Significance
The transition from foraging to herding and farming influenced human health, but the impact of regional differences in trajectories of cultural change on human biology are poorly resolved. We investigate long-term trends in human stature and body mass of 3,507 skeletons from 366 archaeological sites in seven regions with varying trajectories of Holocene subsistence change. We observe declines in body size that preceded the transition to agriculture, and significant regional variation following the transition. Holocene statures and body mass remained relatively stable in primary regions of domestication; however, in areas such as Central and Northern Europe where non-native crops were difficult to establish, increases in stature and body mass coincide with the timing of selective sweeps for lactase persistence.
Keywords: bioarchaeology, agriculture, health, domestication, human adaptation
Abstract
Evidence for a reduction in stature between Mesolithic foragers and Neolithic farmers has been interpreted as reflective of declines in health, however, our current understanding of this trend fails to account for the complexity of cultural and dietary transitions or the possible causes of phenotypic change. The agricultural transition was extended in primary centers of domestication and abrupt in regions characterized by demic diffusion. In regions such as Northern Europe where foreign domesticates were difficult to establish, there is strong evidence for natural selection for lactase persistence in relation to dairying. We employ broad-scale analyses of diachronic variation in stature and body mass in the Levant, Europe, the Nile Valley, South Asia, and China, to test three hypotheses about the timing of subsistence shifts and human body size, that: 1) the adoption of agriculture led to a decrease in stature, 2) there were different trajectories in regions of in situ domestication or cultural diffusion of agriculture; and 3) increases in stature and body mass are observed in regions with evidence for selection for lactase persistence. Our results demonstrate that 1) decreases in stature preceded the origins of agriculture in some regions; 2) the Levant and China, regions of in situ domestication of species and an extended period of mixed foraging and agricultural subsistence, had stable stature and body mass over time; and 3) stature and body mass increases in Central and Northern Europe coincide with the timing of selective sweeps for lactase persistence, providing support for the “Lactase Growth Hypothesis.”
The transition from hunting and gathering to agricultural subsistence, characterized by the development of human control over the reproduction and evolution of plants and animals, has long been interpreted as the most significant shift in human interaction with the natural world (1). A general characteristic of this transition is the reduction in dietary breadth and dependence on one or a few highly productive domesticated plants that may lead to associated nutritional deficiency and a greater proportion of dietary carbohydrates relative to protein (2). Such nutritional changes are a component of the dominant paradigm of our understanding of the transition to agriculture, that it engendered a negative impact on human health which was exacerbated by sedentism, greater population density and frequencies of infectious disease, and the increased prevalence of zoonotic diseases associated with domestic animals (3, 4). A decrease in stature in the transition from hunting and gathering to farming has been documented in a wide range of contexts (5); however, many studies treat the transition as a rapid and discrete dichotomous contrast between foraging and farming subsistence strategies. This masks the complexity of the underlying social and biological transitions (6). In many regions, foragers are known to have extensively managed wild plants prior to full agriculture, and the process of plant domestication and the associated shift to crop farming is associated with hundreds or thousands of years of cultural and domesticate coevolution, during which many farming populations practiced mixed subsistence strategies and continued hunting and foraging. These trends suggest that dichotomous contrasts between foragers and farmers may be problematic, and the phenotypic consequences of the transition to agriculture should be considered using very deep diachronic skeletal series that document human biological change over long-time spans within the late Pleistocene and Holocene. Studies adopting this approach demonstrate that in some regions, initial declines in health indicators and stature are followed by a recovery (7, 8), or conversely that body size decreases predated the origins of agriculture (9). Recently an innovative study integrating estimates of the genetic component of stature based on ancient DNA and direct measures of human stature from European skeletal remains, demonstrated that a decline in stature between the Upper Paleolithic and Mesolithic and subsequent increase between the Neolithic and Bronze Age, was supported by both lines of evidence (10).
An important aspect of the domestication of animals is what has been termed the Secondary Products Revolution, the use of domestic animals for byproducts such as milk, wool, or as a source of labor for agricultural subsistence (11). A potential barrier to the consumption of milk among most human populations is the inability to digest the milk sugar lactose. The production of secondary milk products such as yogurt, where fermentation reduces the levels of lactose, or cheese, which separates lactose in whey (12), buffer adverse side effects of lactose consumption and involve a rich array of cultural practices for the reduction of milk (13). Human infants typically produce the intestinal enzyme lactase to enable digestion of dietary lactose in breastmilk but lose this ability during early childhood. There are a variety of single-nucleotide polymorphisms in the gene promoting lactase persistence that demonstrate independent convergent evolution in the ability to digest lactose among some European and Eastern African populations (14), and there has also been selection for lactase persistence in other regions (15, 16), in each case indicating an adaptive response to cultural shifts toward dairying. Milk is a source of insulin-like growth factor I (IGF-I) but IGF-I is degraded during the production of cheese or yogurt. It has been proposed that lactase persistence and milk consumption during childhood development and adulthood elevate circulating concentrations of IGF-I, which coordinate the timing of life-history events and promote skeletal growth (17).
In two recent papers, we have hypothesized that many of the phenotypic and health consequences of cultural and dietary change in the Holocene can be linked through energetic trade-offs, which provide a diversity of phenotypic outcomes in different contexts (18), and an adaptive shift toward milk consumption and lactase persistence may have mediated nutritional stress and “turbo-charged” growth in regions where chronic nutritional deficiency was mediated by dairying (19). There is some evidence that increases in stature in southern Sweden occurred during the early Bronze Age due to a consolidation of an agro-pastoral economy, rather than the onset of farming (20). Collectively, these papers support what we term the Lactase Growth Hypothesis (LGH), which suggests that lactase persistence and the ability to digest primary dairy products and lactose increased available dietary energy, shifted the energetic biology of human growth, and fueled regional differences in human body size.
In this paper, we investigate long-term diachronic trends in human stature and body mass in seven regions with different trajectories of the transition from hunting and gathering to agriculture, including the Levant, Southern, Central, and Northern Europe, the Nile Valley, South Asia, and China to address the following questions:
-
1.
Does the pattern of body size variation provide support for a Holocene reduction in body size associated with the transition to, and intensification of, agriculture?
-
2.
Does the pattern of body size variation throughout the Holocene differ between regions of in situ domestication compared with those where there was adoption of nonnative domesticates and more dynamic interactions between foragers and farmers?
-
3.
Are increases in adult stature found in regions and periods where there is evidence for strong past selection for lactase persistence?
The Origins and Diffusion of Agriculture and Animal Domesticates.
The earliest evidence for the transition to agriculture occurs in the Levant region of the Eastern Mediterranean. The late Epipaleolithic “Natufian” (14,500–11,600 cal B.P.) of the Levant is often interpreted as providing the earliest archaeological signature of the transition due to the extensive exploitation of wild grains and grindstones, stone architecture, and a variety of organized site structures (21), including the earliest evidence for bread (22). Many of the features associated with the Natufian are actually found at Epipaleolithic sites as early as 20 kya representing long-term trends toward sedentism, architecture, and exploitation of grain among hunter-gatherers (23); however, there is evidence of intensification of cereal cultivation associated with the Younger Dryas cooling at approximately 13,000 B.P. (24). The Pre-Pottery Neolithic A period shows the first evidence for larger human settlements with permanent architecture and intensive use and storage of grains by 11 kya (25). The Neolithization process was in full swing by 9 kya (26), yet the importance of hunting and gathering to subsistence in these periods remains evident (27), and illustrates the complexity of subsistence transitions in this region.
While these late Pleistocene and early Holocene cultures in Southwest Asia reflect the earliest transition to farming, it is now well established that agriculture and the domestication of animals originated independently in different regions of the world at different times throughout the first half of the Holocene (28), while agriculture also spread through migration and cultural diffusion into other regions including Europe (29). The adoption of agriculture in Europe was geographically and temporally variable. In Southern Europe, the process occurred fairly rapidly between 8 and 7.5 kya as climatic conditions were favorable for Southwest Asian domesticates, and was driven by a combination of demic diffusion (the migration of farmers into new territory) and cultural change by existing Mesolithic hunter-gatherer populations (30–32). The adoption of agriculture in Central Europe was primarily driven by demic diffusion from the Balkans (33), but there is evidence that this process was more gradual because of the rich aquatic resources in river systems such as the Danube (34), and possible challenges of establishing agriculture based upon Southwest Asian crops in more northern climes. The Linearbandkeramik culture, representing the early Neolithic of Central Europe, marks a crucial period in the expansion of agriculture into Central and Northern Europe as there is evidence of a reduction in crop diversity (35), the herding of mature cattle used for milk and secondary by-products (36), and selection for lactase persistence (37) in this population.
In the higher latitude regions of Northern Europe, the adoption of agriculture was limited by climatic factors, which reduced growing seasons and limited the viability of Southwest Asian crops, delayed the establishment of full agriculture, and led to closer contact and interaction between hunter-gatherers and farmers (38). The earliest Neolithic in the Baltics at ca. 7400 B.P. was characterized by population continuity from the Mesolithic, continued hunting and gathering, and the introduction of pottery. There appears to have been no significant shift in diet at the time, and broadscale agriculture was not established until ca. 4000 B.P. in the region (39). The adoption of agriculture in Scandinavia was characterized by genetic discontinuity and long-term persistence of hunting and gathering and distinct genetic lineages of foragers and farmers, with a gradual cultural diffusion and dietary shifts by 4000 B.P. (40). The process of Neolithization in Britain was similar to other regions of Northern Europe in that the process was delayed relative to other European regions, but it differed from Scandinavia and the Eastern Baltics as it appears to have been characterized by the replacement of Mesolithic foragers by diffusion of continental farmers (41). In central and northern Europe, the Neolithic transition is associated with animal herding and milk consumption (40, 42, 43) because crops were precarious (38). The delayed transition to agriculture across Northern Europe is associated with evidence for a selective sweep for increased lactase persistence genotypes (MCM6 allele) that occurred from ca. 6000–2000 B.P. (44) with evidence for strong selection after 3000 B.P. (45).
The origin and diffusion of dairying in the Levant and Europe is reasonably well resolved. There is evidence for widespread use of dairy products from lipid residues in Neolithic pottery throughout Europe (46) and increased dairy production in northern latitudes (47). The use of pottery often indicates a greater emphasis on delayed return resources that take time to process through activities like fermentation, and the storage and trade of food (48). The production of secondary milk products such as yogurt or cheese, which could be consumed without the presence of a lactase persistence (LP) allele, likely accounts for much of this evidence (49). The very earliest evidence for the direct consumption of milk (ca. 5000 B.P.), via whey protein in dental calculus, comes from Northern Europe (50). Selection for increased frequencies of genetic variants that lead to the persistence of lactase production into adulthood in Central and Northern Europe demonstrates the dependence of human populations on direct dairy products such as milk and that Neolithic culture drove human evolution in marginal environments (51). These shifts within Central Europe are the likely starting point of selection on FADS1 and lactase associated with the demic diffusion of farmers (31, 44). Recent evidence suggests that selection for LP in Central and Northern Europe may have been in response to famine or pathogen exposure, and the pattern of LP persistence alleles reflected in ancient DNA (aDNA) corresponds with incident solar radiation (46), patterns which may be explained in relation to the challenges of establishing crop species at higher latitudes (52) or increased prevalence of zoonotic diseases (53, 54). While no direct associations between LP alleles and phenotypic variation have been found in contemporary UK Biobank data, representing populations buffered from severe caloric restriction or high disease prevalence (46), the relationship between LP and phenotype in the past is unknown.
The process of transition toward agricultural lifeways differed in other regions. In the Nile Valley, agriculture was introduced as a relatively complete package from the Levant at ca. 7500 B.P. (28). While there were independent domestication events of species such as pearl and finger millet, sorghum, and African rice within sub-Saharan Africa, none of these species were indigenous to the Nile region. There is evidence for the presence of herd animals on the same timescale (55), however, there are variable patterns of lactase persistence genotypes in the region today, which have been attributed to past selection and gene flow from Southwest Asia (56, 57). In China, there is evidence for the in situ domestication of common and foxtail millet between 10,300 and 8700 cal B.P. (58) and the domestication of rice between 6900 and 6600 B.P. in the lower Yangtze River (59); however, the dietary shift toward dependence on cereal crops was a gradual process distributed over millennia (28). Although there is evidence for early and rapid domestication of pigs in the lower Yangtze (60), the adoption of domesticated animals commonly used in dairying did not occur until the late Holocene (61). The prevalence of lactase persistence phenotypes within China remains low today (15), although there are slightly higher frequencies in the North (16). The evidence for long-term dietary change within South Asia is particularly complex with considerable spatial and temporal variation. The earliest pottery and domestic rice are present by 9 kya, but evidence for significant sedentary villages and agricultural dependence occurs only after 4 kya (62) following the mid-Holocene movement of crops from both the Western Eurasia and China (63). There is evidence for the independent domestication of cattle in the Indus region ca. 7 kya and convergent evolution for lactase persistence in South Asia, with the highest frequencies in the northwest parts of the region, but very low frequencies in southern and eastern areas of the Indian subcontinent (64). It is also notable that Indian pastoralists maintain greater stature than higher caste individuals, which has been attributed to milk consumption (65).
The question of body size variation as a reflection of diet and health in the past has been of long-standing interest to bioarchaeologists. While documented declines in Neolithic estimated statures have been linked to lower predicted statures based on genetics (66), adult body size also reflects developmental plasticity and life history variation (67). Stature itself is not really a trait, but rather a consequence of growth, which ultimately reflects variation in strategies for energy allocation throughout development. Body mass likewise indicates investment in lean and fat tissue, although unlike stature, these can respond to ecological stresses through adult life. Improved growth is generically a good marker of health because many aspects of somatic maintenance benefit from better growth in early life, whereas defense against pathogens and early reproduction reduces energy for linear growth and lean tissue deposition. Applying a life history perspective to growth provides insights into the likely role of infectious disease and pathogens in reductions in stature in prehistory, as there are multiple routes to generate the adult phenotype which extend beyond diet but also include allocation of energy to immune function or reproduction, potentially mediated by fat deposition (18).
Results
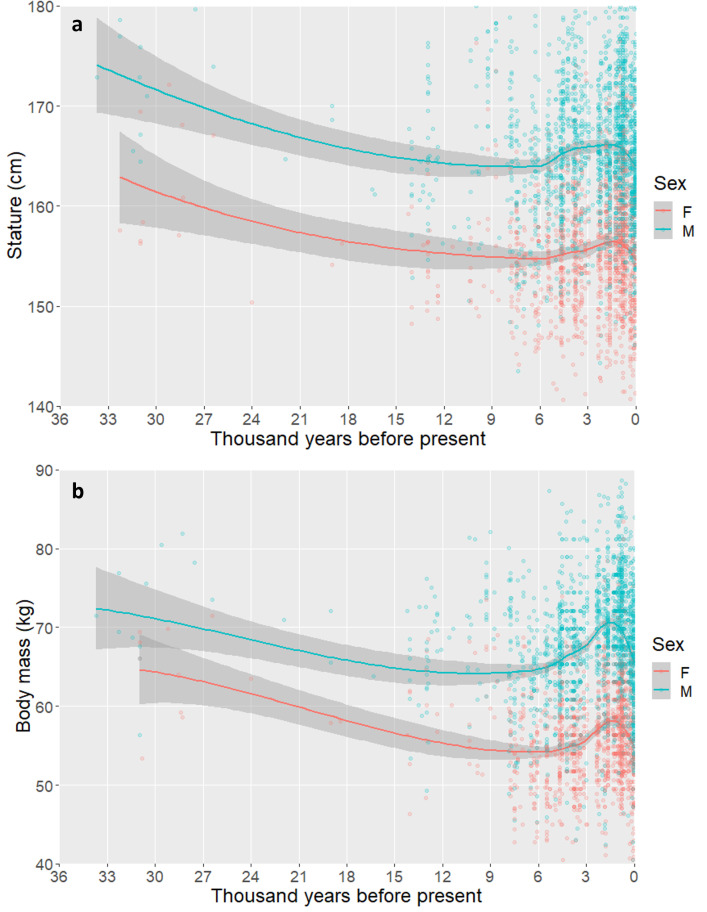
The pooled dataset including all seven regions indicates there is evidence for a long-term reduction in mean stature and body mass from the Upper Paleolithic to the mid-Holocene that is evident before the adoption of agricultural subsistence in most regions. Mean statures reach their minimum between 8 and 6 kya (Fig. 1) and are followed by mid-Holocene rebounds in stature and body mass. When compared between temporal intervals, there are declines in both stature and body mass in the terminal Pleistocene (20–15 kya), followed by another reduction ca. 8–7 kya (SI Appendix, Fig. S2) and a pattern of diversification of phenotypes in the second half of the Holocene. Our dataset does not have uniform representation of different regions across the full temporal range, as earlier assemblages are dominated by Southwest Asian and European samples, so some of these results of pooled analyses may be influenced by data aggregation and require further study as we address regional comparisons below.
Fig. 1.
Scatterplots with Lowess lines illustrating broad patterns of (A) stature variation, illustrating a general decline over 30 kya, followed by stability through the terminal Pleistocene and early Holocene, in the period characterized by the origin and diffusion of agriculture in most regions; (B) body mass which declines until the period of the broad adoption of agriculture following 7 kya. Shaded regions represent 95% confidence intervals around fit lines.
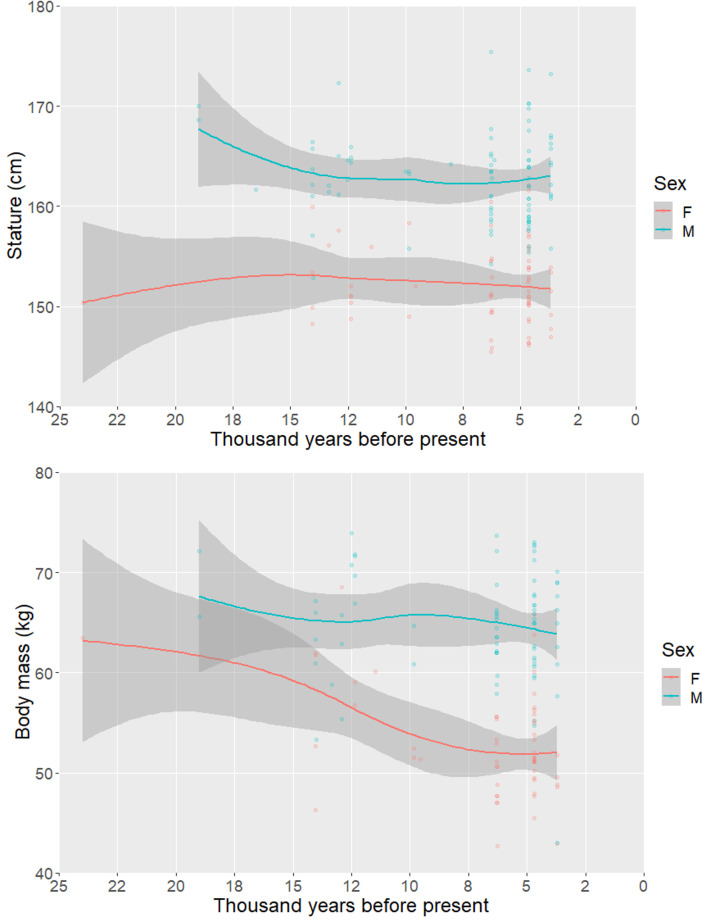
Similar trends are also visible on regional scales. In the Levant, the region with the earliest evidence for the transition to agriculture, there is a significant decline in male stature from ca. 20 kya to 9 kya (Fig. 2 and SI Appendix, Fig. S3), by which time the newly Neolithic lifeways are fully established, followed by general stability until 3 kya. Female stature is relatively stable across this time frame, but body mass declines, while male body mass remains relatively consistent across this chronological series.
Fig. 2.
Scatterplots with Lowess lines illustrating long-term trends in (A) stature and (B) body mass in the Levant, a region representing the earliest transition to agriculture and the in situ domestication of numerous plant and animal species. Shaded regions represent 95% confidence intervals around fit lines.
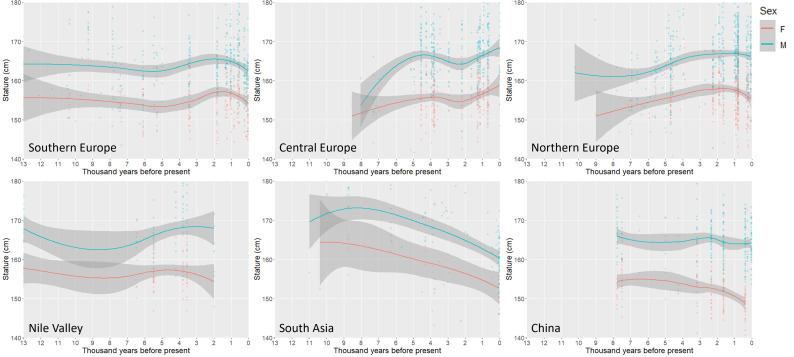
A comparison of trends in stature across the past 10 kya in other regions is presented in Fig. 3. Southern Europe is characterized by a general decline between 10 and 6 kya, followed by relative stability through the mid-Holocene (SI Appendix, Fig. S4). In Central Europe, there is a marked and significant increase in male stature between 8 and 5 kya, and a general increase in female stature across the same time frame (SI Appendix, Fig. S5A). Both males and females in Northern Europe are also characterized by a general increase in stature from 7 kya, with males peaking ca. 3 kya and females ca. 2 kya (SI Appendix, Fig. S5A). Stature trends across the same time frame in the Nile are more variable and show no specific long-term trends, while in China statures are generally consistent throughout the Holocene, except for a decline among females after 3 kya (SI Appendix, Fig. S6). A contrasting pattern is observed in South Asia where there is a significant decline in both male and female stature throughout the Holocene.
Fig. 3.
Scatterplots with LOWESS lines illustrating temporal variation in stature in Southern, Central, and Northern Europe, the Nile Valley, South Asia, and China. Lowess smoothing parameter = 0.8 except as follows: Northern Europe = 0.9, Nile = 1.1, South Asia = 1.0. Shaded regions represent 95% confidence intervals around fit lines.
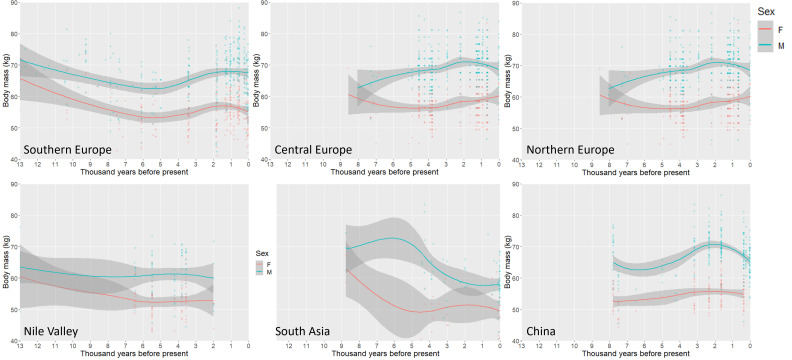
A regional comparison of Holocene body mass trends (Fig. 4) illustrates a consistent pattern of initial decline in Southern Europe followed by a period of relative stability. In Central Europe, there is a general increase in male body mass between 4.5 and 2 kya, while female mass is relatively stable throughout the Holocene. In Northern Europe, there are early Holocene declines in both male and female body mass that reach their low point approximately 5 kya and are followed by increases by 2 kya. When these patterns are contrasted with other regions, we see relatively little change in the Nile Valley, while in South Asia male body masses increase in the first half of the Holocene in a period where female mass appears to decline. Here, estimated male masses fall considerably after 4 kya. In China, there appears to be relative stability in body mass in the early part of the Holocene, followed by increases among males from 5 to 2 kya, and among females from 3 to 1 kya.
Fig. 4.
Scatterplots with LOWESS lines illustrating temporal variation in body mass in Southern, Central and Northern Europe, the Nile Valley, South Asia, and China. Lowess smoothing parameter: Southern Europe = 0.9, Central Europe = 0.8, Northern Europe = 0.9, Nile Valley = 1.1, South Asia = 1.1, China = 1.0. Shaded regions represent 95% confidence intervals around fit lines.
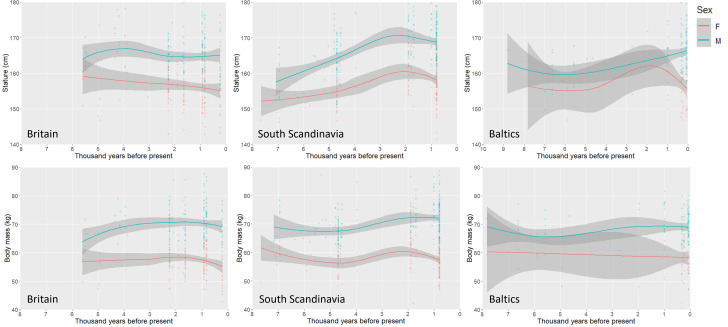
Noting that the most significant long-term increases in stature occur in Central and Northern Europe where there is evidence for strong selection acting upon lactase persistence during the mid-Holocene, we consider specific trends in subregions of Northern Europe, Britain, southern Scandinavia, and the eastern Baltics, over the past 8,000 y (Fig. 5 and SI Appendix, Fig. S5 A and B). In Britain, there is relatively minor and nonsignificant temporal variation in stature through time, while male body mass generally increases from ca. 5 to 2 kya. In Scandinavia, there are marked and significant increases in male stature between 7 and 4 kya. A less pronounced increase in female stature is observed on a longer timescale, between 7 and 2 kya. Body masses in the region are consistent among early Holocene males but females show a decrease through the mid-Holocene. Both sexes show increases in body mass between 5 and 2 kya, but the trend is more pronounced among females. In the Baltics increases in stature are expressed in both males and females between 6 and 2 kya while body masses are relatively consistent throughout the Holocene.
Fig. 5.
Long-term trends in stature (above) and body mass (below) in subregions of Northern Europe. Lowess smoothing parameters for stature: Britain = 1.0, Scandinavia = 1.0, Baltics = 1.0. Lowess smoothing parameters for body mass: Britain = 0.9, South Scandinavia = 1.0, Baltics = 1.0. Shaded regions represent 95% confidence intervals around fit lines.
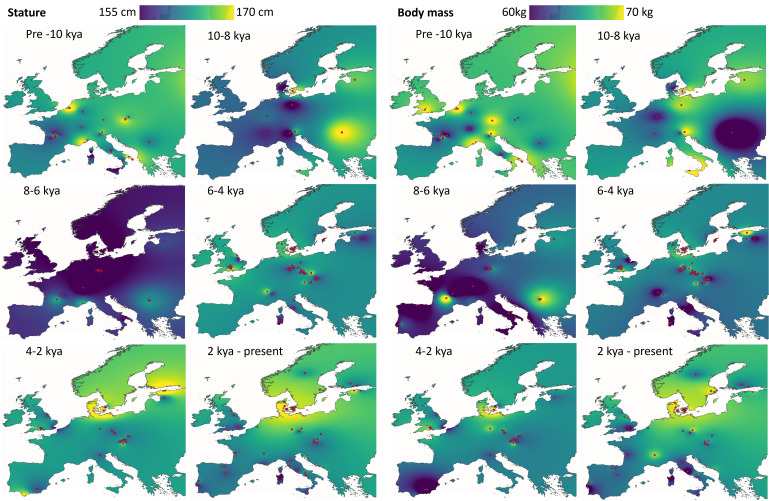
To investigate the spatiotemporal patterning of body size variation throughout Europe in greater detail, we generated heat maps of mean statures and body masses (Fig. 6 and SI Appendix, S8–S12). The results demonstrate fairly uniform stature across Europe before 10 kya and a general decline between 10 to 6 kya, followed by increases that are most pronounced in Northern Europe and Southern Scandinavia. Body mass trends follow a broadly similar pattern, with much of Europe characterized by estimated body masses above 65 kg before 10 kya, followed by declines in much of Western Europe through to 6 kya. Increases in body mass are observed in Central Europe from 6 to 4 kya and across most of Northern Europe from 4 kya to the present. The period from 10 to 6 kya is predominantly before the transition to agriculture in central and northern regions but includes hunter-gatherers, farmers, and others with variable or transitional subsistence strategies, suggesting that further analyses with an expanded dataset are required to contextualize this trend.
Fig. 6.
Heatmaps of spatiotemporal variation in stature and body mass throughout Holocene Europe. Red points denote site locations.
Discussion
In this study, we investigated long-term trends in human stature and body mass relative to late Pleistocene and Holocene cultural change in seven different regions. We analyzed data by chronological and geographical information rather than cultural labels, given the significant spatiotemporal and regional variation in cultural characteristics attributed to terms such as the Neolithic, opting instead to discuss the broader timescales upon which the process of the transition to domesticated plants and animals was enacted. The results demonstrated that in most regions body size decreased before the earliest manifestations of agriculture, regional patterns of phenotypic variation over time are variable, and this spatiotemporal variation in stature and body mass is not directly associated with the onset of the Neolithic. Given their timing, these trends cannot simply be explained by subsistence changes related to the reliance on domesticated plants and animals. We also noted recent phenotypic diversification that is most pronounced in the last 2,000 years, which requires further study but may stem from a combination of demographic expansion, genetic diversification, and socio-economic inequality.
It is worth noting that the long-term trends in the Levant, where the earliest transition to agriculture was observed as a complex process over millennia (23, 26), demonstrated relatively stable stature and body mass trends over time. The Levant is a region characterized by long-term population continuity and the in situ domestication of numerous species of indigenous plants (68) and animals (69) over an extended period of the terminal Pleistocene and early Holocene (70). The transition to agriculture in this region represented a long period of mixed hunting and gathering and cultivation of crops and domesticates that were well adapted to local environmental conditions. Similarly, there was no significant change in stature through time in China after plant domestication, and an increase in body mass among males during the later Holocene. This is a region that is also characterized by population continuity, local domesticates, a very long period of mixed foraging and farming rather than an abrupt agricultural transition, and high levels of environmental productivity.
It is important to note that our approach to comparing population trends by region may confound local impacts of migrations and gene flow, such as the well documented increase in steppe ancestry among northern Europeans, which may have influenced north-south gradients in human stature (71), and similar population movements in other regions likely influenced the complexity and timing of cultural and phenotypic changes. In South Asia, for example, we noted long-term reductions in stature and body mass throughout the Holocene. The region, however, exhibits a high degree of ecological diversity and is characterized by the adoption of different domesticates that originated in East Asia, Western Asia, and Africa in different regions of the Indian Subcontinent. There is also geographic variation in lactase persistence phenotypes that complicate the pattern here. Similarly, in the Nile Valley, another region characterized by the adoption of plant and animal domesticates from other regions, results are highly variable and likely confounded by the complexity of migration history in the region. At present, there are insufficient data to match aDNA evidence for ancestry with direct phenotypic measures on the broad scale presented in this paper. However, it is likely that underlying genetic variation and changes in the sociocultural environment, including diet, underpin phenotypic change. Further research will be required to clarify long-term spatiotemporal trends in phenotypic and genetic variation.
We also aimed to test the LGH by determining whether the geographic and temporal timing of selection for LP phenotypes is associated with increases in stature and body mass. The most significant mid-Holocene increases in stature and body mass occurred in Northern Europe between 7 and 4 kya and these were preceded by increases in stature in Central Europe that occurred between ∼8 and 5 kya. These regions are linked in providing evidence for mid-Holocene selective sweeps in genetic variants associated with LP, providing preliminary support for the LGH. Within Northern Europe, modest increases in body mass were noted in Britain among males, but the most significant trends toward increased stature and body mass were found in the Baltic and southern Scandinavian regions. Heat map results demonstrate how the current patterns of stature and mass variation in Europe were established throughout the mid to late Holocene. While size increases were noted in regions where there is evidence of natural selection in response to dairying, we noted different trends among males and females with more significant increases in stature generally expressed among men and more significant variation in body mass among women. We suggest this is explained by greater plasticity among men, particularly in stature, in response to environmental and cultural fluctuations, while women’s phenotypic variation is better able to buffer environmental stress (72) via sexual dimorphism in body mass that reflect lifelong differences in energetics and somatic investment (73). There is evidence that males show greater stunting in response to early life undernutrition (74), which would lead to greater variation in adult male statures. While skeletal methods of body mass estimation do not generally reflect late-life accrual of body mass (75), both lean mass (funding fetal growth) and fat mass (funding lactation) are components of maternal fitness, and substantial variability in these tissues emerges prior to reproduction, suggesting that body mass variation is more directly linked to female fitness than stature (76). Phenotypic plasticity may have also been expressed most strongly late in development, where IGF-I factors in dairy milk may have directly fueled growth differences and sexual dimorphism (17, 19). In general, we note that while the timing of size increases corresponds with selective sweeps in lactase persistence (44), it is unclear whether phenotypic variation reflects underlying genetic variation or whether phenotypic plasticity precedes later genetic adaptation, but there is growing evidence that the latter is an important mechanism of adaptability (77).
Overall, our results provide provisional evidence for greater phenotypic stability in regions of in situ domestication and where the transition to agriculture was gradual over millennia. The dispersal of farmers into novel environments where foreign domesticates may have struggled to establish appears to have led to greater phenotypic diversity in human populations. In Central and Northern Europe, where the cultural response to environmental stress involved the direct consumption of milk, this appears to buffer environmental stress and may have fueled the patterns of human growth and body size observed in the late Holocene. The trends observed in this paper may be a direct or indirect consequence of shifts in energy allocation to somatic growth associated with the digestion of lactose but may otherwise be influenced by patterns of disease load during growth, changes in weaning patterns, population density, migration, or genetic drift. A life-history framework may help to understand how the interaction of such factors influences somatic investment (18). We note that there are other regions where LP genetic variants are found in high frequencies, including the Mongolian Steppe (78), and convergent evolution of MCM6 in East Africa (14) with what may be a stronger directional selection among the Maasai (79). At present, we do not have data of sufficient resolution to investigate whether ancient selection and dairying fueled phenotypic change in these regions. However, our results suggest that the transition to agriculture may have had regionally specific influences on human populations that can be elucidated through analyses of long-term diachronic trends in human-culture-environment interactions. Long-term trends are best investigated through broadscale integration of bioarchaeological and phenotypic data with aDNA, paleoecology, and archaeological data that account for the spatiotemporal complexity of Holocene cultural and dietary transitions.
Materials and Methods
In this study, we compare body size estimates from skeletal remains derived from 366 archaeological sites in the Levant, Northern, Central and Southern Europe, the Nile Valley, South Asia, and China (SI Appendix, Fig. S1 A–D and Table S1) spanning the period from 34,300 B.P. to the present. Stature estimates for 3,507 individual skeletons were derived from maximum femur length, and when femora were not preserved, tibial lengths, using regression equations derived from ecogeographically matched populations (SI Appendix, Tables S2 and S3). Body mass estimates, based on femoral head diameters or the maximum breadth of the tibial plateau were calculated for 3,342 individuals using published equations (SI Appendix, Table S4). We view the terminology surrounding categories such as Mesolithic, Epipaleolithic, and Neolithic to be broadly problematic because of regional differences in their use and spatiotemporal variation in the specific dietary and cultural correlates of such categories which may lead to spurious dichotomization of cultural variables. We opt, instead, to analyze data chronologically by calibrated radiocarbon dates, both as a continuous variable and grouped into millennial cohorts and tested for significant differences using univariate ANOVA with Hochberg GT2 post hoc tests (SI Appendix, Tables S5–S32). We interpret these results in the context of regional variation in late Pleistocene and Holocene cultural trajectories.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
The authors would like to thank three reviewers for comments that improved this manuscript and all curators who granted access to archaeological material used in this study. Funding was received from the European Research Council (617627 to J.T.S. and 323727 to C.M.), the National Science Foundation (BCS-0642297 and BCS-0642710 to C.B.R.), the Leverhulme/Isaac Newton funded Early Career Fellowship (ECF-2015-520 [Leverhulme]/Minute 1508(c) [INT] to E.P.), the National Geographic Society Research and Exploration Grant (8495-08 to L.M.), and the British Academy International Partnership and Mobility Grant PM140125 to E.P. and V.M.-T.
Author contributions
J.T.S., E.P., C.B.R., and J.C.K.W. designed research; J.T.S., E.P., C.B.R., M.B., M.A.G., F.-J.L., L.M., V.M.-T., E.P., M.R., and J.C.K.W. performed research; J.T.S., E.P., C.B.R., M.B., M.A.G., F.-J.L., L.M., C.M., V.M.-T., E.P., M.R., Y.Y.S., S. Stefanovic, S. Stoddart, and G.Z. contributed new reagents/analytic tools; J.T.S. and E.P. analyzed data; and J.T.S., E.P., C.B.R., and J.C.K.W. wrote the paper.
Competing interest
C.S.L. and C.B.R. are coauthors on a chapter in a forthcoming book.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
Data used in this study, some of which has been previous published (80, 81) are publicly available through Borealis (DOI: https://doi.org/10.5683/SP3/RTPPWX), (82).
Supporting Information
References
- 1.Childe V. G., Man Makes Himself (Watts and Co., 1936). [Google Scholar]
- 2.Cordain L., et al. , Plant-animal subsistence ratios and macronutrient energy estimations in worldwide hunter-gatherer diets. Am. J. Clin. Nutr. 71, 682–692 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Cohen G. J., Armelagos M. N., Paleopathology at the Origins of Agriculture (Academic Press, 1984). [Google Scholar]
- 4.Larsen C. S., Biological changes in human populations with agriculture. Annu. Rev. Anthropol. 24, 185–213 (1995). [Google Scholar]
- 5.Mummert A., Esche E., Robinson J., Armelagos G. J., Stature and robusticity during the agricultural transition: Evidence from the bioarchaeological record. Econ. Hum. Biol. 9, 284–301 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Pinhasi R., Stock J. T., Human Bioarchaeology of the Transition to Agriculture (John Wiley & Sons, 2011). [Google Scholar]
- 7.Starling A. P., Stock J. T., Dental indicators of health and stress in early Egyptian and Nubian agriculturalists: A difficult transition and gradual recovery. Am. J. Phys. Anthropol. 134, 520–528 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Stock J. T., et al. , “Body size, skeletal biomechanics, mobility and habitual activity from the late palaeolithic to the mid-dynastic Nile Valley” in Human Bioarchaeology of the Transition to Agriculture, Pinhasi R., Stock J. T., Eds. (Wiley-Blackwell, 2011), pp. 347–367. [Google Scholar]
- 9.Niskanen M., Ruff C. B., Holt B., Sladek V., Berner M., “Temporal and geographic variation in body size and shape of europeans from the late pleistocene to recent times” in Skeletal Variation and Adaptation in Europeans: Upper Paleolithic to the Twentieth Century, Ruff C., Ed. (Wiley Blackwell, 2018), pp. 49–90. [Google Scholar]
- 10.Cox S. L., Ruff C. B., Maier R. M., Mathieson I., Genetic contributions to variation in human stature in prehistoric Europe. Proc. Natl. Acad. Sci. U.S.A. 116, 21484–21492 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenfield H. J., The secondary products revolution: The past, the present and the future. World Archaeol. 42, 29–54 (2010). [Google Scholar]
- 12.Curry A., Archaeology: The milk revolution. Nature 500, 20–22 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Sibbesson E., Reclaiming the rotten: Understanding food fermentation in the Neolithic and beyond. Environ. Archeol. 27, 111–122 (2019). [Google Scholar]
- 14.Tishkoff S. A., et al. , Convergent adaptation of human lactase persistence in Africa and Europe. Nat. Genet. 39, 31–40 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ségurel L., Bon C., On the evolution of lactase persistence in humans. Annu. Rev. Genomics Hum. Genet. 18, 297–319 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Itan Y., Jones B. L., Ingram C. J., Swallow D. M., Thomas M. G., A worldwide correlation of lactase persistence phenotype and genotypes. BMC Evol. Biol. 10, 36 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiley A. S., The evolution of lactase persistence: Milk consumption, insulin-like growth factor i, and human life-history parameters. Q. Rev. Biol. 93, 319–345 (2018). [Google Scholar]
- 18.Wells J. C. K., Stock J. T., Life history transitions at the origins of agriculture: A model for understanding how niche construction impacts human growth, demography and health. Front. Endocrinol. (Lausanne) 11, 325 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells J. C. K., Pomeroy E., Stock J. T., Evolution of lactase persistence: Turbo-charging adaptation in growth under the selective pressure of maternal mortality? Front. Physiol. 12, 696516 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tornberg A., Stature and the Neolithic transition– Skeletal evidence from southern Sweden. J. Archaeol. Sci. Rep. 17, 58–67 (2018). [Google Scholar]
- 21.Bar-Yosef O., The Natufian culture in the Levant, threshold to the origins of agriculture. Evol. Anthropol. 6, 159–177 (1998). [Google Scholar]
- 22.Arranz-Otaegui A., Gonzalez Carretero L., Ramsey M. N., Fuller D. Q., Richter T., Archaeobotanical evidence reveals the origins of bread 14,400 years ago in northeastern Jordan. Proc. Natl. Acad. Sci. U.S.A. 115, 7925–7930 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maher L. A., Richter T., Stock J. T., The pre-Natufian Epipaleolithic: Long-term behavioral trends in the Levant. Evol. Anthropol. 21, 69–81 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Hillman G., Hedges R., Moore A., Colledge S., Pettitt P., New evidence of lateglacial cereal cultivation at Abu Hureyra on the euphrates. Holocene 11, 383–393 (2001). [Google Scholar]
- 25.Kuijt I., Finlayson B., Evidence for food storage and predomestication granaries 11,000 years ago in the Jordan Valley. Proc. Natl. Acad. Sci. U.S.A. 106, 10966–10970 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Twiss K. C., The Neolithic of the Southern Levant. Evol. Anthropol. 16, 24–35 (2007). [Google Scholar]
- 27.Rosen A. M., Rivera-Collazo I., Climate change, adaptive cycles, and the persistence of foraging economies during the late Pleistocene/Holocene transition in the Levant. Proc. Natl. Acad. Sci. U.S.A. 109, 3640–3645 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belwood P., First Farmers: The Origins of Agricultural Societies (Blackwell Publishing, 2005). [Google Scholar]
- 29.Pinhasi R., Fort J., Ammerman A. J., Tracing the origin and spread of agriculture in Europe. PLoS Biol. 3, e410 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeder M. A., Domestication and early agriculture in the Mediterranean Basin: Origins, diffusion, and impact. Proc. Natl. Acad. Sci. U.S.A. 105, 11597–11604 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathieson S., Mathieson I., FADS1 and the timing of human adaptation to agriculture. Mol. Biol. Evol. 35, 2957–2970 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vander Linden M., Silva F., Dispersals as demographic processes: Testing and describing the spread of the Neolithic in the Balkans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 376, 20200231 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fort J., Demic and cultural diffusion propagated the Neolithic transition across different regions of Europe. J. R. Soc. Interface 12, 20150166 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jovanović J., Power R. C., de Becdelièvre C., Goude G., Stefanović S., Microbotanical evidence for the spread of cereal use during the Mesolithic-Neolithic transition in the Southeastern Europe (Danube Gorges): Data from dental calculus analysis. J. Archaeol. Sci. 125, 105288 (2021). [Google Scholar]
- 35.Colledge S., Conolly J., Shennan S., The evolution of Neolithic farming from SW Asian origins to NW European limits. Eur. J. Archaeol. 8, 137–156 (2005). [Google Scholar]
- 36.Gillis R. E., et al. , The evolution of dual meat and milk cattle husbandry in Linearbandkeramik societies. Proc. Biol. Sci. 284, 20170905 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Itan Y., Powell A., Beaumont M. A., Burger J., Thomas M. G., The origins of lactase persistence in Europe. PLOS Comput. Biol. 5, e1000491 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Betti L., et al. , Climate shaped how Neolithic farmers and European hunter-gatherers interacted after a major slowdown from 6,100 BCE to 4,500 BCE. Nat. Hum. Behav. 4, 1004–1010 (2020). [DOI] [PubMed] [Google Scholar]
- 39.Jones E. R., et al. , The neolithic transition in the baltic was not driven by admixture with early european farmers. Curr. Biol. 27, 576–582 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malmström H., et al. , Ancient DNA reveals lack of continuity between neolithic hunter-gatherers and contemporary Scandinavians. Curr. Biol. 19, 1758–1762 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Brace S., et al. , Ancient genomes indicate population replacement in Early Neolithic Britain. Nat. Ecol. Evol. 3, 765–771 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Copley M. S., et al. , Dairying in antiquity. III. Evidence from absorbed lipid residues dating to the British Neolithic. J. Archaeol. Sci. 32, 523–546 (2005). [Google Scholar]
- 43.Copley M. S., et al. , Direct chemical evidence for widespread dairying in prehistoric Britain. Proc. Natl. Acad. Sci. U.S.A. 100, 1524–1529 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allentoft M. E., et al. , Population genomics of stone age Eurasia. bioRxiv 36, 2022.05.04.490594 (2022). [Google Scholar]
- 45.Burger J., et al. , Low prevalence of lactase persistence in bronze age Europe indicates ongoing strong selection over the last 3,000 years. Curr. Biol. 30, 4307–4315.e13 (2020). [DOI] [PubMed] [Google Scholar]
- 46.Evershed R. P., et al. , Dairying, diseases and the evolution of lactase persistence in Europe. Nat. 2022, 1–10 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cubas M., et al. , Latitudinal gradient in dairy production with the introduction of farming in Atlantic Europe. Nat. Commun. 11, 1–9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Craig O. E., Prehistoric fermentation, delayed-return economies, and the adoption of pottery technology. Curr. Anthropol. 62, S233–S241 (2021). [Google Scholar]
- 49.Salque M., et al. , Earliest evidence for cheese making in the sixth millennium BC in northern Europe. Nature 493, 522–525 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Warinner C., et al. , Direct evidence of milk consumption from ancient human dental calculus. Sci. Reports 4, 1–6 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gerbault P., et al. , Evolution of lactase persistence: An example of human niche construction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366, 863–877 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Betti L., et al. , Climate shaped how Neolithic farmers and European hunter-gatherers interacted after a major slowdown from 6,100 BCE to 4,500 BCE. Nat. Hum. Behav. 4, 1004–1010 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Ledger M. L., et al. , Intestinal parasites at the Late Bronze Age settlement of Must Farm, in the fens of East Anglia, UK (9th century B.C.E.). Parasitology 146, 1583–1594 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Ledger M. L., et al. , Parasite infection at the early farming community of Çatalhöyük. Antiquity 93, 573–587 (2019). [Google Scholar]
- 55.Brass M., Early North African cattle domestication and its ecological setting: A reassessment. J. World Prehist. 31, 81–115 (2018). [Google Scholar]
- 56.Hollfelder N., Babiker H., Granehäll L., Schlebusch C. M., Jakobsson M., The genetic variation of lactase persistence alleles in Sudan and South Sudan. Genome Biol. Evol. 13, evab065 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hassan H. Y., et al. , Genetic diversity of lactase persistence in East African populations Genetics. BMC Res. Notes 9, 1–7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu H., et al. , Earliest domestication of common millet (Panicum miliaceum) in East Asia extended to 10,000 years ago. Proc. Natl. Acad. Sci. U.S.A. 106, 7367–7372 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fuller D. Q., et al. , The domestication process and domestication rate in rice: Spikelet bases from the lower Yangtze. Science 323, 1607–1610 (2009). [DOI] [PubMed] [Google Scholar]
- 60.Dong N., Yuan J., Rethinking pig domestication in China: Regional trajectories in central China and the Lower Yangtze Valley. Antiquity 94, 864–879 (2020). [Google Scholar]
- 61.Larson G., Fuller D. Q., The evolution of animal domestication. Annu. Rev. Ecol. Evol. Syst. 45, 115–136 (2014). [Google Scholar]
- 62.Fuller D. Q., Murphy C., Overlooked but not forgotten: India as a center for agricultural domestication. Gen. Anthropol. 21, 1–8 (2014). [Google Scholar]
- 63.Stevens C. J., et al. , Between China and South Asia: A Middle Asian corridor of crop dispersal and agricultural innovation in the Bronze Age. Holocene 26, 1541–1555 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gallego Romero I., et al. , Herders of Indian and European cattle share their predominant allele for lactase persistence. Mol. Biol. Evol. 29, 249–260 (2012). [DOI] [PubMed] [Google Scholar]
- 65.Guntupalli A. M., Baten J., The development and inequality of heights in North, West, and East India 1915–1944. Explor. Econ. Hist. 43, 578–608 (2006). [Google Scholar]
- 66.Marciniak S., et al. , An integrative skeletal and paleogenomic analysis of stature variation suggests relatively reduced health for early European farmers. Proc. Natl. Acad. Sci. U.S.A. 119, e2106743119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wells J. C. K., Stock J. T., Re-examining heritability: Genetics, life history and plasticity. Trends Endocrinol. Metab. 22, 421–428 (2011). [DOI] [PubMed] [Google Scholar]
- 68.Asouti E., Fuller D. Q., From foraging to farming in the southern Levant: The development of Epipalaeolithic and Pre-pottery Neolithic plant management strategies. Veg. Hist. Archaeobot. 21, 149–162 (2012). [Google Scholar]
- 69.Conolly J., et al. , Meta-analysis of zooarchaeological data from SW Asia and SE Europe provides insight into the origins and spread of animal husbandry. J. Archaeol. Sci. 38, 538–545 (2011). [Google Scholar]
- 70.Fuller D. Q., Willcox G., Allaby R. G., Early agricultural pathways: Moving outside the ‘core area’ hypothesis in Southwest Asia. J. Exp. Bot. 63, 617–633 (2012). [DOI] [PubMed] [Google Scholar]
- 71.Mathieson I., et al. , Genome-wide patterns of selection in 230 ancient Eurasians. Nature 528, 499–503 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stinson S., Sex differences in environmental sensitivity during growth and development. Am. J. Phys. Anthropol. 28, 123–147 (1985). [Google Scholar]
- 73.Wells J. C. K., Sexual dimorphism in body composition across human populations: Associations with climate and proxies for short- and long-term energy supply. Am. J. Hum. Biol. 24, 411–419 (2012). [DOI] [PubMed] [Google Scholar]
- 74.Thurstans S., et al. , Boys are more likely to be undernourished than girls: A systematic review and meta-analysis of sex differences in undernutrition. BMJ Glob. Health 5, e004030 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Young M., Johannesdottir F., Poole K., Shaw C., Stock J. T., Assessing the accuracy of body mass estimation equations from pelvic and femoral variables among modern British women of known mass. J. Hum. Evol. 115, 130–139 (2018). [DOI] [PubMed] [Google Scholar]
- 76.Wells J. C. K., Life history trade-offs and the partitioning of maternal investment: Implications for health of mothers and offspring. Evol. Med. Public Health 2018, 153–166 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Levis N. A., Pfennig D. W., Evaluating ‘plasticity-first’ evolution in nature: Key criteria and empirical approaches. Trends Ecol. Evol. 31, 563–574 (2016). [DOI] [PubMed] [Google Scholar]
- 78.Segurel L., et al. , Why and when was lactase persistence selected for? Insights from Central Asian herders and ancient DNA. PLoS Biol. 18, e3000742 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schlebusch C. M., Sjodin P., Skoglund P., Jakobsson M., Stronger signal of recent selection for lactase persistence in Maasai than in Europeans. Eur. J. Hum. Genet. 21, 550–553 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ruff C. B., Ed., Skeletal Variation and Adaptation in Europeans: Upper Paleolithic to the Twentieth Century (Wiley Blackwell, Hoboken, NJ, 2017), pp. 493. [Google Scholar]
- 81.Stock J. T., et al. , “Body Size, Skeletal Biomechanics, Mobility and Habitual Activity from the Late Palaeolithic to the Mid-Dynastic Nile Valley” in Human Bioarchaeology of the Transition to Agriculture, R. Pinhasi, J. T. Stock, Eds. (Wiley-Blackwell, Hoboken, NJ, 2011), pp. 347–367. [Google Scholar]
- 82.Stock J. T., et al. , Subsistence Transitions Body Size Dataset. Borealis. https://borealisdata.ca/dataset.xhtml?persistentId=doi:10.5683/SP3/RTPPWX. Accessed 1 November 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
Data used in this study, some of which has been previous published (80, 81) are publicly available through Borealis (DOI: https://doi.org/10.5683/SP3/RTPPWX), (82).