Abstract
Nodular fasciitis (NF) is a benign soft tissue lesion that can occur anywhere in the body. Its occurrence in the breast is a rare phenomenon, but is clinically important to distinguish from a malignant tumor as they both present as lesions of the breast. In this report, we discuss a case of NF of the breast in an postmenopausal woman who presented with an asymmetry of the breast on an annual screening mammogram followed by diagnostic imaging and a core biopsy. Ultimately, excision of the lesion (1.2 cm) was the definitive treatment for this patient and histological evaluation confirmed the diagnosis of NF. Additionally, we review the most recent literature on this topic discussing the significance to better understand the characteristics and best treatment course for breast NF. Results from a review of 11 breast NF cases demonstrated that the average age at diagnosis is 49, the mean diameter of lesions was 1.33 cm, and lesions were more frequently identified in the right upper breast. The clinical features of breast NF may present similarly to that of a malignant tumor. Accurate diagnosis with immunohistochemistry staining or USP6 FISH analysis is critical to prevent misdiagnosis and overtreatment. Clinician awareness, surgical treatment, and patient education are important for best management of breast NF.
Keywords: Nodular fasciitis, Subcutaneous pseudosarcomatous fasciitis, Benign breast lesion, Spindle-cell lesion
Background
Nodular (pseudosarcomatous) fasciitis (NF) is a rare benign soft tissue lesion that was first reported in 1955 by Konwaler as a circumscribed, reactive, and subcutaneous tumor-like lesion [1]. A classical case of NF would present as either a 30-50-year-old male or female with a relatively small (<4 mm), possibly tender and fast growing lesion on either the arms, head and neck, trunk, or legs [2], [3], [4]. Although it can present at any age and develop in soft tissue anywhere in the body, its prevalence is not well known and available data are unreliable with 2 reports documenting it to be 0.8% and 11% [5,6]. Regardless, it is clinically important since NF shares similar clinicopathological characteristics as soft tissue sarcoma and can potentially be misdiagnosed [7,8].
NF in the breast is rare with an estimated 28 cases (1970-2015) being reported [9]. However, it is a known diagnostic entity acknowledged by the World Health Organization in 2012 as a benign mesenchymal breast tumor and should be included in the differential [10]. Due to its rarity and ambiguity, it can be difficult to diagnose and may be overlooked in older patients as the prevalence is higher in younger to middle aged adults. This report describes a case of breast NF in a postmenopausal woman and discusses a review of recent literature.
Case presentation
A 68-year-old African American female presented to the clinic for a surgical consultation following an abnormality found on her annual bilateral mammogram screening (Fig. 1A) which revealed a focal asymmetry at the right upper breast seen best on mediolateral oblique view. The right breast BI-RADS was category 0, which prompted additional imaging and left breast BI-RADS was category 2. The breast tissues were heterogeneously dense. The patient has not had any abnormal screenings nor any breast problems in the past and has a stable benign lesion in the left breast. She denies any trauma in the recent past, breast pain, nipple discharge, or skin changes. Physical examination revealed no palpable masses, tenderness, or other abnormalities except for an asymmetry of the breasts, left breast smaller than right which is her baseline.
Fig. 1.
Mammogram imaging of upper right breast. (A) Mammogram screening shows a focal asymmetry best seen on MLO view. (B) Diagnostic mammogram imaging with spot compression confirms screening with persisting asymmetry within the superior right breast.
The patient's current medical conditions include chronic kidney disease stage 3 with secondary hyperparathyroidism, hypertension, hyperlipidemia, irritable bowel syndrome with a history of gout. The patient has no family history of breast cancer but her father, 2 paternal aunts, and a paternal uncle all have a history of colon cancer. To her knowledge, no genetic testing has been done in her family. She is postmenopausal, has no children, and is a former smoker.
Follow up diagnostic (Fig. 1B) and ultrasound imaging (Figs. 2A and B) of the right breast revealed a complex cyst at the upper breast 12:00/8 cm measuring 0.3 × 0.8 × 0.2 cm and an adjacent benign simple cyst at 12:00/8 cm. The BI-RADS category was 4A, low suspicion for malignancy. To follow, an ultrasound-guided biopsy of the complex cyst was performed. Histopathology of the core biopsy tissue showed hypocellular proliferation of bland spindled cells admixed with a collagenous and focally myxoid stroma (Figs. 3A and B). No breast glandular parenchyma was present. Immunohistochemical staining showed that the cells were cytokeratin 5/6 (−) and p63 (−). The spindle cells were also negative for nuclear beta-catenin staining but positive for cytoplasmic beta-catenin staining. Based on this information, differential diagnoses at this stage included NF, and less likely fibromatosis, myofibroblastoma, and other reactive/reparative spindle cell proliferations.
Fig 2.
Ultrasonography imaging of the upper right breast. (A) Ultrasound findings show a complex cyst (left) at 12:00/8 cm measuring 0.3 × 0.8 × 0.2 cm. Radial view of complex cyst. (B) Antiradial view of complex cyst.
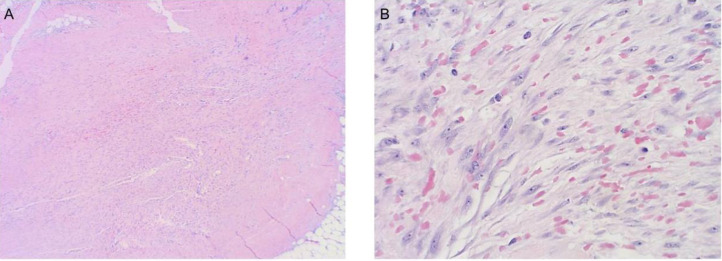
Fig. 3.
Histology images of core biopsy tissue. (A) Low power view showing the fascicular arrangement of the bland spindle cells within the fibroadipose tissue with extravasated red blood cells. (B) High power view showing bland spindle cells within a background of myxoid stroma, extravasated red blood cells and rare lymphocytes.
However, the exclusion of malignancy could not be definitively made based on the biopsy report and the patient was recommended for surgical excision of the lesion for examination of the entire lesion. At this time, the patient and her husband were quite anxious about the possibility of the lesion being malignant. A right breast excision was successfully performed with negative margins. The final pathology report confirmed the diagnosis of NF (1.2 cm). Microscopic evaluation of the sample revealed cells that are beta-catenin (+) cytoplasm, beta-catenin (−) nuclear, p63 (−), CK AE1/AE3 (−), and SMMS-1 (−).
Discussion
NF is a rare benign soft tissue lesion made up of fibroblastic/myofibroblastic cells that arises in subcutaneous tissue. It has a self-limiting course that either spontaneously resolves or can be surgically excised with little to no risk of recurrence [11]. NF in the breast is a rare occurrence but over the past 3 decades, it has increasingly been recognized as clinically important to distinguish from breast cancer to avoid misdiagnosis and overtreatment [12]. In this report, we discuss a case of breast NF (0.8 cm by ultrasound, 1.2 cm by excision) in a 68-year-old female which was initially identified as a focal asymmetry on annual screening mammography.
Epidemiology
The most up-to-date literature on breast NF is summarized in a comprehensive review by Paliogiannis et al. [9] which discussed findings from 22 cases in January 1970 to October 2015. Additionally, in an analysis of 272 samples (2004-2014), the largest series of NF to date, 9 cases in the breast were identified making up 3.3% of the cases [4]. To assess the most recent literature on breast NF, we conducted a search on PubMed from November 2015 to July 2022 which resulted in a list of 30 cases included in 10 articles as seen in Table 1. Studies by Zhang et al. and Cloutier et al. were excluded in the following analysis as disaggregated data was inaccessible and the remaining 11 cases were included in the review [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]. All patients were women and the mean age was 49 years (29-88). Two-thirds of patients denied any pain or tenderness in 10 cases and no patients reported any history of trauma in 9 of the cases. The mean diameter of the lesion computed during imaging scans was 1.33 cm (0.5-3.2) in 11 patients. Location of the lesions were more frequently seen in the right (n = 7, 63.6%) vs left (n = 4, 36.4%) breast and occurred in the following regions: upper (n = 5) > lower (n = 3) > inner=outer=central (n = 1 each). The patient in our case was older than the average age and had a smaller lesion on imaging than the average diameter in our review; however, the location of the lesion was congruent with what was reported.
Table 1.
Demographic and clinical characteristics breast nodular fasciitis cases (November 2015-July 2022).
| Case | Ref | Age (years) | Pain | Trauma | Size (cm) | Location | Immunohistochemistry staining (unless noted otherwise) | Treatment |
|---|---|---|---|---|---|---|---|---|
| 1 | Kang et al. [13] | 48 | Yes | No | 3.2 (US) | R-UO | CYTOLOGY*Positive: smooth muscle actin, smooth muscle myosin and calponin Negative: AE1/AE3, CAM5.2, S100, caldesmon, desmin, myogenin, CD31 FISH USP6 (18%, cut off 9-10%) |
FNA, Spontaneously resolved |
| 2 | Moghimi et al. [14] | 43 | No | - | 1 (US) | L-UO | Diagnosis based off of histology | Excision biopsy |
| 3 | Hayashi et al. [12] | 88 | - | No | 1.8 (US) | R-LO |
Positive: α-smooth muscle actin (α-SMA) and vimentin Negative: pancytokeratin, cytokeratin 14, estrogen receptor, progesterone receptor, β-catenin, cluster of differentiation 34 (CD34), desmin, S100, and synaptophysin |
Excision biopsy |
| 4 | Knight et al. [15] | 69 | Yes | No | 0.8 (US) | L-inner |
Positive: smooth muscle actin Negative: multiple CK antibodies |
Core biopsy, observation (3years with no progression) |
| 5 | Erinanc et al. [16] | 48 | Yes | No | 1.3 (US) | L-central |
Positive: smooth muscle actin and vimentin Negative: desmin, S100, CD34 |
Excision biopsy |
| 6 | Shimizu et al. [17] | 53 | No | - | 0.5 (US) | R-upper | Diagnosis based off of histology | Excision biopsy |
| 7 | Zhang et al. [18] (7 cases) |
15-51 (mean 36) | - | No | 1-3.5cm | Upper x4 Lower x2 Axillary x1 |
Positive: smooth muscle actin (SMA, 6/6), CD10 (2/3) Negative: desmin, β-catenin, CD34, extended-spectrum cytokeratin (CKpan), epithelial membrane antigen (EMA), S- 100 protein, p63 and ALK-1 Ki-67: positive index 5% to 15% FISH USP6 gene rearrangement was found in 6 cases MYH9-USP6 gene fusion was detected in 2 cases |
Surgical resection (6 cases) Core biopsy, spontaneously resolved (1 case) |
| 8 | Cloutier et al. [19] (12 cases) |
15-61 (median 32) | - | - | 0.4-5.8cm (median 0.8) | - |
Positive: SMA Negative: for pan-cytokeratin, p63, desmin, CD34, and nuclear beta-catenin Targeted RNA sequencing performed on 11 cases identified (USP6 gene fusions in 8/11) Break-apart USP6 FISH (1 case) MYH9-USP6 rearrangement (4 cases) |
5/12 elected observation, self-resolution (3/5) |
| 9 | Zhao et al. [20] (2 cases) |
29 | No | No | 0.8 (US) | L-UO |
Positive: SMA, CD10 Negative: CK5/6, AE1/3, p63, desmin, S100, beta-catenin (nuclear) FISH USP6 (23%, cutoff 15%) |
Excision biopsy |
| 55 | No | No | 1.1 (MRI) | R-LI |
Positive: SMA, CD10 Negative: CK5/6, AE1/3, p63, desmin, S100, beta-catenin (nuclear) FISH USP6 (25%, cutoff 15%) |
Excision Biopsy | ||
| 10 | Lin et al. [21] (3 cases) |
43 | No | No | 1 (US) | R-UI | - | Excision Biopsy |
| 37 | No | No | 2.2 (ABUS) | R-LI | - | Core biopsy, Surgical Excision | ||
| 29 | No | No | 0.9 (ABUS) | R-outer | - | Core biopsy, Surgical excision |
ABUS, automated breast ultrasound screening; FNA, fine-needle aspiration; L, left; LI, lower inner; LO, lower outer; R, right; UI, upper inner; UO, upper outer; US, ultrasound.
All patients are female.
Diagnosis
Breast NF has a heterogeneous clinicopathologic course, but is commonly diagnosed on histology as α-smooth muscle actin-positive spindle cells in fibro-myxoid stroma with a tissue culture-like appearance [22]. It can be distinguished from other spindle cell lesions of the breast using immunohistological stains. In our case, a negative nuclear beta-catenin staining excluded desmoid-type fibromatosis, negative SMMS-1 excluded sclerosing adenosis, and negative CK5/6, CK AE1/AE3, and p63 staining decreased the likelihood of a malignant diagnosis. Other stains utilized among cases in our analysis include a negative stain for ER, desmin, and CD34 to rule out myofibroblastoma and negative staining for S-100 to rule out schwannoma/neurofibromas. Additionally, a positive finding of USP6 gene rearrangement with fluorescence in situ hybridization can further support the diagnosis of NF, which is a relatively recent method introduced [23]. A USP6 fluorescence in situ hybridization test was utilized in 5 papers listed in Table 1.
Treatment
Despite the nature of breast NF to spontaneously resolve and have an extremely low recurrence rate, surgical excision is still commonly performed to exclude the possibility of malignancy and for curative treatment. Majority of cases (n = 7, 63.3%) in our review used excisional biopsy as their treatment of choice with only 3 cases performing core biopsies (2/3 followed with surgical excision), and 1 with fine-needle aspiration. In this report, a core biopsy was performed initially due to the suspicion for malignancy rather than a benign lesion. In the case of a benign lesion, an excisional biopsy may be preferred to reduce the amount of procedures performed and undue anxiety and pain for the patient.
Conclusion
Breast NF is a rare and benign lesion that commonly presents in middle aged females. It is often fast growing, may or may not be associated with pain or history of trauma and can present similarly to malignant and nonmalignant pathologies making it an important differential to keep in mind. Since the World Health Organization classification of breast NF along with the emerging reports of breast NF cases, the number of diagnoses will inadvertently increase. The understanding and awareness of the epidemiology, clinical and histopathological course, and most effective treatment for breast NF will allow clinicians to be more equipped moving forward to manage and counsel patients appropriately on a historically challenging diagnosis.
Patient consent
Written informed consent was obtained for publication of this case report. A copy is available for review by the editorial office of this journal.
Footnotes
Competing Interests: The authors have no conflicts of interest to declare.
References
- 1.Konwaler BE, Keasbey L, Kaplan L. Subcutaneous pseudosarcomatous fibromatosis (fasciitis) Am J Clin Pathol. 1955;25(3):241–252. doi: 10.1093/ajcp/25.3.241. [DOI] [PubMed] [Google Scholar]
- 2.Meister P, Bückmann FW, Konrad E. Nodular fasciitis (analysis of 100 cases and review of the literature) Pathol Res Pract. 1978;162(2):133–165. doi: 10.1016/S0344-0338(78)80001-6. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu S, Hashimoto H, Enjoji M. Nodular fasciitis: an analysis of 250 patients. Pathology. 1984;16(2):161–166. doi: 10.3109/00313028409059097. [DOI] [PubMed] [Google Scholar]
- 4.Lu L, Lao IW, Liu X, Yu L, Wang J. Nodular fasciitis: a retrospective study of 272 cases from China with clinicopathologic and radiologic correlation. Ann Diagn Pathol. 2015;19(3):180–185. doi: 10.1016/j.anndiagpath.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Kransdorf MJ. Benign soft-tissue tumors in a large referral population: distribution of specific diagnoses by age, sex, and location. AJR Am J Roentgenol. 1995;164(2):395–402. doi: 10.2214/ajr.164.2.7839977. [DOI] [PubMed] [Google Scholar]
- 6.Ramnani BG, Kumar A, Chandak S, Ranjan A, Patel MK. Clinicopathological profile of benign soft tissue tumours: a study in a tertiary care hospital in Western India. J Clin Diagn Res. 2014;8(10):FC01–FC04. doi: 10.7860/JCDR/2014/8690.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khanna V, Rajan M, Reddy T, Alexander N, Surendran P. Nodular fasciitis mimicking a soft tissue sarcoma—a case report. Int J Surg Case Rep. 2018;44:29–32. doi: 10.1016/j.ijscr.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rani D, Gupta A. Cytological diagnosis and misdiagnosis of nodular fasciitis. J Cytol. 2019;36(4):196–199. doi: 10.4103/JOC.JOC_112_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paliogiannis P, Cossu A, Palmieri G, Scognamillo F, Pala C, Nonnis R, et al. Breast nodular fasciitis: a comprehensive review. Breast Care (Basel) 2016;11(4):270–274. doi: 10.1159/000448185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan PH, Ellis IO. Myoepithelial and epithelial-myoepithelial, mesenchymal and fibroepithelial breast lesions: updates from the WHO classification of tumours of the breast 2012. J Clin Pathol. 2013;66(6):465–470. doi: 10.1136/jclinpath-2012-201078. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein KE, Lattes R. Nodular (pseudosarcomatous) fasciitis, a nonrecurrent lesion: clinicopathologic study of 134 cases. Cancer. 1982;49(8):1668–1678. doi: 10.1002/1097-0142(19820415)49:8<1668::aid-cncr2820490823>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi S, Yasuda S, Takahashi N, Okazaki S, Ishibashi K, Kitada M, et al. Nodular fasciitis of the breast clinically resembling breast cancer in an elderly woman: a case report. J Med Case Rep. 2017;11(1):57. doi: 10.1186/s13256-017-1219-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang A, Kumar JB, Thomas A, Bourke AG. A spontaneously resolving breast lesion: imaging and cytological findings of nodular fasciitis of the breast with FISH showing USP6 gene rearrangement. BMJ Case Rep. 2015;2015 doi: 10.1136/bcr-2015-213076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moghimi M, Yazdian Anari P, Vaghefi M, Meidany A, Salehi H. Nodular fasciitis of the breast. Iran J Radiol. 2016;13(1):e18774. doi: 10.5812/iranjradiol.18774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knight JA, Hunt KN, Carter J. Nodular fasciitis of the breast in an elderly woman. Radiol Case Rep. 2017;12(4):642–644. doi: 10.1016/j.radcr.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erinanc H, Türk E. The rare benign lesion that mimics a malignant tumor in breast parenchyma: nodular fasciitis of the breast. Case Rep Pathol. 2018;2018 doi: 10.1155/2018/1612587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimizu TK, Gibbs P, Orrison S, Ma A. Nodular fasciitis of the breast. Breast J. 2020;26(5):1054–1055. doi: 10.1111/tbj.13734. [DOI] [PubMed] [Google Scholar]
- 18.Zhang YH, Qiu Y, Zhang Z, Chen HJ, Zhang HY. Nodular fasciitis of the breast: a clinicopathological and genetic analysis of seven cases. Zhonghua Bing Li Xue Za Zhi. 2021;50(5):476–481. doi: 10.3760/cma.j.cn112151-20201230-00989. Chinese. [DOI] [PubMed] [Google Scholar]
- 19.Cloutier JM, Kunder CA, Charville GW, Hosfield EM, García JJ, Brown RA, et al. Nodular fasciitis of the breast: clinicopathologic and molecular characterization with identification of novel USP6 fusion partners. Mod Pathol. 2021;34(10):1865–1875. doi: 10.1038/s41379-021-00844-4. [DOI] [PubMed] [Google Scholar]
- 20.Zhao M, Yin X, Wen Y, Ru G, Meng X. Nodular fasciitis of the breast: report of two cases illustrating the diagnostic implications for USP6 gene rearrangement and brief review of the literature. Exp Mol Pathol. 2021;123 doi: 10.1016/j.yexmp.2021.104690. [DOI] [PubMed] [Google Scholar]
- 21.Lin W, Bao L. Nodular fasciitis of the breast: the report of three cases. BMC Womens Health. 2022;22(1):54. doi: 10.1186/s12905-022-01631-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magro G, Salvatorelli L, Puzzo L, Piombino E, Bartoloni G, Broggi G, et al. Practical approach to diagnosis of bland-looking spindle cell lesions of the breast. Pathologica. 2019;111(4):344–360. doi: 10.32074/1591-951X-31-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erickson-Johnson MR, Chou MM, Evers BR, Roth CW, Seys AR, Jin L, et al. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91(10):1427–1433. doi: 10.1038/labinvest.2011.118. [DOI] [PubMed] [Google Scholar]