Significance
Global modeling studies suggest increased species arrivals from lower latitudes and local extirpations at high latitudes due to global warming. Our analysis of 20,670 standardized scientific trawls with 193 fish species from the northeast Atlantic and Arctic Oceans found an increase in species richness in the region correlated with an increase in sea bottom temperature. Some Arctic species declined in probability of occurrence over time, but some increased. This, together with the increase in southern-latitude species led to an enrichment of the Arctic and sub-Arctic marine fauna attributed to climate change from 1994 to 2020.
Keywords: climate warming, species richness, biodiversity, Arctic Ocean, demersal fish
Abstract
Observed range shifts of numerous species support predictions of climate change models that species will shift their distribution northward into the Arctic and sub-Arctic seas due to ocean warming. However, how this is affecting overall species richness is unclear. Here we analyze 20,670 scientific research trawls from the North Sea to the Arctic Ocean collected from 1994 to 2020, including 193 fish species. We found that demersal fish species richness at the local scale has doubled in some Arctic regions, including the Barents Sea, and increased at a lower rate at adjacent regions in the last three decades, followed by an increase in species richness and turnover at a regional scale. These changes in biodiversity correlated with an increase in sea bottom temperature. Within the study area, Arctic species’ probability of occurrence generally declined over time. However, the increase in species from southern latitudes, together with an increase in some Arctic species, ultimately led to an enrichment of the Arctic and sub-Arctic marine fauna due to increasing water temperature consistent with climate change.
Climate warming constitutes one of the main faces of climate change and is having a direct impact on species, communities, and ecosystems (1, 2). In the oceans, the average increase in temperature in the last 140 y has been of 1 °C (3, 4). Marine ectotherms occupy most of their potential latitudinal range with regard to thermal tolerance and therefore move to higher latitudes following the displacement of their thermal niche (5, 6). However, these changes can occur at different rates across species, depending on traits such as their dispersal potential, thermal niche and capacity to exploit new resources, which may lead to changing community composition (1, 7). Understanding how these changes occur is crucial for effective conservation and management strategies. Yet, to date, consistent empirical evidence of a generalization of these shifts in the Arctic fish community is lacking.
Arctic and sub-Arctic ecosystems are among the most rapidly warming regions in the world, with some areas warming four times faster than, and seas warming twice, the global average (4, 8, 9), and their species composition could be changing accordingly (10). Until now, a doubling in species richness has been reported in some areas of the North Sea, though not in others, and increases of smaller magnitude have also been reported around North America (11–13). However, fish community analyses are mostly restricted to nonpolar latitudes, and they rarely exceed the 62°N of the north Bering’s Sea. Studies focusing on areas above 62°N only exist in the Barents Sea, where some boreal species arrived recently (10, 14, 15) (SI Appendix, Table S1). Of these, one study examined distributional shifts in a fish community of 49 species from 2004 to 2017, and it reported an increase of less than 50% in species richness (15). This represents the only work reporting empirical evidence of a regional increase in marine fish species richness with climate change in Arctic latitudes. Thus, although an increase in species richness is predicted into the Arctic Ocean, and several model projections exist in the literature, including species extirpations (16–18), the empirical evidence is limited temporally and taxonomically, and lacks a long-term correlation with climate warming.
In the Norwegian and Barents Seas, recent warming and increased Atlantic water inflow events have been recorded, with a decline in sea-ice cover in the northern Barents Sea (19–21). As a consequence, profound effects on the geographical distribution and productivity of commercial fishing stocks are expected (22–24). In fact, species turnover was projected to increase in the area in the next decades, resulting from some local species extirpations and the arrival of warmer-water species (17, 25, 26). Here, we report three decades of field data to test these predictions.
Recent changes in species distributions in the Norwegian Sea include consistent northward expansions of the Atlantic mackerel (Scomber scombrus) and the European Hake (Merluccius merluccius), and the predicted northward expansion of bluefin-tuna (Thunnus thynnus), among other species (27–30). Even stronger are the changes reported in the Arctic region of the Barents Sea, where at least 11 boreal (sub-Arctic) species have been recently recorded (14, 31). Similarly, studies in the Bering Sea found localized increases in biodiversity and suggested that areas with increased species richness and climatic stability were climate refugia (32). Several recent expansions of benthic species distributional ranges, such as that of the red king crab (Paralithodes camtschaticus) or the snow crab (Chionoecetes opilio), are rapidly altering benthic communities, and are also affecting demersal fish trophodynamics (33, 34). However, how these examples may materialize into a wider trend in the demersal fish fauna of the Arctic region due to climate change has not been investigated. Moreover, accounting for both species gains and losses needs to cover a sufficiently large region to avoid boundary effects.
Here, we test the commonness of these shifts into the Arctic across the demersal fish community of a wider latitudinal range (from 56 to 82°N), for more species and a longer time period (27 y) than previous studies.
We developed our analysis in Norwegian-Barents Seas, and the adjacent areas in the North Sea and around Svalbard with the aim of understanding changes in biodiversity across the whole region. To do so we explored changes in three scales of biodiversity: alpha diversity (average local species richness), beta diversity and its component turnover (excluding species richness effect), and gamma diversity (total regional species richness) (35–37). Each of these measures provides information on biological diversity at a particular scale, and when combined provide a complete measure of biological diversity across the whole study area.
To analyze the change in local diversity (alpha diversity) at each sampling site over time, we used generalized additive models (GAMs) (a semi-parametric statistical modeling technique that allows for nonlinear effect of variables, as is the case of time). We then explored the contribution of environmental variables to local species richness and made annual projections of changes in local species richness using the nonparametric algorithm of boosted regression trees (BRTs) (selected because BRT models can easily accommodate the inclusion of a large number of environmental variables, including those with relatively high level of collinearity) (38, 39). To analyze the change in beta diversity, which accounts for the variability between sites, we calculated the pairwise mean diversity using the Bray–Curtis dissimilarity metric. However, this difference can arise from simply having different species richness (i.e., number of species at each site) or from having different species composition (i.e., different species at each site), and to discern among these two sources of variability between sites, we also calculated Nestedness and Turnover, which are the two components of beta diversity (37). Finally, to calculate gamma diversity, or the overall species richness in the study area, we used rarefaction species accumulative curves, which consider the relationship between sampling effort and species found during this sampling (40).
Results
Alpha Diversity.
A total of 193 demersal fish species were recorded between 1994 to 2020 across the whole study area (Fig. 1 and SI Appendix, Table S2). To study the change in species richness per trawl considering differences in sampling effort across time, GAM models with year, latitude, and sampling effort were fitted at each study area to species count data. In the main study area (Norwegian-Barents Seas), the model predicted a 66% increase in the average number of species per trawl (alpha diversity), from 8.0 species/trawl in 1994 (95% confidence interval CI 7.8, 8.1), to 13.4 species/trawl in 2020 (95% CI 13.1, 13.6) (P < 0.05, deviance explained (DV) explained = 14%) (Fig. 2 and Table 1). The increase in species richness was correlated with changes in sea bottom temperature (Pearson r = 0.59, P < 0.05, SI Appendix, Supplementary Results). Increasing species richness was also found for the adjacent areas in the North Sea and around Svalbard, though no significant correlation was detected with sea bottom temperature (SBT) at those regions (Table 1 and SI Appendix, Fig. S1).
Fig. 1.
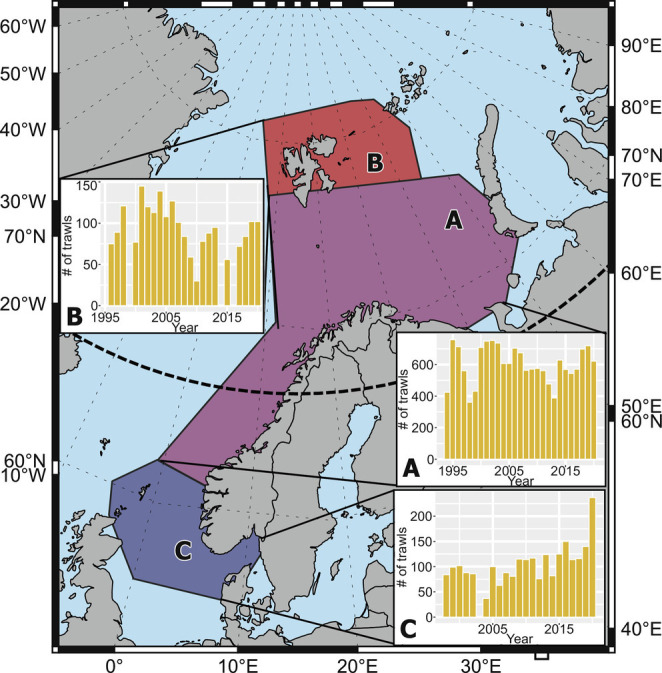
Study area and histograms of the temporal distribution of the number of trawls in the present study. A: Norwegian & Barents Sea; B: Svalbard; C: North Sea. Dashed lines are latitude and longitude, and thick black dashed line is the polar circle (66°N).
Fig. 2.
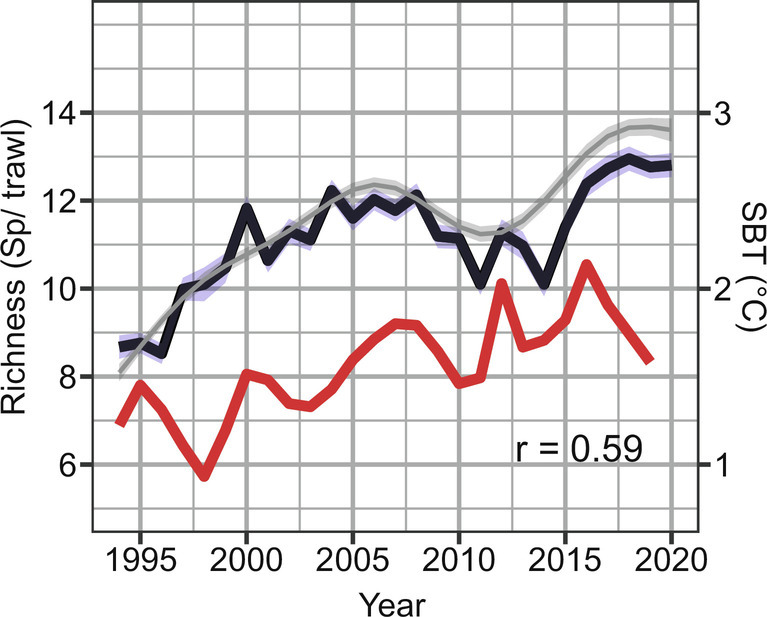
Average number of species per trawl (alpha diversity) across the Norwegian-Barents Sea. Black line represents mean species richness per trawl with 95% CI in blue. Grey smoothed line and light grey 95% CI represents the marginal effect of Year for constant sampling effort from a GAM model using Year and swept area as an offset (Table 1). Red line indicates changes in mean sea bottom temperature across the main study area (correlation with mean alpha diversity r = 0.59).
Table 1.
Average species richness per trawl (alpha diversity) in the first and last year of sampling predicted from a GAM with Year and Latitude as smooth predictors, and swept area as a logarithmic predictor (%DV = % Deviance explained)
| Region | First year of data | Richness first year [lower, upper 95% CI] | Last year of data | Richness last year [lower, upper 95% CI] | % increase [lower, upper 95% CI] | No. of trawls | % DV |
|---|---|---|---|---|---|---|---|
| Norwegian & Barents Sea | 1994 | 8.0 [7.8, 8.1] | 2020 | 13.4 [13.1, 13.6] | 66 [61, 75] | 16,283 | 16 |
| North Sea | 1998 | 11.2 [10.6, 11.8] | 2020 | 16.4 [15.6, 17.2] | 46 [32, 6] | 2,338 | 42 |
| Svalbard | 1996 | 6.9 [6.3, 7.5] | 2020 | 12.8 [12.2, 13.5] | 87 [63, 115] | 2,065 | 12 |
The more relaxed assumptions of the nonparametric BRT allowed the inclusion of the whole study area in one model (which facilitates spatiotemporal projections), and the inclusion of 17 explanatory variables. The resulting model presented a good fit to the data (correlation with independent data r = 0.63, DV explained = 38%, P < 0.05) and predicted local increases in species richness up to 125% in some regions from 1994 to 2019. Among the explanatory variables included in the BRT model, “Depth” was the variable that explained the most deviance in the data, followed by “year” and “bottom temperature” (SI Appendix, Fig. S2). The model projected an increase in species richness at higher latitudes, especially high in the Arctic region of the Barents Sea, where species richness more than doubled in some regions, during the study period (Fig. 3 and SI Appendix, Fig. S3).
Fig. 3.
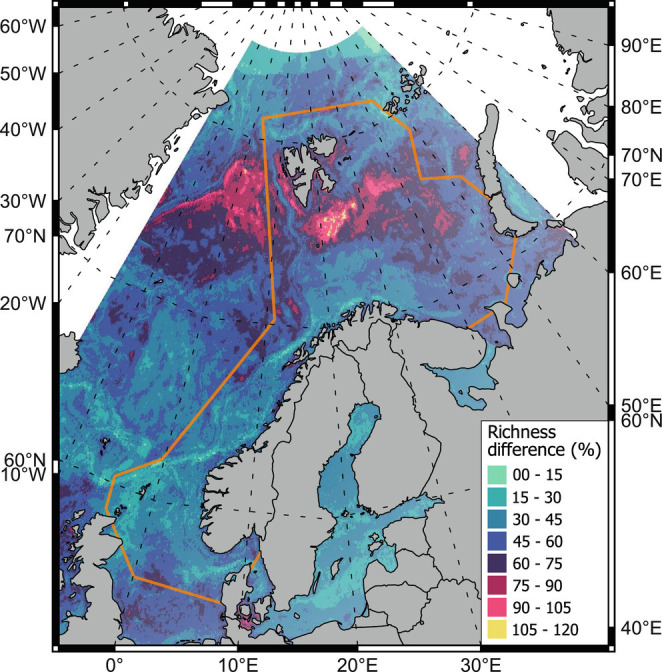
Difference between mean species richness from 1994 to 1996 and 2017 to 2019 expressed as percentage of change. The orange polygon is the study area boundary. Dashed lines are latitude and longitude.
Gamma Diversity.
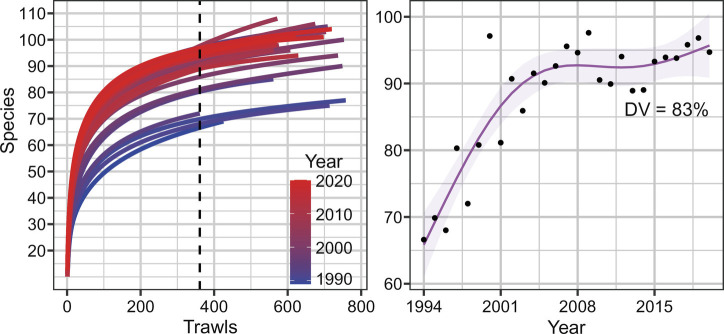
The overall species richness in the main study area (gamma diversity) increased 45% during the study period, from 65 species in 1994 to 1994 species in 2020, following a similar temporal pattern to alpha diversity (Fig. 4 and Table 2 and SI Appendix, Fig. S4). Very similar increases were reported in adjacent areas (47% in the North Sea and 45% in Svalbard, Table 2 and SI Appendix, Fig. S4). GAM models for total species richness at each region, with year and both annual mean trawl-swept area and total mean swept area only selected year as relevant explanatory variables. All additional estimators, including Chao index, incidence based index, and first- and second-order jackknife estimators, calculated across the study area, detected an increase in gamma diversity across time, of similar magnitude (SI Appendix, Fig. S5).
Fig. 4.
Left: Annual species accumulation (rarefaction) curves in the main region Norwegian-Barents Sea. Dashed line marks the minimum common number of trawls. Right: Annual mean accumulated species richness within the minimum common number of trawls.
Table 2.
Total species richness predicted from GAM with year as the only explanatory variable at each region (gamma diversity) in the first and last years of data, at minimum common annual number of trawls (%DV = %Deviance explained)
| Region | First year of data | Richness first year [95% CI] | Last year of data | Richness last year [95% CI] | % increase [95% CI] | Annual trawls | % DV |
|---|---|---|---|---|---|---|---|
| Norwegian & Barents Sea | 1994 | 66 [61, 71] | 2020 | 96 [91, 101] | 45 [29, 65] | 361 | 83 |
| Svalbard | 1996 | 33 [29, 37] | 2020 | 48 [44, 52] | 45 [20, 77] | 30 | 69 |
| North Sea | 1998 | 40 [37, 42] | 2020 | 58 [51, 57] | 47 [32, 65] | 37 | 81 |
Beta Diversity.
Pairwise mean total beta diversity in the main study area did not significantly change with time (Fig. 5). However, the turnover contribution to beta diversity, which is not affected by species richness, increased until 2008, declined until 2014, and increased afterward, with an overall increase of 16% (95% CI 6, 27) (Fig. 5 and SI Appendix, Fig. S6 and Table 3). Similarly, total beta diversity in the adjacent region of Svalbard increased until 2005 and declined afterwards. However, the turnover increased linearly across time in this region. In the North Sea, both the total beta diversity and turnover declined with time.
Fig. 5.
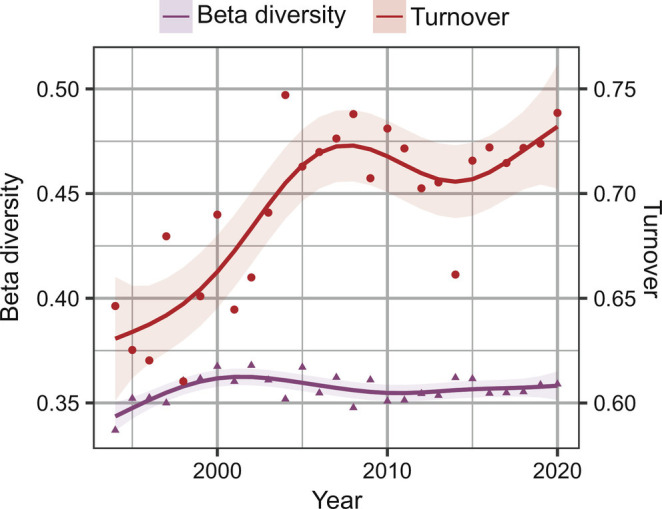
Annual mean beta diversity and turnover (richness independent), red line) in the main region across time, calculated from mean pairwise Jaccard dissimilarity index.
Table 3.
Mean total beta diversity and turnover predicted from GAM at first and last year of data at each region, at minimum common annual number of trawls (%DV = %Deviance explained)
| Region | P | First year of data | Estimation first year [95% CI] | Last year of data | Estimation last year [95% CI] | % increase [95% CI] | Annual trawls | % DV |
|---|---|---|---|---|---|---|---|---|
| Norwegian & Barents Sea | B | 1994 | 0.35 [0.34, 0.36] | 2020 | 0.36 [0.35, 0.37] | 4 [−3, 9] | 361 | 54 |
| T | 0.63 [0.60, 0.66] | 0.73 [0.70, 0.76] | 16 [6, 27] | 75 | ||||
| Svalbard | B | 1996 | 0.32 [0.31, 0.33] | 0.34 [0.32, 0.35] | 5 [−4, 15] | 30 | 69 | |
| T | 0.61 [0.57, 0.64] | 0.75 [0.71, 0.79] | 24 [15, 38] | 50 | ||||
| North Sea | B | 1998 | 0.34 [0.32, 0.36] | 0.32 [0.29, 0.34] | −7 [−19, 6] | 37 | 55 | |
| T | 0.68 [0.63, 0.74] | 0.62 [0.55, 0.69] | −9 [−25 ,9] | 63 |
The GAM models for temporal change in beta diversity and turnover did not select trawl-swept area as a relevant explanatory for the main study area or the adjacent regions. Thus, when the increasing species richness is accounted for, species turnover, as well as alpha and gamma diversity, increased over the years.
Species’ Trends.
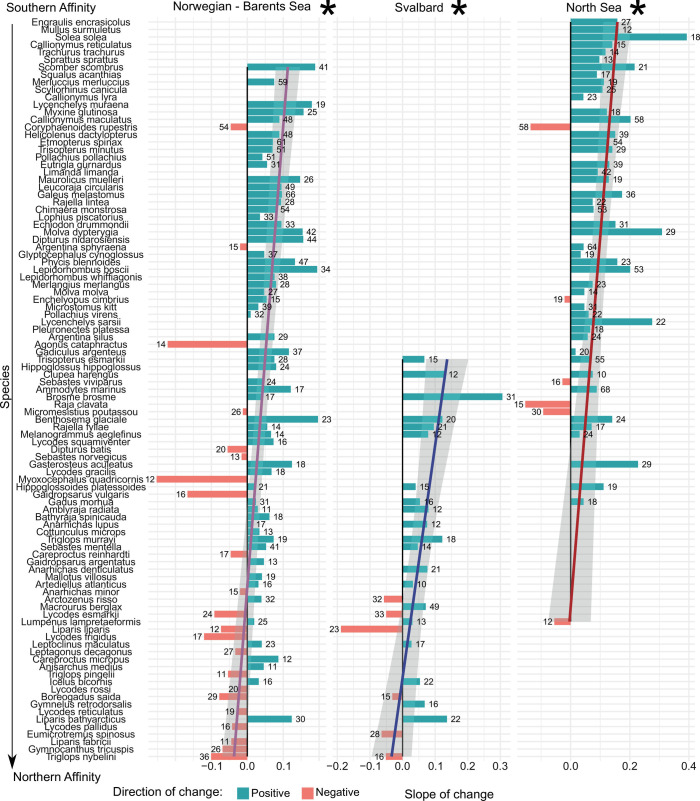
From the 193 species in our study, 99 species showed a significant temporal trend (increasing or decreasing) in their probability of occurrence with time, in at least one of the studied areas (Fig. 6). Of these, 71 species showed only positive trends, and 23 species showed only negative trends in at least one studied area, while five species showed positive and negative trends depending on the study area (Fig. 6). Thus, while no trend was detected for about half of the species, 76% of species showing a significant consistent trend increased. The number of species increasing was consistently higher than the number of species decreasing over time, across all regions. Species declining mostly inhabited high mean latitudes, and linear regression identified a negative effect of mean latitude on the temporal change in species probability of occurrence in the main study area, and around Svalbard (Fig. 6). Among the 67 species only found in the main region and/or Svalbard (Arctic region), 18 species showed a significant change in their probability of occurrence with time, six increased and 12 declined.
Fig. 6.
Change in the probability of species’ occurrence with time. Length of the bars correspond to the Slope of the effect of Year on species probability of occurrence, using a GAM model including swept area, latitude, and depth as smooth predictors, and Year as a linear predictor. Species are ordered by their mean latitude of occurrence during the study period. Linear regression on the effect of Year with mean latitude was conducted and plotted, and asterisks indicate significance (P < 0.05).
Discussion
Following global trends in warming, sea bottom temperature data suggests an increase of 0.3 °C per decade in our study area from 1993 to 2019, and this increase was several times higher in some regions of the Arctic Barents Sea (9). Here we show that this has been accompanied by a significant increase of the demersal marine fish biodiversity during the last three decades, in alpha, gamma, and turnover beta diversity. Considering previous studies focusing on smaller areas and fewer species (15, 32), this study indicates an ecologically significant increase in species richness in the Atlantic side of the Arctic in concert with climate warming. Consequently, species are expanding their range poleward, directly increasing the local species richness in the Barents Sea, in line with other results in near-Arctic regions (11), and with predicted poleward shifts in the North and Arctic Seas (16). This increase in biodiversity was concentrated in two different periods, and interrupted by a decline period in alpha, beta and gamma diversity between 2007 to 2014, as suggested by Ellingsen et al. (41).
Even though there have been studies showing consistent changes in biodiversity patterns and beta diversity at a global scale, whether a systematic loss of local (alpha) diversity has or will take place remains uncertain (42–44). Several species are changing their distributional range, mostly expanding poleward, which leads to redistributions of global biodiversity and local increases in biodiversity (11, 42). In an analysis of 50,000 marine species, Chaudhary et al. (5) found that thousands of species had shifted from equatorial to mid-latitudes, and we confirm that this shift has continued into higher northern latitudes, leading to two times more demersal fish species in the study area. Increases in alpha diversity were already reported in some regions of the Arctic Ocean, albeit smaller areas and/or shorter time periods (15, 32). With this study, we show that the increase in species richness is not localized, but widespread across the region and concomitant with the increase in bottom temperature.
We identified sea bottom temperature as the third most relevant variable in our models, after depth and year. The fact that year remains an important variable, and that the BRT model explains 36% of the overall deviance in species richness, suggests that other processes and variables are needed to fully explain the changes in species richness across the study area during the study period. For example, our analysis is based on presence-absence data, and for this reason alpha diversity is statistically dependent on abundance, because increased abundance in the sampling tends to result in more species observed as a result of increased sampling effort. Although we do take into account the sampling effort with every trawl swept area, this remains a potential source of uncertainty that needs to be considered.
Most studies on species richness consider only gamma (total) or alpha (average) diversity. When beta diversity is considered it is most commonly measured as Jaccard’s coefficient, which is not independent of species richness (45). Here, we find that while both alpha and gamma diversity are significantly increasing, so is species turnover, but not total beta diversity (Fig. 5). Thus, the spatial heterogeneity of biodiversity is increasing as well as biodiversity overall. However, the beta-diversity decomposition also have been affected by varying sampling efficiency across sites and years, which we did not test in this study.
Over the study area, we found that 71 species showing a significant increase in probability of occurrence with time. When species’ mean latitude was used as an indicator of the Arctic affinity of each species, it proved statistically significant to model the effect of year in species probability of occurrence in the Norwegian and Barents Seas, and also around Svalbard. Although this a clear indicator of a decline of several Arctic species, not all species occupying high mean latitudes showed a decline in probability of occurrence with time. Some high-latitude species increased substantially, perhaps because they benefited from changes in food-web interactions. This also suggests a partial coexistence between boreal and Arctic species that, together with the increase of lower-latitude species, leads to a consistent increase in biodiversity, in line with previous results on fishes and crustaceans (46, 47), but not with a previous hypothesis of a synchronous species extirpation in the western Barents and Norwegian Seas (17, 25). If this trend is maintained in the following decades, we may observe enriched communities in the Arctic, which may be sustained by the projected increase in net primary productivity of up to 50% (26, 48). Our results have direct implications for the management of marine resources in the region, where proactive mitigation and adaptation actions to changes in species occurrence can help prevent future negative impacts in the socioeconomic activities of the area, or inform about new opportunities. However, the wider effects of changing fish biodiversity on marine food webs and socioecological system remain to be investigated. Our results have direct implications for the management of marine resources in the region, where proactive mitigation and adaptation actions to changes in species occurrence can help prevent future negative impacts in the socioeconomic activities of the area, and inform about new opportunities. Closer monitoring of individual species decreasing in abundance is already necessary to detect these changes as soon as possible. However, the wider effects of changing fish biodiversity on marine food webs and socioecological system remain to be investigated.
Materials and Methods
Study Area.
The data analyzed here were selected from a research trawl surveys database published by the Institute of Marine Research between 1980 and 2020 in an attempt to gather all the scientific trawl surveys conducted by the institute in a single and open access resource (49). These trawls were mostly restricted to the continental shelf and slope from the north of the North Sea into the Arctic Ocean, mostly from 56°N to 81°N Latitude, and from 2°W to 50°E Longitude (49). The whole area had a marked temperature gradient, with average bottom water temperatures over 8 °C in the North Sea to −1 °C in the northern region of the Barents Sea. Because of variation in the temporal and spatial distribution of the data, we considered one main study area, with the longest time period (Norwegian & Barents Sea) and two adjacent areas (Svalbard and North Sea), which contain a shorter temporal period (Fig. 1).
The database initially contained 60,355 research trawls from several surveys between 1980 and 2020. We excluded data associated with broken gear, had incomplete metadata (data without reporting depth, or coordinates, or type of gear), or were questionable (e.g., shrimp trawl opening of several km). We restricted the analysis to shrimp trawling data using Campelen 1800 shrimp trawl with 20 mm mesh size, a maximum of 5 km trawling distance and 60 m of trawl opening, from 30 m to 700 m depth. We only included fish species (classes Actinopterygii, Elasmobranchii, Holocephali, Myxini and Petromyzonti). Invertebrates were not included in the study. After data standardization and selection, 20,670 surveys from 1994 to 2020 remained.
Depending on the data spatial coverage over time, and based on the biogeographical realms that have been described in the area (50), we divided the study area into a main area containing most of the data and the longest temporal coverage, the Norwegian-Barents Sea, and two adjacent areas with more limited data: the area around Svalbard and the North Sea (Fig. 1). Although the latitudinal and longitudinal distribution of trawls across time was relatively homogeneous at each region, the swept area of each trawl presented a weak negative trend with time for the Norwegian-Barents Sea and Svalbard regions, which was accounted for within the statistical modelling (SI Appendix, Figs. S7 and S8).
Environmental Variables.
Spatially explicit environmental co-variates were collated from three sources: Copernicus Marine Service database, Bio-Oracle and MARSPEC database (51–53). Some of the environmental variables were available as annual estimates [e.g., sea surface temperature (SST)] whereas others were available as long-term averages (e.g., bottom nitrate concentration) (SI Appendix, Table S3).
Variables from the Copernicus Marine Service were available for each year from 1993 to 2019. These include estimates of SST, SBT, sea surface salinity, ice concentration and sea surface currents (northward and eastward components), and were obtained from the “GLOBAL_REANALYSIS_PHY_001_031” dataset (53) (SI Appendix, Table S3). Because these layers were only available from 1993 to 2019, we limited the analyses to this period. Long-term averages (2000 to 2014) of nitrate, iron, dissolved oxygen, surface and bottom productivity, and temperature range were obtained from Bio-Oracle. Bathymetry was obtained from the MARSPEC database (51, 52). Variables from Bio-Oracle and MARSPEC were downloaded using the “sdmpredictors” package from R software (54).
A distance to coast layer was created using the “raster” package, also in R (55). All the environmental layer resolutions were matched by downscaling to the lowest original resolution (0.083° Longitude × 0.083° Latitude). Variables from year, latitude, longitude, and annual number of trawls were also manually created. The shapefiles for the ocean and land were obtained from the Marine Regions database (56) and maps were created using QGIS software (57).
The change in SBT with time was calculated as the annual mean across each study area (Fig. 2 and SI Appendix, Methods). SBT thus reflects the likely average temperature individual fish may have experienced over time rather than temperature when sampled.
Biodiversity Measures.
Species richness is typically measured as alpha (local) and gamma (regional) diversity, while the extent of differentiation of communities along habitat (spatial and/or temporal) gradients, is beta diversity (36, 58). The most commonly used measures of beta diversity, such as the Jaccard index, are influenced by species richness, which should be accounted for where species richness significantly varies. We thus report both total beta diversity and a species-richness-independent index here called turnover, following (35). The mathematical difference between beta diversity and turnover is called nestedness, and arises where sites with less species are a subset of species from neighboring sites with more species. Thus, we report four measures of diversity: alpha, gamma, total beta, and turnover.
Alpha Diversity.
We assessed alpha diversity in the study area as the annual mean species richness per trawl (species/trawl) using presence data only. GAMs were used to explore the changes in species richness with time, correcting for the different swept area by adding it as a logistic explanatory variable. The model construction was done using the package “mgcv” within the R software (59, 60).
We used BRTs to explore the drivers of these changes, and to make spatial projections of the species richness in the study area. A threshold of 0.9 correlation coefficient was selected due to the robustness of the procedure to collinearity (38) (SI Appendix, Methods).
The model calibration was conducted using 75% of the trawls, and the remaining 25% were used for model validation. To improve the model calibration and reduce computational requirements, we conducted a 50 times bootstrapping process (SI Appendix, Methods). Each model was limited to 20,000 trees, and tree complexity was set to 3 to allow for interactions between the explanatory variables. The learning rate was adjusted to 0.01, bag fraction to 0.3, and other parameters were set to their defaults (38). Pearson correlations were used for model validation. Species richness was projected annually for each bootstrap, taking the mean as the final annual projection.
To study the effect of each environmental variable on species richness, we built partial dependence plots using the R package “devEMF” and basic R from the BRT model. These showed the mean species richness predicted across each environmental variable gradient, while all the other variables were kept at their means (59, 61).
To explore the spatial changes in alpha diversity over time, we statistically predicted the geographic distribution of species richness in each year from 2017 to 2019 and 1994 to 1996 from the BRT model, and calculated the difference between the mean of each period. We used the “predict” function of the “dismo” package, and the package “raster” from R (38, 55, 59, 62). Maps were created with QGIS (57).
Beta Diversity.
Beta diversity and its turnover component were calculated using the mean pairwise Jaccard dissimilarity index (ßJ) from presence-only data, which can be divided into two components: species replacement (Repl.) and nestedness (Nest.) (35, 37, 63):
where a refers to the number of species present in both sites, and b and c are the species present only in either site. Pairwise means each trawl is compared with every other trawl in a year and the average dissimilarity calculated.
Here we report total beta diversity (ßJ) and the relative contribution of species replacement, to beta diversity, which we refer to as turnover:
To account for differing sample sizes between years, we conducted a bootstrapping process. That is, we randomly selected a subsample of the smallest sample size over years, calculated its total beta diversity and turnover, and repeated this process 200 times for each year (35). We report the mean of these bootstrapping calculations in Fig. 5 and SI Appendix, Fig. S6 (35). All calculations were carried out using the functions beta.div.comp, from the adespatial package (64).
To explore the trend of these changes with time and to account for the negative trend of swept area with time (SI Appendix, Fig. S3), we fitted a GAM model with year and annual mean swept area as explanatory variables to the annual mean pairwise total beta diversity and turnover.
Gamma Diversity.
To study changes in gamma diversity, we constructed species accumulation curves (SAC) for each year across our study regions (40, 65). To eliminate the bias that a selected order of the locations may have on the overall SAC, we used the function “specaccum,” in the “vegan” package, set the parameter method = “random” to randomize the order of site addition, and permutated the process 200 times (65, 66). We report the mean species richness per trawl of all random permutations. We then assessed the change in gamma diversity with time, standardized to the minimum common number of sites, fitting a GAM model with year and annual swept area as the explanatory variables.
To test whether the results were robust to alternative measures of richness, we used the package “SpadeR” to calculate nine different species richness indices with time, each of them with different statistical assumptions, including Chao indices, incidence-based indices, and first and second order jackknife estimators (67, 68).
Individual Species Contributions.
To study which species drove the changes in biodiversity, we fitted GAMs to the presence data of each species with smooth parameters for depth, swept area and latitude, and fixed effect of year, using a binomial distribution. Each species biomass-weighted mean latitude was calculated from the complete area to arrange the species by their Arctic affinity, and simple linear regression was used to assess the effect of mean latitude on the temporal change in species probability of occurrence.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
C.G.-V. would like to thank Eric Molina for his interest in the work and helpful discussion. M.C. would like to acknowledge partial funding from the Spanish National Project ProOceans (PID2020-118097RB-I00) and institutional support of the “Severo Ochoa Centre of Excellence” accreditation (CEX2019-000928-S). F.S. would like to acknowledge partial funding from the National Institute of Water and Atmospheric Research (NIWA) Coasts & Oceans Research Programme 5 (SCI 2020/21).
Author contributions
C.G.-V., F.S., M.C., and M.J.C. designed research; C.G.-V. performed research; C.G.-V. analyzed data; F.S. contributed to and critically revised the manuscript; M.C. and M.J.C. contributed to and critically revised the manuscript. Supervised the whole process.; C.L. contributed to and critically revised the manuscript; and C.G.-V. wrote the paper.
Competing interest
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission. A.B. is a guest editor invited by the Editorial Board.
Data, Materials, and Software Availability
Previously published data were used for this work (Ove Djupevåg (2021) IMR bottom trawl data 1980 to 2020 10.21335/NMDC-328259372). All the code and subset of data is available through GitHub (https://github.com/CescGV)
Supporting Information
References
- 1.Poloczanska E. S., et al. , Global imprint of climate change on marine life. Nat. Clim. Change 3, 919–925 (2013). [Google Scholar]
- 2.Dahlke F. T., Wohlrab S., Butzin M., Pörtner H. O., Thermal bottlenecks in the life cycle define climate vulnerability of fish. Science (80-) 369, 65–70 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Huang Boyin, et al. , (2017): NOAA Extended Reconstructed Sea Surface Temperature (ERSST), Version 5. NOAA National Centers for Environmental Information. doi: 10.7289/V5T72FNM [1/07/2021]. [DOI]
- 4.Pörtner H.-O., “Climate change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change”, Pörtner H.O., et al. , (Cambridge Univ. Press. Press, 2022). [Google Scholar]
- 5.Chaudhary C., Richardson A. J., Schoeman D. S., Costello M. J., Global warming is causing a more pronounced dip in marine species richness around the equator. Proc. Natl. Acad. Sci. U. S. A. 118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sunday J. M., Bates A. E., Dulvy N. K., Thermal tolerance and the global redistribution of animals. Nat. Clim. Change 2, 686–690 (2012). [Google Scholar]
- 7.Pinsky M. L., Selden R. L., Kitchel Z. J., Climate-driven shifts in marine species ranges: Scaling from organisms to communities. Ann. Rev. Mar. Sci. 12, 153–179 (2020). [DOI] [PubMed] [Google Scholar]
- 8.England M. R., Eisenman I., Lutsko N. J., Wagner T. J. W., The recent emergence of Arctic amplification. Geophys. Res. Lett. 48, e2021GL094086 (2021). [Google Scholar]
- 9.Isaksen K., et al. , Exceptional warming over the Barents area. Sci. Rep. 12, 1–18 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fossheim M., et al. , Recent warming leads to a rapid borealization of fish communities in the Arctic. Nat. Clim. Change 5, 673–677 (2015). [Google Scholar]
- 11.Batt R. D., Morley J. W., Selden R. L., Tingley M. W., Pinsky M. L., Gradual changes in range size accompany long-term trends in species richness. Ecol. Lett. 20, 1148–1157 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Hiddink J. G., ter Hofstede R., Climate induced increases in species richness of marine fishes. Glob. Change Biol. 14, 453–460 (2008). [Google Scholar]
- 13.Magurran A. E., Dornelas M., Moyes F., Gotelli N. J., McGill B., Rapid biotic homogenization of marine fish assemblages. Nat. Commun. 6, 1–5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pecuchet L., et al. , Novel feeding interactions amplify the impact of species redistribution on an Arctic food web. Glob. Chang. Biol. 26, 4894–4906 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Frainer A., et al. , Increased functional diversity warns of ecological transition in the Arctic. Proc. R. Soc. B Biol. Sci. 288: 202100547 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pereira H. M., Scenarios for global biodiversity in the 21st century. Science (80-) 330, 1496–1501 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Cheung W. W. L., et al. , Projecting global marine biodiversity impacts under climate change scenarios. Fish Fish. 10, 235–251 (2009). [Google Scholar]
- 18.Molinos J. G., et al. , Climate velocity and the future global redistribution of marine biodiversity. Nat. Clim. Change 6, 83–88 (2016), 10.1038/nclimate2769. [DOI] [Google Scholar]
- 19.Comiso J. C., Large decadal decline of the arctic multiyear ice cover. J. Clim. 25, 1176–1193 (2012). [Google Scholar]
- 20.Mork K. A., Skagseth Ø., Søiland H., Recent warming and freshening of the Norwegian Sea observed by Argo data. J. Clim. 32, 3695–3705 (2019). [Google Scholar]
- 21.Lind S., Ingvaldsen R. B., Furevik T., Arctic warming hotspot in the northern Barents Sea linked to declining sea-ice import. Nat. Clim. Change 8, 634–639 (2018). [Google Scholar]
- 22.Drinkwater K. F., Kristiansen T., A synthesis of the ecosystem responses to the late 20th century cold period in the northern North Atlantic. ICES J. Mar. Sci. 75, 2325–2341 (2018). [Google Scholar]
- 23.Koenigstein S., et al. , Forecasting future recruitment success for Atlantic cod in the warming and acidifying Barents Sea. Glob. Change Biol. 24, 526–535 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Stenevik E. K., Sundby S., Impacts of climate change on commercial fish stocks in Norwegian waters. Mar. Policy 31, 19–31 (2007). [Google Scholar]
- 25.Cheung W. W. L., Watson R., Pauly D., Signature of ocean warming in global fisheries catch. Nature 497, 365–368 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Tittensor D. P., et al. , Next-generation ensemble projections reveal higher climate risks for marine ecosystems. Nat. Clim. Chang. 11, 973–981 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cormon X., Kempf A., Vermard Y., Vinther M., Marchal P., Emergence of a new predator in the North Sea: Evaluation of potential trophic impacts focused on hake, saithe, and Norway pout. ICES J. Mar. Sci. 73, 1370–1381 (2016). [Google Scholar]
- 28.Druon J. N., et al. , Habitat suitability of the Atlantic bluefin tuna by size class: An ecological niche approach. Prog. Oceanogr. 142, 30–46 (2016). [Google Scholar]
- 29.Skaret G., Bachiller E., Langøy H., Stenevik E. K., Mackerel predation on herring larvae during summer feeding in the Norwegian Sea. ICES J. Mar. Sci. 72, 2313–2321 (2015). [Google Scholar]
- 30.Wienerroither R. M., et al. , The marine fishes of Jan Mayen Island, NE Atlantic - past and present. Mar. Biodivers. 41, 395–411 (2011). [Google Scholar]
- 31.Kortsch S., Primicerio R., Fossheim M., Dolgov A. V., Aschan M., Climate change alters the structure of arctic marine food webs due to poleward shifts of boreal generalists. Proc. R. Soc. B Biol. Sci., 282, 20151546 (2015), 10.1098/rspb.2015.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alabia I. D., et al. , Marine biodiversity refugia in a climate-sensitive subarctic shelf. Glob. Chang. Biol. 27, 3299–3311 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Holt R. E., et al. , Snow crab (Chionoecetes opilio), a new food item for North-east Arctic cod (Gadus morhua) in the Barents Sea. ICES J. Mar. Sci. 78, 491–501 (2021). [Google Scholar]
- 34.Lorentzen G., et al. , Current status of the red king crab (Paralithodes camtchaticus) and snow crab (Chionoecetes opilio) industries in Norway. Rev. Fish. Sci. Aquac. 26, 42–54 (2018). [Google Scholar]
- 35.Baselga A., Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 19, 134–143 (2010). [Google Scholar]
- 36.Whittaker R. H., Evolution and measurement of species diversity. Taxon 21, 213–251 (1972). [Google Scholar]
- 37.Legendre P., Interpreting the replacement and richness difference components of beta diversity. Glob. Ecol. Biogeogr. 23, 1324–1334 (2014). [Google Scholar]
- 38.Elith J., Leathwick J. R., Hastie T., A working guide to boosted regression trees. J. Anim. Ecol. 77, 802–813 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Wood S. N., Generalized Additive Models: An Introduction with R, C. and Hall/CRC, Ed. (2017). [Google Scholar]
- 40.Soberón J., Llorent J., El uso de funciones dc acumulación de especies para la prediccion de la rigueza de especies. Conserv. Biol. 7, 480–488 (1993). [Google Scholar]
- 41.Ellingsen K. E., et al. , The rise of a marine generalist predator and the fall of beta diversity. Glob. Chang. Biol. 26, 2897–2907 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Dornelas M., Assemblage time series reveal biodiversity change but not systematic loss. Science (80-.) 344, 296–299 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Butchart S. H. M., Global biodiversity: Indicators of recent declines. Science (80-.) 328, 1164–1168 (2010). [DOI] [PubMed] [Google Scholar]
- 44.Pilotto F., et al. , Meta-analysis of multidecadal biodiversity trends in Europe. Nat. Commun. 11, 1–11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kreft H., Jetz W., A framework for delineating biogeographical regions based on species distributions. J. Biogeogr. 37, 2029–2053 (2010). [Google Scholar]
- 46.Renaud P. E., et al. , Is the poleward expansion by Atlantic cod and haddock threatening native polar cod, Boreogadus saida? Polar Biol. 35, 401–412 (2012). [Google Scholar]
- 47.Węsławski J. M., Legeżyńska J., Włodarska-Kowalczuk M., Will shrinking body size and increasing species diversity of crustaceans follow the warming of the Arctic littoral? Ecol. Evol. 10, 10305–10313 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tittensor D. P., et al. , Global patterns and predictors of marine biodiversity across taxa. Nature 466, 1098–1101 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Djupevåg O., IMR bottom trawl data 1980-2020. Institute of Marine Research (2021), 10.21335/NMDC-328259372. [DOI]
- 50.Costello M. J., et al. , Marine biogeographic realms and species endemicity. Nat. Commun. 81, 1–10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sbrocco E. J., Barber P. H., MARSPEC: Ocean climate layers for marine spatial ecology. Ecology 94, 979 (2013). [Google Scholar]
- 52.Assis J., et al. , Bio-ORACLE v2.0: Extending marine data layers for bioclimatic modelling. Glob. Ecol. Biogeogr. 27, 277–284 (2018). [Google Scholar]
- 53.Gounou A., Drévillon M., Clavier M., PRODUCT USER MANUAL For Global Ocean Reanalysis Products. E.U. Copernicus Mar. Serv, Inf. [Online] (2020) (August 18, 2021). [Google Scholar]
- 54.Dormann C. F., et al. , Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography (Cop.) 36, 27–46 (2013). [Google Scholar]
- 55.Hijmans R. J. (2015). Raster: Geographic Data Analysis and Modeling. R Package Version 2.4-15. http://CRAN.R-project.org/package=raster
- 56.Flanders Marine Institute, Maritime Boundaries Geodatabase: Maritime Boundaries and Exclusive Economic Zones (200NM). version 11le, 10.14284/386 (2019). [DOI] [Google Scholar]
- 57.QGIS Development Team (2021). QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org
- 58.Legendre P., Borcard D., Peres-Neto P. R., Analyzing beta diversity: Partitioning the spatial variation of community composition data. Ecol. Monogr. 75, 435–450 (2005). [Google Scholar]
- 59.R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ [Google Scholar]
- 60.Wood S. N., Augustin N. H., GAMs with integrated model selection using penalized regression splines and applications to environmental modelling. Ecol. Modell. 157, 157–177 (2002). [Google Scholar]
- 61.Johnson P. (2020). _devEMF: EMF Graphics Output Device_. R package version 4.0-2. http://CRAN.R-project.org/package=devEMF
- 62.Hijmans R. J., Phillips S., Leathwick J., Elith J. (2022). _dismo: Species Distribution Modeling_. R package version 1.3-9. http://CRAN.R-project.org/package=dismo
- 63.Podani J., Schmera D., A new conceptual and methodological framework for exploring and explaining pattern in presence - absence data. Oikos 120, 1625–1638 (2011). [Google Scholar]
- 64.Dray S., et al. , (2022). _adespatial: Multivariate Multiscale Spatial Analysis_. R packageversion 0.3-19. 10.1890/11-1183.1 http://CRAN.R-project.org/package=adespatial [DOI]
- 65.Ugland K. I., Gray J. S., Ellingsen K. E., The species-accumulation curve and estimation of species richness. J. Anim. Ecol. 72, 888–897 (2003). [Google Scholar]
- 66.Oksanen J., Package “vegan” title community ecology package (Version 2.5-7, 2020).
- 67.Chao A., Chiu C.-H., “Nonparametric estimation and comparison of species richness” in ELS (JohnWiley & Sons Ltd., 2016), pp. 1–11. [Google Scholar]
- 68.Chao A., Ma K. H., Hsieh T. C., Chiu C.-H., SpadeR: Species-richness prediction and diversity estimation with R. CRAN (2016) (April 20, 2022). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
Previously published data were used for this work (Ove Djupevåg (2021) IMR bottom trawl data 1980 to 2020 10.21335/NMDC-328259372). All the code and subset of data is available through GitHub (https://github.com/CescGV)