Abstract
Endothelial progenitor cell (EPC) transplantation has therapeutic effects in cerebral ischemia. However, how EPCs modulate microglial activity remains unclear. In the study, we explored whether EPCs modulated microglial/macrophage activity and facilitated injured brain repair. Adult male mice (n = 184) underwent transient middle cerebral artery occlusion, and EPCs were transplanted into the brain immediately after ischemia. Microglial/macrophage activity and complement receptor 3 (CR3) expression were evaluated in ischemic brains and cultured microglia. CR3 agonist leukadherin-1 was administrated into mice immediately after ischemia to imitate the effects of EPCs. Synaptophysin and postsynaptic density protein 95 (PSD-95) expressions were detected in EPC- and leukadherin-1 treated mice. We found that EPC transplantation increased the number of M2 microglia/macrophage-phagocytizing apoptotic cells and CR3 expression in ischemic brains at 3 days after ischemia (p < 0.05). EPC-conditional medium or cultured EPCs increased microglial migration and phagocytosis and upregulated CR3 expression in cultured microglia under oxygen-glucose deprivation condition (p < 0.05). Leukadherin-1 reduced brain atrophy volume and neurological deficits at 14 days after ischemia (p < 0.05). Both EPC transplantation and leukadherin-1 increased synaptophysin and PSD-95 expression at 14 days after ischemia (p < 0.05). EPC transplantation promoted CR3-mediated microglial/macrophage phagocytosis and subsequently attenuated synaptic loss. Our study provided a novel therapeutic mechanism for EPCs.
Keywords: Brain, endothelial progenitor cell, ischemia, microglial/macrophages, phagocytosis
Introduction
Ischemic stroke constitutes most strokes, which are the leading cause of permanent disability and the second-leading cause of death worldwide. 1 Currently, thrombolysis and endovascular thrombectomy are the only proven effective treatments. However, less than 15% of the patients can access these treatments because of the limited therapeutic time window and tissue window. 2 Therefore, other potential and alternative therapeutic methods are urgently needed for the treatment of ischemic stroke.
Emerging preclinical and clinical studies have recognized that stem cell therapy as a promising strategy could effectively ameliorate neurological deficits and functionally enhance recovery from ischemic stroke. 3 Endothelial progenitor cells (EPCs) hold particular promise for ischemic therapy due to their wide variety of sources, fewer ethical constraints, and strong capacity for angiogenesis.4–6 However, the exact therapeutic mechanism that limits their clinical translational application has not yet been understood.
Numerous studies have demonstrated that the complement system is activated and exerts protective or detrimental effects during cerebral ischemia. 7 Complement 3 (C3) is the core of the complement signaling pathway and activates the downstream signaling pathway mainly via binding to the receptor C3aR or complement receptor 3 (CR3). 7 CR3 is exclusively expressed on resident microglia in the brain and mediates phagocytosis function.8,9 The C3/CR3 signaling pathway is involved in promoting synapse elimination and phagocytizing debris, contributing to nervous circuit development and functional recovery from neurological diseases.9–11 However, emerging evidence suggests that C3/CR3 signaling is also an etiologic factor in neurodegeneration. 12 Our previous study proved that EPC transplantation inhibited astrocyte release of complement component C3 and regulated the C3/C3aR pathway in microglia, attenuating inflammatory response and neurological function deficit after cerebral ischemia. 13 However, whether EPC transplantation affects CR3 to regulate microglial/macrophage activity and exert its therapeutic function remains unexplored.
In this study, we transplanted EPCs into the brains of a transient middle cerebral artery occlusion (tMCAO) model and explored the effects of EPCs on CR3-mediated microglial/macrophage phagocytosis after cerebral ischemia.
Materials and methods
EPC isolation and identification
The EPC isolation procedure was approved by the Ethics Committee of Shanghai Jiao Tong University in Shanghai, China. The ethical standards were consistent with the Helsinki Declaration of 1975 (as revised in 1983) and written informed consent was obtained from all donors. Human umbilical cord blood samples of five donors were obtained from the International Peace Maternity and Child Health Hospital of China in Shanghai, China. First, umbilical cord blood was diluted in phosphate-buffered saline (PBS) at 1:1. The diluted blood was then layered carefully on top of the lymphocyte separation medium (Yishen, Shanghai, China) and centrifuged for 30 min at 400 × g at 4°C. Finally, mononuclear cells were collected. After washing once with PBS for 10 min at 400 × g at 4°C, the mononuclear cells (1 × 107 per well) were cultured in a 6-well plate coated with collagen type I (Corning) using Endothelial Cell Growth Medium 2 (EGM-2) medium (Lonza, Walkersville, MD) at 37°C. Outgrowth EPC populations within five to eight passages were used for characterization or in subsequent experiments. Green fluorescent protein (GFP)-EPC cell production was performed as described previously. 14
For immunofluorescent staining, EPCs were fixed with 4% paraformaldehyde, followed by incubation in 0.3% Triton-X 100 for 10 min and 10% donkey serum for 1 h at room temperature. The EPCs were then incubated with primary antibodies including CD34 (1:50 dilution, BD Biosciences, Franklin Lakes, NJ) and kinase insert domain receptor (KDR; 1:50 dilution, R&D, Minneapolis, MN), CD133 (1:50 dilution, Abnova, Taipei, China), KDR (1:50 dilution, R&D), and von Willebrand factor (vWF; 1:400 dilution, Abcam, Cambridge, MA) at 4°C overnight. After washing three times, the EPCs were incubated with the following secondary antibodies: Alexa Fluor 594-conjugated donkey anti-mouse and Alexa Fluor 488-conjugated donkey anti-goat, or Alexa Fluor 488-conjugated donkey anti-rabbit (1:500 dilution, Invitrogen, Carlsbad, CA) for 1 h at room temperature. Images were captured using a confocal microscope (Leica, Solms, Germany).
Establishment of a mouse model of transient middle cerebral artery occlusion (tMCAO)
The procedure for laboratory animal use was approved by the Institutional Animal Care and Use Committee of Shanghai Jiao Tong University, Shanghai, China. The animal studies were performed in accordance with Reporting of In Vivo Experiments (ARRIVE) guidelines. All studies were conducted in accordance with the US National Research Council’s Guide for the Care and Use of Laboratory Animals, the US Public Health Service’s Policy on Humane Care and Use of Laboratory Animals and Guide for the Care and Use of Laboratory Animals. We used 8-week-old male ICR mice (n = 210) weighing 25–30 g. A total of 184 mice were randomly divided into five experimental groups: Sham (n = 60), PBS (n = 30), EPC (n = 30), DMSO (n = 32), and LA-1 (n = 32) mice. Twenty-six mice died within 1 week after tMCAO because of severe ischemic brain injury or hemorrhagic transformation (7 mice died in PBS; 5 mice died in EPC; 8 mice died in DMSO; 6 mice died in LA1) (Suppl. Table 1). The tMCAO procedure was performed as previously described.5,15 Briefly, a 6-0 silicone-coated homemade nylon filament was inserted from the external carotid artery and along the internal carotid artery to the opening of the middle cerebral artery. After 90 min, the nylon filament was removed. During the 90 minutes’ occlusion, the animals were anesthetized. The ischemic injured areas included the ipsilateral somatosensory cortex, motor cortex, and striatum. The success of occlusion and reperfusion of cerebral blood flow was confirmed by laser Doppler flowmetry (Moor Instruments, Axminster, Devon, UK). Mice with cerebral blood flow in the ipsilateral hemisphere not reduced to 20% of the baseline were excluded from the study. The sham-operated animals underwent parallel procedures except for the occlusion of the middle cerebral artery.
EPC transplantation
The mice were fixed on a stereotaxic frame (RWD Life Science, Shenzhen, China). A total volume of 10 μl PBS containing 3 × 105 EPCs was injected with a needle through the skull at a rate of 1 μl/minute using a stereotactic brain injection system (RWD Life Science). The injection site was located 2 mm lateral to the bregma and 2.5 mm under the dura, which corresponded to the peri-infarct region of the ipsilateral brain. The needle was maintained for 10 min after the completion of the injection in case of liquid leakage. After needle withdrawal, the skull hole was sealed with bone wax and the skin incision was sutured. The sham-operated animals underwent the same procedure without EPC administration.
Leukadherin-1 injection
The CR3 agonist, leukadherin-1 (LA1, Selleck, Shanghai, China) was dissolved in dimethyl sulfoxide (DMSO) to a final working concentration of 10 mg/ml. The dissolved LA1 was stored at −20°C until use. LA1 was taken out from the freezer, thawed on ice, and injected intraperitoneally into mice (1 mg/kg) immediately after the mice. LA1 injection was performed once daily for the next 3 days after tMCAO. The control mice underwent the identical procedure with the same volume of DMSO solution.
Assessment of deficiencies in neurological function
Two investigators who were blinded to the experimental design and treatment performed the evaluations of neurological function deficiency using modified neurological severity scores (mNSS), which included motor, balance, and reflex tests. The total neurological severity score was 14 (normal score, 0; maximal deficit score, 14). The higher the score, the more severe the neurological deficit. 16
Preparation of brain samples
The mice were euthanized at 1, 3, and 14 days after tMCAO. The brains were perfused with 0.9% saline, followed by 4% paraformaldehyde, and post-fixed overnight in 4% paraformaldehyde. The brains were then dehydrated in 30% sucrose until they sank to the bottom of the liquid and were subsequently stored at −80°C. The brains were cut into 30-μm sections from the anterior commissure to the hippocampus and stained with Cresyl Violet or antibodies. Brains were perfused with 0.9% saline only if the samples were needed for western blotting and real-time PCR.
Measurement of brain atrophy volume
The brains were collected and frozen immediately after euthanizing the mice. A series of 30-μm coronal sections were cut from the anterior commissure to the hippocampus. A total of 20 coronal sections from each brain were mounted on slides, stained with 0.1% Cresyl Violet, and used for the measurement of brain atrophy volume. The distance between adjacent sections was 300 μm. Brain area was measured using ImageJ software (NIH, Bethesda, MD). The atrophy area was calculated by subtracting the area of the normal brain in the ischemic hemisphere from the area of the contralateral hemisphere. The total brain atrophy volume was calculated according to the formula , where h is the distance between two adjacent sections and Sn and Sn+1 are the atrophy areas of two adjacent sections, respectively.
Real-time PCR
Total RNA was extracted from the ischemic brains using TRIzol reagent (Invitrogen) and transcribed to cDNA using a ZymoScript II First Strand cDNA Synthesis Kit (Abconal, Shanghai, China) according to the manufacturers’ recommendations. The sequences of each primer used in the study were summarized in Suppl. Table 2.
Western blot analysis
Equal amounts of proteins were loaded onto a 10% resolving gel for electrophoresis and transferred onto polyvinylidene fluoride membranes (Immobilon-P, Billerica, MA). The membranes were then blocked with 5% non-fat milk and incubated with primary antibodies against CR3 (1:1000 dilution, Abcam), synaptophysin (1:1000 dilution, Abcam), PSD-95 (1:1000 dilution, Millipore, Billerica, MA), and β-actin (1:1000 dilution, Abcam) at 4°C overnight. After washing three times, the membranes were incubated with horseradish peroxidase (HRP)-conjugated anti-mouse or anti-rabbit immunoglobulin G for 1 h at room temperature. After washing three times, the membranes were then reacted with an enhanced chemiluminescent substrate (Thermo Scientific, Waltham, MA). The chemiluminescence results were semi-quantified using ImageJ software (NIH).
Immunohistochemistry
Paraformaldehyde-fixed brain sections were washed three times with PBS and then incubated with 0.3% Triton-X 100 for 10 min at room temperature. After blocking with 10% donkey serum for 1 h at room temperature, the brain sections were incubated with primary antibodies—Iba1 (1:200 dilution, Wako, Osaka, Japan and 1:200 dilution, Novus Biologicals, Centennial, CO), MAP2 (1:200 dilution, Millipore), CR3 (1:200 dilution, Abcam), and synaptophysin (1:200 dilution, Abcam), CD86 (1:200 dilution, Abcam) and Arginase 1 (Arg1) (1:200 dilution, Santa Cruz Biotechnology, Dallas texas, CA) —at 4°C overnight. After washing three times, the brain sections were incubated with fluorescence-conjugated secondary antibodies for 1 h at room temperature. The brain sections were then incubated with 4′, 6-diamidino-2-phenylindole (DAPI, Life Technologies, Mulgrave, VIC, Australia) for 5 min at room temperature and then covered and sealed with mounting medium (Vector Labs, Burlingame, CA) for further analysis.
For apoptosis analysis, frozen brain sections were subjected to deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining. The sections were incubated with the reaction agents of a cell death detection kit (Roche, Penzberg, Germany) in a 24-well plate, according to the manufacturer's instructions. The TUNEL-stained sections were examined by confocal microscopy (Leica). Four fields along the peri-infarct regions were selected for each section.
Cell counting
We used four brain sections for the analysis of Iba1, CD86, Arg1 and TUNEL immunostaining. The distance between each section was 300 μm. The slices were chosen from 1.1, 0.5, −0.1, and −0.7 mm from the bregma. Four fields were selected in each section of the peri-infarct region. The images were examined using a confocal microscope (Leica) under the same conditions. The Iba1-, CD86-, Arg1- and TUNEL-positive cells in each section were manually counted by a blinded investigator.
Preparation of EPC and human umbilical vein endothelial cell (HUVEC)-conditioned medium (CM)
To produce EPC-conditioned medium (EPC-CM), EPCs were cultured in a 10-cm dish (Corning) at a density of 1.5 × 106 cells. Fresh medium (EGM-2) was then added to the culture dish. The EPCs were then incubated at 37°C for 24 h. The EPC-CM was collected and centrifuged at 1000 rpm for 5 min, and the supernatant was sterile filtered and frozen at −20°C until use. EGM-2 served as the control medium (EPC-free).
To produce EPC-O-CM, EPCs were cultured in a mixed medium (glucose-free DMEM: serum-free EGM-2 = 1:2) in a hypoxic chamber, which was used for the oxygen-glucose deprivation (OGD) assay. The methods for OGD were as described previously. 17 BV2 cells in DMEM with glucose and serum-free medium and EPCs in modified OGD medium were placed in a sealed modular incubator chamber (Billups-Rothenberg, San Diego, CA). Air flow (0% O2, 95% N2, and 5% CO2) was then delivered to induce hypoxia. The cells were treated in this chamber for 20 min. After incubation for 24 h at 37°C, the resulting EPC-O-CM, was collected, centrifuged, sterile filtered, and frozen at −20°C until use.
To produce HUVEC-conditioned medium; namely, HUVEC-CM and HUVEC-O-CM, HUVECs were cultured in a 10-cm dish (Corning) under normal or OGD conditions. Endothelial cell medium (ECM) (consisting of basal medium, 5% fetal bovine serum (FBS), and 1% endothelial cell growth factors) was used under normal condition, while the mixed medium (glucose-free DMEM: serum-free ECM = 1:2) was used under OGD condition. The HUVECs were incubated for 24 h at 37°C to collect HUVEC-CM and treated under OGD conditions for 24 h to collect HUVEC-O-CM. HUVEC-CM and HUVEC-O-CM were centrifuged at 1000 rpm for 5 min. The supernatant was sterile filtered and frozen at −20°C until use. Three batches of cells and three batches of CM were used in the experiment, with each batch of CM resulting from three 10-cm dishes (1.5 × 106 cells per dish).
In vitro migration assay
EPCs and BV2 cells were co-cultured using a hanging cell culture transwell (Millipore). BV2 cells with a density of 3 × 104 were seeded in an upper chamber, the bottom of which was made of 8.0 μm pore polycarbonate (Millipore). Co-culture was performed by inserting this upper chamber into a 24-well plate (lower chamber) where EPCs were seeded at approximately 90% confluence or CM was added. Co-cultured cells were incubated at 37°C or treated under OGD conditions for 24 h. Un-transduced microglia on the upper membrane surface were wiped off with a cotton swab, while microglia migrating to the lower membrane surface were stained with hematoxylin (Beyotime, Shanghai, China). Five optical fields per membrane surface were imaged using an optical microscope (Leica). Migrated cells were counted by a blinded investigator.
In vitro phagocytosis assay
BV2 cells were cultured in 24-well plates in DMEM containing 5% FBS. When the cells reached 80% confluency, the medium was replaced with CM and supplemented with lipopolysaccharide (LPS, 200 ng/mL, Sigma). After 12 h of incubation, 10 μl of diluted fluorescent beads (Sigma) was added to the medium. After another 12 h of incubation, the BV2 cells were washed once with PBS to remove extracellular beads. The cells were then stained with Iba1 antibody. The immunostaining procedure was performed as described above in the Immunohistochemistry section. Five fields for each well were examined using a fluorescence microscope (Leica). Iba1-positive cells and the number of beads per cell body were counted by a blinded investigator.
Statistical analysis
Statistical analysis was performed using PASW Statistics for Windows, Version 18.0 (SPSS, Inc., Chicago, IL). Kolmogorov-Smirnov tests were used to assess variable distributions. For parametric analysis, multiple comparisons were evaluated by one-way analysis of variance (ANOVA) followed by Bonferroni (homogeneity of variance) or Tamhane tests (heterogeneity of variance). Unpaired Student’s t-tests were used for comparisons between groups. Kruskal-Wallis and Mann-Whitney U tests were used for nonparametric analysis. All data are presented as mean ± SD. Statistical significance was set at P < 0.05. To better display the action intensity of EPCs, we first averaged the raw data of Control groups, then raw data in all groups divided the average, which became the final data for statistical analysis. In this way, the values of the Control groups were normalized to 1, to which we compared the values of the other groups.
Results
EPC isolation, identification, and transplantation
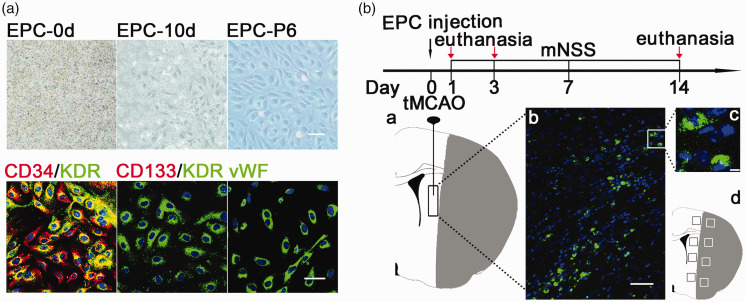
Mononuclear cells were isolated from human umbilical cord blood cells and seeded in a 6-well plate at a density of 1 × 107/well. At 10 days, cobblestone-like EPCs appeared and remained cobblestone-like, with high proliferation ability at passage six. Immunofluorescent staining showed that the EPCs expressed both CD34 (red) and KDR (green), which are considered characteristic markers of EPCs.18,19 The EPCs also expressed the endothelial cell marker vWF (green, Figure 1(a)) and did not express the stem cell marker, CD133 (red, Figure 1(a)). We injected GFP-transfected EPCs into the peri-infarct region of the striatum in the ipsilateral hemisphere of the brain. The results showed that the intensity of GFP fluorescence remained strong in the injected cells, suggesting that the grafted cells remained alive 14 days after transient middle cerebral artery occlusion (tMCAO, Figure 1(b)).
Figure 1.
EPC isolation, identification, and transplantation. (a) Mononuclear cells were cultured on 6-well plates after isolation. Cobblestone-shaped EPCs appeared 10 days after isolation and maintained this shape to passage six. Scale bar = 100 μm. Representative immunofluorescence images of EPCs labeled for CD34 (red) and kinase insert domain receptor (KDR) (green), CD133 (red) and KDR (green), and von Willebrand factor (vWF) (green), respectively. Scale bar = 50 μm. (b) Flowchart of the experimental design. Mice underwent transient middle cerebral artery occlusion (tMCAO) and were randomly assigned to different groups. EPCs were injected into the peri-infarct region of the striatum within 120 minutes after tMCAO. The schematic diagram of the ischemic brain showed the location of the injected EPCs (a); Representative images of injected EPCs labeled by green fluorescent protein (GFP) in the peri-infarct region of the striatum at 14 days after tMCAO (b). Scale bar = 50 μm. Magnification showing strong GFP intensity and clear nuclear (blue) labeling (c). Scale bar = 10 μm. The black boxes indicated four fields from which immunofluorescence images were selected from each section in the peri-infarct regions (d).
EPC transplantation increased the number of microglia/macrophages in ischemic brains
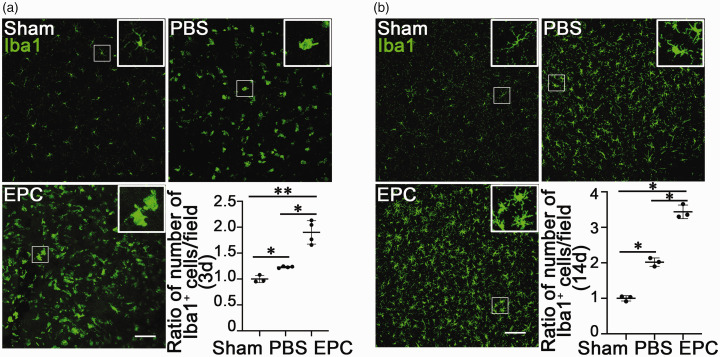
To evaluate the effects of EPC transplantation on microglia/macrophages, we first examined the number of microglia/macrophages in mice following tMCAO. The number of microglia/macrophages was higher in the EPC-treated group than that in the PBS-treated group at 3 (Figure 2(a), p < 0.05) and 14 (Figure 2(b), p < 0.05) days after tMCAO. Our previous study proved that EPC transplantation enhanced the M2 phenotype of microglia-related genes but reduced M1 phenotype-related genes in the mouse brain. 13 We speculated that the increased microglia/macrophages in the EPC-treated mice were M2 microglia/macrophages.
Figure 2.
EPC transplantation enhanced microglial/macrophages number after tMCAO. (a&b) Immunofluorescence images of Iba1+ microglia/macrophages (green) in the peri-infarct region of ischemic brains in the Sham, PBS, and EPC groups at 3 and 14 days after tMCAO, respectively. The bar graphs showed the ratios of the numbers of microglia/macrophages among groups. The data in the Sham group were normalized to 1. Data are shown as mean ± SD. n = 3–4 per group. *p < 0.05, **p < 0.01.
EPC transplantation enhanced M2 microglial/macrophage phagocytosis via CR3
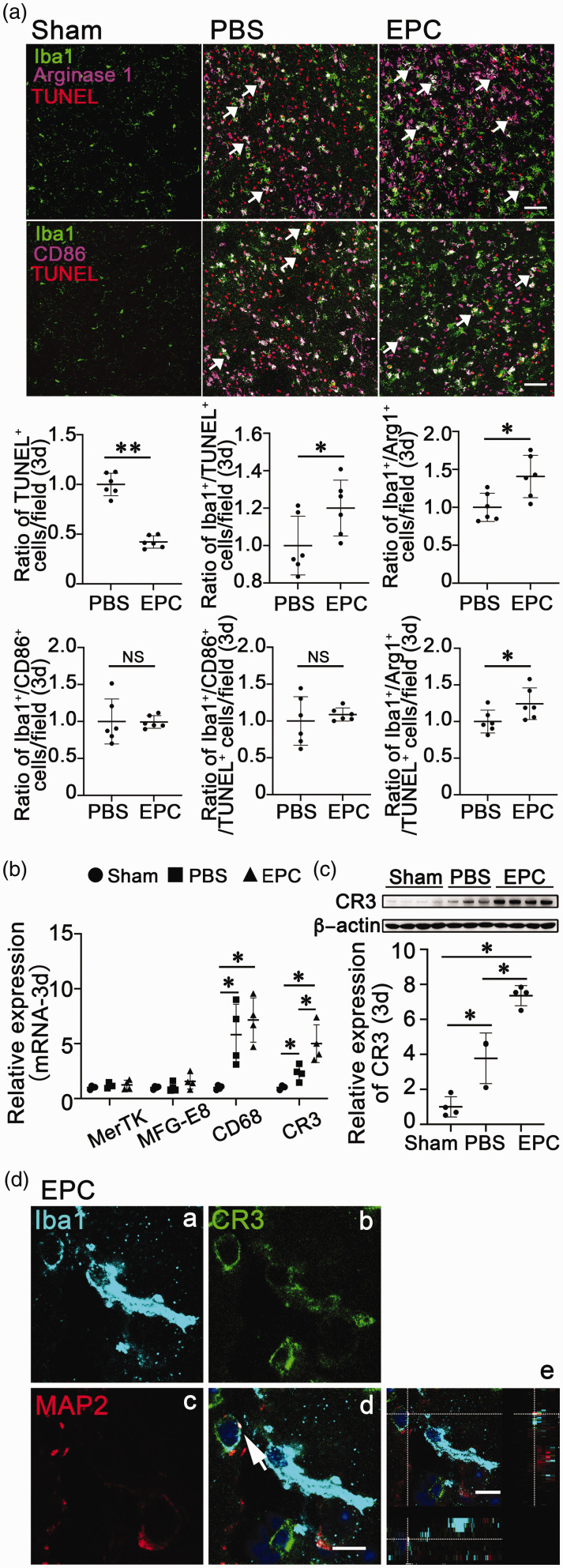
To clarify the function of the increased microglia/macrophages in the EPC-treated mice, we evaluated the ability of microglial/macrophage phagocytosis after tMCAO. We found that there were fewer TUNEL+ cells but more Iba1+/TUNEL+ cells at 3 days after tMCAO in the EPC-treated mice (Figure 3(a), p < 0.05). These results suggested that EPC transplantation promoted microglia/macrophages to phagocytize more apoptotic cells during the acute stage of tMCAO. To further confirm which phenotype of the microglia/macrophages phagocytizing apoptotic cells, we detected CD86 and Arg1, the M1 and M2 marker of microglia/macrophages, in the peri-infarct area at 3 days after tMCAO. We found that the number of Arg1+ M2 microglia/macrophages was higher in the EPC-treated group than that in the PBS-treated group at 3 days after tMCAO (Figure 3(a), p < 0.05), whereas there was no significant difference in the number of CD86+ M1 microglia/macrophages (Figure 3(a), p > 0.05). We also found that there were more Iba1+/Arg1+/TUNEL+ cells in EPC-treated mice brains than that in PBS-treated mice brains (Figure 3(a), p < 0.05). These results suggested that M2 phenotype of microglia/macrophages was the main part of the increased microglia/macrophages and played a major role in phagocytosis for apoptotic cells.
Figure 3.
EPC transplantation promoted M2 microglial/macrophage phagocytosis associated with enhancing complement receptor 3 (CR3). (a) Immunofluorescence images of Iba1Continued.(microglia/macrophages, green), TUNEL (apoptotic cells, red), CD86 (M1 microglia/macrophages, purple), and Arg1 (M2 microglia/macrophages, purple)-labeled cells in the peri-infarct area at 3 days after tMCAO. Scale bar = 50 μm. The bar graphs showed the ratio of TUNEL+ cells, Iba1+/TUNEL+ cells, Iba1+/Arg1+ cells, Iba1+/CD86+ cells, Iba1+/CD86+/TUNEL+ cells and Iba1+/Arg1+/TUNEL+ cells among groups at 3 days after tMCAO, respectively. The data in the PBS group were normalized to 1. Data are shown as means ± SD. n = 5–6 per group. *p < 0.05. **p < 0.01. (b) Quantifications of microglial/macrophage phagocytosis-related gene (MerTK, MFG-E8, CD68, and CR3) expression at 3 days after tMCAO. (c) Detection of CR3 expression by Western blot in of brain samples at 3 days after tMCAO. The bar graph showed the quantification of CR3 expression. The data in the Sham group were normalized to 1. Data are shown as mean ± SD. n = 3–4 per group. *p < 0.05. (d) Immunostaining of Iba1 (light blue, a), CR3 (green, b), and MAP2 (red, c)-labeled cells and merged images (d) of the cells in the mouse brain at 3 days after tMCAO. Scale bar = 10 μm. Confocal image of Iba1, CR3, and MAP2 colocalization in three-dimensional space (e). Scale bar = 10 μm.
Our group and others found that MerTK, MFG-E8, CD68, and CR3 mediated microglial/macrophage phagocytosis during pathophysiological processes, including cerebral ischemia.7,20–22 To further determine which receptor mediated microglial/macrophage phagocytosis, we assessed the presence of MerTK, MFG-E8, CD68, and CR3 during tMCAO. The results showed that the expression of MerTK and MFG-E8 did not change, while the mRNA expression levels of CD68 and CR3 were increased 3 days after tMCAO. EPC transplantation did not affect MerTK and MFG-E8, but further increased CD68 and CR3 mRNA expression (Figure 3(b), p < 0.05). Western blotting confirmed that EPC transplantation enhanced CR3 3 days after tMCAO (Figure 3(c), p < 0.05). CR3 is a specific marker and receptor that mediates microglial phagocytosis in the brain. 7 Iba1, CR3, and MAP2 colocalization in three-dimensional space showed that Iba1+ microglia/macrophages engulfed debris of MAP2-labeled neurons through CR3 (Figure 3(d)). These results suggested that EPC transplantation enhanced microglial/macrophage phagocytosis associated with upregulating CR3.
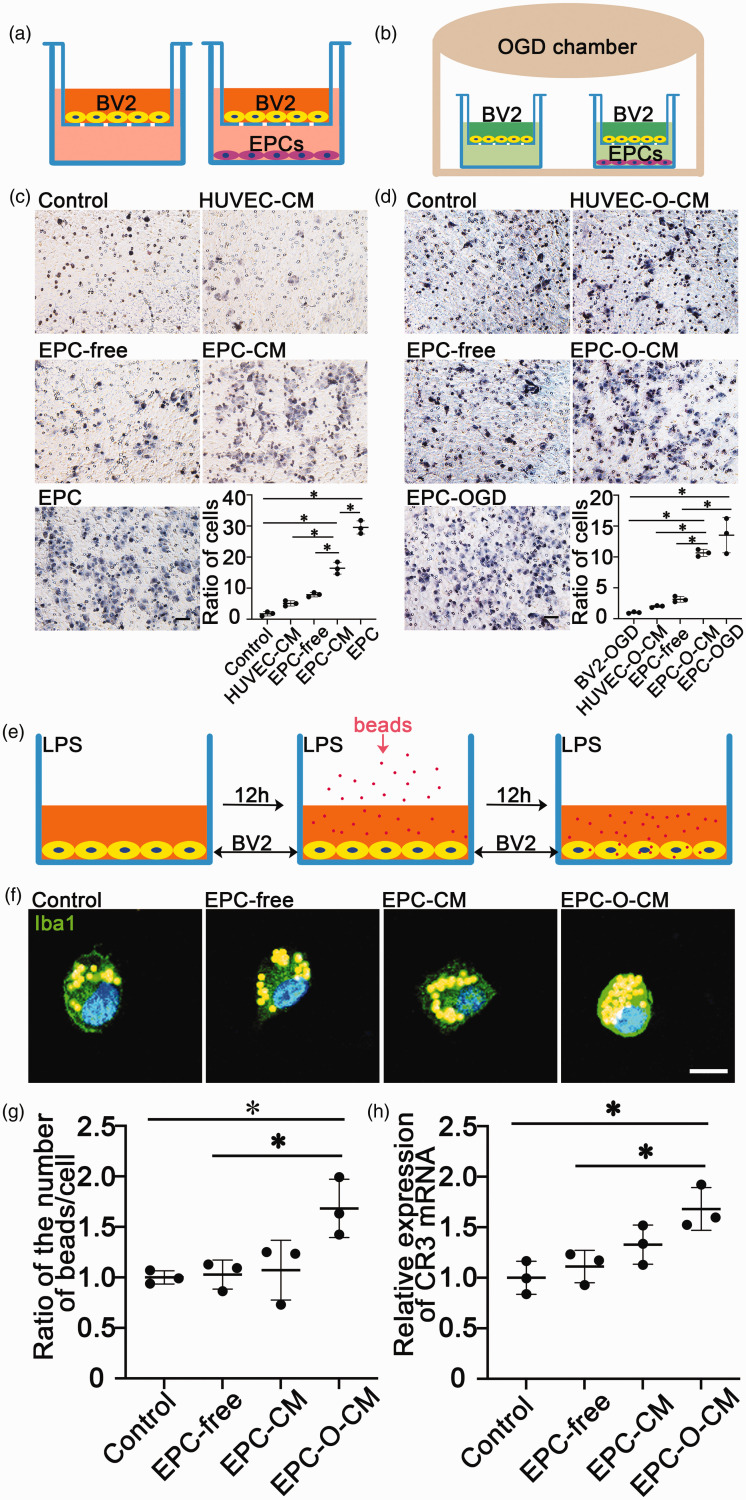
EPC promoted microglial migration and phagocytosis in vitro
To further demonstrate that EPCs could modulate microglial activity, we evaluated microglial migration and phagocytosis after treatment with EPC-CM and EPC-O-CM in vitro. Transwell assays showed that EPC-CM, EPC-O-CM, and EPCs increased the number of migrated microglia under both normal and OGD culture conditions (Figure 4(c) and (d), p < 0.05). These results suggested that EPCs promoted microglial migration. Next, we detected microglial phagocytosis using fluorescent beads treated with EPC-CM and EPC-O-CM under LPS stimulation (Figure 4(e)). The results showed more beads in the cell body of EPC-O-CM-treated microglia (Figure 4(f) and (g), p < 0.05). Real-time PCR showed that EPC-O-CM-treated microglia expressed the highest levels of CR3 mRNA compared to the other groups (Figure 4(h), p < 0.05), confirming that EPCs promoted microglial migration and enhanced microglial phagocytosis associated with upregulating CR3.
Figure 4.
EPC-conditioned medium (EPC-CM) or EPCs promoted microglial migration and phagocytosis. (a&b) Diagram of the transwell assay. BV2 cells were cultured on the transwell in basic culture (Dulbecco's Modified Eagle Medium [DMEM] containing 5% fetal bovine serum [FBS]) medium or in oxygen-glucose deprivation OGD (glucose-free DMEM) conditions in a hypoxic chamber. (c&d) Migrated BV2 cells and quantifications of the number of cells per field among groups. Scale bar = 50 μm. Data from the control group were normalized to 1. Data are shown as mean ± SD. n = 3 per group. *p < 0.01. (e) Diagram of the phagocytosis assay. (f) Images of phagocytized beads (yellow particles) per microglia (green, Iba1) among groups under lipopolysaccharide (LPS) stimulation. Scale bar = 10 μm. (g–h) Quantifications of the number of beads per microglia and the complement receptor 3 (CR3) mRNA of microglia among groups under LPS stimulation. Data from the control group were normalized to 1. Data are shown as mean ± SD. n = 3 per group. *p < 0.05.
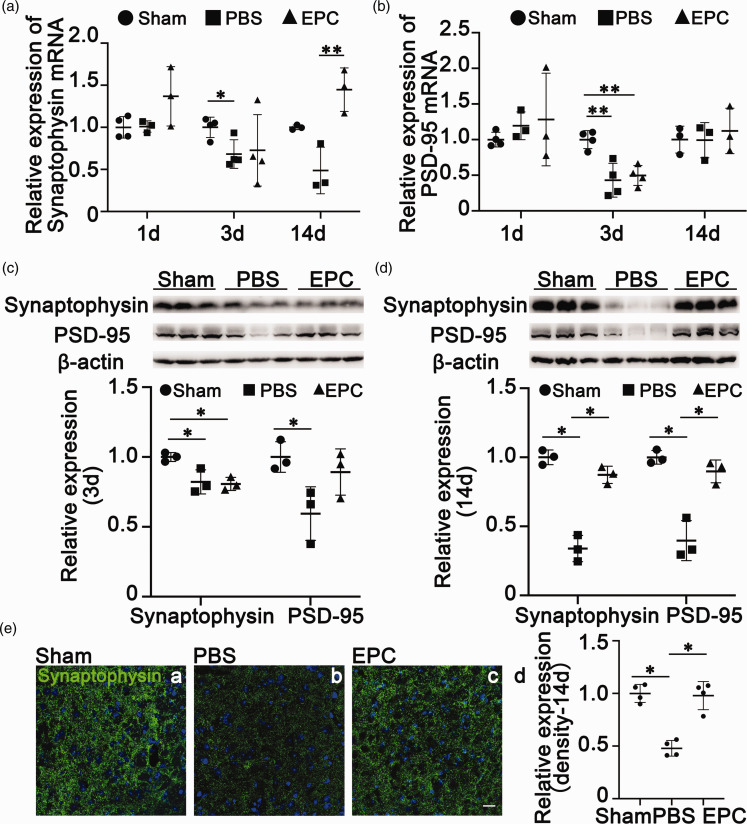
EPC transplantation reduced synaptic loss in the brain after cerebral ischemia
Microglia help synaptic pruning via synapse elimination during nervous system development. 10 To determine whether EPCs enhanced the ability of microglial/macrophage phagocytosis to affect synapse remodeling, we detected synaptophysin and PSD-95 synaptic remodeling genes by real-time PCR. The decreased synaptophysin expression (Figure 5(a), p < 0.05) was not affected by EPC transplantation at 3 days after tMCAO (Figure 5(a), p > 0.05). Synaptophysin expression also showed a decreasing trend at 14 days after tMCAO, while EPC transplantation reversed this tendency and increased synaptophysin expression (Figure 5(a), p < 0.01). EPC transplantation did not affect PSD-95 mRNA expression at 3 and 14 days after tMCAO (Figure 5(b), p > 0.05). Western blotting showed reduced synaptophysin and PSD-95 expression at 3 and 14 days after tMCAO (Figure 5(c) and (d), p < 0.05), while EPC transplantation reversed this decrease (Figure 5(c) and (d), p < 0.05). Immunostaining confirmed reduced synaptophysin levels at 14 days after tMCAO, while EPC transplantation increased synaptophysin expression to a level close to that in the sham group (Figure 5(e), p < 0.05). These results indicated that EPC transplantation reduced synaptic loss during the recovery phase of tMCAO.
Figure 5.
EPC transplantation reduced synaptic loss after transient middle cerebral artery occlusion (tMCAO). (a&b) Quantifications of synaptophysin and postsynaptic density protein 95 (PSD-95) mRNA expression levels in ischemic brains among groups at 1, 3, and 14 days after tMCAO, respectively. The data in the Sham group were normalized to 1. The data are shown as mean ± SD. n = 3–4 per group. *p < 0.05, **p < 0.01. (c&d) Western blots showing levels of synaptophysin and PSD-95 expression in the brain at 14 days after tMCAO. The bar graphs showed the quantifications of synaptophysin and PSD-95 expression levels. The data in the Sham group were normalized to 1. The data are shown as mean ± SD. n = 3 per group. *p < 0.05 and (e) Representative images of synaptophysin immunostaining (green) among groups at 14 days after tMCAO. Scale bar = 20 μm. The bar graphs showed the quantification of the fluorescence intensity of synaptophysin. The data in the Sham group were normalized to 1. The data are shown as mean ± SD. n = 4 per group. *p < 0.05.
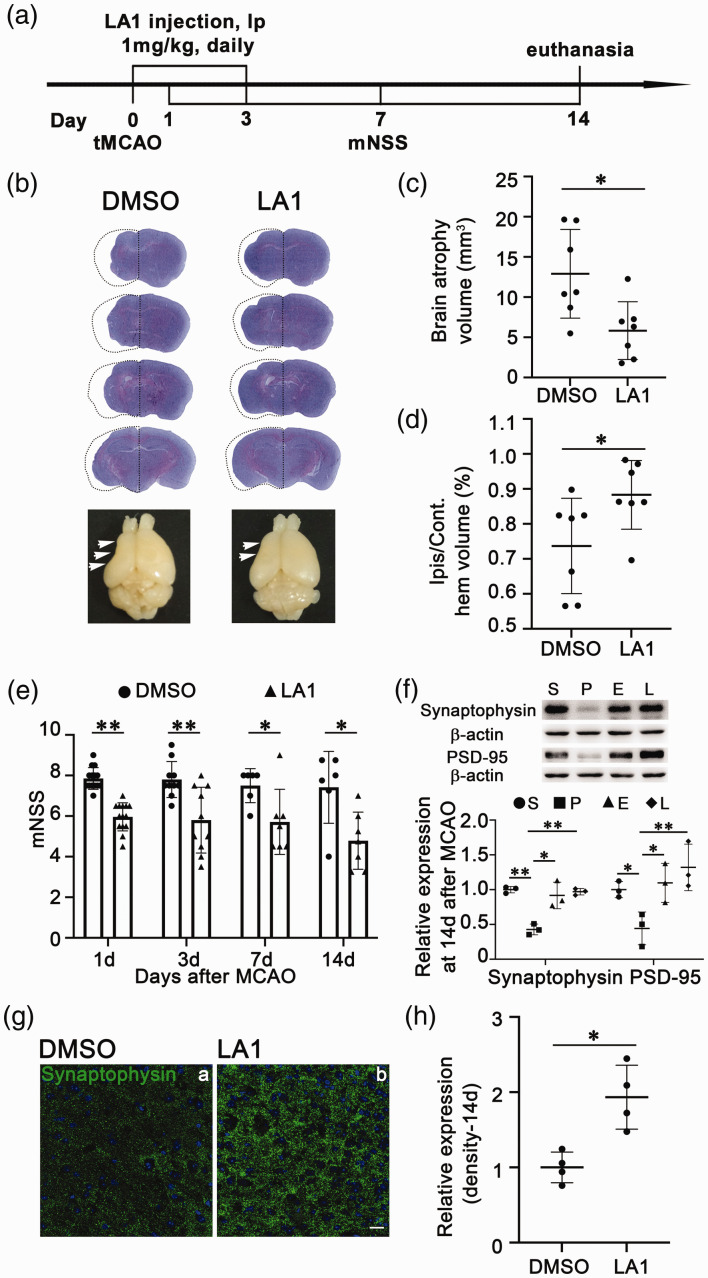
CR3 agonist LA1 reduced neurological deficits and synaptic loss in ischemic mice
To clarify whether EPC transplantation improved neurological function and reduced synaptic loss via CR3 activation of microglia, we intraperitoneally injected LA1 immediately and 3 days after tMCAO. Brain atrophy volume was smaller in the LA1 treated group compared to that in the DMSO control group at 14 days after tMCAO (Figure 6(c) and (d), p < 0.01). To evaluate neurological deficiency after tMCAO, we assessed motor, balance, and reflex functions in mice at 1, 3, 7, and 14 days according to mNSS. The mNSS was lower throughout the examination period in the LA1 treated group compared to that in the control group (Figure 6(e), p < 0.05). These results indicated that the LA1 injection attenuated ischemic brain injury and neurological deficiency. We further observed decreased synaptophysin and PSD-95 expression after tMCAO while EPC treatment reversed this downregulation at 14 days after tMCAO (Figure 6(f), p < 0.05). Synaptophysin and PSD-95 expression was also increased in LA1 injected mice at 14 days after tMCAO (Figure 6(f), p < 0.01). Synaptophysin and PSD-95 expression did not differ significantly between EPC- and LA1-treated mice. Immunostaining confirmed that LA1 injection increased synaptophysin expression to a level close to that in the DMSO control group 14 days after tMCAO (Figure 6(g) and (h), p < 0.05). These results demonstrated that LA1 injection had a similar effect on attenuating brain atrophy volume, neurological deficiency, and synaptic loss after tMCAO compared to EPC transplantation.
Figure 6.
Injection of leukadherin-1 (LA1) reduced brain atrophy, neurological function deficit, and synaptic loss after transient middle cerebral artery occlusion (tMCAO). (a) Flowchart of the experimental design. At 1, 3, and 14 days, mice were euthanized, and their brains were collected for mRNA, protein, and immunostaining. (b) Cresyl violet staining and atrophy for the whole brain. The dashed line showed the original size of the ischemic brain side. (c) Quantification of the atrophy volume between the dimethyl sulfoxide (DMSO) and LA1 groups at 14 days after tMCAO. The data are shown as mean ± SD. n = 7 per group. *p < 0.05. (d) Quantification of the ratios of ipsilateral and contralateral ischemic brain atrophy volumes in the DMSO and LA1 groups. The data are shown as mean ± SD. n = 7 per group. *p < 0.05. (e) Modified neurological severity scores (mNSS) for the DMSO and LA1 groups after tMCAO. The data are shown as mean ± SD. n = 6–14 per group. *p < 0.05, **p < 0.01. (f) Quantification of synaptophysin and postsynaptic density protein 95 (PSD-95) in Western blot. The data from the Sham group were normalized to 1. Data are shown as mean ± SD. n = 3 per group. *p < 0.05, **p < 0.01. S: Sham; P: PBS; E: EPC; L: LA1. (g) Images of synaptophysin immunostaining (green) in the DMSO and LA1 groups at 14 days after tMCAO. Scale bar = 20 μm and (h) Quantification of the fluorescence intensity of synaptophysin. Data from the DMSO group were normalized to 1. Data are shown as mean ± SD. n = 4 per group. *p < 0.05.
Discussion
This study aimed to clarify whether EPC transplantation affects CR3 and regulates microglial/macrophage activity to reduce ischemic brain injury. We demonstrated that EPC transplantation increased microglial/macrophage migration and phagocytosis in the ischemic brain and cultured microglia. EPC transplantation upregulated CR3 expression, which was associated with enhanced microglial/macrophage phagocytosis during the acute stage and reduced synaptic loss during the recovery stage of tMCAO. Furthermore, we demonstrated that the CR3 agonist LA1 mimicked the therapeutic effects of EPCs on diminishing synaptic loss, contributing to alleviating brain injury and ameliorating neurological function after tMCAO.
Iba1 is the most widely used marker to label microglia in the brain and contributes to mediating microglia activity under pathological conditions.23,24 Since it cannot be completely excluded that Iba1 is also staining infiltrating macrophages, the study will refer to microglia/macrophages.
Less invasive delivery routes have been used in experimental stroke models including intra-arterial, intravenous, and intranasal routes, which have potential for clinical translational application.25–27 We chose the route of direct intracerebral injection because the purpose of the study was to prove principle. Our results showed that EPC transplantation reduced TUNEL+ cells and enhanced microglial/macrophage migration and phagocytosis of apoptotic cells during the acute stage of tMCAO. Increasing evidence reported that transplanted EPCs could secrete a series of protective cytokines and growth factors such as VEGF, IGF-1, IL-8, SDF-1, et al. which contributed to cell survival and tissue regeneration after brain ischemic injury.4,5,28 In addition, EPC-CM treatment could protect neurons against OGD-induced cell loss, 23 which indirectly indicated a decrease in neuronal death. Thus, we considered that the reduced TUNEL+ cells after EPC transplantation partly due to reducing cell death after tMCAO. One of the primary functions of microglia/macrophages is to migrate to damaged tissue and phagocytize the debris to limit the inflammatory response and improve late-stage regeneration.29,30 Our previous study also demonstrated that EPC transplantation reduced brain infarct area at the acute stage of tMCAO, which was associated with inhibiting the inflammatory response. 13 In this study, we found that EPC transplantation enhanced the ability of microglial/macrophage phagocytosis of TUNEL+ cells, contributing to functional recovery at the recovery phase of tMCAO. Moreover, this group of microglia/macrophages responsible for phagocytosis was mainly M2 microglia/macrophages, which was also consistent with the function of M2 microglia/macrophages reported in the previous research work. 31 Therefore, we speculated that the enhanced phagocytosis of microglia/macrophages decreased the neuroinflammtion, partially contributing to reduced number of TUNEL+ cells after ischemic injury. In summary, EPC transplantation could attenuate tissue injury and improve neurological function after stroke by multiple pathways and is a potential candidate for clinical translation.
C3 is the core component of complement activation pathways and mainly exerts its functions by binding with C3aR and CR3, two major receptors of C3. 7 Our previous data indicated that EPC transplantation reduced C3 but did not affect C3aR expression after tMCAO. 13 We sought to clarify whether EPC transplantation affected the other crucial receptor of C3, CR3, to promote tissue recovery. We found that CR3 was upregulated in the brain and that EPC transplantation further increased CR3 expression at 3 days after tMCAO, which was associated with enhanced phagocytosis of microglia/macrophages in the ischemic brain. In vitro study showed higher migration and phagocytosis and increased CR3 expression in cultured BV2 microglia cells after EPC-O-CM or EPC treatment during hypoxia. Thus, EPCs modulated microglial/macrophage activity associated with upregulating CR3. However, the use of the BV2 cell line instead of the primary microglial cells is a limitation of the study.
Numerous studies have shown that C3/CR3 signaling mediates microglial clearance of apoptotic neurons, degenerated synapses, and extracellular debris in a variety of neurodegenerative diseases, resulting in protective and beneficial effects.32–36 In cerebral ischemia, the role of C3/CR3 signaling is controversial. In a mouse model of embolic stroke, C3 inhibition suppressed complement-dependent synaptic uptake by microglia, contributing to reduced cognitive decline. 37 However, in a permanent MCAO rat model, the inhibition of CD11b, a subunit of CR3 (CD11b/CD18), with anti-CD11b mAb weakened microglial phagocytosis of myelin debris, resulting in aggravated ischemic brain injury. 38 The results of an in vitro study indicated that CR3 activation in microglia was involved in regulating synaptic activity and plasticity under hypoxia and LPS stimulation conditions. 39 Our study findings supported the notion of CR3 upregulation in microglia under hypoxic conditions and mediation of microglial/macrophage phagocytosis of apoptotic cells during the acute stage of tMCAO. Currently, there are no available specific inhibitors for CR3. We used the CR3 agonist LA1 to clarify whether CR3 activation elicited beneficial effects by affecting microglial/macrophage phagocytosis and synapse remodeling after tMCAO. Synaptophysin and PSD-95 are primary proteins that reflect synapse remodeling.40–42 LA1 injection within 3 days after tMCAO reversed synaptophysin and PSD-95 levels decreased 14 days after cerebral ischemia. Reduced synaptic loss was associated with ischemia-induced brain atrophy and neurological function deficiency 14 days after cerebral ischemia. The results of our study demonstrated that LA1 injection elicited similar protective effects on reducing synaptic loss as EPC transplantation in mice with brain ischemia. In contrast to our expectation, ischemic mice showed no impact on microglial/macrophage phagocytosis at 3 days after LA1 injection (data not shown). We demonstrated that EPC treated mice showed enhanced microglial/macrophage phagocytosis and increased synaptic proteins after tMCAO. The phagocytosis ability of microglia/macrophages was beneficial for the brain tissue recovery. 43 Thus, we believed that synaptic proteins preserved in EPC-treated mice were related to the microglial/macrophage phagocytosis. It is not direct evidence because the specific CR3 inhibitor is not commercially available. Nevertheless, this indirect evidence could partially support those ideas that EPC exerted beneficial effects associated with regulating CR3 expression after cerebral ischemia.
LA1 is a small-molecule allosteric activator of CR3. 44 LA1 activates the subunit CD11b of CR3 by binding to a site distal to the site of ligand binding. 45 LA1 mainly promotes neutrophil adhesion to the inflamed endothelium and restricts further tissue infiltration after injury, exerting anti-inflammatory function. 46 Thus, LA1 treatment could modulate the inflammatory response and decrease tissue injury by modulating macrophage polarization. 47 Both residential microglia and tissue macrophages originate from myeloid cells and share common characteristics. 29 We speculated that LA1 treatment during the acute stage of cerebral ischemia could resolve the acute stroke-induced inflammatory response, contributing to reduced synaptic loss and ischemic brain injury. Whether LA1 treatment affects microglial/macrophage polarization in cerebral ischemia requires further study.
We used LA1 to mimic the effect of EPC transplantation on CR3 activation. We did not observe changes in microglial/macrophage phagocytosis in LA1-injected mice at 3 days after tMCAO, but did observe increased synaptophysin and PSD-95 expression at 14 days after tMCAO, suggesting that LA1 was not useful to assess the direct effect of EPC transplantation on CR3 activation. Some studies used the CD11b antibody to study CR3 function during pathological conditions;44,48 However, blocking CD11b with the CD11b antibody did not affect alteration in the chemotaxis and cytotoxicity of cells expressing CR3, while CD11b activation mimicked CR3 activation induced by pathophysiological conditions. 49 Therefore, the use of CD11b antibody to block and study the role of CR3 in cerebral ischemia is also inappropriate. Much work is needed to develop a CR3-specific and functional agonist to investigate the precise mechanism of EPC transplantation in regulating CR3 and clarify the role of CR3 during cerebral ischemia.
In the brain, CR3 is exclusively expressed on residential microglia to mediate the clearance of debris for tissue remodeling and recovery after ischemic brain injury.7,29 Although the role of CR3 in microglial phagocytosis of injured neurons, damaged tissue, or degenerated myelin has been widely studied in many neurodegenerative diseases, its role in microglia in ischemic stroke remains elusive.11,36,50 We found that EPC transplantation enhanced microglial/macrophage phagocytosis of apoptotic neurons 3 days after cerebral ischemia and upregulated synaptophysin and PSD-95 expression at 14 days after tMCAO. To investigate which receptor mediated microglial/macrophage phagocytosis, we further detected the expression of microglial/macrophage phagocytosis-related genes, including MerTK, MFG-E8, CD68, and CR3. Only CR3 expression was upregulated after EPC transplantation 3 days after tMCAO. Iba1, CR3, and MAP2 colocalization in three-dimensional space showed that CR3 was involved in phagocytizing the debris of damaged neurons. Many circulating cells expressing CR3, including neutrophils, monocytes, eosinophils, natural killer cells, and B and T lymphocytes, can be recruited in the brain after cerebral ischemia resulting from damage to the blood-brain-barrier. 51 Consequently, it is difficult to identify which type of cells was the dominant type with upregulated expression of CR3 and mediated phagocytosis after cerebral ischemia. Residential microglia are mainly responsible for the initial phagocytic response after focal brain ischemia, while most blood-borne macrophages are recruited secondarily to participate in the removal of necrotic tissue. 52 In addition, we transplanted EPCs via direct brain injection, causing a more direct impact on resident brain cells, including microglia, compared to circulating cells. Therefore, EPC transplantation mainly upregulated CR3 expression in resident microglia and activated CR3-mediated phagocytosis of apoptotic cells during the acute stage of cerebral ischemia, creating a less harmful environment for synaptic remodeling and functional recovery after ischemic injury.
In conclusion, we have proposed a novel mechanism for EPC transplantation during MCAO. In this study, EPC transplantation enhanced microglial/macrophage phagocytosis by regulating CR3 expression in the acute stage of tMCAO, contributing to attenuated synaptic loss during the recovery stage of tMCAO. Our results indicated that increasing microglial/macrophage phagocytosis associated with regulating CR3 activity provides a novel therapeutic strategy for cerebral ischemia.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221135841 for Endothelial progenitor cell transplantation attenuates synaptic loss associated with enhancing complement receptor 3-dependent microglial/macrophage phagocytosis in ischemic mice by Yuanyuan Ma, Ze Liu, Lu Jiang, Liping Wang, Yongfang Li, Yanqun Liu, Yongting Wang, Guo-Yuan Yang, Jing Ding and Zhijun Zhang in Journal of Cerebral Blood Flow & Metabolism
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the National Natural Science Foundation of China (81901185, YYM; 81771244, ZJZ; 81771251, GYY; 81974179, ZJZ) and Shanghai Municipal Committee of Science and Technology (Code 2018BR05).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
MYY prepared the figures and wrote the manuscript; LZ performed the immunostaining, helped analyze data and revise the figures; JL performed the animal model and contributed to the analysis of data; WLP and LYQ did the neurological function assessment. LYF helped transplant EPCs into mice and collect brain samples; WYT helped analyze the results; DJ helped instruct the experiment; ZZJ and YGY conceived the study and revised the manuscript.
Supplemental material
Supplemental material for this article is available online.
References
- 1.Paul S, Candelario-Jalil E. Emerging neuroprotective strategies for the treatment of ischemic stroke: an overview of clinical and preclinical studies. Exp Neurol 2021; 335: 113518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet (London, England) 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 3.Kawabori M, Shichinohe H, Kuroda S, et al. Clinical trials of stem cell therapy for cerebral ischemic stroke. IJMS 2020; 21: 7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao YH, Yuan B, Chen J, et al. Endothelial progenitor cells: therapeutic perspective for ischemic stroke. CNS Neurosci Ther 2013; 19: 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen C, Lin X, Wang J, et al. Effect of HMGB1 on the paracrine action of EPC promotes post-ischemic neovascularization in mice. Stem Cells 2014; 32: 2679–2689. [DOI] [PubMed] [Google Scholar]
- 6.Fan Y, Shen F, Frenzel T, et al. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann Neurol 2010; 67: 488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma Y, Liu Y, Zhang Z, et al. Significance of complement system in ischemic stroke: a comprehensive review. Aging Dis 2019; 10: 429–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hickman SE, Kingery ND, Ohsumi TK, et al. The microglial sensome revealed by direct RNA sequencing. Nat Neurosci 2013; 16: 1896–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci 2012; 35: 369–389. [DOI] [PubMed] [Google Scholar]
- 10.Stevens B, Allen NJ, Vazquez LE, et al. The classical complement cascade mediates CNS synapse elimination. Cell 2007; 131: 1164–1178. [DOI] [PubMed] [Google Scholar]
- 11.Schafer DP, Lehrman EK, Kautzman AG, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012; 74: 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tenner AJ, Stevens B, Woodruff TM. New tricks for an ancient system: physiological and pathological roles of complement in the CNS. Mol Immunol 2018; 102: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma Y, Jiang L, Wang L, et al. Endothelial progenitor cell transplantation alleviated ischemic brain injury via inhibiting C3/C3aR pathway in mice. J Cereb Blood Flow Metab 2020; 40: 2374–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Chang S, Li W, et al. cxcl12-engineered endothelial progenitor cells enhance neurogenesis and angiogenesis after ischemic brain injury in mice. Stem Cell Res Ther 2018; 9: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Y, Li Y, Jiang L, et al. Macrophage depletion reduced brain injury following middle cerebral artery occlusion in mice. J Neuroinflammation 2016; 13: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Chopp M, Chen J, et al. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab 2000; 20: 1311–1319. [DOI] [PubMed] [Google Scholar]
- 17.Cai H, Ma Y, Jiang L, et al. Hypoxia response element-regulated MMP-9 promotes neurological recovery via glial scar degradation and angiogenesis in delayed stroke. Mol Ther 2017; 25: 1448–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen CY, Wu RW, Tsai NW, et al. Increased circulating endothelial progenitor cells and improved short-term outcomes in acute non-cardioembolic stroke after hyperbaric oxygen therapy. J Transl Med 2018; 16: 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fadini GP, Baesso I, Albiero M, et al. Technical notes on endothelial progenitor cells: ways to escape from the knowledge Plateau. Atherosclerosis 2008; 197: 496–503. [DOI] [PubMed] [Google Scholar]
- 20.Diaz-Aparicio I, Paris I, Sierra-Torre V, et al. Microglia actively remodel adult hippocampal neurogenesis through the phagocytosis secretome. J Neurosci 2020; 40: 1453–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang LY, Pan J, Mamtilahun M, et al. Microglia exacerbate white matter injury via complement C3/C3aR pathway after hypoperfusion. Theranostics 2020; 10: 74–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao L, Zabel MK, Wang X, et al. Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. EMBO Mol Med 2015; 7: 1179–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwabenland M, Brück W, Priller J, et al. Analyzing microglial phenotypes across neuropathologies: a practical guide. Acta Neuropathol 2021; 142: 923–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lituma PJ, Woo E, O’Hara BF, et al. Altered synaptic connectivity and brain function in mice lacking microglial adapter protein Iba1. Proc Natl Acad Sci USA 2021; 118: e2115539118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dali P, Shende P. Advances in stem cell therapy for brain diseases via the intranasal route. Curr Pharm Biotechnol 2021; 22: 1466–1481. [DOI] [PubMed] [Google Scholar]
- 26.Fang J, Guo Y, Tan S, et al. Autologous endothelial progenitor cells transplantation for acute ischemic stroke: a 4-year follow-up study. Stem Cells Transl Med 2019; 8: 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodríguez-Frutos B, Otero-Ortega L, Gutiérrez-Fernández M, et al. Stem cell therapy and administration routes after stroke. Transl Stroke Res 2016; 7: 378–387. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Ma Y, Miao XH, et al. Neovascularization and tissue regeneration by endothelial progenitor cells in ischemic stroke. Neurol Sci 2021; 42: 3585–3593. [DOI] [PubMed] [Google Scholar]
- 29.Ma Y, Wang J, Wang Y, et al. The biphasic function of microglia in ischemic stroke. Prog Neurobiol 2017; 157: 247–272. [DOI] [PubMed] [Google Scholar]
- 30.Chen W, Zhang Y, Zhai X, et al. Microglial phagocytosis and regulatory mechanisms after stroke. J Cereb Blood Flow Metab 2022; 42: 1579–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao X, Wang H, Sun G, et al. Neuronal interleukin-4 as a modulator of microglial pathways and ischemic brain damage. J Neurosci 2015; 35: 11281–11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Werneburg S, Jung J, Kunjamma RB, et al. Targeted complement inhibition at synapses prevents microglial synaptic engulfment and synapse loss in demyelinating disease. Immunity 2020; 52: 167–182.e7. e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nonaka S, Nakanishi H. Microglial clearance of focal apoptotic synapses. Neurosci Lett 2019; 707: 134317. [DOI] [PubMed] [Google Scholar]
- 34.Norris GT, Smirnov I, Filiano AJ, et al. Neuronal integrity and complement control synaptic material clearance by microglia after CNS injury. J Exp Med 2018; 215: 1789–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu H, Liu B, Frost JL, et al. Complement component C3 and complement receptor type 3 contribute to the phagocytosis and clearance of fibrillar Aβ by microglia. Glia 2012; 60: 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silverman SM, Ma W, Wang X, et al. C3- and CR3-dependent microglial clearance protects photoreceptors in retinitis pigmentosa. J Exp Med 2019; 216: 1925–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alawieh AM, Langley EF, Feng W, et al. Complement-dependent synaptic uptake and cognitive decline after stroke and reperfusion therapy. J Neurosci 2020; 40: 4042–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Wu C, Hou Z, et al. Pseudoginsenoside-F11 accelerates microglial phagocytosis of myelin debris and attenuates cerebral ischemic injury through complement receptor 3. Neuroscience 2020; 426: 33–49. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Malik A, Choi HB, et al. Microglial CR3 activation triggers long-term synaptic depression in the hippocampus via NADPH oxidase. Neuron 2014; 82: 195–207. [DOI] [PubMed] [Google Scholar]
- 40.Rogers JT, Liu CC, Zhao N, et al. Subacute ibuprofen treatment rescues the synaptic and cognitive deficits in advanced-aged mice. Neurobiol Aging 2017; 53: 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlisle HJ, Fink AE, Grant SG, et al. Opposing effects of PSD-93 and PSD-95 on long-term potentiation and spike timing-dependent plasticity. J Physiol 2008; 586: 5885–5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmitt U, Tanimoto N, Seeliger M, et al. Detection of behavioral alterations and learning deficits in mice lacking synaptophysin. Neuroscience 2009; 162: 234–243. [DOI] [PubMed] [Google Scholar]
- 43.Arandjelovic S, Ravichandran KS. Phagocytosis of apoptotic cells in homeostasis. Nat Immunol 2015; 16: 907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dickinson CM, LeBlanc BW, Edhi MM, et al. Leukadherin-1 ameliorates endothelial barrier damage mediated by neutrophils from critically ill patients. J Intensive Care 2018; 6: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Celik E, Faridi MH, Kumar V, et al. Agonist leukadherin-1 increases CD11b/CD18-dependent adhesion via membrane tethers. Biophys J 2013; 105: 2517–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jagarapu J, Kelchtermans J, Rong M, et al. Efficacy of leukadherin-1 in the prevention of hyperoxia-induced lung injury in neonatal rats. Am J Respir Cell Mol Biol 2015; 53: 793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao X, Dong G, Zhu Y, et al. Leukadherin-1-Mediated activation of CD11b inhibits LPS-Induced pro-inflammatory response in macrophages and protects mice against endotoxic shock by blocking LPS-TLR4 interaction. Front Immunol 2019; 10: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmid MC, Khan SQ, Kaneda MM, et al. Integrin CD11b activation drives anti-tumor innate immunity. Nat Commun 2018; 9: 5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsikitis VL, Morin NA, Harrington EO, et al. The lectin-like domain of complement receptor 3 protects endothelial barrier function from activated neutrophils. J Immunol 2004; 173: 1284–1291. [DOI] [PubMed] [Google Scholar]
- 50.Rotshenker S. Microglia and macrophage activation and the regulation of complement-receptor-3 (CR3/MAC-1)-mediated myelin phagocytosis in injury and disease. JMN 2003; 21: 65–72. [DOI] [PubMed] [Google Scholar]
- 51.Lamers C, Plüss CJ, Ricklin D. The promiscuous profile of complement receptor 3 in ligand binding, immune modulation, and pathophysiology. Front Immunol 2021; 12: 662164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schroeter M, Jander S, Huitinga I, et al. Phagocytic response in photochemically induced infarction of rat cerebral cortex. The role of resident microglia. Stroke 1997; 28: 382–386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221135841 for Endothelial progenitor cell transplantation attenuates synaptic loss associated with enhancing complement receptor 3-dependent microglial/macrophage phagocytosis in ischemic mice by Yuanyuan Ma, Ze Liu, Lu Jiang, Liping Wang, Yongfang Li, Yanqun Liu, Yongting Wang, Guo-Yuan Yang, Jing Ding and Zhijun Zhang in Journal of Cerebral Blood Flow & Metabolism