Abstract
Background
To assess the association between the reproductive factors of age at menarche, age at menopause, and reproductive span and the incidence of myocardial infarction (MI) and ischemic stroke (IS).
Methods
We used a population-based retrospective cohort study from the National Health Insurance Service database of Korea including a total of 1,224,547 postmenopausal women. Associations between age at menarche (≤ 12, 13–14 [reference], 15, 16, and ≥ 17 years), age at menopause (< 40, 40–45, 46–50, 51–54 [reference], and ≥ 55 years), and reproductive span (< 30, 30–33, 34–36, 37–40 [reference], and ≥ 41 years) and the incidence of MI and IS were assessed by Cox proportional hazard models with adjustment for traditional cardiovascular risk factors and various reproductive factors.
Results
During a median follow-up of 8.4 years, 25,181 MI and 38,996 IS cases were identified. Late menarche (≥ 16 years), early menopause (≤ 50 years), and short reproductive span (≤ 36 years) were linearly associated with a 6%, 12–40%, and 12–32% higher risk of MI, respectively. Meanwhile, a U-shaped association between age at menarche and risk of IS was found, with a 16% higher risk in early menarche (≤ 12 years) and a 7–9% higher risk in late menarche (≥ 16 years). Short reproductive span was linearly associated with an increased risk of MI, whereas both shorter and longer reproductive spans were associated with an increased risk of IS.
Conclusions
This study demonstrated different patterns of association between age at menarche and incidence of MI and IS: a linear association for MI versus a U-shaped association for IS. Female reproductive factors in addition to traditional cardiovascular risk factors should be considered when assessing overall cardiovascular risk in postmenopausal women.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-023-02757-2.
Keywords: Postmenopausal women, Cardiovascular disease, Age at menarche, Age at menopause, Reproductive span
Background
The burden of cardiovascular disease (CVD) continues to increase globally with 523 million cases and 18.6 million deaths in 2019 [1]. CVD is the leading cause of death for women accounting for 35% of total deaths in women in 2019 [2]. Among women, the notable increase in coronary heart disease risk after menopause has led to the hypothesis that alterations in levels of endogenous sex hormones, especially estrogen, could contribute to chronological changes in CVD risk [3]. There is abundant evidence that estrogen protects against atherosclerosis by promoting vasodilation, reducing fibrosis, and improving mitochondrial function and anti-oxidant activity [4].
Puberty and menopause transition are periods with remarkable changes in hormones, especially estradiol secretion. The timing of menarche and menopause varies depending on several factors, including estrogen levels, around these two turning points [5]. In this context, the relationships between reproductive factors, such as age at menopause [6–11] and age at menarche [6–8, 10, 12–15], with risk of coronary heart disease (CHD) and stroke have been evaluated in several studies (Additional file 1: Table S1). Early menarche is generally associated with an increased risk of CHD and stroke [7, 8, 13, 16], while some studies have reported an increased risk with late menarche [10], a U-shaped association [14, 15], or a null association [12]. Furthermore, there is a possibility that such association may differ according to CVD types. For example, a recent umbrella review reported that early menopause was associated with a 1.5-fold higher risk of ischemic heart disease, but no association was found with stroke [17]. Early menarche (< 12 years) was associated with a 1.2-fold higher risk of stroke, but not with CHD (adjusted hazard ratio [aHR] 1.08, 95% confidence interval [CI] 0.97–1.20) [6].
Reproductive span is a simple way to estimate the lifelong period with endogenous estrogen exposure, calculated by the difference between the timing of menarche and menopause. The association between reproductive span and CVD incidence has been investigated, but mostly in Western populations with conflicting results [18, 19]. A shorter reproductive span is generally associated with a higher risk of CVD events [18]. In a recent pooled analysis from 12 cohort studies, women with a short reproductive span (< 30 years) had a 71% higher risk of CVD [19], both for CHD and stroke. Similar results have been reported in studies of Asian women [10, 12]. However, a study in Korea reported a U-shaped association between reproductive span and incidence of CHD, while a short reproductive span was associated with a higher risk of stroke [10]. In addition, there might be interactions between reproductive factors (e.g., age at menarche and reproductive span) on the incidence of CVD. A previous study suggested that the timing of menarche affected the association between reproductive life span and incidence of CVD events, showing that early menarche (age ≤ 12 years) strengthened the association between short reproductive span and higher risk of CVD [19]. To our knowledge, such associations have not been investigated in an Asian female population.
Therefore, our aim in this study was to evaluate the associations between age at menarche, age at menopause, and reproductive span and incidence of CVD (myocardial infarction [MI] and ischemic stroke) among postmenopausal women using a large Korean population-based database. In addition, we investigated the combined effects of reproductive factors on the incidence of MI and ischemic stroke.
Methods
Data source and study setting
The present study used data from the National Health Insurance Services (NHIS) database of Korea. The KNHIS is a universal health coverage system that provides mandatory universal comprehensive medical care to 97% of the Korean population and an additional medical aid program to the 3% of the population in the lowest income bracket.
The NHIS database contains demographic information (e.g., age, sex, and income) in addition to claim information (general information on specifications, consultation statements, diagnosis statements defined by the International Classification of Disease 10th revision (ICD-10), and prescription statements) [20, 21].
Furthermore, because NHIS recommends free biennial cardiovascular health screening for all Koreans, health screening information (self-questionnaire on health behavior [e.g., past medical history, smoking, and drinking], anthropometric measurements [e.g., body mass index (BMI) and blood pressure], and laboratory test results [e.g., fasting glucose and lipid levels]) is also available for a large proportion of the population [22]. In addition, the National Cancer Screening Program (NCSP) was introduced in 1999, including breast and cervical cancer screening for women [23]. As a part of the NCSP, all Korean women over 40 years are screened for breast cancer biennially, and participants are asked to complete a questionnaire on reproductive factors. This nationwide database is widely used in epidemiological studies [24], including the studies on reproductive factors and various health outcomes [25].
Study population
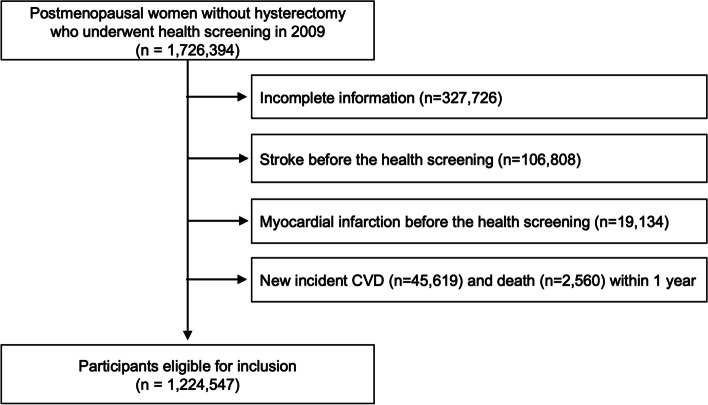
We initially identified 1,726,394 postmenopausal women without hysterectomy who underwent both cardiovascular and breast/cervical cancer screening in 2009. We first excluded 327,726 participants with missing data for at least one variable for complete case analysis (Additional file 1: Table S2). We also excluded participants who had a previous diagnosis of stroke (n = 106,808) or MI (n = 19,134). Participants who had a new CVD diagnosis (n = 45,619) or died (n = 2560) within 1 year after the health screening date were also excluded. A total of 1,224,547 postmenopausal women were included in the final analyses (Fig. 1).
Fig. 1.
Flow chart of the study population
This study was approved by the Institutional Review Board of Samsung Medical Center (IRB File No. SMC 2019–07-045). The review board waived the requirement for written informed consent because publicly open and anonymous data were analyzed in a retrospective manner.
Reproductive factors
According to the NCSP guidelines, participants of cervical and breast cancer screening completed a questionnaire addressing their age at menarche and age at menopause in the form of short answers. Reproductive span was estimated as the difference between age at menarche and age at menopause [25, 26]. Considering the distribution of age at menarche and menopause among Korean women and previous studies [27, 28], we categorized participants as follows: age at menarche (≤ 12, 13–14 [reference], 15, 16, and ≥ 17 years), age at menopause (< 40, 40–45, 46–50, 51–54 [reference], and ≥ 55 years), and reproductive span (< 30, 30–33, 34–36, 37–40 [reference], and ≥ 41 years). The restricted cubic spline curve supported our categorization for each reproductive factor in association with CVD risk (Additional file 2: Fig. S1). Information regarding parity (0, 1, or ≥ 2 children), total lifetime breast feeding history (never, < 6, 6–12, or ≥ 12 total months), hormone replacement therapy (HRT) history (never, < 2, 2–5, or ≥ 5 years), and use of oral contraceptives (OC) (never, < 1, or ≥ 1 year) was also collected.
Study outcomes and follow-up
Endpoints of the study were incident MI and ischemic stroke. Newly diagnosed MI and ischemic stroke cases were identified on the basis of ICD-10 code for MI (ICD-10 codes I21 or I22 during hospitalization or recording of these codes at least twice as an outpatient setting) and ischemic stroke (ICD-10 codes I63 or I64 during hospitalization with claims for brain magnetic resonance imaging or brain computerized tomography) [29]. We defined CVD as the composite of MI and ischemic stroke. The cohort was followed from the health examination date to the date of incident MI, ischemic stroke, death, or until the end of the study period (31 December 2018), whichever came first.
Covariates: traditional cardiovascular risk factors
Subjects were classified as never, ex-, or current smokers. Based on daily alcohol consumption, alcohol consumption was classified as none (0 g/day), mild to moderate (< 30 g/day), or heavy (≥ 30 g/day) [30, 31]. Regular exercise was defined as performing > 30 min of moderate physical activity at least five times per week or > 20 min of strenuous physical activity at least three times per week. Participants were categorized into five groups based on BMI (kg/m2) according to the Asia–Pacific criteria of the World Health Organization as follows: underweight (< 18.5), normal (18.5–23), overweight (23–25), obese (25–30), and severely obese (≥ 30). Baseline comorbidities (hypertension, diabetes mellitus, and dyslipidemia) were identified based on the combination of diagnosis codes (ICD-10) with relevant prescribed medications for each disease and clinical information. Hypertension was defined as a claim with ICD codes I10-I13 or I15 and antihypertensive medication or systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg. Diabetes mellitus was defined as a claim with E11-E14 codes linked to a history of hypoglycemic medication prescriptions or fasting glucose level ≥ 126 mg/dL. Dyslipidemia was defined as claims with code E78 and lipid-lowering medications or total cholesterol level ≥ 240 mg/dL [29]. Income level was based on the monthly insurance premium because insurance contributions in Korea are based on income level and not health risk.
Statistical analyses
Baseline values for all variables were measured in 2009. Continuous variables are presented as means ± standard deviations and categorical variables are presented as numbers and percentages. Hazard ratios (HRs) and 95% confidence interval (CI) values for the associations between reproductive factors and MI, ischemic stroke, and composite CVD were analyzed using Cox proportional hazards models. Multivariable-adjusted proportional hazards models were applied. Model 1 included age and traditional cardiovascular risk factors (income, smoking, alcohol consumption, regular exercise, body mass index, systolic blood pressure, total cholesterol, fasting glucose, hypertension, diabetes mellitus, and dyslipidemia). Model 2 additionally included reproductive factors (age at menarche, age at menopause, parity, duration of breast feeding, duration of HRT, and duration of OC use). Model 3 included reproductive span instead of age at menarche and age at menopause. Separate analyses were undertaken for MI and ischemic stroke. To determine which covariates to include for each reproductive factor (age at menarche, age at menopause, and reproductive factor), we drew a directed acyclic graph (DAG) including all covariates (Additional file 3: Fig. S2, Additional file 4: Fig. S3, Additional file 5: Fig. S4). According to the DAG, we assumed that none of the covariates has a causal relationship with age at menarche and CVD. We assumed that age, BMI, income, smoking status, alcohol consumption, regular exercise, age at menarche, parity, duration of breast feeding, and duration of oral contraceptive were common causes of age at menopause and CVD (confounders) and were included in the model. Regarding the association between reproductive span and CVD, we additionally included age at menopause as a confounder. Other variables (hypertension, diabetes mellitus, dyslipidemia, and duration of HRT) were mediators for the association between reproductive span and CVD, and not included in the model. We further examined the associations between various combinations of the three reproductive factors and the incidence for MI and ischemic stroke. We considered age at menarche 13–14 years, age at menopause of 51–54 years, and reproductive span 37–40 years as reference groups. We also conducted a subgroup analysis by comparing participants 60 years or older with those younger than 60 years to take reproductive factors by year of birth into consideration, i.e., the birth-cohort effect [12, 32].
Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA), and a P value < 0.05 was considered statistically significant.
Results
Baseline characteristics of the study population
The characteristics of the study participants are presented in Table 1. Participants with incident CVD events were likely to be older (66.9 vs 60.8 years) and to have later menarche (16.8 vs 16.4 years), earlier menopause (49.6 vs 50.1 years), and shorter reproductive span (32.8 vs 33.6 years). There was also a higher proportion of never HRT or ever OC users among those participants with incident CVD than those without incident CVD. Participants with incident CVD had more comorbidities including hypertension, diabetes mellitus, and dyslipidemia than those participants who did not develop CVD.
Table 1.
Baseline characteristics of the study participants
| Incident cardiovascular disease (myocardial infarction or ischemic stroke) |
P value | AMD or PR (95% CI) |
||
|---|---|---|---|---|
| No (n = 1,163,480) |
Yes (n = 61,067) |
|||
| Age (years) | 60.8 ± 8.0 | 66.9 ± 8.3 | < 0.0001 | 6.16 (6.10–6.23) |
| Income (quartile) | ||||
| Q1 (lowest) | 269,311 (23.2) | 13,247 (21.7) | < 0.0001 | 1.00 |
| Q2 | 219,661 (18.9) | 10,941 (17.9) | 1.01 (0.99–1.04) | |
| Q3 | 289,069 (24.9) | 14,843 (24.3) | 1.04 (1.02–1.07) | |
| Q4 (highest) | 385,439 (33.1) | 22,036 (36.1) | 1.15 (1.13–1.18) | |
| Smoking status | ||||
| Never | 1,121,142 (96.4) | 57,779 (94.6) | < 0.0001 | 1.00 |
| Ex-smoker | 11,914 (1.02) | 670 (1.1) | 1.09 (1.01–1.17) | |
| Current smoker | 30,424 (2.61) | 2618 (4.3) | 1.62 (1.56–1.68) | |
| Alcohol consumption | ||||
| None | 1,013,068 (87.1) | 54,967 (90.0) | < 0.0001 | 1.00 |
| Mild | 144,190 (12.4) | 5827 (9.5) | 0.75 (0.74–0.78) | |
| Heavy | 6222 (0.50) | 273 (0.5) | 0.82 (0.73–0.92) | |
| Regular exercise | 219,319 (18.9) | 8764 (14.4) | < 0.0001 | 0.73 (0.72–0.75) |
| Body mass index (kg/m2) | 24.1 ± 3.1 | 24.4 ± 3.3 | < 0.0001 | 0.31 (0.28–0.33) |
| < 18.5 | 24,611 (2.1) | 1582 (2.6) | 1.37 (1.31–1.44) | |
| 18.5–23 | 406,916 (35.0) | 18,704 (30.6) | 1.00 | |
| 23–25 | 309,110 (26.6) | 15,474 (25.3) | 1.08 (1.06–1.11) | |
| 25–30 | 375,149 (32.2) | 22,075 (36.2) | 1.26 (1.24–1.29) | |
| ≥ 30 | 47,694 (4.1) | 3232 (5.29) | 1.44 (1.39–1.50) | |
| Systolic BP (mmHg) | 125.2 ± 16.1 | 129.9 ± 17.0 | < 0.0001 | 4.71 (4.58–4.85) |
| Diastolic BP (mmHg) | 76.8 ± 10.1 | 78.7 ± 10.4 | < 0.0001 | 1.94 (1.86–2.02) |
| Fasting glucose (mg/dL) | 99.0 ± 23.0 | 105.3 ± 33.8 | < 0.0001 | 6.30 (6.11–6.49) |
| Total cholesterol (mg/dL) | 209.01 ± 43.8 | 210.1 ± 45.1 | < 0.0001 | 0.96 (0.60–1.32) |
| Comorbidities | ||||
| Hypertension | 491,733 (42.3) | 37,169 (60.9) | < 0.0001 | 2.05 (2.01–2.08) |
| Diabetes mellitus | 134,195 (11.5) | 13,492 (22.1) | < 0.0001 | 2.07 (2.03–2.11) |
| Dyslipidemia | 376,475 (32.4) | 21,960 (36.0) | < 0.0001 | 1.16 (1.15–1.18) |
| Age at menarche (years) | 16.4 ± 1.8 | 16.8 ± 1.8 | < 0.0001 | 0.36 (0.34–0.37) |
| ≤ 12 | 11,793 (1.0) | 373 (0.6) | < 0.0001 | 0.89 (0.80–0.98) |
| 13–14 | 146,603 (12.6) | 5251 (8.6) | 1.00 | |
| 15 | 209,389 (18.0) | 8718 (14.3) | 1.16 (1.12–1.20) | |
| 16 | 244,831 (21.0) | 12,700 (20.8) | 1.43 (1.38–1.47) | |
| ≥ 17 | 550,864 (47.4) | 34,025 (55.7) | 1.68 (1.64–1.73) | |
| Age at menopause (years) | 50.1 ± 4.0 | 49.6 ± 4.5 | < 0.0001 | 0.48 (0.45–0.51) |
| < 40 | 19,301 (1.7) | 1637 (2.7) | < 0.0001 | 1.88 (1.79–1.98) |
| 40–45 | 115,815 (10.0) | 8154 (13.4) | 1.58 (1.54–1.62) | |
| 46–50 | 527,997 (45.4) | 28,694 (47.0) | 1.24 (1.22–1.26) | |
| 51–54 | 434,332 (37.3) | 18,815 (30.8) | 1.00 | |
| ≥ 55 | 66,035 (5.7) | 3767 (6.2) | 1.30 (1.26–1.34) | |
| Reproductive span (years) | 33.6 ± 4.4 | 32.8 ± 4.9 | < 0.0001 | 0.84 (0.80–0.88) |
| < 30 | 156,340 (13.4) | 11,567 (18.9) | < 0.0001 | 1.79 (1.74–1.84) |
| 30–33 | 351,323 (30.2) | 20,109 (32.9) | 1.41 (1.37–1.44) | |
| 34–36 | 471,279 (40.5) | 21,244 (34.8) | 1.15 (1.13–1.18) | |
| 37–40 | 161,421 (13.9) | 6737 (11.0) | 1.00 | |
| ≥ 41 | 23,117 (2.0) | 1410 (2.3) | 1.37 (1.30–1.43) | |
| Parity | ||||
| Nulliparity | 28,295 (2.4) | 990 (1.6) | < 0.0001 | 1.00 |
| 1 | 1,062,402 (91.3) | 57,740 (94.6) | 0.92 (0.86–0.99) | |
| ≥ 2 | 72,783 (6.3) | 2337 (3.8) | 1.52 (1.43–1.62) | |
| Duration of breast feeding (months) | ||||
| Never | 78,590 (6.8) | 2410 (4.0) | < 0.0001 | 1.00 |
| < 6 | 77,652 (6.7) | 2195 (3.6) | 0.92 (0.87–0.98) | |
| 6–12 | 203,318 (17.5) | 7776 (12.7) | 1.24 (1.18–1.30) | |
| ≥ 12 | 803,920 (69.1) | 48,686 (79.7) | 1.92 (1.84–2.00) | |
| Duration of HRT (years) | ||||
| Never | 971,399 (83.5) | 54,552 (89.3) | < 0.0001 | 1.00 |
| < 2 | 112,034 (9.6) | 3709 (6.1) | 0.60 (0.58–0.62) | |
| 2–5 | 45,869 (3.9) | 1537 (2.5) | 0.61 (0.58–0.64) | |
| ≥ 5 | 34,178 (2.9) | 1269 (2.1) | 0.67 (0.64–0.71) | |
| Duration of OC use (years) | ||||
| Never | 978,738 (84.1) | 52,483 (85.9) | < 0.0001 | 1.00 |
| < 1 | 111,850 (9.6) | 4992 (8.2) | 0.84 (0.82–0.86) | |
| ≥ 1 | 72,892 (6.3) | 3592 (5.9) | 0.92 (0.89–0.95) | |
Data are expressed as means ± SD or n (%)
AMD absolute mean difference, PR prevalence ratio, CI confidence interval, BP blood pressure, HRT hormone replacement therapy, OC oral contraceptive
Associations between age at menarche, age at menopause, and reproductive span with CVD events
During a median follow-up of 8.4 years, 61,067 CVD events (25,181 MI and 38,996 ischemic stroke cases) were identified. Deaths during follow-up until the end of the study were 50,990 cases (4.2%) and the rate of dropping out from NHIS due to immigration is low (less than 2300 cases per year). Late menarche was associated with an increased risk of MI compared to 13–14 years at menarche: 16 years (aHR 1.06, 95% CI 1.01–1.11) and ≥ 17 years (aHR 1.06, 95% CI 1.02–1.11) (Table 2, Additional file 6: Fig. S5). Compared to women who experienced menopause at 51–54 years, early menopause was associated with an increased risk of MI: 46–50 years (aHR 1.12, 95% CI 1.09–1.16), 45–45 years (aHR 1.24, 95% CI 1.19–1.29), and < 40 years (aHR 1.40, 95% CI 1.30–1.52). When compared to a reproductive span of 37–40 years, a shorter reproductive span was associated with an increased risk of MI: 34–36 years (aHR 1.12, 95% CI 1.08–1.16), 30–33 years (aHR 1.19, 95% CI 1.15–1.16), and < 30 years (aHR 1.32, 95% CI 1.26–1.38).
Table 2.
Hazard ratios and 95% confidence intervals of myocardial infarction, ischemic stroke, and cardiovascular disease according to reproductive factors (age at menarche, age at menopause, and reproductive span)
| Reproductive factors | Subjects (N) |
Myocardial infarction | Ischemic stroke | Cardiovascular disease | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Events (n) |
IR | Model 1 | Model 2 | Events (n) |
IR | Model 1 | Model 2 | Events (n) |
IR | Model 1 | Model 2 | ||
| Age at menarche (years) | |||||||||||||
| ≤ 12 | 12,166 | 137 | 1.4 | 0.86 (0.72–1.02) | 0.86 (0.72–1.02) | 252 | 2.5 | 1.15 (1.01–1.31) | 1.16 (1.02–1.31) | 373 | 3.7 | 1.03 (0.93–1.14) | 1.03 (0.93–1.15) |
| 13–14 | 151,854 | 2267 | 1.8 | 1 (Ref.) | 1 (Ref.) | 3238 | 2.6 | 1 (Ref.) | 1 (Ref.) | 5251 | 4.2 | 1 (Ref.) | 1 (Ref.) |
| 15 | 218,107 | 3712 | 2.1 | 1.02 (0.97–1.07) | 1.01 (0.96–1.07) | 5418 | 3.0 | 1.01 (0.96–1.05) | 1.00 (0.96–1.04) | 8718 | 4.9 | 1.01 (0.98–1.05) | 1.01 (0.97–1.04) |
| 16 | 257,531 | 5268 | 2.5 | 1.07 (1.01–1.12) | 1.06 (1.01–1.11) | 8074 | 3.8 | 1.08 (1.03–1.12) | 1.07 (1.03–1.11) | 12,700 | 6.1 | 1.08 (1.04–1.11) | 1.07 (1.03–1.10) |
| ≥ 17 | 584,889 | 13,797 | 2.9 | 1.08 (1.03–1.13) | 1.06 (1.02–1.11) | 22,014 | 4.6 | 1.11 (1.07–1.15) | 1.09 (1.05–1.13) | 34,025 | 7.2 | 1.10 (1.07–1.13) | 1.08 (1.05–1.12) |
| P for trend | < 0.0001 | 0.0002 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | |||||||
| Age at menopause (years) | |||||||||||||
| < 40 | 20,938 | 686 | 4.0 | 1.41 (1.30–1.52) | 1.40 (1.30–1.52) | 1048 | 6.2 | 1.32 (1.24–1.40) | 1.31 (1.23–1.39) | 1637 | 9.8 | 1.36 (1.29–1.43) | 1.35 (1.28–1.42) |
| 40–45 | 123,969 | 3372 | 3.3 | 1.24 (1.19–1.30) | 1.24 (1.19–1.29) | 5246 | 5.2 | 1.18 (1.15–1.22) | 1.18 (1.14–1.22) | 8154 | 8.2 | 1.21 (1.18–1.24) | 1.21 (1.17–1.24) |
| 46–50 | 556,691 | 11,771 | 2.6 | 1.12 (1.09–1.16) | 1.12 (1.09–1.16) | 18,400 | 4.1 | 1.12 (1.09–1.14) | 1.12 (1.09–1.14) | 28,694 | 6.4 | 1.12 (1.10–1.14) | 1.12 (1.10–1.14) |
| 51–54 | 453,147 | 7833 | 2.1 | 1 (Ref.) | 1 (Ref.) | 11,860 | 3.2 | 1(Ref.) | 1 (Ref.) | 18,815 | 5.1 | 1 (Ref.) | 1 (Ref.) |
| ≥ 55 | 69,802 | 1519 | 2.6 | 0.99 (0.93–1.04) | 0.98 (0.93–1.04) | 2442 | 4.3 | 1.01(0.97–1.06) | 1.01 (0.97–1.05) | 3767 | 6.7 | 1.00 (0.96–1.03) | 1.00 (0.96–1.03) |
| P for trend | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | |||||||
| Reproductive span (years)a | |||||||||||||
| < 30 | 167,907 | 4735 | 3.5 | 1.33 (1.27–1.39) | 1.32 (1.26–1.38) | 7495 | 5.5 | 1.27 (1.23–1.32) | 1.26 (1.22–1.31) | 11,567 | 8.6 | 1.30 (1.26–1.33) | 1.29 (1.25–1.32) |
| 30–33 | 371,432 | 8250 | 2.7 | 1.20 (1.16–1.25) | 1.19 (1.15–1.24) | 12,909 | 4.3 | 1.17 (1.14–1.21) | 1.17 (1.13–1.20) | 20,109 | 6.7 | 1.19 (1.16–1.22) | 1.18 (1.15–1.21) |
| 34–36 | 401,857 | 7444 | 2.2 | 1.12 (1.08–1.17) | 1.12 (1.08–1.16) | 11,298 | 3.4 | 1.10 (1.07–1.14) | 1.10 (1.06–1.13) | 17,877 | 5.5 | 1.11 (1.08–1.14) | 1.11 (1.08–1.13) |
| 37–40 | 240,950 | 3861 | 1.9 | 1 (Ref.) | 1 (Ref.) | 5842 | 2.9 | 1 (Ref.) | 1 (Ref.) | 9284 | 4.7 | 1 (Ref.) | 1 (Ref.) |
| ≥ 41 | 42,401 | 891 | 2.6 | 1.03 (0.96–1.11) | 1.03 (0.96–1.11) | 1452 | 4.2 | 1.06 (1.00–1.12) | 1.06 (1.00–1.13) | 2230 | 6.5 | 1.05 (1.00–1.10) | 1.05 (1.00–1.10) |
| P for trend | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | |||||||
IR incidence rate per 1000 person-years
Model 1: The full model included age and traditional cardiovascular risk factors (income, smoking, alcohol consumption, regular exercise, body mass index, systolic blood pressure, total cholesterol, fasting glucose, hypertension, diabetes mellitus, and dyslipidemia)
Model 2: The full model included age, traditional cardiovascular risk factors (income, smoking, alcohol consumption, regular exercise, body mass index, systolic blood pressure, total cholesterol, fasting glucose, hypertension, diabetes mellitus, and dyslipidemia), and reproductive factors (age at menarche, age at menopause, parity, duration of breast feeding, duration of hormone replacement therapy, and duration of oral contraceptive use)
aThe full model included reproductive span instead of age at menarche and menopause in model 2
Values in bold indicate statistical significance
In contrast, different associations were observed between age at menarche, reproductive span, and ischemic stroke (Table 2). Both early menarche (≤ 12 years, aHR 1.16, 95% CI 1.02–1.31) and late menarche (16 years, aHR 1.07, 95% CI 1.03–1.11; ≥ 17 years, aHR 1.09, 95% CI 1.05–1.13) were associated with an increased risk of ischemic stroke, compared to 13–14 years at menarche, yielding a U-shaped association between age at menarche and ischemic stroke. In addition, compared to a reproductive span of 37–40 years, both shorter (≤ 36 years) and longer (≥ 41 years) reproductive spans were associated with an increased risk of ischemic stroke: < 30 years (aHR 1.26, 95% CI 1.22–1.31), 30–33 years (aHR 1.17, 95% CI 1.13–1.20), 34–36 years (aHR 1.10, 95% 1.03–1.13), and ≥ 41 years (aHR 1.06, 95% CI 1.00–1.13).
In the sensitivity analysis, (1) the results without lag time showed consistent associations with the main analysis (Additional file 1: Table S3) and (2) the results from the model, which consists of confounders from DAG, showed a consistent association with the main findings while attenuated (Additional file 1: Table S4).
Associations between combinations of the three reproductive factors and CVD risk
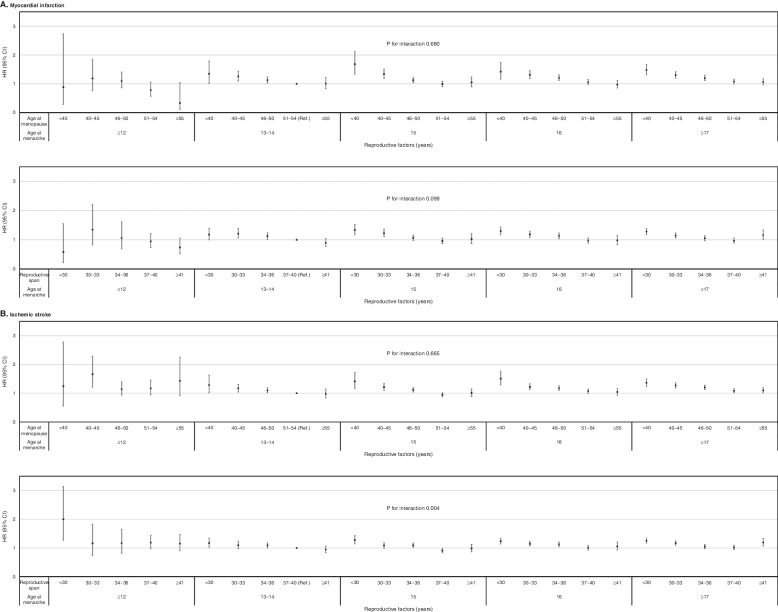
The combination of late menarche and early menopause (< 40 years) was associated with an increased risk of MI: 15 years (aHR 1.69, 95% CI 1.34–2.13), 16 years (aHR 1.44, 95% CI 1.17–1.76), and ≥ 17 years (aHR 1.50, 95% CI 1.33–1.70) (P for interaction = 0.68). Similarly, the combination of a short reproductive span (< 30 years) and late menarche increased the risk of MI: 15 years (aHR 1.34, 95% CI 1.17–2.13), 16 years (aHR 1.44, 95% CI 1.17–1.76), and ≥ 17 years (aHR 1.50, 95% CI 1.33–1.70) (P for interaction = 0.09). There was a significant interaction between reproductive span and age at menarche for ischemic stroke (P for interaction = 0.004). Increased risk of ischemic stroke related to short reproductive span (< 30 years) was strengthened by early age at menarche (≤ 12 years) (aHR 2.00, 95% CI 1.27–3.14). A U-shaped association between age at menarche and risk of ischemic stroke was observed compared to the reference group. The combination of either early or late menarche with a short reproductive span and early menopause was associated with an increased risk of ischemic stroke (Fig. 2 and Additional file 1: Table S2).
Fig. 2.
Associations between age at menarche in combination with age at menopause and reproductive span and the risk of cardiovascular events. HR, hazard ratio adjusted for cardiovascular risk factors (income, smoking, alcohol consumption, regular exercise, body mass index, systolic blood pressure, total cholesterol, fasting glucose, hypertension, diabetes mellitus, and dyslipidemia) and reproductive factors (parity, duration of breast feeding, duration of hormone replacement therapy, and duration of oral contraceptive use); CI, confidence interval, a) the association of combined reproductive factors with myocardial infarction. b) the association of combined reproductive factors with ischemic stroke
Age-stratified analyses
Age-stratified analyses (< 60 years and ≥ 60 years) yielded results consistent with those obtained for the entire study population (Additional file 1: Table S3). The increased risk of MI associated with late menopause and early menopause and short reproductive span was more prominent in the younger age group (< 60 years) than the older age group (≥ 60 years).
Discussion
In this large nationwide study, we investigated the association between reproductive factors and risk of MI and ischemic stroke. We found that late menarche, early menopause, and short reproductive span duration were associated with an increased risk of MI. Meanwhile, a U-shaped association between age at menarche and risk of ischemic stroke was noted, with a 16% higher risk in early menarche (≤ 12 years) and a 7–9% higher risk in late menarche (≥ 16 years). Similarly, both shorter and longer duration reproductive spans were associated with an increased risk of ischemic stroke.
Various patterns of association between age at menarche and CVD incidence have been reported. We found that early menarche was associated with an increased risk of ischemic stroke, but not MI, unlike several pre-existing studies that found a higher risk of both CHD and stroke for those women who had experienced early menarche [6–8, 13]. One possible reason for our findings is that the etiology of MI is largely uniform, namely atherosclerotic plaque rupture with superimposed in situ arterial thrombosis, whereas the etiology of ischemic stroke is heterogenous involving atherothrombotic, cardioembolic, small-vessel disease, and cryptogenic causes [33]. Although estrogen protects against atherosclerosis, it can increase coagulation by activating intrinsic pathway factors thereby increasing the risk of ischemic stroke, but not MI [34].
We found that late menarche was associated with an increased risk of both MI and ischemic stroke. A similar finding was reported in a previous study of Korean women: menarche ≥ 17 years was associated with an aHR of 1.62 (95% CI 1.11–2.36) for MI and an aHR of 1.22 (95% CI 1.09–1.36) for stroke, whereas most other studies have reported no association [6–8, 13]. This difference may partly be explained by differences in disease epidemiology among countries. In Korea, the incidence of ischemic stroke is higher than that of MI (63.3 vs 40.0 per 100,000 person-years in 2013) [35, 36]. In addition, late menarche could be related to low BMI and poor nutrition during puberty. Underweight adolescents were expected to have late menarche (HR 0.27, 95% CI 0.12–0.60), which in turn leads to a higher risk of CVD in adults [37]. In particular, the incidence of underweight is more prevalent in Asian than Western populations [38]. However, late menarche may be linked to higher prepubertal cortisol secretion levels through inhibition of pulsatile luteinizing hormone due to suppression of hypothalamic-pituitary responsiveness to GnRH [39, 40]; high cortisol levels are known to increase the incidence and progression of atherogenesis [41].
In agreement with previous studies [6–11], early age at menopause was associated with an increased risk of both MI and ischemic stroke. A pooled analysis of observational studies reported an increased risk of non-fatal CVD in women who had undergone premature menopause (< 40 years; HR 1.55, 95% CI 1.37–1.73) and early menopause (age 40 to 44 years; HR 1.30, 95% CI 1.22–1.39) [42]. An early decrease in endogenous estrogens adversely affects cardiovascular risk factors such as lipid and blood pressure, negating its protective effects as an endothelial vasodilator.
The association between a shorter reproductive span and a higher risk of MI or ischemic stroke is consistent with the association between age at menopause and MI and ischemic stroke, because the reproductive span is largely determined by age at menopause. Furthermore, a short reproductive span is associated with an increased risk of atrial fibrillation, which in turn can increase the risk of ischemic stroke [43]. We also found that a longer reproductive span was associated with an increased risk of ischemic stroke, but not of MI. The reason for this is unclear, but it may be partly associated with the increased risk of stroke in very early menarche (< 12 years).
The combination of late menarche and early menopause/reproductive span increased the risk of MI in an additive manner, with the lowest risk found in those participants with early menarche and late menopause/long reproductive span. Interestingly, early menarche increased the risk of ischemic stroke regardless of age at menopause and reproductive span in contrast to the associations between these factors and MI [19]. Women who had undergone early menarche and had a short reproductive span had the highest risk of ischemic stroke, perhaps due to the combined effect of improper timing of estrogen production and insufficient estrogen exposure.
The association between reproductive factors and CVD risk was stronger in women less than 60 years compared to women aged ≥ 60 years. This interesting finding may be attributed to the different CVD risks of each reference group. Old age itself is a potent risk factor for CVD risk, and the old age group is more likely to have other traditional risk factors (e.g., hypertension), which results in the smaller effect size of reproductive factors in old-aged women.
Clinical guidelines for primary prevention of CVD in women acknowledge premature menopause (< 40 years) as a risk-enhancing factor in addition to traditional CVD risk factors and suggest that statin therapy be considered in those women who undergo premature menopause [42]. However, the relationships between age at menarche and reproductive span and CVD are still inconsistent and further studies are needed to investigate the relationship between these reproductive factors and the risk of MI and ischemic stroke.
Our study has several strengths including the large study population, adjustment for various reproductive factors besides traditional CVD risk factors, and evaluation of the association between various combinations of the reproductive factors of age at menarche, age at menopause, and reproductive span and MI and ischemic stroke risk. Furthermore, even though age at menarche and age at menopause are strongly interrelated [44], only a few studies have adjusted for both age at menarche and age at menopause, unlike our study [8–11]. However, several limitations need to be considered when interpreting our findings. First, there may have been a recall bias associated with self-reported age at menarche and age at menopause. We consider this unlikely though as a previous study reported a high correlation (r = 0.79) and mean absolute error of 0.62 years between the recalled and original age at menarche [45]. Second, we were not able to include the information on body weight or nutrition status at adolescents even though we suggested underweight as a key factor. Particularly, a dietary pattern is an important confounding factor for the association between reproductive factor and CVD, which could not be considered on this study [46–48]. Further studies including such confounding factor are needed. Third, we included only Korean women; thus, the generalizability of our study findings is limited. Racial/ethnic differences may exist in the associations between reproductive factors and CVD risk because reproductive trajectories vary across different races/ethnicities [49]. Fourth, we did not include the women with established CVD because of concerns about accuracy in identifying the recurrent CVD events. Further studies are needed with a sophisticated operational definition of recurring cases from the viewpoints of secondary prevention.
Conclusions
In conclusion, we demonstrated different patterns of association between age at menarche and MI and ischemic stroke. While late menarche was linearly associated with an increased risk of MI, both early and late menarche were associated with an increased risk of ischemic stroke. Early menopause and short reproductive span were linearly associated with an increased risk of both MI and ischemic stroke, except for a slight increase in the risk of ischemic stroke in women with a reproductive span longer than 40 years. Clinicians need to consider female reproductive factors in addition to traditional CVD risk factors when assessing the overall risk of CVD in postmenopausal women.
Supplementary Information
Additional file 1: Supplementary Tables. Table S1. A summary of previous studies (selected) on the relationship between reproductive factors and cardiovascular disease incidence. Table S2. Information on missing data. Table S3. Hazard ratios and 95% confidence intervals of myocardial infarction, ischemic stroke, and cardiovascular disease according to reproductive factors (age at menarche, age at menopause, and reproductive span) without lag periods. Table S4. Hazard ratios and 95% confidence intervals of myocardial infarction, ischemic stroke, and cardiovascular disease according to reproductive factors (age at menarche, age at menopause, and reproductive span) adjustment for confounders based on directed acyclic graph. Table S5. Age at menarche in combination with age at menopause and reproductive span and the risk of cardiovascular events. Table S6. Hazard ratios and 95% confidence intervals of cardiovascular disease by reproductive factors according to age group.
Additional file 2: Fig. S1. The restricted cubic spline curve for the association of reproductive factors with cardiovascular diseases.
Additional file 3: Fig. S2. Directed acyclic graph illustrating the assumptions about the causal relationship between age at menarche and cardiovascular disease. Red circles indicate confounders, blue circles represent mediators/colliders. The grey circle refers to an unmeasured variable. BMI, body mass index; HRT, hormone replacement therapy; CVD, cardiovascular disease
Additional file 4: Fig. S3. Directed acyclic graph illustrating the assumptions about the causal relationship between age at menopause and cardiovascular disease. Red circles indicate confounders, blue circles represent mediators/colliders. The grey circle refers to an unmeasured variable. BMI, body mass index; HRT, hormone replacement therapy; CVD, cardiovascular disease.
Additional file 5: Fig. S4. Directed acyclic graph illustrating the assumptions about the causal relationship between reproductive span and cardiovascular disease. Red circles indicate confounders, blue circles represent mediators/colliders. The grey circle refers to an unmeasured variable. BMI, body mass index; HRT, hormone replacement therapy; CVD, cardiovascular disease.
Additional file 6: Fig. S5. Hazard ratios (HRs) and 95% confidence intervals (CIs) for cardiovascular events according to menstrual history. aModel 2: The full model included age, cardiovascular risk factors (income, smoking, alcohol consumption, regular exercise, body mass index, systolic blood pressure, total cholesterol, fasting glucose, hypertension, diabetes mellitus, and dyslipidemia) and reproductive factors (age at menarche, age at menopause, parity, duration of breast feeding, duration of hormone replacement therapy, and duration of oral contraceptive use). bModel 3: The full model included age, cardiovascular risk factors (income, smoking, alcohol consumption, regular exercise, body mass index, systolic blood pressure, total cholesterol, fasting glucose, hypertension, diabetes mellitus, and dyslipidemia) and reproductive factors (reproductive span, parity, duration of breast feeding, duration of hormone replacement therapy, and duration of oral contraceptive use)
Acknowledgements
None
Abbreviations
- aHR
Adjusted hazard ratio
- BMI
Body mass index
- CHD
Coronary heart disease
- CI
Confidence interval
- CVD
Cardiovascular disease
- DAG
Directed acyclic graph
- HRs
Hazard ratios
- HRT
Hormone replacement therapy
- ICD-10
International Classification of Disease 10th revision
- IS
Ischemic stroke
- MI
Myocardial infarction
- NCSP
National Cancer Screening Program
- NHIS
National Health Insurance Services
- OC
Oral contraceptives
Authors’ contributions
SMJ, JEY, and DWS contributed to the study design and conception. DWS and DK contributed to data acquisition and analyses. SMJ, JEY, and DWS drafted the manuscript. SMJ, JEY, DWS, KHJ, KH, HL, and DYL contributed to the interpretation of data. SMJ, JEY, DWS, KHJ, KH, HL, and DYL critically revised and approved the final manuscript. DWS had full access to all data analyzed in this study and take responsibility for the integrity of the data and the accuracy of the data analyses. DWS is the manuscript’s guarantor. The corresponding author attests that all listed authors meet the authorship criteria and that no others meeting the criteria have been omitted. The authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
The data that support the findings of this study are available from the Korean National Health Insurance Service (KNHIS) and were used under license for the current study (http://nhiss.nhis.or.kr). Restrictions apply to their availability (data are not publicly available). Data are available with permission of the KNHIS from the authors upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Samsung Medical Center (IRB File No. SMC 2019–07-045).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Su-Min Jeong and Jung Eun Yoo are co-first authors.
References
- 1.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogel B, Acevedo M, Appelman Y, Bairey Merz CN, Chieffo A, Figtree GA, Guerrero M, Kunadian V, Lam CSP, Maas A, et al. The Lancet women and cardiovascular disease Commission: reducing the global burden by 2030. Lancet. 2021;397(10292):2385–2438. doi: 10.1016/S0140-6736(21)00684-X. [DOI] [PubMed] [Google Scholar]
- 3.Menopause and risk of cardiovascular disease Ann Intern Med. 1976;85(4):447–452. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- 4.Iorga A, Cunningham CM, Moazeni S, Ruffenach G, Umar S, Eghbali M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol Sex Differ. 2017;8(1):33–33. doi: 10.1186/s13293-017-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apter D, Vihko R. Premenarcheal endocrine changes in relation to age at menarche. Clin Endocrinol (Oxf) 1985;22(6):753–760. doi: 10.1111/j.1365-2265.1985.tb00165.x. [DOI] [PubMed] [Google Scholar]
- 6.Peters SA, Woodward M. Women’s reproductive factors and incident cardiovascular disease in the UK Biobank. Heart. 2018;104(13):1069–1075. doi: 10.1136/heartjnl-2017-312289. [DOI] [PubMed] [Google Scholar]
- 7.Ley SH, Li Y, Tobias DK, Manson JE, Rosner B, Hu FB, Rexrode KM. Duration of reproductive life span, age at menarche, and age at menopause are associated with risk of cardiovascular disease in women. J Am Heart Assoc. 2017;6(11):e006713. doi: 10.1161/JAHA.117.006713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murakami K, Metoki H, Satoh M, Asayama K, Hosaka M, Matsuda A, Inoue R, Tsubota-Utsugi M, Murakami T, Nomura K, et al. Menstrual factors and stroke incidence in Japanese postmenopausal women: the Ohasama study. Neuroepidemiology. 2016;47(2):109–116. doi: 10.1159/000452220. [DOI] [PubMed] [Google Scholar]
- 9.Hu FB, Grodstein F, Hennekens CH, Colditz GA, Johnson M, Manson JE, Rosner B, Stampfer MJ. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med. 1999;159(10):1061–1066. doi: 10.1001/archinte.159.10.1061. [DOI] [PubMed] [Google Scholar]
- 10.Jung KJ, Kim MR, Yun YD, Kim HC, Jee SH. Duration of ovarian hormone exposure and atherosclerotic cardiovascular disease in Korean women: the Korean Heart Study. Menopause. 2016;23(1):60–66. doi: 10.1097/GME.0000000000000489. [DOI] [PubMed] [Google Scholar]
- 11.Yang L, Lin L, Kartsonaki C, Guo Y, Chen Y, Bian Z, Xie K, Jin D, Li L, Lv J, et al. Menopause characteristics, total reproductive years, and risk of cardiovascular disease among Chinese women. Circ Cardiovasc Qual Outcomes. 2017;10(11):e004235. doi: 10.1161/CIRCOUTCOMES.117.004235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L, Li L, Millwood IY, Peters SAE, Chen Y, Guo Y, Bian Z, Chen X, Chen L, Feng S, et al. Age at menarche and risk of major cardiovascular diseases: evidence of birth cohort effects from a prospective study of 300,000 Chinese women. Int J Cardiol. 2017;227:497–502. doi: 10.1016/j.ijcard.2016.10.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lakshman R, Forouhi NG, Sharp SJ, Luben R, Bingham SA, Khaw KT, Wareham NJ, Ong KK. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab. 2009;94(12):4953–4960. doi: 10.1210/jc.2009-1789. [DOI] [PubMed] [Google Scholar]
- 14.Canoy D, Beral V, Balkwill A, Wright FL, Kroll ME, Reeves GK, Green J, Cairns BJ. Age at menarche and risks of coronary heart and other vascular diseases in a large UK cohort. Circulation. 2015;131(3):237–244. doi: 10.1161/CIRCULATIONAHA.114.010070. [DOI] [PubMed] [Google Scholar]
- 15.Lee JJ, Cook-Wiens G, Johnson BD, Braunstein GD, Berga SL, Stanczyk FZ, Pepine CJ, Bairey Merz CN, Shufelt CL. Age at menarche and risk of cardiovascular disease outcomes: findings from the National Heart Lung and Blood Institute-sponsored women’s ischemia syndrome evaluation. J Am Heart Assoc. 2019;8(12):e012406. doi: 10.1161/JAHA.119.012406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters SA, van der Schouw YT, Wood AM, Sweeting MJ, Moons KG, Weiderpass E, Arriola L, Benetou V, Boeing H, Bonnet F, et al. Parity, breastfeeding and risk of coronary heart disease: a pan-European case-cohort study. Eur J Prev Cardiol. 2016;23(16):1755–1765. doi: 10.1177/2047487316658571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okoth K, Chandan JS, Marshall T, Thangaratinam S, Thomas GN, Nirantharakumar K, Adderley NJ. Association between the reproductive health of young women and cardiovascular disease in later life: umbrella review. BMJ. 2020;371:m3502. doi: 10.1136/bmj.m3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishra S, Chung H-F, Waller M, Mishra G. Duration of estrogen exposure during reproductive years, age at menarche and age at menopause, and risk of cardiovascular disease events, all-cause and cardiovascular mortality: a systematic review and meta-analysis. BJOG. 2021;128(5):809–821. doi: 10.1111/1471-0528.16524. [DOI] [PubMed] [Google Scholar]
- 19.Mishra SR, Chung HF, Waller M, Dobson AJ, Greenwood DC, Cade JE, Giles GG, Bruinsma F, Simonsen MK, Hardy R, et al. Association between reproductive life span and incident nonfatal cardiovascular disease: a pooled analysis of individual patient data from 12 studies. JAMA Cardiol. 2020;5(12):1410–1418. doi: 10.1001/jamacardio.2020.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheol Seong S, Kim YY, Khang YH, Heon Park J, Kang HJ, Lee H, Do CH, Song JS, Hyon Bang J, Ha S, et al. Data resource profile: the National Health Information Database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017;46(3):799–800. doi: 10.1093/ije/dyw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin DW, Cho B, Guallar E. Korean National Health Insurance Database. JAMA Intern Med. 2016;176(1):138. doi: 10.1001/jamainternmed.2015.7110. [DOI] [PubMed] [Google Scholar]
- 22.Shin DW, Cho J, Park JH, Cho B. National General Health Screening Program in Korea: history, current status, and future direction. Precis Future Med. 2022;6(1):9–31. doi: 10.23838/pfm.2021.00135. [DOI] [Google Scholar]
- 23.Yoo KY. Cancer control activities in the Republic of Korea. Jpn J Clin Oncol. 2008;38(5):327–333. doi: 10.1093/jjco/hyn026. [DOI] [PubMed] [Google Scholar]
- 24.Lee YH, Han K, Ko SH, Ko KS, Lee KU. Data analytic process of a nationwide population-based study using national health information database established by National Health Insurance Service. Diabetes Metab J. 2016;40(1):79–82. doi: 10.4093/dmj.2016.40.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoo JE, Shin DW, Han K, Kim D, Yoon JW, Lee DY. Association of female reproductive factors with incidence of fracture among postmenopausal women in Korea. JAMA Netw Open. 2021;4(1):e2030405. doi: 10.1001/jamanetworkopen.2020.30405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin DW, Yu J, Cho J, Lee SK, Jung JH, Han K, Kim SY, Yoo JE, Yeob KE, Kim YY, et al. Breast cancer screening disparities between women with and without disabilities: a national database study in South Korea. Cancer. 2020;126(7):1522–1529. doi: 10.1002/cncr.32693. [DOI] [PubMed] [Google Scholar]
- 27.Yoo JE, Shin DW, Han K, Kim D, Won HS, Lee J, Kim SY, Nam GE, Park HS. Female reproductive factors and the risk of dementia: a nationwide cohort study. Eur J Neurol. 2020;27(8):1448–1458. doi: 10.1111/ene.14315. [DOI] [PubMed] [Google Scholar]
- 28.Jeon KH, Shin DW, Han K, Kim D, Yoo JE, Jeong SM, Cho JH. Female reproductive factors and the risk of lung cancer in postmenopausal women: a nationwide cohort study. Br J Cancer. 2020;122(9):1417–1424. doi: 10.1038/s41416-020-0789-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim MK, Han K, Park YM, Kwon HS, Kang G, Yoon KH, Lee SH. Associations of variability in blood pressure, glucose and cholesterol concentrations, and body mass index with mortality and cardiovascular outcomes in the general population. Circulation. 2018;138(23):2627–2637. doi: 10.1161/CIRCULATIONAHA.118.034978. [DOI] [PubMed] [Google Scholar]
- 30.Jeong SM, Lee HR, Han K, Jeon KH, Kim D, Yoo JE, Cho MH, Chun S, Lee SP, Nam KW, et al. Association of change in alcohol consumption with risk of ischemic stroke. Stroke. 2022;53(8):2488–2496. doi: 10.1161/STROKEAHA.121.037590. [DOI] [PubMed] [Google Scholar]
- 31.Yoo JE, Han K, Shin DW, Kim D, Kim B-s, Chun S, Jeon KH, Jung W, Park J, Park JH, et al. Association between changes in alcohol consumption and cancer risk. JAMA Network Open. 2022;5(8):e2228544–e2228544. doi: 10.1001/jamanetworkopen.2022.28544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahn JH, Lim SW, Song BS, Seo J, Lee JA, Kim DH, Lim JS. Age at menarche in the Korean female: secular trends and relationship to adulthood body mass index. Ann Pediatr Endocrinol Metabol. 2013;18(2):60–64. doi: 10.6065/apem.2013.18.2.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soler EP, Ruiz VC. Epidemiology and risk factors of cerebral ischemia and ischemic heart diseases: similarities and differences. Curr Cardiol Rev. 2010;6(3):138–149. doi: 10.2174/157340310791658785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siegerink B, Govers-Riemslag JW, Rosendaal FR, Ten Cate H, Algra A. Intrinsic coagulation activation and the risk of arterial thrombosis in young women: results from the Risk of Arterial Thrombosis in relation to Oral contraceptives (RATIO) case-control study. Circulation. 2010;122(18):1854–1861. doi: 10.1161/CIRCULATIONAHA.110.943738. [DOI] [PubMed] [Google Scholar]
- 35.Kim JY, Kang K, Kang J, Koo J, Kim D-H, Kim BJ, Kim W-J, Kim E-G, Kim JG, Kim J-M, et al. Executive summary of stroke statistics in Korea 2018: a report from the Epidemiology Research Council of the Korean Stroke Society. J Stroke. 2019;21(1):42–59. doi: 10.5853/jos.2018.03125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim RB, Kim HS, Kang DR, Choi JY, Choi NC, Hwang S, Hwang JY. The trend in incidence and case-fatality of hospitalized acute myocardial infarction patients in Korea, 2007 to 2016. J Korean Med Sci. 2019;34(50):e322. doi: 10.3346/jkms.2019.34.e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee MH, Kim SH, Oh M, Lee KW, Park MJ. Age at menarche in Korean adolescents: trends and influencing factors. Reprod Health. 2016;13(1):121. doi: 10.1186/s12978-016-0240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3(3):141–146. doi: 10.1046/j.1467-789X.2002.00065.x. [DOI] [PubMed] [Google Scholar]
- 39.Breen KM, Karsch FJ. Does cortisol inhibit pulsatile luteinizing hormone secretion at the hypothalamic or pituitary level? Endocrinology. 2004;145(2):692–698. doi: 10.1210/en.2003-1114. [DOI] [PubMed] [Google Scholar]
- 40.Shi L, Wudy SA, Buyken AE, Maser-Gluth C, Hartmann MF, Remer T. Prepubertal glucocorticoid status and pubertal timing. J Clin Endocrinol Metab. 2011;96(6):E891–E898. doi: 10.1210/jc.2010-2935. [DOI] [PubMed] [Google Scholar]
- 41.Walker BR. Glucocorticoids and cardiovascular disease. Eur J Endocrinol. 2007;157(5):545–559. doi: 10.1530/EJE-07-0455. [DOI] [PubMed] [Google Scholar]
- 42.Zhu D, Chung H-F, Dobson AJ, Pandeya N, Giles GG, Bruinsma F, Brunner EJ, Kuh D, Hardy R, Avis NE, et al. Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. Lancet Public Health. 2019;4(11):e553–e564. doi: 10.1016/S2468-2667(19)30155-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang S, Kwak S, Kwon S, Lee H-J, Lee H, Park J-B, Lee S-P, Kim H, Han K, Kim Y-J, et al. Association of total reproductive years with incident atrial fibrillation, and subsequent ischemic stroke in women with natural menopause. Circ Arrhythm Electrophysiol. 2019;12(11):e007428. doi: 10.1161/CIRCEP.119.007428. [DOI] [PubMed] [Google Scholar]
- 44.Mishra GD, Pandeya N, Dobson AJ, Chung H-F, Anderson D, Kuh D, Sandin S, Giles GG, Bruinsma F, Hayashi K, et al. Early menarche, nulliparity and the risk for premature and early natural menopause. Hum Reprod. 2017;32(3):679–686. doi: 10.1093/humrep/dew350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Must A, Phillips SM, Naumova EN, Blum M, Harris S, Dawson-Hughes B, Rand WM. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? Am J Epidemiol. 2002;155(7):672–679. doi: 10.1093/aje/155.7.672. [DOI] [PubMed] [Google Scholar]
- 46.Duan R, Chen Y, Qiao T, Duan R, Chen M, Zhao L, Gong Y, Cheng G. Modern dietary pattern is prospectively associated with earlier age at menarche: data from the CHNS 1997–2015. Nutr J. 2020;19(1):95. doi: 10.1186/s12937-020-00622-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dunneram Y, Greenwood DC, Cade JE. Dietary patterns and age at natural menopause: evidence from the UK Women’s Cohort Study. Maturitas. 2021;143:165–170. doi: 10.1016/j.maturitas.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 48.Fung TT, Willett WC, Stampfer MJ, Manson JE, Hu FB. Dietary patterns and the risk of coronary heart disease in women. Arch Intern Med. 2001;161(15):1857–1862. doi: 10.1001/archinte.161.15.1857. [DOI] [PubMed] [Google Scholar]
- 49.Gold EB, Bromberger J, Crawford S, Samuels S, Greendale GA, Harlow SD, Skurnick J. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153(9):865–874. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Tables. Table S1. A summary of previous studies (selected) on the relationship between reproductive factors and cardiovascular disease incidence. Table S2. Information on missing data. Table S3. Hazard ratios and 95% confidence intervals of myocardial infarction, ischemic stroke, and cardiovascular disease according to reproductive factors (age at menarche, age at menopause, and reproductive span) without lag periods. Table S4. Hazard ratios and 95% confidence intervals of myocardial infarction, ischemic stroke, and cardiovascular disease according to reproductive factors (age at menarche, age at menopause, and reproductive span) adjustment for confounders based on directed acyclic graph. Table S5. Age at menarche in combination with age at menopause and reproductive span and the risk of cardiovascular events. Table S6. Hazard ratios and 95% confidence intervals of cardiovascular disease by reproductive factors according to age group.
Additional file 2: Fig. S1. The restricted cubic spline curve for the association of reproductive factors with cardiovascular diseases.
Additional file 3: Fig. S2. Directed acyclic graph illustrating the assumptions about the causal relationship between age at menarche and cardiovascular disease. Red circles indicate confounders, blue circles represent mediators/colliders. The grey circle refers to an unmeasured variable. BMI, body mass index; HRT, hormone replacement therapy; CVD, cardiovascular disease
Additional file 4: Fig. S3. Directed acyclic graph illustrating the assumptions about the causal relationship between age at menopause and cardiovascular disease. Red circles indicate confounders, blue circles represent mediators/colliders. The grey circle refers to an unmeasured variable. BMI, body mass index; HRT, hormone replacement therapy; CVD, cardiovascular disease.
Additional file 5: Fig. S4. Directed acyclic graph illustrating the assumptions about the causal relationship between reproductive span and cardiovascular disease. Red circles indicate confounders, blue circles represent mediators/colliders. The grey circle refers to an unmeasured variable. BMI, body mass index; HRT, hormone replacement therapy; CVD, cardiovascular disease.
Additional file 6: Fig. S5. Hazard ratios (HRs) and 95% confidence intervals (CIs) for cardiovascular events according to menstrual history. aModel 2: The full model included age, cardiovascular risk factors (income, smoking, alcohol consumption, regular exercise, body mass index, systolic blood pressure, total cholesterol, fasting glucose, hypertension, diabetes mellitus, and dyslipidemia) and reproductive factors (age at menarche, age at menopause, parity, duration of breast feeding, duration of hormone replacement therapy, and duration of oral contraceptive use). bModel 3: The full model included age, cardiovascular risk factors (income, smoking, alcohol consumption, regular exercise, body mass index, systolic blood pressure, total cholesterol, fasting glucose, hypertension, diabetes mellitus, and dyslipidemia) and reproductive factors (reproductive span, parity, duration of breast feeding, duration of hormone replacement therapy, and duration of oral contraceptive use)
Data Availability Statement
The data that support the findings of this study are available from the Korean National Health Insurance Service (KNHIS) and were used under license for the current study (http://nhiss.nhis.or.kr). Restrictions apply to their availability (data are not publicly available). Data are available with permission of the KNHIS from the authors upon reasonable request.