Abstract
Purpose
Non-small-cell lung cancer (NSCLC) comprises approximately 80% of all lung malignancies. The 5-year survival rate of patients with advanced lung cancer who lost their chances of surgery is approximately 15%. Suitable animal models are important in screening individualized treatment plans for patients with lung cancer, evaluating the pre-clinical efficacy of new drugs, and conducting basic research.
Patients and Methods
In this study, we collected malignant pleural effusion (MPE) samples from 31 patients with NSCLC, successfully constructed 11 NSCLC patient-derived xenografts (PDXs), and analyzed the factors affecting their successful establishment. Primary PDX tumors were characterized using histological analysis, immunohistochemistry, short tandem repeat (STR) profiling, and cytogenetic analysis.
Results
The PDXs preserved the histopathology and protein expression pattern of parental tumors. STR analysis revealed the PDX tissue and a tumor tissue of the same individual origin. Statistical analysis showed that the survival time of patients reflected the malignant degree of MPEs to a certain extent, thus affecting the establishment of PDXs. However, the age, gender, and clinical and biochemical indicators of the patients did not affect the establishment of PDX models.
Conclusion
These data suggest that the established NSCLC PDXs preserved the molecular characteristics of primary lung cancer and can serve as a new tool to elucidate the pathogenesis of tumors, explore new treatment methods, and conduct the research and development of new drugs for tumors.
Keywords: PDX, translational model, short tandem repeat profiling, cytogenetic analysis, survival time
Introduction
In February 2018, the National Cancer Center reported lung cancer as the leading cancer in China in terms of morbidity and mortality, with approximately 782,000 cases and 626,000 deaths annually.1 Non-small-cell lung cancer (NSCLC) accounts for approximately 80% of all lung malignancies. Chemotherapy-based comprehensive treatments, including radiotherapy and targeted therapy, are usually adopted for advanced lung cancer when the opportunity for surgery is lost. The 5-year survival rate of these patients is approximately 15%, and the expected survival time is short.2–4 Malignant pleural effusions (MPEs) are a common complication of advanced lung cancer and the manifestation of locally advanced lung cancer. Chemotherapy, targeted therapy, and immunotherapy are important treatments for advanced NSCLC.5,6 At present, most tumors are treated following the standardized scheme in industry guidelines. However, given the heterogeneity of tumors, different tumor types or different patients of the same tumor type exhibit various sensitivities to drugs, and the treatment effects vary greatly.7,8 Suitable animal models are required to screen individualized treatment plans for patients with lung cancer, evaluate the pre-clinical efficacy of new drugs, and conduct basic research. Patient-derived xenografts (PDXs) are a transplanted tumor model formed by implanting tissue blocks, primary cells, and circulating tumor cells derived from tumor patients into immunodeficient mice.9 PDXs are more predictive of clinical outcomes compared with cell line-derived xenografts.10–14 Tissue fragments from tumorectomy or biopsy are usually used to construct PDXs, but they have limited availability or cell viability and cannot be fully preserved.15–18 Limited studies have shown that tumor cells from the pleural effusion of patients with NSCLC can be easily separated and expanded and cultured efficiently. MPEs may be an excellent source of tumor-initiating cells because they can effectively reproduce in vitro and in vivo and can reproduce the natural heterogeneity of tumors.19–21
In this study, we used MPEs as the source of tumor cells for PDX establishment. We collected 31 MPE samples of NSCLC and successfully constructed 11 NSCLC PDXs. The age, gender, clinical and biochemical tests, survival time, and other factors of patients with MPEs were used to analyze the variables affecting tumor formation rate. Our results can serve as a reference for the future establishment of NSCLC PDXs with MPEs as tumor cell sources. The results of this study cannot only provide a reference for the establishment of NSCLC PDXs with MPEs as tumor cell source in the future but also lay a solid foundation for the development of the PDX model of malignant tumor accompanied with pleural effusion and ascites. In addition, the established PDXs can be used to clarify tumor pathogenesis, explore new treatment methods, understand the drug resistance mechanism of NSCLC, test drug sensitivity, and develop new tumor drugs.
Materials and Methods
Patient Characteristics
From September 2017 to January 2019, patients with NSCLC treated in Wuhan Pulmonary Hospital were included in the study, and patient data (Supplementary Table S1), including gender, age, disease stage, clinical examination and biochemical data, survival period, and immunohistochemistry, were collected prospectively. This study was approved by the Clinical Research Ethics Board of Wuhan Pulmonary Hospital (WPH201710), and all patients provided a written informed consent. All protocols adhered to the tenets of the Declaration of Helsinki. All animal studies were approved by the Institutional Animal Care and Use Committee of Nanchang Royo Biotech Co., Ltd (RYE2017090501). Standard animal care and laboratory guidelines are based on “Guidelines for the Care and Use of Laboratory Animals” (National Research Council, 8th edition, 2011).
Collection and Pretreatment of MPEs
In accordance with the TNM pathological classification of patients with lung cancer, we selected NSCLC patients with TNM grade IV and MPEs for enrollment. The process of collecting MPEs from patients with advanced lung cancer by thoracic puncture ensured strict sterility, and all MPEs were confirmed as NSCLC by exfoliative cytology. The collected MPE samples were transported to Nanchang Royo Biotech Co., Ltd. at 2 °C–8 °C within 72 h.
Phosphate-buffered saline (PBS, 10 µL, PB2004Y, China) was added with 10 µL 0.4% trypan blue dye to observe the cell number and viability. The samples were diluted with PBS based on sample viscosity. MPE samples were centrifuged at 300 g for 10 min to enrich the cells. The precipitate was collected and resuspended in PBS, and mononuclear cells were isolated using a lymphocyte separation solution (LTS10771, TBD Science, Tianjin, China) in accordance with the instructions and PBMC Isolation tubes (601,001, TBD Science, Tianjin, China). The interphase was collected, resuspended in PBS, and then centrifuged at 400 g for 10 min to collect cells. The collected cells were counted, and their viability was tested. The cell suspension was collected and transported to the animal room at low temperature (2 °C–8 °C) for inoculation.
Establishment of PDXs
All animals used in this study were 6–8-week-old female BALB/c nude mice (GemPharmatech Co., Ltd., China). The mice were bred in the animal room of Nanchang Royo Biotech Co., Ltd. Under specific pathogen-free conditions, the mice were bred on a commercial mouse diet and provided with a 12 h light-dark cycle.
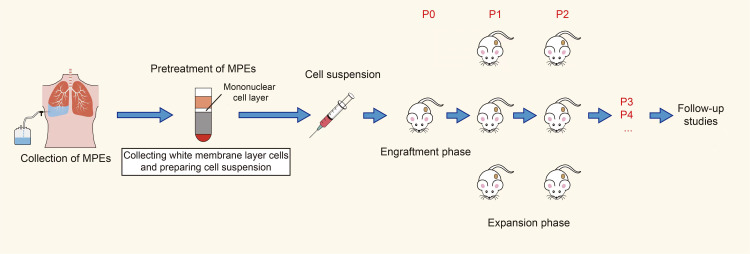
A mixture of 1:1 cell suspension and Matrigel (Corning 354,234) was injected subcutaneously into the right forelimb of the mice in a 200 µL injection volume with a cell density of 1×107. The mouse tumor volume was measured with a vernier caliper twice a week and calculated as follows: V=A*B2/2, where V is the tumor volume, A is the longest tumor diameter, and B is the shortest tumor diameter. When the tumor volume of the NSCLC PDXs reached 1000–1500 mm3, the tumor tissue was surgically stripped, and the mice were euthanized. The surgically removed tumor tissue was cut into 2 mm × 2 mm × 2 mm pieces with a surgical blade and inoculated sterile into the subcutaneous right forelimb of the new immunodeficient mice. The tumor was passaged to P4 using the same method for subsequent experimental study (Figure 1).
Figure 1.
Construction flow of PDX models for NSCLC.
Animals showing signs of skin ulcers, hunched posture, weight loss, vocalization, irritability, or lack of grooming were carefully monitored and euthanized. Euthanasia was performed by carbon dioxide.
Histological and Immunohistochemical (IHC) Analyses
Tissue samples used for hematoxylin–eosin (H&E) staining and immunohistochemistry were derived from lymph node metastases and PDXs from lung cancer patients (No. 11 and No. 27) with MPE. The tumor tissue and PDXs were fixed in formalin and embedded in paraffin. Slides (4 µm) were prepared and stained with H&E for pathological evaluation. The tissue sections were incubated with Cytokeratin 7, P40, and Thyroid Transcription Factor-1 antibodies (Zhong Shan-Golden Bridge Biological Technology Co., Ltd., Beijing, China) at 4 °C overnight. The horseradish peroxidase-IgG secondary antibody was used at 37 °C for 15 min and detected by diaminobenzidine reaction.
Short Tandem Repeat (STR) Profiling and Cytogenetic Analysis
We used QIAamp DNA FFPE Tissue Kit (#56404, Qiagen, China) to isolate DNA from formalin-fixed and paraffin-embedded tissues. The slides were dewaxed with xylene and then washed with ethanol to remove xylene. The sample was lysed overnight under denaturing conditions with proteinase K and then cultured at 90 °C to reverse the cross-linking of formalin. In a suitable solution environment, DNA was bound to the QIAamp MinElute column, and the residual pollutants were washed. Finally, the DNA was eluted. The multiple polymerase chain reaction (PCR) multiplex amplification system (CELL STRTM System) by Beijing HKgene Technology Co., Ltd. was used to analyze 20 STR loci and perform 1 sex locus amplification. The PCR products were analyzed with ABI 3130xl DNA Analyzer (Applied Biosystems). The test results were analyzed with GeneMapper ID-X v1.2 (Applied Biosystems) software.
Statistical Analysis
The association of PDX establishment with covariates was statistically analyzed. Whether the PDXs were successfully constructed was set to terminate the observation event, and factors, such as gender, survival time, and clinical examination and biochemical data, were considered covariates that may affect tumor formation rate. The classification was analyzed by chi-square test or Fisher’s exact test, whereas the analysis of continuous variables was performed to determine whether it conforms to the normal distribution and whether t-test or Mann–Whitney U-test should be used. The Log rank test was used to compare survival curves. Cox proportional hazard regression was used for univariate and multivariate survival analyses. All statistical analyses were performed using SPSS23.0 version. All P values were bidirectional, and statistical significance was considered at P < 0.05.
Results
Establishment of PDXs of NSCLC
In this study, we collected 31 cases of MPE samples of NSCLC with TNM grade IV and MPE and successfully constructed 11 cases of NSCLC with a tumor formation rate of 35.5%. The PDXs were established in nude mice and successfully passaged for other analyses. In this study, we selected the tumor tissues of two patients and their corresponding PDX models for histological and IHC analyses, in which patient NO. 11 was displayed in the results section, and patient NO. 27 was displayed in the Supplementary Materials (Supplementary Figures S1 and S2).
PDXs Preserve the Histopathology and Protein Expression Pattern of the Parental Tumor
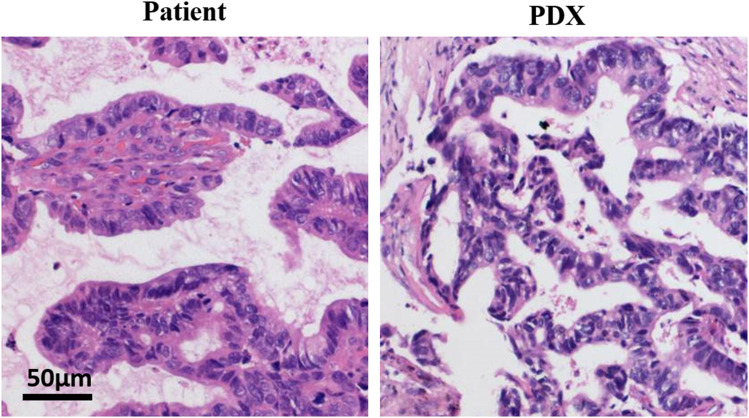
Evaluation of patient tumor showed that irregular glandular tubular cell nests were found in the fibro-fatty tissue. Several were papillary, the epithelial cells were crowded, the nucleus was large and vacuolated, and nuclear divisions were easily observed. Coagulative necrosis was found in certain areas (Figure 2). The tumor evaluation of PDXs showed a typical adenocarcinoma area under the microscope, the cancer tissue was solid nested, and the shape was similar to that of the original tumor (Figure 2). In summary, the established PDXs were consistent with the histological characteristics of the original tumor.
Figure 2.
The PDX models are consistent with the histological. Tumor tissues from patient (NO.11) and the P4 generation PDX model were fixed and examined with H&E staining.
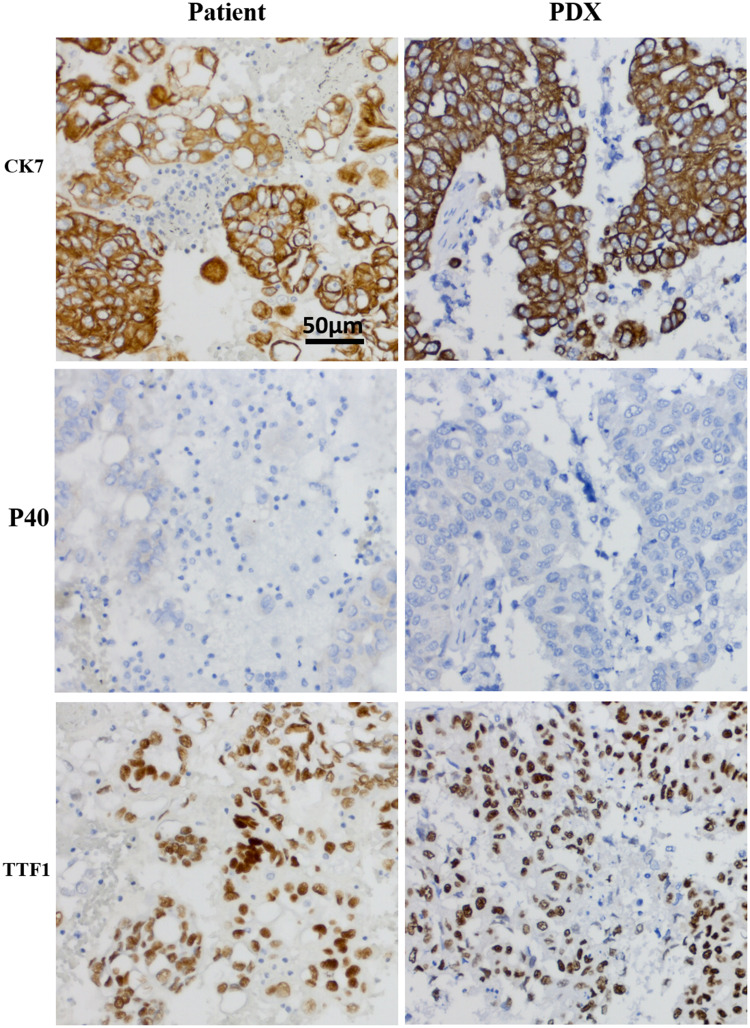
CK7 and TTF-1 are common markers of lung adenocarcinoma, and P40 is a common marker of lung squamous cell carcinoma. CK7, P40, and TTF-1 immunohistochemistry was performed on the patient samples and PDXs. IHC results of PDXs and NSCLC patients, which were CK7(+), P40(-) and TTF-1(+), were the same (Figure 3). The P40 negative result ruled out the possibility of lung squamous cell carcinoma, and CK7 and TTF-1 positive finding confirmed that the established PDXs and original tumor tissues were lung adenocarcinoma. The established PDXs maintained the IHC characteristics of the patients with NSCLC.
Figure 3.
The PDX models have the same protein expression pattern with the original tumor.
Notes: HC images of the lymph node metastasis of patients and P4 generation PDX. Tumor tissues from the patient (NO.11) and the P4 generation PDX were fixed and immunohistochemical with specific antibodies.
In summary, the established PDXs were consistent with the histological features and protein expression pattern of the original tumor.
STR Analysis Revealed PDX Tissue and Tumor Tissue of the Same Individual Origin
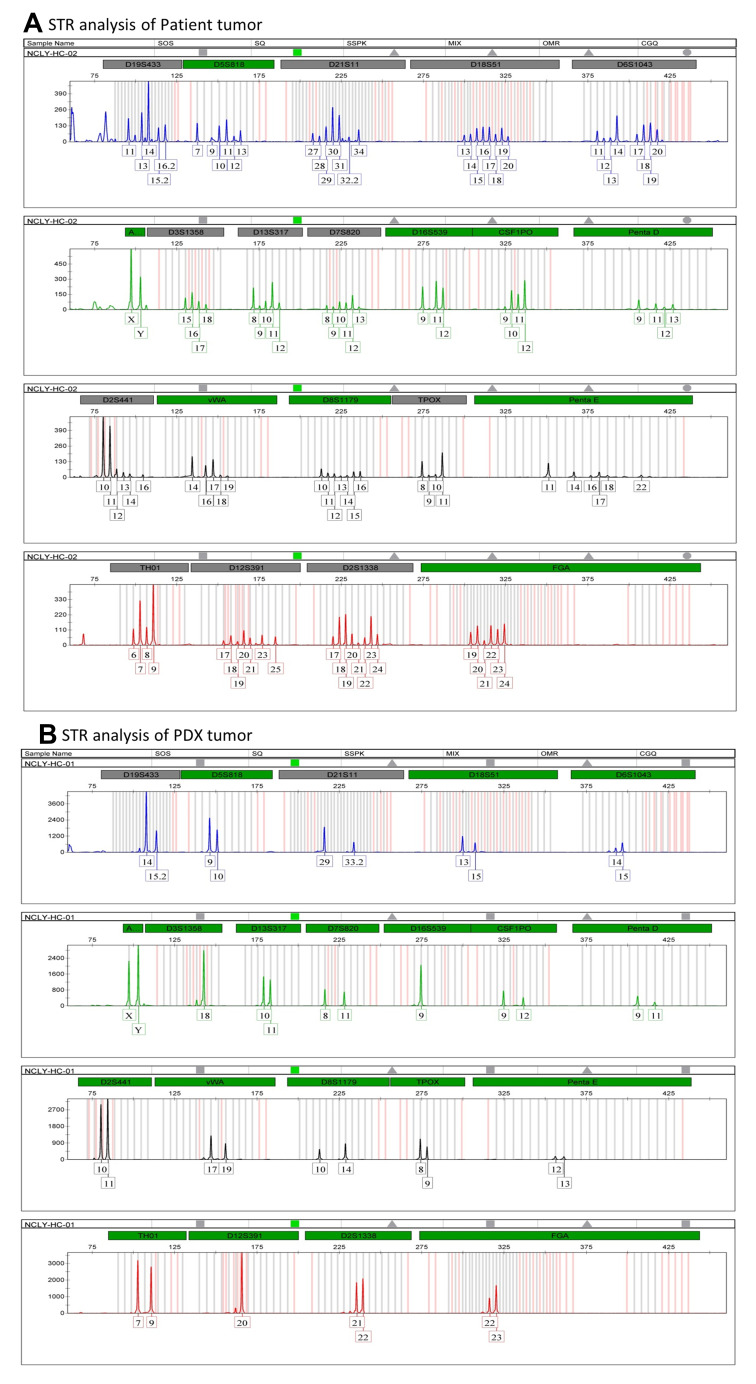
STRs, also known as microsatellite DNA, are mainly used in genetic linkage map analysis, family identification, identity authentication, and other fields. The DNA samples of patients and PDXs were tested for STR genotyping in accordance with the above steps. The STR data showed no cross-contamination of other human cells. STR analysis results showed that the two STR data conformed to the law of inheritance and can be judged as the same individual source (Figure 4).
Figure 4.
PDX tissue and tumor tissue are the same individual origin by STR profile.
Notes: (A) Short tandem repeat (STR) profile of patient tissue. (B) Short tandem repeat (STR) profile of PDX.
Characteristics of Patients and MPEs Did Not Affect the Establishment of PDXs
The PDX tumor formation rate was compared with these characteristics to determine whether any characteristics influence the successful establishment of PDXs (Table 1). The results based on age, gender, tumor metastasis, color, transparency, cell number, total protein content, lactate dehydrogenase (LDH) content, and carcinoembryonic antigen content of the MPEs showed no significant difference in PDX tumor formation rate. Therefore, the age, sex, clinical biochemical indicators, characteristics of MPEs, and other factors did not affect the establishment of PDX.
Table 1.
Baseline Characteristics of Participants in the Study (N = 31)
| All (n=31) | PDX Established (n=11) | PDX Did Not Develop (n=20) | P-value+ | ||||
|---|---|---|---|---|---|---|---|
| Age (years) | 59.81±11.86 | 56.2±13.3 | 61.8±10.9 | 0.213b | |||
| Sex (%) | Male (%) | 18 (58.1%) | 4 (36.4%) | 14 (70%) | 0.128a | ||
| Female (%) | 13 (41.9%) | 7 (63.6%) | 6 (30%) | ||||
| Color of MPEs (%) | Red (%) | 14 (45.2%) | 5 (45.5%) | 9 (45%) | 1.000a | ||
| Yellow (%) | 17 (54.8%) | 6 (54.5%) | 11 (55%) | ||||
| Transparency of MPEs (%) | Cloudy (%) | 28 (90.3%) | 10 (90.9%) | 18 (90%) | 1.000a | ||
| Clear (%) | 3 (9.7%) | 1 (9.1%) | 2 (10%) | ||||
| Cell counts of MPEs (10^6/L) | 1400 (800,3160) | 1800 (500,3500) | 1300 (800,3133) | 0.804c | |||
| Total protein in MPEs (g/L) | 39.08±10.15 | 38.61±7.02 | 39.35±11.68 | 0.851b | |||
| Total protein in venous blood (g/L) | 59.22±5.35 | 58.76±5.44 | 59.48±5.42 | 0.730b | |||
| LDH in MPEs (U/L) | 595 (300,866) | 683 (443,906) | 577 (250,840) | 0.509c | |||
| LDH in venous blood (U/L) | 195 (139,241) | 169 (143,284) | 196 (139,235) | 0.901c | |||
| Ratio of LDH | 3.094±1.962 | 3.552±1.759 | 2.842±2.064 | 0.343b | |||
| Tumor metastasis (%) | Single (%) | 14 (45.2%) | 7 (63.6%) | 7 (35%) | 0.153a | ||
| Multiple (%) | 17 (54.8%) | 4 (36.4%) | 13 (65%) | ||||
| CEA in MPEs (ng/mL) | 359.40 (34.66,1000.00) | 362.20 (73.15,949.70) | 201.37 (16.76,1000.00) | 0.684c | |||
| CEA in venous blood (ng/mL) | 26.49 (7.45,161.2) | 28.19 (10.64,59.12) | 19.28 (5.1,253.56) | 0.710c | |||
Notes: aFisher’s exact test; bStudent’s t-test; cmann–Whitney U-test. +For comparisons of normally distributed variables, non-normal data and categorical variables, Student’s t-test, Mann–Whitney U-test, and Fisher’s exact test were used, respectively.
Abbreviations: TP, total protein; LDH, lactate dehydrogenase; CEA, carcinoembryonic antigen.
Correlation of Engraftability and Clinical Outcome
As a key point, we examined whether the survival of NSCLC patients with MPEs affected the establishment of PDXs. Except for one patient who could not be followed up due to loss of contact, we analyzed the survival of 10 (33.3%) patients in the tumor group and 20 (66.7%) patients in the nontumor group.
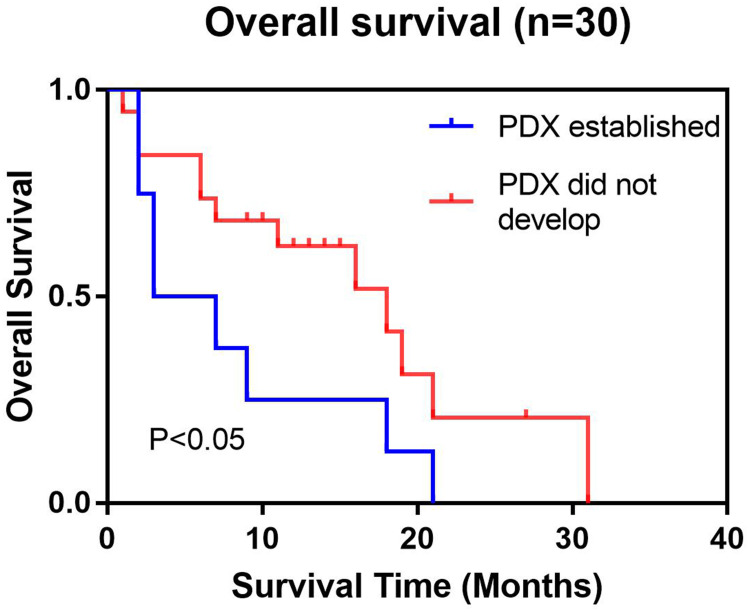
The survival time of patients in the tumor group (median survival = 3 (month), 95% confidence interval (CI): 1.5–4.5) was significantly lower than that of patients in the nontumor group (median survival time = 16 (month), 95%: CI 7.0–25.0). In the Log rank test, P = 0.031 (P < 0.05, Figure 5, Table 2). Thus, the survival time of patients was evidently related to the establishment of PDX.
Figure 5.
The survival rates of the patients corresponding to the successful establishment of PDXs were significantly lower. The survival curves of the groups with successful PDX establishment and those without successful PDX establishment showed significant differences in survival.
Table 2.
Log Rank Test to Compare the Relationship Between Survival Period and Tumor Development
| Univariate Analysis | ||||
|---|---|---|---|---|
| Covariates | N(30) | Event | Median Time of Lifetime (Months) (95% CI) | P |
| PDX established | 10 | 10 | 3 (1.5–4.5) | 0.031 (P<0.05) |
| PDX did not develop | 20 | 13 | 16 (7.0–25.0) | |
Discussion
The establishment of an appropriate preclinical model is essential for translational cancer research. As a preclinical model, PDX has shown advantages in drug screening, biomarker development, and joint clinical trials.22–24 In recent years, the number of PDXs of lung cancer has gradually increased. Previous studies used resected or biopsy tumor tissues to construct PDXs, but the present research used the MPEs of patients with lung cancer because most of patients with MPEs lose the opportunity for operation, and surgical samples cannot be obtained upon diagnosis. Moreover, the number of tumors, including primary NSCLC, and the ratio of transplanted tumors are limited, and surgical debris must be processed quickly, usually with a limited amount of raw tumor material. However, limited studies have shown that tumor cells from the pleural effusion of patients with NSCLC can be easily isolated and expanded and cultured efficiently. Therefore, we used MPEs as the source of tumor cells to establish a NSCLC PDX and achieved better results.
A large number of studies have confirmed that PDXs can preserve well the morphological characteristics and protein expression patterns of tumors, and our experimental results are consistent with their findings.25–27 Therefore, in this study, we selected the tumor tissues of two patients and their corresponding PDXs for histological and IHC analysis. H&E staining results showed that PDXs preserved the histological appearance of the primary tumor. Different pathological types of cancer have various clinical indicators. The IHC markers selected in this study included CK7, P40, and TTF-1. CK7 is the most sensitive immunological marker for lung adenocarcinoma, with a positive rate of nearly 100%. Therefore, CK7 is the first choice for the identification of primary lung adenocarcinoma. TTF-1 positivity also often indicates lung adenocarcinoma; squamous cell carcinoma is generally negative, and P40 is a common marker for lung squamous cell carcinoma. PDX tissue and original tumor p40 were negative, excluding squamous cell carcinoma. Considering the results of immunohistochemistry, we determined that the PDX tissue and original tumor were typical adenocarcinoma. Both presented consistent IHC features. The tumor tissue used for H&E staining and IHC analysis in this study was derived from lymph node metastases of patients with lung cancer instead of MPEs to obtain accurate results. In addition, our models used free cancer cells, which were in a liquid environment, that were extracted from MPEs. However, after the isolated cancer cells formed a solid tumor in the mouse, typical adenocarcinoma areas with solid nests appeared, consistent with the histological characteristics of the original tumor. The free cancer cells in MPEs can still form glandular lumen structures.
The survival period of patients significantly affected the establishment of PDXs. The survival period itself was our key indicator before starting the study. We speculated that the survival period of patients can comprehensively reflect the degree of tumor malignancy to a certain extent. The higher the degree of malignancy, the higher the chance of tumor formation. In addition, statistics showed that whether a PDX was established is a risk factor for survival. This finding inversely proves that the more malignant tumor cell samples are, the shorter the survival time of the patient. Thus, the survival time of patients who successfully established PDXs was significantly shorter than that of patients who failed to establish PDXs.
The color and transparency of MPEs were also our concern. We speculated that the bloody pleural fluid may be caused by the malignant degree of the tumor itself, but the experimental results did not support this assumption. In addition, the cell count of the extracted MPEs did not affect the establishment of PDXs. We speculated that the cell count of the MPE samples can reflect tumor malignancy to a certain extent. Therefore, the cell count of MPEs was determined, and no statistical difference was found. We speculated the small sample size as one of the reasons. In addition, MPEs contain neutrophils, lymphocytes, mesothelial cells, and so on because each patient has different ratios between cells of MPEs, and the cell count of MPEs does not fully reflect the number of cancer cells, which may explain the lack of statistical difference. In the follow-up research, we can further explore this speculation.
In this experiment, we used lymphocyte separation solution to separate mononuclear cells in MPEs, that is, the interphase. These monocytes are not only cancer cells but also epithelial cells, inflammatory cells, and so on. We did not further isolate cancer cells. As a result, we can inoculate a mixture of cells to immunodeficient mice. The advantages of this process are simple operation and low cost. However, whether the mixed cells of nontumor cells affect the tumorigenesis rate of PDXs is unclear. In subsequent experiments, we can use magnetic bead purification or flow sorting to isolate cancer cells and construct a PDX. Then, we can explore the influence of nontumor cells in monocytes on the tumor formation rate of PDXs.
DNA was extracted from the established P4 tissues by PCR and analyzed by electrophoresis (Supplementary Table S2). The samples tested contained genes from mice and humans (Supplementary Figure S3). Thus, the xenotransplantation model we established was derived from human tissues. To further verify the genetic relationship between PDX samples and patient tissue, we selected the tissue of patient No. 11 and the corresponding PDX model for STR testing. The STR results showed that the STR data of PDX and primary tumor conformed to genetic rules, and they can be judged to be from the same individual source.
Conclusion
H&E staining, IHC, PCR and STR of the patient samples and tumor-bearing mouse samples revealed similar morphological and molecular characteristics, which can be judged to have the same individual source. In addition, the patient’s survival rate was negatively correlated with establishment of PDXs. However, Characteristics of patients and MPEs did not affect the establishment of PDXs. Our research confirmed that MPEs are a good source of tumor cells and provided a reference for the establishment of NSCLC PDXs in the study of the pathogenesis and treatment of the disease.
Acknowledgments
This study was supported by the Natural Science Foundation of Jiangxi Province (No. 20192BCD40003 to YQH), the Health Commission of Hubei Province Scientific Research Project (No. WJ2019H428 to ML), and the Health Commission of Wuhan Municipal Scientific Research Project (No. WX19B05 to ML).
Disclosure
Yuanqiao He is an employee of Nanchang Royo Biotech Co, Ltd. The authors report no conflicts of interest in this work.
References
- 1.Chen W, Sun K, Zheng R, et al. Cancer incidence and mortality in China, 2014. Chin J Cancer Res. 2018;30(1):1–12. doi: 10.21147/j.issn.1000-9604.2018.01.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Z, Fillmore CM, Hammerman PS, et al. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014;14(8):535–546. doi: 10.1038/nrc3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffman PC, Mauer AM, Vokes EE. Lung cancer. Lancet. 2000;355(9202):479–485. doi: 10.1016/S0140-6736(00)82038-3 [DOI] [PubMed] [Google Scholar]
- 4.Ettinger DS, Akerley W, Borghaei H, et al. National comprehensive cancer network. Non-small cell lung cancer, version 2.2013. J Natl Compr Canc Netw. 2013;11(6):645–53;quiz 653. doi: 10.6004/jnccn.2013.0084 [DOI] [PubMed] [Google Scholar]
- 5.Morgensztern D, Waqar S, Subramanian J, et al. Prognostic impact of malignant pleural effusion at presentation in patients with metastatic non-small-cell lung cancer. J Thorac Oncol. 2012;7(10):1485–1489. doi: 10.1097/JTO.0b013e318267223a [DOI] [PubMed] [Google Scholar]
- 6.Taghizadeh N, Fortin M, Tremblay A. US hospitalizations for malignant pleural effusions: data from the 2012 national inpatient sample. Chest. 2017;151(4):845–854. doi: 10.1016/j.chest.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 7.Yeh CT, Wu AT, Chang PM, et al. Trifluoperazine, an antipsychotic agent, inhibits cancer stem cell growth and overcomes drug resistance of lung cancer. Am J Respir Crit Care Med. 2012;186(11):1180–1188. doi: 10.1164/rccm.201207-1180OC [DOI] [PubMed] [Google Scholar]
- 8.Huisman C, Smit EF, Giaccone G, Postmus PE. Second-line chemotherapy in relapsing or refractory non-small-cell lung cancer: a review. J Clin Oncol. 2000;18(21):3722–3730. doi: 10.1200/JCO.2000.18.21.3722 [DOI] [PubMed] [Google Scholar]
- 9.Gao H, Korn JM, Ferretti S, et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat Med. 2015;21(11):1318–1325. doi: 10.1038/nm.3954 [DOI] [PubMed] [Google Scholar]
- 10.Byrne AT, Alférez DG, Amant F, et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat Rev Cancer. 2017;17(4):254–268. doi: 10.1038/nrc.2016.140 [DOI] [PubMed] [Google Scholar]
- 11.Kim HR, Kang HN, Shim HS, et al. Co-clinical trials demonstrate predictive biomarkers for dovitinib, an FGFR inhibitor, in lung squamous cell carcinoma. Ann Oncol. 2017;28(6):1250–1259. doi: 10.1093/annonc/mdx098 [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Chen HN, Wang K, et al. Ketoconazole exacerbates mitophagy to induce apoptosis by downregulating cyclooxygenase-2 in hepatocellular carcinoma. J Hepatol. 2019;70(1):66–77. doi: 10.1016/j.jhep.2018.09.022 [DOI] [PubMed] [Google Scholar]
- 13.Fong EL, Wan X, Yang J, et al. A 3D in vitro model of patient-derived prostate cancer xenograft for controlled interrogation of in vivo tumor-stromal interactions. Biomaterials. 2016;77:164–172. doi: 10.1016/j.biomaterials.2015.10.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Zhou L, Xie K, et al. Polylactide-tethered prodrugs in polymeric nanoparticles as reliable nanomedicines for the efficient eradication of patient-derived hepatocellular carcinoma. Theranostics. 2018;8(14):3949–3963. doi: 10.7150/thno.26161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ilie M, Nunes M, Blot L, et al. Setting up a wide panel of patient-derived tumor xenografts of non-small cell lung cancer by improving the preanalytical steps. Cancer Med. 2015;4(2):201–211. doi: 10.1002/cam4.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein AP, Saha S, Liu CZ, et al. Influence of handling conditions on the establishment and propagation of head and neck cancer patient derived xenografts. PLoS One. 2014;9(6):e100995. doi: 10.1371/journal.pone.0100995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roscilli G, De Vitis C, Ferrara FF, et al. Human lung adenocarcinoma cell cultures derived from malignant pleural effusions as model system to predict patients chemosensitivity. J Transl Med. 2016;14:61. doi: 10.1186/s12967-016-0816-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujii E, Kato A, Chen YJ, et al. The status of donor cancer tissues affects the fate of patient-derived colorectal cancer xenografts in NOG mice. Exp Anim. 2015;64(2):181–190. doi: 10.1538/expanim.14-0080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mancini R, Giarnieri E, De Vitis C, et al. Spheres derived from lung adenocarcinoma pleural effusions: molecular characterization and tumor engraftment. PLoS One. 2011;6(7):e21320. doi: 10.1371/journal.pone.0021320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grimshaw MJ, Cooper L, Papazisis K, et al. Mammosphere culture of metastatic breast cancer cells enriches for tumorigenic breast cancer cells. Breast Cancer Res. 2008;10(3):R52. doi: 10.1186/bcr2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basak SK, Veena MS, Oh S, et al. The malignant pleural effusion as a model to investigate intratumoral heterogeneity in lung cancer. PLoS One. 2009;4(6):e5884. doi: 10.1371/journal.pone.0005884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klinghammer K, Walther W, Hoffmann J. Choosing wisely - preclinical test models in the era of precision medicine. Cancer Treat Rev. 2017;55:36–45. doi: 10.1016/j.ctrv.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 23.Wakefield CE, Doolan EL, Fardell JE, et al. The avatar acceptability study: survivor, parent and community willingness to use patient-derived xenografts to personalize cancer care. EBioMedicine. 2018;37:205–213. doi: 10.1016/j.ebiom.2018.10.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinrich MA, Mostafa AMRH, Morton JP, et al. Translating complexity and heterogeneity of pancreatic tumor: 3D in vitro to in vivo models. Adv Drug Deliv Rev. 2021;174:265–293. doi: 10.1016/j.addr.2021.04.018 [DOI] [PubMed] [Google Scholar]
- 25.Park B, Jeong BC, Choi YL, et al. Development and characterization of a bladder cancer xenograft model using patient-derived tumor tissue. Cancer Sci. 2013;104(5):631–638. doi: 10.1111/cas.12123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xin Y, Li S, Jiang Q, et al. Establishment of a jaw fibrosarcoma patient-derived xenograft and evaluation of the tumor suppression efficacy of plumbagin against jaw fibrosarcoma. Front Oncol. 2020;10:1479. doi: 10.3389/fonc.2020.01479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X, Shen C, Wei Z, et al. Patient-derived non-small cell lung cancer xenograft mirrors complex tumor heterogeneity. Cancer Biol Med. 2021;18(1):184–198. doi: 10.20892/j.issn.2095-3941.2020.0012 [DOI] [PMC free article] [PubMed] [Google Scholar]