ABSTRACT
Background
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited kidney disorder and a major cause of kidney failure worldwide. However, its impact on quality-of-life has not been systematically explored.
Methods
The CYSTic-QoL study was an observational study designed to study quality-of-life in adult European ADPKD patients with an estimated glomerular filtration rate (eGFR) ≥30 mL/min/1.73 m2. A total of 465 patients were recruited from six expert European centres with baseline data recorded, including health-related quality-of-life (HRQoL), incorporating a Kidney Disease QoL short form questionnaire (KDQoL-SF, version 1.3), magnetic resonance imaging (MRI) for total kidney volume (TKV) measurements and DNA for genotyping. The cohort was stratified by baseline eGFR, TKV or genotype and correlated with HRQoL scores. Bivariate and multivariate analyses were applied to examine the relationship between HRQoL and variables of interest. KDQoL-SF scores were calculated using an online tool provided by the RAND organization. For 36-item short form values, mean centre scores were normalized to their native populations.
Results
The mean age of participants was 43 years and 55% were female, with a mean eGFR of 77 mL/min/1.73 m2 and height-adjusted TKV (ht-TKV) of 849 mL/min; 66% had PKD1 pathogenic variants. ADPKD patients uniformly reported decreased general health and less energy, with the majority also experiencing poorer physical, mental or emotional health and limitations in social functioning. A total of 32.5% of participants experienced flank pain, which was significantly and negatively correlated with the majority of KDQoL-SF subscales by multivariate analysis. Higher ht-TKV and lower eGFR were negatively associated with decreased energy and poorer physical health, respectively, although not with flank pain.
Conclusion
ADPKD patients suffer from significantly decreased QoL in multiple domains, exacerbated particularly by chronic pain.
Keywords: ADPKD, kidney function, pain, quality-of-life, total kidney volume
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited kidney disease, a major cause of kidney failure and a significant medical and economic burden across Europe [1, 2]. Nevertheless, the impact of this major disease on the quality-of-life (QoL) of patients with preserved kidney function has not been systematically explored.
There have been few published studies on QoL in ADPKD based on sufficiently large and representative patient cohorts. Published data have been limited to clinical trial populations preselected for more severe disease [3], single-centre studies [4–6], the inclusion of patients with late chronic kidney disease (CKD; Category G4 and G5) or already on kidney replacement therapy (KRT) [7, 8] and the use of multiple health-related quality-of-life (HRQoL) instruments of varying sensitivity and specificity. Indeed, a systematic review performed in 2017 concluded that only nine published studies could be included based on the 36-item short form (SF-36) questionnaire: of note, three of these were based on randomized controlled trials [9].
Recent studies have concluded that pain is an important but neglected symptom in ADPKD. An international Delphi survey, followed by a consensus meeting between patients, caregivers and medical professionals led by the Standardized Outcomes in Nephrology Polycystic Kidney Disease initiative, identified four core outcomes that should be considered in future trials: kidney function, mortality, cardiovascular disease and pain [10, 11]. Of note, pain was the highest-ranked patient-reported outcome, reflecting its importance to patients, yet it has been variably measured in a minority (24%) of clinical trials to-date [12]. Indeed, the prevalence of pain as a major and troubling clinical symptom that could adversely affect QoL in ADPKD patients is unknown [13].
The CYSTic Consortium was established in 2017 to build a longitudinal international observational cohort of patients with ADPKD to facilitate prospective studies of factors influencing the natural history of ADPKD, the impact of the disease on individual patients and the economic costs on health-care systems in adult patients with an estimated glomerular filtration rate (eGFR) ≥30 mL/min/1.73 m2. In this article we report the results of baseline QoL in participants recruited to six European expert ADPKD centres (CYSTic-QOL study).
MATERIALS AND METHODS
Patient recruitment and centre participation
More than 450 patients were initially recruited from six expert centres across Europe (Belgium, France, Italy, the Netherlands, Spain and the UK) with baseline clinical data recorded, including HRQoL (KDQoL-SF version 1.3 questionnaire), abdominal magnetic resonance imaging (MRI) for total kidney volume (TKV) measurements and DNA for genotyping. Each study centre consented to transfer their data to an electronic database (Askimed) utilized by the study. The study was approved by a regional ethics committee (18/EE/0247) and by the study sponsor, Sheffield Teaching Hospitals NHS Foundation Trust. Ethics approval was also obtained by each participating centre within their own country.
Inclusion and exclusion criteria
Baseline inclusion criteria were age ≥18 years, eGFR ≥30 mL/min/1.73 m2 [Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula], a clinical diagnosis of ADPKD based on imaging and positive family history (modified Pei–Ravine criteria) [14] and written informed consent. Exclusion criteria were the use of KRT before enrollment (dialysis and allograft) or anticipated to receive such therapy within 12 months after enrollment, participation in a clinical trial aiming to modify disease outcome ≤1 year before enrollment and significant cardiac failure (i.e. New York Heart Association stage IV). Clinical and laboratory measurements were calculated and expressed as mean values with standard deviations (SDs).
Questionnaires
Demographic and socio-economic data were collected by the use of a specifically designed questionnaire and included information such as age, gender, marital status, ethnicity, body mass index (BMI), hypertension, flank pain, smoking status, level of education attained and employment status.
HRQoL
HRQoL data were collected using the KDQoL-SF version 1.3 questionnaire, which combines the disease-specific KDQoL and the generic SF-36 [15]. A total of 12 subscales are included in the KDQoL and 8 in the SF-36: 2 optional subscales (dialysis staff encouragement and patient satisfaction) in the KDQoL were omitted as there were no patients on dialysis. The SF-36 is composed of 36 questions organized into eight multi-item scales. Each scale is directly transformed into a 0–100 score on the assumption that each question carries equal weight. The higher the score, the lower the disability, i.e. a score of 100 is equivalent to no disability and a score of 0 is equivalent to maximum disability. There is no total score generated for the KDQoL-SF, although a physical component score (PCS) and a mental component score (MCS) can be generated for the SF-36. The PCS is derived from the first four SF-36 domains (general health, physical functioning, role limitations–physical, bodily pain) and the MCS is derived from the second four domains (emotional well-being, role limitations–emotional, social functioning and energy/vitality).
Data from the six study centres were analysed both separately and collectively. The scores are presented as means and SDs and the number in parentheses shown against each subscale represents the number of items in the subscale. To understand centre-dependent variation, we compared the two composite scores, PCS and MCS, with their country-specific general population norms [16–20]. Population norms for France were applied to Belgium due to the lack of published information.
Genotyping
All consented patients underwent molecular genetic testing by Sanger sequencing for PKD1 and PKD2 and/or a targeted next-generation sequencing (NGS) panel. Patients with no clear pathogenic variant detected after Sanger sequencing or targeted NGS were screened for large rearrangements using multiplex ligation-dependent probe amplification and array-based comparative genomic hybridization.
TKV analysis
MRI was performed using a standardized MRI protocol without the use of intravenous contrast. TKV analysis was performed by individual centres from T2 or T1-weighted (Bergamo) coronal MR images using a variety of available methods, including manual segmentation (Sheffield, Groningen, Brussels, Bergamo), semi-automated manual contouring using ITK-SNAP (Brest) [21] or by applying the ellipsoid formula (Barcelona) [22]. TKV values were corrected for patient height (m) to generate ht-TKV (mL/m).
Patient stratification
Patients were then stratified into three groups based on known measures or predictors of disease severity, i.e. baseline eGFR (Group 1: >90, Group 2: 60–90, Group 3: 30–59 mL/min/1.73 m2), ht-TKV (Group 1: <500, Group 2: 501–1000, Group 3: >1000 mL/m) and genotype (Mutation Group 1: PKD1-truncating, Mutation Group 2: PKD1-non-truncating, Mutation Group 3: PKD2-no mutation detected). The eGFR groups correlated with CKD-EPI-defined CKD classes 1–3. For TKV we divided the cohort approximately into tertiles after adjustment for height; a ht-TKV >650 mL/m cut-off has a predictive value for the onset of Stage 3 CKD [23]. The genotype groups have non-overlapping Kaplan–Meier survival curves for end-stage renal disease [24].
Statistical analysis
For each centre, all centre patient characteristics as well as clinical and laboratory measurements were analysed and reported as both means and SDs for continuous data or as numbers and percentages for categorical data. HRQoL scores and age were summarized using measures of central tendency and dispersion. Continuous data were examined and tested for normality to decide whether parametric or non-parametric tests were to be used. Analysis of variance (ANOVA), Student's t-test chi-squared test or Fisher's exact test, were performed as appropriate to compare for statistically significant differences between groups and Tukeys post hoc tests were performed to indicate which of the groups were significantly different.
To select which variables to include in multiple regression analysis, binary regression was performed using all of the variables of interest from Table 1 as independent variables and each of the subscales from the KDQoL-SF as dependent variables. All the results with a significance of P ≤ .25 were then included in the multiple regression analysis. Bonferroni correction was applied for multiple testing. A P-value of <.05 was chosen to indicate statistical significance for the final results.
Table 1.
Sociodemographic and clinical characteristics of participants
| Variable | All centres (N = 465) | UK (n = 77) | Spain (n = 84) | Italy (n = 41) | France (n = 48) | Belgium (n = 99) | Netherlands (n = 116) |
|---|---|---|---|---|---|---|---|
| Age (years), mean (SD) | 43.2 | 43.4 | 42.7 | 44.0 | 47.4 | 41.0 | 43.1 |
| (12.8) | (14.0) | (10.4) | (10.8) | (13.5) | (14.0) | (12.4) | |
| Female, n (%) | 256 | 41 | 48 | 22 | 27 | 59 | 59 |
| (55.1) | (53.2) | (57.1) | (53.7) | (56.3) | (59.6) | (50.9) | |
| Married (yes), n (%) | 243 | 45 | 38 | 21 | 26 | 46 | 67 |
| (52.3) | (58.4) | (45.2) | (51.2) | (54.2) | (46.5) | (57.8) | |
| Ethnicity (White European), n (%) | 448 | 71 | 82 | 41 | 48 | 96 | 110 |
| (96.3) | (92.%) | (97.6) | (100.0) | (100.0) | (97.0) | (94.8) | |
| BMI, mean (SD) | 26.0 | 29.0 | 24.8 | 24.5 | 24.5 | 24.8 | 26.9 |
| (6.5) | (8.9) | (3.2) | (12.0) | (4.5) | (4.9) | (4.9) | |
| Highest education (university), n (%) | 159 | 21 | 35 | 7 | 23 | 34 | 39 |
| (34.2) | (27.3) | (41.7) | (17.1) | (47.9) | (34.3) | (33.6) | |
| Smoker, (yes), n (%) | 75 | 6 | 13 | 6 | 12 | 11 | 27 |
| (16.1) | (7.8) | (15.5) | (14.6) | (25.0) | (11.1) | (23.3) | |
| Flank pain (yes), n (%) | 151 | 40 | 19 | 12 | 16 | 33 | 40 |
| (32.5) | (51.9) | (22.6) | (29.3) | (33.3) | (33.3) | (34.5) | |
| Hypertension (yes), n (%) | 273 | 43 | 38 | 22 | 35 | 56 | 79 |
| (58.7) | (55.8) | (45.2) | (53.7) | (72.9) | (56.6) | (68.1) | |
| Employment (full-time), n (%) | 250 | 43 | 45 | 27 | 31 | 52 | 52 |
| (53.8) | (55.8) | (53.6) | (65.9) | (64.6) | (52.5) | (44.8) | |
| eGFR (mL/min/1.73 m2), mean (SD) | 76.7 | 69.0 | 81.2 | 79.4 | 66.8 | 86.3 | 73.9 |
| (25.2) | (20.8) | (22.7) | (21.6) | (26.3) | (23.1) | (28.5) | |
| ht-TKV, mean (SD) | 848.9 | 664.5 | 801.9 | 923.5 | 1093.5 | 772.4 | 913.8 |
| (621.5) | (459.6) | (513.7) | (579.9) | (655.7) | (663.8) | (688.5) | |
| TKV, mean (SD) | 1489.0 | 1149.1 | 1480.1 | 1585.6 | 1861.7 | 1323.3 | 1629.1 |
| (1108.4) | (794.6) | (1098.3) | (983.7) | (1128.5) | (1112.8) | (1240.2) | |
| PKD1 (%) | 65.8 | 74.0 | 61.9 | –a | 91.7 | 58.6 | 76.7 |
aExcluded due to large proportion of missing data.
RESULTS
Patient characteristics
The sociodemographic and baseline clinical characteristics of the 465 participants in the CYSTic I cohort are shown in Table 1. The mean age of the cohort was 43.2 years (SD 12.8) and included slightly more females (55%) than males. They were predominantly white Europeans (96%) and slightly more than half were married (52%). A minority were smokers (16%) and 54% were in full-time employment. In all, 33% reported experiencing flank pain and 57% had been diagnosed with hypertension. The mean BMI of the cohort was 26.0 kg/m2 (SD 6.5), eGFR 76.7 mL/min/1.73 m2 (SD 25.2), ht/TKV 848.9 mL/m (SD 621.5) and TKV 1489.0 mL (SD 1108.4). There were no significant differences between the study centres for age, gender, ethnicity, marital status and hypertension. However, there were significant centre differences by ANOVA for other factors such as BMI, university education, smoking history, flank pain, full-time employment, eGFR, ht-TKV and total TKV.
Total and centre-specific HRQoL scores
Data from each study centre were analysed separately and collectively to generate the scores (Supplementary data, Table S1). For the 10 subscores comprising the KDQoL, all study centres recorded high scores indicating good overall HR-QoL. However, some differences between centres were detected for individual subscales.
For the subscales that make up the SF-36, mean scores were high except for general health [53.70 (SD 14.82)] and energy/vitality [47.41 (SD 20.66)]. For the latter, centre scores were lower than the norm except for the UK [58.93 (SD 21.51)] and Spain [65.12 (SD 20.12)]. For the SF-36, Italy recorded lower scores for most of the subscales compared with other study centres.
Standard population-based reference values (1998 USA) for both the PCS and MCS scales are set to 50 points with an SD of 10 points [25]. Using these reference values, we observed that the mean values for both the PCS and MCS are within these parameters, with Spain having the highest PCS [51.58 (SD 7.56)] and MCS [51.54 (SD 7.55)]. The lowest PCS values were recorded in Italy [45.15 (SD 8.30)], with the Netherlands [43.59 (SD 5.68)] recording the lowest MCS values.
HRQoL scores stratified by eGFR
Baseline eGFR information was available for 92% of patients (n = 428). Table 2 summarizes the relationship between the 20 HRQoL subscores stratified by eGFR (groups 1–3). Patients with a lower eGFR were also less likely to be in full-time employment and reported lower sexual function on the KDQoL. Significant negative associations, which reflect a reduced QoL, for three subscores in the SF-36 relating to physical health (physical functioning, role limitations–physical and PCS) were found in relation to decreasing eGFR.
Table 2.
KDQoL-SF scores by eGFR group
| KDQoL | eGFR Group 1 (>90 mL/min/1.73 m2) (n = 150) | eGFR Group 2 (60–90 mL/min/1.73 m2) (n = 168) | eGFR Group 3 (30–60 mL/min/1.73 m2) (n = 110) | P-value |
|---|---|---|---|---|
| Symptom/problem | 85.03 | 86.81 | 84.14 | .246 |
| Effects of kidney disease | 92.42 | 93.49 | 90.62 | .127 |
| Burden of kidney disease | 86.88 | 85.07 | 81.66 | .075 |
| Work | 81.56 | 87.65 | 72.90 | .001 |
| Cognitive function | 83.99 | 83.87 | 84.63 | .935 |
| Social interaction | 80.38 | 80.70 | 81.05 | .949 |
| Sexual function | 94.76 | 88.83 | 86.03 | .002 |
| Sleep | 70.30 | 67.91 | 66.67 | .271 |
| Social support | 77.50 | 79.14 | 81.00 | .566 |
| Overall health | 75.96 | 75.37 | 71.12 | .053 |
| SF-36 | ||||
| Physical function | 92.70 | 90.77 | 83.03 | <.000 |
| Role–physical | 88.48 | 86.09 | 78.01 | .021 |
| Pain | 77.27 | 76.43 | 72.78 | .226 |
| General health | 54.57 | 54.04 | 51.12 | .150 |
| Emotional well-being | 65.24 | 65.26 | 64.13 | .789 |
| Role–emotional | 75.65 | 75.83 | 70.68 | .288 |
| Social function | 81.91 | 80.00 | 76.29 | .170 |
| Energy/vitality | 49.24 | 47.27 | 43.35 | .074 |
| PCS | 50.37 | 49.67 | 47.16 | .005 |
| MCS | 46.26 | 46.17 | 45.52 | .747 |
Statistically significant values in bold (P < .05).
HRQoL scores stratified by ht-TKV
TKV information was available for 87% of patients (n = 406). Supplementary data, Table S2 summarizes the relationship between the 20 HRQoL subscores when stratified by ht-TKV (groups 1–3). Surprisingly, only one SF-36 subscore (energy/vitality) showed a significantly negative association, and therefore a worse QoL, with increasing ht-TKV.
HRQoL scores stratified by genotype
Genotyping information was available in 89% of patients (n = 415). One centre (Bergamo) had minimal genotyping information available and was therefore excluded from the analysis. Overall, 66% of patients had PKD1 mutations and 17.6% had PKD2 mutations. There was variation noted between centres: Brest had the highest percentage of PKD1 patients (91.7%) while Brussels had the lowest (58.6%); the percentage of PKD2 patients varied accordingly. Among the genotyped patients, 6.2% remained genetically unresolved (no mutation detected).
Supplementary data, Table S3 summarizes the relationship between the 20 HRQoL subscores when stratified by genotype mutation groups 1–3. Patients in mutation group 1 (PKD1-truncating) had significantly lower energy/vitality scores that are associated with decreased QoL, mirroring the change seen with ht-TKV group 3 with the largest kidneys (Supplementary data, Table S2). Differences in social support and sexual function were also unexpectedly found between the three mutation groups.
Multivariate analysis for flank pain, smoking and gender with HRQoL scores
To select the variables to include in multiple regression analysis, binary regression was performed using all of the variables of interest from Table 1 as independent variables and each of the subscales from the KDQoL-SF as dependent variables. Using this approach, we identified flank pain, reported by almost one-third of patients (32.5%), as the variable is negatively associated with the highest number (i.e. 10/20 KDQoL-SF individual subscores), suggesting an inferior QoL, including the two SF-36 composite scores, PCS and MCS (Table 3).
Table 3.
Significant multivariate results for KDQoL-SF in patients experiencing flank pain
| KDQoL subscales | Unstandardized coefficients | 95% CI for coefficients | Part correlation (part R2) | t-test for coefficients | P-value for coefficients | Bonferroni correction | ||
|---|---|---|---|---|---|---|---|---|
| β | SE | Lower | Upper | |||||
| Symptom/problem list | –5.217 | 1.472 | –8.112 | –2.323 | –0.183 (–0.177) | –3.544 | <.000 | 0.000 |
| Effects of kidney disease | –5.510 | 1.279 | –8.027 | –2.994 | –0.222 (–0.225) | –4.308 | <.000 | 0.000 |
| Burden of kidney disease | –9.399 | 1.978 | –13.289 | –5.508 | –0.245 (–0.240) | –4.751 | <.000 | 0.000 |
| Cognitive function | –5.337 | 1.922 | –9.117 | –1.558 | –0.145 (–0.142) | –2.777 | .006 | 0.042 |
| Quality of social interaction | –4.864 | 1.767 | –8.339 | –1.388 | –0.144 (–0.142) | –2.752 | .006 | 0.030 |
| Sleep | –7.584 | 2.037 | –11.590 | –3.579 | –0.194 (0.188) | –3.724 | <.000 | 0.000 |
| SF–36 subscales | ||||||||
| Overall health | –5.402 | 1.940 | –9.219 | –1.585 | –0.157 (–0.151) | –2.785 | .006 | 0.072 |
| Physical functioning | –5.677 | 1.740 | –9.099 | –2.256 | –0.164 (–0.151) | –3.262 | .001 | 0.008 |
| Role limitations–physical | –12.252 | 3.173 | –18.489 | –6.014 | –0.207 (–0.191) | –3.862 | <.000 | 0.000 |
| Pain | –8.572 | 2.239 | –12.973 | –4.170 | –0.192 (0.185) | –3.829 | <.000 | 0.000 |
| General health | –3.760 | 1.646 | –6.998 | –0.522 | –0.128 (0.124) | –2.285 | .023 | 0.184 |
| PCS | –4.550 | 1.302 | –7.121 | –1.979 | –0.263 (0.258) | –3.495 | .001 | 0.010 |
SE, standard error.
Statistically significant values in bold (P < .05).
In addition, smoking was reported by 16.1% of patients and was negatively associated with 3/20 KDQoL-SF subscores (i.e. symptom/problem list, burden of kidney disease and MCS) (Supplementary data, Table S4). Finally, we noted positive associations for female gender in two KDQoL-SF subscores (i.e. sleep and pain) (Supplementary data, Table S5). The highest correlations (negative or positive) were reported for physical limitation in those with flank pain, the burden of kidney disease for smokers and pain for female gender (Table 3, Supplementary data, Tables S4–S5).
HRQoL scores in groups with or without flank pain
When the cohort was divided into those who reported flank pain (n = 140) and those who did not (n = 289), significant differences were found in 14/20 KDQoL-SF categories, 4 more domains compared with multivariate analysis (Fig. 1, Table 4). Absolute differences between the mean values of both groups were >3 for all significant subscores, with the greatest differences (>10) detected for pain and role limitations–physical. Both groups were comparable in baseline characteristics apart from a lower percentage of females in the group reporting pain (20.6% versus 32.9%; Table 4).
FIGURE 1:
KDQoL-SF scores in patients reporting flank pain (n = 140) and those with no pain (n = 289). Only subscores with significant differences are shown (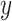 -axis).
-axis). 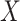 -axis scale from 40 to 100.
-axis scale from 40 to 100.
Table 4.
Baseline characteristics of groups reporting pain and no pain
| Variables | Pain [n = 144 (31.0%)] (Group 1) | No pain [n = 305 (65.6%)] (Group 2) | P-value ANOVA |
|---|---|---|---|
| Age (years), mean (SD) | 43.72 (12.79) | 44.23 (12.97) | .885 |
| Female, n (%) | 96 (20.6) | 153 (32.9) | .003 |
| Married (yes), n (%) | 81 (17.4) | 156 (33.5) | .967 |
| Smoking (yes), n (%) | 24 (5.2) | 48 (10.3) | .099 |
| BMI, mean (SD) | 25.94 (6.19) | 26.03 (6.73) | .986 |
| eGFR (mL/min/1.73 m2), mean (SD) | 75.49 (25.51) | 77.13 (25.34) | .726 |
| PKD1-truncating, n (%) | 58 (40.3) | 113 (37.0) | .794a |
| PKD1-non-truncating, n (%) | 42 (29.2) | 87 (28.5) | |
| PKD2-no mutation detected, n (%) | 35 (24.3) | 79 (25.9) | |
| Genotype unknown, n (%) | 9 (6.3) | 26 (8.5) | |
| TKV, mean (SD) | 1320 (833) | 1527 (1152) | .322 |
| ht-TKV, mean (SD) | 815 (542) | 870 (647) | .603 |
All percentages have been calculated from the total participants (n = 465). Missing data for 16 participants (3.4%).
aPearson chi-squared.
Statistically significant values in bold (p < 0.05).
Centre-specific HRQoL mean scores normalized to country-specific normative scores
The normative values (0–100) used in the KDQoL and SF-36 are based on US population norms [25]. Although good correlations have been found between US and European populations, significant variance in some domains has also been noted [26, 27]. To investigate observed centre-specific differences for HRQoL scores relevant to pain, we calculated differences between centre PCS and MCS scores with available country-specific SF-36 composite scores for their normal population (normative scores) [28]. For PCS and MCS, 5/6 and 6/6 centres reported overall negative scores and therefore decreased QoL, compared with their normal populations (Fig. 2). The largest differences (>3) were observed for MCS in 4/6 countries, while for PCS this was observed in only 2/6 countries (Italy, France). The smallest differences for both PCS and MCS were observed in the UK and Spain.
FIGURE 2:
Individual centre SF-36 mean subscores normalized to population norms for PCS and MCS.
DISCUSSION
In this large multicentre study of ADPKD patients with an eGFR ≥30 mL/min/1.73 m2 recruited from six major European countries, we report significant impairment in QoL measures affecting multiple domains using a validated HRQoL questionnaire. ADPKD patients consistently report poorer general health, less energy, poorer physical mental and emotional health and limitations in social functioning. These differences are clearly distinct aetiologically from those related to late-stage kidney disease (eGFR <30 mL/min/1.73 m2) or KRT. A moderate reduction in eGFR (30–60 mL/min/1.73 m2) was itself associated with poorer physical health, decreased sexual health and decreased ability to work in full-time employment compared with patients with near-normal eGFR (60–90, >90 mL/min/1.73 m2). These differences related to declining kidney function have been reported in a previous study [6], although not in others [3, 8]. However, the negative findings were either based on patients recruited into two clinical trials (HALT A and B) [3] or had a significant number of older patients (26%, mean age 52 years) with an eGFR >30 mL/min/1.73 m2 [8], which could have confounded the analysis.
An unexpected finding was the frequency of flank pain, a symptom reported by 32.5% of participants, which was independent of kidney function (eGFR), kidney size (TKV) or genotype. The pain was significantly negatively correlated with 10/20 subscores of the KDQoL-SF questionnaire reflecting a reduced QoL in these areas. Additional differences in both sexual and social function were noted when patient groups with and without pain were compared. Although patients with the largest kidneys (ht-TKV >1000 mL/m) and associated genotype (PKD1-truncating) reported less energy compared with those with smaller kidneys, there was no linear relationship between kidney volume and pain, as previously reported [3]. Our study nonetheless reveals that kidney pain is common, independent of kidney volume, function or genotype, but associated with significantly poorer QoL in patients experiencing it.
The variation in individual QoL scores between centres led us to question whether some of the differences observed could relate to differences between the native European populations. Using published general population country-specific composite scores, we found negative differences and therefore decreased QoL for PCS in five of six centres and MCS in all six centres [28]. However, if a threshold difference of >3 is applied for clinical significance, fewer centres were found to be different from their population norms, especially for PCS (two of six). The greater differences in MCS (four of six) suggest that taken as a whole, this group of patients is affected more by the domains contributing to MCS rather than PCS. The UK and Spain both showed the least difference for PCS and MCS, whether applying standard (US) or country-specific normative values. Similarly, the lowest scores for PCS and MCS were recorded in Italy and the Netherlands, respectively, regardless of whether standard or country-specific normative values were used. These comparisons lead us to conclude that the differences noted between the centres are not obviously related to country-specific differences.
The factors leading to poorer QoL in ADPKD patients with an eGFR ≥30 mL/min/1.73 m2 are multiple. The two major factors identified, moderate decline in eGFR and flank pain, account for some, though not all, of the measured changes. It seems likely that other specific psychosocial factors such as loss of a first-degree relative due to ADPKD, worry about transmitting the disease to the next generation (genetic ‘guilt’), which were not measured in the KDQoL-SF instrument, are important and merit further study [6]. Other physical factors (e.g. significant polycystic liver volume enlargement) that were not measured in this study have been found to contribute to poorer QoL in other cohorts [9]. Finally, it would be of interest to determine whether the availability of a disease-modifying treatment (tolvaptan), which has since become available in all six countries represented in this cohort, has had a measurable effect on QoL reporting.
Our study has some limitations. First, this was a cross-sectional study and therefore causal relationships between the associations found must remain speculative. There was also significant variability between centres for some baseline characteristics (Table 1), which may have reduced the power of the study to detect important differences. Conversely, this is presently the largest international academic-initiated observational study in ADPKD and our current findings should be relevant to more than one country, at least in Europe. Second, the participants were largely racially homogeneous (White Caucasian), so our results may not apply to other ethnic populations. Third, we were not able to exclude the potential contribution of advanced polycystic liver disease to QoL in some patients. Finally, the KDQoL-SF instrument did not include detailed questions regarding pain, analgesic use or psychosocial risk. All these issues could be explored in future studies with an amended protocol including different racial groups.
In summary, we report significantly decreased QoL in multiple domains within a large and representative cohort of European ADPKD patients. A significant contributing factor appears to be flank pain. We suggest that a greater awareness of pain as a common symptom experienced by ADPKD patients is needed and further research into better ways of managing pain in ADPKD should be a priority.
Supplementary Material
ACKNOWLEDGEMENTS
We thank all participants for their generous contributions to the success of this study.
Contributor Information
Jean Winterbottom, Academic Nephrology Unit, Department of Infection, Immunity and Cardiovascular Disease, University of Sheffield, Sheffield, UK; Sheffield Kidney Institute, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK.
Roslyn J Simms, Academic Nephrology Unit, Department of Infection, Immunity and Cardiovascular Disease, University of Sheffield, Sheffield, UK; Sheffield Kidney Institute, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK.
Anna Caroli, Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Bergamo, Italy.
Emilie Cornec-Le Gall, Brest University, Inserm, UMR 1078, GGB, CHU Brest, Brest, France.
Nathalie Demoulin, Cliniques Universitaires Saint-Luc, Université Catholique de Louvain Medical School, Brussels, Belgium.
Monica Furlano, Inherited Kidney Disorders, Nephrology Department, Fundació Puigvert, IIB Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain.
Esther Meijer, Department of Nephrology, University Medical Centre Groningen, Groningen, The Netherlands.
Olivier Devuyst, Cliniques Universitaires Saint-Luc, Université Catholique de Louvain Medical School, Brussels, Belgium.
Ron T Gansevoort, Department of Nephrology, University Medical Centre Groningen, Groningen, The Netherlands.
Yannick Le-Meur, Brest University, Inserm, UMR 1227, LBAI, CHU Brest, Brest, France.
Norberto Perico, Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Bergamo, Italy.
Roser Torra, Inherited Kidney Disorders, Nephrology Department, Fundació Puigvert, IIB Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain.
Albert C M Ong, Academic Nephrology Unit, Department of Infection, Immunity and Cardiovascular Disease, University of Sheffield, Sheffield, UK; Sheffield Kidney Institute, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK.
FUNDING
Establishment of the CYSTic cohort was funded in part by an unrestricted educational grant from Otsuka Europe and research grants from the Sheffield Kidney Research Foundation and the PKD Charity (UK).
AUTHORS’ CONTRIBUTIONS
All authors obtained consent, recruited patients, and collected and contributed data from their centre. J.W. performed the primary data analysis. A.C.M.O. obtained funding and supervised and coordinated the study. J.W. and A.C.M.O. wrote the article. All authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
R.T. is a member of the CKJ editorial board. The results presented in this article have not been published previously in whole or part except in abstract form.
REFERENCES
- 1. Ong AC, Devuyst O, Knebelmann B et al. Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet 2015; 385: 1993–2002 [DOI] [PubMed] [Google Scholar]
- 2. Spithoven EM, Kramer A, Meijer E et al. Analysis of data from the ERA-EDTA Registry indicates that conventional treatments for chronic kidney disease do not reduce the need for renal replacement therapy in autosomal dominant polycystic kidney disease. Kidney Int 2014; 86: 1244–1252 [DOI] [PubMed] [Google Scholar]
- 3. Miskulin DC, Abebe KZ, Chapman AB et al. Health-related quality of life in patients with autosomal dominant polycystic kidney disease and CKD stages 1–4: a cross-sectional study. Am J Kidney Dis 2014; 63: 214–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Barros BP, Nishiura JL, Heilberg IP et al. Anxiety, depression, and quality of life in patients with familial glomerulonephritis or autosomal dominant polycystic kidney disease. J Bras Nefrol 2011; 33: 120–128 [PubMed] [Google Scholar]
- 5. Rizk D, Jurkovitz C, Veledar E et al. Quality of life in autosomal dominant polycystic kidney disease patients not yet on dialysis. Clin J Am Soc Nephrol 2009; 4: 560–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simms RJ, Thong KM, Dworschak GC et al. Increased psychosocial risk, depression and reduced quality of life living with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 2016; 31: 1130–1140 [DOI] [PubMed] [Google Scholar]
- 7. Suwabe T, Ubara Y, Mise K et al. Quality of life of patients with ADPKD—Toranomon PKD QOL study: cross-sectional study. BMC Nephrol 2013; 14: 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eriksson D, Karlsson L, Eklund O et al. Health-related quality of life across all stages of autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 2017; 32: 2106–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Neijenhuis MK, Kievit W, Perrone RD et al. The effect of disease severity markers on quality of life in autosomal dominant polycystic kidney disease: a systematic review, meta-analysis and meta-regression. BMC Nephrol 2017; 18: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cho Y, Tong A, Craig JC et al. Establishing a core outcome set for autosomal dominant polycystic kidney disease: report of the Standardized Outcomes in Nephrology–Polycystic Kidney Disease (SONG-PKD) Consensus Workshop. Am J Kidney Dis 2021; 77: 255–263 [DOI] [PubMed] [Google Scholar]
- 11. Cho Y, Rangan G, Logeman C et al. Core outcome domains for trials in autosomal dominant polycystic kidney disease: an international Delphi survey. Am J Kidney Dis 2020; 76: 361–373 [DOI] [PubMed] [Google Scholar]
- 12. Sautenet B, Cho Y, Gutman T et al. Range and variability of outcomes reported in randomized trials conducted in patients with polycystic kidney disease: a systematic review. Am J Kidney Dis 2020; 76: 213–223 [DOI] [PubMed] [Google Scholar]
- 13. Torra R, Perez-Gomez MV, Furlano M. Autosomal dominant polycystic kidney disease: possibly the least silent cause of chronic kidney disease. Clin Kidney J 2021; 14: 2281–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pei Y, Obaji J, Dupuis A et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol 2009; 20: 205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hays RD, Kallich JD, Mapes DL et al. Development of the Kidney Disease Quality of Life (KDQOL) instrument. Qual Life Res 1994; 3: 329–338 [DOI] [PubMed] [Google Scholar]
- 16. Bowling A, Bond M, Jenkinson C et al. Short form 36 (SF-36) Health Survey questionnaire: which normative data should be used? Comparisons between the norms provided by the Omnibus Survey in Britain, the Health Survey for England and the Oxford Healthy Life Survey. J Public Health Med 1999; 21: 255–270 [DOI] [PubMed] [Google Scholar]
- 17. Leplege A, Ecosse E, Verdier A et al. The French SF-36 Health Survey: translation, cultural adaptation and preliminary psychometric evaluation. J Clin Epidemiol 1998; 51: 1013–1023 [DOI] [PubMed] [Google Scholar]
- 18. Apolone G, Mosconi P. The Italian SF-36 Health Survey: translation, validation and norming. J Clin Epidemiol 1998; 51: 1025–1036 [DOI] [PubMed] [Google Scholar]
- 19. Alonso J, Regidor E, Barrio G et al. [Population reference values of the Spanish version of the Health Questionnaire SF-36]. Med Clin (Barc) 1998; 111: 410–416 [PubMed] [Google Scholar]
- 20. Aaronson NK, Muller M, Cohen PD et al. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol 1998; 51: 1055–1068 [DOI] [PubMed] [Google Scholar]
- 21. Yushkevich PA, Piven J, Hazlett HC et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 2006; 31: 1116–1128 [DOI] [PubMed] [Google Scholar]
- 22. Irazabal MV, Rangel LJ, Bergstralh EJ et al. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol 2015; 26: 160–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bhutani H, Smith V, Rahbari-Oskoui F et al. A comparison of ultrasound and magnetic resonance imaging shows that kidney length predicts chronic kidney disease in autosomal dominant polycystic kidney disease. Kidney Int 2015; 88: 146–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cornec-Le Gall E, Audrezet MP, Chen JM et al. Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol 2013; 24: 1006–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ware JE Jr., Gandek B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol 1998; 51: 903–912 [DOI] [PubMed] [Google Scholar]
- 26. Ware JE Jr, Kosinski M, Gandek B et al. The factor structure of the SF-36 Health Survey in 10 countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol 1998; 51: 1159–1165 [DOI] [PubMed] [Google Scholar]
- 27. Gandek B, Ware JE Jr, Aaronson NK et al. Tests of data quality, scaling assumptions, and reliability of the SF-36 in eleven countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol 1998; 51: 1149–1158 [DOI] [PubMed] [Google Scholar]
- 28. Ware JE Jr., Gandek B, Kosinski M et al. The equivalence of SF-36 summary health scores estimated using standard and country-specific algorithms in 10 countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol 1998; 51: 1167–1170 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





