Abstract
Background
In the field of neurorehabilitation, robot-assisted therapy (RAT) and virtual reality (VR) have so far shown promising evidence on multiple motor and functional outcomes. The related effectiveness on patients’ health-related quality of life (HRQoL) has been investigated across neurological populations but still remains unclear. The present study aimed to systematically review the studies investigating the effects of RAT alone and with VR on HRQoL in patients with different neurological diseases.
Methods
A systematic review of the studies evaluating the impact of RAT alone and combined with VR on HRQoL in patients affected by neurological diseases (i.e., stroke, multiple sclerosis, spinal cord injury, Parkinson’s Disease) was conducted according to PRISMA guidelines. Electronic searches of PubMed, Web of Science, Cochrane Library, CINAHL, Embase, and PsychINFO (2000–2022) were performed. Risk of bias was evaluated through the National Institute of Health Quality Assessment Tool. Descriptive data regarding the study design, participants, intervention, rehabilitation outcomes, robotic device typology, HRQoL measures, non-motor factors concurrently investigated, and main results were extracted and meta-synthetized.
Results
The searches identified 3025 studies, of which 70 met the inclusion criteria. An overall heterogeneous configuration was found regarding the study design adopted, intervention procedures and technological devices implemented, rehabilitation outcomes (i.e., related to both upper and lower limb impairment), HRQoL measures administered, and main evidence. Most of the studies reported significant effects of both RAT and RAT plus VR on patients HRQoL, whether they adopted generic or disease-specific HRQoL measures. Significant post-intervention within-group changes were mainly found across neurological populations, while fewer studies reported significant between-group comparisons, and then, mostly in patients with stroke. Longitudinal investigations were also observed (up to 36 months), but significant longitudinal effects were exclusively found in patients with stroke or multiple sclerosis. Finally, concurrent evaluations on non-motor outcomes beside HRQoL included cognitive (i.e., memory, attention, executive functions) and psychological (i.e., mood, satisfaction with the treatment, device usability, fear of falling, motivation, self-efficacy, coping, and well-being) variables.
Conclusions
Despite the heterogeneity observed among the studies included, promising evidence was found on the effectiveness of RAT and RAT plus VR on HRQoL. However, further targeted short- and long-term investigations, are strongly recommended for specific HRQoL subcomponents and neurological populations, through the adoption of defined intervention procedures and disease-specific assessment methodology.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12955-023-02097-y.
Keywords: Health-related quality of life, Neurorehabilitation, Robot-assisted therapy, Virtual reality, Systematic review
Background
Neurological diseases result in a broad spectrum of motor, functional, and cognitive impairments. Consequently, they represent the leading cause of disability and the second leading cause of death worldwide [1]. Contextually to populations growing and ageing, the burden of neurological disorders continues to increase globally. Therefore, urgent solutions from interdisciplinary rehabilitation, innovative approaches and technology-enabled smart healthcare strategies are needed [2]. Notably, in recent decades, the field of neurorehabilitation has shown a growing interest in high technologies, like robotics and virtual reality (VR), due to their potential and multipurpose application to patients’ recovery pathways [3, 4].
Robot-assisted therapy (RAT), for example, has been extensively used in accordance with the principles of motor relearning and neuroplasticity with the purpose of better maximizing afferent input from peripheral joints and providing task-specific stimulation to the central nervous system [3]. In targeting both upper and lower limb impairment, RAT has so far shown several advantages, including the implementation of repetitive, intensive, and task-oriented rehabilitation exercises through a smaller workforce, optimized and customized treatment, and real-time quantitative assessment and monitoring of motor disability [5]. For this purpose, different robotic devices have been used so far (i.e., exoskeletons, end-effectors, soft-robots) [6], reporting promising evidence on diverse rehabilitation outcomes related to both upper (e.g., arm range of motion, hand grip and strength, dexterity) and lower (e.g., gait, balance, mobility) limb impairment. Notably, prior systematic reviews and meta-analyses have reported informative effects in different acute and chronic conditions, including stroke [7, 8], traumatic brain injury (TBI) [9], spinal cord injury (SCI) [10], multiple sclerosis (MS) [11], and Parkinson’s Disease (PD) [12], corroborating the added value of integrating robotic technologies in standard rehabilitation programs across clinical populations.
Although RAT has been shown to enable effective training, deeper investigation to shed light on its broader potential is still needed. For instance, further studies have highlighted the effects of robotic devices when combined with additional technological systems, like VR [13]. VR is defined as a system based on computer-simulated 3D environments (i.e., virtual environments—VEs) that allow the user to interact with virtual objects by the integration of visual, auditory, and haptic feedback [14]. It is acknowledged that three main factors characterize VR, namely immersion, sense of presence, and interactivity. Immersion refers to the degree to which the VE can provide multisensory stimuli originating from a high degree of matching between the cues generated by the systems and the user's actions. Therefore, immersion in and interactivity with VEs affects users' perception, ultimately determining their sense of presence. VR systems can be distinguished into fully-immersive, semi-immersive, and non-immersive devices, depending on the extent to which the interaction with the VE blocks out user’s real-world perception. Examples of fully-immersive VR devices are tools like head-mounted displays (HMD) and cave automatic virtual environment (CAVE). Among the semi-immersive devices, large monitors or projectors provide the users moderate immersion, while non-immersive tools include simpler devices such as PCs or tablets. Through the implementation of diverse VEs at different levels of immersion VR has so far offered multimodal stimulation and multisensory feedback during training. This has not only provided patients with more engaging therapy sessions, contributing to enduring practice and strengthening performance awareness, but it has also enabled deeper stimulation, ultimately inducing cortical and subcortical synaptic-level changes that are essential for motor relearning [15].
Nevertheless, it must be acknowledged that, although robotic and VR devices have so far shared high technological impact, they profoundly differ from each other when considering technical and interactivity issues. Therefore, the applicability of these technological devices and their efficacy still need to be better understood, especially when they are implemented independently or in combination and when they are used with specific neurological populations.
Overall, it must be noted that the introduction of RAT alone or in combination with VR has mainly provided more robust conclusions when taking into account outcomes like motor improvement and functional status change [5, 16]. However, the broader effectiveness of this innovative recovery procedure, including its long-term impact, still needs to be more deeply investigated and confirmed. Health-related quality of life (HRQoL) has so far been considered as an indicator of therapy effectiveness, since it facilitates observing patients’ perceived improvement in terms of their physical and mental state, especially when taking into consideration patients’ real-life environments and health domains. Nevertheless, prior studies have predominantly explored HRQoL as an additional outcome, with the main objective to extend the investigation of RAT efficacy to the secondary effects resulting from motor improvement. Similarly, concurrent investigations have been carried out to include cognitive and psychosocial outcomes. For example, alongside motor recovery, recent studies have highlighted positive changes on depression and anxiety symptoms, increased perceived well-being, improved coping strategies, and enhanced executive function performance [13, 17–19].
Following this line, the adoption of a multidimensional approach in the field of neurorehabilitation, where multiple patient’s health-related domains and their complementarity are considered, still remains an open challenge [20]. Accordingly, it is of paramount concern to provide clearer and wider overview of RAT effects, in particular targeting a wide range of neurological diseases and disorders and by giving more centrality to patient’s non-motor characteristics is of paramount concern. This would not only help future clinical trials to better orient their investigation on disease-specific outcomes, but it would also better address the effects of RAT beyond motor improvement.
For this purpose, a systematic review was conducted on studies investigating the impact of RAT, alone or combined with VR, on HRQoL in patients affected by neurological diseases. Specifically, the present study aimed at describing the evidence on short- and long-term post-intervention changes regarding HRQoL, including how this outcome was evaluated across neurological populations. In addition, a secondary systematic synthesis was conducted on the non-motor outcomes (e.g., cognitive, psychological) that were investigated concurrently.
Methods
A preliminary check on registered or ongoing similar systematic reviews was conducted on the International Prospective Register of Systematic Review (PROSPERO) platform. No results were provided and, thus, the systematic review protocol was registered (ref. CRD42022367228).
The current study belongs to a broader project called PHTinRehab Study (Perception of High Technology in Rehabilitation: a prospective real-life Study on usability, effectiveness, and health-related quality of life), approved by the Ethics Committee of the Clinical Scientific Institutes Maugeri IRCCS (February 2021, protocol n. 2517CE).
Search strategy and studies selection
The preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed [21]. Preliminary electronic searches of PubMed, Web of Science, Cochrane Library, CINAHL, Embase, and PsychINFO were performed on 11th January 2022 by applying the following search query for all databases: (rehabilitation) AND ((robot*) OR (virtual reality)) AND (quality of life). Even though the present study aimed to retrieve records specifically focused on neuromotor rehabilitation, the general term ‘rehabilitation’ was preferred to ensure full retrieval despite its applicability to different clinical fields (e.g., cardiac-pulmonary rehabilitation, cognitive rehabilitation). Therefore, the identification of the eligible studies was checked throughout the records screening procedure. Furthermore, to optimize studies identification according to the eligibility criteria (Table 1), additional mutual filters (i.e., year of publication timespan, article language) were defined consistently for each electronic database. During the systematic review process, a reference management and bibliography-creating software (EndNote Web) was implemented.
Table 1.
Eligibility criteria
| Inclusion criteria |
| HRQoL evaluation following RAT or RAT plus VR-based neurorehabilitation |
| Adult patients affected by neurological diseases |
| Quantitative and qualitative studies |
| Original research published in English in a peer-reviewed journal |
| Year of publication timespan: 2000–2021 |
| Exclusion criteria |
| Other fields of rehabilitation (e.g., VR-based cognitive rehabilitation) |
| Healthy participants or patients affected by psychiatric disorders |
| Conference papers, proceedings, study protocols, reviews, commentaries, editorials, position papers |
Risk of bias assessment
The National Institutes of Health (NIH) Quality Assessment Tool for Controlled Intervention Studies and for the Before–After (Pre–Post) Studies With No Control Group were used to evaluate the methodological quality of the records included [22]. The first one comprised 14 items assessing the quality of randomization, treatment allocation, blinding, similarity of study groups at baseline, dropout management, adherence to treatment, outcome measures validity, power calculation, hypothesis testing, and intention-to-treat analysis. The second checklist comprised 12 items evaluating the quality of the study question, eligibility criteria, sample representativeness, intervention description, outcome measures validity, blinding, follow-up rate, and statistical analyses. For both checklists, each item was rated as yes, no, or not reported, assigning one point for every ‘yes’ answer. Based on this evaluation, studies were classified according to quality rating: ‘Poor’, ‘Fair’, or ‘Good’ (details on the NIH Quality Assessment questions and the scoring procedures can be found in the supplementary material). The studies were evaluated by three researchers (F.Z., N.F., R.A.) working independently. Any discrepancies were discussed until a consensus was reached. The studies evaluated as having a higher risk of bias were not excluded, but their quality was considered in the interpretation of the results, as appropriate [23].
Data extraction and synthesis
Following eligibility criteria discussion and agreement of all authors, progressive exclusion of the non-eligible records was performed starting from the title, then the abstract, and finally the full-text. Two authors (F.Z. and N.F.) completed the entire review process working independently. Disagreements were solved through periodically planned discussions involving all authors. Each identified article was then screened multiple times until sufficient understanding of the study aim, design, and outcomes was obtained. Of the included studies, a wide range of data was then extracted and reported in a structured table. Due to its extent, it was provided as supplementary material. The table includes: author(s), year of publication, nation where the study was conducted, the nation’s Human Development Index (HDI) [24] and the related rank, professional specialty field of the research group, study design (including if studies were multicentric, pilot, funded, if they included any follow-up evaluations and related duration), patients’ characteristics (i.e., disease, type of hospitalization, sample size, mean age, ethnicity, any prior experience using technological devices), study purpose, rehabilitation outcomes, commercial name of technological devices, robotic device typology (i.e., exoskeleton, end-effector, soft-robot), level of VR immersion (i.e., non-immersive, semi-immersive, fully-immersive), intervention characteristics (i.e., duration in weeks, total number of sessions, session duration in minutes), HRQoL, psychological variables, motor and functional outcome measures used, and main results. Descriptive statistics were calculated on the main characteristics of the studies, whereas the main results were meta-synthetized through narrative analysis. No specific statistical software or tool was implemented for data synthesis.
Results
Flow of studies through the review
From the initial electronic search, a total of 4640 records were retrieved. After duplicates were identified and removed, 3025 studies underwent the screening procedure. A total of 2832 records were excluded by title and abstract screening. The remaining 193 were screened by full-text. Of these, 127 met all the inclusion criteria. Additional records were not identified by further hand searching. Figure 1 shows the flow diagram of the studies selected, including the reasons for exclusion. Most of the excluded records were considered as off-topic (n = 1538). These were not strictly focused on RAT, but belonged to different fields of rehabilitation (e.g., traditional treatment exclusively, cognitive rehabilitation). Others specifically involved non-adult patients (n = 70), whereas other records (n = 1276) were identified as non-original research (e.g., study protocols, proceedings). Of the included studies, a total of 57 reported results on the effects of VR-based rehabilitation exclusively. The remaining provided evidence on RAT alone (n = 52) or in combination with VR (n = 18). The present work specifically describes the results from the implementation of RAT alone or plus VR. Thus, the final number of studies included in this systematic review is 70. The results on the effects of VR will be presented in a separate work.
Fig. 1.
Flow chart of the review process
Risk of bias
Of the studies evaluated with the Controlled Intervention Studies checklist (n = 48), most (n = 28, 58.3%) were classified as having ‘fair’ methodological quality. 33.4% of the studies met most of the criteria and were evaluated as ‘good’ (n = 16), whereas only 4 studies (8.3%) were labelled as ‘poor’. The remaining 22 studies were assessed with the Before-After (Pre-Post) Studies With No Control Group scale. The majority of these (n = 16, 72.7%) had ‘fair’ methodological quality, while four (18.2%) were classified as ‘good’ and the final two (9.1%) as ‘poor’ quality.
Overall, only 11.4% of the included studies (n = 70), were therefore evaluated as having a potentially high risk of bias. Details on the evaluation for each study are reported as supplementary material.
Characteristics of the included studies
A full report of the data extracted from the included studies is presented in a structured synoptic table (Additional file 1: Append ix 1) [13, 25–93].
Design
The main characteristics regarding the design of the included studies are summarised in Tables 2 and 3. Most (65.7%) were published in the last five years and were conducted in European countries (55.7%). Data extracted on the research groups’ specialty fields denote a multidisciplinary contribution to the investigation on the current topic, with the majority of the research groups having their main expertise in rehabilitation medicine (92.6%), neurology (41.4%), engineering (25.7%), neuroscience (14.3%), and psychology (12.9%). Most of the studies (62.9%) were randomised controlled trials (RCTs), followed by pre-post clinical trials without a control group (31.4%) and quasi-experimental studies (5.7%). More than half of the studies included follow-up evaluations (55.7%), were single-centered (75.7%), and were funded (61.4%). In conclusion, almost one study out of three was a pilot (28.6%).
Table 2.
Main characteristics of the included studies (n = 70)
| Year of publication | n (%) | Nationa | n (%b) | Research group specialty field | n (%b) |
|---|---|---|---|---|---|
| 2016–2021 | 46 (65.7) | Europe | 39 (55.7) | Rehabilitation Medicine | 65 (92.6) |
| 2011–2015 | 18 (25.7) | America | 21 (30.0) | Neurology | 29 (41.4) |
| 2000–2010 | 6 (8.6) | Asia | 17 (24.3) | Engineeringc | 18 (25.7) |
| Oceania | 2 (2.9) | Neuroscience | 10 (14.3) | ||
| Occupational Physiatry | 9 (12.9) | ||||
| Psychology | 9 (12.9) | ||||
| Physiology | 6 (8.6) | ||||
| Geriatrics and orthopedics | 5 (7.1) | ||||
| Public Health | 3 (4.3) |
aEurope (i.e., Denmark, Germany, Italy, Netherlands, Norway, Romania, Spain, Sweden, Switzerland, Turkey, United Kingdom), America (i.e., Canada, United States of America), Asia (i.e., China, India, Israel, Japan, South Korea, Taiwan), Oceania (Australia)
bNon-cumulative percentages
cBiomedical, Mechanical, Computer Engineering
Table 3.
Study design characteristics of the included studies (n = 70)
| Study design | n (%) | Follow-up | n (%) | Funding | n (%) | Multicentric | n (%) | Pilot Study | n (%) |
|---|---|---|---|---|---|---|---|---|---|
| RCTs | 44 (62.9) | Yesa | 39 (55.7) | Yes | 43 (61.4) | Yes | 17 (24.3) | Yes | 20 (28.6) |
| Pre-post Clinical Trial (no control group) | 22 (31.4) | No | 31 (42.3) | No | 27 (38.6) | No | 53 (75.7) | No | 50 (71.4) |
| Non-RCT | 4 (5.7) |
aFollow-up range: 1–36 months
Participants
Table 4 shows the main characteristics of participants. The sample size of the included studies ranged from 3 to 224 patients (total of all studies, n = 2956). The 37.1% of the studies included fewer than 30 participants and, according to risk of bias assessment, only 17.2% reported that sample size was sufficiently large to detect results with appropriate statistical power. Most of the participants included were outpatients (44.3%), suffered from stroke (54.3%), MS (21.4%), SCI (15.7%), or PD (15.7%). Finally, only four studies reported participants’ ethnicity [54, 58–60].
Table 4.
Participants’ characteristics in the included studies (n = 70)
| Patients | n (%) | Disease | n (%) |
|---|---|---|---|
| Outpatients | 31 (44.3) | Stroke | 38 (54.3) |
| Inpatients | 15 (21.4) | Multiple Sclerosis | 15 (21.4) |
| Both | 3 (4.3) | Spinal Cord Injury | 11 (15.7) |
| Not defined | 21 (30.0) | Parkinson’s Disease | 3 (4.3) |
| Othera | 3 (4.3) |
aHereditary Spastic Paraplegia, Neuromuscular diseases, Orthopedics
Intervention
Robotic and VR devices typology, including the main characteristics of the interventions are summarized in Table 5. Overall, 74.3% of the studies implemented RAT exclusively, while the remaining 25.7% coupled RAT with VR systems. Of the latter, all used non-immersive VR devices, except for one study that also implemented semi-immersive VE [61]. In particular, the studies on RAT mainly implemented exoskeletal devices (59.6%), and particularly targeted rehabilitation outcomes related to lower limb functioning (71.2%), such as gait, balance, mobility, and muscle strength. The studies investigating the effects of RAT plus VR implemented exoskeletal or end-effector robotic devices (44.4%), and mainly provided rehabilitation exercises to target upper limb outcomes (72.2%), including manual dexterity, reaching and grasping abilities, shoulder pain, and spasticity. Regarding intervention duration, RAT alone lasted longer on average when compared to RAT plus VR in terms of overall therapy duration (in weeks), total number of sessions, and session duration (in minutes).
Table 5.
Main characteristics of the technological devices and of the intervention in RAT (n = 52) and RAT plus VR (n = 18) studies
| RAT | RAT plus VR | Overall | |
|---|---|---|---|
| Robot typology, n (%a) | |||
| Exoskeleton | 31 (59.6) | 8 (44.4) | 39 (55.7) |
| End-effector | 21 (40.4) | 8 (44.4) | 29 (41.4) |
| Soft-robotics | – | 3 (16.6) | 3 (4.3) |
| Targeted extremities, n (%) | |||
| Upper Limbs | 15 (28.8) | 13 (72.2) | 28 (40.0) |
| Lower Limbs | 37 (71.2) | 4 (22.2) | 41 (58.6) |
| Both | – | 1 (5.6) | 1 (1.4) |
| Intervention, mean ± SD (range) | |||
| Overall duration (weeks) | 6.5 ± 3.7 (2–24) | 4.8 ± 1.5 (3–8) | 6.0 ± 3.3 (2–24) |
| n. of sessions | 22.0 ± 10.2 (6–60) | 19.6 ± 8.9 (8–40) | 21.4 ± 9.9 (6–60) |
| Session duration (min) | 60.1 ± 30.9 (20–180) | 55.7 ± 39.7 (30–180) | 58.9 ± 33.4 (20–180) |
aNon-cumulative percentage
HRQoL outcome measures
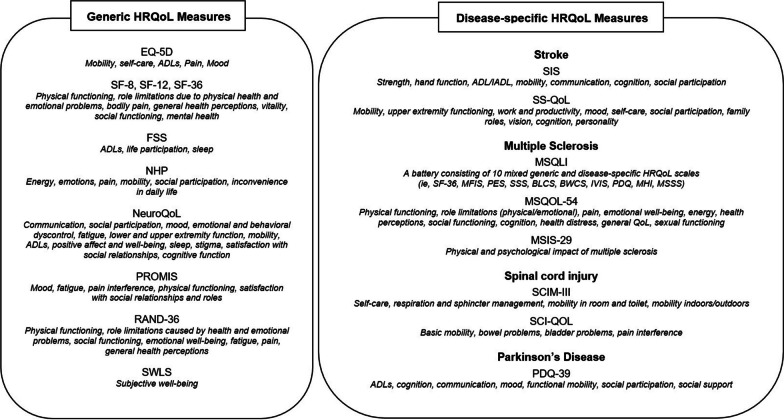
A summary of the HRQoL measures used in the included studies is reported in Fig. 2. Notably, both generic and disease-specific HRQoL measures were administered. Most of the studies (55.7%) implemented disease-specific scales. Only three studies [81, 87, 89] provided a mixed evaluation. Different factors explaining HRQoL were investigated. Overall, these were associated with different health-related domains, including perceived physical functioning, emotional state, self-care, mood, pain, social participation, autonomy in the ADLs, and cognition.
Fig. 2.
Summary of the generic and disease-specific HRQoL measures and their subcomponents investigated in the studies included. BLCS Bladder Control Scale, BWCS Bowel Control Scale, EQ-5D EuroQoL-5 Dimensions, FSS Fatigue Severity Scale, IVIS Impact of Visual Impairment Scale, MFIS Modified Fatigue Impact Scale, MHI Mental Health Inventory, MSIS-29 Multiple Sclerosis Impact Scale, MSQLI Multiple Sclerosis Quality Of Life Inventory, MSQOL-54 Multiple Sclerosis Quality Of Life-54, MSSS Modified Social Support Survey, Neuro-QoL Quality of life in Neurological Conditions, NHP Nottingham Health Profile, PDQ Perceived Deficits Questionnaire, PDQ-39 Parkinson’s Disease questionnaire-39, PES Pain Effects Scale, PROMIS The Patient-Reported Outcomes Measurement Information Systems, RAND-36 Research and Development Corporation-36, SCI-QOL Spinal Cord Injury-Quality of Life Independence, SCIM-III Spinal Cord Independence Measure-Version 3, SF-8 SF-12, SF-36, Short Form Health Survey, SIS Stroke Impact Scale, SS-QOL Stroke Specific Quality Of Life Scale, SSS Sexual Satisfaction Scale, SWLS Satisfaction With Life Scale
HRQoL changes in RAT
More than half of the studies (63.4%) reported significant effects on HRQoL following RAT alone. Of these, significant post-intervention between-groups changes were mostly found in stroke patients [32–34, 44, 46, 53, 65, 93], and in only one study in patients with PD [59]. Exclusive significant within-groups effects were found across clinical populations, namely stroke [42, 43, 45, 47, 54, 56, 58, 60, 84, 86, 87], MS [67, 72, 76, 82, 88, 89], SCI [30, 31, 39, 73], PD [68], and other neurological diseases [27, 63]. The 51.9% of the studies included follow-up evaluations. Of these, only two studies [44, 53] (both on patients with stroke) found significant between-groups effects over time (up to 3 months), while other studies on patients with stroke [47, 56, 60, 84, 86, 87] or MS [67, 89] showed only significant within-groups long-term effects (up to 36 months). Notably, most of the studies that reported significant effects implemented disease-specific HRQoL measures (60.6%). Similarly, 68.4% of the studies reporting no significant changes used generic HRQoL scales.
HRQoL changes in RAT plus VR
The majority (66.7%) of the studies combining RAT with VR reported significant post-treatment changes on HRQoL. Significant between-groups effects emerged in patients with stroke only [13, 70, 91], while exclusive significant within-groups changes were observed in patients with stroke [49, 57, 66, 69, 79, 83] and MS [61, 64, 85]. No studies on the effects of RAT plus VR in patients with PD were retrieved. Moreover, only 33.3% of the studies on RAT plus VR included longitudinal evaluations. Of these, one study on patients with stroke [70] found significant between-groups effects over one month after intervention, while other studies on patients with stroke [57, 66, 69, 79] or MS [64] showed significant within-groups improvements up to 2 months after treatment. Similarly to studies on RAT alone, most (83.3%) of the significant effects were found when disease-specific HRQoL measurements were adopted.
Non-motor outcomes concurrently investigated
Of the included studies, 32.9% conducted a psychological, cognitive and/or formative evaluation. Five studies investigated anxiety and depression symptoms [27, 63, 67, 68, 74], while ten assessed depression symptomatology only [13, 33, 41, 50, 54, 55, 58, 81, 82]. Seven studies conducted a cognitive assessment, especially concerning memory, attention, and executive functions abilities [13, 33, 34, 64, 68, 77, 83]. Other studies investigated patient satisfaction with the treatment [73, 77], perceived technological device usability [75], fear of falling [51], motivation and self-efficacy [71], coping abilities [33], and perceived well-being [33, 88].
Discussion
A systematic review of the studies investigating the effects of RAT alone and RAT plus VR on HRQoL in patients with neurological diseases was conducted. From each study, a wide range of data were extracted, including the methodology adopted to measure HRQoL across neurological populations and a summary of the psychological outcomes concurrently evaluated. At the end of the review process, a total of 70 studies were included and synthetized. Of these, 52 studies were on RAT exclusively, while the remaining 18 examined HRQoL changes after implementing VR systems, too.
Over the last two decades, the number of studies including HRQoL as a rehabilitation outcome has been increasing sensibly. Indeed, most of the included studies date back to within the last five years, denoting a rapid and recent growing interest in investigating HRQoL and its subcomponents along motor outcomes. This reflected the composition of the research groups and their specialty field. Although it was observed that the majority had expertise in rehabilitation medicine and neurology, authors’ contributions came from different fields, such as engineering, neuroscience, occupational physiatry, physiology, geriatrics, public health, and psychology. The increase found in the number of studies and the heterogeneity of expertise area may be attributable to the increasing necessity and interest in adopting multidisciplinary approaches to study neurorehabilitation processes, especially when multiple outcomes that are not strictly related to motor improvement are considered and technological and innovative procedures are implemented [20]. Besides, the introduction of high technologies has however raised cost-effectiveness issues, particularly when referring to robotic devices. Prior evidence regarding the economic evaluations of technology-based rehabilitation interventions has so far drawn controversial conclusions within different neurological populations [94, 95]. Certainly, the implementation of robotics in rehabilitation programs entails higher costs when compared to more standard and conventional treatments, leading research on this topic to require financial support, especially when the feasibility of the technological devices is tested. This may explain why most of the included studies were funded and one study out of three was a pilot. In support of this, it was also observed that most of the studies implementing also VR received funding and provided preliminary evidence of technology deployment.
Further heterogeneity was also found in terms of patients’ characteristics, intervention, and robotic typologies used. Most of the patients had stroke, while others presented MS or SCI. Stroke prevalence is not surprising considering the nowadays global estimation of cases (70% over 64 years) with an increasing burden in both sexes [96, 97]. Regarding MS and SCI, it is widely recognized that both pathologies result in irreversible motor dysfunction often highly correlated to progressive disability and gait impairment [98, 99]. Accordingly, the number of cases observed in this systematic review reflects the clinical potential of RAT that has been so far exploited to target lower limb deficits (e.g., robot-assisted gait training—RAGT).
Furthermore, sample size varied extensively across the studies included. Although a considerable number of patients was included in this systematic review, more than one study out of three involved fewer than 30 participants leading to statistically underpowered results in most cases. Even though it must be noted that most of these studies were pilots, future research involving larger sample size to obtain more robust results is strongly recommended.
Similarly, it should be also highlighted that only four studies have reported patients’ ethnicity. As recommended by prior studies, future research is encouraged to report participants’ ethnic diversity for different reasons, including the possibility to provide increased results generalizability [100]. Lastly, informative findings regarding the intervention and the technology type implemented were observed. Overall, the studies varied considerably in the global intervention duration, total number of therapy sessions and individual session duration. Notably, differences in intervention procedures between RAT and RAT plus VR were found. On average, the rehabilitation programs coupling RAT to VR were shorter, suggesting that the concurrent implementation of VR systems may add complexity to recovery procedures, ultimately raising feasibility and usability issues [101]. In support of this, it was observed that all the studies on RAT plus VR used non-immersive VEs (e.g., PC monitors). It is acknowledged that the implementation of VR devices providing more immersive VEs was associated with a stronger likelihood for the patient to experience adverse effects like nausea, dizziness, or oculomotor disturbances, that in turn may represent factors potentially affecting RAT delivery and efficacy, as well as patients’ treatment acceptability. Nevertheless, although immersion is not strictly related to sense of presence and stronger engagement, it is recognized that it can lead patients to experience positive emotions and increased motivation during the treatment [102]. This would contribute to enhancing patients’ adherence to the therapy, ultimately optimizing its efficacy. Accordingly, future studies should more deeply explore the role of VR and its key characteristics (i.e., interactivity, immersion, and sense of presence) when combined with robotic devices and their effects on diverse non-motor outcomes, including HRQoL.
Mixed results on HRQoL were found. Regardless of the study design adopted, most of the studies reported significant effects following RAT alone or RAT plus VR. In both cases, the majority provided significant within-group improvements, and a lower percentage of studies reported significant post-treatment comparisons with a control group. Informative findings were then observed when examining HRQoL results with reference to the neurological population investigated, assessment methodology adopted, and study design. Although most of the studies provided significant evidence, both intervention typologies provided between-group effects in patients with stroke mainly. Evidence on the other pathologies (i.e., MS, SCI, and PD) were found with no significant differences to a control group. Moreover, of the studies coupling RAT to VR, only one involved patients affected by SCI and none on patients with PD were retrieved. Therefore, not only more studies involving larger sample sizes that allow study group comparisons are needed, but future research on this topic should also extend the investigation to neurological populations that have been understudied so far. Deeper observations of RAT effects on HRQoL are also needed to point out possible explanations about non-significant results. It must be noted that one study out of three found no significant effects in any neurological population. One possible explanation may be related to the methodology adopted to assess HRQoL. A large number of tools to assess a wide range of health-related components was retrieved and grouped into two main categories, namely generic and disease-specific HRQoL measures. The comparison between these two categories has been extensively discussed [103]. Although generic measures are designed to be broadly applied across different types and severity of diseases, they may generate contradictory evidence in content validity resulting in lower responsiveness in certain clinical populations. Disease-specific measures, on the other hand, might be more sensitive and representative when investigating HRQoL outcomes in specific clinical subgroups. In the present systematic review, it was observed that most of the studies that provided non-significant results adopted generic tools, whereas most of the significant effects were detected when disease-specific scales were implemented. Future studies should take this finding into account and, for example, adopt both measure types when assessing HRQoL to point out any internal consistency issues and advance more generalizable conclusions. Lastly, concerning the study design, it was noted that the studies reporting significant changes at follow-up evaluations (up to 36 months after treatment) were mainly on patients with stroke and, again, adopted a disease-specific HRQoL assessment approach. This supports the importance of the choice of HRQoL measures when targeting effects over time and, moreover, the necessity to extend longitudinal investigations to other neurological populations.
One last finding of this systematic review regards the psychological and cognitive outcomes investigated along HRQoL. Of these, only which variables and the related adopted measures were presented to give more centrality to the results on HRQoL. From data synthesis, it emerged that psychological outcomes such as anxiety and depression symptoms, coping abilities, motivation, or perceived device usability were mainly investigated in studies on RAT alone. Interestingly, patients’ cognitive aspects were mainly evaluated when VR was also applied to the treatment. This distribution is not surprising if we consider the impact that the exposure to VEs may have particularly on patients’ cognitive status given the potential of VR to provide multimodal stimulation and multisensory feedbacks during therapy. Nevertheless, future research should consider comprehensive evaluations of patients’ health domains in order to better understand their inter-relationships regardless of the type of intervention. Furthermore, future works are needed to better deepen also the relationship between the psychological and cognitive variables and specific HRQoL subcomponents.
Limitations and future steps
Overall, the studies included in this systematic review made it possible to describe informative and promising evidence on robotic and VR devices application to neurorehabilitation and their effects on patients’ HRQoL. However, some limitations were identified too. The heterogeneity observed among the studies concerning the design, participants’ characteristics, intervention (i.e., rehabilitation outcomes, robotic device typology, duration), and HRQoL measures adopted made it especially difficult to draw robust conclusions. Moreover, this heterogeneity prevented advancing definitive interpretations on the added value of integrating VR to RAT. More studies combining the two technological devices are needed in order to provide more generalisable insights. Differently, the present review shed light on understudied neurological populations and, moreover, it allowed to note that, in the field of neurorehabilitation, HRQoL was investigated taking into account multiple and different health domains, ranging from motor and functional status to psychosocial related factors. The complexity of HRQoL as a construct, along with the other characteristics of the included studies, made the overall studies configuration too mixed to identify subgroups or subsets and, therefore, to perform meta-analyses as well. Accordingly, future research aiming at studying HRQoL improvements should take this heterogeneity into account and provide deeper investigations on specific HRQoL subcomponents and neurological populations adopting precise intervention procedures and an adequate assessment methodology. Following this line, multidisciplinary approaches are strongly recommended to optimally address the complexity of HRQoL and to extend knowledge on RAT effectiveness in patients’ everyday life.
Supplementary Information
Additional file 1: Appendix 1. Summary of the data extraction and results of methodological quality assessment.
Acknowledgements
Not applicable.
Abbreviations
- RAT
Robot-assisted therapy
- VR
Virtual reality
- HRQoL
Health-related quality of life
- VE
Virtual environment
- ADLs
Activities of daily living
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- TBI
Traumatic brain injury
- SCI
Spinal cord injury
- MS
Multiple sclerosis
- PD
Parkinson’s disease
Author contributions
FZ and PS contributed to the conceptualization of the study. FZ, NF, and RA conducted formal analysis. FZ wrote the original draft, while RA, AP, AG, MD, and PS provided supervision. All authors reviewed, edited, and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Feigin VL, Nichols E, Alam T, Bannick MS, Beghi E, Blake N, et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . World Report on Ageing and Health. Luxembourg: World Health Organization; 2015. [Google Scholar]
- 3.Molteni F, Gasperini G, Cannaviello G, Guanziroli E. Exoskeleton and end-effector robots for upper and lower limbs rehabilitation: narrative review. PM&R. 2018;10:S174–S188. doi: 10.1016/j.pmrj.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Voinescu A, Sui J, Stanton FD. Virtual reality in neurorehabilitation: an umbrella review of meta-analyses. JCM. 2021;10(7):1478. doi: 10.3390/jcm10071478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iandolo R, Marini F, Semprini M, Laffranchi M, Mugnosso M, Cherif A, et al. Perspectives and challenges in robotic neurorehabilitation. Appl Sci. 2019;9(15):3183. [Google Scholar]
- 6.Giansanti D. The rehabilitation and the robotics: Are they going together well? Healthcare. 2020;9(1):26. doi: 10.3390/healthcare9010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chien WT, Chong YY, Tse MK, Chien CW, Cheng HY. Robot-assisted therapy for upper-limb rehabilitation in subacute stroke patients: a systematic review and meta-analysis. Brain Behav. 2020;10(8):e01742. doi: 10.1002/brb3.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nedergård H, Arumugam A, Sandlund M, Bråndal A, Häger CK. Effect of robotic-assisted gait training on objective biomechanical measures of gait in persons post-stroke: a systematic review and meta-analysis. J NeuroEng Rehabil (JNER) 2021;18(1):1–22. doi: 10.1186/s12984-021-00857-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Postol N, Marquez J, Spartalis S, Bivard A, Spratt NJ. Do powered over-ground lower limb robotic exoskeletons affect outcomes in the rehabilitation of people with acquired brain injury? Disabil Rehabil Assist Technol. 2019;14(8):764–775. doi: 10.1080/17483107.2018.1499137. [DOI] [PubMed] [Google Scholar]
- 10.Fang CY, Tsai JL, Li GS, Lien ASY, Chang YJ. Effects of robot-assisted gait training in individuals with spinal cord injury: a meta-analysis. Biomed Res Int. 2020;2020. [DOI] [PMC free article] [PubMed]
- 11.Calabrò RS, Cassio A, Mazzoli D, Andrenelli E, Bizzarini E, Campanini I, et al. What does evidence tell us about the use of gait robotic devices in patients with multiple sclerosis? A comprehensive systematic review on functional outcomes and clinical recommendations. Eur J Phys Rehabil Med. 2021;57(5):841–849. doi: 10.23736/S1973-9087.21.06915-X. [DOI] [PubMed] [Google Scholar]
- 12.Alwardat M, Etoom M, Al Dajah S, Schirinzi T, Di Lazzaro G, Sinibaldi Salimei P, et al. Effectiveness of robot-assisted gait training on motor impairments in people with Parkinson’s disease: a systematic review and meta-analysis. Int J Rehabil Res. 2018;41(4):287–296. doi: 10.1097/MRR.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 13.Manuli A, Maggio MG, Latella D, Cannavò A, Balletta T, De Luca R, et al. Can robotic gait rehabilitation plus Virtual Reality affect cognitive and behavioural outcomes in patients with chronic stroke? A randomized controlled trial involving three different protocols. J Stroke Cerebrovasc Dis. 2020;29(8):104994. doi: 10.1016/j.jstrokecerebrovasdis.2020.104994. [DOI] [PubMed] [Google Scholar]
- 14.Baus O, Bouchard S. Moving from virtual reality exposure-based therapy to augmented reality exposure-based therapy: a review. Front Hum Neurosci. 2014;8. [DOI] [PMC free article] [PubMed]
- 15.Calabrò RS, Russo M, Naro A, De Luca R, Leo A, Tomasello P, et al. Robotic gait training in multiple sclerosis rehabilitation: Can virtual reality make the difference? Findings from a randomized controlled trial. J Neurol Sci. 2017;377:25–30. doi: 10.1016/j.jns.2017.03.047. [DOI] [PubMed] [Google Scholar]
- 16.De Keersmaecker E, Lefeber N, Geys M, Jespers E, Kerckhofs E, Swinnen E. Virtual reality during gait training: does it improve gait function in persons with central nervous system movement disorders? A systematic review and meta-analysis. NRE. 2019;44(1):43–66. doi: 10.3233/NRE-182551. [DOI] [PubMed] [Google Scholar]
- 17.Calabrò RS, De Cola MC, Leo A, Reitano S, Balletta T, Trombetta G, et al. Robotic neurorehabilitation in patients with chronic stroke: psychological well-being beyond motor improvement. Int J Rehabil Res. 2015;38(3):219–225. doi: 10.1097/MRR.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 18.Fundarò C, Giardini A, Maestri R, Traversoni S, Bartolo M, Casale R. Motor and psychosocial impact of robot-assisted gait training in a real-world rehabilitation setting: a pilot study. PLoS ONE. 2018;13(2):e0191894. doi: 10.1371/journal.pone.0191894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maggio MG, Torrisi M, Buda A, De Luca R, Piazzitta D, Cannavò A, et al. Effects of robotic neurorehabilitation through lokomat plus virtual reality on cognitive function in patients with traumatic brain injury: a retrospective case-control study. Int J Neurosci. 2020;130(2):117–123. doi: 10.1080/00207454.2019.1664519. [DOI] [PubMed] [Google Scholar]
- 20.Lexell J, Brogårdh C. The use of ICF in the neurorehabilitation process. NRE. 2015;36(1):5–9. doi: 10.3233/NRE-141184. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group: Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Heart, Lung, and Blood institute: Study Quality Assessment Tools [Internet]. 2022. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 23.Stone J, Gurunathan U, Glass K, Munn Z, Tugwell P, Doi SAR. Stratification by quality induced selection bias in a meta-analysis of clinical trials. J Clin Epidemiol. 2019;107:51–59. doi: 10.1016/j.jclinepi.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Human Development Report. Human Development Index and its components [Internet]. 2022. Available from: http://hdr.undp.org/en.
- 25.Aprile I, Germanotta M, Cruciani A, Pecchioli C, Loreti S, Papadopoulou D, et al. Poststroke shoulder pain in subacute patients and its correlation with upper limb recovery after robotic or conventional treatment: a secondary analysis of a multicenter randomized controlled trial. Int J Stroke. 2021;16(4):396–405. doi: 10.1177/1747493020937192. [DOI] [PubMed] [Google Scholar]
- 26.Baunsgaard CB, Nissen UV, Brust AK, Frotzler A, Ribeill C, Kalke YB, et al. Exoskeleton gait training after spinal cord injury: an exploratory study on secondary health conditions. J Rehabil Med. 2018;50(9):806–813. doi: 10.2340/16501977-2372. [DOI] [PubMed] [Google Scholar]
- 27.Bertolucci F, Di Martino S, Orsucci D, Ienco EC, Siciliano G, Rossi B, et al. Robotic gait training improves motor skills and quality of life in hereditary spastic paraplegia. NeuroRehabilitation. 2015;36(1):93–99. doi: 10.3233/NRE-141196. [DOI] [PubMed] [Google Scholar]
- 28.Bovolenta F, Goldoni M, Clerici P, Agosti M, Franceschini M. Robot therapy for functional recovery of the upper limbs: a pilot study on patients after stroke. J Rehabil Med. 2009;41(12):971–975. doi: 10.2340/16501977-0402. [DOI] [PubMed] [Google Scholar]
- 29.Capecci M, Pournajaf S, Galafate D, Sale P, Le Pera D, Goffredo M, et al. Clinical effects of robot-assisted gait training and treadmill training for Parkinson’s disease: a randomized controlled trial. Ann Phys Rehabil Med. 2019;62(5):303–312. doi: 10.1016/j.rehab.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 30.Çinar Ç, Yildirim MA, Öneş K, Gökşenoğlu G. Effect of robotic-assisted gait training on functional status, walking and quality of life in complete spinal cord injury. Int J Rehabil Res. 2021;44(3):262–268. doi: 10.1097/MRR.0000000000000486. [DOI] [PubMed] [Google Scholar]
- 31.Çinar Ç, Öneş K, Yildirim MA, Gökşenoğlu G. Comparison of the patients with complete and incomplete spinal cord injury administered robotic-assisted gait training treatment. J Phys Med Rehabil Sci/Fiziksel Tup ve Rehabilitasyon Bilimleri Dergisi. 2020;23(1):12–19. [Google Scholar]
- 32.Conroy SS, Wittenberg GF, Krebs HI, Zhan M, Bever CT, Whitall J. Robot-assisted arm training in chronic stroke: addition of transition-to-task practice. Neurorehabil Neural Repair. 2019;33(9):751–761. doi: 10.1177/1545968319862558. [DOI] [PubMed] [Google Scholar]
- 33.De Luca R, Maresca G, Balletta T, Cannavò A, Leonardi S, Latella D, et al. Does overground robotic gait training improve non-motor outcomes in patients with chronic stroke? Findings from a pilot study. J Clin Neurosci. 2020;81:240–245. doi: 10.1016/j.jocn.2020.09.070. [DOI] [PubMed] [Google Scholar]
- 34.Dundar U, Toktas H, Solak O, Ulasli AM, Eroglu S. A comparative study of conventional physiotherapy versus robotic training combined with physiotherapy in patients with stroke. Top Stroke Rehabil. 2014;21(6):453–461. doi: 10.1310/tsr2106-453. [DOI] [PubMed] [Google Scholar]
- 35.Francisco GE, Yozbatiran N, Berliner J, O’Malley MK, Pehlivan AU, Kadivar Z, et al. Robot-assisted training of arm and hand movement shows functional improvements for incomplete cervical spinal cord injury. Am J Phys Med Rehabil. 2017;96:S171–S177. doi: 10.1097/PHM.0000000000000815. [DOI] [PubMed] [Google Scholar]
- 36.Gandolfi M, Geroin C, Picelli A, Munari D, Waldner A, Tamburin S, et al. Robot-assisted vs. sensory integration training in treating gait and balance dysfunctions in patients with multiple sclerosis: a randomized controlled trial. Front Hum Neurosci. 2014;8:318. doi: 10.3389/fnhum.2014.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gandolfi M, Valè N, Dimitrova EK, Mazzoleni S, Battini E, Benedetti MD, et al. Effects of high-intensity robot-assisted hand training on upper limb recovery and muscle activity in individuals with multiple sclerosis: a randomized, controlled. Single-Blinded Trial Front Neurol. 2018;9:905. doi: 10.3389/fneur.2018.00905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garnier-Villarreal M, Pinto D, Mummidisetty CK, Jayaraman A, Tefertiller C, Charlifue S, et al. Predicting duration of outpatient physical therapy episodes for individuals with spinal cord injury based on locomotor training strategy. Arch Phys Med Rehabil. 2022;103(4):665–675. doi: 10.1016/j.apmr.2021.07.815. [DOI] [PubMed] [Google Scholar]
- 39.Gorman PH, Forrest GF, Asselin PK, Scott W, Kornfeld S, Hong E, et al. The effect of exoskeletal-assisted walking on spinal cord injury bowel function: results from a randomized trial and comparison to other physical interventions. J Clin Med. 2021;10(5):1–11. doi: 10.3390/jcm10050964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hidler J, Nichols D, Pelliccio M, Brady K, Campbell DD, Kahn JH, et al. Multicenter randomized clinical trial evaluating the effectiveness of the Lokomat in subacute stroke. Neurorehabil Neural Repair. 2009;23(1):5–13. doi: 10.1177/1545968308326632. [DOI] [PubMed] [Google Scholar]
- 41.Hornby TG, Campbell DD, Kahn JH, Demott T, Moore JL, Roth HR. Enhanced gait-related improvements after therapist- versus robotic-assisted locomotor training in subjects with chronic stroke: a randomized controlled study. Stroke. 2008;39(6):1786–1792. doi: 10.1161/STROKEAHA.107.504779. [DOI] [PubMed] [Google Scholar]
- 42.Housley SN, Wu D, Richards K, Belagaje S, Ghovanloo M, Butler AJ. Improving upper extremity function and quality of life with a tongue driven exoskeleton: a pilot study quantifying stroke rehabilitation. Stroke Res Treat. 2017;2017:3603860. doi: 10.1155/2017/3603860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsieh Y, Wu C, Lin K, Yao G, Wu K, Chang Y. Dose-response relationship of robot-assisted stroke motor rehabilitation: the impact of initial motor status. Stroke. 2012;43(10):2729–2734. doi: 10.1161/STROKEAHA.112.658807. [DOI] [PubMed] [Google Scholar]
- 44.Hsieh YW, Wu CY, Wang WE, Lin KC, Chang KC, Chen CC, et al. Bilateral robotic priming before task-oriented approach in subacute stroke rehabilitation: a pilot randomized controlled trial. Clin Rehabil. 2017;31(2):225–233. doi: 10.1177/0269215516633275. [DOI] [PubMed] [Google Scholar]
- 45.Huang PC, Hsieh YW, Wang CM, Wu CY, Huang SC, Lin KC. Predictors of motor, daily function, and quality-of-life improvements after upper-extremity robot-assisted rehabilitation in stroke. Am J Occup Ther. 2014;68(3):325–333. doi: 10.5014/ajot.2014.010546. [DOI] [PubMed] [Google Scholar]
- 46.Hung C, Hsieh Y, Wu C, Lin Y, Lin K, Chen C. The effects of combination of robot-assisted therapy with task-specific or impairment-oriented training on motor function and quality of life in chronic stroke. PM&R. 2016;8(8):721–729. doi: 10.1016/j.pmrj.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 47.Hung C, Lin K, Chang W, Huang W, Chang YJ, Chen C, et al. Unilateral vs bilateral hybrid approaches for upper limb rehabilitation in chronic stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2019;100(12):2225–2232. doi: 10.1016/j.apmr.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 48.Juszczak M, Gallo E, Bushnik T. Examining the effects of a powered exoskeleton on quality of life and secondary impairments in people living with spinal cord injury. Top Spinal Cord Inj Rehabil. 2018;24(4):336–342. doi: 10.1310/sci17-00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kayabınar E, Özalp M, Koçyiğit MF, As İ, Elbasan B. The effects of robotic and conventional gait training in addition to neurodevelopmental treatment on balance, mobility, and health-related quality of life in patients with stroke. Neurolsci Neurophysiol. 2019;36(2):112–119. [Google Scholar]
- 50.Kim JH, Ko MH, Park JW, Lee HJ, Nam KY, Nam YG, et al. Efficacy of electromechanically-assisted rehabilitation of upper limb function in post-stroke patients: a randomized controlled study. J Rehabil Med Clin Commun. 2021;4:1000074. doi: 10.2340/20030711-1000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim HS, Park JH, Lee HS, Lee JY, Jung JW, Park SB, et al. Effects of wearable powered exoskeletal training on functional mobility, physiological health and quality of life in non-ambulatory spinal cord injury patients. J Korean Med Sci. 2021;36(12):e80. doi: 10.3346/jkms.2021.36.e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kozlowski AJ, Fabian M, Lad D, Delgado AD. Feasibility and safety of a powered exoskeleton for assisted walking for persons with multiple sclerosis: a single-group preliminary study. Arch Phys Med Rehabil. 2017;98(7):1300–1307. doi: 10.1016/j.apmr.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 53.Kumar S, Yadav R, Afrin A. The effectiveness of a robotic tilt table on the muscle strength and quality of life in individuals following stroke: a randomised control trial. Int J Ther Rehabil. 2020;27(12):1–9. [Google Scholar]
- 54.Kutner NG, Zhang R, Butler AJ, Wolf SL, Alberts JL. Quality-of-life change associated with robotic-assisted therapy to improve hand motor function in patients with subacute stroke: a randomized clinical trial. Phys Ther. 2010;90(4):493–504. doi: 10.2522/ptj.20090160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leblebicier MA, Saracoglu I, Yaman F, Sahın E. Effect of robot-assisted gait training on quality of life and depression in patients with hemiplegia. Ann Clin Anal Med. 2021;12(10):1088–1092. [Google Scholar]
- 56.Lee Y, Lin K, Cheng H, Wu C, Hsieh Y, Chen C. Effects of combining robot-assisted therapy with neuromuscular electrical stimulation on motor impairment, motor and daily function, and quality of life in patients with chronic stroke: a double-blinded randomized controlled trial. J Neuroeng Rehabil. 2015;12:96. doi: 10.1186/s12984-015-0088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee SH, Lee JY, Kim MY, Jeon YJ, Kim S, Shin JH. Virtual reality rehabilitation with functional electrical stimulation improves upper extremity function in patients with chronic stroke: a pilot randomized controlled study. Arch Phys Med Rehabil. 2018;99(8):1447–1453.e1. doi: 10.1016/j.apmr.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 58.Linder SM, Rosenfeldt AB, Bay RC, Sahu K, Wolf SL, Alberts JL. Improving quality of life and depression after stroke through telerehabilitation. Am J Occup Ther. 2015;69(2):1–9. doi: 10.5014/ajot.2015.014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lo AC, Chang VC, Gianfrancesco MA, Friedman JH, Patterson TS, Benedicto DF. Reduction of freezing of gait in Parkinson’s disease by repetitive robot-assisted treadmill training: a pilot study. J Neuroeng Rehabil. 2010;7:51. doi: 10.1186/1743-0003-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG, et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. New Engl J Med. 2010;362(19):1772–1783. doi: 10.1056/NEJMoa0911341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manuli A, Maggio MG, Tripoli D, Gulli M, Cannavo A, La Rosa G, et al. Patients’ perspective and usability of innovation technology in a new rehabilitation pathway: an exploratory study in patients with multiple sclerosis. Multiple Sclerosis Related Disord. 2020;44. [DOI] [PubMed]
- 62.McGibbon C, Sexton A, Gryfe P, Dutta T, Jayaraman A, Deems-Dluhy S, et al. Effect of using of a lower-extremity exoskeleton on disability of people with multiple sclerosis. Disabil Rehabil Assist Technol. 2021;1–8. [DOI] [PubMed]
- 63.Miura K, Tsuda E, Kogawa M, Ishiyama H, Maeda K, Kuzuhara K, et al. Effects of gait training with a voluntary-driven wearable cyborg, Hybrid Assistive Limb (HAL), on quality of life in patients with neuromuscular disease, able to walk independently with aids. J Clin Neurosci. 2021;89:211–215. doi: 10.1016/j.jocn.2021.04.038. [DOI] [PubMed] [Google Scholar]
- 64.Munari D, Fonte C, Varalta V, Battistuzzi E, Cassini S, Montagnoli AP, et al. Effects of robot-assisted gait training combined with virtual reality on motor and cognitive functions in patients with multiple sclerosis: a pilot, single-blind, randomized controlled trial. Restor Neurol Neurosci. 2020;38(2):151–164. doi: 10.3233/RNN-190974. [DOI] [PubMed] [Google Scholar]
- 65.Mustafaoglu R, Erhan B, Yeldan I, Gunduz B, Tarakci E. Does robot-assisted gait training improve mobility, activities of daily living and quality of life in stroke? A single-blinded, randomized controlled trial. Acta Neurol Belg. 2020;120(2):335–344. doi: 10.1007/s13760-020-01276-8. [DOI] [PubMed] [Google Scholar]
- 66.Nijenhuis SM, Prange GB, Amirabdollahian F, Sale P, Infarinato F, Nasr N, et al. Feasibility study into self-administered training at home using an arm and hand device with motivational gaming environment in chronic stroke. J Neuroeng Rehabil. 2015;12:89. doi: 10.1186/s12984-015-0080-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ozsoy-Unubol T, Ata E, Cavlak M, Demir S, Candan Z, Yilmaz F. Effects of robot-assisted gait training in patients with multiple sclerosis: a single-blinded randomized controlled study. Am J Phys Med Rehabil. 2021. [DOI] [PubMed]
- 68.Paker N, Bugdayci D, Goksenoglu G, Sen A, Kesiktas N. Effects of robotic treadmill training on functional mobility, walking capacity, motor symptoms and quality of life in ambulatory patients with Parkinson’s disease: a preliminary prospective longitudinal study. NeuroRehabilitation. 2013;33(2):323–328. doi: 10.3233/NRE-130962. [DOI] [PubMed] [Google Scholar]
- 69.Park M, Ko MH, Oh SW, Lee JY, Ham Y, Yi H, et al. Effects of virtual reality-based planar motion exercises on upper extremity function, range of motion, and health-related quality of life: a multicenter, single-blinded, randomized, controlled pilot study. J Neuroeng Rehabil. 2019;16(1). [DOI] [PMC free article] [PubMed]
- 70.Park JH, Park G, Kim HY, Lee JY, Ham Y, Hwang D, et al. A comparison of the effects and usability of two exoskeletal robots with and without robotic actuation for upper extremity rehabilitation among patients with stroke: a single-blinded randomised controlled pilot study. J Neuroeng Rehabil. 2020;17(1):137. doi: 10.1186/s12984-020-00763-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Piira A, Lannem AM, Gjesdal K, Knutsen R, Jørgensen L, Glott T, et al. Quality of life and psychological outcomes of body-weight supported locomotor training in spinal cord injured persons with long-standing incomplete lesions. Spinal Cord. 2020;58(5):560–569. doi: 10.1038/s41393-019-0401-2. [DOI] [PubMed] [Google Scholar]
- 72.Pilutti LA, Lelli DA, Paulseth JE, Crome M, Jiang S, Rathbone MP, et al. Effects of 12 weeks of supported treadmill training on functional ability and quality of life in progressive multiple sclerosis: a pilot study. Arch Phys Med Rehabil. 2011;92(1):31–36. doi: 10.1016/j.apmr.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 73.Platz T, Gillner A, Borgwaldt N, Kroll S, Roschka S. Device-training for individuals with thoracic and lumbar spinal cord injury using a powered exoskeleton for technically assisted mobility: achievements and user satisfaction. Biomed Res Int. 2016;2016:1–10. doi: 10.1155/2016/8459018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Postol N, Spratt NJ, Bivard A, Marquez J. Physiotherapy using a free-standing robotic exoskeleton for patients with spinal cord injury: a feasibility study. J Neuroeng Rehabil. 2021;18(1):180. doi: 10.1186/s12984-021-00967-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rozevink SG, van der Sluis CK, Garzo A, Keller T, Hijmans JM. HoMEcare aRm rehabiLItatioN (MERLIN): telerehabilitation using an unactuated device based on serious games improves the upper limb function in chronic stroke. J Neuroeng Rehabil. 2021;18(1):48. doi: 10.1186/s12984-021-00841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schwartz I, Sajin A, Moreh E, Fisher I, Neeb M, Forest A, et al. Robot-assisted gait training in multiple sclerosis patients: a randomized trial. Mult Scler J. 2012;18(6):881–890. doi: 10.1177/1352458511431075. [DOI] [PubMed] [Google Scholar]
- 77.Schwickert L, Klenk J, Stähler A, Becker C, Lindemann U. Robotic-assisted rehabilitation of proximal humerus fractures in virtual environments: a pilot study. Z Gerontol Geriatr. 2011;44(6):387–392. doi: 10.1007/s00391-011-0258-2. [DOI] [PubMed] [Google Scholar]
- 78.Sconza C, Negrini F, Di Matteo B, Borboni A, Boccia G, Petrikonis I, et al. Robot-assisted gait training in patients with multiple sclerosis: a randomized controlled crossover trial. Medicina (Kaunas). 2021;57(7). [DOI] [PMC free article] [PubMed]
- 79.Shin JH, Kim MY, Lee JY, Jeon YJ, Kim S, Lee S, et al. Effects of virtual reality-based rehabilitation on distal upper extremity function and health-related quality of life: a single-blinded, randomized controlled trial. J Neuroeng Rehabil. 2016;13:17. doi: 10.1186/s12984-016-0125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stolz R, Nayyar R, Louie J, Bower KJ, Paul SK, Ng L. The effectiveness of a novel cable-driven gait trainer (Robowalk) combined with conventional physiotherapy compared to conventional physiotherapy alone following stroke: a randomised controlled trial. Int J Rehabil Res. 2019;42(4):377–384. doi: 10.1097/MRR.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 81.Straudi S, Manfredini F, Lamberti N, Martinuzzi C, Maietti E, Basaglia N. Robot-assisted gait training is not superior to intensive overground walking in multiple sclerosis with severe disability (the RAGTIME study): a randomized controlled trial. Mult Scler J. 2020;26(6):716–724. doi: 10.1177/1352458519833901. [DOI] [PubMed] [Google Scholar]
- 82.Straudi S, Fanciullacci C, Martinuzzi C, Pavarelli C, Rossi B, Chisari C, et al. The effects of robot-assisted gait training in progressive multiple sclerosis: a randomized controlled trial. Mult Scler. 2016;22(3):373–384. doi: 10.1177/1352458515620933. [DOI] [PubMed] [Google Scholar]
- 83.Taravati S, Capaci K, Uzumcugil H, Tanigor G. Evaluation of an upper limb robotic rehabilitation program on motor functions, quality of life, cognition, and emotional status in patients with stroke: a randomized controlled study. Neurol Sci. 2022;43(2):1177–1188. doi: 10.1007/s10072-021-05431-8. [DOI] [PubMed] [Google Scholar]
- 84.Timmermans AAA, Lemmens RJM, Monfrance M, Geers RPJ, Bakx W, Smeets RJEM, et al. Effects of task-oriented robot training on arm function, activity, and quality of life in chronic stroke patients: a randomized controlled trial. J Neuroeng Rehabil. 2014;11:45. doi: 10.1186/1743-0003-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tramontano M, Morone G, De Angelis S, Casagrande Conti L, Galeoto G, Grasso MG. Sensor-based technology for upper limb rehabilitation in patients with multiple sclerosis: a randomized controlled trial. Restor Neurol Neurosci. 2020;38(4):333–341. doi: 10.3233/RNN-201033. [DOI] [PubMed] [Google Scholar]
- 86.Uivarosan D, Tit DM, Iovan C, Nistor-Cseppento DC, Endres L, Lazar L, et al. Effects of combining modern recovery techniques with neurotrophic medication and standard treatment in stroke patients. Sci Total Environ. 2019;679:80–87. doi: 10.1016/j.scitotenv.2019.05.070. [DOI] [PubMed] [Google Scholar]
- 87.van Nunen MPM, Gerrits KHL, Konijnenbelt M, Janssen TWJ, de Haan A. Recovery of walking ability using a robotic device in subacute stroke patients: a randomized controlled study. Disabil Rehabil Assist Technol. 2015;10(2):141–148. doi: 10.3109/17483107.2013.873489. [DOI] [PubMed] [Google Scholar]
- 88.Vaney C, Gattlen B, Lugon-Moulin V, Meichtry A, Hausammann R, Foinant D, et al. Robotic-assisted step training (lokomat) not superior to equal intensity of over-ground rehabilitation in patients with multiple sclerosis. Neurorehabil Neural Repair. 2012;26(3):212–221. doi: 10.1177/1545968311425923. [DOI] [PubMed] [Google Scholar]
- 89.Wier LM, Hatcher MS, Triche EW, Lo AC. Effect of robot-assisted versus conventional body-weight-supported treadmill training on quality of life for people with multiple sclerosis. J Rehabil Res Dev. 2011;48(4):483–492. doi: 10.1682/jrrd.2010.03.0035. [DOI] [PubMed] [Google Scholar]
- 90.Wu J, Dodakian L, See J, Burke Quinlan E, Meng L, Abraham J, et al. Gains across WHO dimensions of function after robot-based therapy in stroke subjects. Neurorehabil Neural Repair. 2020;34(12):1150–1158. doi: 10.1177/1545968320956648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu C, Yang C, Chuang L, Lin K, Chen H, Chen M, et al. Effect of therapist-based versus robot-assisted bilateral arm training on motor control, functional performance, and quality of life after chronic stroke: a clinical trial. Phys Ther. 2012;92(8):1006–1016. doi: 10.2522/ptj.20110282. [DOI] [PubMed] [Google Scholar]
- 92.Wu M, Landry JM, Kim J, Schmit BD, Yen SC, Macdonald J. Robotic resistance/assistance training improves locomotor function in individuals poststroke: a randomized controlled study. Arch Phys Med Rehabil. 2014;95(5):799–806. doi: 10.1016/j.apmr.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yadav R, Kuma S, Yadav S. Robotic tilt table exercises versus conventional exercises in rehabilitation of hemiplegic patients. Int J Ther Rehabil. 2018;25(9):475–480. [Google Scholar]
- 94.Winser S, Lee SH, Law HS, Leung HY, Bello UM, Kannan P. Economic evaluations of physiotherapy interventions for neurological disorders: a systematic review. Disabil Rehabil. 2020;42(7):892–901. doi: 10.1080/09638288.2018.1510993. [DOI] [PubMed] [Google Scholar]
- 95.Lo K, Stephenson M, Lockwood C. The economic cost of robotic rehabilitation for adult stroke patients: a systematic review. JBI Database Sys Rev Implement Rep. 2019;17(4):520–547. doi: 10.11124/JBISRIR-2017-003896. [DOI] [PubMed] [Google Scholar]
- 96.Guzik A, Bushnell C. Stroke epidemiology and risk factor management. CONTINUUM Lifelong Learn Neurol. 2017;23(1):15–39. doi: 10.1212/CON.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 97.Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. 2017;120(3):439–448. doi: 10.1161/CIRCRESAHA.116.308413. [DOI] [PubMed] [Google Scholar]
- 98.Comber L, Galvin R, Coote S. Gait deficits in people with multiple sclerosis: a systematic review and meta-analysis. Gait Posture. 2017;51:25–35. doi: 10.1016/j.gaitpost.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 99.Fehlings M, Singh A, Tetreault L, Kalsi-Ryan S, Nouri A. Global prevalence and incidence of traumatic spinal cord injury. CLEP. 2014;309. [DOI] [PMC free article] [PubMed]
- 100.McCambridge AB, Elkins MR. If we can’t see race and ethnicity in research, How will we see racial inequality? J Physiother. 2021;67(2):82–83. doi: 10.1016/j.jphys.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 101.Zanatta F, Giardini A, Pierobon A, D’Addario M, Steca P. A systematic review on the usability of robotic and virtual reality devices in neuromotor rehabilitation: patients’ and healthcare professionals’ perspective. BMC Health Serv Res. 2022;22(1):523. doi: 10.1186/s12913-022-07821-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Howard MC. A meta-analysis and systematic literature review of virtual reality rehabilitation programs. Comput Hum Behav. 2017;70:317–327. [Google Scholar]
- 103.the LIVSFORSK network, Haraldstad K, Wahl A, Andenæs R, Andersen JR, Andersen MH, et al. A systematic review of quality of life research in medicine and health sciences. Qual Life Res. 2019;28(10):2641–50. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Appendix 1. Summary of the data extraction and results of methodological quality assessment.
Data Availability Statement
Not applicable.