Abstract
Environmental causes of cardiovascular diseases (CVDs) are global health issues. In particular, an association between metal exposure and CVDs has become evident but causal evidence still lacks. Therefore, this symposium at the Society of Toxicology 2022 annual meeting addressed epidemiological, clinical, pre-clinical animal model-derived and mechanism-based evidence by five presentations: 1) An epidemiologic study on potential CVD risks of individuals exposed occupationally and environmentally to heavy metals; 2) Both presentations of the second and third were clinical studies focusing on the potential link between heavy metals and pulmonary arterial hypertension (PAH), by presenting altered blood metal concentrations of both non-essential and essential metals in the patients with PAH and potential therapeutic approaches; 3) Arsenic-induced atherosclerosis via inflammatory cells in mouse model; 4) Pathogenic effects on the heart by adult chronic exposure to very low-dose cadmium via epigenetic mechanisms and whole life exposure to low dose cadmium via exacerbating high-fat-diet-lipotoxicity. This symposium has brought epidemiologists, therapeutic industry, physicians, and translational scientists together to discuss the health risks of occupational and environmental exposure to heavy metals through direct cardiotoxicity and indirect disruption of homeostatic mechanisms regulating essential metals, as well as lipid levels. The data summarized by the presenters infers a potential causal link between multiple metals and CVDs and defines differences and commonalities. Therefore, summary of these presentations may accelerate the development of efficient preventive and therapeutic strategies by facilitating collaborations among multidisciplinary investigators.
Keywords: Epidemiological evidence, Metal toxicities, Cardiovascular diseases, Pulmonary hypertension, arsenics, cadmium
1. Introduction
Cardiovascular diseases (CVDs) are the leading cause of death worldwide and they disproportionally affect people living in different communities and environments. Each year, the American Heart Association (AHA), in conjunction with the National Institutes of Health and other government agencies, brings together in a single document of the most up-to-date statistics related to heart disease, stroke, and cardiovascular risk factors (Tsao et al., 2022). CVDs produce immense health and economic burdens in the United States (U.S.) and globally. In terms of economic costs of CVDs, the average annual direct and indirect cost of CVDs in the U.S. was about $378.0 billion from 2017 to 2018; the estimated direct costs of CVDs in the U.S. increased from $103.5 billion from 1996 to 1997 to $226.2 billion from 2017 to 2018. CVDs accounted for the highest direct cost ($99.6 billion) from 2017 to 2018 in inpatient hospital stays. Globally, in 2020, about 19 million deaths were attributed to CVDs, which amounted to an increase of 18.7% from 2010. In the Cardiovascular Lifetime Risk Pooling Project including 30,447 participants from seven U.S. cohort studies, among individuals ≥60 years of age with low cardiovascular health (CVH), the 35-year risk of CVDs was the highest in white males (65.5%), followed by white females (57.1%), black females (51.9%), and black males (48.4%) (Tsao et al., 2022). In the AHA’s My Life Check–Life’s Simple 7, the CVD risk factors include core health behaviors (smoking, physical activity, diet, and weight) and health factors (cholesterol, blood pressure (BP), and glucose control) (Tsao et al., 2022). Except for these seven major risk factors, social determinants of health (SDoH), which encompass the economic, social, environmental, and psychosocial factors that influence health, also play significant roles in the development of CVD risk factors, particularly for the disparate prevalence among different populations (Althoff et al., 2022; Islam et al., 2022; Jilani et al., 2021; Powell-Wiley et al., 2022).
In recent decades, observational and experimental research on risks for CVDs have increased regarding exposures to environmental and occupational toxicants including heavy metals such as inorganic arsenic (IAs), cadmium (Cd), and lead (Pb). Globally, heavy metals are found in all environmental systems (air, ground and surface water, soil/sediment, and particulate matters) and have been documented at elevated levels in certain occupational settings (smelting, metal manufacturing, and mining) (OSHA, 2022.). Observational research has evaluated environmental and occupational exposures to heavy metals as a risk factor for CVDs via multiple routes including inhalation and ingestion, which has been supported by experimental mechanistic models in animal and molecular studies. Recent research has expanded into metals and examined the cardiotoxic effects of exposure to multiple metals that typically co-exist in environmental systems or occupational settings (Guo et al., 2022; Zhang et al., 2016). In the largest global study of heavy metals exposure and CVDs (37 countries), findings demonstrated that exposure to IAs, Pb, and Cd was directly associated with increased CVD incidence and mortality in a dose-response manner (Chowdhury et al., 2018; Nigra et al., 2016).
This symposium provides an update on observational and experimental findings for heavy metal exposures and risks for CVDs. The summary is divided into four parts including: i) a review on the associated risk for CVDs from exposures to heavy metals in the environmental and occupational settings; ii) new clinical findings from pilot studies on metal concentrations and risks for pulmonary arterial hypertension (PAH), and iii) new insight on experimental animal models to address arsenic (As)-induced atherosclerosis with its underlying mechanisms, iv) Cd (Cd)-induced cardiac toxicity. This summary brings together current and new science that infers a causal relationship between chronic heavy metal exposures and CVDs, thus identifying potential sources for clinical and public health interventions to reduce CVD risk by mitigating exposures to heavy metals.
2. Epidemiology evidence of the association between exposure to environmental metals and CVDs
2.1. Update for the general status
Heavy metal(oids) (herein: metals), such as As, Pb, Cd, and tungsten (W), are naturally occurring and found globally in environmental matrices including groundwater, surface water, soil and sediment, and ambient air. Environmental exposure to heavy metals especially As, Cd, and Pb have been associated with CVDs in observational studies and further evidenced through mechanistic investigations in experimental studies. Suggested mechanisms of cardiotoxicity due to exposure to As, Cd, Pb, or W include oxidative stress and lipid peroxidation (Ercal et al., 2001; Pi et al., 2002; Wasel and Freeman, 2018; Wu et al., 2001) inflammation (As, Cd, Pb) (Cortes et al., 2021; Moon et al., 2012; Tellez-Plaza et al., 2013), platelet aggregation (As, Cd)(Kumar and Bhattacharya, 2000; Lee et al., 2002; Moon et al., 2012; Tellez-Plaza et al., 2013), endothelial dysfunction and promotion of atherosclerosis [As (Lee et al., 2003), Cd (Tellez-Plaza et al., 2013), W (Nigra et al., 2018)], vascular smooth muscle cell dysfunction (As (Moon et al., 2012) and Cd (Tellez-Plaza et al., 2013), and inactivation of nitric oxide (As, Cd and Pb)(Kao et al., 2003; Moon et al., 2012; Pineda-Zavaleta et al., 2004; Tellez-Plaza et al., 2013).
Over the past century, industrialization has increased human activities such as mining, smelting, agriculture, and manufacturing, leading to increased levels of metals in environmental systems and thus increased exposure in humans (Bradl et al., 2005; Chowdhury et al., 2018; Nigra et al., 2018; Tchounwou et al., 2012). Observational studies have shown an increased risk for CVDs at low-moderate levels commonly found throughout the world. The extent of human exposure and increase in levels found in environmental systems creates a growing concern on the impact on CVD health in potentially millions of people. Evidence has identified independent risk for CVDs and related subclinical outcomes associated with As exposure (Abhyankar et al., 2012; Bhatnagar, 2006; Domingo-Relloso et al., 2019; James et al., 2015; Lahm et al., 2018; Moon et al., 2013; Shiue and Hristova, 2014), Cd (Agarwal et al., 2011; Franceschini et al., 2017; Hassoun, 2021; Navas-Acien et al., 2004; Rich et al., 1987; Ruopp and Cockrill, 2022; Simonneau et al., 2019; Tellez-Plaza et al., 2008), Pb (Agarwal et al., 2011; Hassoun, 2021; Navas-Acien et al., 2007; Weisskopf et al., 2009), and W (Agarwal et al., 2011; Nigra et al., 2018; Tchounwou et al., 2012; Wasel and Freeman, 2018). These metals are also known to co-occur in environmental systems potentially increasing cardiotoxic effects due to exposure. Therefore, following are the examples for some metals.
2.1.1. Arsenic
Arsenic is found throughout the world with the highest natural levels occurring in areas with historical or current volcanic activity (ATSDR, 2016). Arsenic is additionally released into the environment through industrial emissions, mining, and smelting. Arsenic can exist in multiple forms in the environment, with the inorganic forms (arsenate and arsenite) being the most concerning for CVDs risk (Chen et al., 2011; Chen et al., 2013; Farzan et al., 2015; James et al., 2015; Jomova et al., 2011; Xu et al., 2020). Exposure to inorganic As (IAs) is most common through drinking water (ATSDR, 2016; Cosselman et al., 2015; Naujokas et al., 2013), diet, and in certain industrial areas, ambient air (ATSDR, 2016; Navas-Acien and Nachman, 2013; Schmidt, 2014, 2015). Exposure to IAs in ambient air can range from 40 nanograms in rural non-industrialized areas to up to 600 nanograms in industrial areas (WHO, 2000). Inhaled IAs can contribute to the overall exposure however ingestion has been the main route of exposure of focus in research. IAs exposure through the diet may exceed the minimum risk levels when IAs-containing foods are independently consumed in high amounts or in combination (Brandon et al., 2014; Gundert-Remy et al., 2015; Mantha et al., 2017; Nigra et al., 2017; Sobel et al., 2020). The largest contribution of IAs from the diet is from rice and grains grown in contaminated water or soil (Gundert-Remy et al., 2015; Nigra et al., 2017). Seafood also contains As however predominantly in the less toxic organic forms of As (Brandon et al., 2014; Gundert-Remy et al., 2015; Mantha et al., 2017). Limited studies have suggested the contribution of IAs in diet to CVD risk, however the evidence is inconclusive due to the lower gastrointestinal absorption rate (10–35%) compared to IAs ingested in drinking water (80–90%) (Council, 1999; Karagas et al., 2019; Misbahuddin, 2003; Sobel et al., 2020). It is hypothesized that IAs exposure through diet would have similar cardiotoxic effects as IAs exposure through drinking water, however discerning the contribution of IAs from rice in populations also exposed to IAs in drinking water is complicated (Karagas et al., 2019), thus most observational research has focused on exposure to IAs in drinking water. It is estimated that approximately 200 million people are exposed to IAs in drinking water above the World Health Organization guideline (10 μg/L) (Moon et al., 2013).
Several mechanistic pathways for As risk for CVDs have been explored including up-regulation of inflammatory signals (Bunderson et al., 2004; Simeonova et al., 2003; Tsai et al., 2002), enhanced oxidative stress (Barchowsky et al., 1999; Pi et al., 2002), alterations in nitric oxide metabolites (Pi et al., 2000), endothelial and smooth muscle cell proliferation (Pi et al., 2005), angiogenesis and increased apoptosis (Lee et al., 2003; Soucy et al., 2004; Soucy et al., 2005), and enhanced arterial thrombosis, platelet aggregation, and arterial vasoconstriction (Kao et al., 2003; Kumar and Bhattacharya, 2000; Lee et al., 2002). Current research is exploring other mechanistic pathways including how As enhances the formation of atherosclerotic lesions and how As can inhibit lipid efflux through macrophages (see Section 3 below).
Decades of research have established chronic exposure to IAs in drinking water is associated with multiple CVD endpoints (Council, 1999; Moon et al., 2013), especially coronary heart disease (Gundert-Remy et al., 2015; James et al., 2015) in multiple countries including the United States, China, Bangladesh, and Italy (D’Ippoliti et al., 2015; Farzan et al., 2015; Wade et al., 2015). Bangladesh is an area with 45 years of moderate-high IAs exposure (>100 μg/L) in drinking water. In a case-cohort study, a positive association was found between IAs levels >108 μg/L in drinking water and incident CVD (HR = 1.49; 95% CI:1.06,2.11) and incident coronary heart disease (HR = 1.54; 95% CI: 1.02, 2.31) (Chen et al., 2013; Misbahuddin, 2003). In the United States, the current EPA maximum contaminant level of IAs in drinking water is 10 μg/L with CVDs as a critical endpoint in support of the standard (Moon et al., 2013). Evidence of an association between low-moderate levels of IAs and CVD have been demonstrated in the Strong Heart American Indian population, which identified an association between baseline urine As species with CVD incidence and mortality over a 20-year follow-up (Chowdhury et al., 2018). In the San Luis Valley Diabetes Study, using a proportional hazards model adjusting for known CVD risk factors, the risk of CHD increased with time-weighted average exposure to IAs based on residential drinking water (HR = 1.43, 95% CI = 1.06–1.81 per 15 μg/L increase). The risk of CHD increased in a dose-dependent fashion with no evidence of a threshold (p-for linear trend p = 0.0007) (James et al., 2015). Other large studies have supported findings from these two studies. In a 2017 meta-analysis by Moon et al. (Moon et al., 2017), moderate exposure to As in drinking water (20 μg/L) was positively associated with outcomes CVD incidence (RR = 1.09; 95%CI: 1.03,1.14), ischemic attack (OR = 1.11; 95%CI: 1.05,1.17), and stroke incidence (RR = 1.08; 95%CI: 1.00,1.17). Past and ongoing research continue to show that IAs in drinking water is increasingly regarded as a potentially intervenable CVD risk factor.
2.1.2. Cadmium
Cadmium is a highly toxic metal found in all environmental systems especially soil (ATSDR, 2012; Cosselman et al., 2015). Cd is readily absorbed by vegetation from the soil where it bioaccumulates (ATSDR, 2012; Jarup and Akesson, 2009). Cd is also found in beef and other meat products due to consumption of contaminated plants and biomagnification (Di Bella et al., 2020). Human exposure to Cd is ubiquitous across all populations primarily through diet and tobacco smoking (Jarup et al., 1998). Smoking tobacco is the greatest source of Cd exposure in smokers (ATSDR, 2012; Lin et al., 2021) and has been associated with CVD. After ingestion, Cd has a long half-life (15–30 years) due to bioaccumulation in the kidney and bone (Dorian et al., 1992; Jarup and Akesson, 2009; Nordberg, 2007). This long-term accumulation can have cardiotoxic effects through several mechanisms including lipid peroxidation (Ercal et al., 2001; Lin et al., 2021), oxidative stress, and atherogenesis due to renal hypertension (Tellez-Plaza et al., 2013; Tellez-Plaza et al., 2008), which the section below will introduce more.
Observational studies have shown an increased risk for CVDs with Cd exposure estimated through urinary biomarkers (Lloyd-Jones et al., 2010; Navas-Acien et al., 2004; Navas-Acien et al., 2005; Poulsen et al., 2021; Roger et al., 2012) in populations with low-moderate exposure. National studies in NHANES, suggest a positive association between urine Cd (ATSDR, 2012; Jarup et al., 1998; Tellez-Plaza et al., 2008; Whittemore et al., 1991) and hypertension and cardiovascular mortality among men (Agarwal et al., 2011; Lin et al., 2021). Research by Tellez-Plaza et al. found a hazard ratio of 2.32 (1.61,3.35) with corrected urine Cd (0.57 μg/g) levels and all CVD mortality (Tellez-Plaza et al., 2008) and documented a 9.2% attributable risk for CVD mortality with Cd exposure. Systematic reviews of urinary Cd exposure groups and CVD endpoints have demonstrated a 36% increased risk when comparing the highest and lowest exposure groups (Nigra et al., 2016; Tellez-Plaza et al., 2013; Tinkov et al., 2018). In a review (Tellez-Plaza et al., 2013), for instance, urine Cd measurements were significantly associated with pooled relative risks (95% confidence interval) for CVD (1.36 (95% CI: 1.11, 1.66)), coronary heart disease (1.30 (95% CI: 1.12, 1.52)), stroke (1.18 (95% CI: 0.86, 1.59)), and peripheral arterial disease (1.49 (95% CI: 1.15, 1.92)). Exposure to Cd is a global burden with an established risk for multiple CVD endpoints. Prioritizing reduction in Cd exposure is recognized by many national and international health organizations.
2.1.3. Lead
Lead is one of the most prevalent and widely distributed elements in environmental systems (Hertz-Picciotto and Croft, 1993; Schwartz, 1995). Exposure to Pb has decreased over the past few decades with the removal from gasoline and paint, however the presence of Pb in drinking water and soil remains a public health concern. Extensive research has focused on acute Pb exposures and neurocognitive outcomes especially in children, however the past three decades has seen increased research examining low-moderate exposures to Pb and the associated risk for CVD. Systematic reviews of blood Pb levels suggested an associated positive risk for hypertension and increased blood pressure (Hertz-Picciotto and Croft, 1993; Meyer et al., 2008; Nawrot and Staessen, 2006; Staessen et al., 1995; Vaziri and Sica, 2004) with a combined estimated increase in blood pressure (1.0 mmHg (0.5 to 1.4 mmHg) systolic and 0.6 mmHg (0.4 to 0.8 mmHg) diastolic) with doubling of blood Pb levels (Nawrot et al., 2002). Coupled with this is supportive experimental research highlighting the role of oxidative stress as a main mechanism (Paithankar et al., 2021; Vaziri and Sica, 2004). These studies are sufficient to infer a causal effect of Pb exposure and hypertension, however findings from research on other CVD endpoints have been limited and inconclusive, therefore, the potential effects of metals on PAH will be discussed in the below section.
Overall global and domestic trends in Pb exposure have decreased over the past four decades (Meyer et al., 2008); however, CVD trends have continued to increase in general populations (Roth et al., 2020). Special considerations should be made for occupational cohorts with Pb exposure or communities with high Pb exposure. Pb exposure is decreasing however remains a global burden. Given the hypertensive effects of Pb exposure and the subsequent risk for CVD it should be a priority to reduce or mitigate exposure at all ages.
2.1.4. Tungsten
Tungsten is a metal that is found in soil and water systems and commonly co-occurs with IAs, Cd, and Pb, however most human exposure is through occupational settings (Nigra et al., 2018). Past research on W and CVD have suggested stroke (Agarwal et al., 2011; Tyrrell et al., 2013), peripheral arterial disease (PAD) (Navas-Acien et al., 2005), and high blood pressure (Shiue and Hristova, 2014) as associated health outcomes – however, generalizability is limited. While it is understood to have coagulant properties, the toxicology of the metal is still not well known (ATSDR, 2005; Byrne et al., 1997). Recent studies have examined potential increases in CVD risk with exposure to W based on proposed mechanism that W can interfere with binding sites for molybdenum, an essential metal for cellular health (Nigra et al., 2018) and oxidative stress (Lemus and Venezia, 2015; Nigra et al., 2018). Other epidemiologic studies on CVD endpoints, especially hypertension, have been limited and findings have been inconclusive (Lemus and Venezia, 2015; Nigra et al., 2018). The frequent co-occurrence with other heavy metals and their suspected augmentation by W (Bolt and Mann, 2016) is a common limitation to exposure related evidence. Additional research on W’s mechanistic effects is needed.
2.2. Potential effects of metals on PAH
PAH (group 1) is a rare disease with an annual incidence of about 2 to 5 cases per million and a prevalence of 10.6 cases per million adults with a higher predominance among women (Hassoun, 2021; Ruopp and Cockrill, 2022). It is characterized by an elevated mean pulmonary artery pressure (>20 mmHg), elevated pulmonary vascular resistance (≥3 WU), with a normal pulmonary artery wedge pressure (≤15 mmHg) as measured by right heart catheterization during rest with exclusion of other groups that can cause precapillary pulmonary hypertension (PH) (Simonneau et al., 2019). It can be idiopathic, heritable, drug- and toxin-induced, or associated with other conditions such as connective tissue disease, HIV infection, portal hypertension, congenital heart disease or schistosomiasis (Simonneau et al., 2019). In contrast to the National Institutes of Health registry that started in 1981 with a mean age of 36 years, more recent registries included older patients with a noted mean age of 53 years in the United States Registry to Evaluate Early and Long-term PAH Disease Management (REVEAL) (Badesch et al., 2010; Rich et al., 1987). The disease involves progressive pulmonary vascular remodeling that results in right ventricular (RV) failure and death. A significant number of group 1 PAH patients (46.5% in REVEAL) are still labelled as “idiopathic”, and we still do not fully understand the precise mechanisms of the different RV responses to increased afterload in setting of PAH with some patients developing “adaptive” while others develop “maladaptive” responses (Ryan and Archer, 2014).
In vitro and in vivo experimental models and clinical data showed that oxidative stress, an imbalance between the generation of reactive oxygen species (ROS) and the biological system’s ability to detoxify these ROSs or to repair the resulting damages, appears to be a significant mediator and predictor of more severe outcomes in PAH (Lahm et al., 2018). Although environmental exposures to the nonessential metals, such as As, Pb, and Cd, are considered key determinants of oxidative stress and CVDs (Bhatnagar, 2006; Domingo-Relloso et al., 2019; Ercal et al., 2001; Navas-Acien et al., 2004; Nigra et al., 2016; Wang et al., 2004), current studies suffer from multiple drawbacks with most studies being cross sectional in design and the relationship between metal exposure/essential metal dyshomeostasis and PAH and RV dysfunction is less investigated (Yang et al., 2020).
A small study found an association between isolated RV failure and self-reported exposure to occupational dusts (Lagat et al., 2014). In addition, patients with exposure to biomass fuel were found to have increased RV diameters, RV volumes, and PH when compared to controls (Rich et al., 2008). A previous study also showed that ambient pollutant particles can constrict isolated arterial rings, decrease pulmonary arterioles diameter in animals and intratracheal instillation of ambient pollutant particles increase the resistance of pulmonary artery in isolated buffer-perfused lungs (Huang and Ghio, 2006).
Understanding the molecular mechanisms of nonessential metal toxicities and essential metal dyshomeostasis in PAH/RV dysfunction would have a significant impact on exploring potential therapeutic options. For instance, metal toxicity by either generating ROS via Fenton reaction, or by competing/replacing essential metals from essential metal-containing proteins, transcription factors and enzymes to alter their functions, could be central mechanisms behind metal toxicity and essential metal imbalance-associated PAH/RV dysfunction (Paithankar et al., 2021). In fact, the association between altered iron (Fe) homeostasis and PAH have attracted attentions recently, because treatment of Fe deficiency in PAH improved patients’ exercise tolerance, quality of life, hospitalization rates and mortality (Sonnweber et al., 2020). Furthermore, intracellular Fe deficiency in mouse pulmonary artery smooth muscle cells directly led to PAH and RV failure, demonstrating a potential causal relationship (Lakhal-Littleton et al., 2019).
Our own pilot clinical study enrolled 20 PAH patients and 10 healthy controls, completed dietary surveys, and measured 31 metals in whole-blood, plasma and urinary samples using an X Series II quadrupole inductively coupled plasma mass spectrometry (ICP-MS). We found that intake of sulforaphane (SFN)-rich vegetables was significantly less in PAH patients when compared to controls (p < 0.05). PAH patients had higher whole-blood levels of nonessential metals such as Cd, chromium (Cr), Pb, silver, and As, and urinary levels of Cd, Cr, and As. Meanwhile, PAH patients showed lower concentrations of plasma essential metals, manganese (Mn) and Fe, and higher urinary Mn, Zn, and Fe concentrations when compared to controls (Huang et al., 2021).
In addition, antimony blood and plasma levels were significantly higher in PAH patients when compared to controls, and there was a trend for higher blood and plasma antimony levels in idiopathic PAH when compared to the non-idiopathic PAH group. Furthermore, the plasma antimony level was significantly associated with several prognostic invasive hemodynamic markers that reflect the RV function and disease severity in PAH patients including mean right atrial pressure, cardiac output, cardiac index, pulmonary vascular resistance, and mixed venous oxygen saturation (El-Kersh et al., 2022). Previous studies showed an association between antimony exposure and cardiac adverse effects (Guo et al., 2016; Jiang et al., 2021; Tan et al., 2020; Tirmenstein et al., 1995). In animal studies, antimony exposure was associated with cardiac myocyte granular degeneration, mitochondrial swelling, and endoplasmic reticulum dilation (Tirmenstein et al., 1995). In a large population-based study, a higher level of urinary antimony was linked to an increased risk of self-reported congestive heart failure and heart attacks (Guo et al., 2016). The mechanisms of these cardiac adverse events could be related to the effects of antimony on calcium homeostasis and/or an increased oxidative stress that may be induced by environmental antimony exposure (Fig. 1) (Jiang et al., 2021; Tirmenstein et al., 1995). However, it is difficult to conclude a causal relationship between metals and PAH from clinical studies alone, and our preliminary results promoted us to further explore direct roles of individual metals on PAH and/or RV dysfunction and contributions of the oxidative stress pathway.
Fig. 1. Proposed mechanism of antimony (Sb) adverse effects in pulmonary arterial hypertension (PAH).
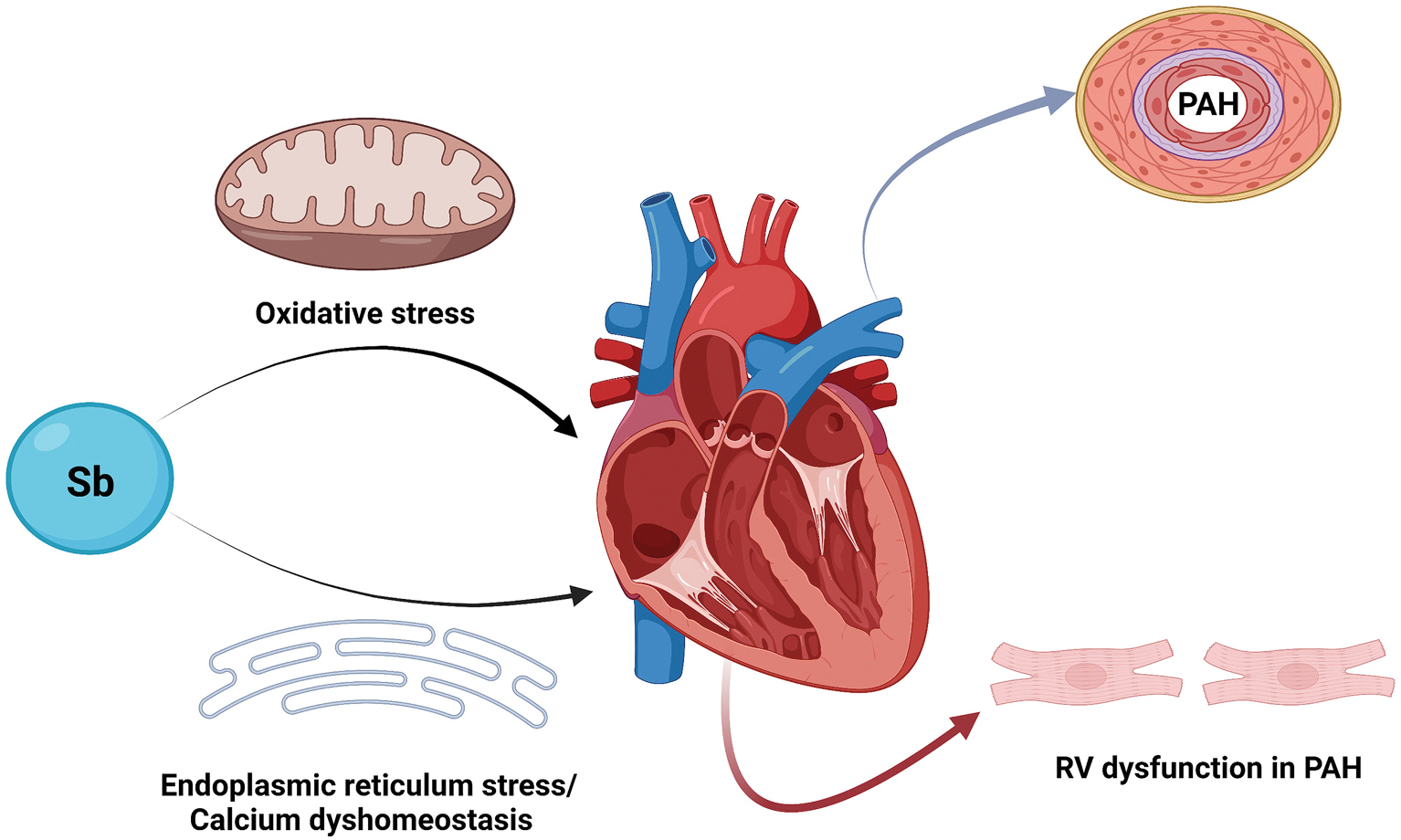
Environmental antimony exposure can potentially increase oxidative stress resulting in redox imbalance, contribute to disturbance of right ventricular (RV) calcium homeostasis, and increase RV endoplasmic reticulum stress which can result in worsening RV dysfunction and maladaptive RV response in PAH. (Created with BioRender.com).
3. Experimental and mechanistic studies on the effects of As and Cd
3.1. Atherosclerosis and As
As exposure is linked to increased CVDs as mentioned above, including atherosclerosis, which is the gradual occlusion of arteries due to accumulation of a fibro-fatty plaque. When the plaque becomes less stable, it can rupture and fragments can block arterial flow to the heart, resulting in myocardial infarct. In human populations, As exposure is linked to cardiovascular outcomes and mortality (Chen et al., 2011; Engel and Smith, 1994; Lisabeth et al., 2010; Meliker et al., 2007; Ruiz-Navarro et al., 1998; Sohel et al., 2009; Wade et al., 2009; Zierold et al., 2004). Several different mouse models have been used to investigate As-induced atherosclerosis, including the apolipoprotein E knock-out (apoE−/−) (Negro Silva et al., 2017; Simeonova et al., 2003; States et al., 2005) and low-density lipoprotein receptor (LDL-R−/−) mice (Bunderson et al., 2004). These models respond to pro-atherogenic stimuli and the cellular and non-cellular components of the plaque mimic that in humans (Getz and Reardon, 2012). Of importance, these models appear to reflect the consequences of human exposures and have provided important insight into As concentrations (Makhani et al., 2018), key windows of early-life exposure (Srivastava et al., 2007), and sex-specific differences (Negro Silva et al., 2021). Multiple overlapping mechanisms have been proposed for As-induced atherosclerosis, including activation of endothelial cells leading to dysfunction (Farzan et al., 2022; Straub et al., 2008; Xu et al., 2017), increased reactive oxygen species (Lemaire et al., 2015; Straub et al., 2008), inflammation (Chen et al., 2007; Wu et al., 2003), and dyslipidemia (Lemaire et al., 2014).
Macrophages play a key role in generation of the atherosclerotic plaque, as they respond to and engulf increased oxidized lipids in the subendothelial space, becoming foam cells. These foam cells can propagate the immune response at the plaque, further recruiting other cell types, such as T lymphocytes, dendritic cells, and neutrophils (Ilhan and Kalkanli, 2015). Macrophages are highly responsive to stimuli in their microenvironment and are polarized in gene expression and response. This polarization exists along a spectrum, whose extremes are characterized by classically activated, pro-inflammatory macrophages on one end and wound-healing, pro-resolving macrophages on the other (Mantovani and Locati, 2009). Pro-inflammatory macrophages predominate in accelerating plaque development, while wound-healing macrophages are more abundant in regressing plaques (Barrett, 2020).
Macrophages are sensitive to As (Fig. 2). In vitro, As increases ROS and alters macrophage-specific gene expression (Bourdonnay et al., 2009a). Importantly, many of these changes are reversible upon removal of As (Bourdonnay et al., 2009b), suggesting that As-induced changes in macrophages that promote atherosclerosis could also be reversible. In order to understand the effects of As on polarized macrophages, murine bone marrow-derived macrophages were polarized to pro-inflammatory macrophages with interferon γ (IFNγ) or wound-healing macrophages with interleukin-4 (IL-4) in the presence or absence of As and gene expression assessed by RNA sequencing. Surprisingly, As has different effects depending on the polarization state of the macrophage. Only a small number of genes were modulated in both IFNγ and IL-4 polarized macrophages, and the majority of the common genes were changed in opposite directions. As inhibited liver X receptor (LXRα) signaling specifically in IFNγ polarized macrophages, while MHC class II gene expression was decreased in IL-4 polarized macrophages. These data suggest that As will affect macrophages differently depending upon polarization state.
Fig. 2. Arsenic causes different pro-atherogenic changes depending on the type of macrophages.
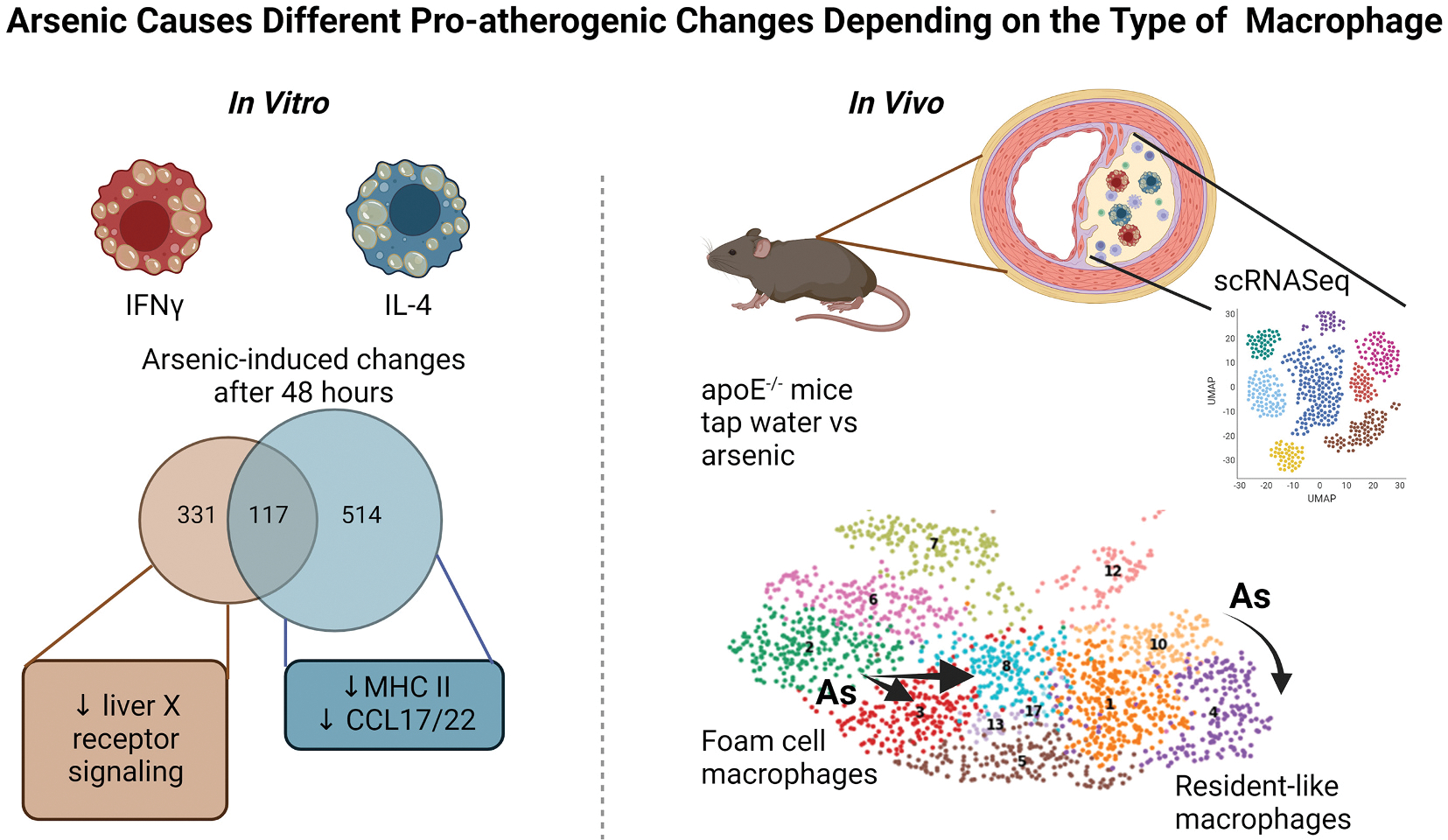
In vitro, macrophages polarized with either IFNγ or IL-4 for 48 h in the presence or absence of arsenic were analyzed by bulk RNA sequencing. Arsenic induced different gene expression changes depending on the polarization. For example, arsenic decreased liver X receptor signaling in IFNγ-stimulated macrophages, but not in IL-4-stimulated macrophages. Arsenic decreased MHC II and CCL17/22 in IL-4-stimulated macrophages, but not IFNγ. In vivo, cells from the atherosclerotic plaques of ApoE−/− mice (tap water versus 200 ppb arsenic for 13 weeks) were isolated and analyzed by single cell RNA sequencing (scRNASeq). Foam cell macrophages in control animals (green cluster 2) increased heterogeneity (to red cluster 3 and light blue cluster 8). Resident-like macrophages changed from yellow cluster 10 (control) to purple cluster 4 (arsenic). However, these changes were distinct and dependent upon the original cluster from which they were derived. (Figure created with Biorender).
While in vitro polarized macrophages are useful tools, they may not faithfully reflect all the macrophages at the plaque. Recent single-cell RNA sequencing experiments in both apoE−/− mice and LDL-R−/− mice have revealed several different types of macrophages, although none correlate with the IFNγ/IL-4 polarized macrophages described in vitro (Zernecke et al., 2020). In contrast, these studies have identified three different macrophage populations namely 1) resident-like, 2) inflammatory-like, and 3) foamy/Trem2hi macrophages. Resident-like macrophages are likely derived from macrophages seeded in the adventitia of aorta and other organs during early stages of embryonic development (Cochain et al., 2018; Ensan et al., 2016). Inflammatory macrophages are characterized by elevated expression of inflammatory cytokines and chemokines, such as Ccl2, Ccl3, Ccl4, Cxcl1 and Cxcl2, and are likely derived from blood monocytes (Cochain et al., 2018; Kim et al., 2018b; Lin et al., 2019). The foamy/Trem2hi cluster displays high levels of Mmp12, Mmp14, Itgax (CD11c), and markers of lipid loading (Abcg1, Trem2, Fabp4), lysosomal cathepsins (Ctsd, Ctsl) Cd9, and Spp1 (osteopontin) (Cochain et al., 2018; Ensan et al., 2016). Thus, we asked whether As exposure changed macrophage populations within the plaque of apoE−/− mice. We identified the three main clusters of macrophages but found that each cluster was changed independently by As. This mirrored our in vitro findings in that depending on the macrophage subtype, As changed the gene expression specific to that subtype.
However, we have yet to identify the contribution of each macrophage subtype and the arsenic-induced gene expression changes to the generation and/or resolution of the atherosclerotic plaque. The early-life period is important for generation and seeding of tissue resident macrophages (Nobs and Kopf, 2021), but is also a window of exposure to arsenic that increases atherosclerosis later in life in mice (Negro Silva et al., 2021; Srivastava et al., 2007). The macrophage profile in atherosclerotic plaques of adult mice following limited early-life exposure is unknown. Other variables such as sex and diet should also be considered. Finally, we hope to use this model to understand possible interventions. Do macrophage changes reverse upon cessation of arsenic exposure? Chelation therapy has shown promise in reducing CVD mortality (Lamas and Ergui, 2016; Lamas et al., 2013), however it is unknown whether this involves changes to the atherosclerotic plaque components, including macrophages. Comparison with human data should also enhance our understanding of relevance of these mouse models, although recent data suggest that at least DNA methylation changes associated with As and As-CVD are similar between humans and mice (Domingo-Relloso et al., 2022).
3.2. Cardiac pathogenic effects of Cd
As mentioned above, Cd is a naturally occurring heavy metal without known biological function in humans, but its toxic impact has been ranked as the top 7 on the Agency for Toxic Substances and Disease Registry (ATSDR) list of environmental chemical hazards (ATSDR, 2012). The primary source of Cd exposure in the non-smoking, general population is ingestion of contaminated food and water, with an average daily intake of between 4 and 26 μg/day (Choudhury et al., 2001; Kim et al., 2018a; Martorell et al., 2011). However, extensive studies have been done for the hepatic and renal toxicities with acute and chronic and high and low doses of Cd exposure. Compared to hepatic and renal toxicities, the cardiac toxicities of Cd, particularly at chronic and low doses were relatively less investigated.
3.2.1. Cardiac effect of chronic exposure of adult mice and rats to very low-dose Cd
We have treated young adult C57BL/6 male mice (6-week-old) with low-dose Cd by i.p. injection of CdCl2 (20 nmol/kg in saline) every other day for 4 weeks. Then, all mice were sacrificed at the 56th week following the 4-week Cd exposure (i.e.: 60th week since the first dose of Cd), as illustrated in Fig. 3A. Exposure to such low-dose Cd overtly increased left ventricle (LV) end-systolic diameter (LVESD) and suppressed fractional shortening without affecting heart rate, LV wall thickness and LV end-diastolic diameter (LVEDD). Chronic Cd exposure significantly suppressed peak shortening and maximal velocity of shortening/relengthening (α dL/dt) without affect duration of shortening and relengthening (TR90) in mouse cardiomyocytes, measured by in vitro isolated primary cardiomyocytes (Turdi et al., 2013). In contrast with this study, female C57BL/6 J mice (> 8 weeks old) randomly received drinking water with and without Cd (100 mg/L, 100 ppm) for 12 weeks (Fig. 3A), but no significant pathological changes were observed in the heart of Cd-treated female C57BL/6 J mice except for increased TUNEL positive cells (Turkcan et al., 2015).
Fig. 3. Cardiac effects of low-dose Cd exposure with and without the second stress, HFD or Western diet.
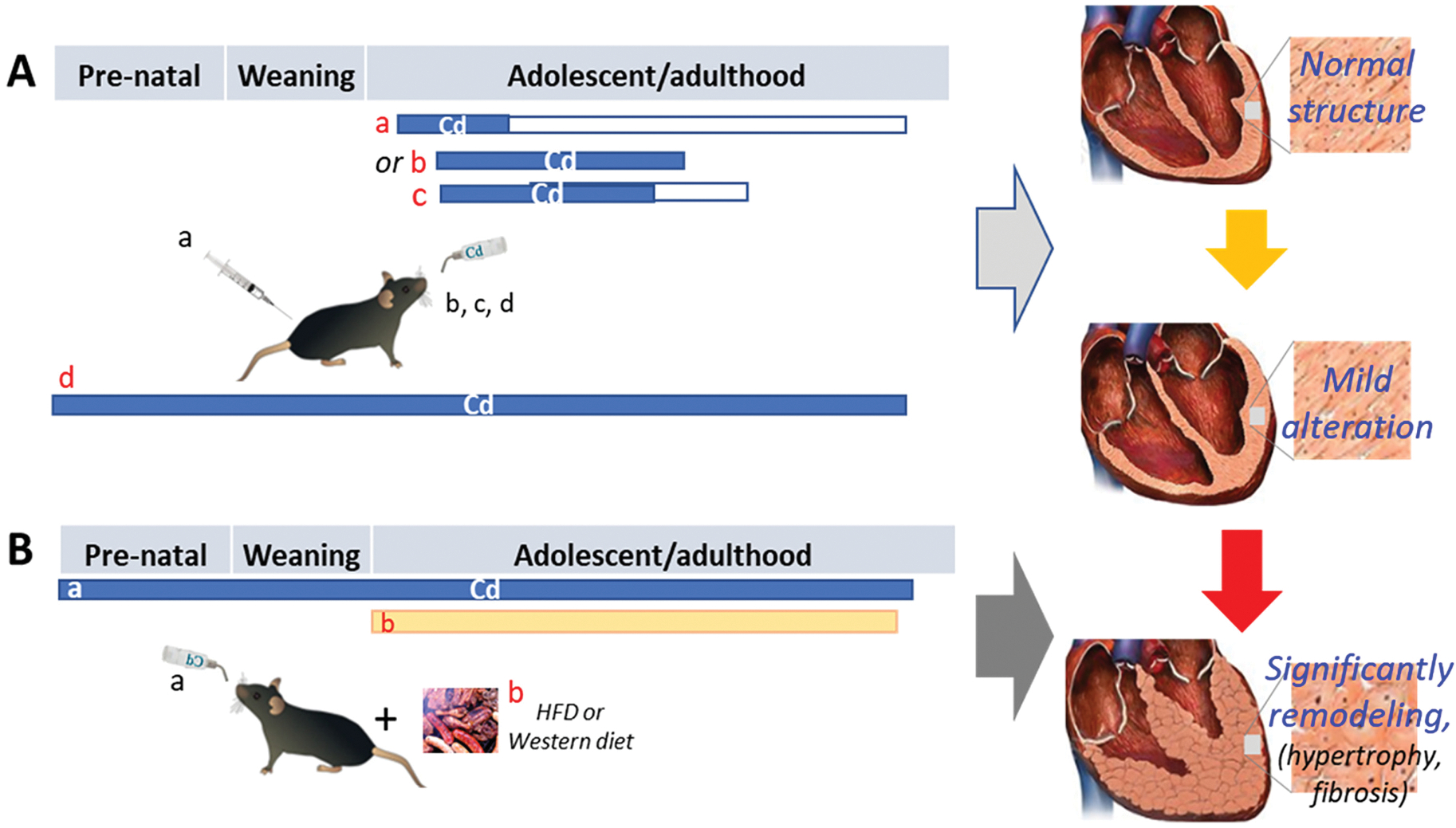
The experimental designs were briefly described for a few studies, for which mice and rats were chronically exposed to low-dose Cd (blue bar) either intraperitoneally or in the drinking water at different stages of life for various durations. In panel A: a, starting at 6 week (wks) old and continuing for 4 wks only, followed by abstaining Cd for 56 wks (white box); b, starting at 8 wks old and continuing for 12 wks; c, starting at 8 wks old and continuing for 10 wks, followed by abstaining Cd for 4 wks; d, whole-life Cd exposure until sacrifice. All four strategies caused only mild cardiac pathogenesis without significant cardiac dysfunction. In Panel B: the exacerbated effects of whole-life exposure to low-dose Cd in the drinking water (blue bar) on post-weaning HFD- or Western diet (yellow bar)-induced cardiac remodeling and dysfunction. This illustration was made based on the experimental models and outcomes by Turdi et al. (Panel A-a), Turkcan et al. (Panel A-b), Das et al. (Panel A-c), Liang et al. (Panel A-d, Panel B, Western diet), and Zhou et al. (Panel A-d, Panel B, HFD).
In a study with rats investigating for the role of chronic Cd exposure in modulating cardiac matrix metalloproteinases (MMPs) in the heart, adult male Sprague-Dawley rats were exposed to 15 ppm CdCl2 in drinking water for 10 weeks followed by withdrawal of Cd for 4 weeks (Fig. 3A). Gene expression of inflammatory mediators (IL-1β, IL-6, IL-10, TNF-α and NF-κB), as well as protein expression of matrix metalloproteinases (MMPs), MMP-2 and MMP-9 and their respective inhibitors- TIMP-1 and TIMP-2, and gelatinolytic activity of MMP-2 and MMP-9 were determined. At the protein level, Cd caused a differential effect on the expression and activity of gelatinases and their endogenous inhibitors in an exposure-dependent manner: the administered Cd dose induced an inflammatory response until week 10 that slightly diminishes after 4 weeks. This study suggests that Cd-induced imbalance in the MMP-TIMP system in the cardiac tissue probably by Cd-induced cardiac inflammation, which might contribute to certain pathologies (Das et al., 2021).
Taken together, these mice and rats chronically exposed to low doses of Cd in IP or drinking water did not consistently show significant abnormalities in terms of their cardiac function and pathological structure even though there were some subtle pathogenic changes and fibrotic responses. Whether these inconsistent results among above studies (Das et al., 2021; Turkcan et al., 2015) are due to the difference of species, strain, and sex of animals as well as Cd administration routes and doses need further investigation.
3.2.2. Whole-life exposure to low-dose Cd
The cardiac effects of prenatal to the adulthood exposure to low-dose Cd was also examined recently by a couple of studies. Female C57BL/6 J mice were exposed to 0, 0.5 ppm and 5 ppm Cd, in form of CdCl2, in drinking water for two weeks prior to mating with the same strain of male mice, and continually exposed to these doses of Cd during the breeding and pregnant period. Then, the offspring (F1) of the parents with and without Cd exposure received the same spectrum of treatments as their parents (F0) until 13 or 27 weeks of age, shown in Fig. 3A. These mice did not show significant cardiac dysfunction (neither diastolic nor systolic) or structural changes (Zhou et al., 2022). There was also no significantly pathophysiological remodeling, including hypertrophy and fibrosis (Liang et al., 2019; Zhou et al., 2022). These two studies suggested that chronic whole-life exposure to low-dose Cd did not cause significantly pathogenic effects on the heart in terms of functional and pathological changes. This is a little different from the exposure to adults that showed subtle inflammation and contractility decrease.
3.2.3. Exacerbating effect of chronic pre-exposure to low-dose Cd on the second challenges
An early study showed in female C57BL/6 J mice (8 weeks old) receiving Cd via drinking water at 100 mg/L (10 ppm CdCl2) for 12 weeks (Fig. 3A), Cd induced an inflammatory response and cell death in cardiac tissue. However, disease progression leading to the development of cardiac fibrosis depended on lipid concentrations, as a HFD significantly accelerated Cd deposition in organs, especially in the heart (Turkcan et al., 2015). Based on these results, the authors hypothesized that the combination of two cardiovascular risk factors, hypercholesterolemia and increased cardiac Cd concentrations, as seen in obese smokers, might accelerate and aggravate the pathological processes otherwise induced individually (Turkcan et al., 2015). These studies strongly suggest that the cardiac precondition or co-treatment can significantly affect its sensitivity to Cd-induced damage.
Pre-exposure of animals to chronic low-dose Cd, to mimic the situation of individuals who are residentially exposed to environmentally contaminated low-dose Cd, was investigated for whether it also enhances subsequent challenges-induced cardiac pathogenesis by our group and others. Considering that childhood obesity, which is prevalent in developed countries, is a metabolic risk factor for CVDs, the effects of lifelong, low-dose Cd exposure on post-weaning HFD-induced cardiac remodeling was investigated with C57BL/6 J mice exposed to Cd (0.5 and 5.0 ppm) via drinking water from conception to sacrifice along with offspring mice fed with a HFD (42% kcal from fat) for an additional 10 weeks (Fig. 3B). Exposure to 5 ppm Cd increased cardiac metallothionein (MT) protein levels only in female mice, regardless of dietary intake. Histological analysis revealed that 5 ppm Cd exposure combined with a HFD induced cardiac hypertrophy and fibrosis only in female mice. Biochemical examination showed elevated expression of cardiac hypertrophy markers, atrial natriuretic peptide (ANP) and fibrotic markers collagen 1A1 (COL1A1) protein levels along with COL1A1, COL1A2, and COL3A1 mRNA levels as well as profibrotic markers plasminogen activator inhibitor 1 (PAI-1), connective tissue growth factor (CTGF), and fibronectin (FN) in HFD-fed female mice pre-exposed to 5 ppm Cd. This study showed that lifelong, low-dose Cd exposure enhanced post-weaning HFD-induced cardiac hypertrophy and fibrosis in female but not male mice (Liang et al., 2019).
However, the HFD used in the above study was 42% kcal from fat, called Western diet, rather than the commonly used diet (60% kcal from fat). Therefore, we conducted a similar study, for which females were exposed to Cd-containing (0, 0.5, or 5 ppm) drinking water before breeding; the pregnant mice and dams with offspring continually drank the same Cd-containing water. After weaning, the offspring were continued on the same regime as their parents and fed either a HFD (60% kcal from fat) or ND for 24 weeks (Fig. 3B). This showed a dose-dependent Cd accumulation in the hearts of male and female offspring along with decreased cardiac zinc and copper levels only in female offspring. Exposure to 5 ppm, but not 0.5 ppm, Cd significantly enhanced HFD cardiac effects only in female mice, shown by worsened cardiac systolic and diastolic dysfunction (ejection fraction, mitral E-to-annular e’ ratio), increased fibrosis, hypertrophy, and inflammation, compared to the HFD group. These results suggest that whole-life 5 ppm Cd exposure significantly increases the susceptibility of female offspring to HFD-induced cardiac remodeling and dysfunction (Zhou et al., 2022).
However, the opposite finding was also reported when the second hit was changed. For instance, rats were given Cd at 1 or 2 mg/kg I.P. injection and at 48 h later, hearts were isolated from control and different doses of Cd treated rats were subject to ischemic and reperfusion (I/R) in the ex vivo Langendorff model, the I/R-induced cardiac damage was prevented in the heart from 2 mg/kg Cd-treated rats with significant induction of MT compared to control and l mg/kg Cd-treated rats (Devaux et al., 2009). Therefore, whether there is synergistic or preventive effect of exposure to Cd in combination with other challenges, such as obesity or I/R, may be mediated by different mechanisms due to the difference between chronic and acute models as wells species and Cd exposure route differences.
3.2.4. Cd-exposure changing the individual susceptibility to subsequent stress-induced cardiac effect may be mediated by its epigenetic modification
Although the mechanism of synergistic toxicity or preventive action mentioned above are unclear, DNA methylation seems to play an important role. Movassagh et al. have identified at least three loci of angiogenesis-related genes where methylation correlated with overt differential expression of corresponding genes in myopathic hearts in patients with end-stage heart failure. These findings suggest that altered DNA methylation may be responsible for, at least in part, characteristic gene expression changes in heart failure (Movassagh et al., 2010). In our animal experimental study, we have demonstrated the potential role of increased DNA methylation, as simultaneously administration of the DNA methylation inhibitor 5-AZA could prevent the subtle cardiac dysfunction and overt interstitial fibrosis (collagen deposition) in adult mice at 60 weeks after a 4-week exposure to low-dose Cd (Turdi et al., 2013).
However, there was also evidence that DNA hypomethylation at the neonatal stage alters gene expression patterns in the heart, leading to development of a cardiac I/R-sensitive phenotype later in life. This is because when DNA methylation inhibitor 5-AZA was administered in newborn rats from postnatal day 1–3, cardiac function and related key genes measured at 2-week and 2-month old exhibited that 5-AZA treatment induced an age- and sex-dependent inhibition of global and gene-specific DNA methylation levels in LV, resulting in a long-lasting growth restriction, and enhanced I/R-induced cardiac dysfunction and injury in adults as compared with the saline controls (Zhang et al., 2020); however, whether this increased susceptibility to I/R is also the case that prenatal to whole-life exposure to Cd enhances post-weaning HFD-induced cardiac effects needs further exploration.
4. Conclusive and prospective remarks
This multidisciplinary review provides an update on cardiovascular effects of exposure to non-essential metals such as As, Pb, Cd, and CVDs. We also present findings on metal levels in PAH patients, a significantly less investigated disease. These experimental and observational research updates highlight the continued needs for further research on non-essential metals commonly found in the environment and certain occupational settings and various CVDs, particularly the disparate features of these CVDs among different regions and populations, which may be due to the geographic differences in metal contamination or occupational impact.
By applying pre-clinical models, translational research could provide the link between multiple metals and CVDs and define differences and commonalities of different metals in CVDs. These studies further confirm the direct effect of metals on the specific disease pathogenesis, such as atherosclerosis and cardiac toxicity. Although there remain more questions than answers in the cardiovascular toxicity of these non-essential metals, these new pre-clinical studies and clinical observations may stimulate more mechanistic studies and accelerate the development of efficient preventive and therapeutic strategies by facilitating collaborations among investigators.
Directions for future research should be considered: (1) Cd-mediated epigenetic modification in CVDs has been extensively investigated, but its effect on the cardiac toxicities under different conditions in terms of Cd exposure times of life stage such as prenatal, postnatal, young or adult, sexes of the individuals exposed to Cd, and Cd doses and routes remain further investigation; (2) atherogenic mechanisms of As and how they differ by sex, window of exposure, and diet; (3) translating dose-response studies in animal models into human risk estimates. Although investigating the effects directly caused by non-essential metals are important, the indirect effect of these non-essential metals by disturbing the essential metal homeostasis may also be important.
Acknowledgments
The authors of this review were supported in part by the University of Louisville Executive Vice President for Research and Innovation Internal Grant (JH, LC); University of Louisville School of Medicine Basic Grant (JH, LC); National Institute of Environmental Health Sciences (P30ES030283 to JH, LC; 1R21ES021831 to KAJ); Gilead Sciences COMMIT COVID-19 RFP Program grant (Gilead IN-US-983–6063 to JH); National Center for Advancing Translational Sciences grant (1U18TR003787–01 to JH). Dr. El-Kersh has received institutional research grants from UT and J&J Actelion, has participated in advisory boards for UT and J&J Actelion, and has acted as a consultant for Acceleron and UT. Dr. Mann is supported by funding from the Canadian Institutes of Health Research (PJT-166142), the Heart and Stroke Foundation Canada (G-17–0018365), and the National Institute of Environmental Health Sciences (1 P42 ES033719–01).
Footnotes
CRediT authorship contribution statement
Jiapeng Huang: Writing – original draft, Writing – review & editing. Karim El-Kersh: Visualization, Writing – original draft, Writing – review & editing. Koren K. Mann: Visualization, Writing – original draft, Writing – review & editing. Katherine A. James: Conceptualization, Writing – original draft, Writing – review & editing. Lu Cai: Conceptualization, Visualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
No conflict interest needs to be claimed.
Data availability
No data was used for the research described in the article.
References
- Abhyankar LN, Jones MR, Guallar E, Navas-Acien A, 2012. Arsenic exposure and hypertension: a systematic review. Environ. Health Perspect. 120, 494–500. 10.1289/ehp.1103988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Zaman T, Tuzcu EM, Kapadia SR, 2011. Heavy metals and cardiovascular disease: results from the National Health and nutrition examination survey (NHANES) 1999–2006. Angiology 62, 422–429. 10.1177/0003319710395562. [DOI] [PubMed] [Google Scholar]
- Althoff T, Nilforoshan H, Hua J, Leskovec J, 2022. Large-scale diet tracking data reveal disparate associations between food environment and diet. Nat. Commun. 13, 267. 10.1038/s41467-021-27522-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR, 2005. Agency for Toxic Substances and Disease Registry: Toxicological Profile for Tungsten. US Department of Health and Human Services. Case No. 7440–33-7, Accessed Aug 3, 2022. Doi: https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=806&tid=157. [Google Scholar]
- ATSDR, 2012. Agency for Toxic Substances and Disease Registry: Toxicological Profile for Cadmium. US Department of Health and Human Services. Case No. 7440–43-9, Accessed June 20, 2022. doi: https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=48&tid=15. [Google Scholar]
- ATSDR, 2016. Agency for Toxic Substances and Disease Registry: Toxicological Profile for Arsenic. US Department of Health and Human Services. Case No. 7440–38-2, Accessed June 20, 2022. doi: https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=22&tid=3. [Google Scholar]
- Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, et al. , 2010. Pulmonary arterial hypertension: baseline characteristics from the REVEAL registry. Chest 137, 376–387. 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- Barchowsky A, Klei LR, Dudek EJ, Swartz HM, James PE, 1999. Stimulation of reactive oxygen, but not reactive nitrogen species, in vascular endothelial cells exposed to low levels of arsenite. Free Radic. Biol. Med. 27, 1405–1412. 10.1016/s0891-5849(99)00186-0. [DOI] [PubMed] [Google Scholar]
- Barrett TJ, 2020. Macrophages in atherosclerosis regression. Arterioscler. Thromb. Vasc. Biol. 40, 20–33. 10.1161/ATVBAHA.119.312802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar A, 2006. Environmental cardiology: studying mechanistic links between pollution and heart disease. Circ. Res. 99, 692–705. 10.1161/01.RES.0000243586.99701.cf. [DOI] [PubMed] [Google Scholar]
- Bolt AM, Mann KK, 2016. Tungsten: an emerging toxicant, alone or in combination. Curr. Environ. Health Rep. 3, 405–415. 10.1007/s40572-016-0106-z. [DOI] [PubMed] [Google Scholar]
- Bourdonnay E, Morzadec C, Fardel O, Vernhet L, 2009a. Redox-sensitive regulation of gene expression in human primary macrophages exposed to inorganic arsenic. J. Cell. Biochem. 107, 537–547. 10.1002/jcb.22155. [DOI] [PubMed] [Google Scholar]
- Bourdonnay E, Morzadec C, Sparfel L, Galibert MD, Jouneau S, Martin-Chouly C, et al. , 2009b. Global effects of inorganic arsenic on gene expression profile in human macrophages. Mol. Immunol. 46, 649–656. 10.1016/j.molimm.2008.08.268. [DOI] [PubMed] [Google Scholar]
- Bradl HB, MyiLibrary Elsevier S, Technology C, MyiLibrary, 2005. Heavy Metals in the Environment : [Origin, Interaction and Remediation]. Elsevier Academic Press, Amsterdam. [Google Scholar]
- Brandon EF, Janssen PJ, de Wit-Bos L, 2014. Arsenic: bioaccessibility from seaweed and rice, dietary exposure calculations and risk assessment. Food Addi.t Contam. Part A Chem. Anal. Control Expo. Risk Assess 31, 1993–2003. 10.1080/19440049.2014.974687. [DOI] [PubMed] [Google Scholar]
- Bunderson M, Brooks DM, Walker DL, Rosenfeld ME, Coffin JD, Beall HD, 2004. Arsenic exposure exacerbates atherosclerotic plaque formation and increases nitrotyrosine and leukotriene biosynthesis. Toxicol. Appl. Pharmacol. 201, 32–39. 10.1016/j.taap.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Byrne JV, Hope JK, Hubbard N, Morris JH, 1997. The nature of thrombosis induced by platinum and tungsten coils in saccular aneurysms. AJNR Am. J. Neuroradiol. 18, 29–33 doi. [PMC free article] [PubMed] [Google Scholar]
- Chen SC, Tsai MH, Wang HJ, Yu HS, Chang LW, 2007. Involvement of substance P and neurogenic inflammation in arsenic-induced early vascular dysfunction. Toxicol. Sci. 95, 82–88. 10.1093/toxsci/kfl136. [DOI] [PubMed] [Google Scholar]
- Chen Y, Graziano JH, Parvez F, Liu M, Slavkovich V, Kalra T, et al. , 2011. Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: prospective cohort study. BMJ 342, d2431. 10.1136/bmj.d2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wu F, Liu M, Parvez F, Slavkovich V, Eunus M, et al. , 2013. A prospective study of arsenic exposure, arsenic methylation capacity, and risk of cardiovascular disease in Bangladesh. Environ. Health Perspect. 121, 832–838. 10.1289/ehp.1205797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury H, Harvey T, Thayer WC, Lockwood TF, Stiteler WM, Goodrum PE, et al. , 2001. Urinary cadmium elimination as a biomarker of exposure for evaluating a cadmium dietary exposure–biokinetics model. J. Toxicol. Environ. Health A. 63, 321–350. 10.1080/15287390152103643. [DOI] [PubMed] [Google Scholar]
- Chowdhury R, Ramond A, O’Keeffe LM, Shahzad S, Kunutsor SK, Muka T, et al. , 2018. Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 362, k3310. 10.1136/bmj.k3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochain C, Vafadarnejad E, Arampatzi P, Pelisek J, Winkels H, Ley K, et al. , 2018. Single-cell RNA-Seq reveals the transcriptional landscape and heterogeneity of aortic macrophages in murine atherosclerosis. Circ. Res. 122, 1661–1674. 10.1161/CIRCRESAHA.117.312509. [DOI] [PubMed] [Google Scholar]
- Cortes S, Zuniga-Venegas L, Pancetti F, Covarrubias A, Ramirez-Santana M, Adaros H, et al. , 2021. A positive relationship between exposure to heavy metals and development of chronic diseases: a case study from Chile. Int. J. Environ. Res. Public Health 18. 10.3390/ijerph18041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosselman KE, Navas-Acien A, Kaufman JD, 2015. Environmental factors in cardiovascular disease. Nat. Rev. Cardiol. 12, 627–642. 10.1038/nrcardio.2015.152. [DOI] [PubMed] [Google Scholar]
- Council NR, 1999. Arsenic in Drinking Water. National Academy Press, Washington, D. C., pp. xvii, p. 310. [Google Scholar]
- Das SC, Varadharajan K, Shanmugakonar M, Al-Naemi HA, 2021. Chronic cadmium exposure alters cardiac matrix metalloproteinases in the heart of sprague-dawley rat. Front. Pharmacol. 12, 663048 10.3389/fphar.2021.663048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux S, Maupoil V, Berthelot A, 2009. Effects of cadmium on cardiac metallothionein induction and ischemia-reperfusion injury in rats. Can. J. Physiol. Pharmacol. 87, 617–623. 10.1139/y09-046. [DOI] [PubMed] [Google Scholar]
- Di Bella C, Traina A, Giosue C, Carpintieri D, Lo Dico GM, Bellante A, et al. , 2020. Heavy metals and PAHs in meat, milk, and seafood from Augusta area (southern Italy): contamination levels, dietary intake, and human exposure assessment. Front. Public Health 8, 273. 10.3389/fpubh.2020.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ippoliti D, Santelli E, De Sario M, Scortichini M, Davoli M, Michelozzi P, 2015. Arsenic in drinking water and mortality for cancer and chronic diseases in Central Italy, 1990–2010. PLoS One 10, e0138182. 10.1371/journal.pone.0138182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo-Relloso A, Grau-Perez M, Briongos-Figuero L, Gomez-Ariza JL, Garcia-Barrera T, Duenas-Laita A, et al. , 2019. The association of urine metals and metal mixtures with cardiovascular incidence in an adult population from Spain: the Hortega follow-up study. Int. J. Epidemiol. 48, 1839–1849. 10.1093/ije/dyz061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo-Relloso A, Makhani K, Riffo-Campos AL, Tellez-Plaza M, Klein KO, Subedi P, et al. , 2022. Arsenic exposure, blood DNA methylation, and cardiovascular disease. Circ. Res. 101161CIRCRESAHA122320991 10.1161/CIRCRESAHA.122.320991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorian C, Gattone VH 2nd, Klaasen CD, 1992. Renal cadmium deposition and injury as a result of accumulation of cadmium-metallothionein (CdMT) by the proximal convoluted tubules–A light microscopic autoradiography study with 109CdMT. Toxicol. Appl. Pharmacol. 114, 173–181. 10.1016/0041-008x(92)90066-2. [DOI] [PubMed] [Google Scholar]
- El-Kersh K, Hopkins CD, Wu XY, Rai SN, Cai L, Huang JP, 2022. Plasma level of antimony correlates with pulmonary arterial hypertension severity. Curr. Res. Toxicol. 3 doi:ARTN 100080 10.1016/j.crtox.2022.100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel RR, Smith AH, 1994. Arsenic in drinking water and mortality from vascular disease: an ecologic analysis in 30 counties in the United States. Arch. Environ. Health 49, 418–427 doi. [DOI] [PubMed] [Google Scholar]
- Ensan S, Li A, Besla R, Degousee N, Cosme J, Roufaiel M, et al. , 2016. Self-renewing resident arterial macrophages arise from embryonic CX3CR1(+) precursors and circulating monocytes immediately after birth. Nat. Immunol. 17, 159–168. 10.1038/ni.3343. [DOI] [PubMed] [Google Scholar]
- Ercal N, Gurer-Orhan H, Aykin-Burns N, 2001. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem. 1, 529–539. 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- Farzan SF, Chen Y, Rees JR, Zens MS, Karagas MR, 2015. Risk of death from cardiovascular disease associated with low-level arsenic exposure among long-term smokers in a US population-based study. Toxicol. Appl. Pharmacol. 287, 93–97. 10.1016/j.taap.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzan SF, Eunus HM, Haque SE, Sarwar G, Hasan AR, Wu F, et al. , 2022. Arsenic exposure from drinking water and endothelial dysfunction in Bangladeshi adolescents. Environ. Res. 208, 112697 10.1016/j.envres.2022.112697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini N, Fry RC, Balakrishnan P, Navas-Acien A, Oliver-Williams C, Howard AG, et al. , 2017. Cadmium body burden and increased blood pressure in middle-aged American Indians: the Strong Heart Study. J. Hum. Hypertens. 31, 225–230. 10.1038/jhh.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz GS, Reardon CA, 2012. Animal models of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 32, 1104–1115. 10.1161/ATVBAHA.111.237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundert-Remy U, Damm G, Foth H, Freyberger A, Gebel T, Golka K, et al. , 2015. High exposure to inorganic arsenic by food: the need for risk reduction. Arch. Toxicol. 89, 2219–2227. 10.1007/s00204-015-1627-1. [DOI] [PubMed] [Google Scholar]
- Guo J, Su LL, Zhao XY, Xu ZP, Chen GD, 2016. Relationships between urinary antimony levels and both mortalities and prevalence of cancers and heart diseases in general US population, NHANES 1999–2010. Sci. Total Environ. 571, 452–460. 10.1016/j.scitotenv.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Guo LC, Lv Z, Ma W, Xiao J, Lin H, He G, et al. , 2022. Contribution of heavy metals in PM2.5 to cardiovascular disease mortality risk, a case study in Guangzhou, China. Chemosphere 297, 134102. 10.1016/j.chemosphere.2022.134102. [DOI] [PubMed] [Google Scholar]
- Hassoun PM, 2021. Pulmonary arterial hypertension. N. Engl. J. Med. 385, 2361–2376. 10.1056/NEJMra2000348. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Croft J, 1993. Review of the relation between blood lead and blood pressure. Epidemiol. Rev. 15, 352–373. 10.1093/oxfordjournals.epirev.a036125. [DOI] [PubMed] [Google Scholar]
- Huang YC, Ghio AJ, 2006. Vascular effects of ambient pollutant particles and metals. Curr. Vasc. Pharmacol. 4, 199–203. 10.2174/157016106777698351. [DOI] [PubMed] [Google Scholar]
- Huang JP, Hopkins CD, Wessel C, Chen O, Akca O, Cai L, et al. , 2021. Metallomics profile in pulmonary arterial hypertension patients. Chest 160, 2299a–2300a. 10.1016/j.chest.2021.07.2005. [DOI] [Google Scholar]
- Ilhan F, Kalkanli ST, 2015. Atherosclerosis and the role of immune cells. World J. Clin. Cases. 3, 345–352. 10.12998/wjcc.v3.i4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam SJ, Kim JH, Baltrus P, Topel ML, Liu C, Ko YA, et al. , 2022. Neighborhood characteristics and ideal cardiovascular health among black adults: results from the Morehouse-Emory cardiovascular (MECA) Center for Health Equity. Ann. Epidemiol. 65 10.1016/j.annepidem.2020.11.009, 120 e121–120 e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James KA, Byers T, Hokanson JE, Meliker JR, Zerbe GO, Marshall JA, 2015. Association between lifetime exposure to inorganic arsenic in drinking water and coronary heart disease in Colorado residents. Environ. Health Perspect. 123, 128–134. 10.1289/ehp.1307839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarup L, Akesson A, 2009. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 238, 201–208. 10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Jarup L, Berglund M, Elinder CG, Nordberg G, Vahter M, 1998. Health effects of cadmium exposure–a review of the literature and a risk estimate. Scand. J. Work Environ. Health 24 (Suppl. 1), 1–51 doi. [PubMed] [Google Scholar]
- Jiang X, Yu W, Wu S, Tang L, Zhong G, Wan F, et al. , 2021. Arsenic (III) and/or Antimony (III) induced disruption of calcium homeostasis and endoplasmic reticulum stress resulting in apoptosis in mice heart. Ecotoxicol. Environ. Saf. 220, 112394 10.1016/j.ecoenv.2021.112394. [DOI] [PubMed] [Google Scholar]
- Jilani MH, Javed Z, Yahya T, Valero-Elizondo J, Khan SU, Kash B, et al. , 2021. Social determinants of health and cardiovascular disease: current state and future directions towards healthcare equity. Curr. Atheroscler. Rep. 23, 55. 10.1007/s11883-021-00949-w. [DOI] [PubMed] [Google Scholar]
- Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, et al. , 2011. Arsenic: toxicity, oxidative stress and human disease. J. Appl. Toxicol. 31, 95–107. 10.1002/jat.1649. [DOI] [PubMed] [Google Scholar]
- Kao YH, Yu CL, Chang LW, Yu HS, 2003. Low concentrations of arsenic induce vascular endothelial growth factor and nitric oxide release and stimulate angiogenesis in vitro. Chem. Res. Toxicol. 16, 460–468. 10.1021/tx025652a. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Punshon T, Davis M, Bulka CM, Slaughter F, Karalis D, et al. , 2019. Rice intake and emerging concerns on arsenic in rice: a review of the human evidence and methodologic challenges. Curr. Environ. Health Rep. 6, 361–372. 10.1007/s40572-019-00249-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Melough MM, Vance TM, Noh H, Koo SI, Chun OK, 2018a. Dietary cadmium intake and sources in the US. Nutrients 11. 10.3390/nu11010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Shim D, Lee JS, Zaitsev K, Williams JW, Kim KW, et al. , 2018b. Transcriptome analysis reveals nonfoamy rather than foamy plaque macrophages are proinflammatory in atherosclerotic murine models. Circ. Res. 123, 1127–1142. 10.1161/CIRCRESAHA.118.312804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SV, Bhattacharya S, 2000. In vitro toxicity of mercury, cadmium, and arsenic to platelet aggregation: influence of adenylate cyclase and phosphodiesterase activity. In Vitr. Mol. Toxicol. 13, 137–144. 10.1089/109793300440721. [DOI] [PubMed] [Google Scholar]
- Lagat DK, DeLong AK, Wellenius GA, Carter EJ, Bloomfield GS, Velazquez EJ, et al. , 2014. Factors associated with isolated right heart failure in women: a pilot study from western Kenya. Glob. Heart 9, 249–254. 10.1016/j.gheart.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahm T, Douglas IS, Archer SL, Bogaard HJ, Chesler NC, Haddad F, et al. , 2018. Assessment of right ventricular function in the research setting: knowledge gaps and pathways forward. An official American thoracic society research statement. Am. J. Respir. Crit. Care Med. 198, e15–e43. 10.1164/rccm.201806-1160ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhal-Littleton S, Crosby A, Frise MC, Mohammad G, Carr CA, Loick PAM, et al. , 2019. Intracellular iron deficiency in pulmonary arterial smooth muscle cells induces pulmonary arterial hypertension in mice. Proc. Natl. Acad. Sci. U. S. A. 116, 13122–13130. 10.1073/pnas.1822010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas GA, Ergui I, 2016. Chelation therapy to treat atherosclerosis, particularly in diabetes: is it time to reconsider? Expert. Rev. Cardiovasc. Ther. 14, 927–938. 10.1080/14779072.2016.1180977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas GA, Goertz C, Boineau R, Mark DB, Rozema T, Nahin RL, et al. , 2013. Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA 309, 1241–1250. 10.1001/jama.2013.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MY, Bae ON, Chung SM, Kang KT, Lee JY, Chung JH, 2002. Enhancement of platelet aggregation and thrombus formation by arsenic in drinking water: a contributing factor to cardiovascular disease. Toxicol. Appl. Pharmacol. 179, 83–88. 10.1006/taap.2001.9356. [DOI] [PubMed] [Google Scholar]
- Lee MY, Jung BI, Chung SM, Bae ON, Lee JY, Park JD, et al. , 2003. Arsenic-induced dysfunction in relaxation of blood vessels. Environ. Health Perspect. 111, 513–517. 10.1289/ehp.5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire M, Lemarie CA, Flores Molina M, Guilbert C, Lehoux S, Mann KK, 2014. Genetic deletion of LXRalpha prevents arsenic-enhanced atherosclerosis, but not arsenic-altered plaque composition. Toxicol. Sci. 142, 477–488. 10.1093/toxsci/kfu197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire M, Negro Silva LF, Lemarie CA, Bolt AM, Flores Molina M, Krohn RM, et al. , 2015. Arsenic exposure increases monocyte adhesion to the vascular endothelium, a pro-atherogenic mechanism. PLoS One 10, e0136592. 10.1371/journal.pone.0136592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemus R, Venezia CF, 2015. An update to the toxicological profile for water-soluble and sparingly soluble tungsten substances. Crit. Rev. Toxicol. 45, 388–411. 10.3109/10408444.2014.1003422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Young JL, Kong M, Tong Y, Qian Y, Freedman JH, et al. , 2019. Gender differences in cardiac remodeling induced by a high-fat diet and lifelong, low-dose cadmium exposure. Chem. Res. Toxicol. 32, 1070–1081. 10.1021/acs.chemrestox.8b00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JD, Nishi H, Poles J, Niu X, McCauley C, Rahman K, et al. , 2019. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight 4. 10.1172/jci.insight.124574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HC, Hao WM, Chu PH, 2021. Cadmium and cardiovascular disease: an overview of pathophysiology, epidemiology, therapy, and predictive value. Rev. Port. Cardiol. (Engl Ed) 40, 611–617. 10.1016/j.repce.2021.07.031. [DOI] [PubMed] [Google Scholar]
- Lisabeth LD, Ahn HJ, Chen JJ, Sealy-Jefferson S, Burke JF, Meliker JR, 2010. Arsenic in drinking water and stroke hospitalizations in Michigan. Stroke 41, 2499–2504. 10.1161/STROKEAHA.110.585281. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. , 2010. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 121, 586–613. 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- Makhani K, Chiavatti C, Plourde D, Negro Silva LF, Lemaire M, Lemarie CA, et al. , 2018. Using the apolipoprotein E knock-out mouse model to define atherosclerotic plaque changes induced by low dose arsenic. Toxicol. Sci. 166, 213–218. 10.1093/toxsci/kfy201. [DOI] [PubMed] [Google Scholar]
- Mantha M, Yeary E, Trent J, Creed PA, Kubachka K, Hanley T, et al. , 2017. Estimating inorganic arsenic exposure from U.S. rice and total water intakes. Environ. Health Perspect. 125, 057005 10.1289/EHP418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Locati M, 2009. Orchestration of macrophage polarization. Blood 114, 3135–3136. 10.1182/blood-2009-07-231795. [DOI] [PubMed] [Google Scholar]
- Martorell I, Perello G, Marti-Cid R, Llobet JM, Castell V, Domingo JL, 2011. Human exposure to arsenic, cadmium, mercury, and lead from foods in Catalonia, Spain: temporal trend. Biol. Trace Elem. Res. 142, 309–322. 10.1007/s12011-010-8787-x. [DOI] [PubMed] [Google Scholar]
- Meliker JR, Wahl RL, Cameron LL, Nriagu JO, 2007. Arsenic in drinking water and cerebrovascular disease, diabetes mellitus, and kidney disease in Michigan: a standardized mortality ratio analysis. Environ. Health 6, 4. 10.1186/1476-069X-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PA, Brown MJ, Falk H, 2008. Global approach to reducing lead exposure and poisoning. Mutat. Res. 659, 166–175. 10.1016/j.mrrev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Misbahuddin M, 2003. Consumption of arsenic through cooked rice. Lancet 361, 435–436. 10.1016/S0140-6736(03)12416-6. [DOI] [PubMed] [Google Scholar]
- Moon K, Guallar E, Navas-Acien A, 2012. Arsenic exposure and cardiovascular disease: an updated systematic review. Curr. Atheroscler. Rep. 14, 542–555. 10.1007/s11883-012-0280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon KA, Guallar E, Umans JG, Devereux RB, Best LG, Francesconi KA, et al. , 2013. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease. A prospective cohort study. Ann. Intern. Med. 159, 649–659. 10.7326/0003-4819-159-10-201311190-00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon KA, Oberoi S, Barchowsky A, Chen Y, Guallar E, Nachman KE, et al. , 2017. A dose-response meta-analysis of chronic arsenic exposure and incident cardiovascular disease. Int. J. Epidemiol. 46, 1924–1939. 10.1093/ije/dyx202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movassagh M, Choy MK, Goddard M, Bennett MR, Down TA, Foo RS, 2010. Differential DNA methylation correlates with differential expression of angiogenic factors in human heart failure. PLoS One 5, e8564. 10.1371/journal.pone.0008564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, et al. , 2013. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ. Health Perspect. 121, 295–302. 10.1289/ehp.1205875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Nachman KE, 2013. Public health responses to arsenic in rice and other foods. JAMA Intern. Med. 173, 1395–1396. 10.1001/jamainternmed.2013.6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Selvin E, Sharrett AR, Calderon-Aranda E, Silbergeld E, Guallar E, 2004. Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation 109, 3196–3201. 10.1161/01.CIR.0000130848.18636.B2. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A, Silbergeld EK, Sharrett R, Calderon-Aranda E, Selvin E, Guallar E, 2005. Metals in urine and peripheral arterial disease. Environ. Health Perspect. 113, 164–169. 10.1289/ehp.7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ, 2007. Lead exposure and cardiovascular disease–a systematic review. Environ. Health Perspect. 115, 472–482. 10.1289/ehp.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrot TS, Staessen JA, 2006. Low-level environmental exposure to lead unmasked as silent killer. Circulation 114, 1347–1349. 10.1161/CIRCULATIONAHA.106.650440. [DOI] [PubMed] [Google Scholar]
- Nawrot TS, Thijs L, Den Hond EM, Roels HA, Staessen JA, 2002. An epidemiological re-appraisal of the association between blood pressure and blood lead: a meta-analysis. J. Hum. Hypertens. 16, 123–131. 10.1038/sj.jhh.1001300. [DOI] [PubMed] [Google Scholar]
- Negro Silva LF, Lemaire M, Lemarie CA, Plourde D, Bolt AM, Chiavatti C, et al. , 2017. Effects of inorganic arsenic, methylated arsenicals, and arsenobetaine on atherosclerosis in the mouse model and the role of As3mt-mediated methylation. Environ. Health Perspect. 125, 077001 10.1289/EHP806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negro Silva LF, Makhani K, Lemaire M, Lemarie CA, Plourde D, Bolt AM, et al. , 2021. Sex-specific effects of prenatal and early life inorganic and methylated arsenic exposure on atherosclerotic plaque development and composition in adult ApoE−/− mice. Environ. Health Perspect. 129, 57008. 10.1289/EHP8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigra AE, Ruiz-Hernandez A, Redon J, Navas-Acien A, Tellez-Plaza M, 2016. Environmental metals and cardiovascular disease in adults: a systematic review beyond lead and cadmium. Curr. Environ. Health Rep. 3, 416–433. 10.1007/s40572-016-0117-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigra AE, Nachman KE, Love DC, Grau-Perez M, Navas-Acien A, 2017. Poultry consumption and arsenic exposure in the U.S. population. Environ. Health Perspect. 125, 370–377. 10.1289/EHP351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigra AE, Howard BV, Umans JG, Best L, Francesconi KA, Goessler W, et al. , 2018. Urinary tungsten and incident cardiovascular disease in the strong heart study: an interaction with urinary molybdenum. Environ. Res. 166, 444–451. 10.1016/j.envres.2018.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobs SP, Kopf M, 2021. Tissue-resident macrophages: guardians of organ homeostasis. Trends Immunol. 42, 495–507. 10.1016/j.it.2021.04.007. [DOI] [PubMed] [Google Scholar]
- Nordberg G, 2007. Handbook on the Toxicology of Metals. Academic Press, Amsterdam, Boston: pp. xlvii, 975 p. [Google Scholar]
- OSHA. Toxic Metals - Overview Occupational Safety and Health Administration, Accessed June 20, 2022. doi: https://www.osha.gov/toxic-metals.
- Paithankar JG, Saini S, Dwivedi S, Sharma A, Chowdhuri DK, 2021. Heavy metal associated health hazards: an interplay of oxidative stress and signal transduction. Chemosphere 262, 128350. 10.1016/j.chemosphere.2020.128350. [DOI] [PubMed] [Google Scholar]
- Pi J, Kumagai Y, Sun G, Yamauchi H, Yoshida T, Iso H, et al. , 2000. Decreased serum concentrations of nitric oxide metabolites among Chinese in an endemic area of chronic arsenic poisoning in inner Mongolia. Free Radic. Biol. Med. 28, 1137–1142. 10.1016/s0891-5849(00)00209-4. [DOI] [PubMed] [Google Scholar]
- Pi J, Yamauchi H, Kumagai Y, Sun G, Yoshida T, Aikawa H, et al. , 2002. Evidence for induction of oxidative stress caused by chronic exposure of Chinese residents to arsenic contained in drinking water. Environ. Health Perspect. 110, 331–336. 10.1289/ehp.02110331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi J, Yamauchi H, Sun G, Yoshida T, Aikawa H, Fujimoto W, et al. , 2005. Vascular dysfunction in patients with chronic arsenosis can be reversed by reduction of arsenic exposure. Environ. Health Perspect. 113, 339–341. 10.1289/ehp.7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda-Zavaleta AP, Garcia-Vargas G, Borja-Aburto VH, Acosta-Saavedra LC, Vera Aguilar E, Gomez-Munoz A, et al. , 2004. Nitric oxide and superoxide anion production in monocytes from children exposed to arsenic and lead in region Lagunera, Mexico. Toxicol. Appl. Pharmacol. 198, 283–290. 10.1016/j.taap.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Poulsen AH, Sears CG, Harrington J, Howe CJ, James KA, Roswall N, et al. , 2021. Urinary cadmium and stroke - a case-cohort study in Danish never-smokers. Environ. Res. 200, 111394 10.1016/j.envres.2021.111394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell-Wiley TM, Baumer Y, Baah FO, Baez AS, Farmer N, Mahlobo CT, et al. , 2022. Social determinants of cardiovascular disease. Circ. Res. 130, 782–799. 10.1161/CIRCRESAHA.121.319811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. , 1987. Primary pulmonary hypertension. A national prospective study. Ann. Intern. Med. 107, 216–223. 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- Rich DQ, Freudenberger RS, Ohman-Strickland P, Cho Y, Kipen HM, 2008. Right heart pressure increases after acute increases in ambient particulate concentration. Environ. Health Perspect. 116, 1167–1171. 10.1289/ehp.11230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. , 2012. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation 125, e2–e220. 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. , 2020. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J. Am. Coll. Cardiol. 76, 2982–3021. 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Navarro ML, Navarro-Alarcon M, Gonzalez-de Lopez, la Serrana H, Perez-Valero V, Lopez-Martinez MC, 1998. Urine arsenic concentrations in healthy adults as indicators of environmental contamination: relation with some pathologies. Sci. Total Environ. 216, 55–61. 10.1016/s0048-9697(98)00136-3. [DOI] [PubMed] [Google Scholar]
- Ruopp NF, Cockrill BA, 2022. Diagnosis and treatment of pulmonary arterial hypertension: a review. JAMA 327, 1379–1391. 10.1001/jama.2022.4402. [DOI] [PubMed] [Google Scholar]
- Ryan JJ, Archer SL, 2014. The right ventricle in pulmonary arterial hypertension: disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circ. Res. 115, 176–188. 10.1161/CIRCRESAHA.113.301129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CW, 2014. Low-dose arsenic: in search of a risk threshold. Environ. Health Perspect. 122, A130–A134. 10.1289/ehp.122-A130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CW, 2015. In search of “just right”: the challenge of regulating arsenic in rice. Environ. Health Perspect. 123, A16–A19. 10.1289/ehp.123-A16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J, 1995. Lead, blood pressure, and cardiovascular disease in men. Arch. Environ. Health 50, 31–37. 10.1080/00039896.1995.9955010. [DOI] [PubMed] [Google Scholar]
- Shiue I, Hristova K, 2014. Higher urinary heavy metal, phthalate and arsenic concentrations accounted for 3–19% of the population attributable risk for high blood pressure: US NHANES, 2009–2012. Hypertens. Res. 37, 1075–1081. 10.1038/hr.2014.121. [DOI] [PubMed] [Google Scholar]
- Simeonova PP, Hulderman T, Harki D, Luster MI, 2003. Arsenic exposure accelerates atherogenesis in apolipoprotein E(−/−) mice. Environ. Health Perspect. 111, 1744–1748. 10.1289/ehp.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. , 2019. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 53 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel MH, Sanchez TR, Jones MR, Kaufman JD, Francesconi KA, Blaha MJ, et al. , 2020. Rice intake, arsenic exposure, and subclinical cardiovascular disease among US adults in MESA. J. Am. Heart Assoc. 9, e015658 10.1161/JAHA.119.015658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohel N, Persson LA, Rahman M, Streatfield PK, Yunus M, Ekstrom EC, et al. , 2009. Arsenic in drinking water and adult mortality: a population-based cohort study in rural Bangladesh. Epidemiology 20, 824–830. 10.1097/EDE.0b013e3181bb56ec. [DOI] [PubMed] [Google Scholar]
- Sonnweber T, Pizzini A, Tancevski I, Loffler-Ragg J, Weiss G, 2020. Anaemia, iron homeostasis and pulmonary hypertension: a review. Intern. Emerg. Med. 15, 573–585. 10.1007/s11739-020-02288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy NV, Klei LR, Mayka DD, Barchowsky A, 2004. Signaling pathways for arsenic-stimulated vascular endothelial growth factor-a expression in primary vascular smooth muscle cells. Chem. Res. Toxicol. 17, 555–563. 10.1021/tx034193q. [DOI] [PubMed] [Google Scholar]
- Soucy NV, Mayka D, Klei LR, Nemec AA, Bauer JA, Barchowsky A, 2005. Neovascularization and angiogenic gene expression following chronic arsenic exposure in mice. Cardiovasc. Toxicol. 5, 29–41. 10.1385/ct:5:1:029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, D’Souza SE, Sen U, States JC, 2007. In utero arsenic exposure induces early onset of atherosclerosis in ApoE−/− mice. Reprod. Toxicol. (Elmsford, NY) 23, 449–456. 10.1016/j.reprotox.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staessen JA, Roels H, Lauwerys RR, Amery A, 1995. Low-level lead exposure and blood pressure. J. Hum. Hypertens. 9, 303–328 doi. [PubMed] [Google Scholar]
- States JC, Srivastava S, Sen U, Miller HL, D’Souza SE, 2005. Arsenic exposure accelerates atherogenic changes in ApoE−/− mice. Soc. Toxicol. 84, 141. New Orleans. [Google Scholar]
- Straub AC, Clark KA, Ross MA, Chandra AG, Li S, Gao X, et al. , 2008. Arsenic-stimulated liver sinusoidal capillarization in mice requires NADPH oxidase-generated superoxide. J. Clin. Invest. 118, 3980–3989. 10.1172/JCI35092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Q, Ma J, Zhou M, Wang D, Wang B, Nie X, et al. , 2020. Heavy metals exposure, lipid peroxidation and heart rate variability alteration: association and mediation analyses in urban adults. Ecotoxicol. Environ. Saf. 205, 111149 10.1016/j.ecoenv.2020.111149. [DOI] [PubMed] [Google Scholar]
- Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ, 2012. Heavy metal toxicity and the environment. Exp. Suppl. 101, 133–164. 10.1007/978-3-7643-8340-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez-Plaza M, Navas-Acien A, Crainiceanu CM, Guallar E, 2008. Cadmium exposure and hypertension in the 1999–2004 National Health and Nutrition Examination Survey (NHANES). Environ. Health Perspect. 116, 51–56. 10.1289/ehp.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez-Plaza M, Jones MR, Dominguez-Lucas A, Guallar E, Navas-Acien A, 2013. Cadmium exposure and clinical cardiovascular disease: a systematic review. Curr. Atheroscler. Rep. 15, 356. 10.1007/s11883-013-0356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkov AA, Filippini T, Ajsuvakova OP, Skalnaya MG, Aaseth J, Bjorklund G, et al. , 2018. Cadmium and atherosclerosis: a review of toxicological mechanisms and a meta-analysis of epidemiologic studies. Environ. Res. 162, 240–260. 10.1016/j.envres.2018.01.008. [DOI] [PubMed] [Google Scholar]
- Tirmenstein MA, Plews PI, Walker CV, Woolery MD, Wey HE, Toraason MA, 1995. Antimony-induced oxidative stress and toxicity in cultured cardiac myocytes. Toxicol. Appl. Pharmacol. 130, 41–47. 10.1006/taap.1995.1006. [DOI] [PubMed] [Google Scholar]
- Tsai SH, Liang YC, Chen L, Ho FM, Hsieh MS, Lin JK, 2002. Arsenite stimulates cyclooxygenase-2 expression through activating IkappaB kinase and nuclear factor kappaB in primary and ECV304 endothelial cells. J. Cell. Biochem. 84, 750–758. 10.1002/jcb.10096. [DOI] [PubMed] [Google Scholar]
- Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. , 2022. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation 145, e153–e639. 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- Turdi S, Sun W, Tan Y, Yang X, Cai L, Ren J, 2013. Inhibition of DNA methylation attenuates low-dose cadmium-induced cardiac contractile and intracellular Ca(2+) anomalies. Clin. Exp. Pharmacol. Physiol. 40, 706–712. 10.1111/1440-1681.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkcan A, Scharinger B, Grabmann G, Keppler BK, Laufer G, Bernhard D, et al. , 2015. Combination of cadmium and high cholesterol levels as a risk factor for heart fibrosis. Toxicol. Sci. 145, 360–371. 10.1093/toxsci/kfv057. [DOI] [PubMed] [Google Scholar]
- Tyrrell J, Galloway TS, Abo-Zaid G, Melzer D, Depledge MH, Osborne NJ, 2013. High urinary tungsten concentration is associated with stroke in the National Health and nutrition examination survey 1999–2010. PLoS One 8, e77546. 10.1371/journal.pone.0077546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri ND, Sica DA, 2004. Lead-induced hypertension: role of oxidative stress. Curr. Hypertens. Rep. 6, 314–320. 10.1007/s11906-004-0027-3. [DOI] [PubMed] [Google Scholar]
- Wade TJ, Xia Y, Wu K, Li Y, Ning Z, Le XC, et al. , 2009. Increased mortality associated with well-water arsenic exposure in Inner Mongolia, China. Int. J. Environ. Res. Public Health 6, 1107–1123. 10.3390/ijerph6031107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade TJ, Xia Y, Mumford J, Wu K, Le XC, Sams E, et al. , 2015. Cardiovascular disease and arsenic exposure in Inner Mongolia, China: a case control study. Environ. Health 14, 35. 10.1186/s12940-015-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Fang J, Leonard SS, Rao KM, 2004. Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic. Biol. Med. 36, 1434–1443. 10.1016/j.freeradbiomed.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Wasel O, Freeman JL, 2018. Comparative assessment of tungsten toxicity in the absence or presence of other metals. Toxics 6. 10.3390/toxics6040066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Jain N, Nie H, Sparrow D, Vokonas P, Schwartz J, et al. , 2009. A prospective study of bone lead concentration and death from all causes, cardiovascular diseases, and cancer in the department of veterans affairs normative aging study. Circulation 120, 1056–1064. 10.1161/CIRCULATIONAHA.108.827121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittemore AS, DiCiccio Y, Provenzano G, 1991. Urinary cadmium and blood pressure: results from the NHANES II survey. Environ. Health Perspect. 91, 133–140. 10.1289/ehp.9191133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2000. Arsenic. In: Air Quality Guidelines for Europe. Regional Office for Europe. World Health Organization. Accessed June 20, 2022. doi:https://www.euro.who.int/__data/assets/pdf_file/0014/123071/AQG2ndEd_6_1_Arsenic.PDF#:~:text=Particulate%20arsenic%20compounds%20may%20be%20inhaled%2C%20deposited%20in,in%20cities%20without%20substantial%20industrial%20emission%20of%20arsenic. [Google Scholar]
- Wu MM, Chiou HY, Wang TW, Hsueh YM, Wang IH, Chen CJ, et al. , 2001. Association of blood arsenic levels with increased reactive oxidants and decreased antioxidant capacity in a human population of northeastern Taiwan. Environ. Health Perspect. 109, 1011–1017. 10.1289/ehp.011091011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MM, Chiou HY, Ho IC, Chen CJ, Lee TC, 2003. Gene expression of inflammatory molecules in circulating lymphocytes from arsenic-exposed human subjects. Environ. Health Perspect. 111, 1429–1438 doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Liu S, Aodengqimuge, Wang H, Hu M, Xing C, et al. , 2017. Arsenite induces vascular endothelial cell dysfunction by activating IRE1alpha/XBP1s/HIF1alpha-dependent ANGII signaling. Toxicol. Sci. 160, 315–328. 10.1093/toxsci/kfx184. [DOI] [PubMed] [Google Scholar]
- Xu L, Polya DA, Li Q, Mondal D, 2020. Association of low-level inorganic arsenic exposure from rice with age-standardized mortality risk of cardiovascular disease (CVD) in England and Wales. Sci. Total Environ. 743, 140534 10.1016/j.scitotenv.2020.140534. [DOI] [PubMed] [Google Scholar]
- Yang AM, Lo K, Zheng TZ, Yang JL, Bai YN, Feng YQ, et al. , 2020. Environmental heavy metals and cardiovascular diseases: status and future direction. Chronic. Dis. Transl. Med. 6, 251–259. 10.1016/j.cdtm.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernecke A, Winkels H, Cochain C, Williams JW, Wolf D, Soehnlein O, et al. , 2020. Meta-analysis of leukocyte diversity in atherosclerotic mouse aortas. Circ. Res. 127, 402–426. 10.1161/CIRCRESAHA.120.316903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ji X, Ku T, Li G, Sang N, 2016. Heavy metals bound to fine particulate matter from northern China induce season-dependent health risks: a study based on myocardial toxicity. Environ. Pollut. 216, 380–390. 10.1016/j.envpol.2016.05.072. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Young JL, Cai L, Tong YG, Miao L, Freedman JH, 2020. Corrigendum to “Chronic exposure to arsenic and high fat diet induces sex-dependent pathogenic effects on the kidney” [Chem. Biol. Interact. 310 (2019) 108719]. Chem. Biol. Interact. 317, 108967. 10.1016/j.cbi.2020.108967. [DOI] [PubMed] [Google Scholar]
- Zhou W, Young JL, Men H, Zhang H, Yu H, Lin Q, et al. , 2022. Sex differences in the effects of whole-life, low-dose cadmium exposure on postweaning high-fat diet-induced cardiac pathogeneses. Sci. Total Environ. 809, 152176 10.1016/j.scitotenv.2021.152176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierold KM, Knobeloch L, Anderson H, 2004. Prevalence of chronic diseases in adults exposed to arsenic-contaminated drinking water. Am. J. Public Health 94, 1936–1937. 10.2105/ajph.94.11.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


