Abstract
Renal cell carcinoma (RCC) is a major pathological type of kidney cancer and is one of the most common malignancies worldwide. The unremarkable symptoms of early stages, proneness to postoperative metastasis or recurrence, and low sensitivity to radiotherapy and chemotherapy pose a challenge for the diagnosis and treatment of RCC. Liquid biopsy is an emerging test that measures patient biomarkers, including circulating tumor cells, cell-free DNA/cell-free tumor DNA, cell-free RNA, exosomes, and tumor-derived metabolites and proteins. Owing to its non-invasiveness, liquid biopsy enables continuous and real-time collection of patient information for diagnosis, prognostic assessment, treatment monitoring, and response evaluation. Therefore, the selection of appropriate biomarkers for liquid biopsy is crucial for identifying high-risk patients, developing personalized therapeutic plans, and practicing precision medicine. In recent years, owing to the rapid development and iteration of extraction and analysis technologies, liquid biopsy has emerged as a low cost, high efficiency, and high accuracy clinical detection method. Here, we comprehensively review liquid biopsy components and their clinical applications over the past 5 years. Additionally, we discuss its limitations and predict its future prospects.
Keywords: Renal cell carcinoma, Liquid biopsy, Circulating tumor cells, Cell-free tumor DNA, Diagnosis, Prognosis, Treatment monitoring
Background
Kidney cancer is a common, substantial lesion of the kidney, accounting for approximately 2.2% of all cancer incidences, and its occurrence continues to increase [1]. Renal cell carcinoma (RCC) is the main pathological type of kidney cancer and constitutes over 90% of all kidney cancers. RCC can be classified into three distinct types based on specific histopathological and genetic features, of which clear-cell RCC (ccRCC) is the most common (80–90%), followed by papillary and suspicious cell carcinoma (15% and 5%, respectively) [2]. Surgery is the preferred treatment for RCC; however, 20–40% of patients with RCC experience recurrence and metastasis after surgery [3]. Therapeutic strategies based on radiation and cytotoxic chemotherapy agents have limited therapeutic effects on RCC [3, 4]. The advent and development of targeted agents, including agents targeting mammalian target of rapamycin (mTOR) and receptor tyrosine kinase signaling, have made therapeutic advances, but are prone to drug resistance [5–8]. In recent years, the prognosis of patients with RCC has improved significantly with the introduction of immune checkpoint inhibitor (ICI) therapy [9]. Many factors induce metastasis and recurrence of RCC and lead to death, with the impact of late diagnosis and lack of effective disease surveillance and drug efficacy prediction methods being the most notable. Therefore, the development of a non-invasive screening tool and the identification of appropriate biomarkers for RCC are urgently necessary.
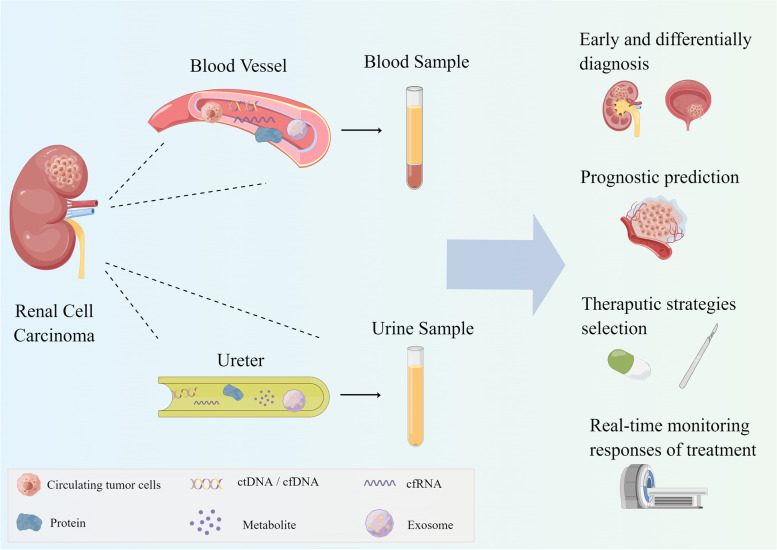
The concept of “liquid biopsy”, a non-invasive exanimation method, has attracted increasing attention [10, 11]. Through collecting and analyzing circulating tumor cells (CTCs) and other tumor markers—cell-free tumor DNA (ctDNA) or circulating DNA (cfDNA), cell-free RNA (cfRNA), exosomes, and tumor derived metabolites and proteins in the blood, urine, pleural fluid, and ascites—liquid biopsy has been widely used in cancers such as liver cancer [12], lung cancer [13], ovarian cancer [14], bladder cancer [15], and other cancers. In RCC, blood and urine are the main samples for liquid biopsy (Fig. 1). With the advantage of non-invasiveness, liquid biopsies can be used to continuously collect samples from patients to detect disease progression or predict drug efficacy, which has the potential to be developed as a screening tool compared to conventional tissue biopsies, which can only provide localized lesions with more invasiveness.
Fig. 1.
Samples and components for liquid biopsy of RCC. Liquid biopsy for RCC mainly uses blood and urine samples comprising CTCs, cfDNA/ctDNA, cfRNA, proteins, metabolites, and exosomes. Due to its non-invasiveness, liquid biopsy can be used to collect patient information continuously and plays multiple roles at different stages of disease. It can screen patients with RCC in the healthy population and identify urological masses for differential diagnosis. Before treatment, liquid biopsy can predict the risk of progression to identify high-risk patients and predict the response of patients to various treatments, which helps to select the appropriate treatment plan. After treatment, liquid biopsy allows real-time monitoring of patient outcomes and prevention of postoperative recurrence and metastasis
In recent years, the rapid update and iteration of technical means in liquid biopsy have made it available for wide application in the clinical diagnosis of RCC. Therefore, liquid biopsy may become a routine method for the diagnosis and prediction of RCC prognosis in the future. Here, we review the technical methods and clinical applications of liquid biopsy in RCC over the past 5 years.
Components for liquid biopsy
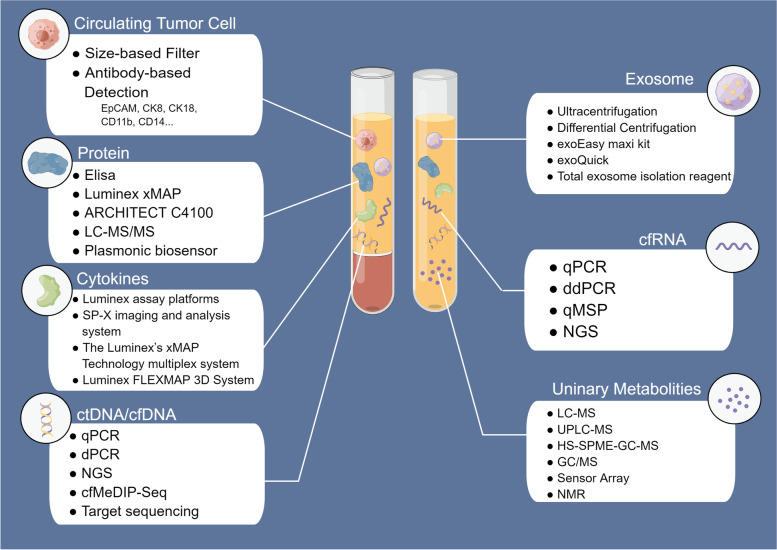
Collection of blood or urine from patients with RCC and subsequent analysis of the status of CTCs, ctDNA, cfRNA, proteins, metabolites, and exosomes can enable the construction of clinical models for the screening, disease monitoring, differential diagnosis, and treatment evaluation of Patients with RCC. This in turn can contribute to the selection of treatment strategies and the practice of personalized medicine. Commonly used probes include those for gene expression and mutation, protein levels, and nucleic acid methylation status (Fig. 2).
Fig. 2.
Commonly used techniques for extraction or analysis of RCC liquid biopsies in recent years. CTC isolation mainly includes size-based and antibody-based methods. cfDNA and ctDNA are separated by patients’ genomic alterations. PCR and sequencing are commonly used to detect and analyze mutations, size, expression, and methylation levels. ddPCR and targeting sequencing enable analysis of specific rare DNA with high sensitivity. With the development and diffusion of NGS, researchers can perform high-throughput analysis of cfDNA/ctDNA at a reduced cost. Similar to cfDNA/ctDNA, cfRNA is commonly analyzed by qPCR, ddPCR, methylation-specific quantitative PCR (qMSP), and NGS. Metabolite analysis is mainly performed using MS-based methods, while NMR and inductors have also been used in recent years. Protein analysis mainly depended on ELISA, the standard method for protein level measurements. Some automated analyzers with low cost and high efficiency are commercially available, which have potential for large-scale clinical applications. As an important field of proteomics, circulating cytokine assays use commercial detection platforms or technologies more frequently than Elisa, which enable a rapid detection of multiple cytokines in the blood. Up to now, there is no standard method for the extraction of exosomes. The most commonly used methods in recent years are ultracentrifugation and differential centrifugation. Meanwhile, some extraction reagents are used, such as exoQuick kit, exoEasy maxi kit and Total exosome isolation reagent
CTCs
CTCs are cancer cells that circulate in the peripheral blood after being naturally excreted from primary or metastatic tumors. Despite their small quantities, CTCs can promote tumor metastasis and progression, which may play a major role in tumor metastasis and recurrence [16, 17]. CTCs have shown considerable clinical value in various cancers [18, 19].
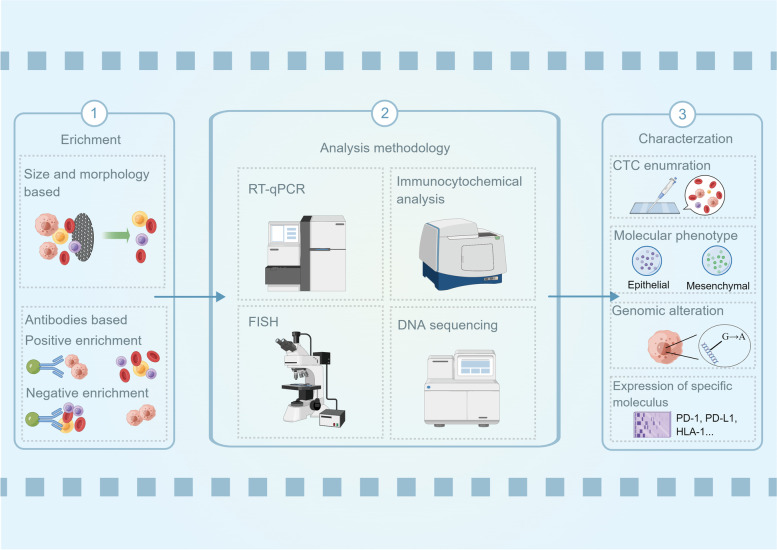
CTC analysis can be divided into enrichment, detection, and characterization (Fig. 3) [20]. No standardized enrichment method for CTCs currently exists. Common CTC enrichment methods can be classified as antibody-based, size-based, and density-based. In the last 5 years, epithelial cell adhesion molecule (EpCAM)-based antibody enrichment methods have become very common in CTC analysis using magnetic beads with antibody functions [21, 22]. The CellSearch system, developed based on EpCAM antibodies, is the first and only FDA-approved clinical method for capturing and counting CTCs [23]. In RCC, the use of a single EpCAM antibody has limited enrichment capacity for CTCs; therefore, carbonic anhydrase IX, XII (CAIX, CAXII), and cytokeratin antibodies were used to capture CTCs in conjunction with EpCAM antibodies [24, 25]. Moreover, multiple clusters of differentiation (CD)-based antibodies were used to identify and exclude other cells, such as myeloid cells (CD11b and CD14) and plasma B cells (CD235a) [24]. In addition, size-based isolation methods have frequently been used in recent years. Filtration membrane-based separations mainly capture cells larger than 8 μm, which can reduce the loss of CTCs and improve their utilization rate [26, 27]. Currently, in addition to the CellSearch System, a variety of technologies, platforms, and products have been developed according to the two methods, including the VERSA Platform [28], FISHMAN-R flow cytometer (On-Chip Biotechnologies, Japan), and Can Patrol CTC enrichment technique (SurExam, China), which were developed according to the antibody-based method, and the CTC-BIOPSY system (Youzhiyou, China) and ISET [29], which were developed according to the size-based method. A previous study compared the capture efficiency of the CellSearch System and ISET for CTCs and concluded that ISET was more suitable for CTC isolation in RCC [30]. CTC detection is mainly achieved using immunocytochemical analysis, including immunohistochemical staining and immunofluorescence. Morphological or image analysis is mainly used as supplementary analysis for immunohistochemical staining. In addition to enumeration, CTCs can also be further characterized by measuring the expression and mutation of specific genes or proteins for more accurate evaluation. Common methods include immunohistochemical analysis, quantitative polymerase chain reaction (qPCR), fluorescence in situ hybridization, and sequencing.
Fig. 3.
Isolation, detection, and characterization of CTCs and the associated clinical value. CTCs are firstly isolated from peripheral blood by size-, morphology-, and antibody-based methods. Then, several methods can be used to detect CTCs from isolating products or further characterize CTCs by analyzing cellular components or morphology. CTC analysis can provide a range of information such as CTC enumeration, molecular phenotypes, mutations, proteomics and metabolomics to analyze patient heterogeneity, drug resistance and risk of progression, which is widely used for early and differential diagnosis, prognostic assessment, treatment schedule selecting, and evaluation of treatment efficacy
Notably, several new enrichment methods for CTCs in RCC have been proposed to improve CTC isolation efficiency, such as slit-filter-based CTC isolation methods and antibody-based enrichment methods using CD45-negative/G250-positive expression; however, high-quality studies with large sample sizes are needed to test their efficacy [31–34].
cfDNA/ctDNA
Normally, DNA in plasma is referred to as cfDNA, which is released by necrotic or apoptotic cells, and the DNA released by CTCs is called ctDNA [35–37]. Compared with cfDNA, ctDNA levels are very low and contain smaller fragments [38, 39]; as such, it has been reported that the analysis of cfDNA is more suitable for liquid biopsies [40]. In RCC, a study reported that the fragment size of ctDNA correlates with patient prognosis and has a significant clinical value [41]. ctDNA can only be distinguished from cfDNA based on tumor-specific genomic alterations, which hinders ctDNA/cfDNA analysis. Moreover, the short half-life of cfDNA, which can only exist from 16 min to 2.5 h, makes its extraction quite difficult [42].
DNA molecules contain considerable biological information, including variant sites, expression levels, fragment sizes, and methylation levels. Many studies have focused on VHL, a tumor suppressor that suppresses the progression of RCC through the hypoxia-inducible factor (HIF)/VHL pathway, which is thought to be closely associated with hereditary RCC [43, 44]. The cfDNA research methods include real-time qPCR (RT-qPCR), digital PCR (dPCR), and DNA sequencing. As a relatively traditional method, RT-qPCR can determine information such as the expression, mutation, and methylation levels of target DNA molecules. Compared to RT-qPCR, dPCR, including droplet digital PCR (ddPCR) and BEAMing, has higher sensitivity and applicability for rare ctDNA [45]. DNA sequencing includes targeted sequencing and next-generation sequencing (NGS) for whole-gene sequencing. The former possesses higher sensitivity, while the latter has a broader detection range [46].
Mutation analysis is currently the most widely used method for ctDNA detection through the detection of single nucleotide variation (SNV), copy number variation, indel (insertion-deletion), and fusions. Several studies have shown that some cfDNA/ctDNA has high mutation frequency in patients with RCC, such as VHL, TP53, BAP1, and PBRM1 [47–51]. However, a study detecting the SNV and indels of ctDNA showed that the low mutational frequency of a single ctDNA may not be sufficient for liquid biopsy [48]. Therefore, comprehensive utilization of multiple ctDNA variation statuses, or a combination with other information such as cfDNA/ctDNA levels or fragment size, may be more feasible for clinical use. Moreover, it has become common to calculate microsatellite instability and tumor mutation burden (TMB) based on DNA variation to predict patient prognosis and drug responses independently or in combination with other mutational analyses [52, 53]. Some studies have focused on the calculation of TMB of ctDNA/cfDNA, called blood TMB (b-TMB), rather than the tissue TMB (t-TMB) used in traditional biopsies. Most of these studies have been conducted on lung cancer and other cancers, but not on RCC [54–57]. Although many studies have reported significant correlations between b-TMB and patient clinical progression and drug response, some studies have shown b-TMB is inconsistent with t-TMB [58, 59]. In recent years, with the development of epigenetics, the pathogenic mechanisms of DNA methylation have been explored further. Using cell-free methylated DNA immunoprecipitation and high-throughput sequencing (cfMeDIP-seq) assays and target sequencing, researchers can measure the methylation levels of specific or untargeted DNA, which have gradually shown their high sensitivity and specificity and have the potential to become key clinical indicators [60, 61]. Regarding the sample selection, it has been demonstrated that ctDNA levels are higher in plasma than in serum and are, therefore, more suitable for DNA analysis [62]. Notably, cfDNA is also present in urine, possibly produced by the direct entry of tumor cells or their breakdown products into the urinary tract [63].
cfRNA
In contrast to cfDNA or ctDNA, cfRNA does not enter the circulation after cell lysis but is secreted by normal and tumor cells [64–66]. Owing to the shortened cell cycle and elevated metabolic level of tumor cells, they produce and secrete more transcripts into the blood or urine [11]. The stability of RNA is lower than that of DNA because of many biological factors, such as RNA hydrolases and metal ions [67–69]. Compared to naked RNA, cfRNA binds to proteins and lipoproteins, which can improve its stability [70, 71], with a half-life of minutes to hours [72], whereas naked RNA is only stable for about 15 s [73]. Meanwhile, some RNAs are encapsulated by extracellular vesicles (EVs) secreted into the circulation by cells, such as exosomes, whose half-life is also significantly higher than that of naked RNA [74, 75].
The vast majority of studies in the last 5 years have focused on the clinical application of microRNAs (miRNAs) rather than mRNAs in liquid biopsies. miRNAs have been shown to play a regulatory role in RCC by promoting or inhibiting tumorigenesis and progression [76–78]. Previously, no single miRNA was found to be sufficiently sensitive and specific to be used as a biomarker for RCC. Therefore, panels consisting of multiple miRNAs are often used as models for RCC diagnosis and evaluation. The methods used to analyze cfRNA were similar to those used to analyze cfDNA. Currently, qPCR and ddPCR are mostly used to verify the expression levels. Notably, in the last 5 years, methylation level analysis was also used with cfRNA, but the result was not as significant as that of DNA [79]. This may be because the experiment was performed only for a single miRNA.
In addition to miRNAs, various other non-coding RNAs have been identified as regulators of RCC and have been explored for their clinical value as biomarkers for liquid biopsies. Long non-coding RNAs (lncRNAs) are a class of non-coding RNAs consisting of more than 200 nucleotides that modulate gene transcription by recruiting chromatin-modifying complexes [80]. Moreover, they interact with mRNAs, miRNAs, and proteins to construct a complex network that also regulates gene transcription and post-transcriptional modifications [81]. Many studies have demonstrated that lncRNAs can influence the tumorigenesis and progression of RCC, such as PVT1 and RCAT1 [82, 83]. Some studies have suggested that lncRNAs have a stronger specificity than other RNA species; therefore, they are more suitable biomarkers in liquid biopsies [84]. Notably, the FDA has approved the detection of PCA3 in urine as a diagnostic method for early prostate cancer, which further emphasizes the significant potential of blood or urine lncRNAs [85]. Circular RNAs (circRNAs) are another class non-coding RNA derived from back splicing and have a wide range of functions, such as targeting and regulating downstream miRNAs as well as influencing the transcription and splicing of parental genes [86]. As circRNAs have become important targets for exploring cancer mechanisms in recent years, an increasing number of differentially expressed circRNAs have been identified in RCC and identified to mediate cancer growth, metastasis, and invasion [87, 88]. It has been suggested that they are highly abundant in multiple body fluids and have a long half-life owing to their resistance to RNase. PIWI-interacting RNAs (piRNAs) are a class of small non-coding RNAs comprising 24–31 nucleotides that have recently been identified in germ cells [89]. Several studies have demonstrated its regulatory role in various cancers. One study showed that overexpression of piR-32051, piR-39894, and piR-43607 was associated with metastasis and worse overall survival in RCC, but their specific functions need to be further clarified [90]. Recently, several experiments have attempted to detect the differential expression of circulating piRNAs between RCC and normal populations, but the possibility of using liquid biopsy markers needs to be explored in more studies [91, 92].
Metabolomics and proteomics
Cellular metabolites have long been thought to participate in and modulate biosynthetic pathways [93, 94] and regulate biological behavior as signaling molecules [95, 96]. Previous studies have shown that multiple enzymes related to metabolic pathways are involved in the pathogenesis of kidney cancer, such as ferredoxin hydratase (FH) and succinate dehydrogenase (SDH) [97–99]; changing the response of tumors to environmental alterations. Therefore, the detection of changes in circulating metabolite profiles can reveal cancer-induced abnormalities in biometabolic pathways and associated biological behaviors, thus effectively differentiating Patients with RCC from healthy populations, distinguishing ccRCC from other subtypes of RCC, and evaluating treatment efficiency and prognosis. Currently, the analysis of metabolites mainly depends on mass spectrometer (MS)-based methods, including liquid chromatography-MS (LC–MS), gas chromatography-MS (GC–MS), matrix-assisted laser desorption/ionization-MS (MALDI), and related derivative techniques such as ultra-performance LC–MS (UPLC-MS) or headspace solid-phase microextraction coupled with GC–MS (HS–SPME–GC–MS) [100–104]. By accurately measuring compound masses and inferring chemical composition and structure, MS has shown high sensitivity and specificity for probing compounds, becoming the preferred method for structural identification of analytes in complex mixtures. Moreover, nuclear magnetic resonance (NMR) is a commonly used method for the analysis of metabolites [105]. Compared to MS, NMR has lower sensitivity and specificity but can provide more detailed structural information. However, metabolite profiles vary widely among individuals, and although some compounds, such as 2-hydroxyglutarate (2HG), have been co-detected in several clinical trials [100, 106], the panel of compounds used in most experiments is inconsistent.
Human blood and urine are rich in proteins, including carcinoembryonic antigens (CEA), tissue-specific secreted proteins, and intracellular proteins released by tissue injury or death [107, 108]. Similar to metabolite profiling, cancer-associated proteins frequently play an important role in the activation of specific tumorigenesis pathways; thus, their detection can reveal the mechanisms of carcinogenesis or be applied as therapeutic targets. Examples include 78 kDa glucose-regulated protein (GRP78), which affects cellular stress, and programmed cell death protein 1 (PD-1) and programmed cell death ligand 1 (PD-L1), which regulate immune checkpoints (ICs) [109–111]. Therefore, proteomics has the potential to identify important biomarkers in liquid biopsies. Several proteins, such as methemoglobin and CEA, have been used for cancer screening [112, 113], and one of the most widely studied proteins in RCC is kidney injury molecule 1 (KIM 1) [114–116]. Currently, multiple cytokines are tested to assess and monitor the efficacy of treatment in patients with RCC. In addition, as direct regulators of immune function, several chemokines have been used to evaluate the response to immunotherapy and anti-angiogenic therapy, especially IL-6 and IL-8 [117, 118]. In RCC, IL-6 specifically targets IL-6R and gp130 on the cell surface [119], thereby activating various cancer-related signaling pathways, such as the JAK-STAT pathway [120]. Meanwhile, IL-8 binds to IL-8R to recruit immune cells to influence the tumor microenvironment (TME), thereby inhibiting anti-tumor immune function [121]. In addition, as a direct therapeutic target, serum VEGF levels can directly reflect the therapeutic responses and drug resistance of patients, which has been adopted in many studies [122]. In addition, other cytokines such as HGF, IFN-α, and TNF-α have also been widely tested for their clinical value. In the last 5 years, most clinical trials have used enzyme-linked immunosorbent assay (ELISA) to determine protein levels, which is currently the gold standard tool [123]. ELISA can quantify proteins with relatively high sensitivity and a wide dynamic detection range [124]. However, ELISA requires manual operation using kits, and its detection efficiency cannot meet the needs of wide clinical applications, which entails the development and promotion of automatic analysis technology and platforms. The Luminex xMAP system is an automated protein detection platform combining the sandwich immunoassay format with flow cytometry, with a general sensitivity in the range of 1–10 pg/mL and a detection range of approximately 3–4 orders of magnitude [125, 126]. Similarly, the ARCHITECT C4100 Analyzer (Abbott France SA, France) is an automatic immunoassay system that is highly concordant with ELISA for the detection of neutrophil gelatinase-associated lipocalin (NGAL) in urine [127]. In addition, Hu et al. developed a plasma biosensor based on gold nanorods with high refractive index sensitivity. This method has a detection range of 50 pg/mL to 5 mg/mL and is less influenced by admissibility factors, making it promising for application in routine clinical examination [128]. In cytokine detection, researchers are mainly adopting commercially available platforms or products instead of ELISA to improve both the efficiency and convenience to meet the needs of clinical practice. Common analysis platforms or techniques for cytokine includes Luminex assay platforms (Assay Gate, USA), Luminex immune-bead technology and a high-sensitivity kit (Invitrogen/Biosource, USA), Luminex FLEXMAP 3D System (Fisher-Scientific, USA), etc.
Exosomes
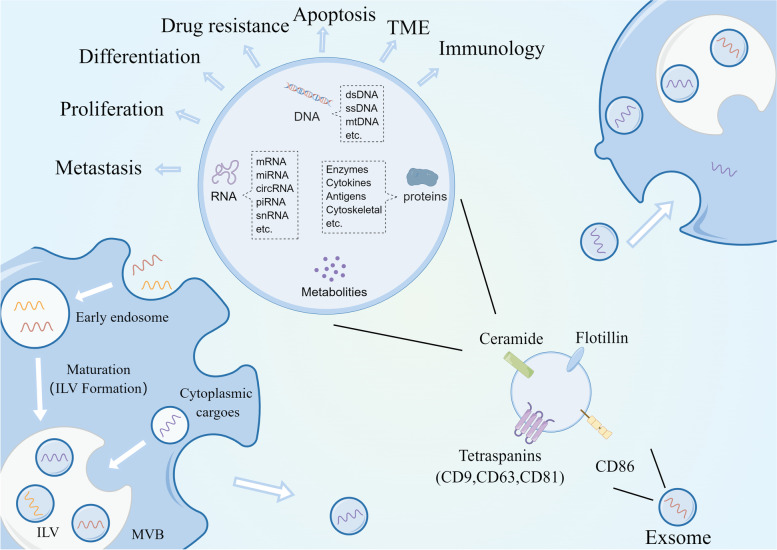
Exosomes are EVs that are enriched with a variety of substances, including DNA, miRNA, mRNA, cellular metabolites, and proteins [129, 130], and are widely distributed in blood, urine, and saliva [131, 132]. Exosomes are secreted by multiple cells and regulate intercellular communication by delivering specific molecules to the receptor cells [133–135]. Several previous studies have demonstrated that exosomes influence RCC progression, including proliferation, metastasis, regulation of the TME, and drug resistance [136–139]. Exosomes originate from intraluminal vesicles (ILVs) released from multivesicular bodies (MVBs). Briefly, cell membranes sprout inward and wrap specific cellular components to form early endosomes (EE), which then mature into MVBs enriched with ILVs. The MVBs also receive cargo from the cytoplasm. MVBs with low cholesterol content are degraded, whereas others with high cholesterol content are transported to the cell membrane, where they fuse with the cell membrane and release ILVs, which are known as exosomes [140, 141]. These exosomes are enriched with cell surface proteins on the membranes such as Tetraspanins (CD9, CD81, CD63), CD86, integrins and ceramide, which are used as exosome markers and recognized by target cells (Fig. 4) [142, 143]. In recent years, exosomes have become a focal point in cancer research and are widely used in liquid biopsies of various cancers [14, 144–146].
Fig. 4.
Exosomal biogenesis and regulatory mechanisms. The cell membrane wraps specific cellular material inward to form EEs which mature into MVBs. Meanwhile, MVBs also receive specific cargo from the cytoplasm. Different materials within the MVBs are separated by the membrane to form ILVs. Low-cholesterol MVBs are degraded, while high-cholesterol MVBs fuse with the cell membrane to release ILVs into the circulation. The released ILVs are called exosomes. The exosomes are enriched with cell surface proteins on the membranes such as Tetraspanins (CD9, CD81, CD63), CD86, integrins and ceramide, which are used as exosome markers and recognized by target cells. It is reported that exosomes regulate tumor differentiation, proliferation, apoptosis, EMT, drug resistance, and the TME by transmitting intercellular messages
A variety of methods are currently used for isolating exosomes, with ultracentrifugation predominating, including differential centrifugation, density gradient ultracentrifugation, and other ultracentrifugation-based methods [147]. Differential centrifugation is the most commonly used separation method because of its simplicity and efficiency. However, differential centrifugation cannot effectively distinguish other impurities, such as cellular debris, proteins, or other EVs, including cellular microvesicles and apoptotic vesicles, resulting in lower product purity [148]. Density gradient differential centrifugation separates impurities by adding media of similar density to the separated material and extending the centrifugation time [149, 150], which can significantly improve the purity of the separated products. However, this also means that it is more time-consuming and difficult to operate, and prolonged centrifugation may cause structural damage [151]. Immunoaffinity-based methods to isolate exosomes are also very prevalent [152], and a number of commercial reagents or methods have been developed, such as the exoEasy Maxi Kit (QIAGEN, USA), total exosome isolation reagent, and EpCAM isolation beads (Invitrogen, USA). By using antibodies or antibody-characterized magnetic beads, higher-purity exosomes can be captured and purified; however, limitations such as low capture rate and high cost still exist [153]. In recent years, as exosome research has intensified, an increasing number of methods have emerged, including ultrafiltration, membrane affinity chromatography, and size exclusion chromatography; many studies have compared the associated advantages and disadvantages [154–156]. Based on the progression of the above methods, many kits have been developed and are commercially available, such as exoQuick (Qiagen, Netherlands), exoEasy (System Biosciences, USA), Total exosome isolation reagent (Invitrogen, USA), etc. These kits make the extraction and enrichment of exosomes less demanding in equipment, and enable the extraction of larger scale exosomes in less time, which make it more feasible to extend exosome detection to clinic. The extraction efficiency and accuracy of various kits are inconsistent due to differences in their extracting methods and techniques, and several studies have tested and compared them [157–159]. Three key issues still limit the wider clinical application of exosomes: first, how to improve the capture rate of exosomes, second, how to make exosome isolation more cost-effective, and third, how to ensure the purity of exosomes.
Clinical application of liquid biopsy in RCC
In recent years, with the continuous development of detection techniques for liquid biopsy components, an increasing number of clinical trials are being conducted to apply this new non-invasive test in various clinical practices for RCC. Depending on the purpose, we divided the trials into three categories: diagnostic (including early diagnosis and differential diagnosis), prognostic, and therapeutic. In the following section, we review the clinical trials on liquid biopsy for RCC in the last 5 years.
Diagnosis of RCC
Early diagnosis of cancer can provide more options for treatment strategies for patients, thus improving patient survival and reducing the consumption of healthcare resources. In addition, identifying cancer from benign tumors and distinguishing between different types of malignant tumors are also crucial for disease control [160]. Therefore, many studies have attempted to use liquid biopsy as a routine method for the clinical diagnosis of RCC (Table 1). As CTCs have long been considered the culprit of tumor metastasis and recurrence, their significance in tumor diagnosis is very limited compared to other components. Only one study in the last 5 years was reported to differentiate between responding and progressing Patients with RCC based on the cytokeratin expression level of CTCs [24]. Multiple mutations were found for cfDNA/ctDNA in the plasma. In 2020, a study detected mutations in 17 of 51 patients with metastatic RCC (mRCC). Among them, the most frequently mutated gene was VHL with a frequency of 41%, followed by BRCA1-associated protein 1 (BAP1) (39%) and recombinant polybromo 1 (PBRM1) (17%) [49]. These ctDNA genetic mutations in the plasma were consistent with the DNA mutations in the corresponding tissues, with a concordance of 77%. However, a study by Sumiyoshi et al. reported that 13 VHL variants were found in 12 of 56 patients with ccRCC (21.6%) with a median variant frequency of 0.78%, while only eight out of 28 patients (28.6%) had plasma VHL variants with VHL mutations in tumor tissue. Owing to its low detection rate, the authors concluded that the analysis of single cfDNA/ctDNA is not applicable to liquid biopsies [48]. In contrast, DNA methylation analysis may be more accurate in patients with RCC, as proposed by two studies conducted in 2020. Lasseter et al. first identified 21 candidate variants in 11 out of 40 patients with mRCC (28%). Based on the methylation levels of 21 cfDNAs, the team constructed methylation scores for the diagnosis of a cohort of 72 individuals (38 Patients with RCC, 34 healthy controls). The results showed that all 34 patients were successfully probed (sensitivity, 100%; specificity, 88%) [61]. Another study further identified 300 differentially methylated regions in plasma cfDNAs and constructed methylation scoring models for diagnosis. According to the scoring model, 67 of 69 Patients with RCC (97.1%) were accurately diagnosed with an area under the receiver operating characteristic curve of 0.990 [60]. Interestingly, the diagnostic model was also applied to urinary cfDNA, with an area under the curve (AUC) of 0.858. Furthermore, the model showed significant differential diagnostic ability. In addition to specific cfDNA analysis, the overall plasma cfDNA level has diagnostic value. According to Yamamoto et al., the AUC of cfDNA levels for detecting RCC was 0.762 (sensitivity, 63.0%; specificity, 78.1%) [41].
Table 1.
Diagnostic application of liquid biopsy in recent 5 years
| Region | Year | Sample | Detection Method | Cohorts | Detected Abnormality |
Practice in clinical |
Result | Ref | |
|---|---|---|---|---|---|---|---|---|---|
| CTC | USA | 2021 | Peripheral blood |
VERSA Platform, Immunofluorescence |
29 RCC patients |
CK ( +) CTC counts |
Distinguishing progressing and responding patients |
AUC 0.79, Sensitivity 73% and Specificity 100% | [24] |
|
ctDNA/ cfDNA |
Japan | 2021 | Plasma |
NGS dPCR |
56 ccRCC patients 31 healthy control |
VHL |
Detection of RCC patients |
13 VHL mutations were found in 12 of 56 ccRCC patients (21.6%) with median variant frequency of 0.78% VHL cfDNA mutations were found in 8 of 28 patients (28.6%) with VHL tumor DNA mutations Patients with VHL cfDNA mutations tended to show a worse OS |
[48] |
| USA | 2020 |
Plasma Urine |
cfMeDIP–seq NGS |
99 RCC patients 28 healthy controls 15 UBC patients |
300 DMRs |
Detection of RCC patients |
67/69 RCC samples (97.1%) were of a higher median methylation score than all control samples with a mean AUC of 0.990 Same analyses were carried out to urine cfDNA from patients with RCC and healthy controls, with the mean AUC of 0.858 |
[60] | |
|
Distinguishing RCC and UBC |
Using methylation score to compare patients with RCC and UBC, resulting in a mean AUC of 0.979 | ||||||||
| USA | 2020 | Plasma |
cfMeDIP–seq Target sequencing |
Cohort 1: 40 mRCC patients Cohort 2: 38 RCC patients 34 healthy controls |
Methylation level of 21 cfDNA variants |
Detection of mRCC patients |
cfDNA variant analysis via targeted sequencing detected 21 candidate variants in 11 of 40 mRCC patients (28%), which can improve the sensitivity combined with tumor DNA variant analysis All of 34 mRCC patients are detected through cfMeDIP–seq (sensitivity 100%, specificity 88%), compared with that cfDNA variant analysis identified variants in 7 patients (21%) |
[61] | |
| Canada | 2020 | Plasma | Target sequencing | 55 mRCC patients | VHL, BAP1, PBRM1 et al | Detection of mRCC patients | 17 of 51 mRCC patients detected cfDNA variants. The most frequent mutated genes are VHL, BAP1 and PBRM1 (the frequency is 41%, 39%, 17%, respectively). The concordance of mutated genes profiling between cfDNA variants in plasma and tumor DNA variants in matched tissues is 77% | [49] | |
| Japan | 2018 | Plasma |
qPCR Microfluidics-based platform |
92 RCC patients 41 healthy controls |
Plasma cfDNA level |
Detection of RCC patients |
AUC 0.762, Sensitivity 63.0% and Specificity 78.1% | [41] | |
| cfRNA | Portugal | 2022 | Plasma | ddPCR |
124 RCC patients 15 oncocytomas patients 64 healthy controls |
miR-21-5p miR-155-5p |
Detection of RCC patients |
Sensitivity 89.52%, specificity 54.69% and accuracy 77.66% | [161] |
| 124 RCC patients |
miR-21-5p miR-155-5p |
Detection of early stages RCC | Sensitivity 92.42%, specificity 34.38% and accuracy 63.85% | ||||||
|
124 RCC patients 15 oncocytomas patients |
miR-126-3p miR-200b-3p |
Distinguishing ccRCC and other RCC subtypes |
Sensitivity 80.46%, specificity 56.76% and accuracy 73.39% | ||||||
| Canada | 2021 | Urine | qPCR |
76 ccRCC patients 8 benign renal tumor patients 16 healthy contrls" |
Circ-EGLN3 Circ-SOD2 |
Detection of RCC patients |
69% of samples detected urinary circEGLN3 and 60% of samples detected urinary circACAD11 circEGLN3 levels were significantly different between the healthy controls versus ccRCC patients (P < 0.05) The AUC of circEGLN3 and circSOD2 was of 0.71 and 0.68, respectively, for distinguishing cancer patients versus non-neoplastic patients Urinary circEGLN3 level of ccRCC patients was lower than that of healthy controls, while tissue circEGLN3 level was higher of ccRCC patients |
[174] | |
| China | 2021 | Serum | qPCR |
123 RCC patients 118 healthy controls |
miR-21-5p miR-150-5p miR-145-5p miR-146a-5p |
Detection of RCC patients |
AUC 0.938, sensitivity 90.79%, specificity 93.75% | [162] | |
| China | 2020 | Serum | qPCR |
113 RCC patients 79 healthy controls |
LncRNA-C00886 |
Detection of RCC patients |
AUC 0.803, sensitivity 67.09%, specificity 89.87% | [172] | |
| Detection of early stages RCC patients | AUC 0.800, sensitivity 71.05%, specificity 89.87% | ||||||||
| Detection of non-metastasis RCC patients | AUC 0.830, sensitivity 73.33%, specificity 89.87% | ||||||||
| Portugal | 2020 | Urine | qMSP |
Cohort 2: 38 ccRCC patients 15 metastasis ccRCC patients 57 healthy controls Cohort 3: 171 ccRCC patients 85 healthy controls |
Methylation level of miR-30a-5p |
Detection of ccRCC patients Detection of metastasis ccRCC patients |
Cohort 2: AUC 0.6873, sensitivity 83%, specificity 53% Cohort 3: AUC 0.6702, sensitivity 63%, specificity 67% Cohort 2: AUC 0.7684, sensitivity 80%, specificity 71% |
[79] | |
| China | 2020 | Serum | qPCR |
146 RCC patients 150 healthy controls |
miR-224-5p miR-34b-3p miR-182-5p |
Detection of RCC patients |
AUC 0.855, sensitivity80.3%, specificity66.3% | [163] | |
| China | 2020 | Serum | qPCR |
Testing cohort: 70 RCC patients 70 healthy controls Validating cohort: 40 RCC patients 40 healthy controls |
miR-20b-5p, miR-30a-5p, miR-196a-5p |
Detection of RCC patients |
Testing cohort: AUC 0.949, sensitivity 92.8%, specificity 80.0% Validating cohort: AUC 0.938, sensitivity 92.5%, specificity 80.0% |
[164] | |
| Canada | 2020 | Urine | qPCR |
30 oncocytomas patients 26 progressive ccRCC-SRM patients 24 non-progressive ccRCC-SRM patients |
9 miRNAs miR-328-3p |
Distinguishing RCC- SRM and oncocytoma Detection of ccRCC patients |
9 urinary miRNAs were differentially expressed between renal oncocytoma (≤ 4 cm) and ccRCC-SRMs (pT1a; ≤ 4 cm), where miR-432-5p and miR-532-5p showed the most measurable discriminatory ability (AUC 0.71, AUC 0.70, respectively) miR-328-3p was significantly down-regulated in progressive ccRCC-SRMs and showed significant discriminatory ability (AUC: 0.68) |
[79] | |
| China | 2018 | Serum | qPCR |
46 RCC patients 46 healthy controls |
LncRNA-GIHCG |
Detection of RCC patients |
AUC 0.920, sensitivity 87%, specificity 84.8% | [173] | |
| Detection of early stage RCC patients | AUC 0.886, sensitivity 80.7%, specificity 84.8% | ||||||||
| Ukraine | 2018 | Urine | qPCR |
52 RCC patients 15 oncocytoma patients 15 healthy controls |
miR-15a | Distinguishing RCC and benign renal tumor | AUC 0.955, sensitivity 100%, specificity 98.1% | [169] | |
| Germany | 2018 | Serum | qPCR |
86 ccRCC patients 55 benign renal tumor patients 28 healthy controls |
miR-122-5p miR-206 |
Detection of ccRCC patients | AUC 0.733, sensitivity 57.1%, specificity 83.8% | [165] | |
| Protein | India | 2021 | Serum | Elisa |
60 RCC patients 60 non-tumor controls |
GRP78 |
Detection of RCC patients |
AUC 0.739, sensitivity 71.7%, specificity 66.7% | [179] |
| USA | 2021 | Plasma | Elisa |
143 mRCC patients 137 18–25 years old healthy controls 252 50–80 years old healthy controls |
hPG80 | Detection of mRCC patients |
Compared to 18–25 years old healthy group: AUC 0.93, accuracy 0.89 Compared to 50–80 years old healthy group: AUC 0.84, accuracy 0.77 |
[180] | |
| Canada | 2020 | Urine | LC–MS/MS |
27 oncocytoma (≤ 4 cm) patients 23 progressive ccRCC-SRM patients 21 non-progressive ccRCC-SRM patients 20 healthy controls |
GLRx、CST3、SLC9A3R1、HSPE1、FKBP1a、EEF1G et al |
Detection of early-stage ccRCC patients |
GLRx (AUC = 0.72, P = 0.0047) showed the most significant discriminatory ability between ccRCC-SRM and healthy controls, followed by SLC9A3R1 (AUC = 0.70), HSPE1 (AUC = 0.70), FKBP1A (AUC = 0.65) and EEF1G (AUC = 0.65) (P < 0.05) Diagnostic model based on the expression of 7 proteins (DDT, EEF1G, EPB41L3, HSPE1, MUC4, RAP1B and SLC9A3R1) showed the most significant discriminatory ability (AUC: 0.82), outperforming all single protein markers |
[178] | |
|
Distinguishing renal oncocytoma (≤ 4 cm) and early-stage ccRCC |
C12orf49 (AUC = 0.77, P = 0.0001) showed the most significant discriminatory ability between ccRCC-SRM and renal oncocytoma, followed by EHD4 (AUC = 0.64, p = 0.049) Diagnostic model based on the expression of 3 proteins (C12orf49、EHD4 and PPA1) showed the most significant discriminatory ability (AUC: 0.85), outperforming all single protein markers |
||||||||
|
Distinguishing progressive and non-progressive early-stage ccRCC |
EPS8L2 (AUC = 0.76, p = 0.0037) showed the most significant discriminatory ability between progressive and non-progressive ccRCC-SRM, followed by CHMP2A (AUC = 0.70, p = 0.034), PDCD6IP (AUC = 0.68), CNDP2 (AUC = 0.63) and CEACAM1 (AUC = 0.66)(P < 0.05) Diagnostic model based on the expression of 2 proteins (EPS8L2 and CCT6A) showed the most significant discriminatory ability (AUC = 0.81), outperforming all single protein markers |
||||||||
| USA | 2019 | Urine | Plasmonic biosensor |
20 RCC patients 20 healthy controls 8 BLCA patients 10 diabetic nephropathy patients |
PLIN-2 |
Detection of RCC patients |
Median urine PLIN-2 concentrations in ccRCC patients (43 ng/mL) were significantly higher (P < 0.001) than healthy groups (0.3 ng/mL), BLCA patients (0.5 ng/mL) and diabetic nephropathy patients (0.6 ng/mL) | [128] | |
| Metabolites | Portugal | 2021 | Urine | HS–SPME–GC–MS |
75 ccRCC patients 75 health control |
6 volatiles metabolites |
Detection of RCC patients |
The diagnostic model was consisted of 6 volatile metabolites The diagnostic ability of ccRCC patients: AUC 0.869, sensitivity 83%, specificity 79%, accuracy 79% The diagnostic ability of stage I ccRCC patients: AUC 0.799, sensitivity 84%, specificity 73%, accuracy 76% The diagnostic ability of stage III-IV ccRCC patients: AUC 0.911, sensitivity 83%, specificity 84%, accuracy 84% |
[101] |
| Italy | 2021 | Urine |
GC/MS Gas sensor array |
40 ccRCC patients 8 healthy controls |
8 volatiles metabolites |
Detection of ccRCC patients |
8 volatile metabolites was differentially expressed in at least 70% ccRCC patients and consisted as a diagnostic model Analyzed through GC/MS, the diagnostic ability of the model: AUC 0.979, sensitivity 85.7%, specificity 100%, accuracy 92.9% (training cohort); AUC 0.875, sensitivity 83.3%, specificity 100%, accuracy 91.7% (testing cohort) Analyzed through Gas Sensor Array, the diagnostic ability of the model: AUC 0.979, sensitivity 100%, specificity 85.7%, accuracy 92.9% (training cohort); AUC 0.906, sensitivity 100%, specificity 83.3%, accuracy 91.7% (testing cohort) |
[102] | |
| Germany | 2021 | Urine |
LC–MS NMR |
41 early stage RCC patients 29 advanced stage RCC patients |
16 urinary metabolites | Distinguishing early and advanced stage RCC patients | A model consisting of 16 metabolites was used for distinguishing early and advanced stage RCC patients: AUC 0.95, sensitivity 80%, specificity 91%, accuracy 86% | [105] | |
| China | 2020 | Urine | LC–MS |
39 RCC patients 22 benign renal tumor patients 68 healthy controls |
6 urinary metabolites |
Distinguishing RCC and benign renal tumor | A model consisting of 3 metabolites (cortolone, testosterone and l-2-aminoadipate adenylate) was used for benign and malignant renal tumor distinction: AUC 0.868, sensitivity 75%, specificity 100% (tenfold cross-validation of testing cohort) | [175] | |
|
Detection of RCC patients |
A model consisting of 3 metabolites (aminoadipic acid, 2-(formamido)-N1-(5-phospho-d-ribosyl) acetamidine and alpha-N-phenylacetyl-l-glutamine) was used for detection RCC patients: AUC 0.841, sensitivity 75%, specificity 88.6% (tenfold cross-validation of testing cohort) | ||||||||
| Japan | 2020 | Urine | LC–MS |
69 stage I-II RCC patients 18 stage III-IV RCC patients 60 benign renal tumor patients |
9 urinary metabolites |
Distinguishing RCC and benign renal tumor | A model consisting of 5 metabolites (L-glutamic acid, lactate, D-sedoheptulose 7-phosphate, 2-hy-droxyglutarate and myoinositol) was used for detection RCC patients: AUC 0.966, sensitivity 93.1%, specificity 95% | [176] | |
| Poland | 2020 | Urine | AuNPET LDI MS |
50 RCC patients 50 healthy controls |
15 urinary metabolites |
Detection of RCC patients |
15 urinary metabolites were identified abnormal distribution in RCC patients' urine (7 upregulation and 8 downregulation), where 3,5-Dihydroxyphenylvaleric acid showed the most significant diagnostic value (AUC 0.844) A model consisting of all 15 metabolites was used for detecting RCC patients: AUC 0.915, efficiency 88%, efficiency 86% |
[103] | |
| China | 2019 | Urine | UPLC-MS |
146 BLCA patients 115 RCC patients 142 healthy controls |
16 urinary metabolites |
Detection of RCC patients |
A model consisting of 6 metabolites (α-CEHC, β-cortolone, deoxyinosine, flunisolide, 11b,17a,21-trihydroxypreg-nenolone and glycerol tripropanoate) was used for distinguishing cancer patients from healthy controls: AUC 0.950 (discovering group); AUC 0.867 (external validating group) | [104] | |
|
Distinguishing BLCA and RCC patients without hematuria |
A model consisting of 6 metabolites (4-ethoxymethylphenol, prostaglandin F2b, thromboxane B3, hydroxybutyrylcarnitine, 3-hydroxyphloretin and N'-formylkynurenine) was used for distinguishing BLCA and RCC patients without hematuria: AUC 0.829 in discovering group; AUC 0.76 in external validating group | ||||||||
|
Distinguishing BLCA and RCC patients with hematuria |
A model consisting of 4 metabolites (1-hydroxy-2-oxopropyl tetrahydropterin, 1-acetoxy-2-hydroxy-16-heptadecyn-4-one, 1,2dehydrosalsolinol and L-tyrosine) was used for distinguishing BLCA and RCC patients with hematuria: AUC 0.913 (discovering group) | ||||||||
| China | 2019 | Urine | LC–MS |
100 RCC patients 34 benign renal tumor patients 129 healthy controls |
18 urinary metabolites |
Detection of RCC patients |
A model consisting of 9 metabolites (N-Jasmonoyltyrosine, Tetrahydroaldosterone-3-glucuronide, Androstenedione, Dopamine 4-sulfate, 3-Methylazelaic acid, Cortolone-3-glucuronide, 7alpha-hydroxy-3-oxochol-4-en-24-oic Acid, Cortolone-3-glucuronide and Lithocholyltaurine) was used for distinguishing cancer patients from healthy controls: AUC 0.905 (testing cohort); AUC 0.885 (validating cohort) N'-formylkynurenine showed a significant discriminating ability of detecting RCC patients (AUC 0.808, sensitivity 84.8%, specificity 83.8%) |
[177] | |
|
Distinguishing RCC and benign renal tumor |
A model consisting of 3 metabolites (L-3-hydroxykynurenine, 1,7-dimethylguanosine and tetrahydroaldosterone-3-glucuronide) was used for distinguishing RCC patients from benign renal tumor patients: AUC 0.834 in testing cohort; AUC 0.816 for tenfold cross-validation | ||||||||
| Distinguishing early and late stages of RCC | A model consisting of 5 metabolites (thymidine, cholic acid glucuronide, alanyl-proline, isoleucyl-hydroxyproline, and myristic acid) was used for distinguishing early (stage I-II) from late stages (stage III-IV) of RCC: AUC 0.881 in testing cohort; AUC 0.813 for tenfold cross-validation | ||||||||
| Exosome | Spain | 2021 | Plasma |
Differential ultracentrifugation, qPCR, NGS, dPCR |
13 RCC patients 15 healthy controls |
Exosomal mtDNA VH1, CγB |
Detection of RCC patients |
dPCR and qPCR demonstrated that VH1 and CγB were of a significant discrimination ability for RCC and healthy group (F phase) VH1: AUC = 0.825, P < 0.0001 for VH1-short; AUC = 0.833, P < 0.0001 for VH1-long CγB: AUC = 0.755, P < 0.0001 for CγB-short; AUC = 0.810, P < 0.0001 for CγB-long |
[183] |
| China | 2020 | Plasma |
exoEasy maxi kit, qPCR |
22 RCC patients 16 healthy controls |
Exosomal miRNA miR-92a-1-5p, miR-149-3p, miR-424-3p |
Detection of RCC patients |
Compared with healthy controls, the levels of exsomal miR-149-3p and miR-424-3p were significantly up-regulated, while miR-92a-1-5p was down-regulated miR-149-3p: AUC 0.7188, sensitivity 75.0%, specificity 72.7% miR-424-3p: AUC 0.7727, sensitivity 75.0%, specificity 81.8% miR-149-3p: AUC 0.8324, sensitivity 87.5%, specificity 77.3% |
[181] | |
| China | 2019 | Urine |
Differential ultracentrifugation, Agilent 2100 Bioanalyzer, NGS |
70 early‐stage ccRCC patients 30 early‐stage PC patients 30 early‐stage BLCA patients 30 healthy controls |
Exosomal miRNA miR-30c-5p |
Detection of ccRCC patients |
Exosomal miRNA miR-30c-5p levels in ccRCC patients' urine were significantly lower than those in healthy controls, where was no significant differences between BLCA cancers, PC cancers and healthy controls The diagnostic value of exosomal miR-30c-5p for ccRCC patients: AUC 0.819, sensitivity 68.57%, specificity 100% |
[182] | |
| China | 2018 | Serum |
Total exosome isolation reagent, EpCAM isolation beads, Flow cytometry |
82 ccRCC patients received nephrectomy 80 healthy controls |
Exosomal miRNA miR-210, miR-1233 |
Detection of RCC patients |
Exosomal miRNA miR-210 and miR-1233 levels in ccRCC patients' serum were significantly lower than those in healthy controls miR-210: AUC 0.69, sensitivity 70%, specificity 62.2% miR-1233: AUC 0.82, sensitivity 81%, specificity 76% |
[153] |
Urine samples have been used more often in clinical trials of cfRNA than cfDNA/ctDNA. Most trials constructed diagnostic panels consisting of several cfRNAs to detect Patients with RCC [161–165]. Some of these diagnostic panels had excellent diagnostic ability; for example, panels composed of miR-21-5p, miR-150-5p, miR-145-5p, and miR-146a-5p had an AUC of 0.938 (sensitivity, 90.79%; specificity, 93.75%) [162]. Interestingly, miR-21-5p was also included in a diagnostic panel in another clinical trial, which was used to differentiate between benign and malignant tumors and to probe Patients with RCC at early stages [161]. miR-21-5p has been reported to play an important role in various cancers and may be associated with mesenchymal–epithelial transition, suggesting its feasibility for clinical liquid biopsies [166–168]. Moreover, several miRNA panels have differential diagnostic value. Among them, miR-126-3p was able to distinguish ccRCC from other pathological types of RCC with an accuracy of 71.77% (sensitivity, 78.16%; specificity, 56.76%), and the accuracy of the combination of miR-126-3p and miR-200-3p increased to 73.39% (sensitivity, 80.46%; specificity 56.76%) [161]. Single miRNAs are more efficient and economical than panels comprising multiple miRNAs. Yulian Mytsyk et al. showed that miR-15a had significant differential diagnostic value for benign tumors and RCC, with an AUC of 0.955 (sensitivity, 100%; specificity, 98.1%) [169]. However, the sample size in this trial was small, and a larger cohort is needed to test its reliability. Another study identified nine urinary miRNAs that were differentially expressed between small renal cancer masses and eosinophilia, a benign renal tumor, where miR-432-5p and miR-532-5p had the most significant discriminatory value, with AUCs of 0.71 and 0.70, respectively [170]. In addition to cell-free miRNA levels, miRNA methylation level is also a potential diagnostic indicator. The methylation level of miR-30a-5p in urine can be measured to detect RCC and identify patients with mRCC with an AUC of 0.855 (sensitivity, 80.3%; specificity, 66.3%) [79]. Previous studies have demonstrated that miR-30a-5p inhibits cancer progression by regulating GRP78 and zinc finger E-box binding homeobox [171, 172], which has the potential to be a biomarker in liquid biopsies and has been used in diagnostic panels in recent studies [164]. In addition to miRNAs, two lncRNAs are also used as diagnostic markers, namely lncRNA-GIHCG and lncRNA-C00886, with AUCs of 0.920 and 0.803, respectively [173, 174]. Among these, lncRNA-GIHCG was found to be significantly decreased post-surgery and has potential in the monitoring of surgical outcomes. Differential expression of circRNA has also been detected in urine and used as a diagnostic indicator; however, it faces three problems: first, the detection rate is insufficient in Patients with RCC; second, the AUC is low compared to other RNA species; and third, the differential expression of serum circRNA is inconsistent with tissue circRNA [175]. Metabolomic changes are mainly reflected in urine, and several clinical trials have constructed diagnostic models to detect Patients with RCC by identifying differentially expressed metabolites therein [101–105, 176–178]. Based on the differential expression of metabolites, several studies have assembled large and complex diagnostic panels with significant diagnostic value and a very high AUC [103, 105, 178]. However, these studies were not without their shortcomings; overly complex diagnostic models are difficult to scale up to clinical application and are weakly reproducible, comprising strong inconsistencies across patients. Sato et al. used five differentially expressed metabolites to detect ccRCC in a cohort of 87 patients with ccRCC and 60 patients with benign urologic disease, with an AUC of 0.966 (sensitivity, 93.1%; specificity, 95%) [177]. Similarly, Zhan Wang et al. performed diagnostic analysis using a panel of six metabolites, resulting in an AUC of 0.950 in the test group and 0.867 in the validation group [104]. This trial further explored the ability to distinguish between bladder cancer and RCC. By using hematuria to classify patients, the trial improved the accuracy of the results and reduced the complexity of the diagnostic panel. The method of classifying patients according to their symptoms may be a useful way to avoid complex diagnostic panels.
Metabolomics can reflect altered pathways in RCC as well as altered renal function, which could contribute to further revealing the pathogenesis and explaining clinical symptoms, such as the relationship between tryptophan and immune regulation [178]. However, related studies have explored this topic to a limited extent and more in-depth analyses are needed. Unlike metabolomic analysis, protein analysis typically uses a single protein to diagnose patients. Only one study used multiple proteins to probe patients with early stage RCC and to discriminate between benign and malignant small renal masses [179]. Although its diagnostic value is not as remarkable as that of metabolomics-based approaches, single biomarker analysis significantly improves its feasibility for clinical application. Proteins used in the last 5 years of research include GRP78, human circulating progastrin 80 (hPG80), and perilipin-2 (PLIN-2) [128, 180, 181]. Among them, probing patients with mRCC based on circulating hPG80 levels showed the most significant diagnostic value [181].
With the increasing understanding of exosome oncogenesis and the continuous development of isolation techniques, the diagnostic value of exosomes is becoming an important tool for cancer diagnosis. In the last 5 years, several studies have extracted exosomal nucleic acids, including miRNA and mtDNA, to diagnose patients with RCC. In contrast to cfRNAs, exosomes typically prefer to use a single miRNA as a biomarker rather than a panel [153, 182, 183]. Multiple exosomal miRNAs have shown significant diagnostic value, including miR-149-3p (AUC, 0.8324; sensitivity, 87.5%; specificity, 77.3%), miR-30c-5p (AUC, 0.819; sensitivity, 68.57%; specificity, 100%), and miR-1233 (AUC, 0.82; sensitivity, 81%; specificity, 76%). Notably, miR-15a is different as a cfRNA between Patients with RCC and a healthy population, but not as an exosomal miRNA [153]. Moreover, Arance et al. demonstrated the potential of mtDNA for use in clinical practice [184]. Notably, this study discussed the difference in the purity of mtDNA in different phases of differential centrifugation to facilitate subsequent analysis, but further work with a larger sample size is needed.
Prognosis of RCC
Apart from its diagnostic value, liquid biopsy can also predict patients’ grades, stages, and survival to identify high-risk patients and predict their risk of metastasis and recurrence (Table 2). CTCs and cfDNA/ctDNA are correlated with overall survival (OS) or progression-free survival (PFS). Basso et al. supposed that patients with CTCs above 3 had a significantly shorter OS (13 vs. 52.8 months) and PFS (5.8 vs. 15 months) than patients with CTCs below 3 [185]. Moreover, both studies demonstrated that shorter cfDNA fragments were remarkably associated with shorter PFS (P = 0.004 and P = 0.006, respectively) [41, 50]. Notably, cfDNA mutation status, fragment size, and the proportion of cfDNA fragments (PCF) were associated with prognosis in the mRCC patient group (P = 0.010, P = 0.011, and P = 0.007, respectively) but not in the metastasis-free Patients with RCC group [50]. Additionally, cfRNA and protein levels were used to assess patient survival. Serum miR-122-5p and miR-206 levels (all P < 0.005) and urinary miR-328-3p levels (hazard ratio (HR) = 0.29, P = 0.042) were also significantly associated with OS [165, 170]. Among them, serum levels of miR-122-5p and miR-206 were also associated with the grading, staging, and distant metastasis of ccRCC [165]. Kohli et al. showed that patients with plasma hPG80 levels above 4.5 pM had significantly shorter OS than the rest of the patients (12 months vs. 31.2 months; P = 0.0031) [181]. Adding the index to the IMDC prognostic scores improved risk prediction ability. Gigja Gudbrandsdottir et al. reported that the chemokines IL-27 and IL-6, and gp30, the receptor for IL-6, have predictive abilities for survival time in patients with RCC. However, the predictive abilities vary in tumors of different sizes [117]. In addition, urinary NGAL levels implicated a predicting ability of risk of death (HR = 5.5, P = 0.005), where its HR for cancer-specific death in patients with levels above 2.19 ng/mmol was 7.2 (P = 0.001) [186].
Table 2.
Prognostic application of liquid biopsy in recent 5 years
| Region | Year | Detected Abnormality |
Sample | Detection Method | Cohorts | Practice in clinical | Result | Ref | |
|---|---|---|---|---|---|---|---|---|---|
| CTC | Italy | 2021 | Enumeration | Peripheral blood | CellSearch System | 195 metastasis RCC patients | Predicting patients' survival |
Patients with at least 3 CTCs had a shorter OS of 13.8 months versus 52.8 months in those with fewer than 3 CTCs Patients had at least 3 CTCs, with a median PFS of 5.8 versus 15 months in the remaining patients |
[184] |
| China | 2019 | Beclin-1 | Peripheral blood |
Can Patrol CTC enrichment technique, RNA ISH |
58 metastasis-free RCC patients 11 metastasis RCC patients |
Predicting patients' metastasis |
The number of preoperative Beclin1-positive CTCs in the metastatic group was significantly higher than the number of Beclin1-negative CTCs (P < 0.05) The difference between the number of Beclin1-positive CTCs and Beclin1-negative CTCs in the metastasis-free group was not statistically significant (P > 0.05) |
[25] | |
|
ctDNA/ cfDNA |
Japan | 2019 |
Status and fragment size of cfDNA or ctDNA |
Plasma |
NGS ddPCR |
53 RCC patients | Predicting patients' survivals |
ctDNA status was associated with PFS and CSS (P = 0.061, P < 0.01, respectively) cfDNA fragment size was significantly associated with PFS and CSS (p = 0.004, p = 0.011, respectively) |
[50] |
| Japan | 2018 | Plasma cfDNA fragment size | Plasma |
qPCR Microfluidics-based platform |
92 RCC patients 41 healthy controls |
Predicting patients' survivals | Shorter cfDNA fragment size was negatively associated with progression-free survival (p = 0.006) | [41] | |
| cfRNA | Canada | 2020 | miR-328-3p | Urine | qPCR |
30 oncocytomas patients 26 progressive ccRCC-SRM patients 24 non-progressive ccRCC-SRM patients |
Predicting patients' survivals | Patients with high miR-328-3p expression levels had significantly longer OS (HR = 0.29, p = 0.042) compared to patients with low miR-328-3p expression levels | [79] |
| Germany | 2018 |
miR-122-5p miR-206 |
Serum | qPCR |
86 ccRCC patients 55 benign renal tumor patients 28 healthy controls |
Predicting patients' grade and metastasis |
miR-122-5p levels were significantly increased in metastasized ccRCCs (cM0 vs. cM1; p = 0.045) and advanced Fuhrman Grade (G1/2 vs. G3/4; p = 0.001) Serum miR-206 expression was significantly increased in advanced pT-stage (pT1/2 vs. pT3/4; p = 0.006) and metastasized ccRCC (cM0 vs. cM1; p = 0.002) |
[165] | |
| Predicting patients' survivals | Univariate Cox Regression analysis showed that elevated miR-122-5p and miR-206 serum levels were correlated with a shorter period of progression-free, cancer-specific, and overall survival (all P < 0.005) | ||||||||
| Protein | India | 2021 | GRP78 | Serum | Elisa |
60 RCC patients 60 non-tumor controls |
Predicting the metastasis of RCC | GRP78 expression was significantly higher in RCC patients with metastatic than in those without metastasis (P < 0.001). Predicting ability of metastasis or non-metastasis RCC: AUC 0.954, sensitivity 100%, specificity 90.4% | [179] |
| Predicting the grade of RCC | Median level of serum GRP78 increases with higher grade of RCC (P < 0.001). Predicting ability of high or low-grade RCC: AUC 0.948, sensitivity 92%, specificity 83% | ||||||||
| Norway | 2021 | IL-6, IL-27, IL-31, OSM, CNT-f, IL-6Rα, gp130 | Serum |
Luminex immune-bead technology and a high-sensitivity kit |
159 RCC patients with nephron sparing surgery, a radical nephrectomy or a cyto-reductive nephrectomy | Predicting patients' recurrence |
Kaplan–Meier analysis showed IL-27 had a significantly predictive ability of recurrence (P = 0.026) Cox multivariate regression consisted of IL-6 and IL-27 showed that IL-6 had a significantly predictive ability of recurrence (P = 0.004) but not IL-27 (P = 0.082) Kaplan–Meier analysis showed both of IL-27 and IL-6 had significantly predictive abilities of recurrence for ccRCC patients withlarge tumors (diameter > 7.0 cm) (P = 0.014, P = 0.026, respectively) |
[117] | |
| Predicting patients' survivals |
Kaplan–Meier analysis and multivariate regression analysis showed IL-6 had a significantly predictive ability of disease-specific survival (DSS) (P < 0.026, P = 0.001, respectively) Kaplan–Meier analysis showed IL-6 could predict DSS for patients with a tumor diameter from a 4 to 7 cm and > 7 cm (P = 0.001, P = 0.02, respectively), and IL-27 could predict DSS for patients with a tumor diameter > 7 cm (P = 0.025) Kaplan–Meier analysis showed IL-6 could predict OS for ccRCC patients (P = 0.001) but not IL-27 (P = 0.066) Kaplan–Meier analysis showed IL-6 could predict DSS for patients with a tumor diameter from a 4 to 7 cm but not > 7 cm (P = 0.018, P = 0.063, respectively), while gp130 could only predict DSS for patients with a tumor diameter > 7 cm (P = 0.001) |
||||||||
| USA | 2021 | Hpg80 | Plasma | Elisa | 89 RCC patients | Refining IMDC prognostic scores |
Patients with high hPG80 levels (> 4.5 pM) had a shorter OS than other patients (12 vs. 31.2 months, respectively; p = 0.0031) Adding hPG80 levels > 4.5 pM in the IMDC risk scores showed better significance slightly and more refined discriminating ability (p = 0.0046) |
[180] | |
| France | 2021 | SAA2, CFB | Plasma | Elisa |
59 mRCC patients with sunitinib or bevacizumab treatment |
Predicting the metastasis of RCC | Combination of Fuhrman grade and levels of SAA2 and CFB showed better ability of predicting time to relapse. The model combining Fuhrman grade and CFB showed the best C-Index (C-Index = 0.7273), followed by the three covariates together (C-Index = 0.7163) and Fuhrman grade alone (C-Index = 0.6948) | [186] | |
| France | 2021 | NGAL | Urine | ARCHITECT C4100 | 50 RCC patients | Evaluating post-operative risk of progression and death | ccRCC patients with NGAL level above 2.19 ng/mmol had a 5-time fold higher risk of progression than patients with NGAL level below the threshold (HR = 5.5, p = 0.005). The HR of cancer specific death for patients with a level of NGAL above 2.19 ng/mmol was of 7.2 (p = 0.001) | [185] | |
| Italy | 2020 | PD-1, PD-L1, BTN3A1 | Plasma | Elisa |
Testing cohort: 21 mccRCC patients with nivolumab treatment Validating cohort: 20 mccRCC patients with nivolumab treatment 15 localized ccRCC patients |
Predicting metastasis of ccRCC patients |
The mean levels of plasma immune checkpoint levels in metastatic ccRCC patients were significantly higher than localized RCC patients (PD-1: 2.79 vs. 1.54 ng/mL, p = 0.003; PD-L1: 0.62 vs. 0.49 ng/mL, p = 0.03) Plasma immune checkpoint levels were correlated to number and localization of metastatic sites |
[187] | |
| USA | 2019 | PLIN-2 | Urine | Plasmonic biosensor |
20 RCC patients 20 healthy controls 8 BLCA patients 10 diabetic nephropathy patients |
Predicting the size of RCC tumor |
The urine PLIN-2 concentrations were associated to tumor size which Spearman correlation coefficient is 0.59 (P < 0.009) | [128] | |
| Metabolites | Japan | 2022 | 5 urinary metabolites | Urine | LC–MS/MS |
56 ccRCC patients 10 benign urological tumor |
Predicting the recurrence | A model consisting of 5 metabolites (lactic acid, glycine, 2-HG, succinic acid, and kynurenic acid) showed significant predicting value for ccRCC recurrence (AUC 0.894, sensitivity 88.9%, specificity 88.0%) and T3 ccRCC patients (AUC 0.903, sensitivity 88.9%, and specificity 100%) | [100] |
| Japan | 2020 | 9 urinary metabolites | Urine | LC–MS |
69 stage I-II RCC patients 18 stage III-IV RCC patients 60 benign renal tumor patients |
Predicting the clinical stage | A model consisting of 4 metabolites (L-kynurenine, L-glutamine, fructose 6-phosphate and butyrylcarnitine) was used for predicting III-IV stage of RCC patients: AUC 0.837, sensitivity 88.5%, specificity 75.4% | [176] | |
| Exosome | Spain | 2021 |
Exosome mtDNA VH1, CγB, HBB |
Plasma |
Differential ultracentrifugation, qPCR, NGS, dPCR |
13 RCC patients 15 healthy controls |
Predicting the metastasis of RCC |
qPCR showed that VH1-short, VH1-long and CγB-short (B phase) were of a significant difference in metastatic group and non-metastatic group (p = 0.020, p = 0.035, p = 0.078, respectively) dPCR showed that VH1-short (B phase) and CγB-short (C phase) were of a significant difference in metastatic group and non-metastatic group (p = 0.069, p = 0.037, respectively) dPCR and qPCR showed that HBB-long were of a more significant difference in metastatic group and non-metastatic group in most phases |
[183] |
Furthermore, liquid biopsy can predict the grades, metastasis, and recurrence of RCC. A 2019 study classified CTCs into Beclin 1-positive and Beclin 1-negative CTCs by measuring the cellular levels of Beclin 1 [25]. This trial showed that the number of pre-operative Beclin 1-positive CTCs was significantly higher than that of Beclin 1-negative CTCs in patients in the metastatic group (P < 0.05), while there was no significant difference in the non-metastatic group. In addition, protein analysis showed excellent predictive ability for RCC grade and stage. Many proteins, including GRP78, Serum Amyloid A2 (SAA2), Complement Factor B (CFB), circulating cytokines, immune checkpoints in plasma, and PLIN-2 in urine, show measurable differences among patients with different progressions [180, 181, 187, 188]. Briefly, GRP78 can predict patient grades and metastasis [180]; SAA2 and CFB were associated with patient recurrence, where combining Fuhrman grade with CFB levels showed the best C-index to predict recurrence [187]; IL-6 and IL-27 can predict recurrence of RCC, but as with survival prediction their abilities to predict recurrence vary across tumor sizes [117]; plasma immune checkpoints, including PD-1, PD-L1, and BTN3A1, can predict metastasis, which is correlated with the number and location of metastases [188]; and urinary PLIN-2 levels can predict tumor size [186]. Compared to protein, metabolite analysis has been less reported in prognosis prediction, with only two studies in the last 5 years constructing metabolite models to predict patient grades and metastasis [100, 177]. Finally, mtDNA in different phases of exosomes is strongly associated with RCC metastasis. dPCR and qPCR showed that mtDNA HBB-long had the most significant difference between the metastatic and non-metastatic groups in most phases. However, this conclusion needs to be supported by larger patient datasets [184].
Monitoring and predicting responses of RCC therapy
Surgery is the preferred treatment strategy for patients with RCC owing to its insensitivity to chemotherapy and radiotherapy. In recent years, many adjuvant therapies have demonstrated important value in improving patient survival [189]. Therefore, it is crucial to select appropriate indicators to predict treatment sensitivity, as well as to monitor variations in patients with RCC before and after treatment in real time to evaluate their efficacy. In the last 5 years, several clinical trials of liquid biopsy have focused on the monitoring of surgery, ICI, and anti-angiogenic therapy (Table 3). Song et al. showed that the number of CTCs decreased sharply after surgery in patients with RCC, with a more significant reduction in the pre-operative high CTC group (21 of 24 patients, 87.5%) than in the low CTC group (9 of 17 patients) [26]. Haga et al. showed that the number of postoperative CTCs was significantly correlated with tumor diameter (P = 0.0004) and surgical approach (P = 0.016), and pre-operative CTCs were significantly higher in patients with stage IV disease [190]. By classifying CTCs into epithelial CTCs, mesenchymal CTCs, and mixed CTCs and analyzing their Beclin-1 expression in patients with mRCC, Wang et al. reported that Beclin-positive mixed CTCs at 6 and 12 months postoperatively were significantly higher than pre-operative and 6 months postoperative CTCs, respectively [25]. This implies that CTCs could be a useful tool for monitoring postoperative metastasis and recurrence. In addition, cell-free nucleic acids have obvious potential for monitoring postoperative outcomes. Plasma GAPDH and hTERT cfDNA levels decreased significantly postoperatively and correlated with the risk of postoperative progression and death [191]. One study reported that each fg/mL of postoperative GAPDH cfDNA and hTERT cfDNA increased the risk of progression by 1.04 and 1.2 in Patients with RCC, respectively. In addition, each fg/mL of postoperative GAPDH cfDNA increased the risk of progression by 14.9 in Patients with RCC with metastasis. However, this study showed that postoperative GAPDH cfDNA and hTERT cfDNA levels were not independent risk factors in Patients with RCC without metastasis, and their levels at 1 year post-operation were not correlated with patient OS or PFS. Moreover, cell-free miR-15a levels decreased remarkably in patients undergoing nephrectomy. On the eighth postoperative day, the mean level decreased by 99.53% (P < 0.01) [169]; interestingly, the expression level correlated with tumor size. Combined with its diagnostic value, miR-15a may be a key molecule in liquid biopsies of RCC. As a traditional marker in liquid biopsies, plasma protein KIM 1 can also predict postoperative risk. Xu et al. showed that high postoperative baseline levels of KIM 1 were significantly associated with shorter disease-free survival (DFS) and OS in patients [192]. Adding it as a complementary measure to the SSIGN score or the UISS score improved the monitoring ability of postoperative metastasis (likelihood ratio test P = 0.078 and P = 0.0022, respectively). In addition, the levels of exosomal miR-210 and miR-1233 significantly decreased postoperatively (P = 0.004 and P = 0.008, respectively) [153].
Table 3.
Monitoring treatment and predicting response by liquid biopsy in recent 5 years
| Region | Year | Detected Abnormality |
Sample | Detection Method | Cohorts | Practice in clinical | Result | Ref | |
|---|---|---|---|---|---|---|---|---|---|
| CTC | China | 2021 | CTC counts | Peripheral blood | CTC-BIOPSY system | 41 RCC patients | Monitoring the postoperative condition of patients | • In the high CTC group, CTC counts decreased in 21 of 24 (87.5%) patients 1 week after surgery compared with the low CTC group (52.9%) | [26] |
| USA | 2021 | PD-L1 | Peripheral blood |
VERSA Platform, Immunofluorescence |
20 RCC patients treated with ICI | Evaluating the responses of ICI therapy | • PD-L1 expression in CAXII single positive CTC correlates with the efficacy of ICI treatment. Detection of progressing patients with ICI therapy: AUC 0.77, sensitivity 67% and specificity 88% | [24] | |
| HLA-1 | Peripheral blood |
VERSA Platform, Immunofluorescence |
22 RCC patients treated with TKI | Evaluating the responses of TKI therapy | • PD-L1 expression in CAXII single positive CTC correlates with the efficacy of TKI treatment. Detection of progressing patients with TKI therapy: AUC 0.83, sensitivity 100% and specificity 88% | ||||
| Japan | 2020 | CTC counts | Peripheral blood | FISHMAN-R flow cytometer |
54 RCC patients treated with NE or RE |
Evaluating the responses of surgery | • Postoperative CTCs was significantly correlated with tumor diameter (P = 0.0004) and surgical approach (P = 0.016) | [189] | |
| China | 2019 | Beclin-1 | Peripheral blood |
Can Patrol CTC enrichment technique, RNA ISH |
58 metastasis-free RCC patients 11 metastasis RCC patients |
Monitoring the postoperative metastasis |
• Beclin1-positive epithelial CTCs in the metastatic group at 12 months postoperatively was significantly higher than preoperatively • Beclin1-positive mesenchymal CTC in the metastatic group at 6 months postoperatively was significantly higher than preoperatively • Beclin1-positive mesenchymal CTC in the metastatic group at 6 months postoperatively was significantly higher than preoperatively; at 12 months postoperatively was significantly higher than 6 months postoperatively and preoperatively |
[25] | |
|
ctDNA/ cfDNA |
Italy | 2022 | TP53 | Plasma | NGS |
12 mccRCC patients with immunotherapy 36 mccRCC patients with TKI therapy |
Evaluating the responses of TKI and immune therapy |
• The most frequently mutated genes in cfDNA were TP53 (43%) and PDGFRA (21%), followed by mTOR, PI3K, BRAF etc. The used NGS panel did not include VHL • Patients with at ctDNA > 0.883 ng/μl had a shorter PFS and OS versus those with ctDNA > 0.883 ng/μl in overall population (P < 0.001, P < 0.008, respectively). The results were consistent with patients treated immunotherapy and TKI separately (P < 0.0365, P < 0.0035, respectively) • Patients with TP53 mutation have a shorter PFS than those who do not (P = 0.04) • Comprehensively evaluated of both ctDNA level and TP53 mutation status, patients with high cfDNA and mutated TP53 had the worst PFS, while patients with low cfDNA and no TP53 mutations had the longer PFS (P = 0.004) |
[51] |
| Predicting the best response to TKI and immunotherapy |
• cfDNA level was associated with best response in the overall population (P = 0.006), which is consistent with patients with immunotherapy (P = 0.004) and TKIs (P = 0.003) • cfDNA cut point of ≥ 2.19 ng/μl for early progressors: Youden’s 0.75, sensitivity 100%, specificity 75% • cfDNA cut point of ≤ 1.35 ng/μl for long progressors: Youden’s 0.556, sensitivity 78%, specificity 78% |
||||||||
| Japan | 2022 |
VHL, TP53, ATM, MET |
Plasma | NGS |
11 ccRCC patients with ICI therapy |
Predicting response to ICI therapy in mRCC patients |
• The commonly mutated genes were VHL (30.0%), TP53 (20.0%), ATM (10.0%), and MET (10.0%) • The coincidence rate of VHL (9 of 14 patients), TP53(2 of 14 patients) and MET (2 of 14 patients) between plasma ctDNA and tumor tissue DNA is 55.6%, 100%, 50%, respectively • For ICI-treated patients, ctDNA decreased in 4 of 5 responders and increased in 5 of 6 non-responders. A longer PFS is showed in the ctDNA-decreased group than ctDNA-increased group |
[47] | |
| Spain | 2021 | GAPDH, hTERT | Plasma | qPCR |
82 RCC patients 20 healthy controls |
Evaluating surgery effects | • After nephrectomy, the mean level of GAPDH cfDNA was 16.9 fg/ml, which was significantly lower than preoperative level (29.3 fg/ml, P < 0.0001) | [190] | |
|
Predicting patients' risk of death |
• Univariate Cox Regression analysis showed that each fg/ml of GAPDH cfDNA increased the risk of progression by 14.8 postoperatively in mRCC patients • Each fg/ml of GAPDH cfDNA and hTERT cfDNA increased the risk of progression by 1.04 and 1.23 postoperatively, respectively |
||||||||
| Japan | 2019 | VHL, TP53, mTOR, TSC1, BAP1et al | Plasma |
NGS ddPCR |
53 RCC patients |
Monitoring responses to surgery and TKI therapy in RCC patients |
• The mutant allele frequency (MAF) of VHL, TP53 and other ctDNA decreased postoperatively, which reflected the changes of tumor burden • Patients with short fragment sizes of cfDNA showed significantly worse responsiveness (P = 0.011). For TKI-treated patients, positive ctDNA was significantly associated with weaker effect (P = 0.049), and short fragment sizes of cfDNA tended to be associated with worse outcome (P = 0.090) |
[50] | |
| cfRNA | Ukraine | 2018 | miR-15a | Urine | qPCR |
52 RCC patients 15 oncocytoma patients 15 healthy controls |
Evaluating surgery effects |
• The tumor size related to the expression of miR-15a (Pearson correlation coefficient 0.873) • The mean expression of miR-15a in patients with nephrectomy decreased by 99.53% (P < 0.01) on the 8th day postoperatively |
[169] |
| China | 2018 | lnc-GIHCG | Serum | qPCR |
20 RCC patients with total nephrectomy |
Evaluating surgery effects | • Serum GIHCG level significantly decreased in postoperatively compared than preoperatively (P < 0.001) | [173] | |
| Protein | USA | 2021 | KIM-1 | Plasma | microbead-based assay |
418 ccRCC patients with total nephrectomy |
Predicting patients' postoperative survivals |
• Higher post-nephrectomy baseline of KIM-1 was related to worse DFS, with a survival time ratio of 0.65 (in univariable lognormal AFT model) and 0.56 (in variable lognormal AFT model) for the 75th vs 25th percentile of baseline KIM-1 (p = 0.0004, P < 0.001, respectively) • Higher post-nephrectomy baseline was related to worse OS in a multivariable AFT model, with a survival time ratio 0.71 for 75th vs 25th percentile of KIM-1 (P < 0.001) |
[191] |
| Predicting patients' postoperative recurrence risk | • Added to either the SSIGN score or the UISS score, baseline KIM 1 improved the predictive value for recurrence after nephrectomy of both models (likelihood ratio test p = 0.078, p = 0.0022, respectively) | ||||||||
| UK | 2021 | CA9, HGF, MET, Gas6, Ax1, VEGF, VEGFR2, IL-8 | Plasma |
Luminex assay platforms Elisa |
330 advanced RCC patients with cabozantinib 330 advanced RCC patients with everolimus |
Predicting the survival and responses of cabozantinib |
• The multivariable analysis showed that baseline levels of HGF were independent prognostic biomarker of PFS for cabozantinib, and HGF, GAS6, VEGF were independent prognostic biomarker for OS with cabozantinib • Decrease of AXL level were independent prognostic biomarker of PFS for cabozantinib, and decrease of levels of HGF, GAS6 were both independent prognostic biomarker for improved OS with cabozantinib |
[193] | |
| Predicting the survival and responses of everolimus |
• The multivariable analysis showed baseline levels of HGF were independent prognostic biomarker of longer PFS for everolimus. No biomarkers were independently prognostic for OS with everolimus • Decrease of HGF level were independent prognostic biomarker of PFS and OS for everolimus |
||||||||
| USA | 2021 | 23 angiokines, including Ang-2, CD-73, HER-3, HGF, IL-6, OPN etc | Plasma |
SP-X imaging and analysis system from Quanterix Ella System (Protein Simple) |
53 non-ccRCC patients with everolimus 46 non-ccRCC patients with sunitinib |
Predicting the survival and treatment benefit of RCC therapy |
Exception of HER-3, SDF-1, TGFb-R3 and BMP-9, higher angiokine levels were associated with worse PFS (HR > 1) The univariate analysis showed that exception of HER-3, SDF-1, TGFb-R3, VEGF-R1, and VEGF-R2, higher angiokine levels were associated with worse OS (HR > 1) The multivariable analysis showed that HGF, OPN, TIMP-1, TSP-2, and VCAM-1 were independently associated with OS with HRs of 1.5, 1.3, 1.7, 1.8, and 2.2, respectively None of the angiokines are statistically significant to predictive benefit for patients receiving either sunitinib or everolimus |
[122] | |
| Spain | 2021 | CXCL10, CXCL11, HGF, IL-6 | Serum | The Luminex’s xMAP Technology multiplex system |
51 mRCC patients with sunitinib therapy 4 mRCC patients with pazopanib therapy 5 mRCC patients with both |
Predicting patients' survivals with anti-angiogenic therapy |
• High basal HGF levels (over 649.1 pg/mL) were significantly correlated to worse PFS (P = 0.003) and OS (P = 0.0034). A reduction of HGF levels during the treatment was related to a lower PFS (p = 0.017), but not with OS • CXCL11 levels were significantly higher in patients who did not respond to treatment (P < 0.05) than in those who responded. High levels of CXCL11 (above 39.4 pg/mL) were significantly related to shorter PFS (P = 0.0003) and OS (P = 0.001). Patients with a reduction of CXCL11 levels after 3 months treatment had a significantly lower OS (P = 0.027), but not PFS |
[192] | |
| France | 2021 | SAA2, CFB | Plasma | Elisa |
59 mRCC patients with sunitinib or bevacizumab treatment |
Predicting survivals of mRCC patients with TKI therapy | • The levels of SAA2 and CFB can subdivide the cohort with anti-angiogenic treatment into 3 different groups according to PFS and OS: CFB low/SAA2 low (PFS 13.23 months, OS: 20.8 months), CFB low/SAA2 high or CFB high/ SAA2 low (PFS 9.87 months, OS 16.52 months), and CFB high/SAA2 high (PFS 2.8 months, OS 8.33 months) | [186] | |
| USA | 2021 | 30 cytokines, including IL-6, IL-1RA, CSF, IFN-γ, IL-12, VEGF etc | Plasma |
Luminex FLEXMAP 3D System |
33 mccRCC patients with immunotherapy 23 mccRCC patients with TKI therapy |
Predicting and monitoring response to ICI therapy in mRCC patients |
• 17 patients are identified in clinical benefits (CB) group and 16 patients are identified in non-clinical benefits (NCB) group • No significant cytokine differences were observed between CB and NCB patients |
[195] | |
| Predicting and monitoring response to TKI therapy in mRCC patients |
• 13 patients are identified in clinical benefits CB group and 10 patients are identified in non-clinical benefits NCB group • Patients in CB group had lower median levels of IL-6 (8.4 vs 13.5 pg/mL, p = 0.02), IL-1RA (178 vs 248 pg/mL, p = 0.03), and G-CSF (23.9 vs 38.3 pg/mL, p = 0.02) compared with patients in NCB group in pretreatment |
||||||||
| USA | 2020 | Ang-1, Ang-2, HGF, CXCL10, IL2, IL6, IL8, IL10, CXCL9, NGAL, OPN, TGFb, VEGF etc | Serum | Luminex instrument |
52 advanced RCC patients with combined axitinib and pembrolizumab treatment |
Predicting the survival and treatment benefit of RCC therapy |
• Higher baseline of CXCL10 was correlated with objective response rate (ORR) (unadjusted P value = 0.0197) • Lower EOT level of CEACAM1, GRO-a, HGF, and TIMP-1 was correlated with objective response rate (ORR) (unadjusted P value = 0.0026, 0.0495, 0.0112, 0.0044, respectively) • At baseline, CEACAM1 levels ≥ median were associated with better PFS (P = 0.085). C2D1, GRO-a and HGF levels were associated with better PFS (P = 0.0034, P < 0.001, respectively). At EOT, HGF and TIMP-1 levels < median were associated with better PFS (P = 0.0034, 0.014, respectively) |
[194] | |
| Italy | 2020 | PD-1, PD-L1, BTN3A1 | Plasma | Elisa |
Testing cohort: 21 mccRCC patients with nivolumab treatment Validating cohort: 20 mccRCC patients with nivolumab treatment 15 localized ccRCC patients |
Predicting survivals of mccRCC patients with immunotherapy |
• The mean pre-treatment levels of plasma ICs in long-responder group (> 18 months) was significantly higher than all patients (PD-1: 13.25 vs. 2.00 ng/mL, p = 0.01; PD-L1: 1.09 vs. 0.64 ng/mL, p = 0.02; BTN3A1: 11.03 vs. 6.84 ng/mL, p = 0.03) • High level of plasma PD-1 (> 2.11 ng/mL), PD-L1 (> 0.66 ng/mL) and BTN3A1 (> 6.84 ng/mL) were correlated to a shorter median PFS (PD-1: 20.7 vs. 6.9 months, P < 0.0001; PD-L1: 19 vs. 9 months, P < 0.0001; BTN3A1: 17.5 vs. 8.4 months, p = 0.002) • After 18 months of immunotherapy, plasma PD-1 and PD-L1 levels were lower than baseline in patients with FPS longer than 18 months (PD1: 1.23 vs. 13.25 ng/mL; PD-L1: 0.73 vs. 1.09 ng/mL) • The predictive value of PD1, PD-L1 and BTN3A1 in validating cohort: PD1: AUC = 1.0, P < 0.001; PD-L1: AUC = 0.944, P < 0.001; BTN3A1: AUC = 0.833, P < 0.03 |
[187] | |
| Exosome | China | 2018 |
Exsomal miRNA miR-210, miR-1233 |
Serum |
Total exosome isolation reagent, EpCAM isolation beads, Flow cytometry |
10 ccRCC patients received surgical tumor removal | Monitoring responses to surgery | • The levels of exosomal miR-210 and miR-1233 were significantly lower in postoperative than in preoperative samples (p = 0.004, p = 0.008, respectively) | [153] |
In addition to surgery, the use of liquid biopsy in predicting the responses to tyrosine kinase inhibitors (TKIs) and ICI therapy deserves attention. Bade et al. highlighted the use of CTCs in the evaluation of responses to ICI and TKI therapy [24]. This study divided patients receiving TKI and ICI therapy into responding and progressive groups and differentiated them using characterized CTCs. The results showed that HLA-1 and PD-1 expression in CAXII single-positive CTCs could effectively identify patients in the progressive phase (AUC = 0.77 and 0.83, respectively), thereby predicting sensitivity to adjuvant therapy and helping to select a suitable drug. A study in 2022 identified the most commonly mutated genes in 11 patients with ccRCC receiving ICI therapy, including VHL (30.0%), TP53 (20.0%), ATM (10.0%), and MET (10.0%). They then explored the concordance between plasma ctDNA and tumor DNA [47]. The results showed that ctDNA decreased in four of five responders and increased in five of six non-responders. Interestingly, patients with elevated ctDNA levels had shorter PFS than those with decreased ctDNA levels. Another recent study also evaluated the mutation status of patients, showing that TP53 had the highest mutation frequency (43%), which was significantly higher than the former results. This study showed that ctDNA levels could not only predict survival time after treatment but also distinguish early response from long-term response; they could also be combined with TP53 mutation status to assess patient risk [51]. Yamamoto et al. concluded that the frequency of ctDNA mutations was significantly reduced postoperatively. In addition, shorter size and higher levels of ctDNA were associated with adverse effects and sensitivity in patients receiving TKI therapy (P = 0.049 and P = 0.090, respectively) [50].
Protein analysis is widely used to assess and monitor the efficacy of ICI and anti-angiogenic therapy. To assess the response to anti-angiogenic therapy, Esteban et al. explored protein levels in patients with mRCC receiving anti-angiogenic therapy, including sunitinib and pazopanib [193]. High levels of HGF were significantly associated with poorer PFS (P = 0.003) and OS (P = 0.0034) in patients receiving anti-angiogenic therapy. HGF levels were higher in treatment-effective patients than in treatment-ineffective patients at baseline (P < 0.05), but there was no difference after three months of treatment. Similarly, CXCL11 levels were significantly associated with poor PFS (P = 0.003) and OS (P = 0.0034) and non-response to anti-angiogenic therapy (P < 0.05). The reduction in CXCL11 after treatment was associated with shorter OS in patients (P = 0.027) but not PFS. In addition, Cooley et al. classified patients receiving anti-angiogenic therapy (sunitinib and bevacizumab) into three categories according to SAA2 and CFB levels [187]. The results showed that the CFB low/SAA2 low group had the best survival (PFS: 13.23 months, OS: 20.8 months); in contrast, the CFB high/SAA2 high group had the worst survival (PFS: 2.8 months, OS: 8.33 months). Moreover, as the direct target of ICI therapy, the level of immune checkpoints in plasma is closely related to the response to ICI treatment [188]. Patients who responded for a long time (> 18 months) to ICI therapy had significantly higher plasma levels of immune checkpoints; however, patients with RCC with high levels of immune checkpoints had a lower median survival. This result indicates a strong association between plasma immune checkpoint proteins and ICI therapy. As a fundamental component of circulating proteomics, a large number of angiokines and chemokines are involved in the evaluation of cancer therapy. Several results have shown that HGF can significantly differentiate the survival time and status of patients with RCC receiving anti-angiogenic therapy and immunotherapy [117, 122, 193–195]. Currently, HGF is demonstrating its clinical potential as a biomarker in a variety of drugs, including cabozantinib [194], sunitinib [122, 193], axitinib [195], pazopanib [193], pembrolizumab [195] and everolimus [122, 194]. In addition, OPN and IL-6 have been widely reported and may be considered biomarkers for therapeutic selection and evaluation[122, 196]. Many other cytokines have been reported with the difference of baseline levels and dynamic changes during treatment between patients with good versus poor prognosis, but there is a lack of consistency between the results. It is worth noting that there are few studies focusing on the combined use of drugs at present, and all of them concentrated on the combined use of antiangiogenic therapy and immunotherapy, while there is a lack of research on the combined use of multiple anti-angiogenic therapy or immunotherapy. Besides, Daniela Vargová et al. analyzed urinary cytokines in 60 RCC patients [197]. The results showed that the baseline level of platelet-derived growth factor (PDGF), IL-15, MIP-1ß, etc. were higher in patients with RCC than healthy controls, and the cytokines decreased at the third day postoperatively. However, the study did not do a more in-depth analysis.
Limitations and prospects
Commonly used metrics in RCC include CTCs, ctDNA/cfDNA, cfRNA, exosomes, and tumor-derived metabolites or proteins. However, these indicators have several limitations. The low level of CTCs in the blood makes their capture and analysis very difficult. Moreover, they have a relatively low diagnostic value. With the popularity of NGS, ddPCR, and methylation analysis, ctDNA/cfDNA is becoming less expensive and more accurate and is widely used in various clinical practices. However, it is still difficult to distinguish ctRNAs from cfRNAs based on genomic alterations. Among cfRNAs, miRNAs are the main molecules used for liquid biopsies. However, the complexity of the miRNA regulatory network makes it difficult to explain the mechanism underlying its role as a biomarker. In addition, many studies have pointed out that the standardization of miRNA analysis, including analytical conditions and methods, still needs to be determined [198, 199]. Although considered ideal components for liquid biopsy, cohort studies of lncRNAs, circRNAs, and piRNAs are insufficient to fully demonstrate their clinical value. Similarly, despite the recent increase in studies on urinary metabolites in liquid biopsies, few studies have been able to reveal the metabolic pathway changes involved, and the metabolites screened showed low consistency. Protein is a traditional molecule used for liquid biopsy, and many automated analytical systems or platforms have emerged in recent years, attempting to detect target proteins with high sensitivity and specificity. However, their accuracy needs to be tested in larger cohorts. Among them, multiple cytokines were used to assess and monitor treatment, and HGF, OPN and IL-6 had shown their predictive abilities. However, there were still a large number of cytokines whose predictive outcomes were inconsistent, as demonstrated in the evaluation of responses of everolimus. In addition, fewer studies have been conducted on cytokines in urine, and current studies lack sufficient attention to the correlation between cytokines in body fluids and the molecular phenotype of tumor cells. Furthermore, the role of exosomes in cancer has received increasing attention, but a standard isolation method that can guarantee both the purity and availability of the product is still lacking.
At the same time, we should recognize that almost all current studies on therapeutic response assessment have focused on a single anticancer drug, resulting in a failure to explore the clinical value of liquid biopsy in treatment regimens based on the combination of multiple drugs. The combined application of immunotherapy and targeted therapy has become clinically prevalent, and further studies should focus on this aspect. In addition, few studies have discussed the possibility of using liquid biopsy with other conventional clinical tests such as imaging tests. As a non-invasive test with potential for dissemination, a novel risk score or guideline, including liquid biopsy with other clinical tests, should be established. Meanwhile, the frontier areas of artificial intelligence and machine learning should be noted and used in combination with liquid biopsies to improve reliability and performance. For the purpose, a global integrated information center should be established to provide comprehensive information on liquid biopsies. Besides, we believe that commercially available testing platforms or kits should be further developed as the ultimate liquid biopsy products to accelerate promotion it to clinical practice. Finally, owing to the clonal diversity of tumors, the genome, transcriptome, proteome, and metabolome have significant interactions between primary and metastatic tumors. This makes it difficult to precisely guide treatment by analyzing a single molecule. Single-cell sequencing can reduce the impact of heterogeneity by identifying the molecular phenotype of captured tumor cells in peripheral blood, and is currently being used in several studies [118, 200]. This also indicates that the combined analysis of multiple liquid biopsy components can yield more information to further facilitate precision medicine.
However, despite several limitations, the advantages of liquid biopsy are obvious and cannot be ignored. A growing number of clinical trials are underway in RCC to improve the accuracy and reliability of liquid biopsy and address these issues. Here, we present the current ongoing clinical trials of liquid biopsy in RCC (Table 4). We believe that future research on liquid biopsy will focus on the following issues: (1) establishing better medical information systems that collect more clinical data to support high-quality trials with large patient cohorts; (2) developing new technologies and analytical platforms to improve measurement efficiency and reduce costs; (3) finding biomarkers with higher sensitivity and specificity; (4) standardizing biomarker isolation techniques and establishing guidelines to increase objectivity and reproducibility of studies; and (5) using a combination of liquid biopsy and other clinical tests and establishing new risk scores or guidelines.
Table 4.
Currently ongoing clinical trials focused on liquid biomarkers in renal cell carcinoma
| NCT Number | Liquid biopsy component | Study Name | Type | Country and number of patients | Recruiting Status | Brief summary |
|---|---|---|---|---|---|---|
| NCT04883827 | CfDNA | Monitoring Disease Burden and Biology Using Tumor Cell Free DNA in Metastatic Kidney Cancer | Observational | United States;150 | Recruiting | This study will assess whether DNA released by kidney cancer into the blood stream and urine of patients can be used to monitor tumor burden and tumor response to treatment in patients receiving immunotherapy |
| NCT04197414 | ctDNA | Development of Urologic Registry for Personalized Medicine in Patients with Urological Malignancy by Analyzing Circulating Tumor DNA | Observational | Korea; 3000 | Recruiting | This study aims to explore the usefulness of ctDNA in plasma and urine for the detection of urologic malignancies, the monitoring of disease progression and the assessment of treatment response |
| NCT05059444 | ctDNA | ORACLE: Observation of Residual Cancer with Liquid Biopsy Evaluation | Observational | United States; 1000 | Recruiting | This study aims to demonstrate the ability of a new ctDNA assay developed by Guardant Health to early detect recurrence in patients with solid tumors (including RCC) |
| NCT03702309 | cfDNA; cfRNA | Liquid Biopsy Evaluation and Repository Development at Princess Margaret | Observational | Canada; 2500 | Recruiting | This study aims to use circulating cell-free nucleic acids, including cfDNA and cfRNA, as a means to non-invasively assess multiple tumor progression and treatment response at multiple time points in a patient's disease course |
| NCT04891055 | CTC; miRNAs | Prospective Translational Study Investigating Predictors of Outcome in Metastatic Renal Cell Carcinoma Patients Treated with Nivolumab (I-Rene Trial) | Observational | Italy; 90 | Recruiting | The study aims to evaluate whether circulating miRNAs or CTCs may be a potential predictor of clinical response and disease progression in metastatic RCC patients treated with Nivolumab |
| NCT03667885 | DNA and mRNAs | Non-Invasive Diagnostics of Small Renal Masses | Observational | Denmark; 160 | Recruiting | Studies 1 and 2 were designed to look for circulating biomarkers in the form of DNA and mRNA contained in microvesicles secreted into the blood by RCCs and to find changes in biomarker levels after surgery |
| NCT04946266 | LncRNA-MFI2-AS1 | Prospective Validation of the Prognostic Value of Long Non-coding MFI2-AS1 RNA in Localized Clear Cell Kidney Cancers | Observational | France; 260 | Recruiting | This study aims to detect the expression of LncRNA-MFI2-AS1 in the plasma of Localized Clear Cell Kidney Cancers to explore its use as a biomarker for pre-tissue analysis diagnosis and patient follow-up |
| NCT04053855 | Urinary exosomes | Evaluation of Urinary Exosomes Presence from Clear Cell Renal Cell Carcinoma | Observational | France; 100 | Recruiting | This study aims to develop a reliable technique to detect tumor exosomes in the urine of ccRCC patients and to provide a new liquid biopsy tool for their early diagnosis |
| NCT05060783 | Glycosaminoglycans | Renal Cancer Detection with Liquid Biopsy | Observational | Denmark; 200 | Recruiting | This study aims to explore the diagnostic role of alterations in plasma and urine glycosaminoglycans for RCC |
| NCT03628859 | Intracellular cytokines | BIOREN (Predictive BIOmarkers in Metastatic RENal Cancer)—A Translational Study on Immunotherapy for Metastatic Renal Cancer | Observational | France; 30 | Recruiting | This study aims to characterize the genetic background of RCCs and their immune environment, to try and identify biomarkers of response and to better understand the mechanisms of resistance to nivolumab in RCC |
| NCT03185039 | MMP2 and MMP9 | Predictive Impact of MMP2 and MMP9 Levels for Patients with Metastatic Kidney Cancer Treated with Anti-angiogenic Agents | Interventional | France; 50 | Recruiting | This study aims to explore plasma levels of MMP2 and MMP9 as predictive biomarkers for treatment with 2 anti-angiogenic drugs (sunitinib or pazopanib) in patients with metastatic kidney cancer |
| NCT05214885 | CA9、NDUFA4L2、ANGPTL4、HILPDA and EGLN3 | Novel Biomarkers of Hypoxia and Metabolism in Clear Cell Renal Cell Carcinoma | Observational | China; 300 | Recruiting | This study aims to detect mRNA and protein levels of five hypoxia- and metabolism-related molecules in blood or urine samples to explore specific tumor biomarker profiles for clinical diagnosis, assessment of ccRCC recurrence, metastasis and prognosis |
| NCT04113486 | NMAP | Expression Levels of Nicotinamide Metabolism-related Protein (NMAP) in Newly Diagnosed Renal Cancer and Non-renal Cancer Populations | Observational | China; 400 | Recruiting | This study aims to observe the difference between NMAP serum levels in primary diagnosed RCC patients and controls and to plot the ROC curve and establish appropriate cut-off values |
| NCT05285579 | Immune-related circulating biomarkers | Predictive Role of Circulating Biomarkers Involved in Angiogenesis in Metastatic Kidney Cancer in the Era of New Therapeutic Associations: Immunotherapies, Anti-angiogenic | Observational | France; 100 | Not yet recruiting | This is a multicenter, exploratory, prospective study to identify angiogenesis and immune-related biomarkers predictive of progression free survival in patients with metastatic or advanced RCC treated by a combination of immunotherapy and antiangiogenic |
| NCT04006405 | Glycosaminoglycans | AURORAX-0087A: Glycosaminoglycan Scores for Surveillance of Recurrence in Leibovich Points ≥ 5 Non-metastatic Clear Cell Renal Cell Carcinoma | Observational | United States; 280 | Recruiting | This is an observational prospective, multicenter, diagnostic test cohort study which aims to predict disease recurrence in non-metastatic ccRCC using glycosaminoglycans scores in blood and urine |
| NCT04712305 | Metabolomics and Proteomics | Urine Metabolomics and Proteomics Profiling to Predict the Responses and Adverse Events of Immuno-Oncology-based Therapy in Patients With Advanced Renal Cell Carcinoma | Observational | Taiwan; 400 | Recruiting | The study aims to identify urinary metabolite and protein markers that can predict anti-tumor efficacy and adverse events in subjects receiving Immuno-Oncology-based therapies for metastatic RCC |
| NCT05112627 | Serum immune markers | Immunophenotyping in Metastatic Renal Cell Carcinoma Patients Receiving Ablative Therapy | Observational | United States; 45 | Recruiting | This study aims to evaluate serum immune markers and peripheral blood mononuclear cell characteristics in patients with metastatic RCC treated with SBRT or PCA, and the impact on their overall distant disease progression |
Conclusion
Currently, liquid biopsy has shown remarkable potential and value for many cancers. In RCC, owing to its advantage of being non-invasive, liquid biopsy can be used for screening in healthy populations or for differential diagnosis of masses of unknown pathological types. Furthermore, for patients with confirmed disease, liquid biopsy can predict survival and progression risk, thus identifying high-risk patients for future research. In addition, liquid biopsy can predict the efficacy of different adjuvant therapies for patients so that personalized treatment plans can be made to practice precision medicine. Additionally, following surgical or adjuvant treatment, liquid biopsy can be used to evaluate the effectiveness of treatment and predict the risk of recurrence or metastasis by continuously monitoring relevant indicators. Owing to its wide range of applications and substantial clinical value, an increasing number of clinical trials are being conducted to find suitable biomarkers and develop more efficient and affordable extraction techniques. Compared to traditional techniques, technological innovations and the maturation of commercial platforms in recent years have improved the isolation efficiency and purity of biomarkers, and many biomarkers have shown their potential. Therefore, despite many unresolved limitations, we believe that liquid biopsy has substantial clinical value and represents a future development direction for cancer diagnosis, prognostic assessment, and treatment monitoring.
Acknowledgements
The material in graphics in this article were supported by Figdraw for free. The manuscript was polished by native speakers of Editage.
Abbreviations
- RCC
Renal cell carcinoma
- CTC
Circulating tumor cells
- cfDNA
Cell-free DNA
- ctDNA
Cell-free tumor DNA
- cfRNA
Cell-free RNA
- ccRCC
Clear cell renal cell carcinoma
- mTOR
Mammalian target of rapamycin
- RTK
Receptor tyrosine kinase
- ICI
Immune checkpoint inhibitor
- EpCAM
Epithelial cell adhesion molecule
- CAIX, CAXII
Carbonic anhydrase IX, XII
- CK
Cytokeratin
- CD
Clusters of differentiation
- FISH
Fluorescence in situ hybridization
- VHL
Von Hippel-Lindau
- GAs
Genomic alterations
- HIF
Hypoxia inducible factor
- RT-qPCR
Real-Time quantitative Polymerase Chain Reaction
- dPCR
Digital PCR
- ddPCR
Droplet digital PCR
- NGS
Next-Generation Sequencing
- cfMeDIP-seq
Cell-free methylated DNA immunoprecipitation and high-throughput sequencing assay
- EVs
Extracellular vesicles
- miRNAs
MicroRNAs
- FH
Ferredoxin hydratase
- SDH
Succinate dehydrogenase
- MS
Mass spectrometer
- LC–MS
Liquid chromatography-MS
- GC–MS
Gas chromatography-MS
- MALDI-MS
Matrix assisted laser desorption/ionization-MS
- UPLC-MS
Ultra-performance LC–MS
- HS–SPME–GC–MS
Headspace solid-phase microextraction coupled with GC–MS
- NMR
Nuclear magnetic resonance
- 2HG
2-Hydroxyglutarate
- ELISA
Enzyme linked immunosorbent assay
- CEA
Carcinoembryonic antigens
- GRP78
78 KDa glucose-regulated protein
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed cell death 1 ligand 1
- KIM-1
Kidney injury molecule 1
- NGAL
Neutropil gelatinase-associated lipocalin
- TME
Tumor microenvironment
- ILVs
Intraluminal vesicles
- MVBs
Multivesicular bodies
- EE
Early endosomes
- BAP1
BRCA1-associated protein 1
- PBRM1
Recombinant polybromo 1
- DMRs
Differentially methylated regions
- ROC
Receiver operating characteristic curve
- AUC
Area under ROC
- MET
Epithelial-mesenchymal transition
- qMSP
Methylation-specific quantitative PCR
- ZEB2
Zinc finger E-box binding homeobox
- BLCA
Bladder cancer
- hPG80
Human Circulating Progastrin 80
- PLIN-2
Perilipin-2
- OS
Overall survival
- PFS
Progression-free survival
- PCF
Proportion of cfDNA fragments
- HR
Hazard ratio
- SAA2
Serum Amyloid A2
- CFB
Complement Factor B
- TKI
Tyrosine kinase inhibitor
- SRMs
Small renal masses
Authors’ contributions
CXN, LSJ, WKF and LMY designed the study. LMY and LL reviewed the information. LMY wrote the manuscript. ZJY and LZY critically reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the 345 Talent Project (Grant No. M0716, Grant No. M0366), the Joint plan of key research and development program of Liaoning Province (Grant No. 2020JH 2/10300137, Grant No. 2020JH 2/10300139), National Natural Science Foundation of China (Grant No. 82072835), Natural Science Foundation of Liaoning Province (Grant No. 2019-MS-360), Shenyang Science and Technology Bureau Plan Projects (Grant No. 20–205-4–076), and Outstanding Scientific Fund of Shengjing Hospital.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have agreed on the contents of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shijie Li, Email: sjli@cmu.edu.cn.
Kefeng Wang, Email: wang.kefeng@hotmail.com.
Xiaonan Chen, Email: chenxn@cmu.edu.cn.
References
- 1.Sung H, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Kovacs G, et al. The Heidelberg classification of renal cell tumours. J Pathol. 1997;183(2):131–133. doi: 10.1002/(SICI)1096-9896(199710)183:2<131::AID-PATH931>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 3.Choueiri TK, Motzer RJ. Systemic Therapy for Metastatic Renal-Cell Carcinoma. N Engl J Med. 2017;376(4):354–366. doi: 10.1056/NEJMra1601333. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh JJ, et al. Renal cell carcinoma. Nat Rev Dis Primers. 2017;3:17009. doi: 10.1038/nrdp.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hahn AW, et al. First-line Treatment of Metastatic Renal Cell Carcinoma: A Systematic Review and Network Meta-analysis. Eur Urol Oncol. 2019;2(6):708–715. doi: 10.1016/j.euo.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Conti A, et al. Progress of molecular targeted therapies for advanced renal cell carcinoma. Biomed Res Int. 2013;2013:419176. doi: 10.1155/2013/419176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ravaud A, Gross-Goupil M. Overcoming resistance to tyrosine kinase inhibitors in renal cell carcinoma. Cancer Treat Rev. 2012;38(8):996–1003. doi: 10.1016/j.ctrv.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Patard JJ, et al. ICUD-EAU International Consultation on Kidney Cancer 2010: treatment of metastatic disease. Eur Urol. 2011;60(4):684–690. doi: 10.1016/j.eururo.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Sharma R, et al. Determinants of resistance to VEGF-TKI and immune checkpoint inhibitors in metastatic renal cell carcinoma. J Exp Clin Cancer Res. 2021;40(1):186. doi: 10.1186/s13046-021-01961-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forshew T, et al. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci Transl Med. 2012;4(136):136ra68. doi: 10.1126/scitranslmed.3003726. [DOI] [PubMed] [Google Scholar]
- 11.Ignatiadis M, Sledge GW, Jeffrey SS. Liquid biopsy enters the clinic - implementation issues and future challenges. Nat Rev Clin Oncol. 2021;18(5):297–312. doi: 10.1038/s41571-020-00457-x. [DOI] [PubMed] [Google Scholar]
- 12.Ye Q, et al. Liquid biopsy in hepatocellular carcinoma: circulating tumor cells and circulating tumor DNA. Mol Cancer. 2019;18(1):114. doi: 10.1186/s12943-019-1043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rolfo C, et al. Liquid Biopsy for Advanced Non-Small Cell Lung Cancer (NSCLC): A Statement Paper from the IASLC. J Thorac Oncol. 2018;13(9):1248–1268. doi: 10.1016/j.jtho.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 14.Zhu JW, Charkhchi P, Akbari MR. Potential clinical utility of liquid biopsies in ovarian cancer. Mol Cancer. 2022;21(1):114. doi: 10.1186/s12943-022-01588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green EA, et al. Clinical Utility of Cell-free and Circulating Tumor DNA in Kidney and Bladder Cancer: A Critical Review of Current Literature. Eur Urol Oncol. 2021;4(6):893–903. doi: 10.1016/j.euo.2021.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Tayoun T, et al. CTC-Derived Models: A Window into the Seeding Capacity of Circulating Tumor Cells (CTCs) Cells. 2019;8(10):1145. doi: 10.3390/cells8101145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabisiewicz A, Grzybowska E. CTC clusters in cancer progression and metastasis. Med Oncol. 2017;34(1):12. doi: 10.1007/s12032-016-0875-0. [DOI] [PubMed] [Google Scholar]
- 18.Vasseur A, et al. Clinical utility of circulating tumor cells: an update. Mol Oncol. 2021;15(6):1647–1666. doi: 10.1002/1878-0261.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alix-Panabières C, Pantel K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021;11(4):858–873. doi: 10.1158/2159-8290.CD-20-1311. [DOI] [PubMed] [Google Scholar]
- 20.Palmela Leitão T, et al. Circulating tumor cell detection methods in renal cell carcinoma: A systematic review. Crit Rev Oncol Hematol. 2021;161:103331. doi: 10.1016/j.critrevonc.2021.103331. [DOI] [PubMed] [Google Scholar]
- 21.Eyvazi S, et al. Antibody Based EpCAM Targeted Therapy of Cancer, Review and Update. Curr Cancer Drug Targets. 2018;18(9):857–868. doi: 10.2174/1568009618666180102102311. [DOI] [PubMed] [Google Scholar]
- 22.Krebs MG, et al. Molecular analysis of circulating tumour cells-biology and biomarkers. Nat Rev Clin Oncol. 2014;11(3):129–144. doi: 10.1038/nrclinonc.2013.253. [DOI] [PubMed] [Google Scholar]
- 23.Riethdorf S, et al. Clinical applications of the Cell Search platform in cancer patients. Adv Drug Deliv Rev. 2018;125:102–121. doi: 10.1016/j.addr.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Bade RM, et al. Development and initial clinical testing of a multiplexed circulating tumor cell assay in patients with clear cell renal cell carcinoma. Mol Oncol. 2021;15(9):2330–2344. doi: 10.1002/1878-0261.12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang ZL, et al. Dynamic changes of different phenotypic and genetic circulating tumor cells as a biomarker for evaluating the prognosis of RCC. Cancer Biol Ther. 2019;20(4):505–512. doi: 10.1080/15384047.2018.1537576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song J, et al. Clinical significance of circulating tumour cells and Ki-67 in renal cell carcinoma. World J Surg Oncol. 2021;19(1):156. doi: 10.1186/s12957-021-02268-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Broncy L, et al. Single-cell genetic analysis validates cytopathological identification of circulating cancer cells in patients with clear cell renal cell carcinoma. Oncotarget. 2018;9(28):20058–20074. doi: 10.18632/oncotarget.25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sperger JM, et al. Integrated Analysis of Multiple Biomarkers from Circulating Tumor Cells Enabled by Exclusion-Based Analyte Isolation. Clin Cancer Res. 2017;23(3):746–756. doi: 10.1158/1078-0432.CCR-16-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laget S, et al. Technical Insights into Highly Sensitive Isolation and Molecular Characterization of Fixed and Live Circulating Tumor Cells for Early Detection of Tumor Invasion. PLoS One. 2017;12(1):e0169427. doi: 10.1371/journal.pone.0169427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bai M, et al. Comparison of two detection systems for circulating tumor cells among patients with renal cell carcinoma. Int Urol Nephrol. 2018;50(10):1801–1809. doi: 10.1007/s11255-018-1954-2. [DOI] [PubMed] [Google Scholar]
- 31.Takagi H, et al. Analysis of the Circulating Tumor Cell Capture Ability of a Slit Filter-Based Method in Comparison to a Selection-Free Method in Multiple Cancer Types. Int J Mol Sci. 2020;21(23):9031. doi: 10.3390/ijms21239031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naoe M, et al. Development of a Highly Sensitive Technique for Capturing Renal Cell Cancer Circulating Tumor Cells. Diagnostics (Basel) 2019;9(3):96. doi: 10.3390/diagnostics9030096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim TH, et al. Detection of circulating tumour cells and their potential use as a biomarker for advanced renal cell carcinoma. Can Urol Assoc J. 2019;13(9):E285–e291. doi: 10.5489/cuaj.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xing T, et al. Candle soot-templated silica nanobiointerface chip for detecting circulating tumour cells from patients with urologic malignancies. RSC Adv. 2018;8(60):34566–34572. doi: 10.1039/C8RA05807E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stroun M, et al. About the possible origin and mechanism of circulating DNA apoptosis and active DNA release. Clin Chim Acta. 2001;313(1–2):139–142. doi: 10.1016/S0009-8981(01)00665-9. [DOI] [PubMed] [Google Scholar]
- 36.Jahr S, et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61(4):1659–1665. [PubMed] [Google Scholar]
- 37.van der Vaart M, Pretorius PJ. The origin of circulating free DNA. Clin Chem. 2007;53(12):2215. doi: 10.1373/clinchem.2007.092734. [DOI] [PubMed] [Google Scholar]
- 38.Mouliere F, et al. Enhanced detection of circulating tumor DNA by fragment size analysis. Sci Transl Med. 2018;10(466):eaat4921. doi: 10.1126/scitranslmed.aat4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Underhill HR, et al. Fragment Length of Circulating Tumor DNA. PLoS Genet. 2016;12(7):e1006162. doi: 10.1371/journal.pgen.1006162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haber DA, Velculescu VE. Blood-based analyses of cancer: circulating tumor cells and circulating tumor DNA. Cancer Discov. 2014;4(6):650–661. doi: 10.1158/2159-8290.CD-13-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto Y, et al. Increased level and fragmentation of plasma circulating cell-free DNA are diagnostic and prognostic markers for renal cell carcinoma. Oncotarget. 2018;9(29):20467–20475. doi: 10.18632/oncotarget.24943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diehl F, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14(9):985–990. doi: 10.1038/nm.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim H, et al. Loss of Von Hippel-Lindau (VHL) Tumor Suppressor Gene Function: VHL-HIF Pathway and Advances in Treatments for Metastatic Renal Cell Carcinoma (RCC) Int J Mol Sci. 2021;22(18):9795. doi: 10.3390/ijms22189795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gossage L, Eisen T, Maher ER. VHL, the story of a tumour suppressor gene. Nat Rev Cancer. 2015;15(1):55–64. doi: 10.1038/nrc3844. [DOI] [PubMed] [Google Scholar]
- 45.Moreno-Manuel A, et al. dPCR application in liquid biopsies: divide and conquer. Expert Rev Mol Diagn. 2021;21(1):3–15. doi: 10.1080/14737159.2021.1860759. [DOI] [PubMed] [Google Scholar]
- 46.Zviran A, et al. Genome-wide cell-free DNA mutational integration enables ultra-sensitive cancer monitoring. Nat Med. 2020;26(7):1114–1124. doi: 10.1038/s41591-020-0915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koh Y, et al. Early dynamics of circulating tumor DNA predict clinical response to immune checkpoint inhibitors in metastatic renal cell carcinoma. Int J Urol. 2022;29(5):462–469. doi: 10.1111/iju.14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sumiyoshi T, et al. Detection of von Hippel-Lindau gene mutation in circulating cell-free DNA for clear cell renal cell carcinoma. Cancer Sci. 2021;112(8):3363–3374. doi: 10.1111/cas.14972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bacon JVW, et al. Plasma Circulating Tumor DNA and Clonal Hematopoiesis in Metastatic Renal Cell Carcinoma. Clin Genitourin Cancer. 2020;18(4):322–331.e2. doi: 10.1016/j.clgc.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto Y, et al. Clinical significance of the mutational landscape and fragmentation of circulating tumor DNA in renal cell carcinoma. Cancer Sci. 2019;110(2):617–628. doi: 10.1111/cas.13906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Del Re M, et al. The amount of DNA combined with TP53 mutations in liquid biopsy is associated with clinical outcome of renal cancer patients treated with immunotherapy and VEGFR-TKIs. J Transl Med. 2022;20(1):371. doi: 10.1186/s12967-022-03557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rizzo A, Ricci AD, Brandi G. PD-L1, TMB, MSI, and Other Predictors of Response to Immune Checkpoint Inhibitors in Biliary Tract Cancer. Cancers (Basel) 2021;13(3):558. doi: 10.3390/cancers13030558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu L, et al. Combination of TMB and CNA Stratifies Prognostic and Predictive Responses to Immunotherapy Across Metastatic Cancer. Clin Cancer Res. 2019;25(24):7413–7423. doi: 10.1158/1078-0432.CCR-19-0558. [DOI] [PubMed] [Google Scholar]
- 54.Schuurbiers M, et al. Biological and technical factors in the assessment of blood-based tumor mutational burden (bTMB) in patients with NSCLC. J Immunother Cancer. 2022;10(2):e004064. doi: 10.1136/jitc-2021-004064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Franses JW, et al. Profile and Predictors of Blood Tumor Mutational Burden in Advanced Hepatocellular Carcinoma. Oncologist. 2022;27(11):e908–e911. doi: 10.1093/oncolo/oyac189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang X, et al. Parallel Analyses of Somatic Mutations in Plasma Circulating Tumor DNA (ctDNA) and Matched Tumor Tissues in Early-Stage Breast Cancer. Clin Cancer Res. 2019;25(21):6546–6553. doi: 10.1158/1078-0432.CCR-18-4055. [DOI] [PubMed] [Google Scholar]
- 57.Wang Z, et al. Assessment of Blood Tumor Mutational Burden as a Potential Biomarker for Immunotherapy in Patients With Non-Small Cell Lung Cancer With Use of a Next-Generation Sequencing Cancer Gene Panel. JAMA Oncol. 2019;5(5):696–702. doi: 10.1001/jamaoncol.2018.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fridland S, et al. Assessing tumor heterogeneity: integrating tissue and circulating tumor DNA (ctDNA) analysis in the era of immuno-oncology - blood TMB is not the same as tissue TMB. J Immunother Cancer. 2021;9(8):e002551. doi: 10.1136/jitc-2021-002551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Friedlaender A, et al. Tissue-Plasma TMB Comparison and Plasma TMB Monitoring in Patients With Metastatic Non-small Cell Lung Cancer Receiving Immune Checkpoint Inhibitors. Front Oncol. 2020;10:142. doi: 10.3389/fonc.2020.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nuzzo PV, et al. Detection of renal cell carcinoma using plasma and urine cell-free DNA methylomes. Nat Med. 2020;26(7):1041–1043. doi: 10.1038/s41591-020-0933-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lasseter K, et al. Plasma cell-free DNA variant analysis compared with methylated DNA analysis in renal cell carcinoma. Genet Med. 2020;22(8):1366–1373. doi: 10.1038/s41436-020-0801-x. [DOI] [PubMed] [Google Scholar]
- 62.El Messaoudi S, et al. Circulating cell free DNA: Preanalytical considerations. Clin Chim Acta. 2013;424:222–230. doi: 10.1016/j.cca.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 63.Siravegna G, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14(9):531–548. doi: 10.1038/nrclinonc.2017.14. [DOI] [PubMed] [Google Scholar]
- 64.Freedman JE, et al. Diverse human extracellular RNAs are widely detected in human plasma. Nat Commun. 2016;7:11106. doi: 10.1038/ncomms11106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dwivedi SKD, et al. Small Non-Coding-RNA in Gynecological Malignancies. Cancers (Basel) 2021;13(5):1085. doi: 10.3390/cancers13051085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Savelyeva AV, et al. Variety of RNAs in Peripheral Blood Cells, Plasma, and Plasma Fractions. Biomed Res Int. 2017;2017:7404912. doi: 10.1155/2017/7404912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parker R, Sheth U. P bodies and the control of mRNA translation and degradation. Mol Cell. 2007;25(5):635–646. doi: 10.1016/j.molcel.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 68.Koczera P, et al. The Ribonuclease A Superfamily in Humans: Canonical RNases as the Buttress of Innate Immunity. Int J Mol Sci. 2016;17(8):1278. doi: 10.3390/ijms17081278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lambert D, Draper DE. Effects of osmolytes on RNA secondary and tertiary structure stabilities and RNA-Mg2+ interactions. J Mol Biol. 2007;370(5):993–1005. doi: 10.1016/j.jmb.2007.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arroyo JD, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108(12):5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wagner J, et al. Characterization of levels and cellular transfer of circulating lipoprotein-bound microRNAs. Arterioscler Thromb Vasc Biol. 2013;33(6):1392–1400. doi: 10.1161/ATVBAHA.112.300741. [DOI] [PubMed] [Google Scholar]
- 72.Tsui NB, Ng EK, Lo YM. Stability of endogenous and added RNA in blood specimens, serum, and plasma. Clin Chem. 2002;48(10):1647–1653. doi: 10.1093/clinchem/48.10.1647. [DOI] [PubMed] [Google Scholar]
- 73.Fleischhacker M, Schmidt B. Circulating nucleic acids (CNAs) and cancer–a survey. Biochim Biophys Acta. 2007;1775(1):181–232. doi: 10.1016/j.bbcan.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 74.Groot M, Lee H. Sorting Mechanisms for MicroRNAs into Extracellular Vesicles and Their Associated Diseases. Cells. 2020;9(4):1044. doi: 10.3390/cells9041044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Voogt WS, Tanenbaum ME, Vader P. Illuminating RNA trafficking and functional delivery by extracellular vesicles. Adv Drug Deliv Rev. 2021;174:250–264. doi: 10.1016/j.addr.2021.04.017. [DOI] [PubMed] [Google Scholar]
- 76.Wu X, et al. CircCYP24A1 hampered malignant phenotype of renal cancer carcinoma through modulating CMTM-4 expression via sponging miR-421. Cell Death Dis. 2022;13(2):190. doi: 10.1038/s41419-022-04623-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Quan J, et al. MiR-23a-3p acts as an oncogene and potential prognostic biomarker by targeting PNRC2 in RCC. Biomed Pharmacother. 2019;110:656–666. doi: 10.1016/j.biopha.2018.11.065. [DOI] [PubMed] [Google Scholar]
- 78.Zhou W, et al. miRNA-133b and miRNA-135a induce apoptosis via the JAK2/STAT3 signaling pathway in human renal carcinoma cells. Biomed Pharmacother. 2016;84:722–729. doi: 10.1016/j.biopha.2016.09.074. [DOI] [PubMed] [Google Scholar]
- 79.Outeiro-Pinho G, et al. MicroRNA-30a-5p(me): a novel diagnostic and prognostic biomarker for clear cell renal cell carcinoma in tissue and urine samples. J Exp Clin Cancer Res. 2020;39(1):98. doi: 10.1186/s13046-020-01600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fang Y, Fullwood MJ. Roles, Functions, and Mechanisms of Long Non-coding RNAs in Cancer. Genomics Proteomics Bioinformatics. 2016;14(1):42–54. doi: 10.1016/j.gpb.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qi X, et al. ceRNA in cancer: possible functions and clinical implications. J Med Genet. 2015;52(10):710–718. doi: 10.1136/jmedgenet-2015-103334. [DOI] [PubMed] [Google Scholar]
- 82.Guo R, et al. LncRNA RCAT1 promotes tumor progression and metastasis via miR-214-5p/E2F2 axis in renal cell carcinoma. Cell Death Dis. 2021;12(7):689. doi: 10.1038/s41419-021-03955-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bohosova J, Kubickova A, Slaby O. lncRNA PVT1 in the Pathogenesis and Clinical Management of Renal Cell Carcinoma. Biomolecules. 2021;11(5):664. doi: 10.3390/biom11050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gibb EA, et al. Human cancer long non-coding RNA transcriptomes. PLoS One. 2011;6(10):e25915. doi: 10.1371/journal.pone.0025915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mugoni V, et al. Circulating RNAs in prostate cancer patients. Cancer Lett. 2022;524:57–69. doi: 10.1016/j.canlet.2021.10.011. [DOI] [PubMed] [Google Scholar]
- 86.Wang Y, et al. Circular RNAs in renal cell carcinoma: implications for tumorigenesis, diagnosis, and therapy. Mol Cancer. 2020;19(1):149. doi: 10.1186/s12943-020-01266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu H, et al. circPTCH1 promotes invasion and metastasis in renal cell carcinoma via regulating miR-485-5p/MMP14 axis. Theranostics. 2020;10(23):10791–10807. doi: 10.7150/thno.47239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen Q, et al. CircRNA cRAPGEF5 inhibits the growth and metastasis of renal cell carcinoma via the miR-27a-3p/TXNIP pathway. Cancer Lett. 2020;469:68–77. doi: 10.1016/j.canlet.2019.10.017. [DOI] [PubMed] [Google Scholar]
- 89.Liu Y, et al. The emerging role of the piRNA/piwi complex in cancer. Mol Cancer. 2019;18(1):123. doi: 10.1186/s12943-019-1052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Y, et al. Piwi-Interacting RNAs (piRNAs) Are Dysregulated in Renal Cell Carcinoma and Associated with Tumor Metastasis and Cancer-Specific Survival. Mol Med. 2015;21(1):381–388. doi: 10.2119/molmed.2014.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao C, et al. Mitochondrial PIWI-interacting RNAs are novel biomarkers for clear cell renal cell carcinoma. World J Urol. 2019;37(8):1639–1647. doi: 10.1007/s00345-018-2575-1. [DOI] [PubMed] [Google Scholar]
- 92.Iliev R, et al. Expression Levels of PIWI-interacting RNA, piR-823, Are Deregulated in Tumor Tissue, Blood Serum and Urine of Patients with Renal Cell Carcinoma. Anticancer Res. 2016;36(12):6419–6423. doi: 10.21873/anticanres.11239. [DOI] [PubMed] [Google Scholar]
- 93.Martínez-Reyes I, Chandel NS. Cancer metabolism: looking forward. Nat Rev Cancer. 2021;21(10):669–680. doi: 10.1038/s41568-021-00378-6. [DOI] [PubMed] [Google Scholar]
- 94.Wang Y, et al. Coordinative metabolism of glutamine carbon and nitrogen in proliferating cancer cells under hypoxia. Nat Commun. 2019;10(1):201. doi: 10.1038/s41467-018-08033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang YP, Lei QY. Metabolite sensing and signaling in cell metabolism. Signal Transduct Target Ther. 2018;3:30. doi: 10.1038/s41392-018-0024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cork GK, Thompson J, Slawson C. Real Talk: The Inter-play Between the mTOR, AMPK, and Hexosamine Biosynthetic Pathways in Cell Signaling. Front Endocrinol (Lausanne) 2018;9:522. doi: 10.3389/fendo.2018.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Isaacs JS, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8(2):143–153. doi: 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 98.Tong WH, et al. The glycolytic shift in fumarate-hydratase-deficient kidney cancer lowers AMPK levels, increases anabolic propensities and lowers cellular iron levels. Cancer Cell. 2011;20(3):315–327. doi: 10.1016/j.ccr.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dong J, et al. Xp11.2 Translocation Renal Cell Carcinoma: Clinical Characteristics and Potential Prognostic Predictors. Dis Markers. 2021;2021:5647933. doi: 10.1155/2021/5647933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morozumi K, et al. Predictive model for recurrence of renal cell carcinoma by comparing pre- and postoperative urinary metabolite concentrations. Cancer Sci. 2022;113(1):182–194. doi: 10.1111/cas.15180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pinto J, et al. Urinary Volatilomics Unveils a Candidate Biomarker Panel for Noninvasive Detection of Clear Cell Renal Cell Carcinoma. J Proteome Res. 2021;20(6):3068–3077. doi: 10.1021/acs.jproteome.0c00936. [DOI] [PubMed] [Google Scholar]
- 102.Murdocca M, et al. Urine LOX-1 and Volatilome as Promising Tools towards the Early Detection of Renal Cancer. Cancers (Basel) 2021;13(16):4213. doi: 10.3390/cancers13164213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arendowski A, et al. Screening of Urinary Renal Cancer Metabolic Biomarkers with Gold Nanoparticles-assisted Laser Desorption/Ionization Mass Spectrometry. Anal Sci. 2020;36(12):1521–1527. doi: 10.2116/analsci.20P226. [DOI] [PubMed] [Google Scholar]
- 104.Wang Z, et al. UPLC-MS based urine untargeted metabolomic analyses to differentiate bladder cancer from renal cell carcinoma. BMC Cancer. 2019;19(1):1195. doi: 10.1186/s12885-019-6354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bifarin OO, et al. Urine-Based Metabolomics and Machine Learning Reveals Metabolites Associated with Renal Cell Carcinoma Stage. Cancers (Basel) 2021;13(24):6253. doi: 10.3390/cancers13246253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shim EH, et al. L-2-Hydroxyglutarate: an epigenetic modifier and putative oncometabolite in renal cancer. Cancer Discov. 2014;4(11):1290–1298. doi: 10.1158/2159-8290.CD-13-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pejcic M, Stojnev S, Stefanovic V. Urinary proteomics–a tool for biomarker discovery. Ren Fail. 2010;32(2):259–268. doi: 10.3109/08860221003599759. [DOI] [PubMed] [Google Scholar]
- 108.Landegren U, Hammond M. Cancer diagnostics based on plasma protein biomarkers: hard times but great expectations. Mol Oncol. 2021;15(6):1715–1726. doi: 10.1002/1878-0261.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54(4):795–809. doi: 10.1016/j.jhep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pauken KE, et al. Emerging concepts in PD-1 checkpoint biology. Semin Immunol. 2021;52:101480. doi: 10.1016/j.smim.2021.101480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yi M, et al. Regulation of PD-L1 expression in the tumor microenvironment. J Hematol Oncol. 2021;14(1):10. doi: 10.1186/s13045-020-01027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang T, Zhang KH. New Blood Biomarkers for the Diagnosis of AFP-Negative Hepatocellular Carcinoma. Front Oncol. 2020;10:1316. doi: 10.3389/fonc.2020.01316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lakemeyer L, et al. Diagnostic and Prognostic Value of CEA and CA19–9 in Colorectal Cancer. Diseases. 2021;9(1):21. doi: 10.3390/diseases9010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee JC, et al. Kidney injury molecule-1 inhibits metastasis of renal cell carcinoma. Sci Rep. 2021;11(1):11840. doi: 10.1038/s41598-021-90919-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Karmakova T, et al. Kidney Injury Molecule 1 (KIM-1): a Multifunctional Glycoprotein and Biological Marker (Review) Sovrem Tekhnologii Med. 2021;13(3):64–78. doi: 10.17691/stm2021.13.3.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ghadrdan E, et al. Evaluation of urinary neutrophil gelatinase-associated lipocalin and urinary kidney injury molecule-1 as biomarkers of renal function in cancer patients treated with cisplatin. J Oncol Pharm Pract. 2020;26(7):1643–1649. doi: 10.1177/1078155220901756. [DOI] [PubMed] [Google Scholar]
- 117.Gudbrandsdottir G, et al. Serum levels of the IL-6 family of cytokines predict prognosis in renal cell carcinoma (RCC) Cancer Immunol Immunother. 2021;70(1):19–30. doi: 10.1007/s00262-020-02655-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yuen KC, et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat Med. 2020;26(5):693–698. doi: 10.1038/s41591-020-0860-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tvedt THA, et al. Interleukin-6 in Allogeneic Stem Cell Transplantation: Its Possible Importance for Immunoregulation and As a Therapeutic Target. Front Immunol. 2017;8:667. doi: 10.3389/fimmu.2017.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26(1):54–74. doi: 10.1016/j.smim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 121.David JM, et al. The IL-8/IL-8R Axis: A Double Agent in Tumor Immune Resistance. Vaccines (Basel) 2016;4(3):22. doi: 10.3390/vaccines4030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Armstrong AJ, et al. Angiokines Associated with Targeted Therapy Outcomes in Patients with Non-Clear Cell Renal Cell Carcinoma. Clin Cancer Res. 2021;27(12):3317–3328. doi: 10.1158/1078-0432.CCR-20-4504. [DOI] [PubMed] [Google Scholar]
- 123.Lequin RM. Enzyme immunoassay (EIA)/enzyme-linked immunosorbent assay (ELISA) Clin Chem. 2005;51(12):2415–2418. doi: 10.1373/clinchem.2005.051532. [DOI] [PubMed] [Google Scholar]
- 124.Cohen L, Walt DR. Highly Sensitive and Multiplexed Protein Measurements. Chem Rev. 2019;119(1):293–321. doi: 10.1021/acs.chemrev.8b00257. [DOI] [PubMed] [Google Scholar]
- 125.Houser B. Bio-Rad’s Bio-Plex® suspension array system, xMAP technology overview. Arch Physiol Biochem. 2012;118(4):192–6. [DOI] [PMC free article] [PubMed]
- 126.Hu R, Wang J. A rapid, multiplexed new technology xMAP liquid chip for detection and identification of pathogens. Wei Sheng Yan Jiu. 2007;36(6):759–762. [PubMed] [Google Scholar]
- 127.Grenier FC, et al. Evaluation of the ARCHITECT urine NGAL assay: assay performance, specimen handling requirements and biological variability. Clin Biochem. 2010;43(6):615–620. doi: 10.1016/j.clinbiochem.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 128.Hu R, et al. Bioplasmonic paper-based assay for perilipin-2 non-invasively detects renal cancer. Kidney Int. 2019;96(6):1417–1421. doi: 10.1016/j.kint.2019.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 130.Jeppesen DK, et al. Reassessment of Exosome Composition. Cell. 2019;177(2):428–445.e18. doi: 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bruschi M, et al. The human urinary exosome as a potential metabolic effector cargo. Expert Rev Proteomics. 2015;12(4):425–432. doi: 10.1586/14789450.2015.1055324. [DOI] [PubMed] [Google Scholar]
- 132.Nik Mohamed Kamal NNS, et al. Plasma- and Saliva Exosome Profile Reveals a Distinct MicroRNA Signature in Chronic Periodontitis. Front Physiol. 2020;11:587381. doi: 10.3389/fphys.2020.587381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10(9):597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hsu C, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189(2):223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Men Y, et al. Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nat Commun. 2019;10(1):4136. doi: 10.1038/s41467-019-11534-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Huang X, et al. Exosomal Circsafb2 Reshaping Tumor Environment to Promote Renal Cell Carcinoma Progression by Mediating M2 Macrophage Polarization. Front Oncol. 2022;12:808888. doi: 10.3389/fonc.2022.808888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hu G, et al. Hypoxia-induced lncHILAR promotes renal cancer metastasis via ceRNA for the miR-613/206/ 1-1-3p/Jagged-1/Notch/CXCR4 signaling pathway. Mol Ther. 2021;29(10):2979–2994. doi: 10.1016/j.ymthe.2021.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fiori ME, et al. Cancer-associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Mol Cancer. 2019;18(1):70. doi: 10.1186/s12943-019-0994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Borrelli C, et al. Drug-Induced Senescent Multiple Myeloma Cells Elicit NK Cell Proliferation by Direct or Exosome-Mediated IL15 Trans-Presentation. Cancer Immunol Res. 2018;6(7):860–869. doi: 10.1158/2326-6066.CIR-17-0604. [DOI] [PubMed] [Google Scholar]
- 140.Gurung S, et al. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal. 2021;19(1):47. doi: 10.1186/s12964-021-00730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Alenquer M, Amorim MJ. Exosome Biogenesis, Regulation, and Function in Viral Infection. Viruses. 2015;7(9):5066–5083. doi: 10.3390/v7092862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lone SN, et al. Liquid biopsy: a step closer to transform diagnosis, prognosis and future of cancer treatments. Mol Cancer. 2022;21(1):79. doi: 10.1186/s12943-022-01543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478)::eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Alimirzaie S, Bagherzadeh M, Akbari MR. Liquid biopsy in breast cancer: A comprehensive review. Clin Genet. 2019;95(6):643–660. doi: 10.1111/cge.13514. [DOI] [PubMed] [Google Scholar]
- 145.Shankar GM, et al. Liquid biopsy for brain tumors. Expert Rev Mol Diagn. 2017;17(10):943–947. doi: 10.1080/14737159.2017.1374854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xiao Y, et al. The potential of exosomes derived from colorectal cancer as a biomarker. Clin Chim Acta. 2019;490:186–193. doi: 10.1016/j.cca.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 147.Konoshenko MY, et al. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. Biomed Res Int. 2018;2018:8545347. doi: 10.1155/2018/8545347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Langevin SM, et al. Balancing yield, purity and practicality: a modified differential ultracentrifugation protocol for efficient isolation of small extracellular vesicles from human serum. RNA Biol. 2019;16(1):5–12. doi: 10.1080/15476286.2018.1564465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Paolini L, et al. Residual matrix from different separation techniques impacts exosome biological activity. Sci Rep. 2016;6:23550. doi: 10.1038/srep23550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lim YJ, Lee SJ. Are exosomes the vehicle for protein aggregate propagation in neurodegenerative diseases? Acta Neuropathol Commun. 2017;5(1):64. doi: 10.1186/s40478-017-0467-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Van Deun, J., et al., The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J Extracell Vesicles, 2014. 3. [DOI] [PMC free article] [PubMed]
- 152.Rupp AK, et al. Loss of EpCAM expression in breast cancer derived serum exosomes: role of proteolytic cleavage. Gynecol Oncol. 2011;122(2):437–446. doi: 10.1016/j.ygyno.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 153.Zhang W, et al. MicroRNAs in Serum Exosomes as Potential Biomarkers in Clear-cell Renal Cell Carcinoma. Eur Urol Focus. 2018;4(3):412–419. doi: 10.1016/j.euf.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 154.Veerman RE, et al. Molecular evaluation of five different isolation methods for extracellular vesicles reveals different clinical applicability and subcellular origin. J Extracell Vesicles. 2021;10(9):e12128. doi: 10.1002/jev2.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Stranska R, et al. Comparison of membrane affinity-based method with size-exclusion chromatography for isolation of exosome-like vesicles from human plasma. J Transl Med. 2018;16(1):1. doi: 10.1186/s12967-017-1374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Enderle D, et al. Characterization of RNA from Exosomes and Other Extracellular Vesicles Isolated by a Novel Spin Column-Based Method. PLoS One. 2015;10(8):e0136133. doi: 10.1371/journal.pone.0136133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Macías M, et al. Comparison of six commercial serum exosome isolation methods suitable for clinical laboratories. Effect in cytokine analysis. Clin Chem Lab Med. 2019;57(10):1539–1545. doi: 10.1515/cclm-2018-1297. [DOI] [PubMed] [Google Scholar]
- 158.Tang YT, et al. Comparison of isolation methods of exosomes and exosomal RNA from cell culture medium and serum. Int J Mol Med. 2017;40(3):834–844. doi: 10.3892/ijmm.2017.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Helwa I, et al. A Comparative Study of Serum Exosome Isolation Using Differential Ultracentrifugation and Three Commercial Reagents. PLoS ONE. 2017;12(1):e0170628. doi: 10.1371/journal.pone.0170628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Januszewicz W, Fitzgerald RC. Early detection and therapeutics. Mol Oncol. 2019;13(3):599–613. doi: 10.1002/1878-0261.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sequeira JP, et al. LiKidMiRs: A ddPCR-Based Panel of 4 Circulating miRNAs for Detection of Renal Cell Carcinoma. Cancers (Basel) 2022;14(4):858. doi: 10.3390/cancers14040858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen X, et al. Identification of a four-microRNA panel in serum for screening renal cell carcinoma. Pathol Res Pract. 2021;227:153625. doi: 10.1016/j.prp.2021.153625. [DOI] [PubMed] [Google Scholar]
- 163.Huang G, et al. A Three-microRNA Panel in Serum: Serving as a Potential Diagnostic Biomarker for Renal Cell Carcinoma. Pathol Oncol Res. 2020;26(4):2425–2434. doi: 10.1007/s12253-020-00842-y. [DOI] [PubMed] [Google Scholar]
- 164.Huang G, et al. Combination of tumor suppressor miR-20b-5p, miR-30a-5p, and miR-196a-5p as a serum diagnostic panel for renal cell carcinoma. Pathol Res Pract. 2020;216(11):153152. doi: 10.1016/j.prp.2020.153152. [DOI] [PubMed] [Google Scholar]
- 165.Heinemann FG, et al. Serum miR-122-5p and miR-206 expression: non-invasive prognostic biomarkers for renal cell carcinoma. Clin Epigenetics. 2018;10:11. doi: 10.1186/s13148-018-0444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tang J, et al. miR-21-5p/SMAD7 axis promotes the progress of lung cancer. Thorac Cancer. 2021;12(17):2307–2313. doi: 10.1111/1759-7714.14060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li X, et al. MiR-21-5p in Macrophage-Derived Exosomes Targets Smad7 to Promote Epithelial Mesenchymal Transition of Airway Epithelial Cells. J Asthma Allergy. 2021;14:513–524. doi: 10.2147/JAA.S307165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li Q, et al. Exosomal miR-21-5p derived from gastric cancer promotes peritoneal metastasis via mesothelial-to-mesenchymal transition. Cell Death Dis. 2018;9(9):854. doi: 10.1038/s41419-018-0928-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mytsyk Y, et al. MicroRNA-15a expression measured in urine samples as a potential biomarker of renal cell carcinoma. Int Urol Nephrol. 2018;50(5):851–859. doi: 10.1007/s11255-018-1841-x. [DOI] [PubMed] [Google Scholar]
- 170.Di Meo A, et al. Prognostic urinary miRNAs for the assessment of small renal masses. Clin Biochem. 2020;75:15–22. doi: 10.1016/j.clinbiochem.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 171.Wang C, et al. MicroRNA-30a-5p Inhibits the Growth of Renal Cell Carcinoma by Modulating GRP78 Expression. Cell Physiol Biochem. 2017;43(6):2405–2419. doi: 10.1159/000484394. [DOI] [PubMed] [Google Scholar]
- 172.Chen Z, et al. The putative tumor suppressor microRNA-30a-5p modulates clear cell renal cell carcinoma aggressiveness through repression of ZEB2. Cell Death Dis. 2017;8(6):e2859. doi: 10.1038/cddis.2017.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xie J, et al. Serum long non-coding RNA LINC00887 as a potential biomarker for diagnosis of renal cell carcinoma. FEBS Open Bio. 2020;10(9):1802–1809. doi: 10.1002/2211-5463.12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.He ZH, et al. Long noncoding RNA GIHCG is a potential diagnostic and prognostic biomarker and therapeutic target for renal cell carcinoma. Eur Rev Med Pharmacol Sci. 2018;22(1):46–54. doi: 10.26355/eurrev_201801_14099. [DOI] [PubMed] [Google Scholar]
- 175.Peter MR, et al. Investigating Urinary Circular RNA Biomarkers for Improved Detection of Renal Cell Carcinoma. Front Oncol. 2021;11:814228. doi: 10.3389/fonc.2021.814228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang M, et al. A pilot investigation of a urinary metabolic biomarker discovery in renal cell carcinoma. Int Urol Nephrol. 2020;52(3):437–446. doi: 10.1007/s11255-019-02332-w. [DOI] [PubMed] [Google Scholar]
- 177.Sato T, et al. Accurate quantification of urinary metabolites for predictive models manifest clinicopathology of renal cell carcinoma. Cancer Sci. 2020;111(7):2570–2578. doi: 10.1111/cas.14440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Liu X, et al. Urine Metabolomics for Renal Cell Carcinoma (RCC) Prediction: Tryptophan Metabolism as an Important Pathway in RCC. Front Oncol. 2019;9:663. doi: 10.3389/fonc.2019.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Di Meo A, et al. Searching for prognostic biomarkers for small renal masses in the urinary proteome. Int J Cancer. 2020;146(8):2315–2325. doi: 10.1002/ijc.32650. [DOI] [PubMed] [Google Scholar]
- 180.Kumar M, et al. Glucose- regulated protein 78 (GRP78) in renal cell carcinoma: A novel biomarker for predicting tumor behavior. Heliyon. 2021;7(6):e07300. doi: 10.1016/j.heliyon.2021.e07300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kohli M, et al. Prognostic Value of Plasma hPG(80) (Circulating Progastrin) in Metastatic Renal Cell Carcinoma. Cancers (Basel) 2021;13(3):375. doi: 10.3390/cancers13030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Xiao CT, et al. MicroRNA Derived from Circulating Exosomes as Noninvasive Biomarkers for Diagnosing Renal Cell Carcinoma. Onco Targets Ther. 2020;13:10765–10774. doi: 10.2147/OTT.S271606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Song S, et al. Urinary exosome miR-30c-5p as a biomarker of clear cell renal cell carcinoma that inhibits progression by targeting HSPA5. J Cell Mol Med. 2019;23(10):6755–6765. doi: 10.1111/jcmm.14553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Arance E, et al. Determination of Exosome Mitochondrial DNA as a Biomarker of Renal Cancer Aggressiveness. Cancers (Basel) 2021;14(1):199. doi: 10.3390/cancers14010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Basso U, et al. Prognostic Role of Circulating Tumor Cells in Metastatic Renal Cell Carcinoma: A Large, Multicenter. Prospective Trial Oncologist. 2021;26(9):740–750. doi: 10.1002/onco.13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ben Khadhra H, et al. ARCHITECT® urine-neutrophil gelatinase-associated lipocalin (u-NGAL) assay as new prognostic marker for clear cell Renal Cell Carcinoma (ccRCC) (preliminary results) Int Urol Nephrol. 2021;53(1):59–67. doi: 10.1007/s11255-020-02604-w. [DOI] [PubMed] [Google Scholar]
- 187.Cooley LS, et al. Experimental and computational modeling for signature and biomarker discovery of renal cell carcinoma progression. Mol Cancer. 2021;20(1):136. doi: 10.1186/s12943-021-01416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Incorvaia L, et al. Baseline plasma levels of soluble PD-1, PD-L1, and BTN3A1 predict response to nivolumab treatment in patients with metastatic renal cell carcinoma: a step toward a biomarker for therapeutic decisions. Oncoimmunology. 2020;9(1):1832348. doi: 10.1080/2162402X.2020.1832348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Larroquette M, et al. Adjuvant therapy in renal cell carcinoma: Current knowledges and future perspectives. Cancer Treat Rev. 2021;97:102207. doi: 10.1016/j.ctrv.2021.102207. [DOI] [PubMed] [Google Scholar]
- 190.Haga N, et al. Perioperative Detection of Circulating Tumor Cells in Radical or Partial Nephrectomy for Renal Cell Carcinoma. Ann Surg Oncol. 2020;27(4):1272–1281. doi: 10.1245/s10434-019-08127-8. [DOI] [PubMed] [Google Scholar]
- 191.Salinas-Sánchez AS, et al. Clinical value of perioperative levels of DNA and mRNA in plasma of patients with renal cell carcinoma. Transl Oncol. 2021;14(2):100999. doi: 10.1016/j.tranon.2020.100999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xu W, et al. Plasma KIM-1 Is Associated with Recurrence Risk after Nephrectomy for Localized Renal Cell Carcinoma: A Trial of the ECOG-ACRIN Research Group (E2805) Clin Cancer Res. 2021;27(12):3397–3403. doi: 10.1158/1078-0432.CCR-21-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Esteban E, et al. Circulating Levels of the Interferon-γ-Regulated Chemokines CXCL10/CXCL11, IL-6 and HGF Predict Outcome in Metastatic Renal Cell Carcinoma Patients Treated with Antiangiogenic Therapy. Cancers (Basel) 2021;13(11):2849. doi: 10.3390/cancers13112849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Powles T, et al. Outcomes based on plasma biomarkers in METEOR, a randomized phase 3 trial of cabozantinib vs everolimus in advanced renal cell carcinoma. BMC Cancer. 2021;21(1):904. doi: 10.1186/s12885-021-08630-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Martini JF, et al. Angiogenic and Immune-Related Biomarkers and Outcomes Following Axitinib/Pembrolizumab Treatment in Patients with Advanced Renal Cell Carcinoma. Clin Cancer Res. 2020;26(21):5598–5608. doi: 10.1158/1078-0432.CCR-20-1408. [DOI] [PubMed] [Google Scholar]
- 196.Chehrazi-Raffle A, et al. Circulating cytokines associated with clinical response to systemic therapy in metastatic renal cell carcinoma. J Immunother Cancer. 2021;9(3):e002009. doi: 10.1136/jitc-2020-002009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Vargová D, et al. Cytokines in Renal Cell Carcinoma: A Step Towards Earlier Detection and Targeted Therapy. Adv Exp Med Biol. 2022;1374:63–72. doi: 10.1007/5584_2021_700. [DOI] [PubMed] [Google Scholar]
- 198.Zaporozhchenko IA, et al. The potential of circulating cell-free RNA as a cancer biomarker: challenges and opportunities. Expert Rev Mol Diagn. 2018;18(2):133–145. doi: 10.1080/14737159.2018.1425143. [DOI] [PubMed] [Google Scholar]
- 199.Oto J, et al. Urinary microRNAs: Looking for a New Tool in Diagnosis, Prognosis, and Monitoring of Renal Cancer. Curr Urol Rep. 2020;21(2):11. doi: 10.1007/s11934-020-0962-9. [DOI] [PubMed] [Google Scholar]
- 200.Xu J, et al. Using single-cell sequencing technology to detect circulating tumor cells in solid tumors. Mol Cancer. 2021;20(1):104. doi: 10.1186/s12943-021-01392-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.