This cohort study characterizes trends in pathways underlying dementia by examining prevalence of postmortem neuropathologies in birth cohorts across 25 years.
Key Points
Question
Have there been trends over time in postmortem neurodegenerative or cerebrovascular neuropathology in older individuals in the US that are similar to observed decreases in clinical dementia in some cohorts?
Findings
In this cohort study of 1554 deceased participants with complete brain autopsies from 2 community-based cohort studies of aging, no differences in global Alzheimer disease pathology or non–Alzheimer disease neurodegenerative pathologies across 4 birth epochs covering 25 years (1905-1930) were found. In contrast, dramatically lower levels of brain atherosclerosis and arteriolosclerosis were found over this period.
Meaning
Since neurodegenerative pathologies have not decreased over time, any reductions in clinical dementia are likely in part associated with improved resilience to pathology.
Abstract
Importance
With rapid aging of the US population, understanding trends over time in dementia occurrence is essential to public health planning and intervention; this understanding includes trends in neuropathologies underlying clinical dementia.
Objective
To characterize trends in pathways underlying dementia by examining prevalence of postmortem neuropathologies in birth cohorts across 25 years.
Design, Setting, and Participants
Two longitudinal cohorts, the Religious Orders Study and the Rush Memory and Aging Project, with autopsy data from 1997 to 2022 with up to 27 years follow-up were analyzed. Deceased individuals with complete postmortem neuropathology evaluations were included, and 177 individuals with most distant (<1905) or recent (>1930) years of birth were excluded.
Exposures
Four categories of year of birth: 1905-1914, 1915-1919, 1920-1924, and 1925-1930.
Main Outcomes and Measures
Outcomes included pathologic diagnosis of Alzheimer disease (AD), global AD pathology, amyloid load, tau tangles, neocortical Lewy bodies, limbic-predominant age-related TDP-43 encephalopathy neuropathological change, atherosclerosis, arteriolosclerosis, gross chronic infarcts, and chronic microinfarcts. For comparison, pathologies in each birth epoch were age-standardized to age distribution of the cohorts. χ2 Tests were used for categorical outcomes, and analysis of variance was used to compare means across birth epochs.
Results
Overall, 1554 participants were examined (510 [33%] male; median [range] age at death, 90 [66-108] years). Participants were distributed fairly evenly across birth epochs (1905-1914: n = 374; 1915-1919: n = 360; 1920-1924: n = 466; 1925-1930: n = 354). Across year of birth groups, no differences were found in prevalence of pathologic AD diagnosis; age-standardized prevalence fluctuated between 62% and 68% in the birth cohorts (χ2 test: P = .76 across birth epochs). Similarly, no differences were found in mean levels of global AD pathology, although there was greater density specifically of tau tangles in later birth cohorts (eg, age-standardized mean [SD], 1.53 [1.20] years for the 1905-1914 cohort and 1.87 [1.47] years for the 1925-1930 cohort; analysis of variance test: P = .01 across birth cohorts). There were no differences over time in other neurodegenerative pathologies. In contrast, atherosclerosis and arteriosclerosis were dramatically lower over time; for example, age-standardized prevalence of moderate to severe atherosclerosis ranged from 54% among those born from 1905-1914 to 22% for 1925-1930 (χ2 test: P < .001 across birth epochs).
Conclusion and Relevance
In this study, few differences in neurodegenerative pathologies were found, but there may be worse levels of tau tangles across birth cohorts over 25 years. This indicates that any improvements over time in clinical dementia observed by cohorts are likely in part associated with improved resilience to pathology rather than reduced AD pathology. Finally, vessel pathologies were markedly lower over birth cohorts, indicating the assocation with brain health of populationwide improvements in several vascular risk factors.
Introduction
Documenting trends over time in disease occurrence is an essential component of public health planning and intervention. With rapid aging of the US population, it is important to understand how diseases of aging, such as dementia, may be changing.
In recent years, several studies have indicated the incidence of dementia in the US may be declining.1,2,3 However, data are not entirely consistent4,5 and research has limitations. Most importantly, studies of dementia cannot clearly establish mechanisms by which disease rates may be changing; to identify risk reduction strategies, characterizing mechanisms is essential.6,7 Few previous cohorts have considered trends in neuropathologies involved in dementia.8,9,10,11 Moreover, existing studies are hard to interpret due to small sample,8 no control for age at death,9,10 or use of hospital-based autopsies.11 Further, all these cohorts were in Europe or Asia and may not mirror US trends.
Thus, we extend current research by examining trends over time in neuropathologies in 2 US cohorts of aging and dementia. Evaluating neuropathology trends provides insight into changes in pathways related to dementia. Additionally, since neuropathology is ubiquitous in aging brains (including those with and without clinical dementia),12,13,14 examining trends in neuropathologies may reflect a wider breadth of disease states than can be observed when focusing on clinical dementia.
Methods
Reporting follows Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Study Populations
The Religious Orders Study15 was initiated in 1994 and includes older priests, nuns, and brothers from across the US, free of known dementia at enrollment. Nearly 1500 participants completed a baseline evaluation as of August 2022. Follow-up rate and autopsies exceed 90%. The Rush Memory and Aging Project15 was established in 1997, with virtually identical design and data collection and includes older men and women from the Chicago, Illinois, area, without known dementia at enrollment; more than 2200 participants completed a baseline evaluation as of August 2022. Follow-up exceeds 90% and autopsy rate exceeds 80%. Participants in both cohorts agreed to annual neurological examinations, neuropsychological testing, and signed an informed consent and Anatomic Gift Act to donate their brains at death. Both studies were approved by an institutional review board of Rush University Medical Center. Here, we examined participants in whom we had an autopsy and complete neuropathologic assessments. To understand demographic characteristics of participants, individuals self-reported race in provided categories.
Definition of Birth Cohorts and Population for Analysis
We focus on calendar year of birth to examine secular trends over time. Specifically, we defined birth year categories such that each (1) included a wide range of ages at death to enable control for age across categories and (2) contained fairly similar sample size. Thus, we created 4 categories: 1905-1914, 1915-1919, 1920-1924, and 1925-1930.
For analyses, there were 1731 participants with autopsies and complete neuropathologic assessments. We excluded 177 with earlier and later years of birth (<1905 or >1930) because ages at death did not adequately overlap with the other birth epochs. This yielded 1554 participants for primary analyses of neuropathologies, with deaths through 2019. In secondary analyses of clinical outcomes, we further excluded 204 participants in whom the last clinical evaluation was more than 2 years prior to death. This yielded 1350 participants for analyses of clinical dementia-related outcomes.
Assessment of Neuropathologic and Health Outcomes
Details of autopsy procedures are described elsewhere.16 Briefly, the brain was removed, one hemisphere frozen, and the other fixed in paraformaldehyde, 4%, for at least 3 days. The fixed hemisphere was cut into 1-cm slabs, and tissue blocks were obtained from prespecified brain regions.
Alzheimer Disease Pathology
Pathologic diagnosis of Alzheimer disease (AD) was rendered according to National Institute on Aging–Alzheimer Association criteria for AD Neuropathologic Changes.17 Brains with intermediate/high neuropathologic change were considered as fulfilling pathologic criteria for diagnosis of AD. We also considered continuous variables assessing levels of AD pathology. We calculated a global pathology score combining summary measures of neuritic plaques, diffuse plaques, and neurofibrillary tangle counts. For this score, we used a modified Bielschowsky silver stain to visualize and count neuritic plaques, diffuse plaques, and neurofibrillary tangles in a 1-mm2 graticule within 5 cortical areas. We converted raw counts to an index by dividing each person’s count by the SD for that count and calculated mean pathologic indices, such that higher scores represent more pathology.
We also obtained molecularly specific estimates of amyloid beta (Aβ) and tau tangles.16,18 We used 8 cortical regions and mean findings across regions to create summary measures of the brain’s load of Aβ and density of tau-labeled tangles; because values were skewed, we used the square root of each. Higher scores represent greater pathology. Briefly, Aβ protein was identified by molecularly specific immunohistochemistry and quantified by image analysis. Neuronal neurofibrillary tangles were identified by molecularly specific immunohistochemistry (antibodies to abnormally phosphorylated tau protein, AT8). Computer-assisted sampling was used to quantify the cortical density (per mm2) of tau immunoreactive tangles.
Other Neurodegenerative Neuropathology
Immunohistochemistry with α-synuclein immunostain (Wako Chemicals) was used for assessment of Lewy bodies in the substantia nigra, 2 limbic sites (entorhinal cortex, anterior cingulate cortex), and 3 neocortical sites (midfrontal cortex, superior or middle temporal cortex, inferior parietal cortex). We classified Lewy body disease as nigral, limbic, or neocortical using a modified version of the staging criteria of McKeith et al.19,20 Neocortical disease required Lewy bodies in frontal, temporal, or parietal cortex and was accompanied by nigral and/or limbic Lewy bodies. A dichotomous variable indicating presence/absence of neocortical Lewy bodies was used here.
To identify limbic-predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC), we assessed transactive response DNA binding protein 43 kDa (TDP-43) pathology in 8 brain regions (amygdala, entorhinal cortex, hippocampus CA1 and subiculum, dentate gyrus, anterior temporal pole, inferior frontal, middle temporal cortex, and midfrontal cortex) using monoclonal antibodies (pS409/410;1:100; MilliporeSignam), which stain pathologically phosphorylated TDP-43 proteins but not normal nuclear TDP-43.21 We classified stage 0 (no presence of TDP-43) and stage 1 (localized to amygdala) as not having LATE-NC and stage 2 (extension to hippocampus and/or entorhinal cortex) and stage 3 (extension to neocortex) as LATE-NC.
Cerebrovascular Pathology
Severity of atherosclerosis was graded evaluating circle of Willis vessels at the base of the brain. Vertebral, basilar, posterior cerebral, middle cerebral, and anterior cerebral arteries were evaluated along with their proximal branches. Severity of atherosclerosis was scaled semiquantitatively (no significant atherosclerosis, mild, moderate, severe) on the basis of involvement of each artery and number of arteries.
Severity of arteriolosclerosis was graded based on concentric hyaline thickening of vessel walls, specifically small arterioles less than approximately 50 μm in diameter. The scale ranged from none to mild if the arterial wall was minimally thickened, moderate if arteriolar wall thickness was up to twice the normal thickness, and severe if wall thickness was more than twice the normal thickness or completely occluded. For atherosclerosis and arteriolosclerosis, we created 2 categories of none/mild and moderate/severe.
Uniform examination of cerebral infarcts was conducted to identify chronic infarcts; we identified brain infarcts visible to the naked eye. Microscopic examination identified chronic microinfarcts. A minimum of 9 regions in 1 hemisphere were examined for microinfarcts on 6-μm paraffin-embedded sections stained with hematoxylin-eosin. We created dichotomous summaries (presence vs absence) for infarct variables.
Clinical Cognitive Outcomes
Annual clinical evaluations included 17 cognitive tests.22 All test scores were transformed to z scores, using baseline means and SDs from the cohorts, and mean z scores were calculated to create a global cognitive composite; here, we examined the last evaluation prior to death. Further, all participants were evaluated annually by an experienced clinician who used cognitive and clinical data to diagnose Alzheimer dementia.23 At death, all clinical data were reviewed for a summary diagnosis, which was made blinded to postmortem data.
Statistical Analysis
For categorical outcomes, we calculated prevalence within each birth year category. To control for confounding by age at death, we computed age-standardized prevalence’s for each year of birth group. Age standardization is the classic statistical technique24 in time-trend analyses, using weighted means to adjust for age (ie, creating a uniform age structure across groups); we standardized the distribution of age at death in the overall analytic population in 6 categories: younger than 82 years, 82 to 85 years, 86 to 89 years, 90 to 93 years, 94 to 97 years, and 98 years or older. For continuous variables, we calculated standardized means and SDs of the weighted data (ie, the SD each group would have had if it had the age distribution of the overall analytic population). Two-sided P values were statistically significant at less than .05. Analyses were done using SAS statistical software version 9.4 (SAS Institute).
For statistical testing across years of birth, we used χ2 tests for categorical outcomes. For continuous variables, we used analysis of variance (ANOVA); Tukey test was applied for post hoc comparisons. Since results were similar for each cohort, analyses presented combine cohorts. Analysis took place between January 2021 and June 2022.
Results
Participants’ year of birth ranged from 1905 to 1930; 374 participants were born from 1905-1914, 360 born from 1915-1919, 466 born from 1920-1924, and 354 born from 1925-1930 (Table). Follow-up time (from enrollment to death) was fairly similar, with only somewhat shorter follow-up in the earliest birth category (median [IQR], 6 [4-10], 8 [5-13], 9 [6-14], 9 [6-15] years of follow-up for the 1905-1914, 1915-1919, 1920-1924, and 1925-1930 birth cohorts, respectively). Median age at death was somewhat younger with each more recent year of birth group: the median (IQR) age at death was 93 (90-98) in the 1905-1914 birth epoch, 92 (87-95) years in the 1915-1919 birth epoch, 90 (85-94) years in the 1920-1924 group, and 87 (82-90) years in those born from 1925-1930. Nonetheless, a large range of ages was represented within each category, and across birth epochs, there was substantial overlap in ages at death (eFigure in Supplement 1). Most other demographic characteristics did not markedly vary across birth groups (Table). Overall, 1513 participants (97%) self-reported as White; 510 (33%) were male; the median (range) age at death was 90 (66-108) years; and the mean (SD) education was approximately 16 (3.6) years in each group. For health characteristics, the mean (SD) number of comorbidities was approximately 2 (0.2) across groups. Prevalence of hypertension was lowest in the earliest birth epoch, then remained fairly steady across later years (217 [58%], 251 [70%], 333 [71%], and 238 [67%] for the 1905-1914, 1915-1919, 1920-1924, and 1925-1930 birth epochs, respectively). Finally, diabetes prevalence was lower in the earlier (range, 50 [13%] to 59 [16%]) than later epochs (range, 124 [27%] to 84 [24%]), consistent with rising rates in the general population.25
Table. Characteristics of Participants: Religious Orders Study and Rush Memory and Aging Project.
| Characteristic | Birth epoch, No. (%) | |||
|---|---|---|---|---|
| 1905-1914 (n = 374) | 1915-1919 (n = 360) | 1920-1924 (n = 466) | 1925-1930 (n = 354) | |
| Time of follow-up from enrollment until death, median (IQR), y | 6 (4-10) | 8 (5-12) | 9 (6-13) | 9 (6-14) |
| Time from last evaluation until death, median (IQR), y | 0.5 (0.2-0.8) | 0.7 (0.4-0.9) | 0.6 (0.3-0.9) | 0.6 (0.4-0.9) |
| Demographics | ||||
| Age at death, median (range), y | 93 (80-108) | 92 (75-105) | 90 (72-101) | 87 (66-95) |
| Male | 107 (29) | 124 (34) | 155 (33) | 124 (35) |
| Female | 267 (71) | 236 (66) | 311 (67) | 230 (65) |
| Years of education, mean (SD) | 16.2 (3.5) | 16.1 (3.8) | 16.3 (3.6) | 16.5 (3.7) |
| Whitea | 371 (99) | 348 (97) | 451 (97) | 343 (97) |
| Rush Memory and Aging Project cohort | 162 (43) | 195 (54) | 278 (60) | 182 (51) |
| Religious Orders Study | 212 (57) | 165 (46) | 188 (40) | 172 (49) |
| Health status as of death | ||||
| No. of comorbidities, mean (SD) | 1.9 (1.3) | 2.1 (1.2) | 2.2 (1.2) | 2.2 (1.3) |
| Hypertension | 217 (58) | 251 (70) | 333 (71) | 238 (67) |
| Diabetes | 50 (13) | 59 (16) | 124 (27) | 84 (24) |
Other race and ethnicity groups are not reported here but include Asian, American Indian, Black, and unknown.
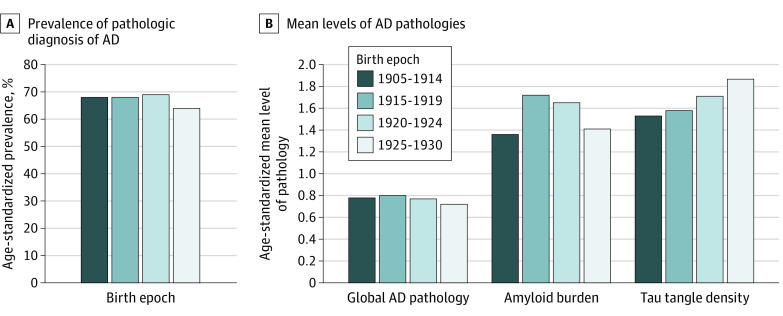
When we examined age-standardized prevalence of pathologic diagnosis of AD across year of birth groups (Figure 1), we found no suggestion of differences; age-standardized prevalence was 68% in those born from 1905-1914, 68% in those born from 1915-1919, 69% in those born from 1920-1924, and 64% in those born from 1925-1930 (χ2 test: P = .76 across year of birth groups). Similarly, we found no differences across birth year categories in mean levels of overall AD pathologic burden. Further, specifically for Aβ load and tau tangle density, there was no evidence that levels were lower over time. For Aβ load, there was some variation (ANOVA test: P < .001 across year of birth groups); those born from 1915-1919 (mean [SD] level of Aβ, 1.72 [1.09]) or 1920-1924 (mean [SD], 1.65 [1.13]) had higher levels than those born from 1905-1914 (mean [SD], 1.36 [1.00]), while those born from 1925-1930 had similar levels (mean [SD], 1.41 [0.98]) to 1905-1914 (post hoc Tukey test comparing pairwise values for year of birth groups; 1915-1919 was higher than 1905-1914 [P < .001] and 1920-1924 was higher than 1905-1914 [P < .001]). For tangle density, we found higher levels over time (ANOVA test for tangle density: P = .01 across birth year categories); those in more recent birth year categories had higher tangle density than those in the earliest category. Specifically, compared with an age-standardized mean (SD) of 1.53 (1.20) for those born from 1905-1914, the mean (SD) was 1.71 (1.39) for the 1920-1924 birth epoch (post hoc testing of these 2 birth epochs: P = .06) and 1.87 (1.47) for the 1925-1930 birth epoch (post hoc testing of these 2 birth epochs: P = .02).
Figure 1. Time Trends in Postmortem Alzheimer Disease (AD) Pathology Across 4 Birth Epochs.
There were 1554 individuals for diagnosis of AD, 1551 for global AD pathology, 1481 for amyloid, and 1511 for tau tangles. Results are age-standardized to the overall distribution of the study population. For pathologic diagnosis of AD, χ2 tests across the birth epochs yielded P = .71. For mean levels of AD pathologies, analysis of variance tests across the birth epochs yielded P = .31 for global AD pathology, P < .001 for amyloid beta burden (in post hoc pairwise comparisons, 1915-1919 and 1920-1924 are higher than 1905-1914), and P = .01 for tangles (in post hoc pairwise comparisons, 1925-1930 is higher than 1905-1914).
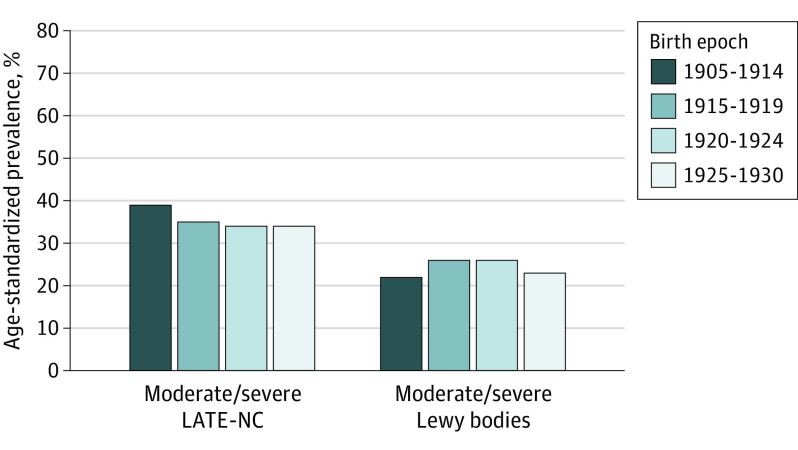
When we examined other neurodegenerative pathologies (Figure 2), there was no evidence of differences in age-standardized prevalence over time. The age-standardized prevalence of LATE-NC was 35% for those born from 1905-1914, 39% for those born from 1915-1919, 34% for those born from 1920-1924, and from 1925-1930 (χ2 test: P = .93 across these year of birth groups). Prevalence of neocortical Lewy body pathology also remained similar across year of birth groups.
Figure 2. Time Trends in Postmortem Neurodegenerative Pathology Across 4 Birth Epochs.
There were 1452 individuals for limbic-predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC) and 1552 for Lewy bodies. Results are age-standardized to the overall distribution of the study population. χ2 Tests across the birth epochs yielded P = .93 for LATE-NC and P = .18 for neocortical Lewy body pathology.
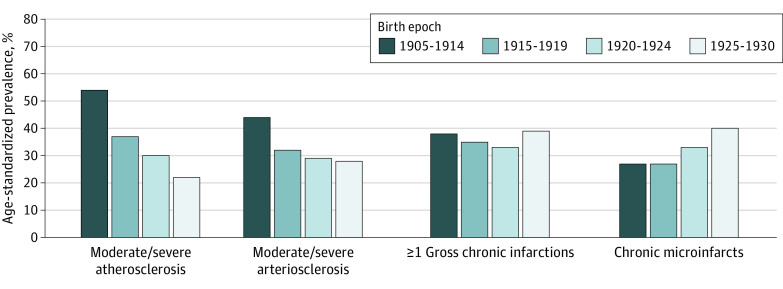
We also considered cerebrovascular pathologies (Figure 3). There were clear differences in age-standardized prevalence of atherosclerosis and arteriolosclerosis over time, such that prevalence was lower with each later birth year category. Age-standardized prevalence of moderate to severe atherosclerosis was 54% among those born from 1905-1914, 37% for 1915-1919, 30% for 1920-1924, and 22% for 1925-1930 (χ2 test: P < .001 across birth year categories). Age-standardized prevalence of moderate/severe arteriolosclerosis ranged from 44% for those born from 1905-1914 to 28% from 1925-1930 (χ2 test: P < .001 across birth year categories). However, infarcts did not follow these trends; there were no differences in age-standardized prevalence of gross chronic infarcts over time (age-standardized prevalence from 33% to 39%; χ2 test: P = .35 across year of birth groups), and chronic microinfarcts appeared more prevalent with later year of birth (age-standardized prevalence ranged from 27% in the 1905-1914 group to 40% in the 1925-1930 group; χ2 test: P = .007 across year of birth groups).
Figure 3. Time Trends in Postmortem Cerebrovascular Pathology Across 4 Birth Epochs.
There were 1546 individuals for atherosclerosis, 1544 for arteriosclerosis, and 1554 for infarcts. Results are age-standardized to the overall distribution of the study population. χ2 Tests across the birth epochs yielded P < .001 for atherosclerosis, P < .001 for arteriosclerosis, P = .35 for gross infarctions, and P = .007 for microinfarcts.
Finally, to enhance understanding of these results, we considered age-standardized levels of global cognitive function prior to death and clinical Alzheimer dementia (data not shown). We found a suggestion of better levels of cognition over time (mean [SD], 1905-1914: −1.07 [1.1] standard units; 1915-1919: −0.93 [1.1]; 1920-1924: −0.90 [1.1]; 1925-1930: −0.89 [1.2]; ANOVA test: P = .05 across year of birth groups). The age-standardized prevalence of Alzheimer dementia appeared to slightly but continuously lower over time (1905-1914: 46%; 1915-1919: 44%; 1920-1924: 42%; 1925-1930: 41%; χ2 test: P = .18 across year of birth groups), although findings did not reach statistical significance.
Discussion
We found no evidence of decreases over time in postmortem AD or other neurodegenerative pathologies, among 2 cohorts with over 1500 autopsies from older individuals born across a quarter of a century. By contrast, we found marked differences over time in atherosclerosis and arteriolosclerosis in these individuals, consistent with documented national trends of decreasing vascular morbidity,26 especially in older persons.27 We did not find lower prevalence of gross chronic infarcts and an apparent increase in chronic microinfarcts over time; this might support suggestions that microinfarcts, in particular, may have a multifactorial etiology.28
Overall, our findings have important implications in terms of understanding dementia. First, we found no association of birth epoch with pathologic AD, global AD pathology, or other degenerative pathologies. Since neurodegenerative pathologies appear to be the strongest pathologic determinants of cognition and clinical dementia,12,29,30 any possible decrease in dementia over time, including our finding of better cognitive function and small (albeit nonsignificant) decreases in clinical Alzheimer dementia, is likely explained by nondegenerative pathways. For example, enhanced resilience to neuropathology over time is plausible. Indeed, several cohorts have reported that controlling for education, a marker of cognitive resilience, attenuates apparent time trends in dementia incidence,6 providing evidence that changes in cognitive resilience may be an effective path to reducing dementia.
Second, although unexpected, we found increases over time in tau tangle density. Tau pathology appears to be a primary pathologic driver of cognitive decline and dementia12,31; in a recent examination of 11 neuropathologies, we reported that tau tangles appeared to explain more variance in cognitive decline than any other pathology.12 Especially given our finding of better cognitive function across birth epochs, this further supports the likelihood of an increase in resilience to pathology over time.
Additionally, our results regarding trends in cerebrovascular pathologies have notable implications. We found substantial decreases in vessel disease over the observed 25-year period. This likely reflects concomitant decreases in clinical vascular morbidity and mortality, which began approximately in the mid-1900s.26 We recently reported that atherosclerosis and arteriosclerosis appeared more broadly involved in cognitive health than other cerebrovascular pathologies32; thus, the dramatic decrease we found in the burden of vessel diseases in the brain could contribute to a modest reduction in clinical dementia. Further, the striking reduction in brain atherosclerosis and arteriolosclerosis highlights the impact on brain aging of nationwide efforts to improve vascular health and the importance of redoubling these efforts since recent data suggest that stroke rates are leveling,33 possibly due to sustained increases nationally in obesity and type 2 diabetes.25
Finally, we did not find reductions over time in infarcts. The prevalence of gross chronic infarcts, generally perceived as the most direct postmortem manifestation of strokes, remained steady. This may be due to combined nationwide reductions in stroke morbidity as well as mortality.26 While reductions in morbidity would lower incidence and prevalence of brain infarcts in the population, this could be countered by reduced poststroke mortality (ie, higher survival rates in those with stroke), enriching the number of persons with disease. Additionally, we focused here on severity of gross infarcts, as this is the primary measure we use to classify postmortem infarcts; in future research, we can consider in more detail aspects such as infarct size, even within gross infarcts.
Unexpectedly, in contrast to the steady prevalence over time of gross infarcts, we observed an increase in microinfarcts. Although this could be due in part to reductions in stroke mortality (as discussed above), there is limited understanding of microinfarcts.27 To date, autopsy remains the primary approach for visualizing the spectrum of microinfarcts, resulting in less research regarding risk factors. In addition to their vascular etiology,34 the higher prevalence we observed may support the suggestion of a broader range of determinants27; as one example, we recently reported that pathogenesis of microinfarcts may be mechanistically linked with amyloid and tau pathologies.35
Strengths and Limitations
There are important strengths of our study. We measured a wide array of pathologies from 2 large, community-based cohorts. Both have been ongoing since the 1990s, with uniform recruitment methods over time, and harmonized identification of pathologies. Follow-up and autopsy rates are high, reducing likelihood of bias, and enabling careful tracking of long-term trends. Indeed, the substantial decreases we found in atherosclerosis and arteriosclerosis over time are reassuring in validating our ability to detect expected trends in the general population.
There are limitations. Similar to all cohort studies, participants represent a select stratum of the general population. Those who participate in research studies are usually healthier; in particular, dementia cohorts exclude those with known dementia at enrollment, and those who died prior to enrollment opportunities (ie, left truncation), potentially hindering the ability to fully understand trends in disease across the general US population; findings for clinical dementia (in our cohort and others) should be interpreted cautiously. However, since neuropathology is so common in aging regardless of health or dementia status, the results here regarding trends in neuropathology likely provide important and relevant insights. Further, both cohorts are conducted via home visits to enhance participation in those with more comorbidities. Another limitation is the small sample of diverse participants. Black and Hispanic or Latinx older persons have higher rates of dementia36 and vascular diseases37; it is possible that time trends in neuropathology may differ in these groups. In future research, we will examine neuropathology in our cohorts of minoritized populations, as they continue to grow and follow-up continues; it is clear that understanding trends over time in neuropathology can provide compelling insights into brain health and dementia prevention.
Conclusions
We found few differences in levels of neurodegenerative pathologies over time. This indicates that any improvements over time in clinical dementia are likely associated with improved resilience to pathology over time.
eFigure. Distributions of Age at Death across Four Birth Epochs: Religious Orders Study and Rush Memory and Aging Project
Data sharing statement
References
- 1.Satizabal CL, Beiser AS, Chouraki V, Chêne G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart study. N Engl J Med. 2016;374(6):523-532. doi: 10.1056/NEJMoa1504327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Derby CA, Katz MJ, Lipton RB, Hall CB. Trends in dementia incidence in a birth cohort analysis of the Einstein Aging study. JAMA Neurol. 2017;74(11):1345-1351. doi: 10.1001/jamaneurol.2017.1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tom SE, Phadke M, Hubbard RA, Crane PK, Stern Y, Larson EB. Association of demographic and early-life socioeconomic factors by birth cohort with dementia incidence among US adults born between 1893 and 1949. JAMA Netw Open. 2020;3(7):e2011094. doi: 10.1001/jamanetworkopen.2020.11094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajan KB, Weuve J, Barnes LL, Wilson RS, Evans DA. Prevalence and incidence of clinically diagnosed Alzheimer’s disease dementia from 1994 to 2012 in a population study. Alzheimers Dement. 2019;15(1):1-7. doi: 10.1016/j.jalz.2018.07.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Power MC, Bennett EE, Turner RW, et al. Trends in relative incidence and prevalence of dementia across non-Hispanic Black and White individuals in the United States, 2000-2016. JAMA Neurol. 2021;78(3):275-284. doi: 10.1001/jamaneurol.2020.4471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu YT, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time: current evidence. Nat Rev Neurol. 2017;13(6):327-339. doi: 10.1038/nrneurol.2017.63 [DOI] [PubMed] [Google Scholar]
- 7.Pase MP, Satizabal CL, Seshadri S. Role of improved vascular health in the declining incidence of dementia. Stroke. 2017;48(7):2013-2020. doi: 10.1161/STROKEAHA.117.013369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrijvers EM, Verhaaren BF, Koudstaal PJ, Hofman A, Ikram MA, Breteler MM. Is dementia incidence declining?: trends in dementia incidence since 1990 in the Rotterdam study. Neurology. 2012;78(19):1456-1463. doi: 10.1212/WNL.0b013e3182553be6 [DOI] [PubMed] [Google Scholar]
- 9.Honda H, Sasaki K, Hamasaki H, et al. Trends in autopsy-verified dementia prevalence over 29 years of the Hisayama study. Neuropathology. 2016;36(4):383-387. doi: 10.1111/neup.12298 [DOI] [PubMed] [Google Scholar]
- 10.Hamasaki H, Honda H, Okamoto T, et al. Recent increases in hippocampal tau pathology in the aging Japanese population: the Hisayama study. J Alzheimers Dis. 2017;55(2):613-624. doi: 10.3233/JAD-160521 [DOI] [PubMed] [Google Scholar]
- 11.Kövari E, Herrmann FR, Bouras C, Gold G. Amyloid deposition is decreasing in aging brains: an autopsy study of 1,599 older people. Neurology. 2014;82(4):326-331. doi: 10.1212/WNL.0000000000000069 [DOI] [PubMed] [Google Scholar]
- 12.Boyle PA, Wang T, Yu L, et al. To what degree is late life cognitive decline driven by age-related neuropathologies? Brain. 2021;144(7):2166-2175. doi: 10.1093/brain/awab092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyle PA, Yu L, Wilson RS, Leurgans SE, Schneider JA, Bennett DA. Person-specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol. 2018;83(1):74-83. doi: 10.1002/ana.25123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Latimer CS, Keene CD, Flanagan ME, et al. Resistance to Alzheimer disease neuropathologic changes and apparent cognitive resilience in the Nun and Honolulu-Asia aging studies. J Neuropathol Exp Neurol. 2017;76(6):458-466. doi: 10.1093/jnen/nlx030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA. Religious orders study and rush memory and aging project. J Alzheimers Dis. 2018;64(s1):S161-S189. doi: 10.3233/JAD-179939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66(12):1837-1844. doi: 10.1212/01.wnl.0000219668.47116.e6 [DOI] [PubMed] [Google Scholar]
- 17.Montine TJ, Phelps CH, Beach TG, et al. ; National Institute on Aging; Alzheimer’s Association . National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123(1):1-11. doi: 10.1007/s00401-011-0910-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004;61(3):378-384. doi: 10.1001/archneur.61.3.378 [DOI] [PubMed] [Google Scholar]
- 19.McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113-1124. doi: 10.1212/WNL.47.5.1113 [DOI] [PubMed] [Google Scholar]
- 20.Wilson RS, Yu L, Schneider JA, Arnold SE, Buchman AS, Bennett DA. Lewy bodies and olfactory dysfunction in old age. Chem Senses. 2011;36(4):367-373. doi: 10.1093/chemse/bjq139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nag S, Yu L, Wilson RS, Chen E-Y, Bennett DA, Schneider JA. TDP-43 pathology and memory impairment in elders without pathologic diagnoses of AD or FTLD. Neurology. 2017;88(7):653-660. doi: 10.1212/WNL.0000000000003610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17(2):179-193. doi: 10.1037/0882-7974.17.2.179 [DOI] [PubMed] [Google Scholar]
- 23.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944. doi: 10.1212/WNL.34.7.939 [DOI] [PubMed] [Google Scholar]
- 24.Ahmad OB, Boschi-Pinto C, Lopez AD, Murray CJL, Lozano R, Inoue M. Age standardization of rates: a new WHO standard. GPE Discussion Series No. 31; World Health Organization. 2001. [Google Scholar]
- 25.Lopez AD, Adair T. Is the long-term decline in cardiovascular-disease mortality in high-income countries over? Evidence from national vital statistics. Int J Epidemiol. 2019;48(6):1815-1823. doi: 10.1093/ije/dyz143 [DOI] [PubMed] [Google Scholar]
- 26.Lackland DT, Roccella EJ, Deutsch AF, et al. ; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; Council on Functional Genomics and Translational Biology . Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke. 2014;45(1):315-353. doi: 10.1161/01.str.0000437068.30550.cf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott CA, Li L, Rothwell PM. Diverging temporal trends in stroke incidence in younger vs older people: a systematic review and meta-analysis. JAMA Neurol. 2022;79(10):1036-1048. doi: 10.1001/jamaneurol.2022.1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith EE, Schneider JA, Wardlaw JM, Greenberg SM. Cerebral microinfarcts: the invisible lesions. Lancet Neurol. 2012;11(3):272-282. doi: 10.1016/S1474-4422(11)70307-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyle PA, Yu L, Leurgans SE, et al. Attributable risk of Alzheimer’s dementia attributed to age-related neuropathologies. Ann Neurol. 2019;85(1):114-124. doi: 10.1002/ana.25380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dodge HH, Zhu J, Woltjer R, et al. ; SMART data consortium . Risk of incident clinical diagnosis of Alzheimer’s disease-type dementia attributable to pathology-confirmed vascular disease. Alzheimers Dement. 2017;13(6):613-623. doi: 10.1016/j.jalz.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonnen JA, Larson EB, Crane PK, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62(4):406-413. doi: 10.1002/ana.21208 [DOI] [PubMed] [Google Scholar]
- 32.Lamar M, Leurgans S, Kapasi A, et al. Complex profiles of cerebrovascular disease pathologies in the aging brain and their relationship with cognitive decline. Stroke. 2022;53(1):218-227. doi: 10.1161/STROKEAHA.121.034814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sidney S, Quesenberry CP Jr, Jaffe MG, et al. Recent trends in cardiovascular mortality in the United States and public health goals. JAMA Cardiol. 2016;1(5):594-599. doi: 10.1001/jamacardio.2016.1326 [DOI] [PubMed] [Google Scholar]
- 34.Arvanitakis Z, Capuano AW, Lamar M, et al. Late-life blood pressure association with cerebrovascular and Alzheimer disease pathology. Neurology. 2018;91(6):e517-e525. doi: 10.1212/WNL.0000000000005951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kapasi A, Leurgans SE, Arvanitakis Z, Barnes LL, Bennett DA, Schneider JA. Aβ (amyloid beta) and tau tangle pathology modifies the association between small vessel disease and cortical microinfarcts. Stroke. 2021;52(3):1012-1021. doi: 10.1161/STROKEAHA.120.031073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brewster P, Barnes L, Haan M, et al. Progress and future challenges in aging and diversity research in the United States. Alzheimers Dement. 2019;15(7):995-1003. doi: 10.1016/j.jalz.2018.07.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention . Stroke facts. Accessed June 6, 2022. https://www.cdc.gov/stroke/facts.htm
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Distributions of Age at Death across Four Birth Epochs: Religious Orders Study and Rush Memory and Aging Project
Data sharing statement