ABSTRACT
Background
It is unknown whether medication status (off and on levodopa) or laboratory versus home settings plays a role in discriminating fallers and non‐fallers in people with Parkinson's disease (PD).
Objectives
To investigate which specific digital gait and turning measures, obtained with body‐worn sensors, best discriminated fallers from non‐fallers with PD in the clinic and during daily life.
Methods
We recruited 34 subjects with PD (17 fallers and 17 non‐fallers based on the past 6 month's falls). Subjects wore three inertial sensors attached to both feet and the lumbar region in the laboratory for a 3‐minute walking task (both off and on levodopa) and during daily life activities for a week. We derived 24 digital (18 gait and 6 turn) measures from the 3‐minute walk and from daily life.
Results
In clinic, none of the gait and turning measures collected during on levodopa state were significantly different between fallers and non‐fallers. In contrast, digital measures collected in the off levodopa state were significantly different between groups, (average turn velocity, average number of steps to complete a turn, and variability of gait speed, P < 0.03). During daily life, the variability of average turn velocity (P = 0.023) was significantly different in fallers than non‐fallers. Last, the average number of steps to complete a turn was significantly correlated with the patient‐reported outcomes.
Conclusions
Digital measures of turning, but not gait, were different in fallers compared to non‐fallers with PD, in the laboratory when off medication and during a daily life.
Keywords: mobility, levodopa, Parkinson's disease, daily life, clinic
Falls represent a significant problem for people with Parkinson's disease (PD) leading to reduced quality of life, 1 hospitalization, 2 , 3 fractures (Paul et al.2 and Thurman, Stevens, and Rao 2006), 4 and increased mortality. 5 Further, ~60% of people with PD fall at least once a year and 39% fall recurrently. 6 Because of these serious consequences, proper fall management has become a priority in PD, and fall prevention requires identification of patients at higher risk for falling.
Because most falls occur while walking and turning, 7 , 8 , 9 understanding gait and turning impairments when patients are both in the off and on levodopa state is very important. Specifically, it is unclear whether the likelihood of falling is affected by levodopa state. For example, it has been proposed that falls occur in the on levodopa state when patients are more active and walk faster with impaired balance control and sometimes, dyskinesia, 10 , 11 whereas others have proposed that falls mostly occur in the off levodopa state because of bradykinesia, rigidity, and impaired motor performance. 12 Characterizing the relationship between gait quality and falls in the off and on levodopa states may help clinicians considering whether a certain medication state would be better to assess fall risk during a clinic visit. 13
In addition to understanding the effects of medication state on fall risk, understanding the effects of environmental setting while evaluating fall risk is important. Specifically, although a clinic/laboratory gait assessments in off and on levodopa state provide information about gait ability under a supervised controlled condition, these assessments may not reflect functional gait performance during daily life. 14 , 15 , 16 It has been shown that gait impairments worsen during daily life in people with PD where multitasking, cluttered environments, and varied challenging conditions are very common. 15 , 17 , 18
Recently, the use of wearable sensor technologies has made it possible to quantify gait in the clinic and during real‐life using the same algorithms. 15 , 16 , 17 , 18 , 19 Although various gait measures obtained with wearable inertial sensors have been shown to discriminate between fallers and non‐fallers in PD (either off or on or daily living conditions), 12 , 20 , 21 , 22 , 23 , 24 , 25 it remains unclear which of these measures are most meaningful under particular medication and test environment conditions. Hence, the aim of this pilot study was to compare the discriminative ability of a wide range of gait and turning parameters in separating fallers from non‐fallers in relation to their levodopa state and environmental settings.
Methods
Participants
Thirty‐four people with idiopathic PD participated in the study. Inclusion criteria for PD were a diagnosis of idiopathic PD from a movement disorders neurologist with the United Kingdom PD Society Brain Bank criteria, Hoehn and Yahr scores of II–IV, and complaints about gait and balance. Exclusion criteria included the inability to follow protocol instructions, other factors affecting gait such as musculoskeletal disorders, uncorrected vision or vestibular problems, or inability to stand or walk in the home without an assistive device. The experimental protocol was approved by the institutional review board of the Oregon Health & Science University (eIRB 15578). All the participants provided informed written consent (Figs. 1 and 2).
FIG. 1.
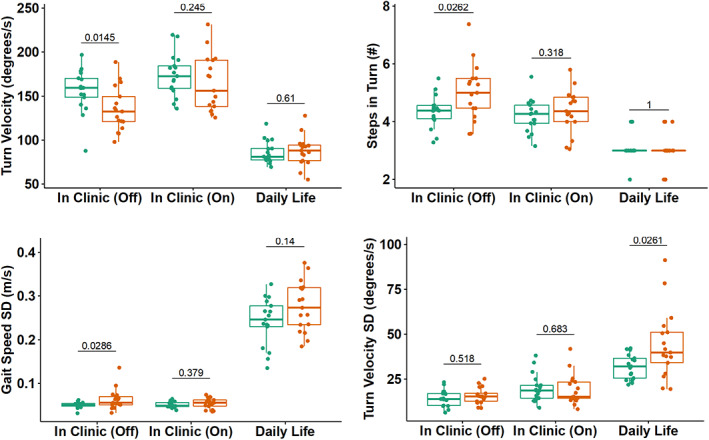
Boxplot of gait and turning measures discriminating fallers from non‐fallers in clinic (on and off levodopa state) and during daily life. Non‐fallers are represented by the color green and the color red represents fallers.
FIG. 2.
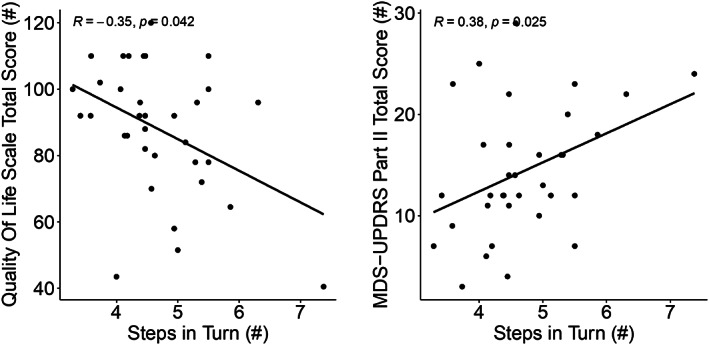
Spearman's correlation between most discriminative objective measures between fallers and non‐fallers to clinical scores.
Laboratory Data Collection
In the laboratory, participants were asked to wear three inertial sensors (Opals by APDM Wearable Technologies‐a Clario company, Portland, OR); one sensor on top of each foot and one over the lower lumbar area with an elastic belt. Each Opal sensor includes a tri‐axial accelerometer, gyroscope, and magnetometer with a sampling rate of 128 Hz. The Opal is lightweight (22 g), has a battery life of 12 hours, and includes 8 GB of storage, that can record over 30 days of data. Participants completed in both the off and on levodopa states: a 3‐min walking task at their natural pace while wearing the Opal sensors and the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS) 26 III motor signs. The off levodopa state was defined as a state after at least 12‐hours of anti‐parkinsonian medication overnight. The on levodopa state was assessed on the same day after the off state testing and 1 hr after a regular dose of levodopa. In addition, self‐reported fall history based on the prior 6 months was collected and participants were classified as fallers (at least one fall) or non‐fallers based on fall history before the study visit. Overall cognition was assessed with the Montreal Cognitive Assessment (MoCA) 27 Finally, patient‐reported scales, including the MDS‐UPDRS, part II, the quality‐of‐life space questionnaires, 28 and the Parkinson's Disease Questionnaire‐39 (PDQ‐39) 29 were collected for each participant.
Daily Life Gait Data Collection
Participants were asked to wear two Opal‐instrumented socks by APDM Wearable Technologies‐a Clario Company and an Opal sensor over the lower lumbar area (at top of pelvis with an elastic belt that clipped together) for a week of continuous monitoring for at least 8 hr/d. The details of the instrumented socks were previously described in Shah et al. 17 Briefly, instrumented socks incorporated the same inertial sensors on top of the foot as the Opal, with the battery separated from the sensor and positioned just above the lateral malleolus. Participants removed the sensors at night to recharge the batteries. Data were stored in the internal memory of the Opals. Participants mailed back the sensors using a pre‐paid mailing box after completion of a week of data collection. Raw data were uploaded to a secure cloud‐based database on Amazon Web Server on return of the devices, processed on the same server and calculated gait metrics were then downloaded to a local computer for further analysis.
Digital Gait and Turning Measures in Clinic
We used the commercial gait analysis algorithms included in Mobility Lab, Version 2 (APDM Wearable Technologies‐a Clario Company) 30 to extract spatial and temporal measures of gait and 180° turning, which have been validated previously. 31 , 32
Digital Gait and Turning Measures During Daily Life
The algorithms used to calculate the measures of gait and turning were the same for the laboratory and daily life data and were detailed previously. 33 In summary, the daily life algorithm first searches for possible bouts of walking, using a time‐domain approach to inertial sensor data from the feet and for turns, based on yaw‐rotational orientation of the pelvis. Second, individual steps are combined into potential bouts of walking, as long as the duration from one step to the next step is no longer than 2.5 s. Finally, each possible bout that contains at least three steps and is at least 3 s in duration is processed with the commercial gait analysis algorithms included in Mobility Lab V2 for prescribed gait tests (APDM). 30 For the gait measures reported in this paper, we calculated a mean and variability across all strides over a week of recording and included only the periods of straight walking, and excluded walking during turns. For turning measures, we used a previously published algorithm to detect and characterize each turn. 34 Briefly, a turn was defined as a trunk rotation around the vertical plane with a minimum of 40°/s, and a start and end of the turn was defined with a threshold of 15°/s. Only turns with durations between 0.5 and 10 s, and turn angles of 40° or more were considered.
In total, for both laboratory and during daily life data, we considered 24 gait and turning measures (including mean and standard deviation). The complete list of measures and definitions are presented in Table S1.
Statistical Analysis
Because of small sample size, Mann–Whitney U test was used to compare the differences between faller and non‐faller groups. Spearman's correlation was used to assess the relation between most discriminative mobility measures discriminating fallers from non‐fallers and severity of PD (such as UDPRS part II, and III, Life Space total score). All statistical analysis was performed using R Version 1.1.456 software. Because of the exploratory nature of this analysis, the statistical significance was set to P < 0.05.
Results
Group Characteristics and Adherence
From a total of 34 people with PD, 17 were fallers and 17 were non‐fallers based on history of falls. Table 1 compares the demographic characteristics between fallers and non‐fallers. There were no significant differences between the groups for demographic characteristics and activity measures from daily life (Table 1).
TABLE 1.
Participant demographic information for non‐faller and faller groups
| Non‐fallers (n = 17) | Fallers (n = 17) | P | |
|---|---|---|---|
| Age (yr) | 66.82 (6.61) | 68.69 (11.10) | 0.29 |
| Disease duration (yr) | 7.29 (5.6) | 9.24 (4.58) | 0.14 |
| H & Y on (n) | 2 (0) | 2.18 (0.53) | 0.164 |
| H & Y off (n) | 2.06 (0.24) | 2.29 (0.59) | 0.153 |
| MDS‐UPDRS part III total score on (n) | 29.47 (8.49) | 32.65 (9.92) | 0.36 |
| MDS‐UPDRS part III total score off (n) | 43.88 (11.3) | 46.18 (10.02) | 0.39 |
| MDS‐UPDRS part III PIGD score on (n) | 2.59 (1.42) | 3.53 (2.62) | 0.34 |
| MDS‐UPDRS part III PIGD score off (n) | 3.53 (1.66) | 5.35 (3.28) | 0.09 |
| MoCA total score (n) | 26.94 (2.38) | 26.88 (2.93) | 0.81 |
| LEDD total score (mg/day) | 1541.94 (2342.53) | 1128.1 (533.18) | 0.36 |
| PDQ‐39 total score (%) | 13.91 (7.3) | 23.3 (14.82) | 0.13 |
| PDQ‐39 mobility score (%) | 11.91 (12.14) | 21.76 (18.68) | 0.11 |
| MDS‐UPDRS dyskinesia on (n) | 0.35 (0.49) | 0.53 (0.51) | 0.31 |
| Activity measures from daily life | |||
| Bouts/hr (n) | 7.82 (3.05) | 7.65 (4.16) | 0.70 |
| Strides/hr (n) | 149.87 (60.95) | 161.07 (94.82) | 0.85 |
| Turns/hr (n) | 20.19 (9.33) | 21.34 (15.71) | 0.97 |
Abbreviations: H & Y, Hoehn and Yahr scale; MDS‐UPDRS Part III, Movement Disorders Society‐Unified Parkinson's Disease Rating Scale, motor sub‐score; MoCA, Montreal Cognitive Assessment; LEDD, levodopa equivalent daily dose; PDQ‐39, Parkinson's Disease Questionnaire‐39.
Digital Gait and Turning Measures Separating Fallers from Non‐Fallers in the Off And On State (in Clinic)
Digital measures, from the 3‐min walk test, that best separated the faller group from the non‐faller group were: average turn velocity (P = 0.014), average number of steps to complete a 180° turn (P = 0.026), and variability of gait speed (P = 0.028) collected in the off levodopa state (See Fig.1 and Table 2 ) on levodopa state.
TABLE 2.
Digital gait and turning measures between fallers and non‐fallers in clinic (off, on levodopa states) and during daily life
| Metric | Off | On | Daily life | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Non‐fallers (n = 17) | Fallers (n = 17) | P | Non‐fallers (n = 17) | Fallers (n = 17) | P | Non‐fallers (N = 17) | Fallers (n = 17) | P | |
| Turn velocity (°/s) | 156.21 (24.73) | 135.26 (25) | 0.01 | 173.79 (23.8) | 164.29 (31.73) | 0.25 | 85.92 (12.91) | 87.64 (16.95) | 0.61 |
| Steps in turn (n) | 4.34 (0.56) | 5.02 (0.98) | 0.03 | 4.2 (0.57) | 4.38 (0.74) | 0.32 | 3.06 (0.56) | 3.06 (0.56) | 1.00 |
| Gait speed SD (m/s) | 0.05 (0.01) | 0.06 (0.02) | 0.03 | 0.05 (0.01) | 0.05 (0.01) | 0.38 | 0.24 (0.05) | 0.28 (0.06) | 0.14 |
| Stride length SD (m) | 0.04 (0.01) | 0.06 (0.03) | 0.05 | 0.05 (0.01) | 0.04 (0.01) | 0.89 | 0.22 (0.05) | 0.27 (0.08) | 0.07 |
| Turn duration(s) | 2.36 (0.36) | 2.63 (0.44) | 0.09 | 2.2 (0.25) | 2.38 (0.33) | 0.15 | 2.24 (0.23) | 2.17 (0.31) | 0.38 |
| Stride time SD (s) | 0.03 (0.01) | 0.03 (0.01) | 0.09 | 0.02 (0) | 0.02 (0.01) | 0.45 | 0.19 (0.05) | 0.16 (0.03) | 0.06 |
| Elevation at mid swing (cm) | 2.19 (0.41) | 2.02 (0.48) | 0.12 | 2.12 (0.45) | 1.99 (0.49) | 0.21 | 2.79 (0.91) | 2.75 (0.6) | 0.87 |
| Foot strike angle SD (°) | 1.57 (0.3) | 1.83 (0.67) | 0.13 | 1.7 (0.56) | 1.78 (0.71) | 0.84 | 6.64 (2.06) | 7.59 (2.08) | 0.25 |
| Double support (%) | 21.23 (4.41) | 21.99 (3.38) | 0.19 | 19.79 (2.92) | 20.66 (2.87) | 0.50 | 23.8 (4.66) | 22.61 (3.11) | 0.31 |
| Stride time (s) | 1.04 (0.06) | 1.06 (0.06) | 0.20 | 1.01 (0.05) | 1.04 (0.07) | 0.17 | 1.15 (0.12) | 1.11 (0.07) | 0.54 |
| Cadence (steps/min) | 116.28 (6.61) | 113.28 (6.08) | 0.21 | 119.13 (6.14) | 115.92 (7.54) | 0.16 | 105.19 (9.78) | 108.32 (7.11) | 0.54 |
| Swing (%) | 39.37 (2.19) | 39.04 (1.68) | 0.23 | 40.1 (1.43) | 39.67 (1.43) | 0.45 | 37.7 (2.21 | 38.34 (1.49) | 0.19 |
| Toe off angle (°) | 32.31 (6.01) | 31.42 (4.7) | 0.41 | 34.29 (4.05) | 33.72 (4.76) | 0.95 | 26.37 (6.52) | 27.56 (5.72) | 1.00 |
| Steps in turn SD (n) | 0.81 (0.24) | 0.86 (0.23) | 0.43 | 0.76 (0.25) | 0.88 (0.25) | 0.16 | 1.86 (0.25) | 1.98 (0.28) | 0.31 |
| Cadence SD (steps/min) | 2.86 (0.92) | 3.4 (2.08) | 0.52 | 2.56 (0.42) | 2.74 (0.79) | 0.84 | 6.79 (1.54) | 6.36 (1.58) | 0.27 |
| Turn velocity SD (°/s) | 14.38 (4.66) | 15.71 (4.53) | 0.52 | 20.06 (7.76) | 19.11 (8.47) | 0.68 | 31.91 (6.87) | 44.23 (19.08) | 0.03 |
| Turn duration SD (s) | 0.32 (0.08) | 0.36 (0.11) | 0.55 | 0.3 (0.08) | 0.33 (0.11) | 0.31 | 0.93 (0.12) | 0.98 (0.22) | 0.89 |
| Foot strike angle (°) | 11.8 (4.61) | 12.77 (5.98) | 0.66 | 14.52 (2.6) | 15.85 (4.08) | 0.22 | 17.22 (4.77) | 17.14 (6.75) | 0.84 |
| Swing SD (%) | 0.92 (0.28) | 1.09 (0.72) | 0.73 | 0.95 (0.38) | 1 (0.57) | 0.86 | 3.33 (0.92) | 3.53 (0.98) | 0.81 |
| Stride length (m) | 1.09 (0.21) | 1.12 (0.23) | 0.79 | 1.2 (0.11) | 1.25 (0.22) | 0.39 | 1.08 (0.21) | 1.16 (0.26) | 0.23 |
| Double support SD (%) | 1.44 (0.38) | 1.58 (1.01) | 0.89 | 1.43 (0.52) | 1.46 (0.58) | 0.73 | 6.02 (2.28) | 7.03 (2.96) | 0.54 |
| Gait speed (m/s) | 1.06 (0.21) | 1.05 (0.21) | 0.92 | 1.19 (0.1) | 1.21 (0.22) | 0.62 | 0.94 (0.23) | 1.05 (0.24) | 0.25 |
| Toe off angle SD (°) | 1.47 (0.42) | 1.52 (0.55) | 0.92 | 1.39 (0.34) | 1.29 (0.26) | 0.41 | 4.64 (1.16) | 4.96 (1.16) | 0.66 |
| Elevation at mid swing SD (cm) | 0.29 (0.07) | 0.34 (0.2) | 0.97 | 0.3 (0.07) | 0.31 (0.1) | 0.95 | 1.98 (0.78) | 2.09 (0.77) | 0.76 |
P<0.05
Digital Gait and Turning Measures Separating Fallers from Non‐Fallers During Daily Life
One measure of variability, specifically, the variability of average turn velocity (P = 0.044), was statistically significant between fallers and non‐fallers during daily life, with fallers showing more variability. (See Fig.1 and Table 2).
Associations with Patient Reported Outcome Measures
Both patient‐reported outcomes, MDS‐UPDRS part II total score (r = 0.38; P = 0.02) and Life Space total score (r = −0.35; P = 0.04) were significantly correlated with the average number of steps to complete a 180° turn during off levodopa state. (See Fig. 2).
Discussion
Our findings demonstrated that levodopa medication state and environment both affect the discriminative ability of gait and turning measures to separate fallers from non‐fallers. Specifically, measures representing the turning quality in fallers seem the most sensitive to discriminate PD fallers from non‐fallers in both the off levodopa state and during daily life. In addition, the number to steps to complete a turn was related to patient‐reported quality of life consistent with capturing meaningful, functional mobility characteristics of the fallers.
In Clinic
Slower turning measures (slower turn velocity and more steps to complete 180° turns) best discriminated PD fallers from non‐fallers while in the off levodopa state. These results are consistent with impairments of turning in PD largely reported in the literature. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 Further, turning characteristics are even more sensitive to early, untreated PD than are characteristics of straight‐ahead, linear gait in a clinical Timed Up and Go Test. 43 Because turning is found to be associated with falls in older adults, 44 , 45 more steps and slower turn velocity to complete a turn in PD may reflect a very cautious turning strategy to assist in balance control. 46 , 47 To the authors' knowledge, this is the first preliminary evidence showing the potential of digital turning measures during off state as marker of falls in PD.
In addition to slow turning characteristics, higher gait speed variability was observed in fallers compared to non‐fallers with PD. Although larger variability in multiple gait measures, such as stride‐time variability, were shown to be associated with fall risk in PD, 12 , 48 , 49 , 50 in our small study stride‐time variability showed a P < .06 in daily life and P < .09 in the off state to discriminate between fallers from non‐fallers.
Overall, we found that off state (vs. on levodopa state) is more informative in detecting fallers. Our findings are in line with the findings of Hoskovcová et al., 24 Foreman et al., 51 and Valkovic et al. 13
During Daily Life
Turning in individuals with PD is characterized by longer turning duration, more steps to complete turns, slower peak and average velocity, and smaller turn angles compared to age‐matched, healthy controls in daily life. 17 , 34 , 44 , 52 , 53 In our study, we found variability of turning velocity during daily activities best discriminated fallers from non‐fallers. In fact, higher variability in various gait and turning measures was observed previously in patients with PD compared to healthy control participants during free living conditions, 18 , 52 , 54 and has been shown to be related to fall risk in PD. 55
Activity measures (ie, number of gait bouts, steps and turns per hour) were similar between fallers and non‐fallers during daily life. This result is consistent with the result of Weiss et al. 55 where the authors found that the number of steps during 3‐day recordings were similar between fallers and non‐fallers.
We found that measures representing slow turning in the off levodopa state showed a significant correlation with patient‐reported outcomes (representing what patients care about) suggesting concurrent (clinical) validity or meaningfulness of the measures. Future studies need to determine the test–retest reliability and sensitivity of the top measures to disease progression to investigate if these measure can be useful for digital endpoints for clinical trials.
There are several limitations of the current study. First, despite the findings suggesting the advantage of off medication testing, these results should be interpreted with caution because of the small sample size. The burden of assessing participants both off and on medication was high; therefore, we have a limited sample size. Second, all participants were first tested in off and then tested in on medication states on the same day, and there was no randomization in testing order based on medication state. Third, in our laboratory, patients only were tested for 180° turns, whereas in daily life we did not have that restriction so it is difficult to directly compare turning measures. Finally, we performed all analyses by taking the average and standard deviation of each measure across all strides over a week. However, in reality, gait speed and other measures are different for gait bouts of different lengths. 17 , 18 , 19 Hence, future research with larger samples, testing in multiple days and randomizing medication states, and looking into the gait and turning measures from a similar bout size in clinic and during daily life are needed to more strongly validate the use of off medication and daily life testing.
Conclusion
Objective measures of turning, specifically slower turning (represented by slower average turn velocity and higher number of steps to complete a turn) during a 3‐min walk when off levodopa and increased variability of average turn velocity during daily life, were most sensitive to discriminate PD fallers from non‐fallers and number of steps to turn 180° during off levodopa state was related to patient‐reported quality of life.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique;
V.V.S.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B.
J.M.: 2C, 3B.
P.C.K.: 1B, 1C, 2C, 3B.
J.G.N.: 2C, 3B.
K.S.: 2C, 3B.
M.E.G.: 2C, 3B.
M.M.: 1A, 1B, 2A, 2C, 3B.
F.B.H.: 1A, 1B, 2A, 2C, 3B.
Disclosures
Ethical Compliance Statement: The experimental protocol was approved by the Institutional Review Board of the Oregon Health & Science University (eIRB 15578). All the participants provided informed written consent. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: This study was supported by the National Institutes of Health grants from National Institute of Aging (R44AG055388 and R43AG044863). OHSU, and V.V.S, J.M., M.E.G., K.S., and F.B.H. are employees of APDM Wearable Technologies‐a Clario company that may have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and managed by Oregon Health & Science University. P.C.K., J.G.N., and M.M. have no potential conflicts to disclose.
Financial Disclosures for the Previous 12 Months: All authors have no potential conflicts to disclose.
Supporting information
Table S1 Gait measures and their definitions.
Acknowledgments
We thank our participants for generously donating their time to participate and also Graham Harker for helping with data collection.
Relevant disclosures and conflict of interest are listed at the end of this article.
References
- 1. Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson's disease: A review of two interconnected, episodic phenomena. Mov Disord 2004;19(8):871–884. 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- 2. Paul SS, Harvey L, Canning CG, Boufous S, Lord SR, Close JCT, Sherrington C. Fall‐related hospitalization in people with Parkinson's disease. Eur J Neurol 2017;24(3):523–529. 10.1111/ene.13238. [DOI] [PubMed] [Google Scholar]
- 3. Temlett JA, Thompson PD. Reasons for admission to hospital for Parkinson's disease. Intern Med J 2006;36(8):524–526. 10.1111/j.1445-5994.2006.01123.x. [DOI] [PubMed] [Google Scholar]
- 4. Thurman, David J. , Judy A. Stevens, Jaya K. Rao. “Practice parameter: assessing patients in a neurology practice for risk of falls (an evidence‐based review): report of the Quality Standards Subcommittee of the American Academy of Neurology.” Neurology 70.6 (2008):473–479. [DOI] [PubMed] [Google Scholar]
- 5. Wenning GK, Ebersbach G, Verny M, et al. Progression of falls in postmortem‐confirmed parkinsonian disorders. Movement disorders: official journal of the Movement Disorder Society 14.6 (1999):947–950. [DOI] [PubMed] [Google Scholar]
- 6. Allen NE, Schwarzel AK, Canning CG. Recurrent falls in parkinson's disease: A systematic review. Parkinson's Dis 2013;2013:1–16. 10.1155/2013/906274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pelicioni PHS, Menant JC, Latt MD, Lord SR. Falls in parkinson's disease subtypes: Risk factors, locations and circumstances. Int J Environ Res Public Health 2019;16(12):2216. 10.3390/ijerph16122216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ashburn A, Stack E, Ballinger C, Fazakarley L, Fitton C. The circumstances of falls among people with Parkinson's disease and the use of falls diaries to facilitate reporting. Disabil Rehabil 2008;30(16):1205–1212. 10.1080/09638280701828930. [DOI] [PubMed] [Google Scholar]
- 9. Cumming RG, Klineberg RJ. Fall frequency and characteristics and the risk of hip fractures. J Am Geriatr Soc 1994;42(7):774–778. 10.1111/j.1532-5415.1994.tb06540.x. [DOI] [PubMed] [Google Scholar]
- 10. McNeely ME, Duncan RP, Earhart GM. Medication improves balance and complex gait performance in Parkinson disease. Gait Posture 2012;36(1):144–148. 10.1016/j.gaitpost.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Curtze C, Nutt JG, Carlson‐Kuhta P, Mancini M, Horak FB. Levodopa is a double‐edged sword for balance and gait in people with Parkinson's disease. Mov Disord 2015;30(10):1361–1370. 10.1002/mds.26269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schaafsma JD, Giladi N, Balash Y, Bartels AL, Gurevich T, Hausdorff JM. Gait dynamics in Parkinson's disease: Relationship to Parkinsonian features, falls and response to levodopa. J Neurol Sci 2003;212(1–2):47–53. 10.1016/S0022-510X(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 13. Valkovič P, Brožová H, Bötzel K, Růžička E, Benetin J. Push and release test predicts better Parkinson fallers and nonfallers than the pull test: Comparison in OFF and ON medication states. Mov Disord 2008;23(10):1453–1457. 10.1002/mds.22131. [DOI] [PubMed] [Google Scholar]
- 14. Galperin I, Hillel I, del Din S, et al. Associations between daily‐living physical activity and laboratory‐based assessments of motor severity in patients with falls and Parkinson's disease. Parkinsonism Relat Disord 2019;62(October 2018):85–90. 10.1016/j.parkreldis.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 15. Hillel I, Gazit E, Nieuwboer A, et al. Is every‐day walking in older adults more analogous to dual‐task walking or to usual walking? Elucidating the gaps between gait performance in the lab and during 24/7 monitoring. Eur Rev Aging Phys Act 2019;16(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Warmerdam E, Hausdorff JM, Atrsaei A, et al. Long‐term unsupervised mobility assessment in movement disorders. Lancet Neurol 2020;19(5):462–470. 10.1016/S1474-4422(19)30397-7. [DOI] [PubMed] [Google Scholar]
- 17. Shah V, Mcnames J, Mancini M, et al. Laboratory versus daily life gait characteristics in patients with multiple sclerosis, Parkinson's disease, and matched controls. J Neuroeng Rehabil 2021;17(159):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. del Din S, Godfrey A, Galna B, Lord S, Rochester L. Free‐living gait characteristics in ageing and Parkinson's disease: Impact of environment and ambulatory bout length. J Neuroeng Rehabil 2016;13(1):1–12. 10.1186/s12984-016-0154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Corrà MF, Atrsaei A, Sardoreira A, et al. Comparison of laboratory and daily‐life gait speed assessment during on and off states in Parkinson's disease. Sensors 2021;21(12):3974. 10.3390/s21123974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gao C, Sun H, Wang T, et al. Model‐based and model‐free machine learning techniques for diagnostic prediction and classification of clinical outcomes in Parkinson's disease. Sci Rep 2018;8(1):7129. 10.1038/s41598-018-24783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rehman RZU, Zhou Y, Del Din S, et al. Gait analysis with wearables can accurately classify fallers from non‐fallers: A step toward better management of neurological disorders. Sensors (Switzerland) 2020;20(23):1–17. 10.3390/s20236992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Creaby MW, Cole MH. Gait characteristics and falls in Parkinson's disease: A systematic review and meta‐analysis. Parkinsonism Relat Disord 2018;57:1–8. 10.1016/j.parkreldis.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 23. Delval A, Betrouni N, Tard C, et al. Do kinematic gait parameters help to discriminate between fallers and non‐fallers with Parkinson's disease? Clin Neurophysiol 2021;132(2):536–541. 10.1016/j.clinph.2020.11.027. [DOI] [PubMed] [Google Scholar]
- 24. Hoskovcová M, Dusek P, Sieger T, et al. Predicting falls in Parkinson disease: What is the value of instrumented testing in off medication state? PLoS ONE 2015;10(10):e0139849. 10.1371/journal.pone.0139849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Din D, Esther M, Pelosin E, et al. Analysis of free‐living gait in older adults with and without Parkinson ’ s disease and with and without a history of falls: Identifying generic and disease‐specific characteristics. J Gerontol Ser A 2017;74(4):500–506. 10.1093/gerona/glx254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society‐sponsored revision of the unified Parkinson's disease rating scale (MDS‐UPDRS): Scale presentation and clinimetric testing results. Mov Disord 2008;23(15):2129–2170. 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 27. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53(4):695–699. www.mocatest. [DOI] [PubMed] [Google Scholar]
- 28. Peel C, Baker PS, Roth DL, Brown CJ, Bodner EV, Allman RM. Assessing mobility in older adults: The UAB study of aging life‐space assessment. Phys Ther 2005;85(10):1008–1019. [PubMed] [Google Scholar]
- 29. Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson's Disease Questionnaire (PDQ‐39): Development and Validation of a Parkinson's Disease Summary Index Score. Age Ageing 1997;26(5):353–357. [DOI] [PubMed] [Google Scholar]
- 30. Mancini M, King L, Salarian A, Holmstrom L, McNames J, Horak FB. Mobility lab to assess balance and gait with synchronized body‐worn sensors. J Bioeng Biomed Sci 2013;Suppl 1:1–5. 10.4172/2155-9538.s1-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Washabaugh EP, Kalyanaraman T, Adamczyk PG, Claflin ES, Krishnan C. Validity and repeatability of inertial measurement units for measuring gait parameters. Gait Posture 2017;55(October 2016):87–93. 10.1016/j.gaitpost.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morris R, Stuart S, Mcbarron G, Fino PC, Mancini M, Curtze C. Validity of mobility lab (version 2) for gait assessment in young adults, older adults and Parkinson's disease. Physiol Meas 2019;40(9):095003. 10.1088/1361-6579/ab4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shah VV, Mcnames J, Mancini M, et al. Quantity and quality of gait and turning in people with multiple sclerosis, Parkinson's disease and matched controls during daily living. J Neurol 2020;267(4):1188–1196. 10.1007/s00415-020-09696-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. El‐Gohary M, Pearson S, McNames J, et al. Continuous monitoring of turning in patients with movement disability. Sensors (Switzerland). 2014;14(1):356–369. 10.3390/s140100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morris ME, Huxham F, Mcginley J, Dodd K, Iansek R. The biomechanics and motor control of gait in Parkinson disease. Clin Biomech 2001;16(6):459–470. [DOI] [PubMed] [Google Scholar]
- 36. Hong M, Perlmutter JS, Earhart GM. A kinematic and electromyographic analysis of turning in people with Parkinson disease. Neurorehabil Neural Repair 2009;23(2):166–176. 10.1177/1545968308320639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Crenna P, Carpinella I, Rabuffetti M, Calabrese E, Mazzoleni P, Nemni R, Ferrarin M. The association between impaired turning and normal straight walking in Parkinson's disease. Gait Posture 2007;26(2):172–178. 10.1016/j.gaitpost.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 38. Mak MKY, Patla A, Hui‐Chan C. Sudden turn during walking is impaired in people with Parkinson's disease. Exp Brain Res 2008;190(1):43–51. 10.1007/s00221-008-1446-1. [DOI] [PubMed] [Google Scholar]
- 39. Hulbert S, Ashburn A, Robert L, Verheyden G. A narrative review of turning deficits in people with Parkinson's disease. Disabil Rehabil 2015;37(15):1382–1389. 10.3109/09638288.2014.961661. [DOI] [PubMed] [Google Scholar]
- 40. Mellone S, Mancini M, King LA, Horak FB, Chiari L. The quality of turning in Parkinson ’ s disease: A compensatory strategy to prevent postural instability? J NeuroEng Rehabil 2016;13:1–9. 10.1186/s12984-016-0147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rehman RZU, Klocke P, Hryniv S, Galna B, Rochester L, Del Din S, Alcock L. Turning detection during gait: Algorithm validation and influence of sensor location and turning characteristics in the classification of parkinson's disease. Sensors (Switzerland). 2020;20(18):1–24. 10.3390/s20185377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shah VV, Curtze C, Mancini M, et al. Inertial sensor algorithms to characterize turning in neurological patients with turn hesitations. IEEE Trans Biomed Eng 2021;68(9):2615–2625. 10.1109/TBME.2020.3037820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zampieri C, Salarian A, Carlson‐Kuhta P, Aminian K, Nutt JG, Horak FB. The instrumented timed up and go test: Potential outcome measure for disease modifying therapies in Parkinson's disease. J Neurol Neurosurg Psychiatry 2010;81(2):171–176. 10.1136/jnnp.2009.173740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leach JM, Mellone S, Palumbo P, Bandinelli S, Chiari L. Natural turn measures predict recurrent falls in community‐dwelling older adults: A longitudinal cohort study. Sci Rep 2018;8(1):1–9. 10.1038/s41598-018-22492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mancini M, Schlueter H, El‐gohary M, et al. Continuous monitoring of turning mobility and its association to falls and cognitive function: A pilot study. J Gerontol A Biol Sci Med Sci 2016;71(8):1102–1108. 10.1093/gerona/glw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wright RL, Peters DM, Robinson PD, Sitch AJ, Watt TN, Hollands MA. Differences in axial segment reorientation during standing turns predict multiple falls in older adults. Gait Posture 2012;36(3):541–545. 10.1016/j.gaitpost.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 47. Thigpen MT, Light KE, Creel GL, Flynn SM. Turning difficulty characteristics of adults aged 65 years or older. Phys Ther 2000;80(12):1174–1187. 10.1093/ptj/80.12.1174. [DOI] [PubMed] [Google Scholar]
- 48. Hausdorff JM. Gait variability: Methods, modeling and meaning. J Neuroeng Rehabil 2005;6:1–6. 10.1186/1743-Received. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Callisaya ML, Blizzard L, Schmidt MD, Martin KL, McGinley JL, Sanders LM, Srikanth VK. Gait, gait variability and the risk of multiple incident falls in older people: A population‐based study. Age Ageing 2011;40(4):481–487. 10.1093/ageing/afr055. [DOI] [PubMed] [Google Scholar]
- 50. Hausdorff JM. Gait dynamics in Parkinson's disease: Common and distinct behavior among stride length, gait variability, and fractal‐like scaling. Chaos 2009;19(2):1–14. 10.1063/1.3147408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Foreman KB, Addison O, Kim HS, Dibble LE. Testing balance and fall risk in persons with Parkinson disease, an argument for ecologically valid testing. Parkinsonism Relat Disord 2011;17(3):166–171. 10.1016/j.parkreldis.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mancini M, El‐Gohary M, Pearson S, et al. Continuous monitoring of turning in Parkinson's disease: Rehabilitation potential. NeuroRehabilitation 2015;37(1):3–10. 10.3233/NRE-151236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Haertner L, Elshehabi M, Zaunbrecher L, et al. Effect of Fear of Falling on Turning Performance in Parkinson ’ s Disease in the Lab and at Home Study. Front Aging Neurosci 2018;10(78):1–8. 10.3389/fnagi.2018.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weiss A, Sharifi S, Plotnik M, Van Vugt JPP, Giladi N, Hausdorff JM. Toward automated, at‐home assessment of mobility among patients with Parkinson disease, using a body‐worn accelerometer. Neurorehabil Neural Repair 2011;25(9):810–818. 10.1177/1545968311424869. [DOI] [PubMed] [Google Scholar]
- 55. Weiss A, Brozgol M, Dorfman M, Herman T, Shema S, Giladi N, Hausdorff JM. Does the evaluation of gait quality during daily life provide insight into fall risk? A novel approach using 3‐day accelerometer recordings. Neurorehabil Neural Repair 2013;27(8):742–752. 10.1177/1545968313491004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Gait measures and their definitions.


