Keywords: brain-derived neurotrophic factor, cell signaling, inflammation, phrenic motor neuron, plasticity
Abstract
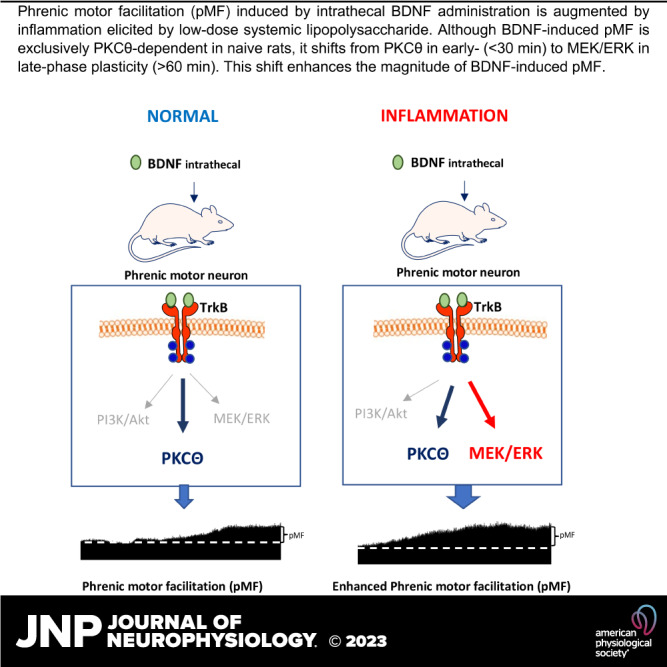
Moderate acute intermittent hypoxia (mAIH) elicits a form of phrenic motor plasticity known as phrenic long-term facilitation (pLTF), which requires spinal 5-HT2 receptor activation, ERK/MAP kinase signaling, and new brain-derived neurotrophic factor (BDNF) synthesis. New BDNF protein activates TrkB receptors that normally signal through PKCθ to elicit pLTF. Phrenic motor plasticity elicited by spinal drug administration (e.g., BDNF) is referred to by a more general term: phrenic motor facilitation (pMF). Although mild systemic inflammation elicited by a low lipopolysaccharide (LPS) dose (100 µg/kg; 24 h prior) undermines mAIH-induced pLTF upstream from BDNF protein synthesis, it augments pMF induced by spinal BDNF administration through unknown mechanisms. Here, we tested the hypothesis that mild inflammation shifts BDNF/TrkB signaling from PKCθ to alternative pathways that enhance pMF. We examined the role of three known signaling pathways associated with TrkB (MEK/ERK MAP kinase, PI3 kinase/Akt, and PKCθ) in BDNF-induced pMF in anesthetized, paralyzed, and ventilated Sprague Dawley rats 24 h post-LPS. Spinal PKCθ inhibitor (TIP) attenuated early BDNF-induced pMF (≤30 min), with minimal effect 60–90 min post-BDNF injection. In contrast, MEK inhibition (U0126) abolished BDNF-induced pMF at 60 and 90 min. PI3K/Akt inhibition (PI-828) had no effect on BDNF-induced pMF at any time. Thus, whereas BDNF-induced pMF is exclusively PKCθ-dependent in normal rats, MEK/ERK is recruited by neuroinflammation to sustain, and even augment downstream plasticity. Because AIH is being developed as a therapeutic modality to restore breathing in people living with multiple neurological disorders, it is important to understand how inflammation, a common comorbidity in many traumatic or degenerative central nervous system disorders, impacts phrenic motor plasticity.
NEW & NOTEWORTHY We demonstrate that even mild systemic inflammation shifts signaling mechanisms giving rise to BDNF-induced phrenic motor plasticity. This finding has important experimental, biological, and translational implications, particularly since BDNF-dependent spinal plasticity is being translated to restore breathing and nonrespiratory movements in diverse clinical disorders, such as spinal cord injury (SCI) and amyotrophic lateral sclerosis (ALS).
INTRODUCTION
Neuroplasticity enables the central nervous system to respond in adaptive (and maladaptive) ways to internal or external stimuli. Neuroplasticity underlies key neural functions, such as learning and memory (1, 2), and it is an intrinsic property of the neural system controlling breathing (3). One well-studied model of respiratory motor plasticity is phrenic long-term facilitation (pLTF) induced by moderate acute intermittent hypoxia (mAIH) (3–5). Understanding respiratory plasticity in different physiological and pathological conditions has important biological implications and is clinically relevant since mAIH represents a simple, safe, and effective means of restoring breathing (and nonrespiratory motor function) in people with chronic spinal cord injury or ALS (6, 7).
Successful translation of mAIH to clinical practice will be enhanced by a comprehensive understanding of mechanisms giving rise to spinal respiratory plasticity and factors that regulate or undermine its expression. One factor that undermines mAIH-induced pLTF is inflammation (8–11). Multiple studies in rodents demonstrate that even a single low-dose administration of lipopolysaccharide (LPS) is sufficient to abolish mAIH-induced pLTF (8, 10, 12). As inflammation is associated with traumatic, ischemic, and degenerative neural injuries (13–17), a greater understanding of mechanisms whereby inflammation constrains plasticity will help to optimize mAIH as a therapeutic modality for people with motor impairment.
To better understand the complex cellular mechanisms giving rise to spinal plasticity, direct application of drugs to the spinal cord can be used versus intermittent hypoxia per se, phrenic motor facilitation (pMF; a more general term that includes AIH-induced pLTF) can be induced by spinal activation of multiple, distinct signaling cascades (18–21). In specific, mAIH-induced pLTF requires cervical spinal 5-HT2 receptor activation (22, 23), ERK MAP kinase activity (22, 24), new synthesis of brain-derived neurotrophic factor [BDNF; (25)], activation of its high-affinity receptor, TrkB (26), and downstream signaling via protein kinase C-θ (PKCθ) (27, 28). As LPS-induced inflammation impairs pMF elicited by spinal 5-HT2, but not TrkB receptor activation (12), inflammation impairs pLTF downstream from serotonin receptors but upstream of BDNF/TrkB signaling. Indeed, BDNF-induced pMF is actually enhanced twofold following LPS administration through unknown mechanisms (12).
Canonical BDNF/TrkB signaling can involve three distinct signaling cascades, including 1) Ras-RAF-MEK-ERK, 2) PI3K-Akt, and/or 3) PLCγ-PKC (29, 30). Factors determining which pathway(s) are activated in a given situation are not fully understood. In naïve rats, BDNF-induced pMF is MEK/ERK and PI3K/Akt independent, but requires PKCθ activation (27). Despite its potential importance, little is known concerning inflammation effects on downstream BDNF/TrkB signaling, regardless of the fact that inflammation is highly prevalent in lung and central nervous system (CNS) disorders that compromise breathing.
Our hypothesis is that with mild inflammation, additional signaling pathways are recruited to elicit pMF beyond PKCθ, amplifying BDNF-induced pMF following systemic LPS administration. To test this hypothesis, we investigated downstream BDNF/TrkB signaling in rats 24 h after low-dose systemic LPS injections (100 µg/kg, ip).
MATERIALS AND METHODS
Animals
All protocols in this study were approved by the University of Wisconsin Animal Care and Use committee. Adult male (3–4 mo) Sprague-Dawley rats (Harlan, Colony 211) were housed in a controlled environment (12-h light/dark cycle; daily humidity and temperature monitoring), with food and water ad libitum. After LPS administration, rats were randomly assigned to groups with spinal delivery of either 1) MEK/ERK inhibitor plus BDNF, 2) PI3K/Akt inhibitor plus BDNF, 3) PKCθ inhibitor plus BDNF, 4) MEK/ERK inhibitor plus artificial cerebrospinal fluid (CSF) + 0.1% BSA (BDNF vehicle), 5) PI3K/Akt inhibitor plus artificial CSF + 0.1% BSA, 6) PKCθ inhibitor plus artificial CSF + 0.1% BSA, 7) 20% DMSO/80% saline (vehicle for MEK/ERK and PI3K/Akt inhibitors) plus BDNF, 8) artificial CSF (vehicle for PKCθ inhibitor) plus BDNF, 9) 20% DMSO/80% saline plus artificial CSF + 0.1% BSA (vehicles only for MEK/ERK and PI3K/Akt groups), and 10) artificial CSF plus artificial CSF + 0.1% BSA (vehicle only for PKCθ group). Because UO126 (MEK/ERK inhibitor) and PI-828 (PI2K/Akt inhibitor) were dissolved in DMSO, experimental groups 7 and 9 served as “control” experiments (inhibitor vehicle + BDNF; and inhibitor vehicle + BDNF vehicle) in Figs. 3 and 4.
Lipopolysaccharide Administration and Experimental Protocol
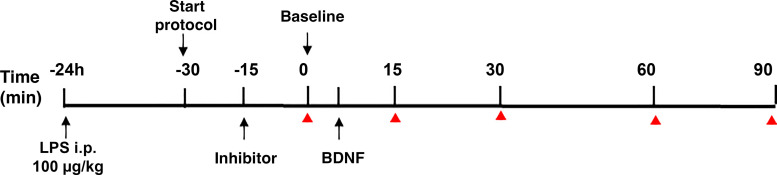
LPS (E. coli 0111:B4) was given at a dose of 100 μg/kg via intraperitoneal (ip) injection ∼24 h before beginning neurophysiological experiments as described previously (10). The experimental protocol including LPS timing, intrathecal drugs, and nerve recordings is described in Fig. 1.
Figure 1.
Experimental protocol. Rats were injected with LPS (100 µg/kg, ip) 24 h before neurophysiology experiments. Rats were anesthetized and prepared for phrenic nerve recordings (start protocol). Blood gases were measured before and after stable nerve recordings were established. Kinase inhibitors were administered, and 20 min allowed before establishing baseline (BL) blood gas values just before intrathecal BDNF injection. Blood samples and phrenic nerve recordings were taken at baseline 15, 30, 60, and 90 min post-BDNF (red triangle). BDNF, brain-derived neurotrophic factor.
Surgical Protocol
Rats were induced with isoflurane and relocated to a temperature-regulated table. Throughout surgical procedures, isoflurane anesthesia continued via nose cone (3.5% in 50% O2, balance N2). Body temperature was measured with a rectal probe and maintained between 36°C–38°C. A tail vein catheter (24 G × 3/4 gauge i.v. catheter, Surflo) was placed to administer infusions (rate: 0.5–1.2 mL/kg/h) during surgery and throughout the experimental protocol. The solution infused to maintain acid-base and fluid balance was 60% lactated Ringer’s solution, 30% HesPan (6% Hetastarch in 0.9% sodium chloride), and 10% bicarbonate (8.4% sodium bicarbonate). Tracheotomy was performed to enable artificial ventilation (Rodent Respirator, model 683, Harvard Apparatus, Holliston, MA; tidal volume 2.5 mL). Two-breath lung hyperinflations were performed regularly by occluding the expiratory outflow transiently to minimize alveolar collapse.
A flow-through CO2 analyzer (Capnogard, Novametrix) was used to monitor end-tidal Pco2 in the expiratory tubing (maintained between 40–45 mmHg). Entrainment of respiratory neural activity with the ventilator was prevented by bilateral vagotomy. A catheter (polyethylene, PE50, Intramedic) was placed in the right femoral artery to monitor blood pressure and draw blood samples for blood-gas analysis (ABL-800 Flex, Radiometer).
Blood samples (0.2–0.4 mL) were drawn for blood gas analysis and a pressure transducer (Gould, P23ID) was used to continuously monitor arterial blood pressure. Blood gases were measured during baseline (BL) before a protocol, and at 15, 30, 60, and 90 min posttreatment. The left phrenic nerve was isolated via dorsal approach, cut distally, partially desheathed, and covered with a saline-soaked cotton ball until protocols began.
C2 laminectomy was performed in all rats. After making a small incision in the dura, two soft silicone catheters (2 Fr; Access Technologies) were inserted caudally 4 mm until the tip rested near the C4 segment. Catheters were attached to a 50-μL Hamilton syringe filled with solutions and were used to deliver drugs (inhibitors, vehicle, and BDNF) to the cervical spinal cord. After surgery, rats were converted to urethane anesthesia (1.85 g/kg) via intravenous infusion over ∼15–20 min. To assure adequate anesthetic depth, rats received a toe pinch with a hemostat, and changes in phrenic nerve activity and blood pressure were assessed as pain indicators. After conversion to urethane, a minimum of 1 h was allowed before initiating an experimental protocol. At the end of protocols, rats were euthanized by urethane overdose.
Neurophysiological measurements.
To paralyze the rats, the neuromuscular blocking agent pancuronium bromide (2.5 mg/kg iv) was used. Mineral oil was added to the phrenic nerve cavity, the left phrenic nerve was completely desheathed, and then placed on bipolar silver electrodes to record nerve activity. Phrenic nerve signals were amplified (gain 10,000×), band-pass filtered (100–10,000 Hz, model 1800, A-M Systems, Carlsborg, WA), rectified, and integrated (Paynter filter, time constant 50 ms, MA-821, CWE). Integrated phrenic nerve bursts were digitized (8 kHz) and analyzed using WINDAQ data-acquisition systems (DATAQ Instruments).
To begin an experimental protocol, the apneic CO2 threshold was determined by lowering end-tidal PCO2 until phrenic nerve activity ceased for ∼1 min. At that time, end-tidal PCO2 was slowly raised to determine the recruitment threshold, when nerve activity resumed. End-tidal PCO2 was raised ∼2 mmHg above the recruitment threshold, and ∼15–20 min were allowed to establish stable baseline nerve activity. Arterial blood samples were drawn ∼5–10 min following drug delivery and throughout the protocol; arterial PCO2 was maintained within 2 mmHg of baseline levels by adjusting inspired CO2 and/or ventilator rate as needed. Arterial partial pressure of oxygen () was kept above 150 mmHg throughout experiments. Arterial partial pressure of carbon dioxide () and during baseline conditions were maintained within predetermined ranges (Table 1) and were similar across all experimental groups. Mean arterial pressure (MAP), temperature, and pH were also similar among groups at baseline and 90 min posttreatment. Thus, , , MAP, temperature, and pH differences among groups did not contribute to differential pMF responses.
Table 1.
Baseline, 30, 60, and 90-min posttreatment
| Experimental Groups | , mmHg | , mmHg | MAP, mmHg | Temp, °C | pH |
|---|---|---|---|---|---|
| LPS +MEK/ERK + BDNF | |||||
| Baseline | 45.6 ± 1.6 | 319.5 ± 11.4 | 122.1 ± 6.9 | 37.2 ± 0.1 | 7.4 ± 0 |
| 30 min | 44.4 ± 0.9 | 314.3 ± 14.4 | 119.8 ± 9.1 | 37.3 ± 0.4 | 7.4 ± 0 |
| 60 min | 44.6 ± 1.0 | 311.0 ± 13.1 | 114.2 ± 6.9 | 37.4 ± 0.3 | 7.4 ± 0 |
| 90 min | 46.1 ± 2.0 | 300.8 ± 17.3 | 112.3 ± 8.4 | 37.4 ± 0.2 | 7.4 ± 0 |
| LPS + MEK/ERK + aCSF | |||||
| Baseline | 45.5 ± 2.0 | 306.8 ± 12.0 | 118.5 ± 11.3 | 37.2 ± 0.1 | 7.4 ± 0 |
| 30 min | 45.3 ± 0.5 | 306.1 ± 13.0 | 109.1 ± 8.6 | 37.5 ± 0.2 | 7.4 ± 0 |
| 60 min | 45.1 ± 0.9 | 298.0 ± 14.1 | 105.4 ± 12.0 | 37.6 ± 0.1 | 7.4 ± 0 |
| 90 min | 45.6 ± 1.7 | 265.4 ± 24.1* | 105.8 ± 6.9 | 37.1 ± 0.1 | 7.4 ± 0 |
| LPS + PI3K/Akt + BDNF | |||||
| Baseline | 48.2 ± 1.4 | 299.8 ± 11.3 | 116.6 ± 11.8 | 37.2 ± 0.1 | 7.4 ± 0 |
| 30 min | 47.6 ± 1.0 | 299.0 ± 15.9 | 105.4 ± 12.0 | 37.2 ± 0.2 | 7.4 ± 0 |
| 60 min | 47.3 ± 1.0 | 297.3 ± 12.4 | 99.4 ± 8.9 | 37.5 ± 0.2 | 7.4 ± 0 |
| 90 min | 47.4 ± 1.2 | 295.0 ± 21.0 | 95.1 ± 15.2 | 37.1 ± 0.1 | 7.4 ± 0 |
| LPS + PI3K/Akt + aCSF | |||||
| Baseline | 45.0 ± 1.3 | 328.4 ± 13.9 | 111.5 ± 8.5 | 37.2 ± 0.1 | 7.4 ± 0 |
| 30 min | 46.1 ± 1.1 | 327.2 ± 7.5 | 108.4 ± 4.3 | 37.6 ± 0.2 | 7.4 ± 0 |
| 60 min | 46.1 ± 1.2 | 317.0 ± 8.9 | 95.4 ± 9.7 | 37.6 ± 0.2 | 7.4 ± 0 |
| 90 min | 45.0 ± 1.1 | 314.2 ± 21.7 | 91.2 ± 8.9 | 37.3 ± 0.2 | 7.4 ± 0 |
| LPS + TIP + BDNF | |||||
| Baseline | 48.9 ± 1.5 | 299.1 ± 8.2 | 114.0 ± 5.1 | 37.2 ± 0.1 | 7.4 ± 0 |
| 30 min | 47.3 ± 1.0 | 303.3 ± 8.9 | 110.2 ± 5.2 | 37.3 ± 0.1 | 7.4 ± 0 |
| 60 min | 47.6 ± 0.6 | 301.3 ± 7.8 | 110.1 ± 4.7 | 37.4 ± 0.2 | 7.4 ± 0 |
| 90 min | 47.4 ± 0.7 | 318.6 ± 6.2* | 98.7 ± 5.8 | 37.3 ± 0.1 | 7.4 ± 0 |
| LPS + TIP + aCSF | |||||
| Baseline | 47.1 ± 1.7 | 343.3 ± 9.7 | 119.3 ± 7.6 | 37.1 ± 0.1 | 7.4 ± 0 |
| 30 min | 48.7 ± 1.9 | 348.3 ± 10.6 | 111.7 ± 4.2 | 37.2 ± 0.2 | 7.4 ± 0 |
| 60 min | 47.8 ± 0.8 | 350.3 ± 6.2 | 105.7 ± 8.5 | 37.3 ± 0.2 | 7.4 ± 0 |
| 90 min | 48.1 ± 0.9 | 350.7 ± 15.2 | 94.4 ± 2.5 | 37.2 ± 0.1 | 7.4 ± 0 |
| LPS + inhibitor vehicle + BDNF | |||||
| Baseline | 48.1 ± 0.9 | 329.6 ± 12.4 | 120.2 ± 3.5 | 37.1 ± 0.1 | 7.4 ± 0 |
| 30 min | 47.1 ± 1.2 | 325.0 ± 8.9 | 119.9 ± 5.5 | 37.0 ± 0.1 | 7.4 ± 0 |
| 60 min | 47.3 ± 1.4 | 326.7 ± 8.5 | 112.4 ± 5.1 | 37.1 ± 0.2 | 7.4 ± 0 |
| 90 min | 47.6 ± 1.0 | 323.0 ± 11.7 | 111.±3.5 | 37.2 ± 0.1 | 7.4 ± 0 |
| LPS + inhibitor vehicle + aCSF | |||||
| Baseline | 46.0 ± 1.9 | 307.7 ± 10.9 | 102.8 ± 6.1 | 37.4 ± 0.2 | 7.4 ± 0 |
| 30 min | 46.4 ± 0.8 | 307.3 ± 8.9 | 94.5 ± 5.6 | 37.4 ± 0.1 | 7.4 ± 0 |
| 60 min | 45.4 ± 1.0 | 303.8 ± 8.5 | 87.5 ± 9.5 | 37.4 ± 0.1 | 7.4 ± 0 |
| 90 min | 46.4 ± 2.2 | 306.1 ± 13.6 | 82.5 ± 16.9 | 37.3 ± 0.2 | 7.4 ± 0 |
Values expressed as means ± SE. There were no significant differences of arterial partial pressure of carbon dioxide (), mean arterial pressure (MAP), temperature, and pH among groups. Differences from baseline within groups (*) are denoted within the table; P < 0.05. aCSF, artificial cerebrospinal fluid; BDNF, brain-derived neurotrophic factor; LPS, lipopolysaccharide; , arterial partial pressure of oxygen.
Pharmacological Treatments
Brain-derived neurotrophic factor.
Recombinant BDNF protein (Promega) was diluted with double distilled water to make stock solution (1 µg/10 µL) and stored at −20°C. The stock was then divided into 4-µL aliquots and stored at −20°C. Stock solutions were not used if stored for more than 1 mo. For intrathecal delivery, BDNF was diluted to 100 ng in 12 µL (in artificial CSF + 0.1% BSA) on the day of use (25). Control groups received artificial cerebrospinal fluid (aCSF) (120 mM NaCl, 3 mM KCl, 2 mM CaCl, 2 mM MgCl, 23 mM NaHCO3, 10 mM glucose bubbled with 95%O2-5%CO2) with 0.1% BSA.
MEK/ERK inhibitor (U0126).
The membrane permeable MEK/ERK inhibitor U0126 [1,4-diamino-2,3-dicyano-1,4-bis(o-aminophenylmercapto) butadiene] (Promega) was dissolved in 100% DMSO (50 mM stock solution), aliquoted, and stored at −20°C until used. On the day of use, it was diluted to a final concentration of 100 μM in 20% DMSO/80% saline (100 μM; 12 μL total, 1 μL/15 s; (31–33).
PI3K/Akt inhibitor (PI-828).
The PI3K/Akt inhibitor PI-828 [1,4-diamino-2,3-dicyano-1,4-bis(o-aminophenylmercapto) butadiene] (Tocris) was dissolved in 100% DMSO (50 mM stock solution), aliquoted and stored at −20°C until used. On the day of use, it was diluted to a final concentration of 100 μM in 20% DMSO/80% saline (100 μM; 12 μL total, 1 μL/15 s; 27).
PKCθ inhibitor (TIP).
Theta inhibitory peptide, TIP (Calbiochem), is a myristoylated peptide mimicking the pseudosubstrate domain of PKCθ. TIP was dissolved in 100% aCSF (1 mM stock solution) and stored at −20°C. On the day of use, it was diluted with aCSF to a concentration of 0.86 mM. The inhibitor was administered (0.86 mM; 12 μL total, 1 μL/15 s) 20 min before BDNF administration. We previously demonstrated this same TIP dose blocks mAIH-induced pLTF (28). For controls, we administered 100% aCSF at the same volume and rate as the inhibitor.
Intrathecal drug administration.
BDNF and other drugs were injected via two silicon catheters placed in the intrathecal space, one for the inhibitor and the other for BDNF. Solutions were administered 20 min before BDNF administration. All solutions were administered with a final volume of 12 μL (1 μL/15 s).
Time Controls
Time control experiments were done to control for potential time-dependent drift in phrenic nerve activity due to the experimental preparation. Time control LPS-treated rats were injected with vehicles for drug and BDNF; drug vehicle was administered before baseline blood/gas measurements and BDNF vehicle was administered 20 min later.
Statistical Analysis
Integrated phrenic nerve burst amplitude was normalized as a percent change from baseline. Phrenic burst frequency was also normalized to baseline levels but expressed as an absolute difference (bursts per minute). Statistical comparisons were made within and between treatment groups using two-way ANOVA (Prism 6; GraphPad Software) for mean arterial pressure (MAP), blood gases (BL and 90 min), temperature, and pH. To detect significant differences between groups, two-way repeated-measures ANOVA was used (Prism 6; GraphPad Software) for phrenic nerve burst amplitude and frequency (BL, 15, 30, 60, and 90 min) with individual comparisons using Tukey’s post hoc test. Significance was set to P < 0.05; results are expressed as mean ± 1 SE.
As there were no statistically significant (or apparent) differences between control groups 7 (20% DMSO/80% saline plus BDNF; n = 4) and 8 (artificial CSF plus BDNF; n = 3), these groups were combined into a single inhibitor vehicle plus BDNF group (n = 7). Groups 9 (20% DMSO/80% saline + artificial CSF + 0.1% BSA; n = 4) and 10 (artificial CSF + artificial CSF + 0.1% BSA; n = 1) were also combined to make a time control group, n = 5; n refers to number of rats.
RESULTS
After LPS, BDNF-Induced pMF Is PKC-θ Dependent in Early but Not Late-Phase pMF
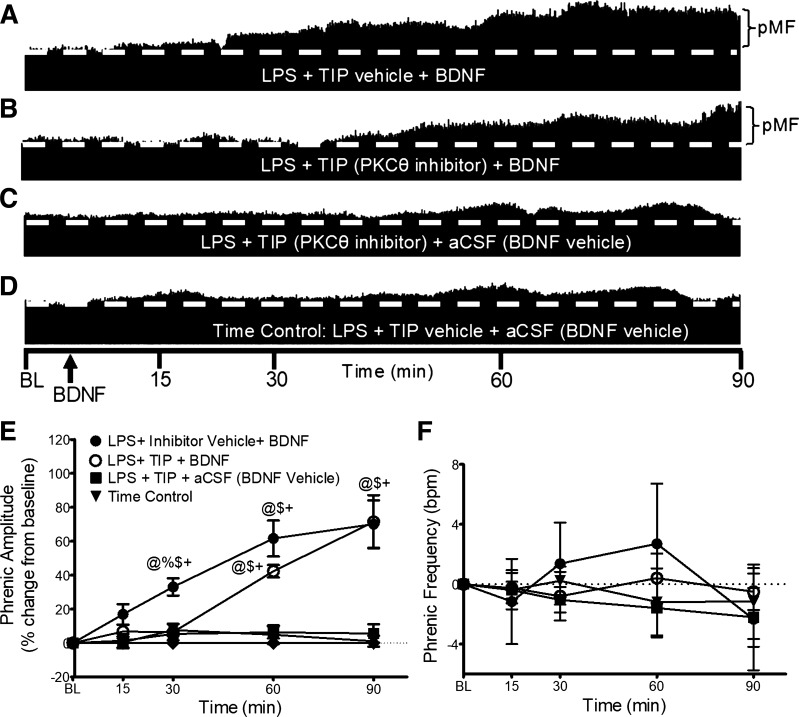
Because BDNF-induced pMF requires PKCθ activity in naïve rats (27), we determined if PKCθ activity is still necessary after LPS administration using theta inhibitory peptide, TIP. Typical neurograms representing integrated phrenic nerve activity are shown in Fig. 2. Although pMF was blocked following TIP administration at 30 min (6 ± 5% vs. 33 ± 5% for TIP vehicle + BDNF; P = 0.0496; n = 5), TIP had no effect on pMF at 60 (42 ± 4%; n = 5) or 90 min (71 ± 16%; n = 5) post-BDNF, demonstrating PKCθ dependence in early but not late phases of BDNF-induced pMF.
Figure 2.
PKCθ inhibitor (TIP) and BDNF-induced pMF. Representative traces of compressed integrated phrenic neurograms (A–D). BDNF-induced pMF after LPS is PKCθ-dependent at 30 min, but not 60 or 90 min. A: post-LPS and intrathecal TIP vehicle (100% aCSF, 12 µL), BDNF elicits robust pMF. B: post-LPS, intrathecal TIP (100 mM/12 µL) 20 min before BDNF impaired pMF at 30 min. At 60 and 90 min post-BDNF, phrenic nerve amplitude is increased. C: with TIP (100 mM/12 µL) plus BDNF vehicle (0.1% BSA and aCSF), pMF is not observed. D: with intrathecal inhibitor vehicle (100% aCSF, 12 µL) plus BDNF vehicle (0.1% BSA and aCSF), pMF is not observed. E: group data for phrenic burst amplitude, expressed as percent change from baseline. LPS + TIP vehicle + BDNF (black circle, n = 8), LPS + PKCθ inhibitor + BDNF (white circles, n = 5), LPS + PKCθ inhibitor + aCSF (squares, n = 4), and LPS + inhibitor vehicle + aCSF (Time Control; black triangles, n = 5) were compared to compare groups at similar time post-BDNF/vehicle injection, and within each group vs. baseline. Phrenic amplitude after LPS + TIP vehicle + BDNF is significantly elevated above the other 3 groups at 30 min only, and above LPS + TIP + aCSF and time control at 60 and 90 min. LPS + TIP vehicle+ BDNF and LPS + PKCθ inhibitor + BDNF were not significantly different at 60 and 90 min post-BDNF. LPS + PKCθ inhibitor + aCSF and time control were not significantly different at any time. Significance is P ≤ 0.05; @significant difference from baseline; %significant difference from LPS + TIP+ BDNF; $significant difference from LPS + TIP + aCSF; and +significant difference from time control. F: phrenic burst frequency, expressed as a change from baseline (burst/min). Phrenic frequency was scaled to equal phrenic amplitude for direct comparison of magnitudes among groups; there were no significant differences between any group at any time; P > 0.05. aCSF, artificial cerebrospinal fluid; BDNF, brain-derived neurotrophic factor; LPS, lipopolysaccharide; pMF, phrenic motor facilitation; TIP, theta inhibitory peptide.
There was no significant difference in phrenic burst frequency at any time in any experimental group (Fig. 2F).
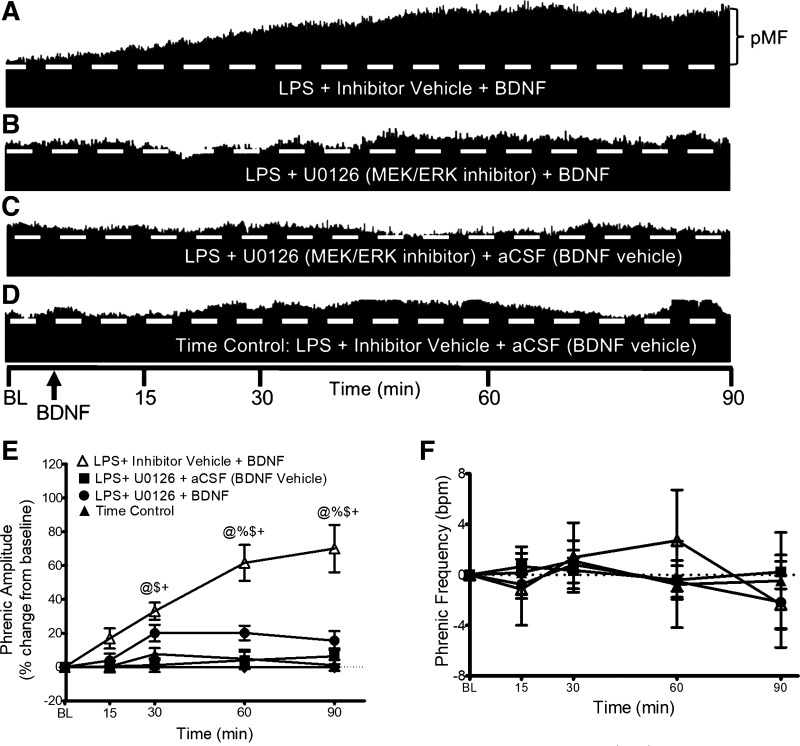
After LPS, BDNF-Induced pMF Is MEK/ERK Dependent in Late-Phase pMF
To determine if MEK/ERK signaling is necessary for BDNF-induced pMF with mild systemic inflammation, we delivered a MEK/ERK inhibitor, U0126, 20 min before BDNF. Typical integrated phrenic neurograms (Fig. 3, A–D) illustrate that MEK/ERK inhibition attenuated pMF at 60 and 90 min post-BDNF. At 15 and 30 min, U0126 had no effect (15 min: 3 ± 4% vs. 16 ± 6% for UO126 vehicle + BDNF; 30 min: 20 ± 5% vs. 33 ± 5% for UO126 vehicle + BDNF). In contrast, pMF following MERK/ERK inhibitor plus BDNF (n = 6) was significantly lower at 60 and 90 min versus pMF without MEK/ERK inhibitor (n = 8) (60 min: 20 ± 4% vs. 47 ± 12% with no inhibitor, P = 0.0255; 90 min: 15 ± 5% vs. 65 ± 21%, P < 0.0001; Fig. 3E). There were no significant differences at any time between groups that did not receive BDNF, demonstrating that the MEK/ERK inhibitor had no independent effect on phrenic nerve activity (Fig. 3E).
Figure 3.
MEK/ERK inhibitor (U0126) and BDNF-induced pMF. Representative traces of compressed integrated phrenic neurograms (A–D). After LPS (100 µg/kg, 24 h post), BDNF-induced pMF is MEK/ERK dependent since pMF at later times (60–90 min) was blocked by U0126. A: after U0126 vehicle (20% DMSO/80% saline), BDNF elicits robust pMF 24 h post-LPS. B: after U0126 (100 mM/12 µL) given 20 min before BDNF in LPS-treated rats, pMF is impaired. C: after U0126 (100 mM/12 µL) plus BDNF vehicle (0.1% BSA and aCSF), pMF is not observed. D: after intrathecal inhibitor vehicle (20% DMSO/80% saline) plus BDNF vehicle (0.1% BSA and aCSF), pMF is not observed. E: group data for phrenic burst amplitude, expressed as a percent increase from baseline. LPS + inhibitor vehicle + BDNF (white triangles, n = 8), LPS + MEK/ERK inhibitor + BDNF (circles, n = 6), LPS + MEK/ERK inhibitor + BDNF vehicle (squares, n = 7), and LPS + inhibitor vehicle + BDNF vehicle (Time Control; black triangles, n = 4) were compared to determine significance between groups, and each group vs. baseline. Rats that received LPS + inhibitor vehicle + BDNF showed elevated phrenic amplitude vs. the other 3 groups at 30, 60 and 90 min. LPS + MEK/ERK inhibitor + BDNF, LPS + MEK/ERK inhibitor + BDNF vehicle and LPS + inhibitor vehicle + BDNF vehicle were not significantly different at any time. Significance is P ≤ 0.05; @significant difference from baseline; %significant difference from LPS + MEK/ERK inhibitor + BDNF; $significant difference from LPS + MEK/ERK inhibitor + BDNF vehicle; and +significant difference from time control. F: phrenic burst frequency, expressed as a change from baseline in burst/min. Phrenic frequency was scaled equal to phrenic amplitude for direct comparison of magnitudes in the groups. There were no significant differences between any group at any time (all P > 0.05). aCSF, artificial cerebrospinal fluid; BDNF, brain-derived neurotrophic factor; LPS, lipopolysaccharide; pMF, phrenic motor facilitation.
There was no difference in phrenic burst frequency at any time in any group (Fig. 3F).
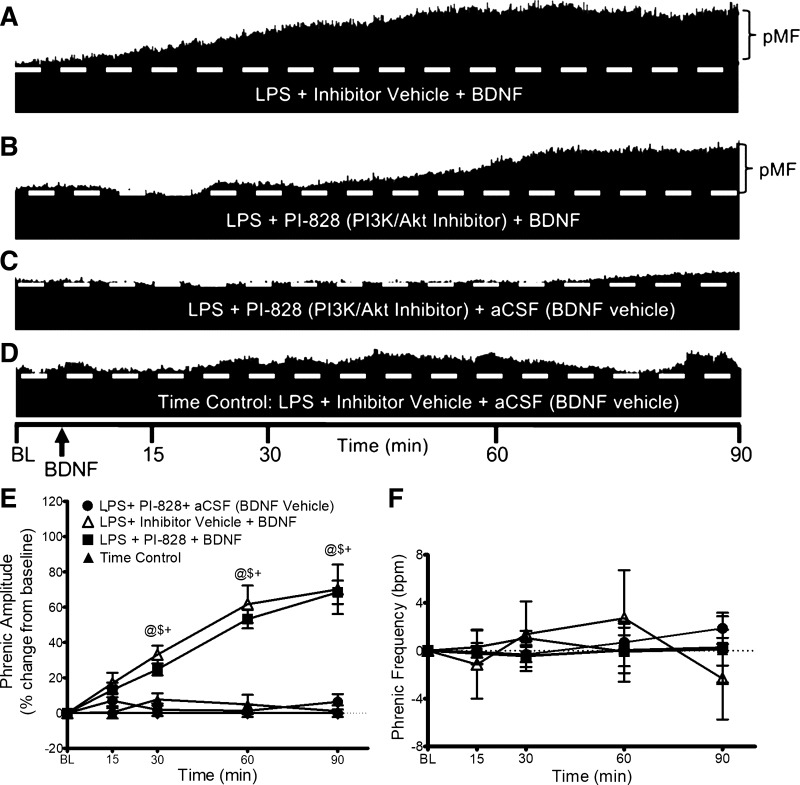
BDNF-Induced pMF Is PI3K/AKT Independent
Similar to mAIH-induced pLTF in naive rats (33), BDNF-induced pMF post-LPS is independent of PI3K/AKT signaling. Representative phrenic neurograms shown in Fig. 4, A–D, illustrate that PI3K inhibition has no impact on BDNF-induced pMF in LPS-treated rats. At 15, 30, 60, and 90 min post-BDNF, PI3K inhibition (n = 5) had no effect on pMF (n = 8) (15 min: 13 ± 1% vs. 16 ± 6%; 30 min: 24 ± 3% vs. 33 ± 5%; 60 min: 53 ± 5% vs. 61 ± 11%; 90 min: 68 ± 6% vs. 70 ± 14% for PI3K/Akt vehicle + BDNF). There were no significant differences at any time between groups that did not receive BDNF (Fig. 4E).
Figure 4.
PI3K/Akt inhibitor (PI-828) and BDNF-induced pMF. Representative traces of compressed integrated phrenic neurograms (A–D). After LPS (100 µg/kg; 24 h post), BDNF-induced pMF remains independent of PI3K/Akt signaling. A: intrathecal inhibitor vehicle (20% DMSO/80% saline) 20 min before intrathecal BDNF exhibits pMF. B: after LPS, BDNF still elicits pMF after intrathecal PI3K/Akt inhibitor (100 mM/12 µL; 20 min before). C: PI3K/Akt inhibitor (PI-828; 100 mM/12 µL; 20 min before) with BDNF vehicle (0.1% BSA and aCSF) does not elicit pMF. D: inhibitor vehicle (20% DMSO/80% saline; 20 min pre) with BDNF vehicle (0.1% BSA and aCSF) does not elicit pMF. E: group data for phrenic burst amplitude, expressed as percent increase from baseline. LPS + inhibitor vehicle + BDNF (white triangles, n = 8), LPS + PI3K/Akt inhibitor + BDNF (squares, n = 5), LPS + PI3K/Akt inhibitor + BDNF vehicle (circles, n = 5), and LPS + inhibitor vehicle + BDNF vehicle (Time Control; black triangles, n = 5) were compared to determine significant differences between groups and vs. baseline. @Significant difference from baseline; $significant difference from LPS + inhibitor vehicle + BDNF; and +significant difference from time control. F: phrenic burst frequency, expressed as a change from baseline in burst/min. Phrenic frequency was scaled equal to phrenic amplitude for direct comparison of magnitudes in the groups. There were no significant differences between any group at any time; P > 0.05. aCSF, artificial cerebrospinal fluid; BDNF, brain-derived neurotrophic factor; LPS, lipopolysaccharide; pMF, phrenic motor facilitation.
No differences were observed in burst frequency at any time or group (Fig. 4F).
DISCUSSION
We demonstrate that mild inflammation elicited by systemic LPS shifted downstream BDNF/TrkB signaling, thereby increasing BDNF-induced pMF. In naïve rats, only PKCθ (not MEK/ERK or PI3K/Akt) is required for BDNF-induced pMF (27). We now demonstrate that 1 day post-LPS, PKCθ activity is necessary for early (30 min), but not late BDNF-induced pMF (60–90 min). In late-phase pMF, MEK/ERK signaling assumes dominance, superseding contributions from PKCθ. PI3K/Akt signaling is not necessary for BDNF-induced pMF in naïve or LPS-treated rats. Although distinct, time-dependent signaling cascades give rise to early versus late hippocampal synaptic plasticity (34), this is the first demonstration of a similar phenomenon in phrenic motor plasticity.
Two metabotropic receptors known to elicit pMF are serotonin 5HT2 and adenosine A2A receptors, which elicit the Q versus S pathways to phrenic motor facilitation; these pathways are named for canonical Gq versus Gs protein signaling of 5HT2 versus A2A receptors, respectively (21). Although the Q pathway dominates with moderate AIH, the S pathway assumes dominance with AIH consisting of severe hypoxic episodes (35). The diversity of ligands capable of initiating pMF likely confers an increased ability to adapt to varied physiological and/or pathophysiological conditions (20, 36). An inflammation-impaired Q pathway may undermine effective compensation for the onset of disease and/or injury. On the other hand, the still-intact S pathway (35) may preserve the ability to compensate for disease/injury.
Here, we demonstrate the capacity to “reroute” from one intracellular signaling cascade to another, potentially compensating for upstream signaling deficits and preserving Q pathway-dependent pMF. When inflammation undermines the capacity for new BDNF protein synthesis, additional signaling pathways downstream from TrkB are recruited in the cell signaling network, increasing the capacity for pMF downstream from BDNF/TrkB signaling. This switch from PKCθ to combined PKCθ (early) plus ERK MAP kinase (late) amplifies the potential for residual BDNF protein to elicit phrenic motor plasticity. Whether or not this switch in downstream signaling confers other advantages remain uncertain, but properties beyond functional plasticity may be enabled by this switch (e.g., synaptogenesis).
Lipopolysaccharide (LPS) is a Toll-like receptor 4 ligand commonly used to model systemic inflammation (8, 9, 37–39). Although LPS does not cross the blood-brain barrier, certain circulating cytokines expressed in response to LPS do cross and activate CNS microglia (40–42). Other mechanisms transmitting systemic inflammation to the CNS are possible, such as the ability of vagal afferent neurons to elicit brainstem inflammation (43–45).
Low-dose LPS increases inflammatory cytokine mRNAs in cervical spinal tissue homogenates or isolated microglia 3 h postinjection, but LPS-induced gene expression has already normalized by 24 h postinjection (10). However, since this study (10) did not report inflammatory cytokine protein levels, it remains possible that they are still elevated 24 h post-LPS. Indeed, microglial proinflammatory cytokine protein levels remain elevated at least 20 h post-LPS (46, 47), and p38-MAPK phosphorylation remains elevated in phrenic motor neurons at least 24 h post-LPS (48). A similar timeline is observed in microglial cell cultures, where proinflammatory mRNA levels peak at ∼3 h, whereas cytokine protein levels remain elevated for at least 24 h post-LPS (49–51).
Although mechanisms recruiting MEK/ERK MAP kinase signaling in BDNF-induced pMF post-LPS are unknown, BDNF/TrkB signaling is highly adaptive and depends on BDNF/TrkB interaction kinetics as well as prevailing neuronal activity (52). As LPS decreases BDNF (53), TrkB receptors may upregulate to compensate for inadequate ligand availability. Although we did not investigate possible TrkB upregulation in this study, it may contribute to increased BDNF-induced pMF post-LPS. On the other hand, recruiting MEK/ERK signaling may be sufficient to amplify pMF without TrkB upregulation. Additional research is needed to distinguish between these possibilities.
Increased availability of adaptor molecules necessary for MEK/ERK pathway activation (e.g., Grb2, SCH, and SOS) could enhance MEK/ERK signaling. Alternately, TrkB translocation to lipid rafts favors ERK-dependent TrkB signaling in in vitro systems. The impact of translocation on PLC/PKC signaling is less clear (54). As inflammation increases basal adenosine levels in the phrenic motor nucleus (55), and A2A receptor activation promotes TrkB translocation to lipid rafts, inflammation may indirectly translocate TrkB to lipid rafts due to elevated extracellular adenosine concentrations, shifting interactions between TrkB and its downstream signaling molecules (56). Adenosine-induced TrkB translocation is ligand-dependent since TrkB must be bound to BDNF, possibly explaining the observation that BDNF-induced pMF initially requires PKCθ in LPS-treated rats, but then shifts toward MEK/ERK dominance as BDNF/TrkB complexes translocate to lipid rafts. Despite precedent for shifts in downstream BDNF/TrkB signaling, additional studies are needed to elucidate mechanisms giving rise to LPS-enhanced BDNF-induced pMF.
Administration of a high dose of the NSAID ketoprofen ∼3 h before mAIH restores pLTF in rats treated with low-dose LPS (9). LPS-induced inflammation undermines the Q pathway upstream from BDNF/TrkB signaling, likely by inhibiting new BDNF protein synthesis (53, 57). However, with systemic inflammation, TrkB activation via BDNF administration recruits additional signaling pathway (i.e., MEK/ERK), thereby enhancing pLTF. The biological significance of this observation and its therapeutic potential needs to be determined, but it opens an interesting path to overcome the detrimental effects of neuroinflammation on phrenic motor plasticity. From the clinical perspective, successful translation of mAIH protocols into therapeutics for improving breathing capacity in patients with SCI is often hindered by ongoing inflammation. Thus, understanding the mechanisms by which inflammation undermines pLTF is crucial.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This study was supported by NIH R01 HL111598 and HL149800; I.M.A-M was supported by an National Heart, Lung, and Blood Institute diversity supplement.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.M.A-M. and G.S.M. conceived and designed research; I.M.A-M. performed experiments; I.M.A-M., M.N., E.A.D., and G.S.M. analyzed data; I.M.A-M. and G.S.M. interpreted results of experiments; I.M.A-M. prepared figures; I.M.A-M. drafted manuscript; I.M.A-M., M.N., E.A.D., and G.S.M. edited and revised manuscript; I.M.A-M., M.N., E.A.D., and G.S.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank K. Burrowes for help in collecting references and contributing to the discussion of shifting cellular mechanisms.
Present address of I. M. Agosto-Marlin: Chondrex Inc. Dept. of Research and Development, 16928 Woodinville-Redmond Rd NE, Suite B-101, Woodinville, WA, USA 98072.
REFERENCES
- 1. Mancini A, de Iure A, Picconi B. Basic mechanisms of plasticity and learning. Handb Clin Neurol 184: 21–34, 2022. doi: 10.1016/B978-0-12-819410-2.00002-3. [DOI] [PubMed] [Google Scholar]
- 2. Wang Y, Fu AKY, Ip NY. Instructive roles of astrocytes in hippocampal synaptic plasticity: neuronal activity-dependent regulatory mechanisms. FEBS J 289: 2202–2218, 2021. doi: 10.1111/febs.15878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26: 239–266, 2003. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol 112: 123–134, 1998. doi: 10.1016/s0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- 5. Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol (1985) 94: 358–374, 2003. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- 6. Sajjadi E, Seven YB, Ehrbar JG, Wymer JP, Mitchell GS, Smith BK. Acute intermittent hypoxia and respiratory muscle recruitment in people with amyotrophic lateral sclerosis: a preliminary study. Exp Neurol 347: 113890, 2022. doi: 10.1016/j.expneurol.2021.113890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vose AK, Welch JF, Nair J, Dale EA, Fox EJ, Muir GD, Trumbower RD, Mitchell GS. Therapeutic acute intermittent hypoxia: a translational roadmap for spinal cord injury and neuromuscular disease. Exp Neurol 347: 113891, 2022. doi: 10.1016/j.expneurol.2021.113891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vinit S, Windelborn JA, Mitchell GS. Lipopolysaccharide attenuates phrenic long-term facilitation following acute intermittent hypoxia. Respir Physiol Neurobiol 176: 130–135, 2011. doi: 10.1016/j.resp.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huxtable AG, Vinit S, Windelborn JA, Crader SM, Guenther CH, Watters JJ, Mitchell GS. Systemic inflammation impairs respiratory chemoreflexes and plasticity. Respir Physiol Neurobiol 178: 482–489, 2011. doi: 10.1016/j.resp.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huxtable AG, Smith SMC, Vinit S, Watters JJ, Mitchell GS. Systemic LPS induces spinal inflammatory gene expression and impairs phrenic long-term facilitation following acute intermittent hypoxia. J Appl Physiol (1985) 114: 879–887, 2013. doi: 10.1152/japplphysiol.01347.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beyeler SA, Hodges MR, Huxtable AG. Impact of inflammation on developing respiratory control networks: rhythm generation, chemoreception and plasticity. Respir Physiol Neurobiol 274: 103357, 2020. doi: 10.1016/j.resp.2019.103357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agosto-Marlin IM, Nichols NL, Mitchell GS. Systemic inflammation inhibits serotonin receptor 2-induced phrenic motor facilitation upstream from BDNF/TrkB signaling. J Neurophysiol 119: 2176–2185, 2018. doi: 10.1152/jn.00378.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shao F, Wang X, Wu H, Wu Q, Zhang J. Microglia and neuroinflammation: crucial pathological mechanisms in traumatic brain injury-induced neurodegeneration. Front Aging Neurosci 14: 825086, 2022. doi: 10.3389/fnagi.2022.825086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dai H-Y, Ji D, Tan C, Sun J, Yao H. Research progress on the role and regulatory mechanism of pathogenic Th17 cells in neuroinflammation. Yi Chuan 44: 289–299, 2022. doi: 10.16288/j.yczz.22-030. [DOI] [PubMed] [Google Scholar]
- 15. Leng F, Edison P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol 17: 157–172, 2021. doi: 10.1038/s41582-020-00435-y. [DOI] [PubMed] [Google Scholar]
- 16. Jurcau A, Simion A. Neuroinflammation in cerebral ischemia and ischemia/reperfusion injuries: from pathophysiology to therapeutic strategies. Int J Mol Sci 23: 14, 2021. doi: 10.3390/ijms23010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Amin J, Erskine D, Donaghy PC, Surendranathan A, Swann P, Kunicki AP, Boche D, Holmes C, McKeith IG, O'Brien JT, Teeling JL, Thomas AJ. Inflammation in dementia with Lewy bodies. Neurobiol Dis 168: 105698, 2022. doi: 10.1016/j.nbd.2022.105698. [DOI] [PubMed] [Google Scholar]
- 18. Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol 104: 251–260, 1996. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- 19. Fields DP, Springborn SR, Mitchell GS. Spinal 5-HT7 receptors induce phrenic motor facilitation via EPAC-mTORC1 signaling. J Neurophysiol 114: 2015–2022, 2015. doi: 10.1152/jn.00374.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Devinney MJ, Huxtable AG, Nichols NL, Mitchell GS. Hypoxia-induced phrenic long-term facilitation: emergent properties. Ann N Y Acad Sci 1279: 143–153, 2013. doi: 10.1111/nyas.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dale-Nagle EA, Hoffman MS, MacFarlane PM, Mitchell GS. Multiple pathways to long-lasting phrenic motor facilitation. Adv Exp Med Biol 669: 225–230, 2010. doi: 10.1007/978-1-4419-5692-7_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tadjalli A, Mitchell GS. Cervical spinal 5-HT(2A) and 5-HT(2B) receptors are both necessary for moderate acute intermittent hypoxia-induced phrenic long-term facilitation. J Appl Physiol (1985) 127: 432–443, 2019. doi: 10.1152/japplphysiol.01113.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci 22: 6239–6246, 2002. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoffman MS, Nichols NL, Macfarlane PM, Mitchell GS. Phrenic long-term facilitation after acute intermittent hypoxia requires spinal ERK activation but not TrkB synthesis. J Appl Physiol (1985) 113: 1184–1193, 2012. doi: 10.1152/japplphysiol.00098.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci 7: 48–55, 2004. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- 26. Dale EA, Fields DP, Devinney MJ, Mitchell GS. Phrenic motor neuron TrkB expression is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation. Exp Neurol 287: 130–136, 2017. doi: 10.1016/j.expneurol.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Agosto-Marlin IM, Mitchell GS. Spinal BDNF-induced phrenic motor facilitation requires PKCθ activity. J Neurophysiol 118: 2755–2762, 2017. doi: 10.1152/jn.00945.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Devinney MJ, Fields DP, Huxtable AG, Peterson TJ, Dale EA, Mitchell GS. Phrenic long-term facilitation requires PKCθ activity within phrenic motor neurons. J Neurosci 35: 8107–8117, 2015. doi: 10.1523/JNEUROSCI.5086-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Duman RS, Voleti B. Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci 35: 47–56, 2012. doi: 10.1016/j.tins.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kowiański P, Lietzau G, Czuba E, Waśkow M, Steliga A, Moryś J. BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell Mol Neurobiol 38: 579–593, 2018. doi: 10.1007/s10571-017-0510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dale EA, Satriotomo I, Mitchell GS. Cervical spinal erythropoietin induces phrenic motor facilitation via extracellular signal-regulated protein kinase and Akt signaling. J Neurosci 32: 5973–5983, 2012. doi: 10.1523/JNEUROSCI.3873-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dale-Nagle EA, Satriotomo I, Mitchell GS. Spinal vascular endothelial growth factor induces phrenic motor facilitation via extracellular signal-regulated kinase and Akt signaling. J Neurosci 31: 7682–7690, 2011. doi: 10.1523/JNEUROSCI.0239-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoffman MS, Nichols NL, Macfarlane PM, Mitchell GS. Phrenic long-term facilitation after acute intermittent hypoxia requires spinal ERK activation but not TrkB synthesis. J Appl Physiol (1985) 113: 1184–1193, 2012. doi: 10.1152/japplphysiol.00098.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pang PT, Nagappan G, Guo W, Lu B. Extracellular and intracellular cleavages of proBDNF required at two distinct stages of late-phase LTP. NPJ Sci Learn 1: 16003, 2016. doi: 10.1038/npjscilearn.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Agosto-Marlin IM, Nichols NL, Mitchell GS. Adenosine-dependent phrenic motor facilitation is inflammation resistant. J Neurophysiol 117: 836–845, 2017. doi: 10.1152/jn.00619.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dale EA, Ben Mabrouk F, Mitchell GS. Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function. Physiology (Bethesda) 29: 39–48, 2014. doi: 10.1152/physiol.00012.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Triantafilou M, Triantafilou K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol 23: 301–304, 2002. doi: 10.1016/s1471-4906(02)02233-0. [DOI] [PubMed] [Google Scholar]
- 38. Lu Y-C, Yeh W-C, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine 42: 145–151, 2008. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 39. Min SS, Quan HY, Ma J, Han J-S, Jeon BH, Seol GH. Chronic brain inflammation impairs two forms of long-term potentiation in the rat hippocampal CA1 area. Neurosci Lett 456: 20–24, 2009. doi: 10.1016/j.neulet.2009.03.079. [DOI] [PubMed] [Google Scholar]
- 40. Gonçalves RA, De Felice FG. The crosstalk between brain and periphery: implications for brain health and disease. Neuropharmacology 197: 108728, 2021. doi: 10.1016/j.neuropharm.2021.108728. [DOI] [PubMed] [Google Scholar]
- 41. Laflamme N, Lacroix S, Rivest S. An essential role of interleukin-1beta in mediating NF-kappaB activity and COX-2 transcription in cells of the blood-brain barrier in response to a systemic and localized inflammation but not during endotoxemia. J Neurosci 19: 10923–10930, 1999. doi: 10.1523/JNEUROSCI.19-24-10923.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rivest S. How circulating cytokines trigger the neural circuits that control the hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology 26: 761–788, 2001. doi: 10.1016/s0306-4530(01)00064-6. [DOI] [PubMed] [Google Scholar]
- 43. Maier SF, Goehler LE, Fleshner M, Watkins LR. The role of the vagus nerve in cytokine-to-brain communication. Ann N Y Acad Sci 840: 289–300, 1998. doi: 10.1111/j.1749-6632.1998.tb09569.x. [DOI] [PubMed] [Google Scholar]
- 44. Goehler LE, Gaykema RP, Nguyen KT, Lee JE, Tilders FJ, Maier SF, Watkins LR. Interleukin-1beta in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems? J Neurosci 19: 2799–2806, 1999. doi: 10.1523/jneurosci.19-07-02799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Blatteis CM, Li S. Pyrogenic signaling via vagal afferents: what stimulates their receptors? Auton Neurosci 85: 66–71, 2000. doi: 10.1016/s1566-0702(00)00221-6. [DOI] [PubMed] [Google Scholar]
- 46. Nikodemova M, Watters JJ. Efficient isolation of live microglia with preserved phenotypes from adult mouse brain. J Neuroinflammation 9: 147, 2012. doi: 10.1186/1742-2094-9-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fernández-Calle R, Vicente-Rodríguez M, Gramage E, Pita J, Pérez-García C, Ferrer-Alcón M, Uribarri M, Ramos MP, Herradón G. Pleiotrophin regulates microglia-mediated neuroinflammation. J Neuroinflammation 14: 46, 2017. doi: 10.1186/s12974-017-0823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tadjalli A, Seven YB, Perim RR, Mitchell GS. Systemic inflammation suppresses spinal respiratory motor plasticity via mechanisms that require serine/threonine protein phosphatase activity. J Neuroinflammation 18: 28, 2021. doi: 10.1186/s12974-021-02074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nakamura Y, Si QS, Kataoka K. Lipopolysaccharide-induced microglial activation in culture: temporal profiles of morphological change and release of cytokines and nitric oxide. Neurosci Res 35: 95–100, 1999. doi: 10.1016/s0168-0102(99)00071-1. [DOI] [PubMed] [Google Scholar]
- 50. Liu J, Hong Z, Ding J, Liu J, Zhang J, Chen S. Predominant release of lysosomal enzymes by newborn rat microglia after LPS treatment revealed by proteomic studies. J Proteome Res 7: 2033–2049, 2008. doi: 10.1021/pr7007779. [DOI] [PubMed] [Google Scholar]
- 51. Nikodemova M, Watters JJ. Outbred ICR/CD1 mice display more severe neuroinflammation mediated by microglial TLR4/CD14 activation than inbred C57Bl/6 mice. Neuroscience 190: 67–74, 2011. doi: 10.1016/j.neuroscience.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Guo W, Nagappan G, Lu B. Differential effects of transient and sustained activation of BDNF-TrkB signaling. Dev Neurobiol 78: 647–659, 2018. doi: 10.1002/dneu.22592. [DOI] [PubMed] [Google Scholar]
- 53. Marefati N, Beheshti F, Vafaee F, Barabadi M, Hosseini M. The effects of incensole acetate on neuro-inflammation, brain-derived neurotrophic factor and memory impairment induced by lipopolysaccharide in rats. Neurochem Res 46: 2473–2484, 2021. doi: 10.1007/s11064-021-03381-3. [DOI] [PubMed] [Google Scholar]
- 54. Suzuki S, Numakawa T, Shimazu K, Koshimizu H, Hara T, Hatanaka H, Mei L, Lu B, Kojima M. BDNF-induced recruitment of TrkB receptor into neuronal lipid rafts: roles in synaptic modulation. J Cell Biol 167: 1205–1215, 2004. doi: 10.1083/jcb.200404106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Marciante AB, Seven YB, Kelly MN, Perim RR, Mitchell GS. Daily fluctuations in spinal adenosine determine mechanisms of respiratory motor plasticity (Preprint). bioRxiv, 2022. doi: 10.1101/2022.12.15.520642. [DOI]
- 56. Assaife-Lopes N, Sousa VC, Pereira DB, Ribeiro JA, Sebastião AM. Regulation of TrkB receptor translocation to lipid rafts by adenosine A(2A) receptors and its functional implications for BDNF-induced regulation of synaptic plasticity. Purinergic Signal 10: 251–267, 2014. doi: 10.1007/s11302-013-9383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schnydrig S, Korner L, Landweer S, Ernst B, Walker G, Otten U, Kunz D. Peripheral lipopolysaccharide administration transiently affects expression of brain-derived neurotrophic factor, corticotropin and proopiomelanocortin in mouse brain. Neurosci Lett 429: 69–73, 2007. doi: 10.1016/j.neulet.2007.09.067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.