Abstract
Background and Objectives
Large-scale observational studies have shown that, in patients with multiple sclerosis (MS), the risk of becoming more severely ill from coronavirus disease 2019 (COVID-19) is determined by older age, male sex, cardiovascular comorbidities, African American ethnicity, progressive disease, recent use of corticosteroids, and B cell–depleting disease-modifying treatment. In contrast, the effect of COVID-19 on the disease course of MS has been studied much less extensively. Our main goal was to explore whether COVID-19 is associated with accelerated clinical disability worsening in patients with MS.
Methods
Since March 2020, demographics and infectious outcome (categorized as ambulatory, hospitalized, and/or death) of patients with MS who developed COVID-19 have been collected at the Belgian National MS Center in Melsbroek. On February 28, 2022, this database was locked and complemented with clinical disability measures—Expanded Disability Status Scale (EDSS), Timed 25-Foot Walk Test (T25FWT), 9-Hole Peg Test (9HPT), and Symbol Digit Modalities Test (SDMT)—that were available from a larger local database, obtained during routine medical follow-up. For each parameter, the first 2 assessments before COVID-19 diagnosis (T0 and T1; T1 is the closest to COVID-19 diagnosis), and the first thereafter (T2), were retrieved.
Results
We identified 234 unique cases of COVID-19. Thirty-one patients were hospitalized (13.2%), and 5 died (2.1%) as a result of their infection. Among survivors with complete EDSS results (N = 138), mean annualized T1-to-T2 EDSS worsening was more pronounced, compared with the respective change between T0 and T1 (0.3 ± 0.9 vs 0.1 ± 0.9, p = 0.012). No such differences were found for the T25FWT, 9HPT, and SDMT scores. Severe COVID-19 (hospitalization) was associated with clinically relevant T1-to-T2 EDSS worsening (OR 2.65, p = 0.042). Vaccination coverage in the total cohort was 53.8%. Being unprotected by vaccination at the time of infection was associated with a worse COVID-19 outcome (hospitalization and/or death; OR 3.52, p = 0.002) but not with clinically relevant T1-to-T2 EDSS worsening.
Discussion
The occurrence and severity of COVID-19 are both associated with clinical disability worsening in patients with MS. Vaccination protects against a more severe course of COVID-19 in this specific population.
Trial Registration Information
The study has been registered at ClinicalTrials.gov (study registration number: NCT05403463).
The coronavirus disease 2019 (COVID-19) is a highly contagious disorder caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Viral spreading is mainly mediated by transmission of respiratory particles from one individual to the other through direct contact or via the airborne route. Although the majority of infections remain asymptomatic or result in a clinical presentation of merely modest severity (e.g., febrile syndrome, headache, upper respiratory tract involvement, and mild pneumonia), more aggressive forms (with hypoxia, respiratory failure, septic shock, and/or multiple organ dysfunction) have been reported in up to 20% of affected individuals. The first official case was identified in the Chinese city of Wuhan in December 2019, and less than 4 months later, COVID-19 was officially declared a pandemic by the World Health Organization.1,2 Daily life has been deeply disrupted ever since and the confirmed death toll has already well exceeded the 6 million mark.3
The clinical course of multiple sclerosis (MS), a chronic inflammatory demyelinating disorder of the CNS, is typically characterized by subacute exacerbations of neurologic dysfunction (termed relapses) and/or by a more slowly yet continuously manifesting accumulation of neurologic disability, classified as progressive disease.4 Systemic infections have been associated with an increased risk of concurrent relapse and MRI activity,5 but their precise effect on the rate of subsequent physical and/or cognitive disability progression remains unknown. Of interest, a population-based retrospective cohort study performed in Canada (using data from 2009 to 2014) demonstrated that, following acute hospitalization regardless of the cause, individuals with MS experienced a significant step worsening of global clinical impairment, as measured with the Expanded Disability Status Scale (EDSS),6 equivalent to 2.5 years of the average time-related disease progression, while the pace of subsequent disability worsening remained unaltered.7
The overarching COVID-19/MS research agenda has, so far, mainly been focusing on (1) defining the risk for having a more severe course of COVID-19, (2) understanding the immunologic response after natural infection and/or vaccination, and (3) deciphering the influence of disease-modifying treatment (DMT) for MS with regard to points (1) and (2) above. Reassuringly, evidence from large-scale observational studies has generally shown that having MS itself does not make affected patients more susceptible to contract COVID-19 or to become severely ill from the infection, compared with the general population. Risk factors are similar in both settings and, for patients with MS, include older age, male sex, cardiovascular comorbidities, African American ethnicity, progressive disease, recent use of corticosteroids, and B cell–depleting DMT.8-13 However, the reverse relationship—i.e., the effect of COVID-19 on clinical disability related to MS—is less well described and therefore forms the subject of our study.
Methods
Study Design
We have conducted a retrospective observational cohort study at the Nationaal Multiple Sclerose Centrum (NMSC) Melsbroek (Belgium), which is a large center specifically focusing on neurologic management, multidisciplinary care, and/or rehabilitation in patients with MS. Our main goal was to investigate the effect of contracting COVID-19, as well as infection severity, on clinical disability worsening in patients with MS. The study has been registered at ClinicalTrials.gov (study registration number: NCT05403463) and followed the Strengthening the Reporting of Observational Studies in Epidemiology data reporting guidelines.
Data Collection
Since March 2020 (i.e., the onset of the first wave of spiking COVID-19 cases in Belgium),3 clinical information of patients followed at the NMSC Melsbroek has been collected in a local database in case of COVID-19 diagnosis, as confirmed by positive PCR testing for SARS-CoV-2. The following variables were recorded: patient identification number and date of COVID-19 diagnosis; age, sex, MS duration, clinical subtype, and DMT regimen at the time of COVID-19 diagnosis; COVID-19 severity (ambulatory care vs hospitalization vs death) and outcome (recovery vs death); and vaccination status (nonvaccinated vs fully vaccinated vs fully vaccinated + booster) at the time of COVID-19 diagnosis. On February 28, 2022, this database was locked for the present study and consisted of 234 unique COVID-19 cases.
The NMSC Melsbroek features a second and more extensive database containing a broad variety of (para)clinical information gathered during routine medical follow-up, which includes regular testing of general disability with the EDSS, leg function/ambulation with the Timed 25-Foot Walk Test (T25FWT), dominant hand function/dexterity using the 9-Hole Peg Test (9HPT), and cognition/information processing speed with the Symbol Digit Modalities Test (SDMT). For each of these parameters, the first 2 available results before COVID-19 diagnosis (labeled T0 and T1, respectively; T1 being the closest to COVID-19 diagnosis), and the first thereafter (labeled T2), were retrieved for each patient in our COVID-19 database (Figure). If clinical measurements were performed during an in-house stay for rehabilitation purposes, only those performed on admission were retained (thus not necessarily the closest to positive COVID-19 testing) to avoid bias due to the effects of intensive exercise treatment. Neurologist-reported relapses and self-reported worsening of neurologic symptoms during the T1-T2 interval were recorded from the medical patient files.
Figure. Sequence of Clinical Evaluations During the Study Period.
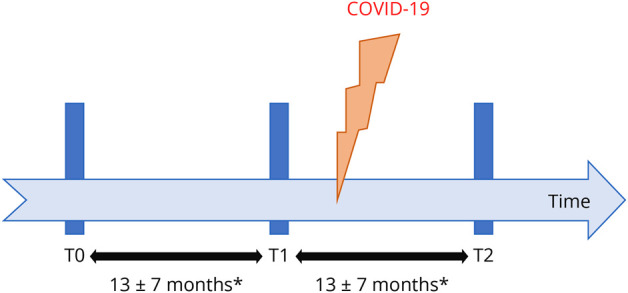
Clinical data were collected at 2 separate moments before (T0 and T1) and 1 (T2) after the diagnosis of the coronavirus disease 2019 in each patient with multiple sclerosis. *Interval duration based on the subcohort with complete Expanded Disability Status Scale data (N = 138).
Evaluations
We have described the basic characteristics of our total cohort of 234 patients with MS who developed COVID-19, including infection severity and related mortality. The difference between the values measured at T0 and T1 (i.e., before COVID-19) was compared, using annualized changes at the individual level (calculated with the following formula: [clinical measure at last date − clinical measure at first date] × 12 months/interval in months) to adjust for any potential variability in interval duration, with the difference between the respective values measured at T1 and T2 (i.e., after COVID-19) for the EDSS, T25WT, 9HPT, and SDMT scores, only in patients who survived COVID-19 and for whom all 3 measurements were available per test. We hereby aimed to answer our primary research question—whether COVID-19 leads to accelerated clinical disability worsening in MS. As secondary outcomes, we have explored whether (1) a relationship exists between COVID-19 severity and T1-to-T2 clinical disability worsening, (2) vaccination status affects COVID-19 severity, and (3) vaccination status influences T1-to-T2 clinical disability worsening.
Standard Protocol Approvals, Registrations, and Patient Consents
Approval for this study was granted by the local ethics committee (institutional authorization number: OG 033; internal reference number: EC2022/07) on April 13, 2022. According to the Belgian law, retrospective studies do not require participant consent.
Statistics
All statistical procedures were performed with GraphPad Prism version 9.3.1 (GraphPad Software; San Diego, CA). All data representing clinical disability (changes) followed a non-normal distribution pattern, as demonstrated by Shapiro-Wilk testing. Group differences were assessed by means of Wilcoxon signed rank or χ2 tests, where appropriate. The effect of COVID-19 severity on clinical disability progression was evaluated using multivariate logistic regression. All reported p values are 2 tailed and were considered statistically significant at the 0.05 level.
Data Availability
Anonymized data supporting the findings of this study will be shared on reasonable request from a qualified investigator and after approval by the ethics committee of the NMSC Melsbroek.
Results
Patient Characteristics and COVID-19 Outcome
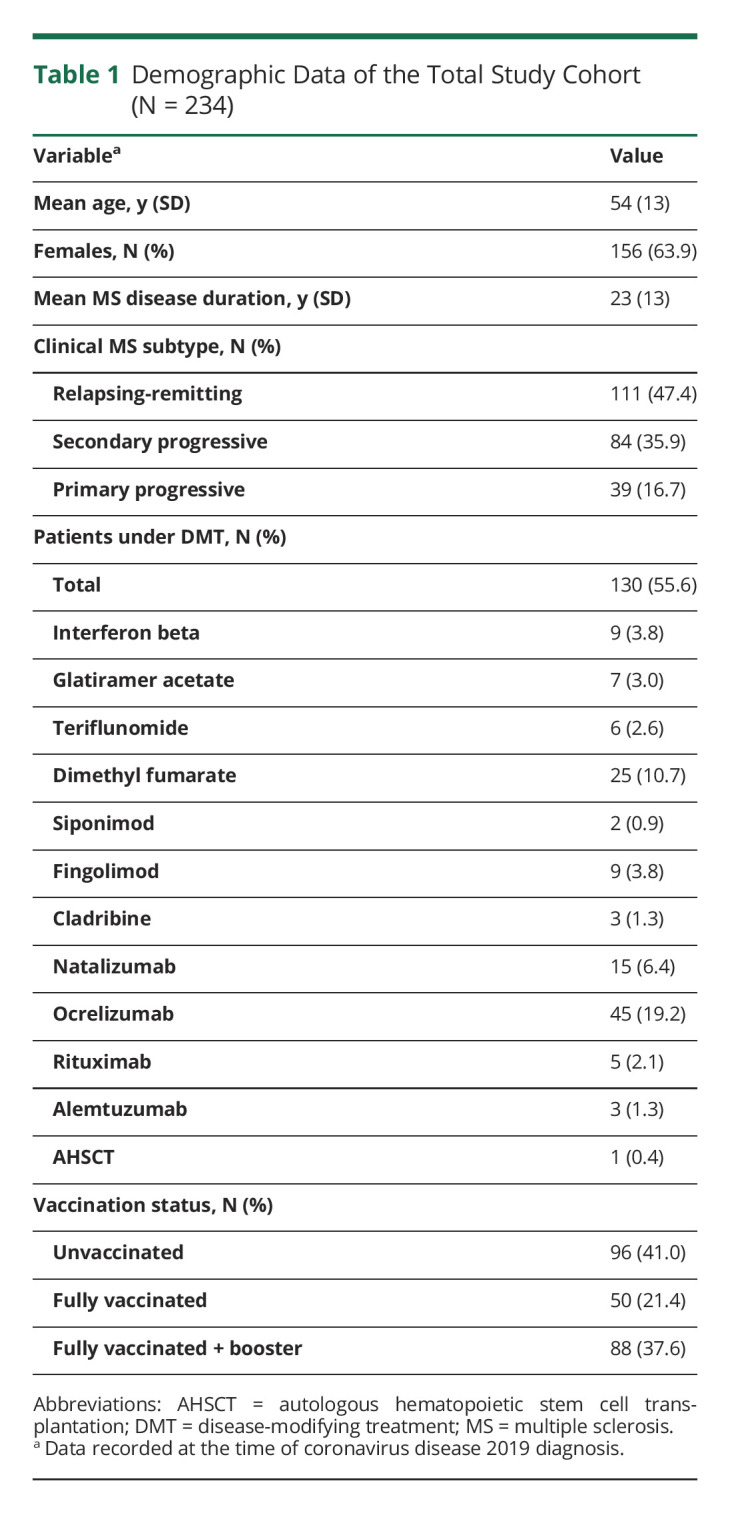
Demographic data of our total cohort (N = 234) are shown in Table 1. Thirty-one patients were hospitalized (13.2%), and 5 died (2.1%) as a result of COVID-19. There was 1 individual from the group of deceased patients who was not admitted to the hospital and died at her residential care center. One patient died of progressive bladder cancer approximately 4 months after infection with SARS-CoV-2, which was considered an event unrelated to COVID-19.
Table 1.
Demographic Data of the Total Study Cohort (N = 234)
COVID-19 and Clinical Disability
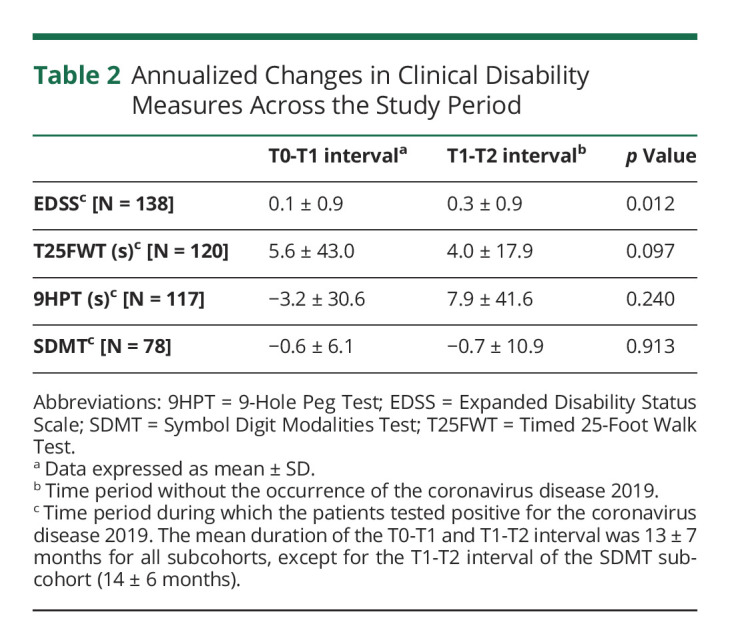
Complete EDSS, T25FWT, 9HPT, and SDMT data sets (i.e., measurements at T0, T1, and T2) were available for 138, 120, 117, and 78 individuals, respectively. A significantly more pronounced annualized EDSS worsening was observed in the T1-T2 interval, compared with the change in the T0-T1 interval. No such differences were found for the other clinical disability measures (Table 2). For the EDSS evaluations, the mean score was 5.5 ± 2.2 at T0, 5.6 ± 2.2 at T1, and 5.8 ± 2.2 at T2, whereas the mean duration of the T0-T1 and T1-T2 interval was 13 ± 7 months for both (the mean time from T1 to COVID-19 was 8 ± 6 months, and the mean time from COVID-19 to T2 was 5 ± 4 months); mean age (54 ± 12 years), sex distribution (99/138 = 71.7% females), mean MS disease duration (24 ± 12 years), MS clinical phenotype distribution (relapsing-remitting: 65/138 = 47.1%, secondary progressive: 51/138 = 37.0%, primary progressive: 22/138 = 15.9%), and DMT coverage (69/138 = 50.0%) were globally comparable between this subcohort and the total cohort.
Table 2.
Annualized Changes in Clinical Disability Measures Across the Study Period
To explore whether the accelerated T0-to-T1 vs T1-to-T2 EDSS worsening was driven by one disease phenotype in particular, this comparative analysis was repeated in patients with relapsing-remitting and progressive MS separately. For those with a relapsing-remitting course (N = 65), annualized T0-to-T1 EDSS worsening was 0.13 ± 0.91, compared with 0.30 ± 0.78 during the T1-T2 interval (p = 0.189). The respective changes were 0.06 ± 0.86 and 0.33 ± 0.92 (p = 0.006) for the patients with progressive MS. These data show a temporal pattern of clinical worsening in both phenotypes, yet one that is more pronounced (5.5- vs 2.3-fold) in the latter. Thus, the statistical effect observed in our total EDSS subcohort of 138 individuals seems to be principally attributable to the subgroup with progressive MS.
Clinically relevant EDSS worsening (defined as a 1.5-point increase if the baseline score was 0, 1.0-point increase if the baseline score ranged from 1.0 to 5.5, or 0.5-point increase if the baseline score was above 5.5)14 was observed in 33/138 (23.9%) patients for the interval during which they tested positive for COVID-19 (T1-T2). Sixteen patients (16/138) developed clinically relevant EDSS worsening, according to the applied definition, in the T0-T1 interval, which is significantly less, compared with those who did so in the T1-T2 interval (33/138, p = 0.007). The proportion of patients who had had a more severe course of COVID-19 (hospitalization) was higher in those who developed clinically relevant T1-to-T2 EDSS worsening, compared with those who did not (9/33 vs 13/105; OR 2.65, 95% CI 1.06–6.84, p = 0.042). COVID-19 severity (hospitalization) was identified as a borderline predictor of clinically relevant T1-to-T2 EDSS worsening (OR 2.73, 95% CI 0.98–7.45, p = 0.050) in a model corrected for EDSS score at T1 and age, sex, and MS subtype (relapsing vs progressive) at the time of COVID-19 infection.
To date, anti-CD20 agents are the only DMT group consistently associated with a worse outcome of COVID-19 in patients with MS.10,11,13 To adjust for their potential confounding effect, analyses that previously yielded statistically significant results were repeated in the EDSS subcohort, after exclusion of all patients under anti-CD20 DMT (i.e., 25 individuals on ocrelizumab and 4 on rituximab). For this adjusted subcohort (N = 109), annualized EDSS worsening was 0.07 ± 0.71 in the T0-T1 interval, compared with 0.27 ± 0.77 in the T1-T2 interval (p = 0.020). The proportion of patients with a more severe COVID-19 course (hospitalization) was higher, yet without reaching statistical significance, in those who developed clinically relevant T1-to-T2 EDSS worsening, compared with those who did not (7/23 vs 13/86; OR 2.46, 95% CI 0.92–6.62, p = 0.092).
In our total cohort (N = 234), there were 17 patients (7.3%) with a neurologist-reported relapse and 96 (41.0%) with self-reported worsening of neurologic symptoms during the T1-T2 interval; 5 patients with a neurologist-reported relapse received pulse treatment with corticosteroids. For the subcohort of patients with available EDSS results (N = 138), the respective numbers were 7 (5.1%) and 63 (44.9%). The proportion of patients who experienced a relapse in the T1-T2 interval was comparable between those who developed clinically relevant EDSS worsening over the same period, compared with those who did not (1/33 vs 6/105; OR 0.52, 95% CI 0.04–3.40, p = 0.540).
Vaccination
At the time of testing positive for COVID-19, 126/234 (53.8%) patients were considered to be protected by vaccination, defined as (1) being fully vaccinated and (2) having tested positive for COVID-19 in the period ranging from 14 days to 6 months after the last administered vaccine dose (which could also be a booster).15 The proportion of unprotected patients was higher (respectively, 23/32 vs 85/202, OR 3.52, 95% CI 1.60–8.25, p = 0.002) in the severe COVID-19 group (hospitalized and/or death), compared with the mild COVID-19 group (ambulatory care). In the group that developed clinically relevant T1-to-T2 EDSS worsening, the proportion of unprotected patients was similar to the group that did not (respectively 17/33 vs 59/105, OR 0.83, 95% CI 0.37–1.77, p = 0.638).
Discussion
In this retrospective observational cohort study, performed at a large tertiary center in Belgium, we have investigated the effect of COVID-19 on clinical disability, as well as the protective properties of vaccination against SARS-CoV-2, in patients with MS. Overall, most of our patients experienced a mild infectious course, with death (2.1%) and hospitalization (13.2%) rates falling in the lower range of percentages previously reported in the literature.8-13 These outcomes were better than predicted by the relatively high age, level of disability, and proportion of patients under DMT with an anti-CD20 agent, which are factors that all have been associated with a worse COVID-19 prognosis in patients with MS.8-13 This beneficial discrepancy might be explained by the substantial vaccination coverage (59%) in our cohort at the time of infection, since, interestingly, mortality (5.6%) and hospitalization (27.8%) rates were substantially higher in the 72 cases of COVID-19 that were diagnosed before March 2021 (i.e., before vaccination against SARS-CoV-2 became available for patients with MS in Belgium). We observed that the occurrence and severity of COVID-19 were both associated with worsening of global disability, as measured with the EDSS, over time in the individual patient trajectory. Mathematically expressed, a more aggressive course of COVID-19 resulted in a more than 2.5-fold increase of the risk of subsequent clinically relevant EDSS deterioration. Our findings are in line with several other studies describing aggravation of MS symptomatology after COVID-19, which appears to be particularly associated with a higher premorbid EDSS score, longer disease duration, and more severe COVID-19 course.16-19 In contrast, a recent nationwide Austrian study did not witness a significantly increased risk for EDSS score worsening after COVID-19 in patients with MS, compared with matched noninfectious controls, but their samples, as opposed to our cohort, were characterized by a slightly more favorable COVID-19 outcome and, more importantly, also by a notably younger age, shorter disease duration, and lower degree of clinical disability.20
Although the precise cause remains unknown, reflection on several hypotheses might help us to better understand the underpinnings of post–COVID-19 neurologic deterioration in patients with MS. First, COVID-19 can be accompanied by a variety of neurologic manifestations, including headache, encephalopathy, stroke, neuropsychiatric symptoms, and hyposmia/dysgeusia. The exact way in which SARS-CoV-2 affects the CNS seems to rely for a large part on secondary immunologic consequences (e.g., blood-brain barrier breakdown, tissue inflammation, hypoxia, and coagulopathy) rather than direct viral invasion.21-23 Second, based on (1) observations in other upper respiratory infections, (2) the accumulating number of acute demyelinating CNS episodes associated with COVID-19, and (3) descriptions of potential inflammatory crossroads between MS and SARS-CoV-2 infection, it has been speculated that COVID-19 may increase the risk of relapses in patients with MS.24-26 However, clinical studies have provided conflicting results and, overall, have not been able to confirm this association.20,27-29 With regard to our cohort, post–COVID-19 relapse activity was comparable with the existing literature but could not be identified as the driving force behind the observed EDSS worsening. Third, about half the participants in our study were classified as having a progressive clinical phenotype, thus subject to the more slowly but continuously evolving neurodegenerative process of MS. In addition, disease progression independent of relapse activity is becoming increasingly recognized as an important contributor to confirmed disability accumulation in relapsing-remitting patients as well.30 The pathologic mechanisms of progressive MS remain to be fully elucidated, but evidence is currently evolving for a prominent role of a particular subset of focal demyelinating lesions with ongoing inflammatory activity at the edges, driven by activated cells of the innate immune system (macrophages and microglia), coined as chronic active or smoldering lesions.31 Of interest, hyperinflammatory dysregulation of the innate immune system and pathologic microgliosis appear to contribute to CNS toxicity in COVID-19,32-34 with direct viral inoculation (human microglia express SARS-CoV-2 entry factors such as angiotensin-converting enzyme 2 and transmembrane protease serine subtype-2) and nod-like receptor pyrin domain containing protein inflammasome activation among the potential mechanisms.35,36 Fourth, the functional decline may also have a more circumstantial origin because the COVID-19 crisis has a generally disruptive effect on health care service delivery, DMT adherence, and rehabilitation treatment in patients with MS,37 all of which are factors expected to culminate in a poorer clinical prognosis.
Efforts to contain and manage the ongoing pandemic heavily depend on large-scale vaccination against SARS-CoV-2. Currently available vaccines operate through mRNA- or nonreplicating adenoviral vector–based immunization against coronavirus spike protein. Their protective effect against various unfavorable COVID-19–related outcomes (e.g., infection, hospitalization, and death) is very well established in the general population38 but has been shown to weaken after 6 months.39,40 Clinical efficacy data obtained specifically in patients with MS are much more scarce. Our study helps to fill that gap by showing that contracting COVID-19 in a state defined as unprotected by vaccination (i.e., individuals who were either unvaccinated or who had received their last dose more than 6 months before positive PCR testing) is associated with a significantly elevated risk of a more aggressive infectious course (hospitalization and/or death, OR 3.52). These findings are in line with recent observations from the Austrian MS-COVID-19 investigators who reported a significantly decreased risk for severe illness after developing COVID-19 (defined as the clinical status at the most severe point requiring hospitalization and additionally meeting at least 1 of 5 prespecified and objective respiratory distress criteria) in fully vaccinated patients with MS (OR 0.21).41 Hesitancy and/or unwillingness to receive vaccination against SARS-CoV-2 ranges between 15% and 30% in patients with MS and is often instilled by safety concerns, despite the fact that the current literature does not show an increased risk for reactogenicity, serious adverse events, or relapse activity.24,25 Our cohort presented with a lower coverage rate, but data collection started well before the rollout of the vaccination campaign in Belgium. At present, COVID-19 vaccination is strongly advised for most people with MS, as it is the general view that potential benefits outweigh any possible risks in the vast majority of cases.24
In conclusion, our study demonstrated that the occurrence and severity of COVID-19 are associated with disability worsening in patients with MS. Vaccination protected against hospitalization and death due to the infection, in the 6 months following administration. These findings help close an actual and relevant knowledge gap. Our analyses were executed on relatively large samples, while diagnostic specificity, cohort characterization, and clinical assessments should be of sufficient quality because data collection took place in a tertiary MS center. Nonetheless, this study is not without limitations. First, the design was retrospective, and although the outcome of each reported COVID-19 case was rigorously verified, we might not have captured every patient who had a fatal outcome. EDSS scoring may be biased by interrater variability, and we cannot clearly explain why COVID-19 (severity) did not affect the other, more objective, clinical test results, aside from a potential overachievement effect during performance testing. Identification of neurologist-reported relapses and patient-reported worsening of neurologic symptoms during the T1-T2 interval was based on the study team's a posteriori interpretation of the medical records, without the use of strict definitions. Second, although the mean time from COVID-19 to T2 was 5 ± 4 months in our EDSS subcohort (N = 138), we cannot exclude the possibility that clinically relevant EDSS worsening from T1 to T2 manifested as an infection-associated effect of short duration, particularly in those with less time between COVID-19 and T2. Follow-up T3 measurements (following the same methodological principles as for defining the T0, T1, and T2 visits; mean T2-T3 interval duration was 13 ± 6 months) are currently available in our database for 46 patients out of the EDSS subcohort. In this group, there were 14 patients with clinically relevant EDSS worsening between T1 and T2, which was sustained at T3 for the majority of them (12/14 = 86%). Third, our logistic regression model assessing the relationship between COVID-19 severity and T1-to-T2 EDSS progression, controlled for factors potentially influencing both the predictor and outcome measure, yielded a result that was only borderline statistically significant. DMT was not included in this model, which can be seen as a limitation of the study. At present, medication directed against CD20 is the only drug class consistently associated with a worse COVID-19 outcome in patients with MS, but their effect on the risk of T1-to-T2 EDSS worsening remains up for debate (i.e., representation of patients with a worse prognosis due to a more aggressive disease course or, in contrary, patients with a better prognosis due to a more potent treatment effect?). Results in the EDSS subcohort in which patients treated with an anti-CD20 agent were excluded (N = 109) showed the same trend as those obtained in the entire EDSS subcohort (N = 138), suggesting (while also taking the increased risk of statistical type II error in this smaller sample into account) that our initial observations were not predominantly driven by the effect of anti-CD20 agents. Finally, we did not use matched controls but relied on multiple longitudinal evaluations in each individual to evaluate the effect of COVID-19 on clinical disability. We believe that the differences in clinical evolution, as witnessed between 2 consecutive time periods of approximately 1 year, are more likely to reflect the effect of COVID-19 than merely the natural history of MS and, moreover, compelling a truly COVID-19–negative MS group risks to be difficult since the number of (asymptomatic) undiagnosed infections is estimated to be high.42,43 In a next phase, it would be interesting to see whether our findings can be validated in future prospective studies, and it may also be worth further exploring if and how MS and COVID-19 pathology converge through innate immune responses.
Glossary
- COVID-19
coronavirus disease 2019
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- MS
multiple sclerosis
- EDSS
Expanded Disability Status Scale
- DMT
disease-modifying treatment
- T25FWT
Timed 25-Foot Walk Test
- 9HPT
9-Hole Peg Test
- SDMT
Symbol Digit Modalities Test
Appendix. Authors
Footnotes
COVID-19 Resources: NPub.org/COVID19
Study Funding
The authors report no targeted funding.
Disclosure
G. Peeters and A. Van Remoortel report no disclosures relevant to the manuscript. G. Nagels is a shareholder of icometrix. J. Van Schependom and M. D'haeseleer report no disclosures to the manuscript. Go to Neurology.org/NN for full disclosures.
References
- 1.Zhu N, Zhang D, Wang W, et al. . A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733. doi. 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19(3):141-154. doi. 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coronavirus Resource Center. Johns Hopkins University & Medicine. Accessed November 6, 2022. coronavirus.jhu.edu. [Google Scholar]
- 4.Dobson R, Giovannoni G. Multiple sclerosis–a review. Eur J Neurol. 2019;26(1):27-40. doi. 10.1111/ene.13819. [DOI] [PubMed] [Google Scholar]
- 5.Correale J, Fiol M, Gilmore W. The risk of relapses in multiple sclerosis during systemic infections. Neurology. 2006;67(4):652-659. doi. 10.1212/01.wnl.0000233834.09743.3b. [DOI] [PubMed] [Google Scholar]
- 6.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444-1452. doi. 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 7.Garland A, Metz LM, Bernstein CN, Peschken CA, Hitchon CA, Marrie RA. Hospitalization is associated with subsequent disability in multiple sclerosis. Mult Scler Relat Disord. 2017;14:23-28. doi. 10.1016/j.msard.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Louapre C, Collongues N, Stankoff B, et al. . Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77(9):1079-1088. doi. 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salter A, Fox RJ, Newsome SD, et al. . Outcomes and risk factors associated with SARS-CoV-2 infection in a North American Registry of patients with multiple sclerosis. JAMA Neurol. 2021;78(6):699-708. doi. 10.1001/jamaneurol.2021.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sormani MP, De Rossi N, Schiavetti I, et al. . Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780-789. doi. 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson-Yap S, De Brouwer E, Kalincik T, et al. . Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology. 2021;97(19):e1870-e1885. doi. 10.1212/WNL.0000000000012753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arrambide G, Llaneza-Gonzalez MA, Costa-Frossard Franca L, et al. . SARS-CoV-2 infection in multiple sclerosis: results of the Spanish Neurology Society Registry. Neurol Neuroimmunol Neuroinflamm. 2021;8(5):e1024. doi. 10.1212/NXI.0000000000001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zabalza A, Cardenas-Robledo S, Tagliani P, et al. . COVID-19 in multiple sclerosis patients: susceptibility, severity risk factors and serological response. Eur J Neurol. 2021;28(10):3384-3395. doi. 10.1111/ene.14690. [DOI] [PubMed] [Google Scholar]
- 14.Meca-Lallana V, Berenguer-Ruiz L, Carreres-Polo J, et al. . Deciphering multiple sclerosis progression. Front Neurol. 2021;12:608491. doi. 10.3389/fneur.2021.608491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022;28(2):202-221. doi. 10.1016/j.cmi.2021.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paybast S, Hejazi SA, Molavi P, Habibi MA, Naser Moghadasi A. A one year follow of patients with multiple sclerosis during COVID-19 pandemic: a cross-sectional study in Qom province, Iran. Mult Scler Relat Disord. 2022;60:103712. doi. 10.1016/j.msard.2022.103712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garjani A, Middleton RM, Hunter R, et al. . COVID-19 is associated with new symptoms of multiple sclerosis that are prevented by disease modifying therapies. Mult Scler Relat Disord. 2021;52:102939. doi. 10.1016/j.msard.2021.102939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michelena G, Casas M, Eizaguirre MB, et al. . Inverted question mark Can COVID-19 exacerbate multiple sclerosis symptoms? A case series analysis. Mult Scler Relat Disord. 2022;57:103368. doi. 10.1016/j.msard.2021.103368. [DOI] [PubMed] [Google Scholar]
- 19.Conway SE, Healy BC, Zurawski J, et al. . COVID-19 severity is associated with worsened neurological outcomes in multiple sclerosis and related disorders. Mult Scler Relat Disord. 2022;63:103946. doi. 10.1016/j.msard.2022.103946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bsteh G, Assar H, Gradl C, et al. . Long-term outcome after COVID-19 infection in multiple sclerosis: a nation-wide multicenter matched-control study. Eur J Neurol. 2022;29(10):3050-3060. doi. 10.1111/ene.15477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77(8):1018-1027. doi. 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spudich S, Nath A. Nervous system consequences of COVID-19. Science. 2022;375(6578):267-269. doi. 10.1126/science.abm2052. [DOI] [PubMed] [Google Scholar]
- 23.Divani AA, Andalib S, Biller J, et al. . Central nervous system manifestations associated with COVID-19. Curr Neurol Neurosci Rep. 2020;20(12):60. doi. 10.1007/s11910-020-01079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollen C, Bernard J. Multiple sclerosis management during the COVID-19 pandemic. Curr Neurol Neurosci Rep. 2022;22(8):537-543. doi. 10.1007/s11910-022-01211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pugliatti M, Berger T, Hartung HP, Oreja-Guevara C, Bar-Or A. Multiple sclerosis in the era of COVID-19: disease course, DMTs and SARS-CoV2 vaccinations. Curr Opin Neurol. 2022;35(3):319-327. doi. 10.1097/WCO.0000000000001066. [DOI] [PubMed] [Google Scholar]
- 26.Bellucci G, Rinaldi V, Buscarinu MC, et al. . Multiple sclerosis and SARS-CoV-2: has the interplay started? Front Immunol. 2021;12:755333. doi. 10.3389/fimmu.2021.755333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barzegar M, Vaheb S, Mirmosayyeb O, Afshari-Safavi A, Nehzat N, Shaygannejad V. Can coronavirus disease 2019 (COVID-19) trigger exacerbation of multiple sclerosis? A retrospective study. Mult Scler Relat Disord. 2021;52:102947. doi. 10.1016/j.msard.2021.102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Etemadifar M, Sedaghat N, Aghababaee A, et al. . COVID-19 and the risk of relapse in multiple sclerosis patients: a fight with no bystander effect? Mult Scler Relat Disord. 2021;51:102915. doi. 10.1016/j.msard.2021.102915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klineova S, Harel A, Straus Farber R, et al. . Outcomes of COVID-19 infection in multiple sclerosis and related conditions: one-year pandemic experience of the multicenter New York COVID-19 Neuroimmunology Consortium (NYCNIC). Mult Scler Relat Disord. 2021;55:103153. doi. 10.1016/j.msard.2021.103153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Portaccio E, Bellinvia A, Fonderico M, et al. . Progression is independent of relapse activity in early multiple sclerosis: a real-life cohort study. Brain. 2022;145(8):2796-2805. doi. 10.1093/brain/awac111. [DOI] [PubMed] [Google Scholar]
- 31.Giovannoni G, Popescu V, Wuerfel J, et al. . Smouldering multiple sclerosis: the 'real MS. Ther Adv Neurol Disord. 2022;15:175628642110667. doi. 10.1177/17562864211066751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matschke J, Lutgehetmann M, Hagel C, et al. . Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19(11):919-929. doi. 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poloni TE, Medici V, Moretti M, et al. . COVID-19-related neuropathology and microglial activation in elderly with and without dementia. Brain Pathol. 2021;31(5):e12997. doi. 10.1111/bpa.12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schurink B, Roos E, Radonic T, et al. . Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe. 2020;1(7):e290-e299. doi. 10.1016/S2666-5247(20)30144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh M, Bansal V, Feschotte C. A Single-cell RNA expression map of human coronavirus entry factors. Cell Rep. 2020;32(12):108175. doi. 10.1016/j.celrep.2020.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan P, Shen M, Yu Z, et al. . SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation. Nat Commun. 2021;12(1):4664. doi. 10.1038/s41467-021-25015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colais P, Cascini S, Balducci M, et al. . Impact of the COVID-19 pandemic on access to healthcare services amongst patients with multiple sclerosis in the Lazio region, Italy. Eur J Neurol. 2021;28(10):3403-3410. doi. 10.1111/ene.14879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dagan N, Barda N, Kepten E, et al. . BNT162b2 mRNA covid-19 vaccine in a nationwide mass vaccination setting. New Engl J Med. 2021;384(15):1412-1423. doi. 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldberg Y, Mandel M, Bar-On YM, et al. . Waning immunity after the BNT162b2 vaccine in Israel. New Engl J Med. 2021;385(24):e85. doi. 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chemaitelly H, Tang P, Hasan MR, et al. . Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. New Engl J Med. 2021;385(24):e83. doi. 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bsteh G, Gradl C, Heschl B, et al. Impact of vaccination on COVID-19 outcome in multiple sclerosis. Eur J Neurol. 2022;29(11):3329, 3336. doi. 10.1111/ene.15488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bohning D, Rocchetti I, Maruotti A, Holling H. Estimating the undetected infections in the Covid-19 outbreak by harnessing capture-recapture methods. Int J Infect Dis. 2020;97:197-201. doi. 10.1016/j.ijid.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russell TW, Golding N, Hellewell J, et al. . Reconstructing the early global dynamics of under-ascertained COVID-19 cases and infections. BMC Med. 2020;18(1):332. doi. 10.1186/s12916-020-01790-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data supporting the findings of this study will be shared on reasonable request from a qualified investigator and after approval by the ethics committee of the NMSC Melsbroek.


