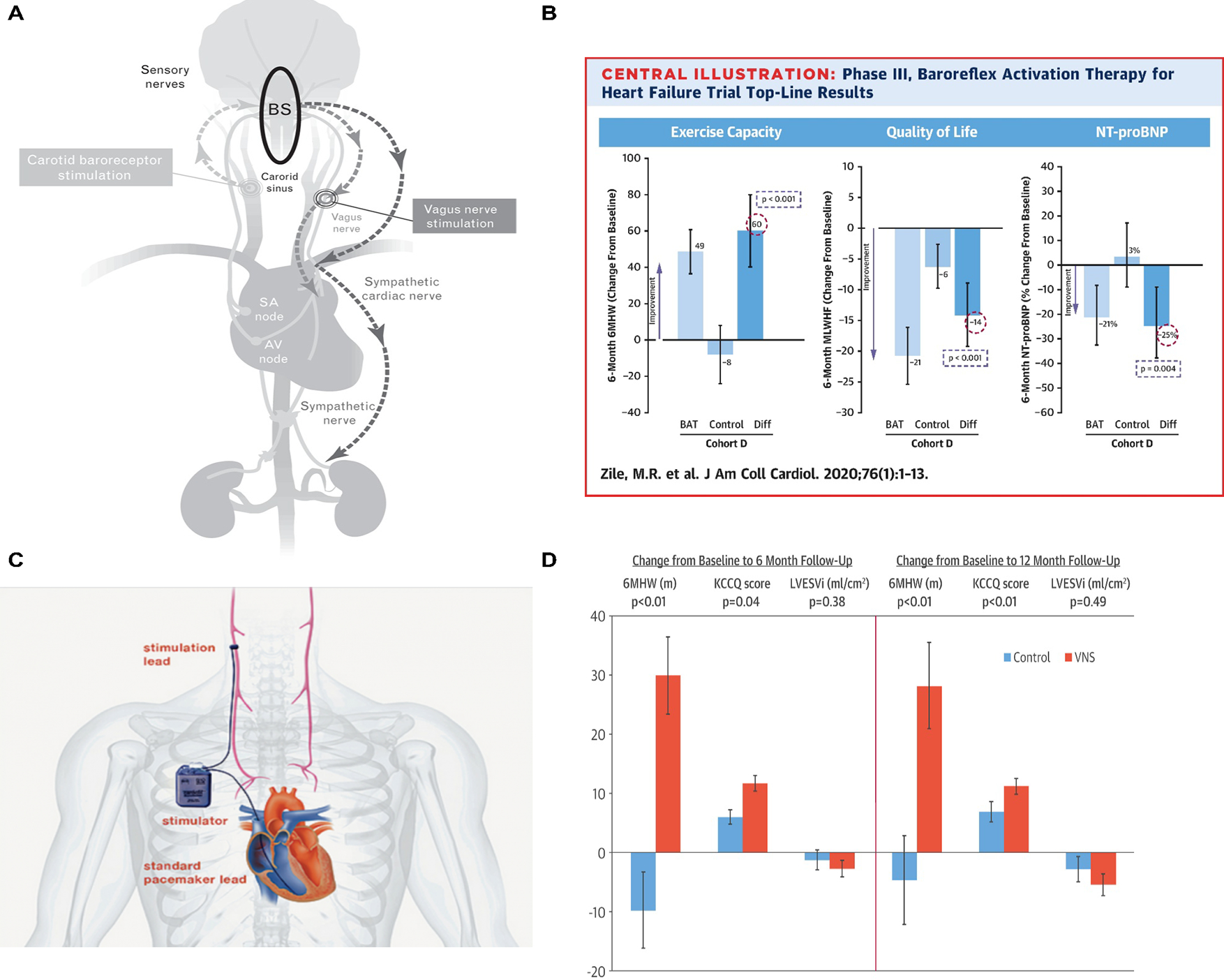
Baroreflex activation therapy and vagus nerve stimulation
Baroreflex activation therapy: (A) The baroreflex signals mainly originate from the carotid sinus and aortic arch (stimulated by arterial distention). These signals inhibit the rostral ventrolateral medulla. Consequently, the sympathetic activity in various organs decreases. From (38). (B) The effect of baroreflex activation therapy on exercise capacity, quality of life, and the level of N-terminal pro-brain natriuretic peptide. From (42).
Vagus Nerve Stimulation: (C) Device-based stimulation of the vagus nerve can counteract the excessive activation of the sympathetic nervous system (SNS) and decreased parasympathetic nervous system (PNS) activity seen in heart failure. (D) INOVATE-HF trial as well as other trials showed that vagus nerve stimulation can improve quality of life. From (49). Abbreviations: BS= brain stem, SA= sino-atrial, AV= atrioventricular.
II. Vagus Nerve Stimulation
Excessive activation of the sympathetic nervous system (SNS) and decreased parasympathetic nervous system (PNS) activity occur as adaptive mechanisms to cardiac injury, congestion and decreased stroke volume (43,44). Chronic SNS stimulation and PNS depression accelerate cardiovascular stress (tachycardia, higher afterload, increased oxygen consumption) and ventricular remodeling (44). The stimulation of the vagus nerve has been introduced in an attempt to counter these long-term deleterious effects (45–47).
Three trials laid the foundation for vagus nerve stimulation (VNS) in HF (Figure 2, Panel C-D). The Autonomic Neural Regulation Therapy to Enhance Myocardial Function in Heart Failure (ANTHEM-HF) study was an uncontrolled, open-label pilot study in which 60 patients with NYHA class II-III symptoms, LVEF ≤40%, and LV end-diastolic diameter (LVEDD) between 50–80 mm were randomized to left versus right cervical Cyberonics VNS system implantation (48). There were significant improvements in LVEF, LV diameter, NYHA class, 6-minute walk distance, and MLHFQ scores. Only one device-related serious adverse event occurred at 6 months. There were 173 device-related, nonserious adverse events with mild dysphonia, cough, and oropharyngeal pain being most common. The Increase of Vagal Tone in Heart Failure (INOVATE-HF) was a randomized, open-label trial of patients with NYHA class III symptoms, LVEF ≤40%, and LVEDD between 50–80 mm who were randomized to VNS (BioControl CardioFit system) versus medical therapy (49). The VNS group improvement in NYHA class, 6 min walk distance, and KCCQ score at 12 months, but there was no improvement in mortality or HF-related clinical events (49). In the Neural Cardiac Therapy for Heart Failure (NECTAR-HF) trial, 96 patients with NYHA class II-III, LVEF ≤35%, LVEDD ≤55 mm, and QRS duration <130 ms were randomized 2:1 to active or inactive VNS; the VNS was switched on in the inactive group at 6 months (50). There was no significant difference in mortality or hospitalization between groups; the trial, did, however, show a reassuring safety profile of VNS (50).
VNS is CE marked and has since received a breakthrough designation and two trials are ongoing, one in HFrEF in 800 patients (Autonomic Regulation Therapy to Enhance Myocardial Function and Reduce Progression of Heart Failure with Reduced Ejection Fraction; ANTHEM-HFrEF; NCT03425422) (51) and the other in HFpEF in 50 patients (Autonomic Neural Regulation Therapy to Enhance Myocardial Function in Patients with Heart Failure and Preserved Ejection Fraction; ANTHEM-HFpEF; NCT03163030) (52).
III. Splanchnic Nerve Modulation
A recently introduced treatment pathway for HF targets the splanchnic vascular compartment. Evidence suggests that even in the absence of an increase of total body fluid, HF exacerbation can occur due to disruption of intravascular fluid distribution (53,54). The splanchnic vascular compartment is a major reservoir for intravascular blood volume and has an important role in volume redistribution in HF (55). Since splanchnic nerves control the arterial and venous vascular tone of this vascular compartment, modulation of these nerves has been attempted as a potential therapy in HF (56–59). Initial investigations of temporary splanchnic nerve blockade (SNB) included small populations of HF patients (Figure 3) (60–62). In hospitalized patients, SNB improved cardiac output and decreased filling pressures (N=11) (61). In ambulatory HF, SNB resulted in a reduction of exercise wedge pressure, improved cardiac index, and favorably changed exercise performance (N=15) (62). The safety and efficacy of SNB was investigated via unilateral surgical resection of the greater splanchnic nerve (N=11). At 3 months, patients demonstrated a reduction in peak exercise wedge pressure (−5.1±5.0 mmHg; p=0.041). Peak oxygen consumption improved by +2.5±3.0 ml/kg/min and +2.5±2.9 ml/kg/min at 6 and 12 months, respectively (both p<0.05) (63). Ongoing efforts are investigating the safety and efficacy of minimally invasive, long-term SNB in HFpEF (NCT04592445 and NCT04575428).
Figure 3.
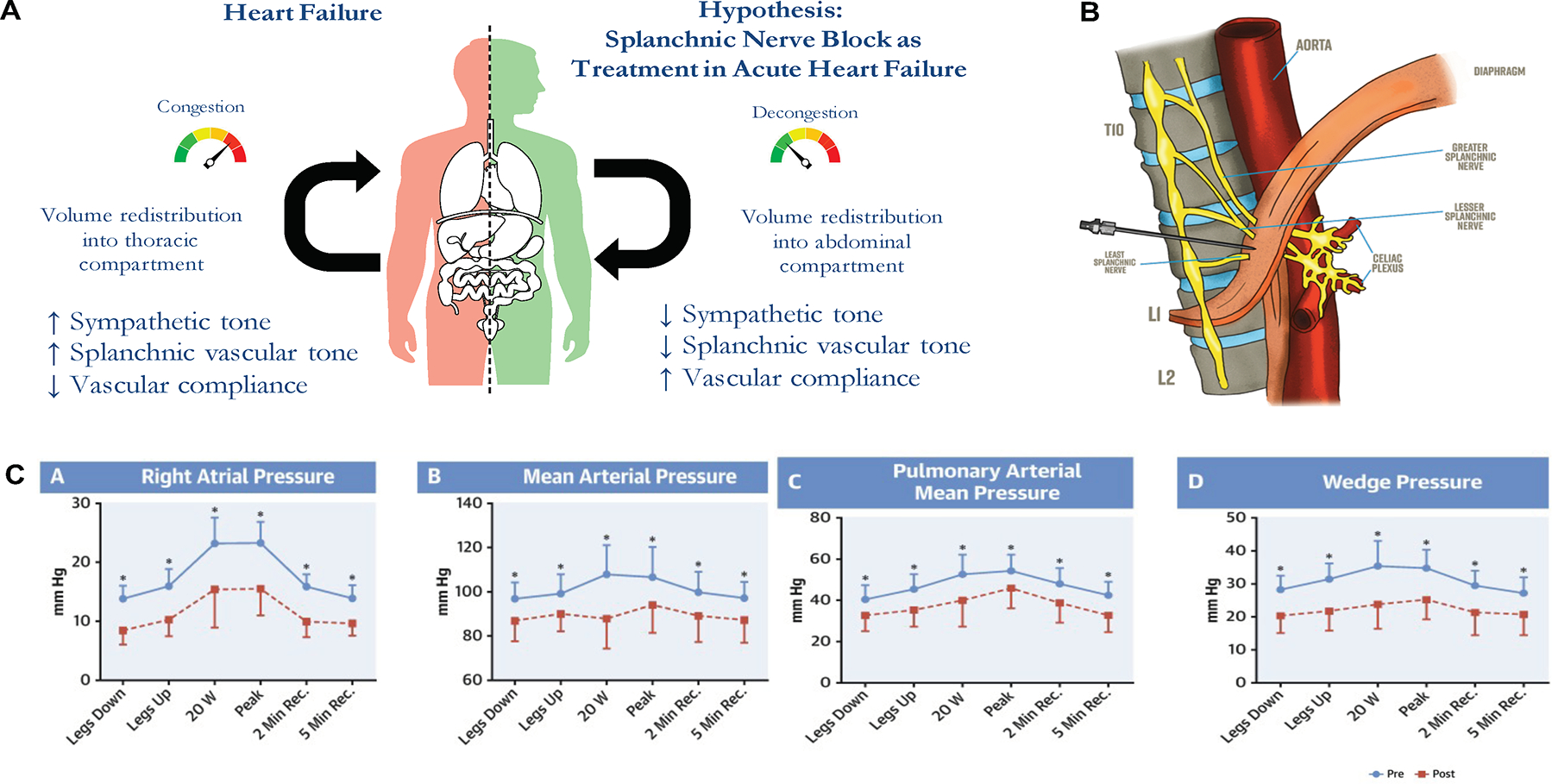
Splanchnic nerve modulation
The splanchnic vascular compartment is a major a reservoir for intravascular blood volume and has an important role in volume redistribution in heart failure (A). Splanchnic nerves control the arterial and venous vascular tone of this vascular compartment; and therefore, splanchnic nerve block can be a potential treatment modality in heart failure (B). Fudim et al showed improvement in right atrial pressure, mean arterial pressure, pulmonary arterial mean pressure, and wedge pressure after a temporary block of the splanchnic nerve in patients with heart failure (C).
ELECTROPHYSIOLOGIC MODULATION
I. Cardiac Contractility Modulation
HFrEF is characterized by abnormal intracellular calcium handling impacting mechanical and electrophysiologic function (64). Most attempts to improve outcomes in HFrEF targeting contractility have failed (65–67). Cardiac contractility modulation (CCM) therapy (OPTIMIZER System by Impulse Dynamics) delivers a biphasic, long-duration (~20 milliseconds), high-voltage (~7.5 V) electrical signal to the septum of the right ventricle during the absolute refractory period (Figure 4, Panel A-B) (68–70). As the delivery of the electric signals occurs during refractory period, it does not result in myocardial contraction but leads to structural and functional changes in the myocardium including molecular changes, enhanced contractility, and decreased left ventricular volume (71). These changes likely occur due to augmentation of intracellular calcium within the cardiomyocyte as a result of increased extracellular calcium influx and calcium-induced calcium release from the sarcoplasmic reticulum (70).
Figure 4.
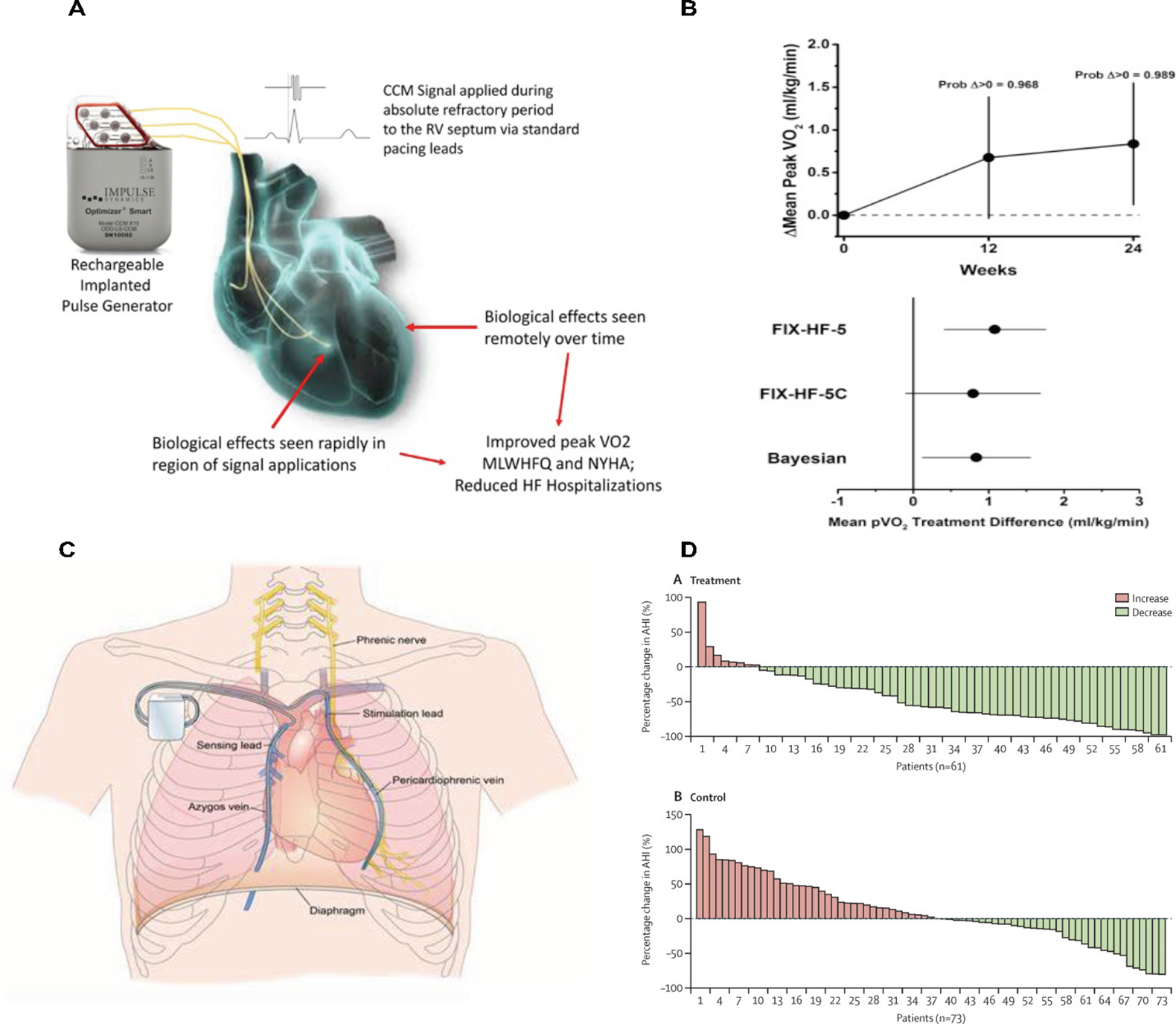
Cardiac contractility modulation and phrenic nerve stimulation
OPTIMIZER System delivers a biphasic, long-duration, high-voltage electrical signals to the septum of the right ventricle during the absolute refractory period, resulting in myocardial changes that lead to enhancement of contractility (A). The results of the OPTIMIZER system on peak VO2 are shown in (B).
Phrenic Nerve Stimulation: (C) Device-based phrenic nerve stimulation during sleep in patients with central sleep apnea leads to diaphragmatic contraction, and thus, restoring normal breathing and stabilizing the level of oxygen and cardon dioxide throughout sleep. (D) Device-based phrenic nerve stimulation (using remedē System) results in improvement in the apnea-hypopnea index (AHI). From Costanzo MR, Ponikowski P, Javaheri S, Augostini R, Goldberg L, Holcomb R, Kao A, Khayat RN, Oldenburg O, Stellbrink C, Abraham WT and remede System Pivotal Trial Study G. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet. 2016;388:974-82.
The OPTIMIZER system is CE marked and was given a breakthrough device designation in 2015, with FDA approval in 2019 based on the results of the FIX-HF-5C (Evaluate Safety and Efficacy of the OPTIMIZER® System in Subjects With Moderate-to-Severe Heart Failure; NCT01381172) trial. This was a randomized trial of 160 patients with NYHA class III-IV symptoms, LVEF 25–45%, and QRS duration <130 ms. Compared to medical therapy, patients with CCM had a lesser decline in peak VO2 (difference of 0.82 ml O2/kg/min, with a 0.989 probability for CCM to be the superior), and improved MLHFQ, NYHA class and 6-minute walk distance at 12 and 24 weeks (71). The CCM group had fewer composite events of cardiac death or HF hospitalization at 24 weeks (2.9 vs 10.8%, p=0.028). Eighty percent of patients remained free of device-related adverse events. The findings of the pivotal CCM trial were supported by a meta-analysis of 4 RCTs showing improved peak VO2, 6-minute walk distance, and MLHFQ (72). Early experience from a European registry indicates benefits on cardiovascular and HF hospitalization (73). Further investigation is planned for patients with HFpEF.
II. Electrical microcurrent therapy
Electrical microcurrent therapy is a novel therapy that consists of an intravenously-placed coil lead in the right ventricle and an intrapericardial patch lead, both of which are connected to a microcurrent generator (C-MIC system by Berlin Heals) implanted in a subcutaneous pocket (74). Microcurrent stimulation of cardiomyocytes results in intracellular and extracellular changes that can lead to reverse remodeling of cardiomyocytes (75). In a recently presented uncontrolled study in 8 HF patients, the C-MIC system resulted in improvement in LVEF from 30.9±4.2% at baseline to 43.3±4.4% after 1 month, decrease in LVEDD from 63.6±3mm at baseline to 55±2.9mm after 2 months, and increase in 6-minute walk distance from 186.8±37.2m at baseline to 416.7m after 1 month (74).
RESPIRATORY MODULATION
I. Phrenic Nerve Stimulation for Central Sleep Apnea
Central sleep apnea (CSA) is characterized by alternating phases of apnea and hyperpnea due to a loss of neural drive to breathe and is estimated to be present in 40% of patients with HFrEF and 20% of HFpEF (76). Patients with HF tend to have a chronic hyperventilatory state (76–78) with episodic falls in PaCO2 below the apnea threshold, and Cheyne Stokes respiration (79,80). CSA contributes to HF progression by oxidative stress, inflammation, and endothelial dysfunction induced by episodes of hypoxia-reoxygenation and sympathetic hyperactivation (81–83).
The Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure (CANPAP) trial investigated continuous positive airway pressure (CPAP) in HFrEF with CSA (84). The trial was terminated early as it did not show any benefit of CPAP. The Treatment of Sleep-Disordered Breathing with Predominant Central Sleep Apnea by Adaptive Servo Ventilation in Patients with Heart Failure (SERVE-HF) trial investigated an adaptive servo-ventilation system in a similar patient population and showed an increase in all-cause and cardiovascular mortality in the servo-ventilation group (85).
An alternative approach targets the phrenic nerve via an implantable, device-based approach (remedē System, Respicardia, Inc.) (CE marked) (Figure 4, Panel C-D). Phrenic nerve stimulation (PNS) during sleep leads to diaphragmatic contraction, restoring normal breathing and stabilizing PaO2 and PaCO2 during sleep (86). The remedé System Pivotal Trial Study was a randomized trial of PNS in 151 patients with moderate to severe CSA (87). The trial showed significant reduction in apnea-hypopnea index (AHI) and improvement in quality of life and oxygenation with PNS at 6 months (87). PNS had consistent effects on CSA severity with improved quality of life in patients with HF (70% of all enrolled) and without HF (88). PNS showed a good safety profile (92% freedom from adverse events at 12 months) (88). Based on these data, the remedē system was FDA approved as a treatment option for moderate to severe CSA in 2017.
II. Asymptomatic diaphragmatic stimulation
Asymptomatic diaphragmatic stimulation (ADS) is a novel therapy that involves trans-abdominal laparoscopic implantation of the VisONE ADS system that delivers diaphragmatic stimulation pulses at asymptomatic outputs gated with cardiac cycles (89). This results in modulation of intrathoracic pressure with enhancement of preload, afterload, and stroke volume. The VisONE Heart Failure Study (NCT03484780) was a pilot, single-arm trial that investigated ADS therapy in 15 patients with LVEF ≤35%, NYHA class II-III symptoms, and no ventricular dyssynchrony. Preliminary data showed improvement in cardiac output, LVEF, and 6-minute walk distance with decrease in heart rate and no complications at 1 month (89). The device was granted a breakthrough device designation status in 2020.
STRUCTURAL INTERVENTIONS
I. Inter-Atrial Shunting
An increase in the left atrial filling pressures with exercise is pathognomonic in HF (90,91). The rise of left atrial and pulmonary pressures is a key determinant of exercise limitation in both HFrEF and HFpEF. Creating a small left to right shunt may reduce exercise-related increases in left atrial pressure (92). A series of devices have been developed to create a permanent controlled left-to-right shunt to decompress the left atrium (Figure 5). Pivotal sham-controlled randomized trials have been initiated to assess the safety and effectiveness of two of these devices.
Figure 5.
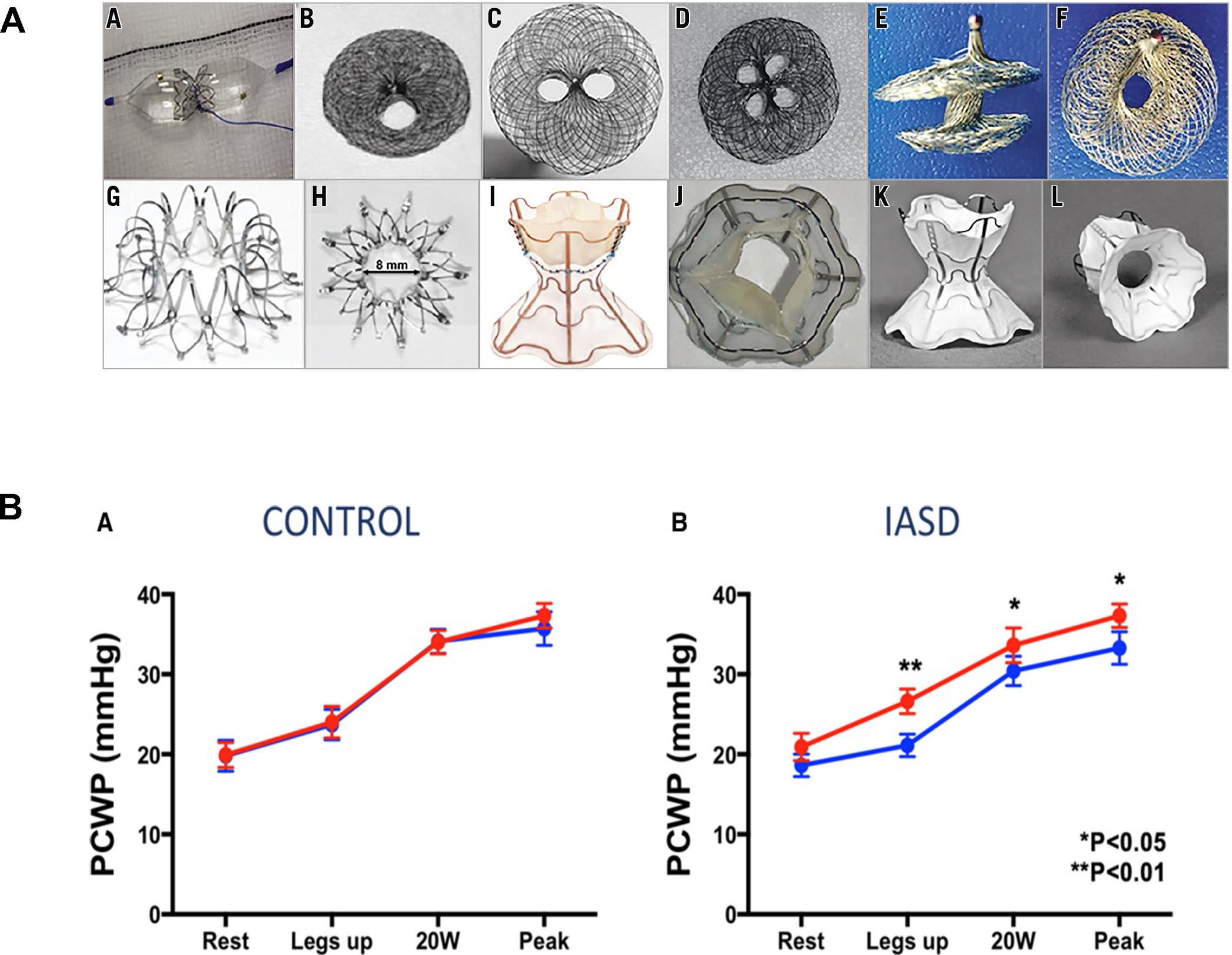
Interatrial shunt devices.
Patients with heart failure typically have increased left atrial pressure with subsequent pulmonary congestion. Creating a permanent, dynamic, controlled left-to-right shunt can decompress the left atrium in heart failure (A). Different interatrial shunt devices are shown in (B). Interatrial shunt devices result in a decrease in the pulmonary capillary wedge pressure, and thus, improvement in pulmonary congestion (C) (94).
The InterAtrial Shunt Device (IASD, Corvia Medical) is a self-expanding metal stent with a double-disc shape and a central opening that is implanted in the atrial septum to creates inter-atrial communication (93,94). The Reduce Elevated Left Atrial Pressure in Patients With Heart Failure (REDUCE LAP-HF; NCT01913613) trial was an open-label, single arm, phase 1 study that evaluated the IASD in 68 patients with HFpEF (93). At 6 months, 58% of the patients had reduced pulmonary capillary wedge pressure (PCWP) during exertion and there were no peri-procedural or device-related complications (93). This was followed by the Reduce Elevated Left Atrial Pressure in Patients With Heart Failure 1 (REDUCE LAP-HF I; NCT02600234) trial, which was a randomized, sham-controlled trial in 94 HFpEF patients (94). IASD treatment decreased PCWP during exercise and there were no complications at 1-month follow-up (94). The device is CE marked and the FDA granted the IASD a breakthrough device designation in 2019. REDUCE LAP-HF II (NCT03088033) is an ongoing randomized, sham-controlled trial studying the efficacy and safety of IASD in 608 subjects with HFpEF.
The V-Wave® interatrial shunt device is an hourglass-shaped device which in its initial design contained a valve to ensure a unidirectional flow from the left to right atrium (95). It was the first inter-atrial shunt device to be studied in HFrEF; with 10 patients implanted, 8 patients had improvement in NYHA class, PCWP and quality of life at 3-month follow-up (96). In a multicenter, single-arm, open-label study with 38 patients, NYHA class II-IV symptoms regardless of LVEF who underwent V-Wave implantation, 78% had improvement in NYHA class and 74% had improved quality of life at 3 months (97). At 12 months, there was only one major adverse neurological or cardiac event (97). However, the device lost efficacy in nearly half of cases because of late valve-related pannus formation. The device is CE marked and the FDA granted the device a breakthrough device designation status in 2019. The Reducing Lung Congestion Symptoms in Advanced Heart Failure (RELIEVE-HF; NCT03499236) study is an ongoing multicenter, randomized trial of the V-Wave device (without the internal valve) in 400 patients with NYHA class III and IV with no restrictions for LVEF.
II. Basilar ventriculoplasty
Accucinch System
The Accucinch Guided Delivery system (Ancora Heart, Inc., Santa Clara, CA.) is a direct device-based, catheter-delivered basilar ventriculoplasty system that involves the implantation of anchors under the posterior mitral annulus resulting in reduction of the septal-free wall dimension, approximation of the papillary muscles and the mitral leaflets, and reduction of the left ventricle volume (Supplemental Figure 2) (98). The FDA has granted this device breakthrough device designation status, and a series of studies are underway to evaluate its safety and performance in patients with HF, with or without functional mitral regurgitation (the CorCinch-EU; NCT03183895, CorCinch-FMR; NCT02806570 and CorCinch-HFrEF; NCT03533517).
III. Left ventricular reconstruction
Ischemic cardiomyopathy and LV remodeling is characterized by expansion and thinning of the LV wall and loss of the typical LV elliptical shape, leading to an increase in the wall tension and inefficient ventricular contraction (99,100). Surgical ventricular reshaping established the concept of LV reconstruction as a potential treatment for selected patients with HF (101–103). The less invasive ventricular enhancement (LIVE) system, also known as the Revivent TC ventricular enhancement system (BioVentrix; San Ramon, CA, USA), consists of a series of tethered anchors that are inserted during a hybrid interventional/surgical procedure on the beating heart that excludes regions of transmural infarction involving the apical-anterior-septal aspect of LV (Supplemental Figure 3) (104). In 82 HFrEF patients with myocardial infarction resulting in LV dilatation and akinetic or dyskinetic wall motion in the anteroseptal, anterolateral, and/or apical regions, implantation of the Revivent TC ventricular enhancement system improved LVEF (29±8% from baseline vs. 34±9% at 12 months, p<0.005) and reduced LV end-systolic (74±28 mL/m2 vs. 54±23 mL/m2, p<0.001) and end-diastolic (106±33 mL/m2 vs. 80±26 mL/m2, p<0.0001) volume index at 12 months (105). Quality of life (MLHFQ 39 vs. 26 points, p<0.001) and 6-minute walk test (363 m vs. 416 m, p<0.001) improved in parallel (105). The Revivent TC ventricular enhancement system is CE marked and the FDA granted a breakthrough device designation status in 2019. The BioVentrix Revivent TC™ System Clinical Study (ALIVE; NCT02931240) is an ongoing IDE approval trial that aims to establish effectiveness and safety of the Revivent TC ventricular enhancement system in 84 treated patients with NYHA class III or IV symptoms, LVEF <45% and LV end-systolic volume index ≥50 mL/m2 compared with 42 concurrent untreated controls.
VOLUME MANAGEMENT
Peritoneal direct sodium removal
Peritoneal direct sodium removal (DSR) is a non-renal approach to prevent and treat volume overload in HF. DSR utilizes a zero-sodium peritoneal solution (10% dextrose) to drive sodium removal as a result of the large gradient between extracellular fluid and the solution (106). In a study in 10 patients receiving peritoneal dialysis (PD) for end-stage renal disease who underwent randomization and crossover to DSR solution or standard PD solution, DSR solution resulted in greater sodium removal (P<0.001) and more fluid removal (106). The RED DESERT (Alfapump Direct Sodium Removal; NCT04116034) study is an ongoing trial to investigate the feasibility and safety of the Alfapump DSR system in HF patients who are resistant to diuretic therapy.
SUMMARY OF EVIDENCE
The following FDA-approved device-based therapies may be considered in patients with HF.
HFrEF
In patients with LVEF 20–50%, moderate-severe or severe functional MR, LVESD of ≤ 7cm and NYHA class II-IV despite maximally tolerated GDMT and CRT if appropriate, MitraClip implantation may be considered.
In patients with LVEF 25–45%, and QRS duration <130 ms. and NYHA class III-IV despite maximally tolerated GDMT, cardiac contractility modulation therapy may be considered.
In patients with LVEF ≤35% and NYHA class II-III despite maximally tolerated GDMT, baroreflex activation therapy may be considered.
HF regardless of LVEF
In patients with moderate to severe CSA regardless of LVEF, phrenic nerve stimulation may be considered.
CONCLUSION
Innovation in device-based therapies has seen a major change in the last decade. A number of device-based therapies have been recently approved for HF and increasingly present an integral building block in the management of different phenotypes of HF. Currently approved and novel therapies that are the pipeline allow in most cases to target a structural or neurohormonal abnormality that are not directly amendable to pharmacological therapeutic interventions. Further, device-based therapies have the advantage to operate independent of patient adherence. Given the more invasive nature and inherent risk of device-based therapies, they are primarily developed and approved for patients with persistent symptomatic or progressive disease despite optimal GDMT. Notably, devices are not replacing pharmacological therapies but rather complement them or fill gaps in areas without any proven medical therapies such as HFpEF. Thus, device-based therapies for HF should be considered complementary to pharmacological therapy.
Supplementary Material
Table 1.
Overview of selected device-based therapies for heart failure.
| Mechanistic target | Device (company) | Suggested solution | Breakthrough designation (Y/N) | CE mark | Trial leading to FDA approval/breakthrough designation and primary endpoint | Ongoing trials |
|---|---|---|---|---|---|---|
| Mitral regurgitation in HF due to annular dilatation, leaflet tethering, papillary muscle desynchrony, and valve tenting | MitraClip™ (Abbott) | Edge-to-edge mitral valve repair | N FDA approved |
Y | COAPT trial • Effectiveness endpoint: all hospitalizations for heart failure within 24 months of follow-up • Safety endpoint: freedom from device-related complications at 12 months |
1. Reshape-HF2 (NCT02444338): open label, randomized, parallel trial to investigate the safety and effectiveness of MitraClip in the treatment of functional mitral regurgitation in patients with NYHA functional class II-IV with the primary outcome being the composite rate of recurrent heart failure hospitalizations and cardiovascular death. 2. EVOLVE-MR (NCT03705312): single-blinded, randomized, parallel trial to investigate the addition of MitraClip to medical treatment in patients with HF and moderate functional mitral regurgitation on left ventricular diastolic remodeling and functional capacity as measured by 6-minute walk test (primary outcomes) |
| Mitral regurgitation in HF due to annular dilatation, leaflet tethering, papillary muscle desynchrony, and valve tenting | Carillon mitral contour system (Cardiac Dimensions) | Indirect mitral annuloplasty system. | N | Y | NA | None |
| Mitral regurgitation in HF due to annular dilatation, leaflet tethering, papillary muscle desynchrony, and valve tenting | • Intrepid (Medtronic) • Tendyne (Abbott) • Sapien M3 • (Edwards Lifesciences) |
Transcatheter mitral valve replacement | N N N |
N Y N |
NA | 1. APOLLO (NCT03242642): open-label, non-randomized, pre-market trial that aims to establish efficacy of the Medtronic Intrepid system in patients with moderate to severe or severe symptomatic MR and LVEF >30% who are not candidates for approved transcatheter repair or conventional MV surgery with the primary outcome of all-cause mortality or HHF after 30 days or KCCQ improvement < 10. 2. SUMMIT (NCT03433274): open-label, randomized, parallel assignment study that aims to investigate safety and efficacy of the Tendyne system in symptomatic patients with moderate to severe or severe MR or severe mitral annular calcification and LVEF ≥25% with the primary outcome being survival free of HHF at 12 months. 3. ENCIRCLE (NCT0415329). Open label, single arm evaluation of Sapien M3 in patient with NYHA ≥ II, MR ≥ 3+. Primary combined endpoint of death and heart failure rehospitalization at 12 months. |
| Tricuspid regurgitation leading to progression of right heart failure and/or right heart failure leading to tricuspid regurgitation | TriClip™ (Abbott) | Edge-to-edge tricuspid valve repair | N | Y | NA | TRILUMINATE Pivotal Trial (NCT03904147): single-blinded, randomized, parallel trial to investigate safety and effectiveness of the TriClip device in symptomatic patients with severe tricuspid regurgitation with the primary outcome being the composite of the number of participants with all-cause mortality or number of participants with tricuspid valve surgery, rate of hospitalization for heart failure, and quality of life assessed using the KCCQ |
| Tricuspid regurgitation leading to progression of right heart failure and/or right heart failure leading to tricuspid regurgitation | EVOQUE (Edwards Lifesciences) Interpid TTVR (Medtronic) |
Transcatheter tricuspid valve replacement system | Y Y |
N N |
NA | TRISCEND (NCT04221490): open-label, single-arm, early feasibility study that aims to assess the safety and performance of the EVOQUE TTVR in symptomatic patients with moderate or greater TR with the primary outcome being freedom from device or procedure-related adverse events at 30 days. The Early Feasibility Study of the Transcatheter Tricuspid Valve Replacement System Transfemoral System (NCT04433065). Single arm, open label study in patient with severe TR. Primary endpoint is the rate of implant or delivery related serious adverse events at 30 days. |
| Excessive activation of the sympathetic nervous system and decreased parasympathetic nervous system activity | Barostim neo System™ (CVRx, Inc) | Baroreceptor stimulation | Y (Expedited Access Pathway) FDA approved |
Y | BeAT-HF • Rate of cardiovascular mortality and heart failure morbidity at study completion • Reduction in NT-proBNP at 6 months • Improvement in 6-minute walk distance at 6 months • Improvement in MLWHF QOL at 6 months • Event-free rate of system- and procedure-related major adverse neurological and cardiovascular events within 6 months |
|
| Excessive activation of the sympathetic nervous system and decreased parasympathetic nervous system activity | VITARIA systems (LivaNova) | Vagus nerve stimulation | Y (Expedited Access Pathway) | Y | ANTHEM-HFrEF (ongoing) • Event-free rate • Cardiovascular mortality and HHF |
1. ANTHEM-HFrEF (NCT03425422): randomized, controlled, open label, parallel trial to investigate the use of autonomic regulation therapy with the VITARIA systems in addition to stable guideline-directed medical therapy in patients with LVEF ≤ 35% and NYHA functional class III, or NYHA functional class II with HHF in the previous 12 months with the primary outcomes being the event-free rate, and cardiovascular mortality and HHF. 2. ANTHEM-HFpEF (NCT03163030): open-label, single-arm trial to investigate the safety and efficacy of VNS with the Cyberonics VNS Therapy System in patients with LVEF ≥40% and NYHA functional class II-III with the primary outcome being the incidence of procedure and device-related complications at 12 months. Secondary outcome is left atrial and ventricular size, 6 minute walk test, quality of life, functional status, autonomic function and blood biomarkers |
| Disruption of intravascular fluid distribution in heart failure | Satera Ablation Systems (Axon Therapies) |
Splanchnic nerve blockade | N | N | NA | 1. Rebalance-HF (NCT04592445): prospective, randomized, sham control, double-blinded study to investigate the safety and effectiveness of catheter-based ablation of the right greater splanchnic nerve in patients with HFpEF with the primary outcome being change in mean PCWP at 1 month and device or procedure-related serious adverse events at 1-month 2. Splanchnic III (NCT04575428): prospective, open-label study to investigate the feasibility and safety of unilateral celiac plexus block using botulinumtoxin in patients with NYHA functional class II-IV HF with the primary outcome being the peak exercise wedge pressure at 4 weeks, peak pulmonary arterial pressure at 4 weeks, and absence of nerve block related complications at 8 weeks. |
| Abnormal intracellular calcium handling of the microcytes | Optimizer Smart system (Impulse Dynamics) | Delivering a biphasic, long-duration, high-voltage electrical signal to the septum of the right ventricle during the absolute refractory period. | Y FDA approved |
Y | FIX-HF-5C Exercise tolerance quantified by peak VO2 measured with cardiopulmonary exercise stress testing at 24 weeks |
PAS (NCT03970343): prospective, non-randomized, single arm, open label, post-market study to investigate the safety of OPTIMIZER in patients with NYHA functional class III and a LVEF of 25–45% with the primary outcome being the incidence of procedure and device-related complications at 1 year. |
| Remodeling of cardiomyocytes | Electrical microcurrent therapy with C-MIC system (Berlin Heals) | Microcurrent stimulation of cardiomyocytes resulting in intracellular and extracellular changes with reverse remodeling of cardiomyocytes | N | N | NA | None |
| Instability of respiratory control and central sleep apnea in heart failure | remedē System (Respicardia, Inc.) | Phrenic nerve stimulation | N FDA approved |
Y | The remedé System Pivotal Trial Study • Effectiveness endpoint: 50% or greater apnea–hypopnea index reduction from baseline to 6 months • Safety endpoint: freedom from serious adverse events related to the procedure, system, or therapy at 12 months |
remedē System Therapy Study (NCT03884660): prospective, open label, non-randomized, post-market study to investigate the safety and effectiveness of remedē system implantation in patients with Moderate to severe central sleep apnea with the primary outcomes being the percentage of patients with related serious adverse events, changes in AHI, changes in daytime sleepiness, impact on the PROMIS-29 questionnaire, impact on the KCCQ in the HF subgroup, and reverse remodeling in the HF subgroup. |
| Alteration of intrathoracic pressure | VisONE asymptomatic diaphragmatic stimulation device (VisCardia) | Diaphragmatic stimulation pulses gated with cardiac cycles leading to modulation of intrathoracic pressure and enhancement of preload, afterload, and stroke volume. | Y | N | N/A | VisONE Heart Failure Study: Pilot Freedom from serious complications or adverse events at 3 months |
| Increase in the left atrial filling pressures with exercise | • InterAtrial Shunt Device (IASD, Corvia Medical) • V-Wave® interatrial shunt device • Occlutech AFR |
Inter-atrial, left-right shunt | Y | Y | REDUCE LAP-HF II (ongoing) • Effectiveness endpoint: exercise pulmonary capillary wedge pressure at 1 month • Safety endpoint: major cardiac, cerebrovascular, and renal adverse events at 1 month RELIEVE-HF (ongoing) • Effectiveness endpoint: composite of death, heart transplant or left ventricular assist device implantation, HHF, and change in six-minute walk distance. • Safety endpoint: MANCE |
1. REDUCE LAP-HF II (NCT03088033): randomized, controlled, triple-blinded, crossover trial to investigate the Corvia Medical, Inc. IASD® System II in patients with ≥ 40 years old, LVEF ≥ 40%, and chronic symptomatic HF despite ongoing stable GDMT with the primary outcome being the composite of cardiovascular mortality or non-fatal, ischemic stroke, HHF, and change in baseline KCCQ at 12 months. 2. RELIEVE-HF (NCT03499236): randomized, controlled, quadruple-blinded study to investigate safety and effectiveness of the V-Wave system in patients with NYHA functional class II or ambulatory class IV HF on GDMT with the primary outcomes being the percentage of treatment group patients experiencing MANCE and the composite of death, heart transplant or left ventricular assist device implantation, HHF, and change in six-minute walk distance. |
| Increase basal left ventricle and mitral annular dimensions | • AccuCinch (Ancora Heart) | Transcatheter direct mitral valve annuloplasty and ventriculoplasty system | Y | N | NA | The CorCinch-EU Study (NCT03183895): prospective, non-randomized, single-arm, study to evaluate safety and efficacy of the AccuCinch system in patients with HF and LVEF of ≥20 to ≤40% with or without functional MR with the primary endpoint being the 30-day major adverse events. |
| Left ventricular remodeling in heart failure | ReVivent TC (BioVentrix) | Left ventricular reconstruction | Y | Y | Less invasive ventricular reconstruction for ischemic heart failure • Combination of the changes in LV end-systolic volume index and end-diastolic volume index and improvement in LV ejection fraction |
ALIVE (NCT02931240): prospective, non-randomized, dual-arm pivotal study to investigate the use of Revivent TC System in patients with LVEF <45%, NYHA functional class II-IV, and LV akinetic and/or dyskinetic scar involving the septum and anterior, apical or anterolateral regions of the LV with the primary outcome being the composite of all cause death, placement of a mechanical support device, and emergent cardiac surgery, prolonged mechanical ventilation, renal failure and stroke through 30 days after the procedure; and the composite of all-cause death, mechanical support device and operation for HF, and bleeding or tamponade through 12 months after the procedure |
| Volume overload | Alfapump DSR (Sequana Medical) | Peritoneal direct sodium removal | Y | N | Zero Sodium Peritoneal Dialysate Protocol Pilot Study (NCT03801226) Protocol discontinuation due to patient discomfort or adverse event |
Alfapump Direct Sodium Removal Feasibility Study (NCT04116034): a single-arm study that aims to investigate the feasibility and safety of the Alfapump direct sodium removal system in patients with heart failure who are resistant to diuretic therapy. The primary outcome is the rate of device related serious adverse events through day 14 and 42 of treatment period |
Abbreviations: AHI= Apnea hypopnea index, DSR= Direct sodium removal, FDA= Food and Drug Administration, GDMT= Guideline directed medical therapy, HF= heart failure, HHF= hospitalization for heart failure, KCCQ= Kansas City Cardiomyopathy Questionnaire, MANCE= Major adverse neurological or cardiovascular event, MR= mitral regurgitation, LV= left ventricle, LVEF= left ventricular ejection fraction, MANCE= Major adverse neurological or cardiac adverse event, NYHA= New York Heart Association, PCWP= pulmonary capillary wedge pressure, IASD= interatrial shunt device, VNS= vagus nerve stimulation
Highlights.
Device therapies have become an integral part of heart failure (HF) management.
The Breakthrough Device Program has expedited the access to device-based HF therapies.
Critical issues related to evidence generation and approval of HF device therapies remain to be addressed.
Disclosures:
Dr. Fudim was supported by NHLBI K23HL151744 from the National Heart, Lung, and Blood Institute (NHLBI), the American Heart Association grant No 20IPA35310955, Mario Family Award, Duke Chair’s Award, Translating Duke Health Award, Bayer and BTG Specialty Pharmaceuticals. He receives consulting fees from AstraZeneca, AxonTherapies, CVRx, Daxor, Edwards LifeSciences, Galvani, NXT Biomedical. Dr. Abraham reports personal fees from Abbott, during the conduct of the study, and during the last 36 months, he received consulting fees from Boehringer Ingelheim, CVRx, Edwards Lifesciences, Impulse Dynamics, and Respicardia, salary support from V-Wave Medical, and research support from the U.S. National Institutes of Health National Heart, Lung, and Blood Institute, all for studies performed within the heart failure arena. Dr. von Bardeleben has received grants from Abbott Structural Heart. Dr. Lindenfeld receives grant funding from AstraZeneca, Volumetrix, and Sensible Medical and consulting fees from AstraZeneca, Abbott, Boehringer Ingelheim, Boston Scientific, CVRx, Edwards LifeSciences, Impulse Dynamics, and VWave. Consulting: AstraZeneca, Abbott, Boehringer Ingelheim, Boston Scientific, CVRx, Edwards LifeSciences, Impulse Dynamics, VWave. Dr. Ponikowski reports consulting fees and speaker’s honoraria from: AstraZeneca, Boehringer Ingelheim, Vifor Pharma, Amgen, Servier, Novartis, Berlin Chemie, Bayer, Pfizer, Cibiem, Coridea, Impulse Dynamics, Renal Guard Solutions, BMS, AbbottVascular Co-PI for the RESHAPE-HF trial: AbbottVascular as well as a research grant: Vifor Pharma. Dr. Sievert consults for 4tech Cardio, Abbott, Ablative Solutions, Ancora Heart, Bavaria Medizin Technologie GmbH, Bioventrix, Boston Scientific, Carag, Cardiac Dimensions, Celonova, Cibiem, CGuard, Comed B.V., Contego, CVRx, Edwards, Endologix, Hemoteq, InspireMD, Lifetech, Maquet Getinge Group, Medtronic, Mitralign, Nuomao Medtech, Occlutech, pfm Medical, Recor, Renal Guard, Rox Medical, Terumo, Vascular Dynamics, Vivasure Medical, Venus, Veryan. Dr. Stone: Speaker or other honoraria from Cook, Terumo, QOOL Therapeutics and Orchestra Biomed; Consultant to Valfix, TherOx, Cardiomech, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Miracor, Neovasc, V-Wave, Abiomed, Ancora, MAIA Pharmaceuticals, Vectorious, Reva, Matrizyme; Equity/options from Ancora, Qool Therapeutics, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, MedFocus family of funds, Valfix. Dr. Anker reports grants from Vifor Int and Abbott, and personal fees from Vifor, Bayer, Boehringer Ingelheim, Novartis, Servier, Abbott, Cardiac Dimensions, Impulse Dynamics, V-Wave and Occlutech. Dr. Butler is a consultant to Abbott, Adrenomed, Amgen, Array, AstraZeneca, Bayer, Berlin Cures, Boehringer Ingelheim, Bristol-Myers Squib, CVRx, G3 Pharmaceutical, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Occlutech, Relypsa, Roche, Sanofi, SC Pharma, V-Wave Limited, and Vifor. Other authors: None.
Abbreviations
- FDA
Food and Drug Administration
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- LVEF
left ventricular ejection fraction
- MLWHQ
Minnesota Living With Heart Failure
- MR
mitral regurgitation
- NT-proBNP
N terminal pro brain natriuretic peptide
References
- 1.US Department of Health and Human Services. Investigational Device Exemptions (IDEs) for Early Feasibility Medical Device Clinical Studies, Including Certain First in Human (FIH) Studies: Guidance for Industry and Food and Drug Administration Staff. 2013.
- 2.Centers for Medicare & Medicaid Services. FY 2020 IPPS/LTCH PPS final rule. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/FY2020-IPPS-Final-Rule-Home-Page (2019).
- 3.Zile MR, Abraham WT, Lindenfeld J et al. First granted example of novel FDA trial design under Expedited Access Pathway for premarket approval: BeAT-HF. Am Heart J 2018;204:139–150. [DOI] [PubMed] [Google Scholar]
- 4.Johnston JL, Dhruva SS, Ross JS, Rathi VK. Early experience with the FDA’s Breakthrough Devices program. Nat Biotechnol 2020;38:933–938. [DOI] [PubMed] [Google Scholar]
- 5. https://www.cms.gov/newsroom/fact-sheets/medicare-coverage-innovative-technology-cms-3372-f.
- 6.Kramer F, Butler J, Shah SJ et al. Real-Life Multimarker Monitoring in Patients with Heart Failure: Continuous Remote Monitoring of Mobility and Patient-Reported Outcomes as Digital End Points in Future Heart-Failure Trials. Digit Biomark 2020;4:45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finkelstein DM, Schoenfeld DA. Combining mortality and longitudinal measures in clinical trials. Stat Med 1999;18:1341–54. [DOI] [PubMed] [Google Scholar]
- 8.Pocock SJ, Ariti CA, Collier TJ, Wang D. The win ratio: a new approach to the analysis of composite endpoints in clinical trials based on clinical priorities. Eur Heart J 2012;33:176–82. [DOI] [PubMed] [Google Scholar]
- 9.Bittl JA, He Y. Bayesian Analysis: A Practical Approach to Interpret Clinical Trials and Create Clinical Practice Guidelines. Circ Cardiovasc Qual Outcomes 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fudim M, Ali-Ahmed F, Patel MR, Sobotka PA. Sham trials: benefits and risks for cardiovascular research and patients. Lancet 2019;393:2104–2106. [DOI] [PubMed] [Google Scholar]
- 11.El Sabbagh A, Reddy YNV, Nishimura RA. Mitral Valve Regurgitation in the Contemporary Era: Insights Into Diagnosis, Management, and Future Directions. JACC Cardiovascular imaging 2018;11:628–643. [DOI] [PubMed] [Google Scholar]
- 12.Delgado V, Bax JJ. Atrial Functional Mitral Regurgitation. Circulation: Cardiovascular Imaging 2017;10:e006239. [DOI] [PubMed] [Google Scholar]
- 13.Baskett RJ, Exner DV, Hirsch GM, Ghali WA. Mitral insufficiency and morbidity and mortality in left ventricular dysfunction. Can J Cardiol 2007;23:797–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ali M, Shreenivas SS, Pratt DN, Lynch DR, Kereiakes DJ. Percutaneous Interventions for Secondary Mitral Regurgitation. Circ Cardiovasc Interv 2020;13:e008998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldman T, Kar S, Elmariah S et al. Randomized Comparison of Percutaneous Repair and Surgery for Mitral Regurgitation: 5-Year Results of EVEREST II. J Am Coll Cardiol 2015;66:2844–2854. [DOI] [PubMed] [Google Scholar]
- 16.Mack MJ, Abraham WT, Lindenfeld J et al. Cardiovascular Outcomes Assessment of the MitraClip in Patients with Heart Failure and Secondary Mitral Regurgitation: Design and rationale of the COAPT trial. Am Heart J 2018;205:1–11. [DOI] [PubMed] [Google Scholar]
- 17.Stone GW, Lindenfeld J, Abraham WT et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N Engl J Med 2018;379:2307–2318. [DOI] [PubMed] [Google Scholar]
- 18.Obadia JF, Messika-Zeitoun D, Leurent G et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N Engl J Med 2018;379:2297–2306. [DOI] [PubMed] [Google Scholar]
- 19.Pibarot P, Delgado V, Bax JJ. MITRA-FR vs. COAPT: lessons from two trials with diametrically opposed results. Eur Heart J Cardiovasc Imaging 2019;20:620–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Praz F, Spargias K, Chrissoheris M et al. Compassionate use of the PASCAL transcatheter mitral valve repair system for patients with severe mitral regurgitation: a multicentre, prospective, observational, first-in-man study. Lancet 2017;390:773–780. [DOI] [PubMed] [Google Scholar]
- 21.Lim DS, Kar S, Spargias K et al. Transcatheter Valve Repair for Patients With Mitral Regurgitation: 30-Day Results of the CLASP Study. JACC Cardiovasc Interv 2019;12:1369–1378. [DOI] [PubMed] [Google Scholar]
- 22.Besler C, Noack T, von Roeder M et al. Transcatheter edge-to-edge mitral valve repair with the PASCAL system: early results from a real-world series. EuroIntervention 2020;16:824–832. [DOI] [PubMed] [Google Scholar]
- 23.Witte KK, Lipiecki J, Siminiak T et al. The REDUCE FMR Trial: A Randomized Sham-Controlled Study of Percutaneous Mitral Annuloplasty in Functional Mitral Regurgitation. JACC Heart Fail 2019;7:945–955. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg SL, Meredith I, Marwick T et al. A randomized double-blind trial of an interventional device treatment of functional mitral regurgitation in patients with symptomatic congestive heart failure-Trial design of the REDUCE FMR study. Am Heart J 2017;188:167–174. [DOI] [PubMed] [Google Scholar]
- 25.McCarthy PM, Kislitsina ON, Malaisrie SC, Davidson CJ. Transcatheter Mitral Valve Replacement with Intrepid. Interv Cardiol Clin 2019;8:287–294. [DOI] [PubMed] [Google Scholar]
- 26.Stanazai Q, Alkhouli M. The intrepid adventure of early transcatheter mitral valve replacement. J Thorac Dis 2018;10:S999–S1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bapat V, Rajagopal V, Meduri C et al. Early Experience With New Transcatheter Mitral Valve Replacement. J Am Coll Cardiol 2018;71:12–21. [DOI] [PubMed] [Google Scholar]
- 28.Muller DWM, Farivar RS, Jansz P et al. Transcatheter Mitral Valve Replacement for Patients With Symptomatic Mitral Regurgitation: A Global Feasibility Trial. J Am Coll Cardiol 2017;69:381–391. [DOI] [PubMed] [Google Scholar]
- 29.Perpetua EM, Reisman M. The Tendyne transcatheter mitral valve implantation system. EuroIntervention 2015;11 Suppl W:W78–9. [DOI] [PubMed] [Google Scholar]
- 30.Goode D, Dhaliwal R, Mohammadi H. Transcatheter Mitral Valve Replacement: State of the Art. Cardiovascular Engineering and Technology 2020;11:229–253. [DOI] [PubMed] [Google Scholar]
- 31.Testa L, Rubbio AP, Casenghi M, Pero G, Latib A, Bedogni F. Transcatheter Mitral Valve Replacement in the Transcatheter Aortic Valve Replacement Era. Journal of the American Heart Association 2019;8:e013352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alperi A, Dagenais F, del Val D et al. Early Experience With a Novel Transfemoral Mitral Valve Implantation System in Complex Degenerative Mitral Regurgitation. JACC: Cardiovascular Interventions 2020;13:2427–2437. [DOI] [PubMed] [Google Scholar]
- 33.Vargas Abello LM, Klein AL, Marwick TH et al. Understanding right ventricular dysfunction and functional tricuspid regurgitation accompanying mitral valve disease. J Thorac Cardiovasc Surg 2013;145:1234–1241 e5. [DOI] [PubMed] [Google Scholar]
- 34.Fender EA, Zack CJ, Nishimura RA. Isolated tricuspid regurgitation: outcomes and therapeutic interventions. Heart 2018;104:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asmarats L, Puri R, Latib A, Navia JL, Rodes-Cabau J. Transcatheter Tricuspid Valve Interventions: Landscape, Challenges, and Future Directions. J Am Coll Cardiol 2018;71:2935–2956. [DOI] [PubMed] [Google Scholar]
- 36.Nickenig G, Weber M, Lurz P et al. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. Lancet 2019;394:2002–2011. [DOI] [PubMed] [Google Scholar]
- 37.Ferguson DW, Abboud FM, Mark AL. Selective impairment of baroreflex-mediated vasoconstrictor responses in patients with ventricular dysfunction. Circulation 1984;69:451–60. [DOI] [PubMed] [Google Scholar]
- 38.Gronda E, Francis D, Zannad F, Hamm C, Brugada J, Vanoli E. Baroreflex activation therapy: a new approach to the management of advanced heart failure with reduced ejection fraction. J Cardiovasc Med (Hagerstown) 2017;18:641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoppe UC, Brandt MC, Wachter R et al. Minimally invasive system for baroreflex activation therapy chronically lowers blood pressure with pacemaker-like safety profile: results from the Barostim neo trial. J Am Soc Hypertens 2012;6:270–6. [DOI] [PubMed] [Google Scholar]
- 40.Georgakopoulos D, Little WC, Abraham WT, Weaver FA, Zile MR. Chronic baroreflex activation: a potential therapeutic approach to heart failure with preserved ejection fraction. J Card Fail 2011;17:167–78. [DOI] [PubMed] [Google Scholar]
- 41.Abraham WT, Zile MR, Weaver FA et al. Baroreflex Activation Therapy for the Treatment of Heart Failure With a Reduced Ejection Fraction. JACC Heart Fail 2015;3:487–496. [DOI] [PubMed] [Google Scholar]
- 42.Zile MR, Lindenfeld J, Weaver FA et al. Baroreflex Activation Therapy in Patients With Heart Failure With Reduced Ejection Fraction. J Am Coll Cardiol 2020;76:1–13. [DOI] [PubMed] [Google Scholar]
- 43.Kishi T Heart failure as an autonomic nervous system dysfunction. Journal of Cardiology 2012;59:117–122. [DOI] [PubMed] [Google Scholar]
- 44.Sobowale CO, Hori Y, Ajijola OA. Neuromodulation Therapy in Heart Failure: Combined Use of Drugs and Devices. J Innov Card Rhythm Manag 2020;11:4151–4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwartz PJ, De Ferrari GM, Sanzo A et al. Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur J Heart Fail 2008;10:884–91. [DOI] [PubMed] [Google Scholar]
- 46.Klein HU, Ferrari GM. Vagus nerve stimulation: A new approach to reduce heart failure. Cardiol J 2010;17:638–44. [PubMed] [Google Scholar]
- 47.Olshansky B, Sabbah HN, Hauptman PJ, Colucci WS. Parasympathetic nervous system and heart failure: pathophysiology and potential implications for therapy. Circulation 2008;118:863–71. [DOI] [PubMed] [Google Scholar]
- 48.Premchand RK, Sharma K, Mittal S et al. Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: results of the ANTHEM-HF trial. J Card Fail 2014;20:808–16. [DOI] [PubMed] [Google Scholar]
- 49.Gold MR, Van Veldhuisen DJ, Hauptman PJ et al. Vagus Nerve Stimulation for the Treatment of Heart Failure: The INOVATE-HF Trial. J Am Coll Cardiol 2016;68:149–58. [DOI] [PubMed] [Google Scholar]
- 50.De Ferrari GM, Stolen C, Tuinenburg AE et al. Long-term vagal stimulation for heart failure: Eighteen month results from the NEural Cardiac TherApy foR Heart Failure (NECTAR-HF) trial. Int J Cardiol 2017;244:229–234. [DOI] [PubMed] [Google Scholar]
- 51.Konstam MA, Udelson JE, Butler J et al. Impact of Autonomic Regulation Therapy in Patients with Heart Failure: ANTHEM-HFrEF Pivotal Study Design. Circ Heart Fail 2019;12:e005879. [DOI] [PubMed] [Google Scholar]
- 52.DiCarlo LA, Libbus I, Kumar HU et al. Autonomic regulation therapy to enhance myocardial function in heart failure patients: the ANTHEM-HFpEF study. ESC Heart Fail 2018;5:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zile MR, Bennett TD, St John Sutton M et al. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation 2008;118:1433–41. [DOI] [PubMed] [Google Scholar]
- 54.Chaudhry SI, Wang Y, Concato J, Gill TM, Krumholz HM. Patterns of weight change preceding hospitalization for heart failure. Circulation 2007;116:1549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fudim M, Hernandez AF, Felker GM. Role of Volume Redistribution in the Congestion of Heart Failure. Journal of the American Heart Association 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bapna A, Adin C, Engelman ZJ, Fudim M. Increasing Blood Pressure by Greater Splanchnic Nerve Stimulation: a Feasibility Study. J Cardiovasc Transl Res 2020;13:509–518. [DOI] [PubMed] [Google Scholar]
- 57.Fudim M, Yalamuri S, Herbert JT, Liu PR, Patel MR, Sandler A. Raising the pressure: Hemodynamic effects of splanchnic nerve stimulation. Journal of applied physiology (Bethesda, Md : 1985) 2017;123:126–127. [DOI] [PubMed] [Google Scholar]
- 58.Gelman S Venous function and central venous pressure: a physiologic story. Anesthesiology 2008;108:735–48. [DOI] [PubMed] [Google Scholar]
- 59.Fudim M, Neuzil P, Malek F, Engelman ZJ, Reddy VY. Greater Splanchnic Nerve Stimulation in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol 2021;77:1952–1953. [DOI] [PubMed] [Google Scholar]
- 60.Fudim M, Ganesh A, Green C et al. Splanchnic nerve block for decompensated chronic heart failure: splanchnic-HF. Eur Heart J 2018;39:4255–4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fudim M, Jones WS, Boortz-Marx RL et al. Splanchnic Nerve Block for Acute Heart Failure. Circulation 2018;138:951–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fudim M, Boortz-Marx RL, Ganesh A et al. Splanchnic Nerve Block for Chronic Heart Failure. JACC Heart Fail 2020;8:742–752. [DOI] [PubMed] [Google Scholar]
- 63.Malek F, Gajewski P, Zymlinski R et al. Surgical ablation of the right greater splanchnic nerve for the treatment of heart failure with preserved ejection fraction: First-in-human clinical trial. European journal of heart failure 2021. [DOI] [PubMed] [Google Scholar]
- 64.Yano M, Ikeda Y, Matsuzaki M. Altered intracellular Ca2+ handling in heart failure. J Clin Invest 2005;115:556–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mebazaa A, Nieminen MS, Packer M et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. JAMA 2007;297:1883–91. [DOI] [PubMed] [Google Scholar]
- 66.Packer M, Carver JR, Rodeheffer RJ et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med 1991;325:1468–75. [DOI] [PubMed] [Google Scholar]
- 67.Chen HH, Anstrom KJ, Givertz MM et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 2013;310:2533–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goliasch G, Khorsand A, Schutz M et al. The effect of device-based cardiac contractility modulation therapy on myocardial efficiency and oxidative metabolism in patients with heart failure. Eur J Nucl Med Mol Imaging 2012;39:408–15. [DOI] [PubMed] [Google Scholar]
- 69.Abraham WT, Nademanee K, Volosin K et al. Subgroup analysis of a randomized controlled trial evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure. J Card Fail 2011;17:710–7. [DOI] [PubMed] [Google Scholar]
- 70.Abi-Samra F, Gutterman D. Cardiac contractility modulation: a novel approach for the treatment of heart failure. Heart Fail Rev 2016;21:645–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abraham WT, Kuck KH, Goldsmith RL et al. A Randomized Controlled Trial to Evaluate the Safety and Efficacy of Cardiac Contractility Modulation. JACC Heart Fail 2018;6:874–883. [DOI] [PubMed] [Google Scholar]
- 72.Giallauria F, Cuomo G, Parlato A, Raval NY, Kuschyk J, Stewart Coats AJ. A comprehensive individual patient data meta-analysis of the effects of cardiac contractility modulation on functional capacity and heart failure-related quality of life. ESC Heart Fail 2020;7:2922–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anker SD, Borggrefe M, Neuser H et al. Cardiac contractility modulation improves long-term survival and hospitalizations in heart failure with reduced ejection fraction. European journal of heart failure 2019;21:1103–1113. [DOI] [PubMed] [Google Scholar]
- 74.Mueller J, Peric M, Kosevic DB et al. ELECTRICAL MICROCURRENT THERAPY IN PATIENTS WITH CHRONIC HEART FAILURE: A SUCCESSFUL DISRUPTIVE TREATMENT APPROACH. Journal of the American College of Cardiology 2020;75:847–847. [Google Scholar]
- 75.Kapeller B, Mueller J, Losert U, Podesser BK, Macfelda K. Microcurrent stimulation promotes reverse remodelling in cardiomyocytes. ESC Heart Fail 2016;3:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Costanzo MR, Khayat R, Ponikowski P et al. Mechanisms and clinical consequences of untreated central sleep apnea in heart failure. J Am Coll Cardiol 2015;65:72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Naughton MT, Benard DC, Liu PP, Rutherford R, Rankin F, Bradley TD. Effects of nasal CPAP on sympathetic activity in patients with heart failure and central sleep apnea. Am J Respir Crit Care Med 1995;152:473–9. [DOI] [PubMed] [Google Scholar]
- 78.Spaak J, Egri ZJ, Kubo T et al. Muscle sympathetic nerve activity during wakefulness in heart failure patients with and without sleep apnea. Hypertension 2005;46:1327–32. [DOI] [PubMed] [Google Scholar]
- 79.Fudim M, Soloveva A. Upright Cheyne-Stokes Respiration in Heart Failure: An Ominous Sign of Cardiovascular Dysregulation. J Am Coll Cardiol 2020;76:2038–2039. [DOI] [PubMed] [Google Scholar]
- 80.Giannoni A, Gentile F, Sciarrone P et al. Upright Cheyne-Stokes Respiration in Patients With Heart Failure. J Am Coll Cardiol 2020;75:2934–2946. [DOI] [PubMed] [Google Scholar]
- 81.Schulz R, Mahmoudi S, Hattar K et al. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med 2000;162:566–70. [DOI] [PubMed] [Google Scholar]
- 82.Lavie L, Lavie P. Molecular mechanisms of cardiovascular disease in OSAHS: the oxidative stress link. Eur Respir J 2009;33:1467–84. [DOI] [PubMed] [Google Scholar]
- 83.Seddon M, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart 2007;93:903–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bradley TD, Logan AG, Kimoff RJ et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005;353:2025–33. [DOI] [PubMed] [Google Scholar]
- 85.Cowie MR, Woehrle H, Wegscheider K et al. Adaptive Servo-Ventilation for Central Sleep Apnea in Systolic Heart Failure. N Engl J Med 2015;373:1095–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Costanzo MR, Augostini R, Goldberg LR, Ponikowski P, Stellbrink C, Javaheri S. Design of the remede System Pivotal Trial: A Prospective, Randomized Study in the Use of Respiratory Rhythm Management to Treat Central Sleep Apnea. J Card Fail 2015;21:892–902. [DOI] [PubMed] [Google Scholar]
- 87.Costanzo MR, Ponikowski P, Javaheri S et al. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet 2016;388:974–82. [DOI] [PubMed] [Google Scholar]
- 88.Costanzo MR, Ponikowski P, Coats A et al. Phrenic nerve stimulation to treat patients with central sleep apnoea and heart failure. Eur J Heart Fail 2018;20:1746–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zuber M, Young R, Shaburishvili T et al. First in Human VisONE Heart Failure Study: Asymptomatic Diaphragmatic Stimulation for Chronic Heart Failure: One Month Results. Journal of Cardiac Failure 2019;25:S183. [Google Scholar]
- 90.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 2010;3:588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Verbrugge FH, Dupont M, Bertrand PB et al. Pulmonary vascular response to exercise in symptomatic heart failure with reduced ejection fraction and pulmonary hypertension. European journal of heart failure 2015;17:320–8. [DOI] [PubMed] [Google Scholar]
- 92.Griffin JM, Borlaug BA, Komtebedde J et al. Impact of Interatrial Shunts on Invasive Hemodynamics and Exercise Tolerance in Patients With Heart Failure. Journal of the American Heart Association 2020;9:e016760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hasenfuss G, Hayward C, Burkhoff D et al. A transcatheter intracardiac shunt device for heart failure with preserved ejection fraction (REDUCE LAP-HF): a multicentre, open-label, single-arm, phase 1 trial. Lancet 2016;387:1298–304. [DOI] [PubMed] [Google Scholar]
- 94.Feldman T, Mauri L, Kahwash R et al. Transcatheter Interatrial Shunt Device for the Treatment of Heart Failure With Preserved Ejection Fraction (REDUCE LAP-HF I [Reduce Elevated Left Atrial Pressure in Patients With Heart Failure]): A Phase 2, Randomized, Sham-Controlled Trial. Circulation 2018;137:364–375. [DOI] [PubMed] [Google Scholar]
- 95.Amat-Santos IJ, Bergeron S, Bernier M et al. Left atrial decompression through unidirectional left-to-right interatrial shunt for the treatment of left heart failure: first-in-man experience with the V-Wave device. EuroIntervention 2015;10:1127–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Del Trigo M, Bergeron S, Bernier M et al. Unidirectional left-to-right interatrial shunting for treatment of patients with heart failure with reduced ejection fraction: a safety and proof-of-principle cohort study. Lancet 2016;387:1290–7. [DOI] [PubMed] [Google Scholar]
- 97.Rodes-Cabau J, Bernier M, Amat-Santos IJ et al. Interatrial Shunting for Heart Failure: Early and Late Results From the First-in-Human Experience With the V-Wave System. JACC Cardiovasc Interv 2018;11:2300–2310. [DOI] [PubMed] [Google Scholar]
- 98.Gooley RP, Meredith IT. The Accucinch transcatheter direct mitral valve annuloplasty system. EuroIntervention 2015;11 Suppl W:W60–1. [DOI] [PubMed] [Google Scholar]
- 99.Nijenhuis VJ, Sanchis L, van der Heyden JAS et al. The last frontier: transcatheter devices for percutaneous or minimally invasive treatment of chronic heart failure. Neth Heart J 2017;25:536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hendriks T, Schurer RAJ, Al Ali L, van den Heuvel AFM, van der Harst P. Left ventricular restoration devices post myocardial infarction. Heart Fail Rev 2018;23:871–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Athanasuleas CL, Buckberg GD, Stanley AW et al. Surgical ventricular restoration in the treatment of congestive heart failure due to post-infarction ventricular dilation. J Am Coll Cardiol 2004;44:1439–45. [DOI] [PubMed] [Google Scholar]
- 102.Dor V, Civaia F, Alexandrescu C, Sabatier M, Montiglio F. Favorable effects of left ventricular reconstruction in patients excluded from the Surgical Treatments for Ischemic Heart Failure (STICH) trial. J Thorac Cardiovasc Surg 2011;141:905–16, 916 e1–4. [DOI] [PubMed] [Google Scholar]
- 103.Michler RE, Rouleau JL, Al-Khalidi HR et al. Insights from the STICH trial: change in left ventricular size after coronary artery bypass grafting with and without surgical ventricular reconstruction. J Thorac Cardiovasc Surg 2013;146:1139–1145 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Loforte A, Alfonsi J, Gliozzi G et al. Less invasive ventricular enhancement (LIVE) as potential therapy for ischaemic cardiomyopathy end-stage heart failure. J Thorac Dis 2019;11:S921–S928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Klein P, Anker SD, Wechsler A et al. Less invasive ventricular reconstruction for ischaemic heart failure. Eur J Heart Fail 2019;21:1638–1650. [DOI] [PubMed] [Google Scholar]
- 106.Rao VS, Turner JM, Griffin M et al. First-in-Human Experience With Peritoneal Direct Sodium Removal Using a Zero-Sodium Solution. Circulation 2020;141:1043–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


