Abstract
Background
Chronic inflammation is a key feature of obesity and a hallmark of colon cancer (CC). The obesity-related hormones leptin and adiponectin alter inflammatory gene profiles in cancer, but their specific role in CC is unclear. We have previously studied the effects of leptin and the macrophage-specific mediator itaconate on M2-like macrophages. This current study evaluates their effects on CC cells.
Methods
HT-29 CC cells (derived from a young patient, stage III CC) were treated with either leptin, adiponectin, 4-octyl itaconate (OI) or dimethyl itaconate (DI). Gene expression after treatment was analyzed at four time points (3, 6, 18, and 24 h).
Results
CCL22 was upregulated after treatment with adiponectin (at 18 h [FC 16.3, p < 0.001]). IL-8 expression increased following both adiponectin (at 3 h [FC 68.1, p < 0.001]) and leptin treatments (at 6 h [FC 7.3, p < 0.001]), while OI induced downregulation of IL-8 (at 24 h [FC −5.0, p < 0.001]). CXCL10 was upregulated after adiponectin treatment (at 6 h [FC 3.0, p = 0.025]) and downregulated by both OI and DI at 24 h, respectively (OI [FC −10.0, p < 0.001]; DI [FC −10.0, p < 0.001]). IL-1β was upregulated after adiponectin treatment (at 3 h [FC 10.6, p < 0.001]) and downregulated by DI (at 24 h [FC −5.0, p < 0.001]). TNF-α expression was induced following adiponectin (at 6 h [FC 110.7, p < 0.001]), leptin (at 18 h [FC 5.8, p = 0.027]) and OI (at 3 h [FC 91.1, p = 0.001]). PPARγ was affected by both OI (at 3 h [FC 10.1, p = 0.031], at 24 h [FC −10.0, p = 0.031]) and DI (at 18 h [FC −1.7, p = 0.033]).
Conclusions
Obesity hormones directly affect inflammatory gene expression in HT29 CC cells, potentially enhancing cancer progression. Itaconate affects the prognostic marker PPARγ in HT29 CC cells. Leptin, adiponectin and itaconate may represent a link between obesity and CC.
Keywords: Colon cancer, Obesity, Inflammation, Metabolic dysfunction, Macrophages
Abbreviations: CCL22, C–C motif chemokine ligand (CCL) 22; FC, fold change; IL, interleukin; PPARγ, peroxisome proliferator activated receptor gamma; TNF, tumor necrosis factor
1. Introduction
Tumor immunology plays an essential role in colon cancer (CC), particularly since both tumor development and progression of CC are directly linked to inflammatory mediators [1]. Cytokine networks can promote cell proliferation, migration, and resistance to apoptosis in CC and are affected by systemic mediators as well as cellular signaling within the tumor microenvironment (TME) [1,2]. As part of innate and adaptive immune cells regulating the pro- and anti-inflammatory gene expression in tumors, cancer cells interact with these cells within the TME and modify their gene expression profile to promote their own growth [1,3]. The obesity-related hormones leptin and adiponectin can alter cytokine profiles in cancer cells and, thereby, either inhibit or promote carcinogenic mechanisms [2]. These effects have been discussed as a potential link between metabolic dysfunction and early-onset CC (EOCC) in young patients less than 50 years old [4,5]. A protective function of adiponectin has been postulated for several types of cancers, inhibiting genes that promote apoptosis and cell migration [2,6]. Opposite effects have been identified for leptin affecting the STAT3-NFκB axis, which is critical in CC [1,2]. The macrophage-specific metabolite itaconate and its cell-permeable derivatives 4-octyl itaconate (OI) and dimethyl-itaconate (DI) are strong inflammatory mediators with a potential role in different types of cancers, but their effects on cytokine expression profiles in CC are poorly understood [7].
The acute inflammatory response is critical for host defense and mediates antitumorigenic effects. In contrast, chronic proinflammatory stress leads to an immunosuppressive environment over time, exerting predominately anti-inflammatory features [8]. Chronic inflammation is a key feature of obesity and is also a hallmark of CC, which is linked to a phenotype characterized by a high M2/M1 macrophage ratio and secretion of immunosuppressive cytokines and chemokines by cells within the TME, such as interleukin (IL)-10 and C–C motif chemokine ligand (CCL) 22 [8]. This induces recruitment of T-helper cells and regulatory T-cells and decreased cytotoxic T-cell activity, thereby inhibiting cancer cell apoptosis [8].
CC cells have been shown to secrete mediators that modify TAM polarization and cytokine expression, further enhancing tumor growth and progression through IL-6 [4,8].
Leptin, adiponectin, and itaconate affect cellular metabolism and marker expression of cancer and immune cells. The two isotypes of adiponectin receptors, adiponectin receptor 1 and 2 (AdipoR1 and AdipoR2), are both expressed in CC cells and are associated with decreased epithelial cell proliferation [9]. Leptin receptor expression as well as expression of the actual hormone leptin is increased in CC as compared to normal colon cells and promote tumor growth [10,11]. Furthermore, leptin can reduce the cytotoxic effects of the common chemotherapeutic agent 5-fluorouracil on CC [12]. A tumor inhibiting role of leptin under certain circumstances is, however, still discussed [13]. We have recently shown that leptin and itaconate can exert cancer-promoting effects on macrophages in vitro [14].
The aim of this study was to define cytokine and gene expression profiles in CC cells as a response to the obesity-related hormones leptin and adiponectin and the macrophage-specific mediator itaconate.
2. Methods
2.1. Cell culture and treatment of HT-29 cells
The HT-29 cell line was purchased from the American Type Culture Collection (ATCC, Manassas, USA). These cells were derived from a 44-year-old woman with stage III CC. Short tandem repeat (STR) analysis was performed to authenticate the HT-29 cell line (ATCC STR Profiling Service, Manassas, USA). Cells were grown at 0.5 × 106 cells/mL in 150 cm2 cell culture flasks (Corning, New York, USA) containing 20 mL of RPMI-1640 growth media (ATCC, Manassas, USA) supplemented with 10% fetal bovine serum (ATCC, Manassas, USA) 1% L-glut, 10,000 units/mL penicillin, and 10 mg/mL streptomycin.
The treatment compounds leptin (BioVendor R&D, Brno, Czech Republic, Catalog no. RD172001100), adiponectin (BioVendor R&D, Brno, Czech Republic, Catalog no. RD172023100), 4-octyl-itaconate (4-OI) (Sigma-Aldrich, St. Louis, USA, Catalog no. SML2338-25MG), and dimethyl itaconate (DI) (Sigma-Aldrich, St. Louis, USA, Catalog no. 592498-100MG) were prepared according to the manufacturer’s instructions. Compounds were diluted, if applicable, using phosphate-buffered saline (PBS) to final treatment concentrations of 300 ng/ml leptin, 20 μg/ml adiponectin, 50 μg/ml 4-OI, and 50 μg/ml DI.
HT29 cells were seeded at a concentration of 0.2 × 106 cells/well into 24-well cell culture plates. Cells were treated in duplicates with either leptin (n = 10), adiponectin (n = 10), 4-OI (n = 10), or DI (n = 10) in a time-response experiment. Negative controls were performed for each individual treatment and time point (n = 10) using 10 μl/ml PBS. Gene expression was measured at 3, 6, 18, and 24 h post-treatment for each compound.
2.2. Gene expression analysis using quantitative real-time PCR
The RNeasy purification kit (Qiagen, Maryland, USA) was used to isolate total RNA from the harvested HT29 cells. RNA was quantified using spectrophotometry (NanoDrop 1000, Thermo Scientific, Massachusetts, USA). We used 20 ng of total RNA paired with high-capacity cDNA reverse transcription kit (Applied Biosystems, California, USA) to perform reverse transcription to cDNA according to the manufacturers protocol. Quantitative real-time PCR (qRT-PCR) was run with Taqman Gene Expression Assays and Fast Advanced Master mix (Applied Biosystems, California, USA) using Step-One Real-Time PCR systems (Applied Biosystems, California, USA). The 18S ribosomal RNA (rRNA) gene was used as the housekeeping gene to normalize results for each target gene listed (Supplemental Table S1).
Gene expression of the following genes was measured (all primers were purchased from Thermo Scientific, Massachusetts, USA): Tumor necrosis factor-alpha (TNF-α), CCL22,IL-1β, C-X-C motif chemokine ligand 10 (CXCL10), peroxisome proliferator-activated receptor γ (PPARγ), IL-8, CCL18, IL-10, cluster of differentiation 80 (CD80), IL-6, aconitate decarboxylase 1 (ACOD1), and nuclear factor κ B (NF-κB) (Supplemental Table S1 lists all primers with their corresponding assay IDs).
2.3. Statistical analysis
Statistical analysis was performed by using a random effect linear regression model for each combination of treatment and gene. The model to predict the CT value includes as predictors all combinations of time point (3, 6, 18, 24 h), treated cells versus negative controls, and target gene (gene of interest versus housekeeping gene 18S). Delta CT (ΔCT) and Delta-delta CT (ΔΔCT) at each of the four time points are estimated as contrasts from the model output. An overall test that considers if any of the four ΔΔCT values are different from zero is run, that is whether the treatment leads to over-expression at any time point at a significance level of α = 0.05. The p-values for the 4 tests of the ΔΔCT at each time point are adjusted to control the false discovery rate (FDR). Fold change (FC) was calculated using the formula 2−ΔΔCT. In case of downregulation of the respective gene, values were inverted according to −1/FC. Statistical calculations were performed using R statistical software, version 4.1.2 [15].
3. Results
Results including ΔCT values and FC are shown in Tables 1–4. Line graphs of significant results are shown in Figs. 1 and 2.
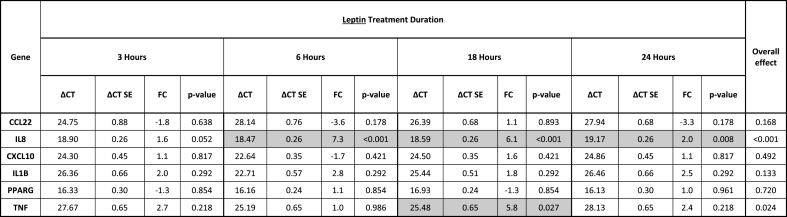
Table 1.
Gene expression of HT-29 colon cancer cells following treatment with leptin. Delta CT (ΔCT) values and fold changes (FC) are listed for all four different time points (3, 6, 18, and 24 h). P-values refer to the test of whether the treated cells are differentially expressed relative to untreated cells (ΔΔCT≠0) at each time point and have been adjusted to control the false discovery rate (FDR). The overall effect considers if the treated cells expressed at any of the 4 time points. Significant results are marked grey (p < 0.05).
Table 4.
Gene expression of HT-29 colon cancer cells following treatment with dimethyl itaconate (DI). Delta CT (ΔCT) values and fold changes (FC) are listed for all four different time points (3, 6, 18, and 24 h). Significant results are marked grey (p < 0.05). P-values refer to the test of whether the treated cells are differentially expressed relative to untreated cells (ΔΔCT≠0) at each time point and have been adjusted to control the false discovery rate (FDR).
Fig. 1.
Time response of significantly altered gene expression (mean ΔCT) following either leptin or adiponectin treatment. Results are plotted for four different time points (3, 6, 18, and 24 h), respectively. Error bars show standard deviations. Significant results at a certain time point compared to the respective control are marked with an asterisk. For leptin treatment (top panel ‘Leptin’), expression results of IL-8 and TNF-α are shown. For adiponectin treatment (bottom panel ‘Adiponectin’), expression results of CCL22, IL-8, CXCL10, IL-1β, and TNF-α are shown. Blue graphs = negative controls, Red graphs = treated cells, IL-8 = interleukin-8; TNF-α = tumor necrosis factor-alpha; CCL22 = C–C motif chemokine ligand 22; CXCL10 = C-X-C motif chemokine ligand 10; IL-1β = interleukin-1 beta. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
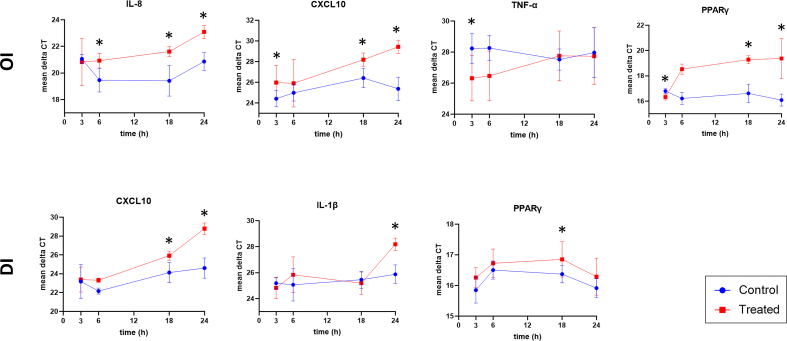
Fig. 2.
Time response of significantly altered gene expression (mean ΔCT) following either 4-octyl itaconate (OI) or dimethyl itaconate (DI) treatment. Results are plotted for four different time points (3, 6, 18, and 24 h), respectively. Error bars show standard deviations. Significant results at a certain time point compared to the respective control are marked with an asterisk. For OI treatment (top panel ‘OI’), expression results of IL-8, CXCL10, TNF-α, and PPARγ are shown. For DI treatment (bottom panel ‘DI’), expression results of CXCL10, IL-1β, and PPARγ are shown. Blue graphs = negative controls, Red graphs = treated cells, IL-8 = interleukin-8; CXCL10 = C-X-C motif chemokine ligand 10; TNF-α = tumor necrosis factor-alpha; PPARγ = peroxisome proliferator-activated receptor gamma; IL-1β = interleukin-1 beta. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
CC cells treated with adiponectin showed increased expression of anti-inflammatory CCL22 after 6 h of treatment, with a peak at 18 h (at 6 h [FC 7.0, p < 0.001], at 18 h [FC 16.3, p < 0.001]) (Table 2, Fig. 1c). All other cell treatments had no effect on CCL22 expression.
Table 2.
Gene expression of HT-29 colon cancer cells following treatment with adiponectin. Delta CT (ΔCT) values and fold changes (FC) are listed for all four different time points (3, 6, 18, and 24 h). Significant results are marked grey (p < 0.05). P-values refer to the test of whether the treated cells are differentially expressed relative to untreated cells (ΔΔCT≠0) at each time point and have been adjusted to control the false discovery rate (FDR).
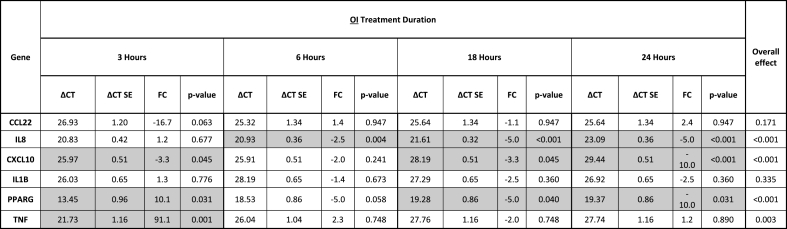
The highest increase in tumor-promoting IL-8 expression was demonstrated early after adiponectin treatment (Table 2, Fig. 1d). The maximum response was shown after 3 h (at 3 h [FC 68.1, p < 0.001], at 6 h [FC 20.3, p < 0.001], at 24 h [FC 4.3, p = 0.002]). Leptin induced a smaller upregulation of IL-8 expression in CC cells (at 6 h [FC 7.3, p < 0.001], at 18 h [FC 6.1, p < 0.001], at 24 h [FC 2.0, p = 0.008]) (Table 1, Fig. 1a). Treatment with OI resulted in downregulation of anti-inflammatory IL-8 expression (at 6 h [FC −2.5, p = 0.004], at 18 h [FC −5.0, p < 0.001], at 24 h [FC −5.0, p < 0.001]) (Table 3, Fig. 2a).
Table 3.
Gene expression of HT-29 colon cancer cells following treatment with 4-octyl itaconate (OI). Delta CT (ΔCT) values and fold changes (FC) are listed for all four different time points (3, 6, 18, and 24 h). Significant results are marked grey (p < 0.05). P-values refer to the test of whether the treated cells are differentially expressed relative to untreated cells (ΔΔCT≠0) at each time point and have been adjusted to control the false discovery rate (FDR).
Proinflammatory CXCL10 expression was slightly upregulated after cell treatment with adiponectin at three time points (at 3 h [FC 2.3, p = 0.044], at 6 h [FC 3.0, p = 0.025], at 24 h [FC 2.5, p = 0.044]) (Table 2, Fig. 1e). In contrast, a predominantly late cellular response with downregulation of CXCL10 was induced by both OI and DI, with a maximum effect at 24 h, respectively (OI at 3 h [FC −3.3, p = 0.045], OI at 18 h [FC −3.3, p = 0.045], OI at 24 h [FC −10.0, p < 0.001]; DI at 18 h [FC −3.3, p = 0.002], DI at 24 h [FC −10.0, p < 0.001]) (Tables 3 and 4, Fig. 2b and e).
Expression of proinflammatory IL-1β was induced early after adiponectin treatment at 3 h only (FC 10.6, p < 0.001) (Table 2, Fig. 1f). Contrary to adiponectin, DI treatment resulted in downregulation at 24 h only (FC −5.0, p < 0.001) (Table 4, Fig. 2f).
An early increase in proinflammatory TNF-α expression in CC cells was demonstrated following adiponectin and OI (adiponectin at 3 h [FC 54.2, p < 0.001], adiponectin at 6 h [FC 110.7, p < 0.001], OI at 3 h [FC 91.1, p = 0.001]) (Tables 2 and 3, Figs. 1g and 2c). Leptin induced a later cellular response with a slight TNF-α upregulation after 18 h (FC 5.8, p = 0.027) (Table 1, Fig. 1b).
The prognostic transcription factor PPARγ was affected by both OI and DI treatment. OI induced an initial upregulation of PPARγ after 3 h of treatment (FC 10.1, p = 0.031), and a later downregulation with a peak at 24 h (at 18 h [FC −5.0, p = 0.040], at 24 h [FC −10.0, p = 0.031]) (Table 3, Fig. 2d). DI showed a slight late downregulation of PPARγ at 18 h of treatment (FC −1.7, p = 0.033) (Table 4, Fig. 2g).
4. Discussion
The expression of certain inflammatory genes in CC is associated with tumor progression, prognosis, and survival. As mediators of inflammation, the obesity-related hormones leptin and adiponectin and the macrophage-specific metabolite itaconate can affect these gene patterns, thereby either promoting or preventing CC development. This study investigated changes in the expression of both pro- and anti-inflammatory markers, as well as the prognostically relevant transcription factor PPARγ in CC following obesity hormone and itaconate treatment.
Leptin and adiponectin are reported to have opposite roles in CC progression, with leptin inducing cancer progression and adiponectin inhibiting tumor growth in vitro [2]. This study showed that this simplistic dichotomous model may not apply to the mechanisms in CC, and that the metabolic state has a complex role in inflammation and CC development.
A limitation of this work is that effects were only shown in vitro using a single CC cell line derived from a human stage III CC. Further studies using CC cells derived from earlier cancer stages are necessary to determine whether there are stage-related effects. EOCC clinically tends to present at more advanced stages and tends to exhibit more aggressive features. The HT-29 CC cell line derived from a young patient was used in an effort to match these criteria [16,17]. Furthermore, cellular responses within the TME are affected by other types of cells that can alter the overall effect on tumor development [18]. Investigating the response of one specific cell type, however, is the basis for further studies using co-culture models and for performing functional studies mimicking the TME.
Another limitation of this study is its focus on gene expression due to its purpose of initially identifying potential metabolic responses. The reported effects on gene expression can serve as a guide for future studies tying together previous findings on metabolism in CC. Such future studies include investigating actual protein production or metabolite accumulation as well as in vivo mechanistic studies including mouse models or patient tissue.
Anti-inflammatory IL-8 production is a key factor in CC development and is associated with enhanced tumor growth, progression, and recurrence through autocrine effects upon cancer cells [19]. Furthermore, IL-8 promotes angiogenesis in CC and mediates proinflammatory effects by functioning as a chemoattractant for neutrophils, thereby inducing the JAK3 pathway [19,20]. Leptin significantly upregulated IL-8 expression in CC cells, but adiponectin resulted in a much higher cellular response with up to 68-fold upregulation following treatment. A similar cellular response was found for TNF-α, with adiponectin inducing expression to a much higher extent as compared to leptin (111-fold versus 6-fold). This suggests that adiponectin might have tumor promoting abilities by upregulating IL-8 expression and by inducing proinflammatory responses through TNF-α in advanced cancer stages.
Both itaconate derivatives downregulated proinflammatory CXCL10 expression, which is a key factor of the anti-tumor T-cell response in CC [21]. Common chemotherapies in CC upregulate CXCL10 expression through IFN-γ signaling by DNA damage, which induces cytotoxic T cell recruitment [21,22]. Itaconate can reduce proinflammatory mechanisms and T-cell infiltration and, therefore, reduce anti-tumorigenic activity by downregulating CXCL10.
A prognostic factor in CC that is associated with patient survival is the expression of the tumor suppressor PPARγ [23]. In this study, both itaconate derivatives OI and DI exerted tumor-enhancing effects associated with poor patient survival by downregulating PPARγ, with OI having a slightly higher impact.
The marker CCL22 is highly expressed in CC and is a chemokine specifically attributed to M2-like macrophages [24]. Tumor cell migration and recruitment of immunosuppressive and tumor-promoting T-lymphocytes can be inhibited by CCL22 suppression, giving this gene an important role in CC progression [24,25]. Adiponectin was the only treatment that significantly affected CCL22 expression by upregulating it up to 16-fold in CC cells. This suggests that adiponectin can enhance tumor progression and metastasis in later disease stages.
We have previously shown that leptin and itaconate can induce macrophage-mediated mechanisms in CC, mediating cancer-promoting effects in macrophages in vitro [14]. The present study shows that the obesity-related hormones leptin as well as adiponectin also have a direct effect on gene expression patterns in CC cells in the absence of macrophages, potentially enhancing cancer progression in CC. Derivatives of the macrophage-specific metabolite itaconate also exert cancer-promoting effects by affecting inflammatory gene expression. Moreover, the direct effect of itaconate treatment on the prognostic marker PPARγ in CC cells suggests that itaconate-related mechanisms can affect patient survival in CC. Leptin and adiponectin as well as macrophage-derived itaconate have a direct effect on CC cell gene expression and provide a link between obesity, metabolic dysfunction, and CC. Further studies are required to investigate gene expression in different cell types within the TME and to identify cancer-promoting pathways, particularly in patients with EOCC.
Declarations
Author contribution statement
Katharina M. Scheurlen: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Dylan L. Snook: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Toriana Alfieri; Andrew B. Littlefield; Joan B. George; Andre Rochet: Performed the experiments; Analyzed and interpreted the data.
Caden Seraphine; Cheyenne N. Cook: Performed the experiments.
Jeremy T. Gaskins: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Susan Galandiuk: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
The Price Institute of Surgical Research, University of Louisville, is financially supported by the John W. Price and Barbara Thruston Atwood Price Trust. Andrew B. Littlefield and Joan B. George were supported by the University of Louisville Cancer Education Program funded by the National Institutes of Health, National Cancer Institute award R25-CA134283.
Data availability statement
Data included in article/supplemental material/referenced in article.
Declaration of interest’s statement
The authors declare no competing interests.
Footnotes
This work was presented in part at the 17th Annual Academic Surgical Congress, February 2nd, 2022, Orlando, Florida.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e13132.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.West N.R., et al. Emerging cytokine networks in colorectal cancer. Nat. Rev. Immunol. 2015;15(10):615–629. doi: 10.1038/nri3896. [DOI] [PubMed] [Google Scholar]
- 2.Riondino S., et al. Obesity and colorectal cancer: role of adipokines in tumor initiation and progression. World J. Gastroenterol. 2014;20(18):5177–5190. doi: 10.3748/wjg.v20.i18.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briukhovetska D., et al. Interleukins in cancer: from biology to therapy. Nat. Rev. Cancer. 2021;21(8):481–499. doi: 10.1038/s41568-021-00363-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheurlen K.M., et al. Metabolic dysfunction and early-onset colorectal cancer - how macrophages build the bridge. Cancer Med. 2020;9(18):6679–6693. doi: 10.1002/cam4.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu P.H., et al. Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol. 2019;5(1):37–44. doi: 10.1001/jamaoncol.2018.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nigro E., et al. Adiponectin and colon cancer: evidence for inhibitory effects on viability and migration of human colorectal cell lines. Mol. Cell. Biochem. 2018;448(1–2):125–135. doi: 10.1007/s11010-018-3319-7. [DOI] [PubMed] [Google Scholar]
- 7.O’Neill L.A.J., Artyomov M.N. Itaconate: the poster child of metabolic reprogramming in macrophage function. Nat. Rev. Immunol. 2019;19(5):273–281. doi: 10.1038/s41577-019-0128-5. [DOI] [PubMed] [Google Scholar]
- 8.Lundholm M., et al. Secreted factors from colorectal and prostate cancer cells skew the immune response in opposite directions. Sci. Rep. 2015;5 doi: 10.1038/srep15651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujisawa T., et al. Adiponectin suppresses colorectal carcinogenesis under the high-fat diet condition. Gut. 2008;57(11):1531–1538. doi: 10.1136/gut.2008.159293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aparicio T., et al. Leptin stimulates the proliferation of human colon cancer cells in vitro but does not promote the growth of colon cancer xenografts in nude mice or intestinal tumorigenesis in Apc(Min/+) mice. Gut. 2005;54(8):1136–1145. doi: 10.1136/gut.2004.060533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Shibli S.M., et al. Expression of leptin and leptin receptors in colorectal cancer-an immunohistochemical study. PeerJ. 2019;7:e7624. doi: 10.7717/peerj.7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartucci M., et al. Obesity hormone leptin induces growth and interferes with the cytotoxic effects of 5-fluorouracil in colorectal tumor stem cells. Endocr. Relat. Cancer. 2010;17(3):823–833. doi: 10.1677/ERC-10-0083. [DOI] [PubMed] [Google Scholar]
- 13.Jimenez-Cortegana C., et al. Leptin, both bad and good actor in cancer. Biomolecules. 2021;11(6) doi: 10.3390/biom11060913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheurlen K.M., et al. Itaconate and leptin affecting PPARgamma in M2 macrophages: a potential link to early-onset colorectal cancer. Surgery. 2022;171(3):650–656. doi: 10.1016/j.surg.2021.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.R_Core_Team R. R Foundation for Statistical Computing; Vienna, Austria: 2020. A Language and Environment for Statistical Computing.https://www.R-project.org [Cited August 11, 2021] Available from: [Google Scholar]
- 16.Read B., Sylla P. Aggressive colorectal cancer in the young. Clin. Colon Rectal Surg. 2020;33(5):298–304. doi: 10.1055/s-0040-1713747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.You Y.N., et al. Young-onset rectal cancer: presentation, pattern of care and long-term oncologic outcomes compared to a matched older-onset cohort. Ann. Surg Oncol. 2011;18(9):2469–2476. doi: 10.1245/s10434-011-1674-7. [DOI] [PubMed] [Google Scholar]
- 18.Peddareddigari V.G., Wang D., Dubois R.N. The tumor microenvironment in colorectal carcinogenesis. Cancer Microenviron. 2010;3(1):149–166. doi: 10.1007/s12307-010-0038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee Y.S., et al. Interleukin-8 and its receptor CXCR2 in the tumour microenvironment promote colon cancer growth, progression and metastasis. Br. J. Cancer. 2012;106(11):1833–1841. doi: 10.1038/bjc.2012.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henkels K.M., et al. IL-8-induced neutrophil chemotaxis is mediated by Janus kinase 3 (JAK3) FEBS Lett. 2011;585(1):159–166. doi: 10.1016/j.febslet.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mowat C., et al. Anti-tumor immunity in mismatch repair-deficient colorectal cancers requires type I IFN-driven CCL5 and CXCL10. J. Exp. Med. 2021;218(9) doi: 10.1084/jem.20210108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song W., et al. The role of CXCL10 in prognosis of patients with colon cancer and tumor microenvironment remodeling. Medicine (Baltim.) 2021;100(38) doi: 10.1097/MD.0000000000027224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogino S., et al. Colorectal cancer expression of peroxisome proliferator-activated receptor gamma (PPARG, PPARgamma) is associated with good prognosis. Gastroenterology. 2009;136(4):1242–1250. doi: 10.1053/j.gastro.2008.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun J., et al. Fucoidan inhibits CCL22 production through NF-kappaB pathway in M2 macrophages: a potential therapeutic strategy for cancer. Sci. Rep. 2016;6 doi: 10.1038/srep35855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anz D., et al. Suppression of intratumoral CCL22 by type i interferon inhibits migration of regulatory T cells and blocks cancer progression. Cancer Res. 2015;75(21):4483–4493. doi: 10.1158/0008-5472.CAN-14-3499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplemental material/referenced in article.