Significance
Heparin is a mixture of sulfated polysaccharides with different sizes of sugar chains and sulfation patterns. As a commonly used anticoagulant drug, heparin also displays protection against sepsis in animal models and sepsis patients, but the benefit of heparin in human clinical trials is controversial. Our hypothesis is that using structurally homogeneous oligosaccharides will improve the efficacy against sepsis and enhance the understanding of the mechanism of action. Here, we demonstrate that an octadecasaccharide (18-mer) inhibits the inflammatory responses of the host animals to achieve protection against sepsis. The findings open a chemical space to design therapeutic agents to treat sepsis.
Keywords: heparin, sepsis, histone, HDL, HMGB1
Abstract
Sepsis is a lethal syndrome manifested by an unregulated, overwhelming inflammation from the host in response to infection. Here, we exploit the use of a synthetic heparan sulfate octadecasaccharide (18-mer) to protect against sepsis. The 18-mer not only inhibits the pro-inflammatory activity of extracellular histone H3 and high mobility group box 1 (HMGB1), but also elicits the anti-inflammatory effect from apolipoprotein A-I (ApoA-I). We demonstrate that the 18-mer protects against sepsis-related injury and improves survival in cecal ligation and puncture mice and reduces inflammation in an endotoxemia mouse model. The 18-mer neutralizes the cytotoxic histone-3 (H3) through direct interaction with the protein. Furthermore, the 18-mer enlists the actions of ApoA-I to dissociate the complex of HMGB1 and lipopolysaccharide, a toxic complex contributing to cell death and tissue damage in sepsis. Our study provides strong evidence that the 18-mer mitigates inflammatory damage in sepsis by targeting numerous mediators, setting it apart from other potential therapies with a single target.
Sepsis impacts millions of people worldwide annually (1), but effective therapeutics approved by regulatory agencies are unavailable (2, 3). Currently, septic patients are treated with antibiotics and supportive care such as fluid resuscitation, but the dysregulated host response remains unaddressed (4). The dysregulated systemic inflammatory response in sepsis is complex, involving many biological factors and signaling pathways. Damage-associated molecular patterns, such as extracellular histones H3 (H3) and high mobility group box 1 (HMGB1), have been recently recognized as critical players leading to uncontrolled inflammation in sepsis and sepsis severity (5–7). Extracellular H3 is found in neutrophil extracellular traps, which are released in response to infection to perform antimicrobial functions. H3 is also released by injured cells in sepsis (8). As positively charged proteins, H3 directly induces endothelial cell death by interacting with phospholipid–phosphodiester in the cell membrane (9). Extracellular H3 amplifies inflammation indirectly by toll-like receptor (TLR) signaling pathways, contributing to organ damage (8). HMGB1 is another cationic protein released from nuclei of damaged cells and activated immune cells. HMGB1 augments cellular injury in septic mice by intracellularly delivering endotoxin (lipopolysaccharide, LPS) to cause cell death. The delivery of LPS by HMGB1 requires the receptor for advanced glycation end-products (10).
Heparan sulfate (HS) is a sulfated polysaccharide widely present on the cell surface and in the extracellular matrix. HS regulates inflammatory responses by interacting with chemokines and controlling the migration of neutrophils (11, 12). HS interacts with histones to neutralize its cytotoxicity to endothelial cells and prevents histone-induced mortality in animal studies (13). HS also binds to HMGB1 to inhibit its pro-inflammatory activity (14). HS has the disaccharide repeating units of glucuronic acid (GlcA) or iduronic acid (IdoA) linked to glucosamine (GlcN), and these residues can carry sulfo groups. The saccharide length and sulfation patterns of HS determine the functions of HS (15). Understanding the relationship between the functions and the saccharide sequences is essential for developing an HS-based therapeutic agent. However, HS isolated from biological sources is a mixture of polysaccharides with different sizes and sulfation patterns. The structural heterogeneity of natural HS adds complexity to studying the relationship between the saccharide sequences and functions.
We have developed a chemoenzymatic method to synthesize pure HS oligosaccharides (16). These pure HS oligosaccharides selectively bind to specific proteins in biological systems, serving as unique molecular probes to investigate the functions of HS. We used oligosaccharides to investigate the mechanism of the anti-inflammatory effect of HS. In one example, an HS octadecasaccharide protects against liver injury caused by acetaminophen overdose. This octadecasaccharide inhibits the pro-inflammatory activity of HMGB1 but does not exhibit anticoagulant activity (17). In another example, an HS dodecasaccharide protects against liver damage by ischemia-reperfusion injury by mitigating thromboinflammation. The dodecasaccharide inhibits the pro-inflammatory activity of HMGB1 and carries anticoagulant activities (18).
In this study, we synthesized three homogeneous HS oligosaccharides, including hexasaccharide (6-mer), dodecasaccharide (12-mer), and octadecasaccharide (18-mer), to investigate their anti-inflammatory activities in septic mouse models. We demonstrate that the treatment of HS 18-mer reduces the inflammatory responses and improves the survival of the polymicrobial septic mice induced by cecal ligation and puncture (CLP) injury, a clinically relevant sepsis model (19). We also employed an endotoxemia mouse model to investigate the mechanism of action 18-mer. Our findings suggest that 18-mer protects against sepsis via at least two mechanisms. In one mechanism, 18-mer directly binds to histone 3 (H3) and neutralizes histone’s cytotoxicity. In the other mechanism, 18-mer promotes the release of apolipoprotein A-I (ApoA-I) from high-density lipoprotein (HDL) to form non-toxic ApoA-I/LPS complexes. ApoA-I reduces the levels of toxic LPS/HMGB1 complexes, which were previously shown to be associated with sepsis lethality (20). Currently, the critical role of dysfunctional HDL, extracellular histones, and HMGB1 has been recognized in preclinical and clinical settings (10, 21, 22). Biologics that individually target HDL, histones, or HMGB1 have also been tested respectively in preclinical trials, yet the outcomes from the treatments are still in the investigational stage (23–25). Our findings set an example of a multi-target approach to dampen the excessive inflammatory response during sepsis and alleviate disease symptoms, which may provide a strategy to treat sepsis.
Results
Synthesis of Structurally Defined HS Oligosaccharides.
Three structurally defined oligosaccharides were used in this study to test the relationship between the length of HS oligosaccharides and the anti-inflammatory effect. The 6-mer, 12-mer, and 18-mer were synthesized using a chemoenzymatic method involving multiple enzymatic modification steps (SI Appendix, Fig. S1A). All three HS have a disaccharide unit of GlcNS-GlcA at the non-reducing end, -IdoA2S-GlcNS- repeating units in the middle, and GlcA-pNP at the reducing end (Fig.1A) (17, 26). The structures and purities of these oligosaccharides were confirmed by mass spectrometry and anion exchange HPLC. None of the oligosaccharides exhibits anticoagulant activity as determined by anti-factor Xa and anti-factor IIa assays (SI Appendix, Fig. S1 B and C). The lack of anticoagulant activity of these oligosaccharides allows us to probe the anti-inflammatory mechanism independent of the anticoagulation effect in a sepsis animal model.
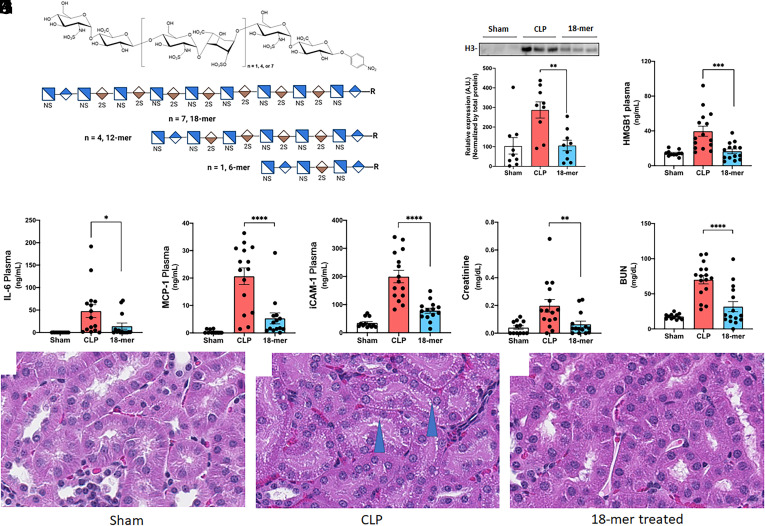
Fig. 1.
18-mer inhibits inflammation in CLP-induced septic mice. (A) Chemical structures of 6-mer, 12-mer, and 18-mer. Short-hand structure for each oligosaccharide is also presented for clarity. Chemoenzymatic synthesis of the HS is shown in SI Appendix, Fig. S1A. (B–H) Mice underwent CLP surgery were administered (S.Q.) 20 mg/kg of 18-mer at 0, 6, 12 h after CLP, and euthanized 24 h after CLP to collect plasma for the following analysis. Concentrations of the biomarkers were individually presented for male and female groups in SI Appendix, Figs. S11 and S12. (B) Circulating H3 in mouse plasma was evaluated by Western analysis. A representative western analysis image is presented on top. The bar graph represents the H3 band intensities from the individual samples. The full image is presented in SI Appendix, Fig. S13. (C) Circulating HMGB1 in mouse plasma was evaluated by ELISA (n = 6 male and 3 female). (D–H) The levels of IL-6, MCP-1, soluble iCAM-1, creatinine, and BUN were tested in mice plasma. The Data was expressed as mean ± SEM and analyzed by one-way ANOVA followed by Dunnett’s multiple comparison test. n = 6 to 10 male and 3 to 5 female. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. (I–K) Photomicrographs of proximal renal tubules. (I) Normal tubules of control mice. (J) 24 h post-CLP, proximal tubular epithelial cells have moderate vacuolation (blue arrowheads) and are less tall than in control mice. (K) 24 h post-CLP with 18-mer treatment, proximal tubular epithelial cells are similar to control mice.
18-mer Reduces Local and Systemic Inflammatory Responses as well as Kidney Damage in CLP-Injured Mice.
We established the CLP mouse model based on the literature reports (27). The CLP model was validated using hematological analysis (SI Appendix, Fig. S2). We hypothesized that the longest synthetic HS, 18-mer, could reduce inflammation in CLP-injured mice (Fig. 1 B–F). Based on our previous studies with synthetic chondroitin sulfates and other studies with nonanticoagulant HS in sepsis (13, 24, 28), we subcutaneously administered 18-mer (20 mg/kg) after CLP surgery to mice at 0, 6, and 12 h, and euthanized 24 h after CLP to collect plasma, peritoneal lavage, and the kidney for analysis. The 18-mer treatment decreased the plasma concentration of histone 3 (H3) and HMGB1 (Fig. 1 B and C). It also significantly reduced the protein levels of inflammatory cytokine (interleukin-6, IL-6), chemokine (monocyte chemoattractant protein-1, MCP-1), and adhesion molecule (intracellular adhesion molecule-1, iCAM-1) in plasma (Fig. 1 D–F) and peritoneal lavage (SI Appendix, Fig. S3 A–C), suggesting that the 18-mer mitigated systemic and local inflammation in the peritoneal space. Peritoneal lavage bacterial counts were performed for CLP mice and 18-mer-treated mice. Although the 18-mer treatment appeared to reduce the bacteria counts, it was not statistically significant (SI Appendix, Fig. S3D). The result suggested that the 18-mer does not display a direct anti-microbial effect in polymicrobial sepsis in vivo. We also conducted a multiplex assay covering 21 biomarkers related to sepsis, showing that the 18-mer had an overall protective effect against sepsis-induced inflammation (SI Appendix, Fig. S3E and Table S3) (29).
The protection from 18-mer treatment against kidney damage was examined. A statistically significant reduction of creatinine and blood urea nitrogen (BUN) was observed in the 18-mer-treated group (Fig. 1 G and H). Moreover, the mRNA levels of IL-6, MCP-1, and iCAM-1 also significantly decreased in the kidney after 18-mer treatment (SI Appendix, Fig. S4 A–C). Histological evaluation of the kidneys in CLP animals revealed that the proximal tubule epithelial cells were shortened in height compared to control animals and had notable cytoplasmic vacuolation. This finding in sepsis models had been previously reported (30). Animals administered the 18-mer did not have this tubular vacuolation, and kidneys were comparable microscopically to control kidneys (Fig. 1 I–K). Additionally, CLP animals had microscopic findings in the tubules at the margin of the inner and outer stripe of the outer medulla in CLP animals (interpreted to involve the thin and thick segment of the loop of Henle). The findings were characterized by intratubular eosinophilic material and cellular debris, which reflected acute tubular degeneration. This region of the kidney is sensitive to ischemic injury, and the findings are consistent with acute hypoperfusion to the kidney which may occur with septicemia. This tubular degeneration was not observed in two of three animals administered the 18-mer, similar to control animals (SI Appendix, Fig. S4 D–F). Taken together, our findings suggest that the treatment of 18-mer exerts renoprotection in CLP-injured mice.
18-mer Neutralizes the Cytotoxicity of Histone 3 (H3).
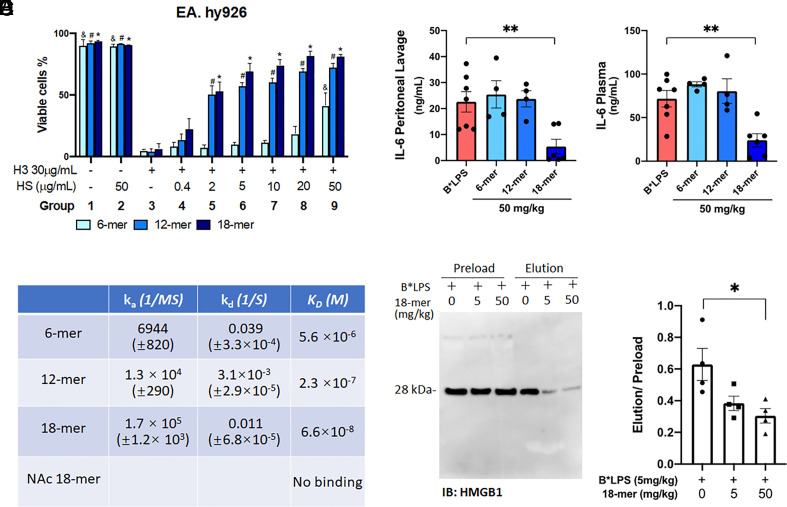
An elevated plasma concentration of extracellular histone has been associated with septic damage (22). Abundant H3 was released to plasma at 24 h in CLP-injured mice; a significant decrease was found in the group treated with 18-mer (Fig. 1B). The effect of size of different HS oligosaccharides on the cytotoxicity of histone (H3) was carried out using the human endothelial cell line EA.hy926. Three oligosaccharides were employed, including 6-mer, 12-mer, and 18-mer (Fig. 2A). The 18-mer treatment improved the cell viability in a dose-dependent manner. While 12-mer improved the cell viability overall, the efficacy is consistently lower than that of 18-mer under different concentrations, and 6-mer was much less effective than that of the 12-mer and 18-mer (Fig. 2A). The binding affinities (KD) to H3 for 18-mer and two shorter oligosaccharides, 6-mer and 12-mer, were determined by surface plasmon resonance (SPR). 18-mer displayed the highest binding affinity (KD = 66 nM) to H3 (Fig. 2B). These data demonstrate that the binding affinity of HS oligosaccharide to H3 correlates with the efficacy of neutralizing extracellular H3’s cytotoxicity. Next, we examined 18-mer’s protection against histone-induced lethality in a mouse model (22, 28). The 18-mer can fully protect against the intravenous injection of histones at a lethal dose (SI Appendix, Fig. S5), suggesting that the saccharide directly affects extracellular histones in vivo.
Fig. 2.
18-mer reduces inflammation by targeting extracellular H3 and the LPS/HMGB1 complex. (A) The viability of endothelial cells EA.hy926 was analyzed by flow cytometry. Cells were treated with 30 μg/mL of H3 and the indicated concentration of HS for an hour before analysis (n = 3). The statistical difference in cell viability with the same HS treatment but the different concentrations is shown compared to the untreated group (group 3) (&P < 0.05 for 6-mer, #P < 0.05 for 12-mer, *P < 0.05 for 18-mer.) (B) The associate rate constant (ka), disassociation constant (kd), and binding affinity (KD) of oligosaccharides to H3 as measured by SPR. (C–F) Plasma and peritoneal lavage were collected from mice 2 h after biotinylated LPS (B*LPS) administration (i.p., 5 mg/kg) with or without the indicated concentration of HS (n = 4 to 7). (C, D) The protein level of IL-6 was evaluated by ELISA. (E, F) The complex of B*LPS/HMGB1 was isolated by affinity pull-down using streptavidin resin from mouse peritoneal lavage, shown as a representative image of immunoblot with HMGB1 (E) and bar graph for the intensity of the band (F). Samples before and after passing streptavidin resin are labeled “preload” and “elution,” respectively. Data expressed as mean ± SEM and analyzed by one-way ANOVA followed by Dunnett’s multiple comparison test. *P < 0.05; **P < 0.01.
The rationales for choosing the structure of the 18-mer that has a repeating IdoA2S-GlcNS disaccharide are primarily based on two factors: 1) neutralization of the toxicity of histone; 2) resistance to the digestion by heparanase. To this end, we tested two other 18-mers, NAc 18-mer and NS6S 18-mer, with different disaccharide repeating units. Unlike the 18-mer, NAc 18-mer did not bind to H3 (Fig. 2B). We also demonstrated that NAc 18-mer did not protect against the toxicity of histones in mice (SI Appendix, Fig. S5). NAc 18-mer consists of a disaccharide repeating unit of GlcA-GlcNAc, containing no sulfo groups, suggesting that sulfation is also important for the protection in addition to the size. Heparanase is reportedly upregulated under sepsis conditions (31). The enzyme degrades HS oligosaccharides to diminish the protective effect against sepsis. Therefore, an oligosaccharide that is resistant to heparanase digestion is desirable. As shown in SI Appendix, Figs. S6A and S7 A and B, the 18-mer is largely resistant to digestion. In a control experiment, NS6S 18-mer was shown to be susceptible to the digestion of heparanase (SI Appendix, Figs. S6B and S7 C and D). NS6S 18-mer has a repeating disaccharide unit of GlcA-GlcNS6S that has a sulfation pattern distinct from that of the 18-mer (SI Appendix, Fig. S7 A and C).
18-mer Attenuates Inflammatory Responses Mediated by LPS/HMGB1 Complex in Endotoxemia Mice but Does Not Disrupt LPS/HMGB1 Complex In Vitro.
HMGB1 is also released under systemic inflammation conditions, including sepsis, and displays pro-inflammatory activity. Elevated plasma concentration of HMGB1 was observed in CLP-injured mice, and the treatment with 18-mer significantly reduced the plasma concentration of HMGB1 (Fig. 1C). HMGB1 forms complexes with bacterial LPS to deliver LPS into the cells, and the intracellular LPS augments pyroptosis through the caspase-11 mediated pathway (20). We previously reported that the 18-mer interacts with HMGB1 with a binding affinity of 186 nM (17). Here, we tested whether the 18-mer reduces the HMGB1-mediated inflammation in endotoxemia mice. In this model, the injection of LPS induces the release of HMGB1 to peritoneal space and subsequent elevation of IL-6 and TNF-α (32). Co-injection of the 18-mer with LPS significantly reduced the concentration of IL-6 in peritoneal lavage and plasma (Fig. 2 C and D) and the concentration of TNF-α in peritoneal lavage and plasma (SI Appendix, Fig. S8 A and B). However, co-injection of 6-mer or 12-mer with LPS showed no reduction in IL-6 and TNF-α, suggesting that the protective effect is HS size-dependent (Fig. 2 C and D and SI Appendix, Fig. S8 A and B). The results confirmed that 18-mer, but not 6-mer or 12-mer, attenuates the inflammatory effect induced by LPS and HMGB1.
We next examined whether 18-mer prevented the formation of the complex of LPS/HMGB1 in vivo (Fig. 2 E and F). In this experiment, we used an endotoxemia mouse model in which the release of HMGB1 was induced via peritoneally administering biotinylated LPS or co-injecting 18-mer and biotinylated LPS. The complex of LPS/HMGB1 was then isolated from peritoneal lavage using an avidin-agarose affinity column followed by immunoblotting for HMGB1. A reduction in LPS/HMGB1 was observed in the co-injection group (Fig. 2 E and F). Coinciding with the LPS/HMGB1 complex reduction, injection with 18-mer reduced concentrations of IL-6 in peritoneal lavage and plasma in a dose-dependent manner (SI Appendix, Fig. S8 C and D). These data suggest that a lower concentration of LPS/HMGB1 complex leads to weaker inflammatory responses from the host.
A potential explanation for the reduction of the LPS/HMGB1 complex by the 18-mer injection is that the 18-mer disassociates the complex. To test the hypothesis, we would need to demonstrate that the 18-mer disrupts the complex of LPS/HMGB1 in an in vitro experiment. This experiment was carried out using the peritoneal lavage harvested from endotoxemia mice that contained the LPS/HMGB1 complex. However, the addition of 18-mer to the lavage failed to decrease the amount of HMGB1 bound to LPS (SI Appendix, Fig. S8E). Like 18-mer, 6-mer did not decrease the complex of LPS/HMGB1. As a positive control, unlabeled LPS effectively displaced HMGB1 from the complex of biotinylated LPS/HMGB1 (SI Appendix, Fig. S8F). Our data suggest that the presence of 18-mer does not cause the disassociation of the LPS/HMGB1 complex. In a separate experiment, no interaction was observed between 18-mer and LPS using a centrifugal ultrafiltration approach (28) (SI Appendix, Fig. S8 G and H), ruling out the possibility that 18-mer reduces the complex of LPS/HMGB1 via interacting with LPS.
18-mer Induces the Formation of ApoA-I/LPS Complex In Vivo.
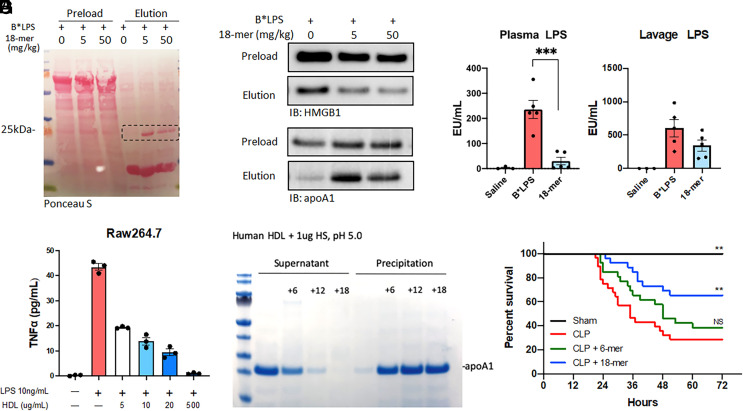
The inability to disrupt the LPS/HMGB1 complex in the in vitro experiment led us to hypothesize that reduction of the LPS/HMGB1 complex by the 18-mer involves other factors. To this end, we analyzed the proteins bound to biotinylated LPS from the endotoxemia mice with and without co-injection of 18-mer. SDS-PAGE analysis with Ponceau S staining clearly revealed an extra protein band (~25 kDa) from the co-injection group (Fig. 3A). Furthermore, the proteomic analysis confirmed that the protein band was ApoA-I (SI Appendix, Table S1 and Fig. S9A).
Fig. 3.
18-mer enlists ApoA-I to reduce inflammation and exert a protective effect against sepsis. (A–D) Plasma and peritoneal lavage were collected from mice 2 h after B*LPS administration (i.p., 5 mg/kg) with or without the indicated concentration of HS (n = 4 to 7). (A) The SDS-PAGE image of the peritoneal lavage stained with Ponceau S. The membrane is the same as Fig. 2E. The protein migrated at 25 kDa was identified as ApoA-I by proteomic analysis. (B) Immunoblot of the level of B*LPS/HMGB1 (Top) and B*LPS/ApoA-I (Bottom) from peritoneal lavage. Samples before and after affinity purification by streptavidin resin are labeled “preload” and “elution,” respectively. The full image is presented in SI Appendix, Fig. S15. (C, D) The concentration of LPS in the plasma and peritoneal lavage (n = 4 to 7). Data expressed as mean ± SEM and analyzed by one-way ANOVA followed by Dunnett’s multiple comparison test. ***P < 0.001(E) The protein level of TNF-a from the cell supernatant of LPS-stimulated Raw264.7, with or without HDL pretreatment (n = 3). (F) The dissociation of human HDL by HS oligosaccharides. Human HDL (200 mg/mL) was incubated with 6-mer, 12-mer, and 18-mer (5 mg/mL) under mildly acidic conditions and centrifuged. The supernatant and precipitation from each reaction were analyzed by SDS-PAGE followed by Coomassie blue staining. (G) The 72-h survival in CLP mice was administered (S.Q.) with saline, 6-mer, and 18-mer at 0, 6, 12, 24, 36, and 52 h. (sham, n=8 male; CLP, n=22 male and 6 female; CLP + 18-mer, n = 20 male and 6 female, CLP + 6-mer, n = 20 male and 6 female). Data analyzed by log-rank test; overall, P = 0.0006; CLP vs. Sham, P = 0.0019; CLP vs. CLP + 18-mer, P = 0.0015; CLP vs. CLP + 6-mer, P = 0.2040. Survival data were individually presented for male and female groups in SI Appendix, Fig. S11.
Three lines of evidence demonstrated that the presence of ApoA-I ameliorates the damage associated with LPS/HMGB1 and LPS alone. First, we discovered that ApoA-I disrupted the LPS/HMGB1 complex in the in vitro experiment. The incubation of recombinant ApoA-I with peritoneal lavage containing biotinylated LPS/HMGB1 complex reduced the binding of HMGB1 to LPS in a dose-dependent manner (SI Appendix, Fig. S9B). Second, we discovered a substantial increase in ApoA-I/LPS complex in the 18-mer co-injected group. In contrast, a decrease in the LPS/HMGB1 complex concentration was observed in this group (Fig. 3B). An inverse correlation between the concentrations of ApoA-I/LPS and LPS/HMGB1 suggests that ApoA-I is used to sequester LPS and to lower the level of LPS/HMGB1 complex. Additionally, the increases in ApoA-I/LPS complex in peritoneal lavage depend on the size of HS oligosaccharides co-injected with LPS. Only the LPS/18-mer co-injection group or, to a lesser extent, the LPS/12-mer, but not the LPS/6-mer co-injection group, showed an increase in ApoA-I/LPS complex (SI Appendix, Fig. S9C). It should be noted that co-injection of 6-mer or 12-mer did not display an anti-inflammatory effect in animals, as demonstrated by comparable levels of IL-6 and TNF-a as in the LPS control group (Fig. 2 C and D and SI Appendix, Fig. S8 A and B). Our data suggest that the ability to elevate the complex of ApoA-I/LPS appears to correlate with anti-inflammatory potency. Third, the plasma concentration of LPS was reduced by 7.8- fold in the 18-mer co-injection group (Fig. 3C). Lower plasma LPS concentrations reduced organ damage. It is known that LPS is cleared in 3 min from blood circulation through the liver when it complexes with HDL (33). It is plausible that 18-mer increases the formation of the ApoA-I/LPS complex and accelerates the clearance of LPS when ApoA-I/LPS enters the circulation. Although there was an approximately 50% reduction in the concentration of lavage LPS from the 18-mer co-injection group, this difference was not statistically significant (Fig. 3D) (P = 0.15). We further measured the concentrations of ApoA-I in peritoneal lavage and plasma from endotoxemia mice and 18-mer co-injection mice. No differences in the concentrations of ApoA-I were observed between the two groups (SI Appendix, Fig. S10 A and B). Taken together, our data suggest that the 18-mer decreases the concentration of the LPS/HMGB1 complex by eliciting the action of ApoA-I.
18-mer Induces the Dissociation of ApoA-I from HDL.
As a primary carrier for ApoA-I, HDL reportedly directly interacts with LPS (34), and thereby HDL should also neutralize the cytotoxicity from LPS. We found that HDL inhibited the release of TNF-α by LPS from raw246.7 cells dose-dependently (Fig. 3E). Notably, high concentrations of HDL (500 mg/mL) completely inhibited the release of TNF-α to background levels, suggesting that HDL effectively neutralizes the cytotoxicity of LPS. Furthermore, HDL was detected in plasma and peritoneal lavage from both endotoxemia and 18-mer co-injection groups (SI Appendix, Fig. S10 C and D). The co-localization of HDL and ApoA-I in the peritoneal space suggests that both HDL and ApoA-I contribute to reducing the toxic effect of LPS/HMGB1.
The impact of 18-mer on the structure of HDL was next investigated. Heparin reportedly caused the dissociation of ApoA-I from HDL particles (35, 36). To this end, we examined whether 18-mer displays a similar effect on HDL using purified human HDL. We incubated 18-mer, 12-mer, and 6-mer each with HDL for one hour before centrifugation. Both supernatants and pellets were subjected to SDS-PAGE analysis (Fig. 3F). SDS-PAGE analysis showed that the addition of 18-mer to HDL moved ApoA-I from the supernatant fraction to the pellet fraction, indicating that 18-mer dissociated ApoA-I from HDL particles. This dissociation depended on oligosaccharide size (Fig. 3F), with 18-mer being the most effective at dissociating ApoA-I from HDL. Our data suggest that HDL undergoes structural changes in the presence of 18-mer.
18-mer Treatment Reduces Lethality in CLP-Injured Mice.
A 72-h survival study with 6-mer and 18-mer treatment was conducted. Compared to CLP-injured mice receiving saline, 18-mer significantly improved the survival, while 6-mer treatment had no significant effect on lethality reduction (Fig. 3G). We also measured the concentrations of ApoA-I and HDL in CLP-injured mice with or without the treatment of 18-mer. A statistically significant reduction in the concentrations of ApoA-I and HDL in peritoneal lavage was observed in the 18-mer treated group (SI Appendix, Fig. S10 E and G), possibly attributed to less injury. However, the plasma concentrations of ApoA-I and HDL showed no difference between the two groups (SI Appendix, Fig. S10 F and H).
Discussion
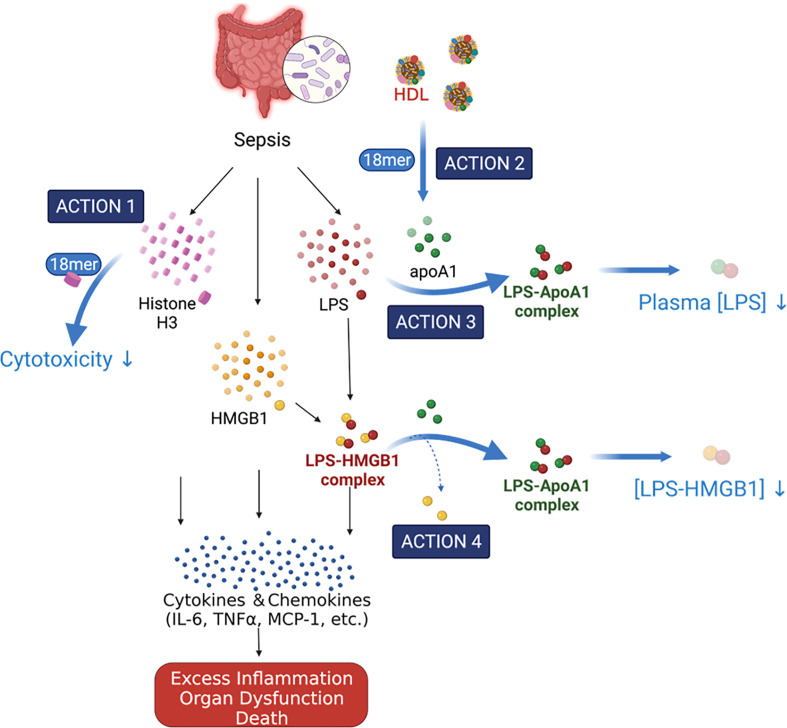
During sepsis, histones and HMGB1 are released from damaged cells and immune cells, fueling inflammation by increasing pro-inflammatory cytokines, chemokines, and adhesion molecules, resulting in a dysregulated host response, organ damage, and death (5). Curbing these excessive and dysregulated inflammatory responses represents a potential strategy for treating sepsis. Extracellular histone and HMGB1 are attractive targets as they control the upstream inflammatory response pathways (4, 22, 37). Several reports emerged in recent years using sulfated glycans, including heparin, chondroitin sulfate E, and synthetic per-O-sulfated maltotrioses and cellobiose to block the pro-inflammatory activities of histone or HMGB1 and thereby to treat sepsis in animal models (13, 24, 28, 32). In this study, we demonstrated that a synthetic non-anticoagulant heparin-based molecule, 18-mer, protected against sepsis-induced inflammation and resulting lethality by simultaneously targeting different pathways. Our findings revealed that the 18-mer engaged at least two targets to protect the animals from damage in sepsis, as illustrated in Fig. 4. First, 18-mer binds to histone H3 and neutralizes its cytotoxicity. Second, 18-mer enlists the actions of ApoA-I to disrupt the LPS-HMGB1 complex and reduce the plasma concentration of LPS.
Fig. 4.
Proposed the mechanism of action by 18-mer in sepsis. Sepsis causes systemic inflammation and releases extracellular H3 and HMGB1 and bacterial LPS. 18-mer displays the protection by directly neutralizing H3 and indirectly targeting HMGB1. 18-mer binds to H3 and neutralizes the cytotoxicity of H3 (Action 1). 18-mer causes the structural changes of HDL and releases ApoA-I (Action 2). ApoA-I binds to LPS to allow a clearance from the circulation to reduce the plasma concentration of LPS (Action 3). ApoA-I displaces HMGB1 from the LPS-HMGB1 complex, which is a pathway to deliver LPS into the cells to cause cell death (Action 4). [Illustration created with BioRender].
The cytotoxicity of extracellular histones is attributed to their interaction with lipid bilayer within the cell membrane leading to its damage (38). Among all the histone isoforms, the cytotoxicity and the correlation to sepsis severity of H3 are the most established (6, 22). The neutralization of the cytotoxic effect of histones by sulfated glycans has been achieved through direct interactions between histones and glycans (13, 24, 28). Here, we demonstrate that 18-mer directly interacts with histone H3 with the binding affinity of 66 nM and improves cell viability after the H3 challenge (action 1 shown in Fig. 4). In another study, Zhang et al. reported that HS oligosaccharides protect against histone-induced lung injury (39). HS oligosaccharides inhibited the activation of toll-like receptor 4 (TLR4)-mediated endothelial cell activations in response to the exposure to a low dose of histone (39). Our data do not rule out the possibility that 18-mer also protects the host from low-concentration histone damage by inhibiting the activation of the TLR4 receptor.
HMGB1 is known to contribute to the pathophysiology of sepsis as established in the endotoxemia and the CLP models. Materials from infectious microbes, such as bacterial LPS, trigger the release of extracellular HMGB1, contributing to cell damage. Extracellular HMGB1 and LPS form a complex, and the LPS-HMGB1 complex facilitates the intracellular delivery of LPS (20). Intracellular LPS activates caspase -11 to cleave gasdermin D into peptides that form gasdermin D pores in the membrane, leading to pyroptosis (40). Blocking the formation of the complex of LPS-HMGB1 should reduce the damage associated with sepsis (32). The authors suggested that heparin attenuates the inflammatory impact of LPS by directly disrupting LPS-HMGB1 (32). As a synthetic heparin mimetic, the 18-mer reduces the concentration of the LPS-HMGB1 complex in the peritoneal lavage of the endotoxemia mice; however, the 18-mer is incapable of directly disrupting the LPS/HMGB1 complex in vitro. Our findings suggest that reducing the complex of LPS/HMGB1 is indirectly achieved. We could not explain the difference in the findings between the two studies.
Our studies show that 18-mer’s protection is mediated through enlisting the ApoA-I action to attenuate the functions of HMGB1 (actions 3 and 4 in Fig. 4). This conclusion points to a mechanism to explain the anti-inflammatory effect of heparin and heparin-like compounds in sepsis. Known for transporting cholesterol from peripheral tissues to the liver and as a vehicle carrying ApoA-I, HDL displays cardioprotection. HDL’s beneficial functions against sepsis have been recently noted (41, 42). The effects of HDL in sepsis are attributed to downregulating macrophage response (34), strengthening endothelial cell barriers (43), and neutralizing LPS (33, 44). In septic patients, dysfunctional HDL concerning decreased concentration, size, and cholesterol efflux capacity has been reported (45, 46). Increasing HDL concentrations or improving its functionality have been suggested as approaches for managing sepsis (42, 47). Our model indicates that 18-mer causes the dissociation of ApoA-I from HDL (action 2 in Fig. 4). ApoA-I binds to LPS to form a non-toxic ApoA-I/LPS complex that benefits the host in two aspects: it accelerates the clearance of LPS from the circulation (action 3 in Fig. 4), and it decreases the formation of the complex of LPS/HMGB1. How 18-mer causes the structural changes in HDL to release ApoA-I to neutralize LPS remains unknown. The study likely requires isolating sufficient HDL from peritoneal lavage of both healthy and septic mice since the composition of HDL is different under inflammatory conditions (41). Such investigations are subject to future study.
A unique structural feature of the 18-mer used in this study is that it is less susceptible to the degradation by heparanase. Heparanase is released in sepsis and is involved in the degradation of endogenous HS to disrupt the glycocalyx on the surface of the endothelium (31). Therefore, it is conceivable that protection in sepsis by an HS oligosaccharide would be diminished after heparanase degradation. Furthermore, heparanase-degraded oligosaccharides, i.e., nonasaccharides (9-mer), in the circulation system reportedly contributed to cognitive impairment in sepsis patients by deactivating long-term potentiation of the hippocampus (48, 49). The 18-mer used in the current study does not contain a heparanase cleavable saccharide sequence (50). Minor cleavage at the non-reducing end of the 18-mer by heparinase unlikely affects the anti-inflammatory effect. This heparanase-resistant 18-mer allows us to maintain its protective effect during treatment while minimizing its side effects on cognitive functions.
HS is a negatively charged polysaccharide that interacts with many proteins. Uncovering the requirement for the size and sulfation patterns of HS for anti-inflammatory activity is essential for further developing therapeutic agents to treat sepsis. A chemoenzymatic synthesis is a powerful tool for preparing structurally homogeneous HS oligosaccharides because it offers short synthetic routes and scalability. The synthetic homogenous HS 6-mer, 12-mer, and 18-mer used in this study provided insights into the mechanism of HS oligosaccharides' protection against damage associated with sepsis. The 12-mer exhibited inhibition of the cytotoxicity of H3 and dissociated ApoA-I from HDL; however, the potencies in both assays were lower than those of the 18-mer. The lower potency in both assays explained that the 12-mer failed to decrease the concentrations of IL-6 and TNF-α in the peritoneal lavage of endotoxemia mice.
Previously, heparin has been tested in clinical trials for sepsis patients (51–53); however, the results were conflicted. A recent paper by Tang et al. showed that the treatment with heparin improved the 28-d survival rate in patients. However, the authors acknowledged that they could not conclude that heparin treatment is protective in all septic patients (32). The causes of sepsis are heterogeneous and, thus, may lead to conflicting clinical outcomes from heparin trials (32, 54). Another limitation of these trials is that investigators used structurally heterogeneous heparin, adding extra complexity to interpreting patient responses to the treatment. Modified heparins or non-anticoagulant heparins were also proposed, yet those compounds are still mixtures and comprise individual saccharide chains that differentially impact biological pathways (13, 32). The availability of homogeneous HS oligosaccharides should remove inconsistent responses resulting from the different chemical structures in heparin, enabling the in-depth mechanistic studies required for developing HS-based therapeutics for better outcomes.
In summary, our studies offer using homogenous HS oligosaccharides to investigate the relationship between HS structure and the multi-faceted functions, including the neutralization of the pro-inflammatory activities of H3 and HMGB1 and the promotion of the anti-inflammatory activities associated with ApoA-I and HDL. Furthermore, our findings open the possibility of investigating whether synergistic protection can be achieved by using a combination of HS saccharides. Ideally, one HS saccharide inhibits the activity of pro-inflammatory proteins, and another HS saccharide promotes the activity of anti-inflammatory proteins. Such an approach would benefit the development of better therapeutics for treating sepsis.
Materials and Methods
HS oligosaccharides, including 6-mer, 12-mer, and 18-mers, were chemoenzymatically synthesized (17, 26). The binding affinity of oligosaccharides to H3 was determined by SPR. The evaluation of HS protection was investigated in the CLP model with various biomarker analysis by ELISA, Luminex multiplex assay, western blot, and qPCR. The mechanistic studies were conducted with the LPS-induced endotoxemia model and pull-down assays, followed by proteomic analysis for identifying apoA-I. All detailed methods can be found in the SI Appendix.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank the University of North Carolina at Chapel Hill (UNC) Michael Hooker Proteomics Center for the proteomic analysis. We thank the UNC Flow Cytometry Core Facility for the access to Becton Dickinson LSRFortessa, and UNC Advanced Analytics Core for help from C. Anderson with the Multiplex assay of biomarker analysis. We also thank N. Hathaway UNC for access to QuantStudio™ 6 Flex Real-Time PCR System. This word is funded by NIH grants (HL094463, HL144970, GM144019, HL157441, HL142604, and AR070179) and Glycan Innovation grants from Eshelman Innovation Institute. Y.-E.L. is a recipient of the predoctoral fellowship from the Government Scholarship to Study Abroad (GSSA) from Taiwan. This work is also supported by GlycoMIP, a National Science Foundation Materials Innovation Platform funded through Cooperative Agreement DMR-1933525. The UNC Flow Cytometry Core Facility is supported in part by the P30 CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center. Research reported in this publication was supported by the Center for AIDS Research award number 5P30AI050410. The UNC Advanced Analytics Core is supported by the P30 DK034987 from NIH.
Author contributions
R.J.L., D.X., R.P., and J.L. designed research; Y.-E.L., Y.X., K.A., F.Z., J. Li, R.S., and C.Y. performed research; Y.X., J. Liu, V.P., and A.M.I. contributed new reagents/analytic tools; Y.-E.L., Y.X., K.A., F.Z., R.S., and J.L. analyzed data; and Y.-E.L., R.S., R.J.L., D.X., R.P., and J.L. wrote the paper.
Competing interest
The authors have stock ownership to disclose. Y.X. and J.L. are founders of Glycan Therapeutics. V.P. and A.M.I. are employees at Glycan Therapeutics. Other authors declare no competing interests. J.L.'s lab at UNC has received a gift from Glycan Therapeutics to support research in glycoscience.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Rudd K. E., et al. , Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the global burden of disease study. Lancet 395, 200–211 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavaillon J. M., Singer M., Skirecki T., Sepsis therapies: Learning from 30 years of failure of translational research to propose new leads. EMBO Mol. Med. 12, e10128 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singer M., et al. , The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315, 801–810 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Poll T., van de Veerdonk F. L., Scicluna B. P., Netea M. G., The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 17, 407–420 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Denning N.-L., Aziz M., Gurien S. D., Wang P., DAMPs and NETs in sepsis. Front. Immunol. 10, 2536 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Gimenez J., et al. , A new mass spectrometry-based method for the quantification of histones in plasma from septic shock patients. Sci. Rep. 7, 1–10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibot S., et al. , High-mobility group box 1 protein plasma concentrations during septic shock. Intensive Care Med. 33, 1347–1353 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Li Y., et al. , Circulating histones in sepsis: Potential outcome predictors and therapeutic targets. Front. Immunol. 12, 972 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abrams S. T., et al. , Circulating histones are mediators of trauma-associated lung injury. Am. J. Respir. Crit. Care Med. 187, 160–169 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng M., Scott M. J., Fan J., Billiar T. R., Location is the key to function: HMGB1 in sepsis and trauma-induced inflammation. J. Leukoc. Biol. 106, 161–169 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Axelsson J., et al. , Inactivation of heparan sulfate 2-O-sulfotransferase accentuates neutrophil infiltration during acute inflammation in mice. Blood 120, 1742–1751 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L., Fuster M., Sriramarao P., Esko J. D., Endothelial heparan sulfate deficiency impairs L-selectin-and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat. Immunol. 6, 902–910 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Wildhagen K. C., et al. , Nonanticoagulant heparin prevents histone-mediated cytotoxicity in vitro and improves survival in sepsis. Blood 123, 1098–1101 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Xu D., Young J., Song D., Esko J. D., Heparan sulfate is essential for high mobility group protein 1 (HMGB1) signaling by the receptor for advanced glycation end products (RAGE). J. Biol. Chem. 286, 41736–41744 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gama C. I., et al. , Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nat. Chem. Biol. 2, 467–473 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Arnold K., Liao Y.-E., Liu J., Potential use of anti-inflammatory synthetic heparan sulfate to attenuate liver damage. Biomedicines 8, 503 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnold K., et al. , Design of anti-inflammatory heparan sulfate to protect against acetaminophen-induced acute liver failure. Sci. Transl. Med. 12, eaav8075 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnold K., et al. , Synthetic anticoagulant heparan sulfate attenuates liver ischemia reperfusion injury. Sci. Rep. 10, 1–10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dejager L., Pinheiro I., Dejonckheere E., Libert C., Cecal ligation and puncture: The gold standard model for polymicrobial sepsis? Trends Microbiol. 19, 198–208 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Deng M., et al. , The endotoxin delivery protein HMGB1 mediates caspase-11-dependent lethality in sepsis. Immunity 49, 740–753.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guirgis F. W., et al. , HDL cholesterol efflux is impaired in older patients with early sepsis: A subanalysis of a prospective pilot study. Shock 50, 66–70 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J., et al. , Extracellular histones are major mediators of death in sepsis. Nat. Med. 15, 1318–1321 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo L., et al. , Replenishing HDL with synthetic HDL has multiple protective effects against sepsis in mice. Sci. Signal. 15, eabl9322 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meara C. H. O., et al. , Neutralizing the pathological effects of extracellular histones with small polyanions. Nat. Commun. 11, 6408 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang H., et al. , Inhibition of HMGB1/RAGE-mediated endocytosis by HMGB1 antagonist box A, anti-HMGB1 antibodies, and cholinergic agonists suppresses inflammation. Mol. Med. 25, 1–13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Y., et al. , Homogeneous low-molecular-weight heparins with reversible anticoagulant activity. Nat. Chem. Biol. 10, 248–250 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rittirsch D., Huber-Lang M. S., Flierl M. A., Ward P. A., Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 4, 31–36 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J., et al. , Enzymatic synthesis of chondroitin sulfate e to attenuate bacteria lipopolysaccharide-induced organ damage. ACS Cent. Sci. 6, 1199–1207 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuchida T., et al. , Protocol for a sepsis model utilizing fecal suspension in mice: Fecal suspension intraperitoneal injection model. Front. Med. 9, 765805 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doi K., Leelahavanichkul A., Yuen P. S., Star R. A., Animal models of sepsis and sepsis-induced kidney injury. J. Clin. Invest. 119, 2868–2878 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt E. P., et al. , The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat. Med. 18, 1217–1223 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang Y., et al. , Heparin prevents caspase-11-dependent septic leathlity indepent of anticoagulant properties. Immunity 54, 454–467 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Yao Z., et al. , Blood-borne lipopolysaccharide is rapidly eliminated by liver sinusoidal endothelial cells via high-density lipoprotein. J. Immunol. 197, 2390–2399 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Nardo D., et al. , High-density lipoprotein mediates anti-inflammatory reprogramming of mancrophages via the transcritional regulator ATF3. Nat. Immunol. 15, 152–160 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tam S.-P., Kisilevsky R., Ancsin J. B., Acute-phase-HDL remodeling by heparan sulfate generates a novel lippprotein with exceptional cholesteroal efflux activity from macrophages. PLoS One 3, e3867 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Digre A., Nan J., Frank M. M., Li J.-P., Heparin intereactions with apoA1 and SAA in inflammation-associated HDL. Biochem. Biophys. Res. Commun. 474, 309–314 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Stevens N. E., et al. , Therapeutic targeting of HMGB1 during experimental sepsis modulates the inflammatory cytokine profile to one associated with improved clinical outcomes. Sci. Rep. 7, 1–14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silvestre-Roig C., et al. , Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature 569, 236–240 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y., et al. , Circulating heparan suflate fragments attneuate histone-induced lung injury independently of histone binding. Shock 48, 666–673 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding J., et al. , Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Morin E. E., Guo L., Schwendeman A., Li X.-A., HDL in sepsis–risk factor and therapeutic approach. Front. Pharmacol. 6, 244 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka S., et al. , High-density lipoproteins during sepsis: From bench to bedside. Crit. Care 24, 134 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galvani S., et al. , HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci. Signal. 8, ra79 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petropoulou P.-I., et al. , Lack of LCAT reduces the LPS-neutralizing capacity of HDL and enhances LPS-induced inflammation in mice. Biochim. Biophys. Acta 1852, 2106–2115 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Tanaka S., et al. , High-density lipoprotein (HDL) particle size and concentration changes in septic shock patients. Ann. Intensive Care 6, 68 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guirgis F. W., et al. , HDL cholesterol efflux is impaired in older patients with early sepsis: A subanalysis of a prospective pilot study. Shock 50, 66–70 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ronsein G. E., Heinecke J. W., Time to ditch HDL-C as a measure of HDL function. Curr. Opin. Lipidol. 28, 414–418 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hippensteel J. A., et al. , Circulating heparan sulfate fragments mediate septic cognitive dysfunction. J. Clin. Invest. 129, 1779–1784 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X., et al. , Circulating heparin oligosaccharides rapidly target the hippocampus in sepsis, potentially impacting cognitive functions. Proc. Natl. Acad. Sci. U.S.A. 116, 9208–9213 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peterson S., Liu J., Deciphering mode of action of heparanase using structurally defined oligosaccharides. J. Biol. Chem. 287, 34836–34843 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jaimes F., et al. , Unfractioned heparin for treatment of sepsis: A randomized clinical trial (The HETRASE Study). Crit. Care Med. 37, 1185–1196 (2009). [DOI] [PubMed] [Google Scholar]
- 52.Fan Y., Jiang M., Gong D., Zou C., Efficacy and safety of low-molecular-weight heparin in patients with sepsis: A meta-analysis of randomized controlled trials. Sci. Rep. 6, 25984 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davidson B. L., Geerts W. H., Lensing A., Low-dose heparin for severe sepsis. N. Engl. J. Med. 347, 1036–1037 (2002). [DOI] [PubMed] [Google Scholar]
- 54.Marshall J. C., Why have clinical trials in sepsis failed? Trends Mol. Med. 20, 195–203 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.