Abstract
Piperazines are important heterocycles in drug compounds. We report the asymmetric synthesis of arylpiperazines by photocatalytic decarboxylative arylation (metallaphotoredox catalysis) then kinetic resolution using n-BuLi/(+)-sparteine. This gave a range of piperazines with very high enantioselectivities. Further functionalizations gave enantioenriched 2,2-disubstituted piperazines, and either N-substituent can be removed selectively. Late-stage functionalizations of enantioenriched piperazine derivatives were demonstrated, including synthesis of a drug compound with glycogen synthase kinase (GSK)-3β inhibitor activity with potential for treating Alzheimer’s disease.
Piperazines are one of the most important saturated nitrogen heterocycles found in small-molecule pharmaceuticals.1 Their druglike properties and synthetic versatility make them excellent candidates in drug discovery programs. For example, imatinib (marketed as Gleevec), a BCR-Abl tyrosine kinase inhibitor, is used in the treatment of multiple cancers with high response rate,2 and gatifloxacin is an important fluoroquinolone antibiotic (Figure 1).3 The majority of the piperazines in pharmaceuticals contain substituents only at the two nitrogen atoms. However, having substitution at a carbon atom of the piperazine ring is very important in drug discovery.4 Examples of substituted piperazines occur in vestipitant, a neurokinin-1 antagonist which is currently in clinical trials for the treatment of anxiety and tinnitus,5 and indinavir, a protease inhibitor used to treat HIV/AIDS.6 Development of methods to access C-substituted piperazines, particularly with control of absolute configuration, would promote structural diversity in small-molecule collections to aid medicinal chemistry.
Figure 1.
Bioactive piperazines.
The most common methods for the formation of 2-substituted piperazines use amino acid starting materials and cyclization chemistry.7 The direct functionalization of the intact piperazine ring is an attractive alternative strategy, allowing late-stage introduction of the substituent.8 One such approach uses lithiation chemistry.9−11 This lithiation–trapping chemistry has provided access to a small selection of 2-substituted piperazines, but extension via transmetalation and Negishi coupling to give 2-arylpiperazines was very low-yielding.11 A recent report of photoredox catalysis has allowed direct arylation or vinylation but there are very few examples, and this is limited to the formation of racemic N-aryl piperazines.12 The direct C–H functionalization of six-membered saturated heterocycles by photocatalysis is problematic due to the poor yields of the product,13,14 so we were attracted to a decarboxylative arylation strategy.15,16 Here we report the successful synthesis of 2-arylpiperazines using photoredox chemistry followed by kinetic resolution using asymmetric lithiation17 to provide highly enantioenriched substituted piperazines. This chemistry allows a new route to 2-arylpiperazines with control of absolute configuration and is showcased with an application to the preparation of a GSK-3β inhibitor with excellent yield and enantioselectivity.
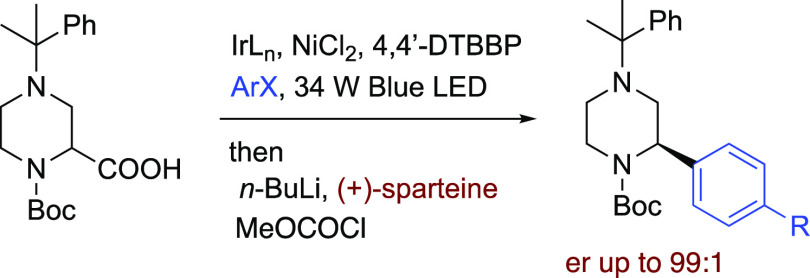
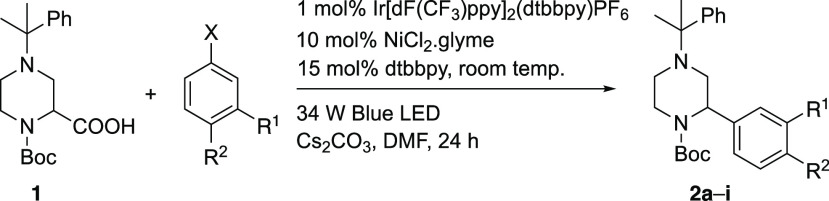
Initial work investigated the possibility of carrying out a direct C–H activation using Ir[dF(CF3)ppy]2(dtbbpy)PF6 as the photocatalyst.13 However, this resulted in low yields of the 2-arylpiperazine products (up to 35% yield using methyl 4-bromobenzoate as the aryl halide) (see the SI). Therefore, the piperazine 1 was formed (see the SI) from the parent piperazine11 using sec-BuLi, TMEDA, and ground dry ice.18 We then explored decarboxylative arylation for the synthesis of a series of 2-arylpiperazines using photocatalysis (Scheme 1).
Scheme 1. Photocatalysis to Give Piperazines 2.
As anticipated from the literature,15,16 the presence of an electron-withdrawing group, such as an acyl or cyano group, gave the best results (Table 1, entries 1, 2, and 5). In the absence of such a group, relatively low yields of the desired products were obtained when starting with the aryl bromide precursor (entries 3, 4, and 6). However, switching to the aryl iodide gave improved results (entries 8 and 9) and even allowed formation of the 2-phenyl derivative 2g (entry 7) and more electron-rich derivatives (entries 11 and 12). Compared with entry 9, a similar yield (48%) of the product 2f was obtained using (instead of the iridium catalyst) the organophotocatalyst 4CzIPn16b and the aryl iodide (entry 10). The reactions were generally performed using 0.6 mmol of acid 1 but could be scaled to 5.7 mmol (2 g) without a significant reduction in yield (2f, 50% yield after 72 h).
Table 1. Yields of Isolated Piperazines 2.
| Entry | X | R1 | R2 | Product | Yield (%) |
|---|---|---|---|---|---|
| 1 | Br | H | COMe | 2a | 80 |
| 2 | Br | H | CO2tBu | 2b | 55 |
| 3 | Br | H | F | 2c | 30 |
| 4 | Br | H | CF3 | 2d | 34 |
| 5 | Br | H | CN | 2e | 70 |
| 6 | Br | H | Cl | 2f | 25 |
| 7 | I | H | H | 2g | 30 |
| 8 | I | H | F | 2c | 55 |
| 9 | I | H | Cl | 2f | 58 |
| 10 | I | H | Cl | 2f | 48a |
| 11 | I | OMe | H | 2h | 42 |
| 12 | I | H | Me | 2i | 35 |
Using 4CzIPn as photocatalyst.
With the 2-arylpiperazines 2 in hand, we were interested in exploring a kinetic resolution protocol developed in our laboratories.17 This chemistry relies on coordination of the chiral base to the carbonyl oxygen atom of the Boc group, so it is important to determine the ratio of rotamers and the rate of rotation of the carbonyl group. From variable-temperature (VT) NMR spectroscopy with the piperazine 2c in d8-THF (Figure 2), the ratio of rotamers is approximately 1:1 and the activation parameters for the rotation were determined as ΔH⧧ ≈ 44 kJ/mol and ΔS⧧ ≈ – 27 J/K·mol. These parameters equate to a barrier to rotation, ΔG⧧ ≈ 49 kJ/mol at −78 °C, and this gives a half-life for rotation of about 2 s at this temperature. These results were supported by density functional theory (DFT) calculations using the B3LYP functional including dispersion interactions and the def2TZVP basis set.19 These calculations indicated a 53:47 ratio of rotamers of 2g and a rotation barrier ΔG⧧ ≈ 50 kJ/mol at −78 °C (see the SI). Hence, despite the presence of both rotamers, the lithiation should be possible due to the relatively fast rate of rotation of the Boc group.
Figure 2.
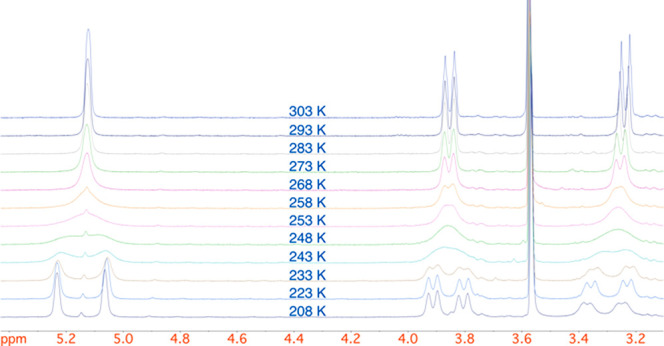
1H VT-NMR spectroscopy of piperazine 2c (400 MHz, d8-THF) showing 5.40–3.10 ppm.
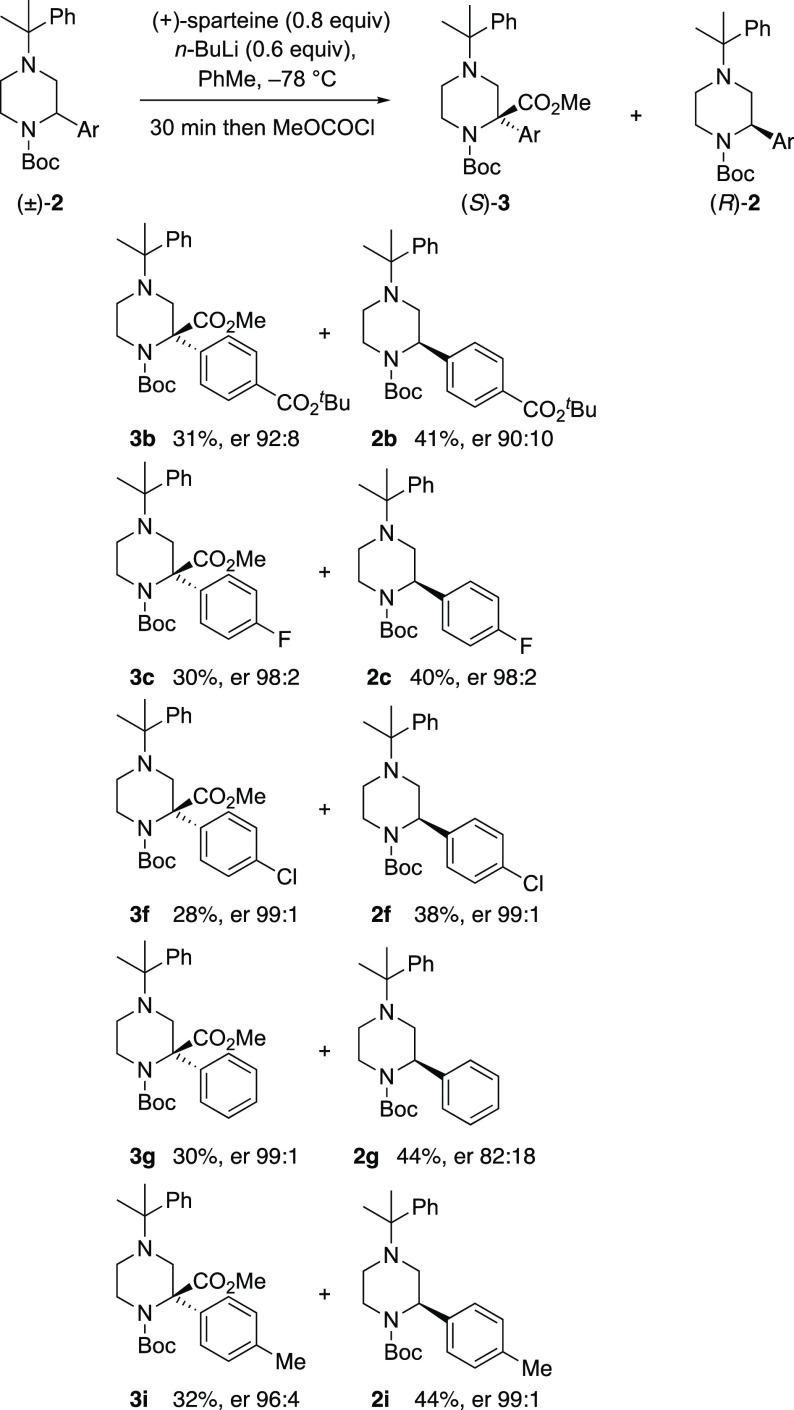
Kinetic resolution of the piperazine derivatives was investigated using (+)-sparteine as the chiral ligand in toluene and adding n-BuLi to this mixture. After optimization, we found very good results for the kinetic resolution using 0.6 equiv of n-BuLi along with 0.8 equiv (+)-sparteine for 30 min (Scheme 2). Trapping the resulting mixture with methyl chloroformate gave the disubstituted products 3b,c,f,g,i, together with recovered piperazines 2b,c,f,g,i with enantiomer ratios (er) up to 99:1. The results indicate a selectivity factor S ≈ 17.20 The resolution tolerated a bulky ester substituent at C-4 of the aromatic ring (2b) but not the nitrile 2e. The 4-fluoro-, 4-chloro-, and 4-methyl-substituted piperazines 2c, 2f, and 2i were excellent substrates. The absolute configuration of the major enantiomer was assigned on the basis of the known preference for BuLi/(+)-sparteine to remove the pro-(R) proton on the carbon atom attached to the N-Boc group,21,22 and this aligns with all previous related examples.17 A model to illustrate the preference is shown (Figure 3), in which the (+)-enantiomer of sparteine when coordinated to BuLi favors lithiation of the (S) enantiomer of the piperazines 2. To favor the other enantiomer, (−)-sparteine could be used.17
Scheme 2. Kinetic Resolution of Selected Piperazines 2.
Figure 3.
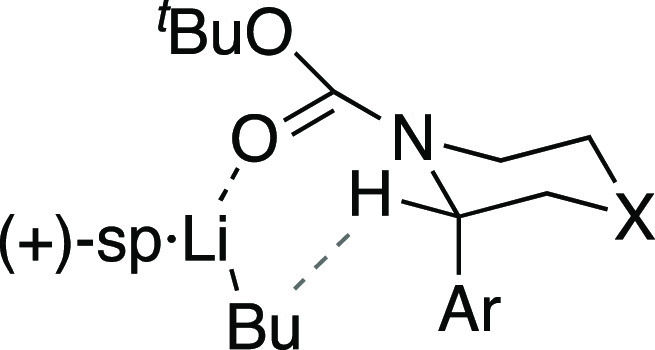
Working model to show preferential abstraction of the proton at C-2 using (+)-sparteine (sp) (X = N-cumyl, this work).
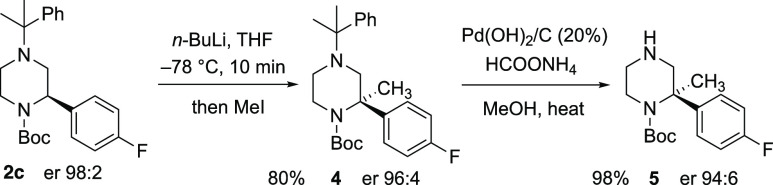
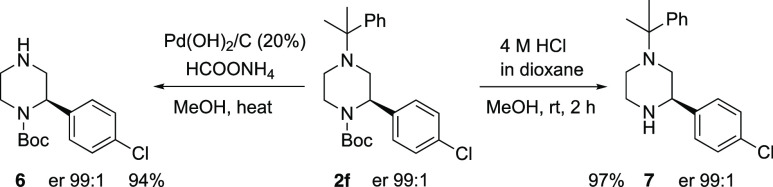
The kinetic resolution reactions resulted in recovered 2-arylpiperazines (38–44% yields) and 2,2-disubstituted piperazines (28–32% yields) with excellent enantioselectivities. The remaining material was, at least in part, the product of ring-opening from β-elimination of the intermediate organolithium species. This side reaction has been noted before with lithiated piperazines, particularly after addition of the electrophile that could coordinate to the distal nitrogen atom.10,11 The bulky N-cumyl group should minimize such an interaction although in our substrates there is a higher propensity for elimination due to formation of a conjugated alkene (styrene). Despite this, we were able to demonstrate successful lithiation–trapping of the enantioenriched recovered piperazines (Scheme 3). For example, treatment of piperazine (R)-2c (er 98:2) with n-BuLi in THF at low temperature for 10 min followed by addition of iodomethane gave the piperazine 4 with high yield and only a slight drop in enantiopurity. The cumyl protecting group on the distal nitrogen atom could be removed easily by hydrogenolysis to give the piperazine 5. To demonstrate the orthogonality of the protecting groups, the N-cumyl and N-Boc groups were cleaved selectively from the piperazine (R)-2f to give the piperazines 6 and 7, respectively, without loss of enantiopurity (Scheme 4).
Scheme 3. Lithiation–Trapping of Piperazine 2c.
Scheme 4. Deprotection of Piperazine 2f.
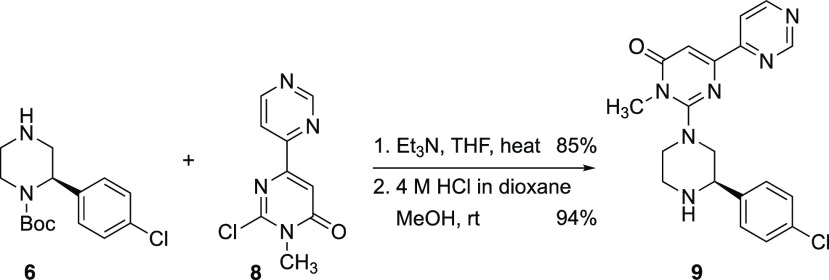
To illustrate the application of this chemistry in synthesis, we prepared the glycogen synthase kinase (GSK)-3β inhibitor 9 (Scheme 5).23 The chloride 8 was prepared according to the literature24 and was coupled with the piperazine 6. This was followed directly by acid-promoted deprotection of the Boc group to give the desired bioactive compound 9. This drug has potential for the treatment of Alzheimer’s disease, and either enantiomer will be accessible depending on the choice of enantiomer of the chiral ligand sparteine in the kinetic resolution chemistry.
Scheme 5. Preparation of the GSK-3β Inhibitor 8.
In conclusion, we have developed a short synthesis of 2-arylpiperazines by photocatalysis. These products are amenable to kinetic resolution with sparteine as the chiral ligand to provide the recovered 2-arylpiperazine and 2,2-disubstituted piperazines with high enantioselectivities. The lithiated intermediates are configurationally stable at low temperature, and this allows the incorporation of electrophiles at C-2 to give other substituted products. The regioselective and stereoselective lithiation–trapping of the 2-arylpiperazines provides a useful way to prepare uncommon, but potentially valuable, 2,2-disubstituted piperazines. The choice of a cumyl group on one nitrogen atom and a Boc group on the other nitrogen atom of the piperazine ring means that the protecting groups are orthogonal and either N-substituent can be cleaved selectively. The chemistry can be applied to the synthesis of enantiomerically enriched bioactive piperazine drug compounds.
Acknowledgments
We would like to thank the Royal Society - SERB for a Newton International Fellowship (grant NIF\R1\191853), the Royal Society (Short Industry Fellowship SIF\R2\202031), the EPSRC (grant EP/R024294/1), and the University of Sheffield. We are grateful to Khalid Doudin (University of Sheffield) for help with the VT-NMR spectroscopy, Anthony J. H. M. Meijer (University of Sheffield) for help with DFT studies, Ilaria Proietti Silvestri (Liverpool ChiroChem) for discussions, and the Faculty of Science mass spectrometry service at the University of Sheffield.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.3c00074.
Experimental procedures and characterization data, NMR spectra, HPLC traces, and DFT calculations (PDF)
Author Contributions
All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- a Taylor R. D.; MacCoss M.; Lawson A. D. G. Rings in drugs. J. Med. Chem. 2014, 57, 5845–5859. 10.1021/jm4017625. [DOI] [PubMed] [Google Scholar]; b Vitaku E.; Smith D. T.; Njardarson J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]; c Mordini A.; Reginato G.; Calamante M.; Zani L. Stereoselective synthesis of polysubstituted piperazines and oxopiperazines. Useful building blocks in medicinal chemistry. Curr. Top. Med. Chem. 2014, 14, 1308–1316. 10.2174/1568026614666140423114013. [DOI] [PubMed] [Google Scholar]; d Romanelli M. N.; Manetti D.; Braconi L.; Dei S.; Gabellini A.; Teodori E. Expert Opin. Drug Discovery 2022, 17, 969–984. 10.1080/17460441.2022.2103535. [DOI] [PubMed] [Google Scholar]
- Stegmeier F.; Warmuth M.; Sellers W. R.; Dorsch M. Targeted cancer therapies in the twenty-first century: lessons from imatinib. Clin. Pharmacol. Ther. 2010, 87, 543–552. 10.1038/clpt.2009.297. [DOI] [PubMed] [Google Scholar]
- Burka J. M.; Bower K. S.; Vanroekel R. C.; Stutzman R. D.; Kuzmowych C. P.; Howard R. S. The effect of fourth-generation fluoroquinolones gatifloxacin and moxifloxacin on epithelial healing following photorefractive keratectomy. Am. J. Ophthalmol. 2005, 140, 83–87. 10.1016/j.ajo.2005.02.037. [DOI] [PubMed] [Google Scholar]
- a Leonard D. M. Ras farnesyltransferase: a new therapeutic target. J. Med. Chem. 1997, 40, 2971–2990. 10.1021/jm970226l. [DOI] [PubMed] [Google Scholar]; b Matsuo M.; Hagiwara D.; Manabe T.; Konishi N.; Shigenaga S.; Murano K.; Matsuda H.; Miyake H.. 1-Benzoyl-2-(indolyl-3-alkyl) piperazine derivatives as neurokinin receptor antagonists. PCT Int. Appl. WO9637489A1, 1996.
- Di Fabio R.; Griffante C.; Alvaro G.; Pentassuglia G.; Pizzi D. A.; Donati D.; Rossi T.; Guercio G.; Mattioli M.; Cimarosti Z.; Marchioro C.; Provera S.; Zonzini L.; Montanari D.; Melotto S.; Gerrard P. A.; Trist D. G.; Ratti E.; Corsi M. Discovery process and pharmacological characterization of 2-(S)-(4-fluoro-2-methylphenyl)piperazine-1-carboxylic acid [1-(R)-(3,5-bis-trifluoromethylphenyl)ethyl]methylamide (vestipitant) as a potent, selective, and orally active NK1 receptor antagonist. J. Med. Chem. 2009, 52, 3238–3247. 10.1021/jm900023b. [DOI] [PubMed] [Google Scholar]
- Vacca J P; Dorsey B D; Schleif W A; Levin R B; McDaniel S L; Darke P L; Zugay J; Quintero J C; Blahy O M; Roth E L-735,524: an orally bioavailable human immunodeficiency virus type 1 protease inhibitor. Proc. Natl. Acad. Sci. U. S. A. 1994, 91, 4096–4100. 10.1073/pnas.91.9.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Gettys K. E.; Ye Z.; Dai M. Recent advances in piperazine synthesis. Synthesis 2017, 49, 2589–2604. 10.1055/s-0036-1589491. [DOI] [Google Scholar]; b Sajadikhah S. S.; Nassiri M. Recent developments in the synthesis of piperazines. Chem. Heterocycl. Compd. 2021, 57, 905–907. 10.1007/s10593-021-02998-0. [DOI] [Google Scholar]; c Gueret R.; Pelinski L.; Bousquet T.; Sauthier M.; Ferey V.; Bigot A. Visible-light-driven carboxylic amine protocol (CLAP) for the synthesis of 2-substituted piperazines under batch and flow conditions. Org. Lett. 2020, 22, 5157–5162. 10.1021/acs.orglett.0c01759. [DOI] [PubMed] [Google Scholar]; d Hsieh S. Y.; Bode J. W. Silicon amine reagents for the photocatalytic synthesis of piperazines from aldehydes and ketones. Org. Lett. 2016, 18, 2098–2101. 10.1021/acs.orglett.6b00722. [DOI] [PubMed] [Google Scholar]
- Ye Z.; Gettys K. E.; Dai M. Opportunities and challenges for direct C-H functionalization of piperazines. Beilstein J. Org. Chem. 2016, 12, 702–715. 10.3762/bjoc.12.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Berkheij M.; van der Sluis L.; Sewing C.; den Boer D. J.; Terpstra J. W.; Hiemstra H.; Iwema Bakker W. I.; van den Hoogenband A.; van Maarseveen J. H. Synthesis of 2-substituted piperazines via direct α-lithiation. Tetrahedron Lett. 2005, 46, 2369–2371. 10.1016/j.tetlet.2005.02.085. [DOI] [Google Scholar]; b McDermott B. P.; Campbell A. D.; Ertan A. First Example of s-BuLi/(−)-Sparteine-Mediated Chiral Deprotonation of a Piperazine and Proof of the Sense of Induction. Synlett 2008, 2008, 875–879. 10.1055/s-2008-1042905. [DOI] [Google Scholar]; c Robinson S. P.; Sheikh N. S.; Baxter C. A.; Coldham I. Dynamic thermodynamic resolution of lithiated N-Boc-N′-alkylpiperazines. Tetrahedron Lett. 2010, 51, 3642–3644. 10.1016/j.tetlet.2010.05.019. [DOI] [Google Scholar]
- a Barker G.; O’Brien P.; Campos K. R. Diamine-free lithiation–trapping of N-Boc heterocycles using s-BuLi in THF. Org. Lett. 2010, 12, 4176–4179. 10.1021/ol1017799. [DOI] [PubMed] [Google Scholar]; b Firth J. D.; O’Brien P.; Ferris L. Synthesis of enantiopure piperazines via asymmetric lithiation–trapping of N-Boc piperazines: unexpected role of the electrophile and distal N-substituent. J. Am. Chem. Soc. 2016, 138, 651–659. 10.1021/jacs.5b11288. [DOI] [PubMed] [Google Scholar]
- Firth J. D.; O’Brien P.; Ferris L. General Procedures for the Lithiation/Trapping of N-Boc Piperazines. J. Org. Chem. 2017, 82, 7023–7031. 10.1021/acs.joc.7b00913. [DOI] [PubMed] [Google Scholar]
- a McNally A.; Prier C. K.; MacMillan D. W. C. Discover of an α-amino C–H arylation reaction using the strategy of accelerated serendipity. Science 2011, 334, 1114–1117. 10.1126/science.1213920. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Noble A.; MacMillan D. W. C. Photoredox α-vinylation of α-amino acids and N-aryl amines. J. Am. Chem. Soc. 2014, 136, 11602–11605. 10.1021/ja506094d. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Prier C. K.; MacMillan D. W. C. Amine α-heteroarylation via photoredox catalysis: a homolytic aromatic substitution pathway. Chem. Sci. 2014, 5, 4173–4178. 10.1039/C4SC02155J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Shaw M. H.; Shurtleff V. W.; Terrett J. A.; Cuthbertson J. D.; MacMillan D. W. C. Native functionality in triple catalytic cross-coupling: sp3 C–H bonds as latent nucleophiles. Science 2016, 352, 1304–1308. 10.1126/science.aaf6635. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Maity B.; Zhu C.; Yue H.; Huang L.; Harb M.; Minenkov Y.; Rueping M.; Cavallo L. Mechanistic insight into the photoredox-nickel-HAT triple catalyzed arylation and alkylation of α-amino Csp3–H bonds. J. Am. Chem. Soc. 2020, 142, 16942–16952. 10.1021/jacs.0c05010. [DOI] [PubMed] [Google Scholar]
- For reviews of photocatalysis, see; a Cullen S. T. J.; Friestad G. K. Synthesis of chiral amines by C–C bond formation with photoredox catalysis. Synthesis 2021, 53, 2319–2341. 10.1055/a-1396-8343. [DOI] [Google Scholar]; b Bell J. D.; Murphy J. A. Recent advances in visible light-activated radical coupling reactions triggered by (i) ruthenium, (ii) iridium and (iii) organic photoredox agents. Chem. Soc. Rev. 2021, 50, 9540–9685. 10.1039/D1CS00311A. [DOI] [PubMed] [Google Scholar]; c Kaur J.; Barham J. P. Site-selective C(sp3)–H functionalizations mediated by hydrogen atom transfer reactions via α-amino/α-amido radicals. Synthesis 2022, 54, 1461–1477. 10.1055/a-1677-6619. [DOI] [Google Scholar]
- Zuo Z.; Ahneman D. T.; Chu L.; Terrett J. A.; Doyle A. G.; MacMillan D. W. C. Merging photoredox with nickel catalysis: Coupling of α-carboxyl sp3-carbons with aryl halides. Science 2014, 345, 437–440. 10.1126/science.1255525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected decarboxylative photocatalysis reactions, see; a Zuo Z.; Cong H.; Li W.; Choi J.; Fu G. C.; MacMillan D. W. C. Enantioselective decarboxylative arylation of α-amino acids via the merger of photoredox and nickel catalysis. J. Am. Chem. Soc. 2016, 138, 1832–1835. 10.1021/jacs.5b13211. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Luo J.; Zhang J. Donor–acceptor fluorophores for visible-light-promoted organic synthesis: photoredox/Ni dual catalytic C(sp3)–C(sp2) cross-coupling. ACS Catal. 2016, 6, 873–877. 10.1021/acscatal.5b02204. [DOI] [Google Scholar]; c Mega R. S.; Duong V. K.; Noble A.; Aggarwal V. K. Decarboxylative conjunctive cross-coupling of vinyl boronic esters using metallaphotoredox catalysis. Angew. Chem., Int. Ed. 2020, 59, 4375–4379. 10.1002/anie.201916340. [DOI] [PubMed] [Google Scholar]; d González-Esguevillas M.; Fernández D. F.; Rincón J. A.; Barberis M.; de Frutos O.; Mateos C.; García-Cerrada S.; Agejas J.; MacMillan D. W. C. Rapid optimization of photoredox reactions for continuous-flow systems using microscale batch technology. ACS Cent. Sci. 2021, 7, 1126–1134. 10.1021/acscentsci.1c00303. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Beil S. B.; Chen T. Q.; Intermaggio N. E.; MacMillan D. W. C. Carboxylic acids as adaptive functional groups in metallaphotoredox catalysis. Acc. Chem. Res. 2022, 55, 3481–3494. 10.1021/acs.accounts.2c00607. [DOI] [PMC free article] [PubMed] [Google Scholar]; f For a palladium-catalyzed C–H activation, see:Jain P.; Verma P.; Xia G.; Yu J.-Q. Enantioselective amine α-functionalization via palladium-catalysed C–H arylation of thioamides. Nat. Chem. 2017, 9, 140–144. 10.1038/nchem.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Cochrane E. J.; Leonori D.; Hassall L. A.; Coldham I. Synthesis and kinetic resolution of N-Boc-2-arylpiperidines. Chem. Commun. 2014, 50, 9910–9913. 10.1039/C4CC04576A. [DOI] [PubMed] [Google Scholar]; b Carter N.; Li X.; Reavey L.; Meijer A. J. H. M.; Coldham I. Synthesis and Kinetic Resolution of Substituted Tetrahydroquinolines by Lithiation then Electrophilic Quench. Chem. Sci. 2018, 9, 1352–1357. 10.1039/C7SC04435F. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Choi A.; El-Tunsi A.; Wang Y.; Meijer A. J. H. M.; Li J.; Li X.; Proietti Silvestri I.; Coldham I. Asymmetric synthesis of 2-arylindolines and 2,2-disubstituted indolines by kinetic resolution. I. Chem.—Eur. J. 2021, 27, 11670–11675. 10.1002/chem.202101248. [DOI] [PMC free article] [PubMed] [Google Scholar]; d El-Tunsi A.; Carter N.; Yeo S.-H.; Priest J. D.; Choi A.; Kobras C. M.; Ndlovu S.; Proietti Silvestri I.; Fenton A. K.; Coldham I. Kinetic resolution by lithiation: highly enantioselective synthesis of substituted dihydrobenzoxazines and tetrahydroquinoxalines. Synthesis 2022, 54, 355–368. 10.1055/a-1638-2478. [DOI] [Google Scholar]; e Choi A.; Meijer A. J. H. M.; Proietti Silvestri I.; Coldham I. Kinetic resolution of 2-aryl-4-methylenepiperidines toward enantioenriched functionalizable piperidine fragments. J. Org. Chem. 2022, 87, 8819–8823. 10.1021/acs.joc.2c00862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien C. J.; Nicewicz D. A. Milled Dry Ice as a C1 Source for the Carboxylation of Aryl Halides. Synlett 2021, 32, 814–816. 10.1055/a-1384-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigend F.; Ahlrichs R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. 10.1039/b508541a. [DOI] [PubMed] [Google Scholar]
- Selectivity factor S = krel = ln[(1 – C)(1 – ee)]/ln[(1 – C)(1 + ee)], where C = conversion and ee = enantiomeric excess; see:; Kagan H. B.; Fiaud J. C. Kinetic Resolution. Top. Stereochem. 1988, 18, 249–330. 10.1002/9780470147276.ch4. [DOI] [Google Scholar]
- For a review, see:; Kasten K.; Seling N.; O’Brien P. Enantioselective Lithiation–Substitution of Nitrogen-Containing Heterocycles. Org. Reactions 2019, 100, 255–328. 10.1002/0471264180.or100.05. [DOI] [Google Scholar]
- a Bailey W. F.; Beak P.; Kerrick S. T.; Ma S.; Wiberg K. B. An Experimental and Computational Investigation of the Enantioselective Deprotonation of Boc-piperidine. J. Am. Chem. Soc. 2002, 124, 1889–1896. 10.1021/ja012169y. [DOI] [PubMed] [Google Scholar]; b Stead D.; Carbone G.; O’Brien P.; Campos K. R.; Coldham I.; Sanderson A. Asymmetric Deprotonation of N-Boc Piperidine: React IR Monitoring and Mechanistic Aspects. J. Am. Chem. Soc. 2010, 132, 7260–7261. 10.1021/ja102043e. [DOI] [PubMed] [Google Scholar]; c Lin W.; Zhang K.-F.; Baudoin O. Regiodivergent enantioselective C–H functionalization of Boc-1,3-oxazinanes for the synthesis of β2- and β3-amino acids. Nat. Catal. 2019, 2, 882–888. 10.1038/s41929-019-0336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui Y.; Uehara F.; Hiki S.; Watanabe K.; Tanaka H.; Shouda A.; Yokoshima S.; Aritomo K.; Adachi T.; Fukunaga K.; Sunada S.; Nabeno M.; Saito K.; Eguchi J.; Yamagami K.; Asano S.; Tanaka S.; Yuki S.; Yoshii N.; Fujimura M.; Horikawa T. Discovery of novel 2-(3-phenylpiperazin-1-yl)-pyrimidin-4-ones as glycogen synthase kinase-3β inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 3726–3732. 10.1016/j.bmcl.2017.06.078. [DOI] [PubMed] [Google Scholar]
- Yao H.; Uras G.; Zhang P.; Shengtao X.; Yin Y.; Liu J.; Qin S.; Li X.; Allen S.; Bai R.; Gong Q.; Zhang H.; Zhu Z.; Xu J. Discovery of Novel Tacrine–Pyrimidone Hybrids as Potent Dual AChE/GSK-3 Inhibitors for the Treatment of Alzheimer’s Disease. J. Med. Chem. 2021, 64, 7483–7506. 10.1021/acs.jmedchem.1c00160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.