Abstract
Objective
To evaluate whether exogenous surfactant therapy may be useful in adult patients with acute lung injury or acute respiratory distress syndrome, using a meta-analysis of published clinical trials.
Design
A comprehensive literature search was performed to identify all randomized clinical trials examining the effects of the treatment of acute lung injury/acute respiratory distress syndrome with exogenous surfactant in adults. The primary outcome measurement was mortality 28 or 30 days after randomization. Secondary outcome measurements included a change in the ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen in the first 24 hours or after 120 hours, the number of ventilation-free days, and any adverse effects. The meta-analysis was performed using the Review Manager 5.0.0 system.
Participants
Randomized clinical trials.
Intervention
Meta-analysis of 9 trials.
Measurements and Main Results
Nine trials involving 2,575 patients were included in the meta-analysis. The analysis showed that treatment with exogenous pulmonary surfactant does not decrease mortality significantly. There was a significant effect of exogenous surfactant treatment on the change in the partial pressure of arterial oxygen/fraction of inspired oxygen ratio in the first 24 hours but this was lost by 120 hours. The duration of ventilation trended lower in surfactant-treated patients but this was not significant. In addition, surfactant-treated patients had a significantly higher risk of adverse effects.
Conclusions
An exogenous surfactant may improve oxygenation over the first 24 hours after administration. However, treatment does not improve mortality and oxygenation over ≥120 hours after administration and results in a high rate of adverse effects. Therefore, the present data suggest that an exogenous surfactant cannot be considered an effective adjunctive therapy in patients with acute lung injury/acute respiratory distress syndrome.
Key Words: randomized control trial, PaO2:FIO2, exogenous surfactant, pulmonary surfactant, adverse effects
ACUTE LUNG INJURY (ALI) and acute respiratory distress syndrome (ARDS) usually are associated with decreased surfactant production and function, which may lead to an increased alveolar surface tension, alveolar collapse, and decreased parenchymal compliance.1, 2, 3, 4 Airway pressures needed to open alveoli that have low surfactant are exceedingly high. Some case-control studies have shown that treatment with exogenous surfactant can improve oxygenation and decrease mortality in ALI/ARDS.5, 6 Theoretically, the exogenous surfactant can decrease surface tension, increase parenchymal compliance, and make ventilation easier, which would lead to a better clinical outcome. Furthermore, the surfactant has important roles in the host immune defense through specific and nonspecific mechanisms, and it should have the ability to protect against ventilator-associated pneumonia.7 However, other research has shown no significant improvement in mortality or oxygenation with exogenous surfactant treatment in patients with ALI/ARDS.7, 8, 9 The different findings may be caused by the differences in the techniques of surfactant administration and the formulation of the surfactant used. Work continues on improving surfactant delivery techniques, but it remains unclear whether the pulmonary effects of surfactant treatment are sufficient to alter the clinical outcome.
A meta-analysis performed >4 years previously investigated the effectiveness of an exogenous pulmonary surfactant in the treatment of adult patients with ALI or ARDS and reported that an exogenous surfactant may improve oxygenation but not mortality.7 In the subsequent 4 years, several more clinical randomized controlled trials (RCTs) assessing the effectiveness of a surfactant in ALI/ARDS have been reported, and this has increased the overall sample. The present meta-analysis used this new larger sample and extra data from the recent trials to further evaluate the effectiveness of surfactant administration to adult patients with ALI/ARDS.
Methods
Eligibility and Search Strategy
A comprehensive literature search was performed to identify trials that compared pulmonary surfactant treatment with standard-of-care therapy in adults diagnosed with ALI or ARDS. The literature was scanned by computerized searches of PubMed, Embase, Cochrane Library, and Chinese Medical Journal Network databases from establishment through March 2011. The search strategy, including the combination of exploded Medical Subject Headings and text words, are provided in the Appendix. The reference literatures of the appropriate trials were hand searched. No language restrictions were enforced.
Study Selection and Data Extraction
Trials included in the meta-analysis were selected for the following inclusion criteria: (1) random allocation to a pulmonary surfactant treatment compared with standard-of-care therapy; (2) exclusively adult patients (>18 years old) diagnosed with ALI or ARDS; and (3) clinical outcomes that evaluated mortality and/or a change in the ratio of partial pressure of arterial oxygen (PaO2) to the fraction of inspired oxygen (FIO2). The methodologic quality was assessed using Review Manager 5.0.0 (Cochrane Collaboration, Oxford, England), which consists of items describing randomization, allocation concealment, blinding, and dropouts. If the number of “no” was ≤1 or the number of “unclear” was ≤2, then the methodologic quality of the study was judged acceptable. Two authors (H.M. and Y.S.) independently reviewed the trials according to the inclusion criteria. If the inclusion or exclusion was uncertain, discussions with coworkers, teachers, or specialists (J.L. and Q.L.) confirmed the choice. The examiners were not blinded to institutions, authors, or journal names. The information was extracted on the type of surfactant; the dose, duration, and delivery method of treatment; the mean age or age range of patients; the sex ratio; the basic PaO2:FIO2 ratio; the Acute Physiology and Chronic Health Evaluation (APACHE) score; and the etiologies of ALI/ARDS. In cases of incomplete or unclear data, authors of the publications were contacted when possible. Data were managed according to the intention-to-treat principle.
Clinical Outcomes
The primary outcome measurement was mortality 28 to 30 days after randomization. The secondary outcome measurements included a change in the PaO2:FIO2 ratio in the first 24 and 120 hours, the number of ventilation-free days, the mean duration of ventilation, and the rate of adverse effects.
Statistical Analysis
Statistical analysis was performed using Review Manager 5.0.0. The odds ratio (OR) and 95% confidence interval (CI) for categoric variables were calculated using a fixed-effect model with the Mantel-Haenszel method. The DerSimonian-Laird random-effect model also was applied to the calculated OR for a significant heterogeneity across studies. The mean difference (MD) and 95% CI were used for continuous variables using the inverse variance method. Statistical heterogeneity was evaluated using the Q statistic with p < 0.1. Statistical significance was considered as p < 0.05.
Results
Eligible Studies
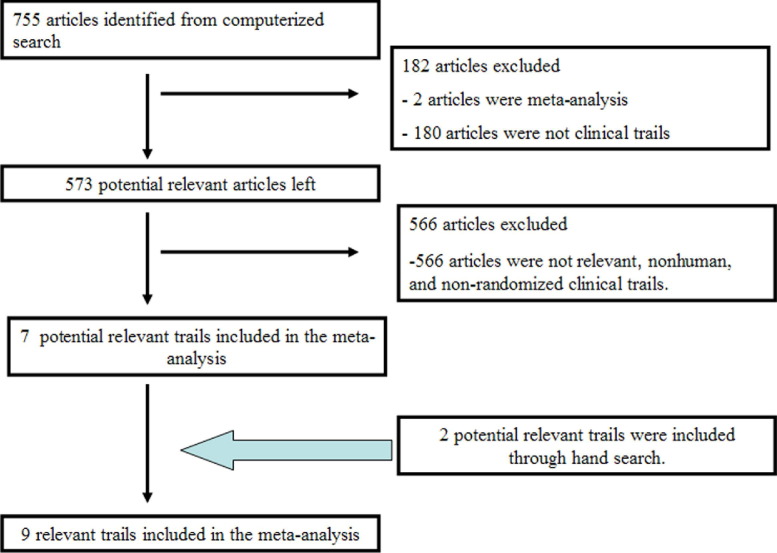
The initial screening identified 757 potentially relevant articles, including 2 from supplemental manual searches of PubMed. After reading the summary or abstract, 2 meta-analyses, 110 reviews, and 547 unrelated, nonhuman, nonadult, non-RCT studies were excluded. Abstracts of 70 non-English-language articles were read and none met the inclusion criteria. Nine of the remaining 28 studies were identified. Searches of the Embase, Cochrane Library, and Chinese Medical Journal Network databases did not identify additional articles (Fig 1).
Fig 1.
Trial selection flowchart indicates the process used for selecting relevant randomized clinical trials included in the present meta-analysis.
Study Characteristics
In total, 2,574 patients were included in the 9 trials8, 9, 10, 11, 12, 13, 14, 15, 16: 759 patients were given a surfactant containing no protein or control treatments,8, 9 1,350 patients were given a surfactant containing protein C or control treatments,11, 12, 13, 16 and 465 patients were given a surfactant containing proteins B and C or control treatments.10, 14, 15 In total, 1,168 patients had direct lung injury12, 15, 16 (Table 1).
Table 1.
Characteristics of Trials Included in the Meta-Analysis
| Study | Year | Design | Patients | Delivery Methods | Type | Dosing | Duration | Deaths: Control; Treated |
|---|---|---|---|---|---|---|---|---|
| Weg et al8 | 1994 | Multicenter: USA, Canada | 34 (C = 17, E = 17) | Aerosol | No protein | DPPC 43.5 mg/kg/d | 120 h | 8; 6 |
| Anzueto et al9 | 1996 | Multicenter: USA, Spain, France | 725 (C = 361, E = 364) | Aerosol | No protein | DPPC 112 mg/kg/d | 120 h | 143; 145 |
| Gregory et al10 | 1997 | Multicenter: USA | 32 (C = 16, E = 16) | Intratracheal | Proteins B + C | 100 mg/kg LBW | 96 h | 7; 3 |
| Spragg et al11 | 2003 | Multicenter: USA, Canada | 28 (C = 13, E = 15) | Intratracheal | Protein C | 1 mg/kg LBW, 4 doses | 24 h | 5; 3 |
| Spragg et al12 | 2004 | Multicenter: USA, Canada, Europe, South Africa | 448 (C = 224, E = 224) | Intratracheal | Protein C | 1 mg/kg LBW, 4 doses | 24 h | 72; 80 |
| I. Tsangaris et al14 | 2007 | University Hospital of Ioannina | 16 (C = 8, E = 8) | Intratracheal | Proteins B + C | Each segmental mg/kg (200/19) | single | 1; 0 |
| Markart et al13 | 2007 | University of Giessen Lung Center | 31 (C = 17, E = 14) | Intratracheal | Protein C | 1 mg/kg, 4 doses (PL 50 mg) | 24 h | 4; 4 |
| Jozef Kesecioglu et al15 | 2009 | Multicenter: North America, Europe, Austria | 418 (C = 210, E = 208) | Intratracheal | Proteins B + C | HL10 large boluses: 3 doses, total 600 mg/kg | 0 h, 12 h, 36 h | 51; 60 |
| Spragg et al16 | 2010 | Multicenter: North America, Europe | 843 (C = 424, E = 419) | Intratracheal | Protein C | rSP-C surfactant, 8 doses | >96 h | 101; 95 |
Abbreviations: C, control group; DPPC, dipalmitoyl phosphatidylcholine; E, experimental group; HL10, exogenous natural porcine surfactant HL 10 as large boluses; LBW, lead body weight; PL, phospholipids; rSP-C, recombinant surfactant protein C–based surfactant.
There were no significant differences between the experimental and control groups in age, sex, basic PaO2:FIO2 ratio, or APACHE score (Table 2).
Table 2.
Baseline Characteristics of Trials Included in the Meta-Analysis
| Pulmonary Surfactant | Usual Therapy | |
|---|---|---|
| Variables | (n = 1,285) | (n = 1,289) |
| Mean age (y) | 51.6 | 51.1 |
| Men/total | 812/1,277 | 819/1,281 |
| Mean basic PaO2:FIO2 ratio | 128.9 (n = 1,285) | 121.6 (n = 1,289) |
| Mean APACHE score | 23.25 (n = 1,269) | 23.35 (n = 1,273) |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; PaO2:FIO2, ratio of partial pressure of arterial oxygen to fraction of inspired oxygen.
The results of the methodologic quality assessment suggested that all 9 trials met the present criteria (Table 3).
Table 3.
Risk of Publication Bias Graph
| Study | Was Allocation Sequence Adequately Generated? | Was Allocation Adequately Concealed? | Was Knowledge-Allocated Intervention Adequately Prevented During the Study? | Were Incomplete Outcome Data Adequately Addressed? |
|---|---|---|---|---|
| Weg et al,8 1994 | Yes | Yes | Yes | Yes |
| Anzueto et al,9 1996 | Yes | Yes | Yes | Yes |
| Gregory et al,10 1997 | Unclear⁎ | Unclear⁎ | Yes | Yes |
| Spragg et al,11 2003 | Yes | Yes | Yes | No |
| Spragg et al,12 2004 | Yes | Unclear⁎ | Yes | Yes |
| Markart et al,13 2007 | Unclear⁎ | Unclear⁎ | Yes | Yes |
| Tsangaris et al,14 2007 | Yes | Unclear⁎ | Yes | Yes |
| Kesecioglu et al,15 2009 | Yes | Yes | Yes | Yes |
| Spragg et al,16 2010 | Yes | yes | Yes | Yes |
Insufficient information from one article about the process to permit a judgment of “yes” or “no.”
Primary Outcome (Mortality at 28 or 30 Days)
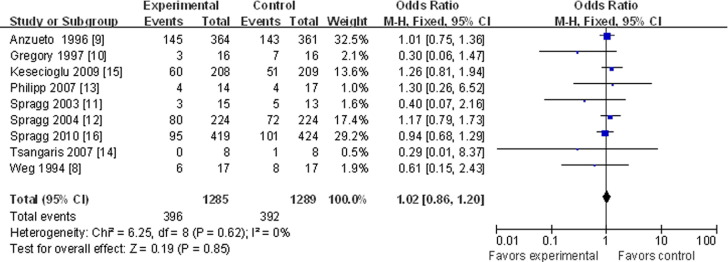
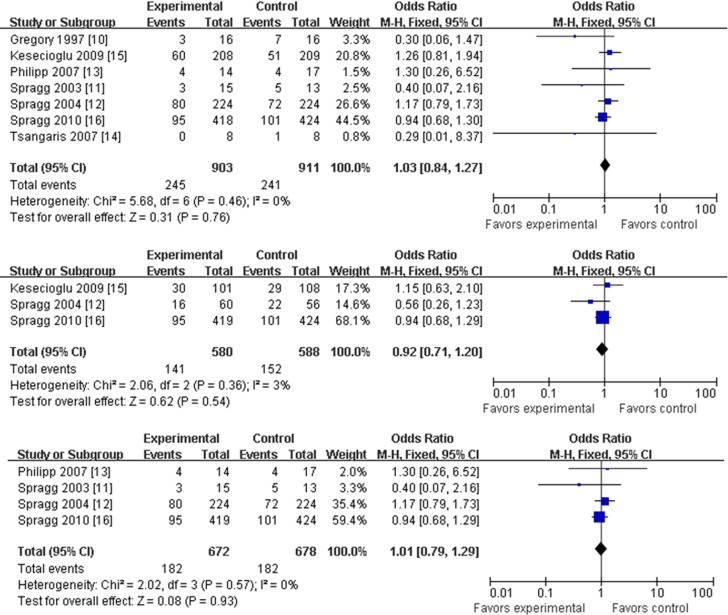
All 9 articles contained 28 or 30-day mortality data.8, 9, 10, 11, 12, 13, 14, 15, 16 Heterogeneity testing showed no bias among the different studies for this outcome (p = 0.62), so a “fixed model” was accepted and used. Meta-analysis showed that treatment with a pulmonary surfactant did not decrease mortality significantly compared with the control-treated groups (OR, 1.02; 95% CI, 0.86-1.20; p = 0.85; Fig 2). Subgroup analysis showed no differences in mortality among the different delivery methods or between treatments with or without a surfactant-containing protein (OR, 1.03; 95% CI, 0.84-1.27; p = 0.76, for trials using a surface protein and an intratracheal delivery method; OR, 0.99; 95% CI, 0.74-1.32; p = 0.93; for trials without a surface protein and an aerosolized delivery method; Fig 3 ). Subgroup analysis also showed no significant differences in outcome among groups with different ARDS etiologies (eg, direct lung injury; OR, 0.92; 95% CI, 0.71-1.20; p = 0.54; Fig 3). Also assessed were the potential differences in mortality between patients given a surfactant containing protein C (OR, 1.01; 95% CI, 0.79-1.29; p = 0.93; Fig 3) and those given proteins B and C (OR, 1.10; 95% CI, 0.73-1.66; p = 0.65).
Fig 2.
Pulmonary surfactant and mortality at 28 or 30 days with odds ratios and 95% confidence intervals. The size of the data markers (squares) is approximately proportional to the statistical weight of each trial. CI, confidence interval; M-H, Mantel-Haenszel.
Fig 3.
Pulmonary surfactant and mortality at 28 or 30 days with odds ratios and 95% confidence intervals for mortality and surface protein with intratracheal delivery methods (top), direct lung injury (middle), and protein C (bottom). The size of the data markers (squares) is approximately proportional to the statistical weight of each trial. CI, confidence interval; M-H, Mantel-Haenszel.
Secondary Outcomes
Mean difference in change in PaO2:FIO2 ratio for initial 24 hours
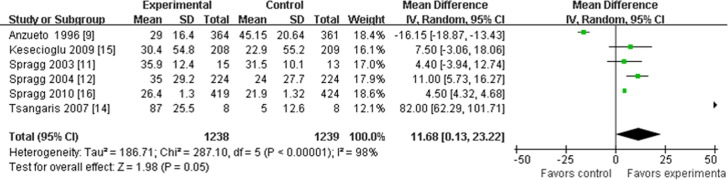
Six articles provided data for this analysis.9, 11, 12, 14, 15, 16 Three trials did not provide the standard deviation at 24 hours; if the data could not be obtained from the authors of these trials, then the standard deviation at baseline was used to replace it approximately.9, 15, 16 Heterogeneity testing was positive for this analysis and could not be eliminated statistically. The heterogeneity may be associated with the different times of data collection up to 24 hours, so only analysis using the “random model” was acceptable to use. Meta-analysis showed that the MD in the change in the PaO2:FIO2 ratio during the initial 24 hours of the experimental groups was improved significantly compared with the control groups (MD, 11.68; 95% CI, 0.13-23.22; p = 0.05; Fig 4).
Fig 4.
Pulmonary surfactant and mean difference in the change of the ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen during 24 hours with mean differences and 95% confidence intervals. The size of the data markers (squares) is approximately proportional to the statistical weight of each trial. CI, confidence interval; IV, inverse variance; SD, standard deviation.
Mean difference in change in PaO2:FIO2 ratio up to 120 hours
Four articles supplied data for this analysis.8, 9, 14, 16 Two trials did not provide the standard deviation over 120 hours; if the data could not be obtained from the authors of these trials, then the standard deviation at baseline was used to replace it approximately.9, 16 Heterogeneity testing was positive and could not be eliminated statistically. The heterogeneity may be associated with different times of data collection and the methods of calculation across the studies, so only analysis using the “random model” was acceptable. Meta-analysis showed that the MD in the change in the PaO2:FIO2 ratio up to 120 hours was not significant between the 2 groups (MD, 2.80; 95% CI, −10.01 to 15.60; p = 0.67).
Mean duration of ventilation
Two articles provided data for this analysis.9, 14 As for the other analyses, heterogeneity testing was positive and could not be eliminated statistically. In this case, the heterogeneity may be associated with different treatment delivery methods and types of treatment. Only analyses using the “random model” were acceptable. Meta-analysis showed a trend for a shorter mean duration of ventilation in the surfactant-treated group (MD, −1.08; 95% CI, −3.00 to 0.85; p = 0.27).
There was only one article supplying sufficient data for the analysis of the number of ventilation-free days, so meta-analysis could not be performed.12
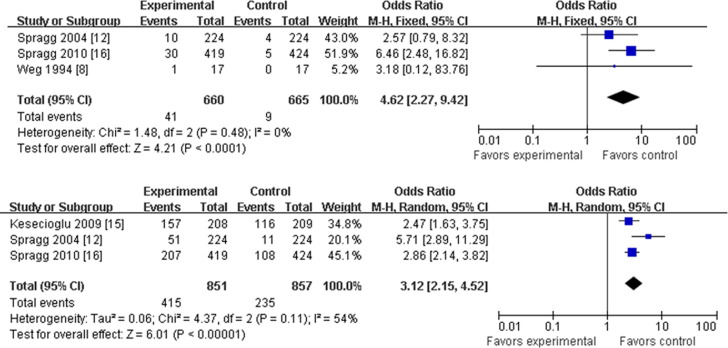
Number of adverse effects
Adverse effects can include treatment-related and other serious adverse events, such as temporary hypoxia, hypotension, and bradyarrhythmia. There were 3 articles that provided data for treatment-related adverse events.8, 12, 16 For these studies, there was no heterogeneity according to the present statistical test, so the “fixed model” was accepted for analysis. Treatment with a pulmonary surfactant was associated with an overall higher rate of treatment-related adverse events (OR, 4.62; 95% CI, 2.27-9.42; p < 0.00001; Fig 5).
Fig 5.
Pulmonary surfactant and rate of adverse effect with odds ratios and 95% confidence intervals. Rates of adverse effects related to treatment (top) and severe complications (bottom) are presented. The size of the data markers (squares) is approximately proportional to the statistical weight of each trial. CI, confidence interval; M-H, Mantel-Haenszel.
Three articles also provided data about other serious adverse events.12, 15, 16 Heterogeneity testing was positive and could not be eliminated statistically. The heterogeneity may be associated with different kinds of events recorded, and only analysis using the “random model” was acceptable. Experimental-treatment groups had higher rates of other serious adverse events compared with control-treated groups (OR, 3.12; 95% CI, 2.15-4.52; p < 0.000 01), which is similar to the treatment-related adverse effects noted earlier (Fig 5).
Discussion
Exogenous surfactant treatment has been shown to be useful in infants with established respiratory distress syndrome. Seger and Soll17 showed in their meta-analysis that infants who receive exogenous surfactant have a decreased risk of pneumothorax, pulmonary interstitial emphysema, bronchopulmonary dysplasia, or death. Another meta-analysis by Duffett et al18 of studies in infants and children also suggested that treatment with exogenous surfactant is associated with decreased mortality, more ventilator-free days, and a shorter duration of ventilation, all without serious adverse events. However, the efficacy of exogenous surfactant treatment in adults is still uncertain.
The previous meta-analysis published in 2006 did not show any significant alterations in outcome through the use of exogenous surfactant.16 Since 2006, 2 large clinical randomized trials also have shown no improvement in mortality, but rather, a possible increase in mortality with no improvement in oxygenation.15, 16 However, some “subgroups” of patients seemed to suggest a more favorable outcome in the surfactant group, such as those in the “protein C-based surfactant-mobility” group and “change in PaO2:FIO2 ratio in the first 24-hour oxygenation” group.7, 19 Given these trends and differences, the authors judged that a new updated meta-analysis, including these subgroups, might provide a new insight into the topic.
The present meta-analysis concentrated on assessing the efficacy of exogenous surfactant treatment in adults with ALI/ARDS. Patients treated with exogenous surfactant had more obvious improvement in the PaO2:FIO2 ratio compared with controls in the first 24 hours after treatment, which was not found to be significant in the previous meta-analysis. However, this improvement in ventilation was no longer apparent in the longer term up to 120 hours. The reason for the early improvements that were negated at later time points may be associated with the half-life and metabolism of the exogenous surfactant. Indeed, the article by Spragg et al16 emphasized that a step in the resuspension process likely resulted in a partial inactivation of the surfactant, and this was cited as a potential reason they determined no improvement in oxygenation. These data also may have a negative impact on this meta-analysis. Therefore, the real effect of exogenous surfactant treatment on the change in oxygenation likely is more significant than can be determined at this time. Moreover, another included study by Tsangaris et al14 may have biased the present meta-analysis because of the small sample. In addition, some data in these studies reported alveolar surface tension at time points after the surfactant treatment. Looking further at these changes in alveolar surface tension and airway pressures may provide more useful insight into the functional activity of the surfactant treatment and its half-life in vivo.13, 16 Unfortunately, oxygenation data could not be obtained from all the studies, even after contacting the primary investigator for this information. Better assessments could be made in the future if these data become available.
Improvements in drug development and in the ability to analyze data using meta-analysis tools have allowed researchers to concentrate on assessing the roles of etiology of ALI/ARDS on outcome and the effects of different formulations of surfactants on outcome. For example, Spragg et al16 and Taut et al19 carried out RCTs using a protein C-based surfactant for the treatment of ALI/ARDS and used these data in a pooled analysis to assess the impact of etiology on outcome. Theoretically, exogenous surfactant treatment can decrease surface tension, increase parenchymal compliance, and make ventilation easier, which would lead to a better clinical outcome in patients with direct lung injury, such as pneumonia. However, these 2 analyses16, 19 and the present analysis agree that no significant difference in mortality is associated with etiology. In the present meta-analysis, studies using protein C were separated from those using proteins B and C, and no differences were found in the outcome of using a protein- compared with a nonprotein-associated surfactant and the control treatment. Further clinical trials assessing these parameters may help to identify any specific benefits.
Three main methods have been described in the literature for the delivery of an exogenous surfactant: aerosolized, intratracheal, and bronchoalveolar lavage fluid. The efficiency of treatment by the aerosolized method is low because the drug does not reach the alveoli effectively. Intratracheal treatment has the advantages of being easy to perform and the ability to administer larger doses, but the surfactant cannot distribute equally within or between the lungs.20 It also has been shown that an exogenous surfactant has difficulty reaching some regions of the lung, where serious injury tends to occur, unless given in larger doses that can counteract the inhibition by plasma and tissue proteins. However, the present meta-analysis showed that neither aerosolized nor intratracheal delivery methods produced significantly different results compared with control groups, and there was no significant difference between the delivery methods. Another delivery method has succeeded in animal tests using bronchoalveolar lavage fluids. This method has the advantage of distributing exogenous surfactant well in the lungs and has been shown to decrease inflammation, increase lung compliance, and increase oxygenation.20 These studies need to be extended and confirmed in humans. In addition to new methods of delivery, more studies should be undertaken to assess the effect of treatment with surfactants combined with other drugs, such as lidocaine, ketamine, antitrypsin, dexamethasone, and phosphodiesterase inhibitors. Early data have suggested that combined treatment may improve the curative effect.20
The new information disclosed by the present meta-analysis compared with previous analyses showed that exogenous surfactant treatment can lead to increased treatment-related and -unrelated adverse events. Further studies are necessary to explore the potential reasons for these adverse effects and to understand the differences seen between adults and infants. One possibility is that intratracheal delivery itself may exacerbate the extent and frequency of adverse effects recorded. More research is required to address these questions before further clinical trials are carried out.
Limitations
As with any meta-analysis, the present study had limitations. Complete data often could not be obtained from the original investigators despite the authors' best efforts, and this very likely affected the final results discussed in this article. In addition, it is difficult to analyze the potential effects of different surfactant preparations, which quite conceivably may have different properties. The authors tried to analyze these different treatments separately within the present study, but this also inevitably reduced the sample size available for analysis in each subgroup. The authors found it difficult to group results from studies together as “one therapy” and they look forward to being able to better analyze groups separately in the future. Other complex analyses existed within the datasets examined, including the timing of treatment; the mode of ventilation before, during, and after treatment; the delivery method; and the dosing schedule used for treatments. In addition, the outcome in patients with ALI/ARDS is governed by many other variables, such as ventilation mode and variables, hemodynamic management, and infectious problems. All these are typical interfering factors in intensive-care patients, which produce a significant impact on the outcome. Although each APACHE score was recorded and no significant difference was found between the 2 groups, some bias owing to the variables listed in the Results could not be avoided. For the present meta-analysis of exogenous surfactant trials, the authors had to make generalizations of some of these factors, and this inevitably oversimplified the disease and the therapy. Subgroup analysis can help to identify possible significant variations, but it decreases the sample number and often cannot be performed.
Conclusions
The present meta-analysis of RCTs shows that exogenous surfactant treatment may improve oxygenation in patients with ALI/ARDS during the first 24 hours after administration and that treatment trends toward a shorter mean duration of ventilation. However, there is no good evidence to suggest that exogenous surfactant treatment improves mortality or longer-term oxygenation over ≥120 hours after administration. Of concern is that exogenous surfactant administration has a high rate of adverse effects from treatment-related complications and other serious events. With these data in mind, exogenous pulmonary surfactant treatment currently cannot be considered an effective adjunctive therapy in adult patients with ALI or ARDS.
Acknowledgments
The authors thank Dr Yu Bai (Shanghai Changhai Hospital) for guidance during this meta-analysis. They also thank Professor Roger G. Spragg for supplying the authors with data and the authors of the trials analyzed in this study for helping to find the required data.
Footnotes
This work was supported in part by the Shanghai Natural Science Foundation (grant 10411951400 to S.F., grant 11ZR1428100 to Q.L.) and Shanghai Tenth People's Hospital (5810 Project 10RD204).
Appendix
The search strategy, including the combination of exploded Medical Subject Headings and text words, follows: ((randomized controlled trial) OR (controlled clinical trial) OR (randomized OR (placebo) OR (drug therapy) OR (randomly) OR (trial) OR (groups)) NOT (animals NOT humans) NOT infants NOT newborn NOT neonate) AND ((ARDS) OR (adult respiratory distress syndrome) OR (acute respiratory distress syndrome) OR (non-cardiogenic pulmonary edema) OR (respiratory insufficiency) OR (systemic inflammatory response syndrome) OR (shock lung) OR (respiratory failure) OR (lung injury) OR (septic shock) OR (sepsis) OR (acute lung injur*) OR (ALI)) AND (((agent) OR (drug)OR (agents) OR (drugs)) AND((Pulmonary Surfactants) OR (Pulmonary Surfactant) OR (Surface-Active Agents) OR (PS))) AND ((treatment) OR (therapy)).
References
- 1.Günther A., Ruppert C., Schmidt R., et al. Surfactant alteration and replacement in acute respiratory distress syndrome. Respir Res. 2001;2:353–364. doi: 10.1186/rr86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Heart and Lung Institute, Task Force on Problems . Department of Health, Education, and Welfare; Washington, DC: 1972. Research Approaches, Needs: The Lung Program; pp. 165–180. [Google Scholar]
- 3.Lewis J.F., Veldhuizen R. The role of exogenous surfactant in the treatment of acute lung injury. Annu Rev Physiol. 2003;65:613–642. doi: 10.1146/annurev.physiol.65.092101.142434. [DOI] [PubMed] [Google Scholar]
- 4.Lewis J.F., Brackenbury A. Role of exogenous surfactant in acute lung injury. Crit Care Med. 2003;31(suppl):S324–S328. doi: 10.1097/01.CCM.0000057911.19145.9F. [DOI] [PubMed] [Google Scholar]
- 5.Walmrath D., Grimminger F., Pappert D., et al. Bronchoscopic administration of bovine natural surfactant in ARDS and septic shock: Impact on gas exchange and haemodynamics. Eur Respir J. 2002;19:805–810. doi: 10.1183/09031936.02.00243402. [DOI] [PubMed] [Google Scholar]
- 6.Bautin A., Khubulava G., Kozlov I., et al. Surfactant therapy for patients with ARDS after cardiac surgery. J Liposome Res. 2006;16:265–272. doi: 10.1080/08982100600850997. [DOI] [PubMed] [Google Scholar]
- 7.Davidson W.J., Dorscheid D., Spragg R., et al. Exogenous pulmonary surfactant for the treatment of adult patients with acute respiratory distress syndrome: Results of a meta-analysis. Crit Care. 2006;10:R41. doi: 10.1186/cc4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weg J.G., Balk R.A., Tharratt R.S., et al. Safety and potential efficacy of an aerosolized surfactant in human sepsis-induced adult respiratory distress syndrome. JAMA. 1994;272:1433–1438. [PubMed] [Google Scholar]
- 9.Anzueto A., Baughman R.P., Guntupalli K.K., et al. Aerosolized surfactant in adults with sepsis-induced acute respiratory distress syndrome: Exosurf Acute Respiratory Distress Syndrome Sepsis Study Group. N Engl J Med. 1996;334:1417–1421. doi: 10.1056/NEJM199605303342201. [DOI] [PubMed] [Google Scholar]
- 10.Gregory T.J., Steinberg K.P., Spragg R., et al. Bovine surfactant therapy for patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1997;155:1309–1315. doi: 10.1164/ajrccm.155.4.9105072. [DOI] [PubMed] [Google Scholar]
- 11.Spragg R.G., Lewis J.F., Wurst W., et al. Treatment of acute respiratory distress syndrome with recombinant surfactant protein C surfactant. Am J Respir Crit Care Med. 2003;167:1562–1566. doi: 10.1164/rccm.200207-782OC. [DOI] [PubMed] [Google Scholar]
- 12.Spragg R.G., Lewis J.F., Walmrath H.D., et al. Effect of recombinant surfactant protein C-based surfactant on the acute respiratory distress syndrome. N Engl J Med. 2004;351:884–892. doi: 10.1056/NEJMoa033181. [DOI] [PubMed] [Google Scholar]
- 13.Markart P., Ruppert C., Wygrecka M., et al. Patients with ARDS show improvement but not normalisation of alveolar surface activity with surfactant treatment: Putative role of neutral lipids. Thorax. 2007;62:588–594. doi: 10.1136/thx.2006.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsangaris I., Galiatsou E., Kostanti E., et al. The effect of exogenous surfactant in patients with lung contusions and acute lung injury. Intensive Care Med. 2007;33:851–855. doi: 10.1007/s00134-007-0597-z. [DOI] [PubMed] [Google Scholar]
- 15.Kesecioglu J., Beale R., Stewart T.E., et al. Exogenous natural surfactant for treatment of acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2009;180:989–994. doi: 10.1164/rccm.200812-1955OC. [DOI] [PubMed] [Google Scholar]
- 16.Spragg R.G., Taut F.J., Lewis J.F., et al. Recombinant surfactant protein C-based surfactant for patients with severe direct lung injury. Am J Respir Crit Care Med. 2011;183:1055–1061. doi: 10.1164/rccm.201009-1424OC. [DOI] [PubMed] [Google Scholar]
- 17.Seger N., Soll R. Animal derived surfactant extract for treatment of respiratory distress syndrome. Cochrane Database Syst Rev. 2009;15 doi: 10.1002/14651858.CD007836. CD007836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duffett M., Choong K., Ng V., et al. Surfactant therapy for acute respiratory failure in children: A systematic review and meta-analysis. Crit Care. 2007;11:R66. doi: 10.1186/cc5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taut F.J., Rippin G., Schenk P., et al. A search for subgroups of patients with ARDS who may benefit from surfactant replacement therapy: A pooled analysis of five studies with recombinant surfactant protein-C surfactant (Venticute) Chest. 2008;134:724–732. doi: 10.1378/chest.08-0362. [DOI] [PubMed] [Google Scholar]
- 20.Li B.B., Ding Z.N. The replacement therapy of pulmonary surfactant for ARDS. International Journal of Anesthesiology and Resuscitation. 2004;25:14–16. [Google Scholar]