ABSTRACT
Candida auris is an emerging multidrug-resistant fungal pathogen that can cause life-threatening infections in humans. Unlike other Candida species that colonize the gut, C. auris efficiently colonizes the skin and contaminates the patient's environment, resulting in rapid nosocomial transmission and outbreaks of systemic infections. As the largest organ of the body, the skin harbors beneficial microbiota that play a critical role to protect from invading pathogens. However, the role of skin microbiota in the colonization and pathogenesis of C. auris remains to be explored. With this perspective, we review and discuss recent insights into skin microbiota and their potential interactions with the immune system in the context of C. auris skin colonization. Understanding microbiota, C. auris, and host interactions in the skin is important to develop microbiome-based therapeutic approaches to prevent and treat this emerging fungal pathogen in humans.
KEYWORDS: C. auris, horizontal transmission, skin microbiota, host defense, microbiome-based therapeutics
PERSPECTIVE
Candida auris, an emerging multidrug-resistant fungal pathogen that predominately colonizes the skin, has been classified as an urgent threat by the U.S. Centers for Disease Control and Prevention (CDC) Antibiotic Threats Report (2019) and ranked in the critical priority group by the World Health Organization (WHO) in a recently released list of fungal priority pathogens (1, 2). C. auris is endemic at high prevalence in some long-term care facilities and acute care settings, where it can spread from patient to patient, resulting in outbreaks and systemic infections (3, 4). Several isolates of C. auris exhibit resistance to all three major classes of FDA-approved antifungal drugs, i.e., azoles, polyenes, and echinocandins. This poses a significant challenge to treat infections caused by this fungal pathogen (5, 6). Unlike most other Candida species, which colonize the gastrointestinal tract, C. auris efficiently colonizes the skin and contaminates the patient's environment (7), which may be related to C. auris’s unusual ability compared to other yeast to cause health care-associated outbreaks. Individuals colonized with C. auris can have a high fungal burden on their skin, which has been positively correlated with environmental contamination, transmission, and outbreaks of infections (7). Because skin colonization likely facilitates C. auris transmission and subsequent invasive disease, understanding the factors regulating C. auris skin colonization is critical to developing novel approaches to prevent and treat this emerging fungal infection in humans.
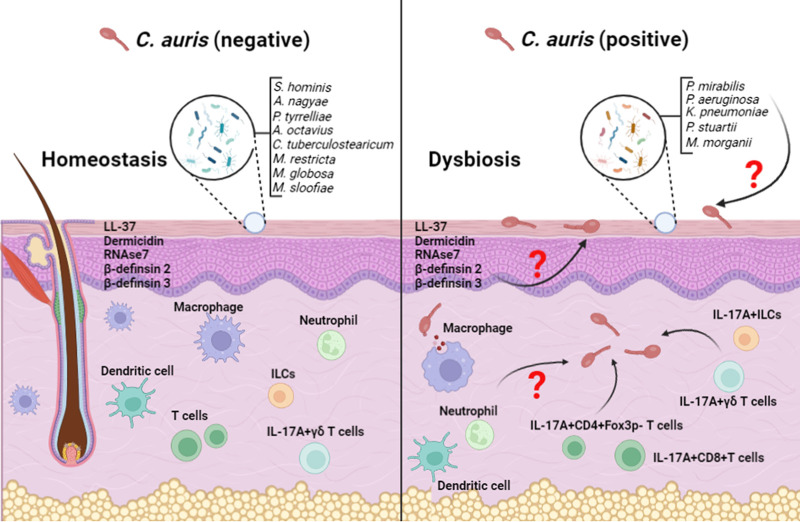
In human skin, C. auris coexists with commensal bacteria and fungi (3). Skin is colonized by a diverse set of commensal organisms (microbiota) that inhibit the growth and colonization of pathogens either by directly secreting small molecules and/or stimulating skin immune factors (8, 9). Results of this interaction can define the colonization level and pathogenesis of skin pathogens in humans. An interesting recent study by Proctor et al. investigated the associations between skin microbiota and C. auris colonization in humans (3). Utilizing 16S rRNA and internal transcribed spacer 1 (ITS1) of the rRNA gene locus sequencing approaches, bacterial and fungal communities were characterized using skin swabs from various body sites in both C. auris-positive and -negative individuals (Fig. 1). C. auris-negative individuals mainly harbored commensal bacteria such as Staphylococcus hominis, Staphylococcus epidermidis, Staphylococcus caprae, Anaerococcus nagyae, Peptoniphilus tyrrelliae, Anaerococcus octavius, and Corynebacterium tuberculostearicum. In contrast, C. auris-positive patients showed a different bacterial community, mainly containing Proteus mirabilis, Pseudomonas aeruginosa, Klebsiella pneumoniae, Providencia stuartii, and Morganella morganii. Fungal members also displayed a considerable shift in C. auris-positive individuals (3, 10). Fungal microbiota in C. auris-negative individuals were dominated by Malassezia species such as Malassezia restricta, Malassezia globosa, Malassezia furfur, and Malassezia arunalokei. On the other hand, the microbiota fungal community of C. auris-positive individuals was dominated by a mixture of Candida species, including C. auris, C. albicans, C. tropicalis, C. glabrata, and others (3, 10). The difference in both commensal bacteria and fungi communities present in C. auris-positive and C. auris-negative patients provides evidence that the host microbiome may play an important role in the colonization of C. auris on the skin. Although microbiota dysbiosis was observed in the skin of C. auris-colonized individuals, it is still not known whether alterations in the microbial community are a consequence of C. auris colonization or whether microbiota dysbiosis contributes to C. auris colonization in the skin.
FIG 1.
The figure illustrates the commensal microbiota and host factors in the C. auris-negative and -positive skin. The homeostatic portion (left) depicts a skin environment that has not been colonized by C. auris, while the dysbiosis (right) portion represents a skin that has been colonized by C. auris. Antimicrobial peptides such as LL-37, dermcidin, human β-defensin 2 and 3, and immune cells such as macrophages, neutrophils, ILCs, and dendritic cells that are integral to the skin immune defense against pathogens are shown in the C. auris-negative and -positive skin.
Commensal microbiota induce innate and adaptive host immune factors to eliminate skin pathogens (11–13). To operate optimally, microbiota, and the immune system need to communicate effectively. Though the role of skin immune factors in C. auris skin colonization is rudimentary, recent studies indicate that skin immune factors such as LL-37 and interleukin 17 (IL-17) inhibit C. auris growth and skin colonization (14, 15). LL-37, a major antimicrobial peptide (AMP) expressed by human keratinocytes, inhibits C. auris growth in vitro (15). LL-37 showed fungistatic and fungicidal activity against C. auris at concentrations of 25 to 100 and 50 to 200 μg/mL, respectively. Furthermore, LL-37 caused fungal cells to undergo extensive surface changes, inhibited the cell cycle, and induced oxidative stress in C. auris (15). Mice express cathelicidin antimicrobial peptide (CAMP), a homologue of human LL-37 peptide. CAMP is regulated by the hypoxia-inducible factor 1 alpha (HIF-1α) transcription factor. Mice lacking CAMP peptide or HIF-1α alone in the skin are highly susceptible to skin bacterial pathogens (16–18). CAMP and HIF-1α are also highly expressed in the intestine, where they are necessary to provide gut microbiota-mediated resistance to C. albicans intestinal colonization (19). On the other hand, skin microbiota such as Staphylococcus epidermidis induce LL-37 (CAMP) in keratinocytes (20). Among three AMPs (LL-37, defensin-1, and defensin-2) induced by S. epidermidis, LL-37 expression was increased 100-fold compared to 10-fold induction of defensin peptides in treatment with S. epidermidis (20). However, currently, the role of microbiota-LL-37 interactions in C. auris skin colonization is not clear. Future studies, such as using CAMP knockout mice, are critical to understanding the role of skin microbiota and AMP regulation in C. auris colonization in the skin.
Another recent study by Huang et al. identified that epicutaneous infection with C. auris elicited different immune cell types (14) (Fig. 1). T helper 17 cells (CD4-positive [CD4+] IL-17A+ and CD4+ IL-17F+), IL-17A- and IL-17F-producing CD8+ T cells, IL-17A-producing γδ T cells, and IL-17A-producing innate lymphoid cells (ILCs) were significantly increased in the skin samples 14 days after infection. Using mice deficient in IL-17R-associated adaptor molecule Act1, which lacks IL-17 responses, the authors demonstrated that IL-17A/IL-17F response is associated with protection against C. auris. Significantly increased fungal load was observed in the skin samples of Act1−/− groups compared to wild-type mice. Further using Rag2−/− (which lacks T cells) and Rag2−/− II2rg−/− (which lacks T cells and ILCs) mouse models, the authors identified that fungal load in skin samples from these mice was significantly increased, indicating the contribution of IL-17A/IL-17F produced by αβ T cells, γδ T cells, and ILCs to control C. auris colonization in the skin. On the other hand, skin microbiota such as S. epidermidis induces IL-17+ T cells to control cutaneous C. albicans skin colonization (21). However, given that host immune response to C. auris differs from C. albicans (22, 23) and there is a difference in immune response elicited by different commensal species of bacteria (21), findings from C. albicans and S. epidermidis cannot be readily extrapolated to C. auris and other microbiota species, respectively. Future studies are necessary to understand microbe-immune cell interactions in the context of C. auris infection.
Given that the skin of C. auris-positive individuals lacks several skin commensal bacteria present in the skin of C. auris-negative individuals (3), understanding the role and mechanisms through which skin microbiota regulate C. auris will expand the knowledge about the microbiota and host immune factors that control C. auris skin colonization. Restoring commensal microbiota in the C. auris-positive and/or individuals highly susceptible to C. auris colonization may be important in establishing a normal microbial ecosystem and preventing C. auris colonization of skin. An expansion of a diverse microbial community will enhance microbial diversity and immune defense that could prevent the selective growth of skin pathogens, including C. auris (11, 13, 24, 25). Furthermore, microbiota synergize with host immune factors to inhibit the colonization of skin pathogens (11, 13, 24, 25). Harnessing the immunomodulatory capacity of symbiotic factors from skin microbiota has a potential therapeutic role against C. auris. However, several questions remain to be answered (Fig. 1). How do skin commensals regulate C. auris colonization? Are the host AMPs (LL37, β-defensin 1, β-defensin 2, β-defensin 3, RNase 7, and dermidicin) and immune cells (innate and adaptive) involved in the defense against C. auris skin colonization? Identification of commensal microbes that regulate C. auris colonization will open the door for novel approaches to prevent C. auris skin colonization, subsequent nosocomial transmission, and mortality due to invasive C. auris infection in humans. For example, skin microbiota such as Staphylococcus hominis are currently in human clinical trials for the treatment of skin bacterial pathogens and atopic dermatitis (9, 12). This supports the feasibility of future interventions using commensal bacteria to prevent and treat C. auris skin colonization. Taken together, identification of bioactive microbial factors and the relevant host immune pathways regulated by specific microbiota will not only increase our understanding the microbiota-mediated mechanisms regulating C. auris skin colonization but also will provide a platform to develop novel antifungal therapeutics.
ACKNOWLEDGMENT
The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control (CDC). A.C. is a Fellow of the CIFAR program Fungal Kingdom: Threats & Opportunities.
Contributor Information
Anuradha Chowdhary, Email: chowdhary.anuradha@gmail.com.
Shankar Thangamani, Email: sthangam@purdue.edu.
Aaron P. Mitchell, University of Georgia
REFERENCES
- 1.Kadri SS. 2020. Key takeaways From the U.S. CDC's 2019 antibiotic resistance threats report for frontline providers. Crit Care Med 48:939–945. doi: 10.1097/CCM.0000000000004371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. 2022. WHO fungal priority pathogens list to guide research, development and public health action. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 3.Proctor DM, Dangana T, Sexton DJ, Fukuda C, Yelin RD, Stanley M, Bell PB, Baskaran S, Deming C, Chen Q, Conlan S, Park M, Welsh RM, Vallabhaneni S, Chiller T, Forsberg K, Black SR, Pacilli M, Kong HH, Lin MY, Schoeny ME, Litvintseva AP, Segre JA, Hayden MK, NISC Comparative Sequencing Program . 2021. Integrated genomic, epidemiologic investigation of Candida auris skin colonization in a skilled nursing facility. Nat Med 27:1401–1409. doi: 10.1038/s41591-021-01383-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rossow J, Ostrowsky B, Adams E, Greenko J, McDonald R, Vallabhaneni S, Forsberg K, Perez S, Lucas T, Alroy KA, Jacobs Slifka K, Walters M, Jackson BR, Quinn M, Chaturvedi S, Blog D, New York Candida auris Investigation Workgroup . 2021. Factors associated with Candida auris colonization and transmission in skilled nursing facilities with ventilator Units, New York, 2016–2018. Clin Infect Dis 72:e753–e760. doi: 10.1093/cid/ciaa1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, Colombo AL, Calvo B, Cuomo CA, Desjardins CA, Berkow EL, Castanheira M, Magobo RE, Jabeen K, Asghar RJ, Meis JF, Jackson B, Chiller T, Litvintseva AP. 2017. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis 64:134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chowdhary A, Prakash A, Sharma C, Kordalewska M, Kumar A, Sarma S, Tarai B, Singh A, Upadhyaya G, Upadhyay S, Yadav P, Singh PK, Khillan V, Sachdeva N, Perlin DS, Meis JF. 2018. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–17) in India: role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J Antimicrob Chemother 73:891–899. doi: 10.1093/jac/dkx480. [DOI] [PubMed] [Google Scholar]
- 7.Sexton DJ, Bentz ML, Welsh RM, Derado G, Furin W, Rose LJ, Noble-Wang J, Pacilli M, McPherson TD, Black S, Kemble SK, Herzegh O, Ahmad A, Forsberg K, Jackson B, Litvintseva AP. 2021. Positive correlation between Candida auris skin-colonization burden and environmental contamination at a ventilator-capable skilled nursing facility in Chicago. Clin Infect Dis 73:1142–1148. doi: 10.1093/cid/ciab327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byrd AL, Belkaid Y, Segre JA. 2018. The human skin microbiome. Nat Rev Microbiol 16:143–155. doi: 10.1038/nrmicro.2017.157. [DOI] [PubMed] [Google Scholar]
- 9.Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, Shafiq F, Kotol PF, Bouslimani A, Melnik AV, Latif H, Kim JN, Lockhart A, Artis K, David G, Taylor P, Streib J, Dorrestein PC, Grier A, Gill SR, Zengler K, Hata TR, Leung DY, Gallo RL. 2017. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med 9:eaah4680. doi: 10.1126/scitranslmed.aah4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang X, Welsh RM, Deming C, Proctor DM, Thomas PJ, Gussin GM, Huang SS, Kong HH, Bentz ML, Vallabhaneni S, Chiller T, Jackson BR, Forsberg K, Conlan S, Litvintseva AP, Segre JA, NISC Comparative Sequencing Program . 2021. Skin metagenomic sequence analysis of early Candida auris Outbreaks in U.S. nursing homes. mSphere 6:e0028721. doi: 10.1128/mSphere.00287-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bier K, Schittek B. 2021. Beneficial effects of coagulase-negative Staphylococci on Staphylococcus aureus skin colonization. Exp Dermatol 30:1442–1452. doi: 10.1111/exd.14381. [DOI] [PubMed] [Google Scholar]
- 12.Nakatsuji T, Hata TR, Tong Y, Cheng JY, Shafiq F, Butcher AM, Salem SS, Brinton SL, Rudman Spergel AK, Johnson K, Jepson B, Calatroni A, David G, Ramirez-Gama M, Taylor P, Leung DYM, Gallo RL. 2021. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nat Med 27:700–709. doi: 10.1038/s41591-021-01256-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belkaid Y, Tamoutounour S. 2016. The influence of skin microorganisms on cutaneous immunity. Nat Rev Immunol 16:353–366. doi: 10.1038/nri.2016.48. [DOI] [PubMed] [Google Scholar]
- 14.Huang X, Hurabielle C, Drummond RA, Bouladoux N, Desai JV, Sim CK, Belkaid Y, Lionakis MS, Segre JA. 2021. Murine model of colonization with fungal pathogen Candida auris to explore skin tropism, host risk factors and therapeutic strategies. Cell Host Microbe 29:210–221.e6. doi: 10.1016/j.chom.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rather IA, Sabir JSM, Asseri AH, Ali S. 2022. Antifungal activity of human cathelicidin LL-37, a membrane disrupting peptide, by triggering oxidative stress and cell cycle arrest in Candida auris. J Fungi 8:204. doi: 10.3390/jof8020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL. 2001. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 17.Peyssonnaux C, Datta V, Cramer T, Doedens A, Theodorakis EA, Gallo RL, Hurtado-Ziola N, Nizet V, Johnson RS. 2005. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J Clin Invest 115:1806–1815. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peyssonnaux C, Boutin AT, Zinkernagel AS, Datta V, Nizet V, Johnson RS. 2008. Critical role of HIF-1alpha in keratinocyte defense against bacterial infection. J Invest Dermatol 128:1964–1968. doi: 10.1038/jid.2008.27. [DOI] [PubMed] [Google Scholar]
- 19.Fan D, Coughlin LA, Neubauer MM, Kim J, Kim MS, Zhan X, Simms-Waldrip TR, Xie Y, Hooper LV, Koh AY. 2015. Activation of HIF-1alpha and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat Med 21:808–814. doi: 10.1038/nm.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bitschar K, Sauer B, Focken J, Dehmer H, Moos S, Konnerth M, Schilling NA, Grond S, Kalbacher H, Kurschus FC, Götz F, Krismer B, Peschel A, Schittek B. 2019. Lugdunin amplifies innate immune responses in the skin in synergy with host- and microbiota-derived factors. Nat Commun 10:2730. doi: 10.1038/s41467-019-10646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naik S, Bouladoux N, Linehan JL, Han S-J, Harrison OJ, Wilhelm C, Conlan S, Himmelfarb S, Byrd AL, Deming C, Quinones M, Brenchley JM, Kong HH, Tussiwand R, Murphy KM, Merad M, Segre JA, Belkaid Y. 2015. Commensal–dendritic-cell interaction specifies a unique protective skin immune signature. Nature 520:104–108. doi: 10.1038/nature14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horton MV, Johnson CJ, Zarnowski R, Andes BD, Schoen TJ, Kernien JF, Lowman D, Kruppa MD, Ma Z, Williams DL, Huttenlocher A, Nett JE. 2021. Candida auris cell wall mannosylation contributes to neutrophil evasion through pathways divergent from Candida albicans and Candida glabrata. mSphere 6:e00406-21. doi: 10.1128/mSphere.00406-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Zou Y, Chen X, Li H, Yin Z, Zhang B, Xu Y, Zhang Y, Zhang R, Huang X, Yang W, Xu C, Jiang T, Tang Q, Zhou Z, Ji Y, Liu Y, Hu L, Zhou J, Zhou Y, Zhao J, Liu N, Huang G, Chang H, Fang W, Chen C, Zhou D. 2022. Innate immune responses against the fungal pathogen Candida auris. Nat Commun 13:3553. doi: 10.1038/s41467-022-31201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, Spencer S, Hall JA, Dzutsev A, Kong H, Campbell DJ, Trinchieri G, Segre JA, Belkaid Y. 2012. Compartmentalized control of skin immunity by resident commensals. Science 337:1115–1119. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stacy A, Belkaid Y. 2019. Microbial guardians of skin health. Science 363:227–228. doi: 10.1126/science.aat4326. [DOI] [PubMed] [Google Scholar]