Keywords: G protein-coupled receptor, schistosomiasis, serotonin, smooth muscle, 5-HT2B receptor
Abstract
The anthelmintic drug praziquantel (PZQ) causes contraction of parasitic schistosomes as well as constriction of blood vessels within the mesenteric vasculature of the host where the adult blood flukes reside. The contractile action of PZQ on the vasculature is mediated by the activation of host serotonergic 5-HT2B receptors (5-HT2BRs). However, the molecular basis for PZQ interaction with these targets and the location of these 5-HT2B receptors in the vessel wall have not been experimentally defined. Evaluation of a PZQ docking pose within the 5-HT2BR orthosteric site, using both Ca2+ reporter and bioluminescence resonance energy transfer (BRET) assays, identified residues F3406.51 and F3416.52 (transmembrane helix 6, TM6) as well as L209EL2 (extracellular loop 2) as critical for PZQ-mediated agonist activity. A key determinant of PZQ selectivity for the 5-HT2B receptor over the 5-HT2A/2C receptors was determined by M2185.39 in transmembrane helix 5 (TM5) of the orthosteric site. Mutation of this residue to valine (M218V), as found in 5-HT2A and 5-HT2C, decreased PZQ agonist activity, whereas the reciprocal mutation (V215M) in 5-HT2C increased PZQ activity. Two-photon imaging in intact mesenteric arterial strips visualized PZQ-evoked Ca2+ transients within the smooth muscle cells of the vessel wall. PZQ also triggered cytoplasmic Ca2+ signals in arterial smooth muscle cells in primary culture that were isolated from mesenteric blood vessels. These data define the molecular basis for PZQ action on 5-HT2B receptors localized in vascular smooth muscle.
INTRODUCTION
The anthelmintic drug praziquantel (PZQ) is used to treat infections caused by parasitic flatworms (1). Notably, PZQ is the key clinical drug used for treating schistosomiasis, a chronic disease resulting from infection with blood flukes known as schistosomes (2, 3). PZQ effects a rapid, spastic paralysis of schistosome worms, with concomitant damage to the worm tegument that aids their immunological clearance from the infected host (1). As a clinical drug PZQ is conventionally used a racemic mixture [(±)-PZQ], with (R)-PZQ action on schistosomes occurring in response to PZQ concentrations in the submicromolar range (4). PZQ is also used to treat a variety of other trematode, cestode, and monogenean infections, with similar effects of PZQ observed on the parasite musculature and surface. The action of PZQ on the parasite is proposed to be mediated through activation of a large-conductance ion channel, known as TRPMPZQ, that is stereoselectively activated by (R)-PZQ with potency in the hundreds of nanomolar range (4–6).
Additionally, PZQ also exerts effects on receptors in the human host, independent of effects on the schistosome parasite. These effects have long been recognized and typically occur in the supramicromolar range (7–12). Some of these host activities are likely relevant to PZQ treatment of the clinical disease: to aid the clearance of the blood flukes or to ameliorate the inflammatory response to schistosome eggs deposited in host tissues. Although the sensitivity of such host responses to PZQ is low (occurring in the supramicromolar range, consistent with the good safety profile of PZQ), these effects may be pathophysiologically relevant, as PZQ concentrations in blood attain such values within the mesenteric and hepatic vasculature, where adult worms reside, before first-pass metabolism of the drug in the liver. One example is PZQ-evoked vasoconstriction of mesenteric arteries, which may contribute to the “hepatic shift” (13) of worms after PZQ treatment. This constriction of mesenteric vessels is mediated by (R)-PZQ interaction with an endogenous host G protein-coupled receptor (GPCR), the 5-hydroxytrptamine 2B receptor (5-HT2B receptor, Ref. 7). (R)-PZQ has been shown to bind to the binding pocket of the human 5-HT2B receptor, where it acts as a partial agonist with micromolar potency (EC50 = ∼8 µM; Ref. 7) as measured by Gq-mediated calcium mobilization in vitro. This action of PZQ is selective for the 5-HT2B receptor compared with 5-HT2A or 5-HT2C receptors, with mesenteric vessel contraction blocked by a selective 5-HT2B receptor antagonist (7).
The interaction of PZQ with the human 5-HT2B receptor is somewhat surprising considering that the 5-HT2B receptor is an aminergic GPCR and PZQ lacks a free amine. Molecular resolution of how a nonamine ligand such as PZQ can activate the 5-HT2B receptor is lacking. Therefore, experimental validation of the proposed PZQ binding pose in the 5-HT2B receptor (7) is important, as it will first, detail the molecular basis by which a nonaminergic chemotype engages a bioaminergic GPCR and second, illuminate how the selectivity of PZQ between different serotonergic GPCRs is achieved (in this case a preference for 5-HT2B over 5-HT2A or 5-HT2C receptors). Such insight will aid understanding of the molecular determinants that govern PZQ association within GPCRs. A further missing piece of data concerns the cellular target(s) of PZQ action in the mesenteric vasculature. In which cell type(s) are the 5-HT2B receptors that are activated by PZQ expressed? The effects of PZQ could be mediated through engagement of endothelial cells, via any other of the cellular components of the vessel wall (such as fibroblasts within the adventitia), through neurons of the mesenteric plexus, or via perivascular adipose tissue.
In this study, we investigated both of these issues by profiling PZQ action at various 5-HT2B receptor mutants to interrogate the (R)-PZQ binding pose and the basis for 5-HT2B selectivity, as well as by defining the cellular localization of PZQ action on 5-HT2B receptors in vascular smooth muscle.
METHODS
Materials and Reagents
Cell culture reagents were from ThermoFisher or Invitrogen. PZQ enantiomers, (R)-PZQ and (S)-PZQ, were resolved following published methods (14). Racemic praziquantel [(±)-PZQ] and other chemicals were sourced from Sigma-Aldrich. A mouse monoclonal anti-FLAG horseradish peroxidase (HRP)-conjugated antibody was sourced from Sigma (catalog no. A8592, RRID:AB_439702). The Flp-In T-REx tetracycline-inducible system was sourced from Invitrogen (catalog no. K6500-01) as well as the Flp-In-293 (catalog no. R750-07) and Flp-In T-REx-293 cell lines (catalog no. R780-07). HEK293 cell lines were authenticated by short tandem repeat (STR) profiling (ATCC) and were screened negative for mycoplasma contamination by monthly scheduled testing (LookOut Mycoplasma PCR Detection Kit; Sigma). The 5-HT2B receptor construct was generated by deleting the vasopressin 2 receptor COOH terminus, TEV cleavage site, and tTA sequence from receptor plasmids sourced from the PRESTO-TANGO library (Addgene kit no. 1000000068; Ref. 15), with the resulting plasmid used as the receptor construct for transfection in Ca2+ reporter and bioluminescence resonance energy transfer (BRET) assays. The Gαq-Rluc8 [Renilla luciferase (RLuc)] construct in pcDNA3.1 was generated in the McCorvy laboratory, pcDNA3.1-Gγ9-GFP2 was a gift from Bryan Roth (Addgene plasmid no. 140991; http://n2t.net/addgene:140991; RRID:Addgene_140991) (16), and pcDNA3.1-Gβ3 was purchased from the cDNA Resource Center (Bloomsburg, PA; catalog no. GNB0300000).
In Silico Modeling
Modeling studies were performed with the Schrodinger computational suite (v. 2021-1 or v. 2022-1) and the Maestro GUI. All modeling was performed with default settings unless otherwise noted. The human 5-HT2B receptor crystal structure in complex with lysergic acid diethylamide (LSD) (PDB: 5TVN) was prepared with the Protein Preparation Wizard and minimized in the OPLS4 force field at pH 7.4. (R)-PZQ and 5-HT were drawn in ChemDraw Professional (v. 2021.0.0), imported into the Maestro GUI (v. 13.1), and prepared with the LigPrep tool in the OPLS4 force field at pH 7.4. The output structures of these ligands were used for subsequent modeling. With a grid generated around the centroid of the ligand (LSD) in 5TVN, initial induced-fit docking (IFD) of (R)-PZQ and 5-HT was performed with the receptor and ligand van der Waals scaling set to 0.30. Residues were optimized within 8.0 Å of the ligand poses, and Glide redocking was performed with the SP protocol. Poses were manually examined, and the highest-ranking pose for each ligand that displayed interactions consistent with functional data was prioritized. Beginning with these initial poses, model refinement was performed for each ligand with IFD with default scaling settings using the XP protocol, a more precise algorithm, for Glide redocking. This iterative refinement resulted in the reported stable poses (see Figs. 1 and 4).
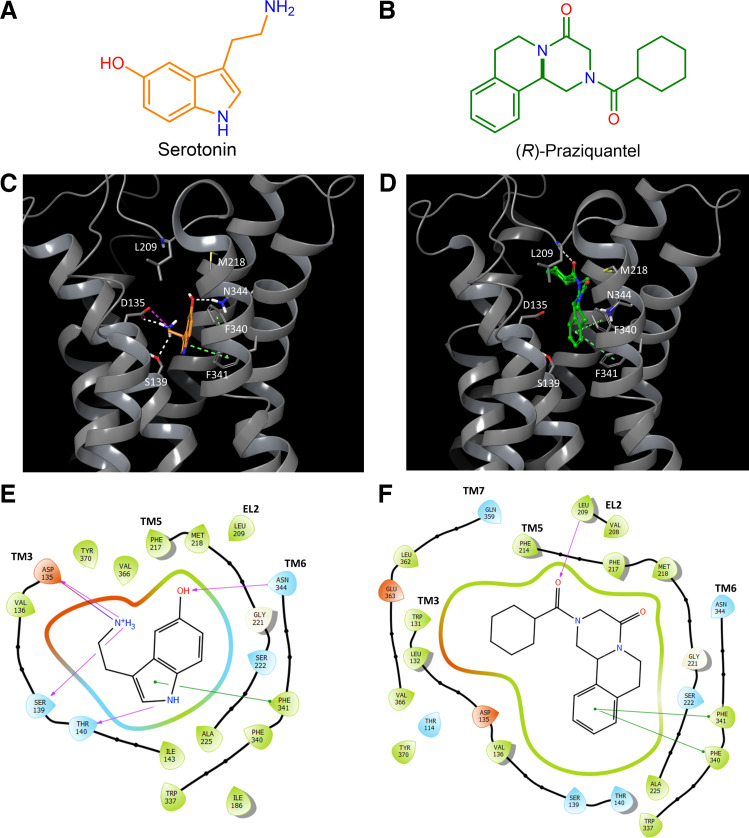
Figure 1.
Comparison of docking of serotonin (5-hydroxytryptamine, 5-HT) and (R)-praziquantel [(R)-PZQ] in the human 5-HT2B receptor. A and B: chemical structures of serotonin (orange; A) and (R)-PZQ (green; B). C: putative binding pose of 5-HT bound to the human 5-HT2B receptor by induced-fit docking. Hydrogen bonds are depicted as white dashes, salt bridges as purple dashes, and π-π or edge-π interactions as green dashes. 5-HT forms a salt bridge with D135, hydrogen bonds with S139 and N344, and π-π interactions with F341. F340 and F341 interact with each other via edge-π interactions. D: putative binding pose of (R)-PZQ bound to the human 5-HT2B receptor by induced-fit docking. The external carbonyl of (R)-PZQ forms a hydrogen bond to the backbone N-H of L209, and (R)-PZQ forms π-π interactions with F340 and F341. E and F: predicted residues that coordinate 5-HT (E) and (R)-PZQ (F) in their proposed binding poses. Hydrogen bonding interactions (purple), salt bridges (blue), and π-π or edge-π interactions (green) are highlighted. EL2, extracellular loop 2; TM, transmembrane helix.
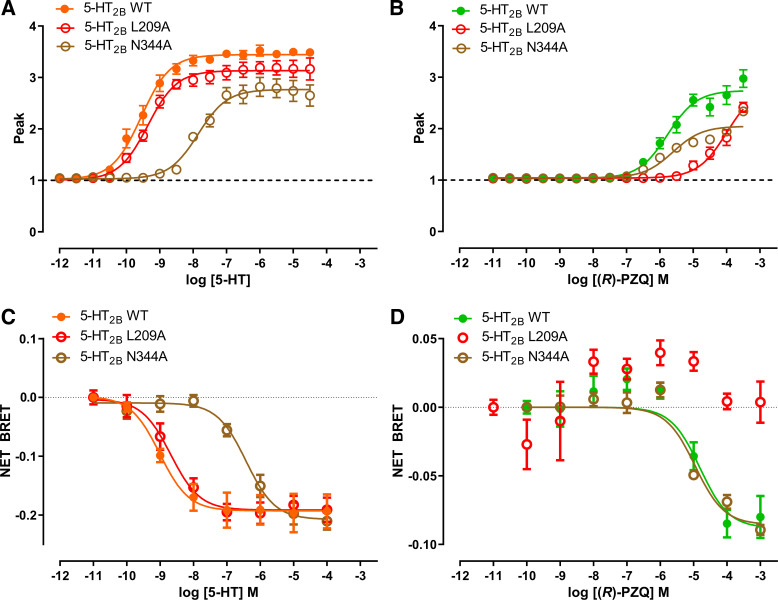
Figure 4.
Functional profiling of residues predicted to interact with the carbonyl groups of (R)-praziquantel [(R)-PZQ]. S139 mutants. A and B: effect of L209A and N344A point mutants on Ca2+ mobilization evoked by various concentrations of 5-hydroxytryptamine (5-HT; A) or (R)-PZQ (B). C and D: effect of L209A and N344A point mutants on bioluminescence resonance energy transfer (BRET) evoked by various concentrations of 5-HT (C) or (R)-PZQ (D). Data points represent mean ± SE from n = 3 independent assays, except for wild type (WT) in C and D (n = 4) and N344A in D (n = 2).
Generation of Stable Cell Lines
Point mutations were made by PCR mutagenesis within the wild-type 5-HT2B receptor (5-HT2BR) coding sequence cloned into a pcDNA5/FRT/TO expression vector (Invitrogen, K6510-20). Mutations were confirmed by sequencing (Retrogen). Stable cell lines expressing individual 5-HT2BR mutants were generated with the Flp-In T-REx-293 tetracycline-inducible system (Invitrogen). The parental cell line Flp-In-293 was routinely passaged in Dulbecco’s modified Eagle’s medium (DMEM) containing blasticidin (10 µg/mL) and Zeocin (100 µg/mL). For transfection, Flp-In TREx-293 cells were plated in 10-cm-diameter petri culture dishes in DMEM medium with no antibiotic selection. When cells reached 60–80% confluence, the Flp recombinase expression vector pOG44 (Invitrogen, V6055-20) and an individual 5-HT2BR-pcDNA5/FRT/TO construct were cotransfected at a 1-to-9 ratio with the Trans-IT2020 (Mirus) transfection reagent in Opti-MEM and left overnight. Medium was replaced 24 h after transfection with fresh medium containing no antibiotics. Cells were then split (48–72 h after transfection) and seeded at low density (∼25% confluence) in a 15-cm-diameter culture dish in DMEM. Antibiotic selection (penicillin 100 U/mL, streptomycin 100 μg/mL, hygromycin B 100 µg/mL, and blasticidin 10 µg/mL) was started when cells reached ∼50–60% confluence, typically within a week. Medium was changed every day during the first week of selection and then every 2–3 days for the following 2–4 wk. At confluence, cells were trypsinized and the polyclonal population replated to allow single colonies to be isolated, using a serial dilution method to generate multiple monoclonal clones for each 5-HT2BR mutant. Once an individual colony grew to confluence, it was passaged, induced (2 µg/mL tetracycline), and screened with a Fluorescence Imaging Plate Reader (FLIPRTETRA; Molecular Devices).
Ca2+ Flux Assay in Stable Cell Lines
Ca2+ flux assays for quantifying (R)-PZQ action at the wild-type 5-HT2B receptor and 5-HT2B receptor mutants were performed by using a FLIPRTETRA to monitor responses from the different tetracycline-induced stable cell lines described above. For 5-HT2B receptor expression, cells were induced (2 µg/mL tetracycline) and seeded in black‐walled clear‐bottomed poly-d-lysine-coated 384‐well plates (∼20,000 cells per well) in 20 μL of DMEM growth medium containing 1% dialyzed fetal bovine serum (dFBS). After 24–48 h, cells were loaded with Ca2+ indicator (20 μL of 2× Fluo‐4 Direct dye, Invitrogen F10471) containing probenecid (2.5 mM) by incubation (30 min at 37°C, followed by an additional 30 min at room temperature). Drug dilutions were prepared in assay buffer (HBSS 20 mM; HEPES, pH 7.4). After dye loading, the assay was performed at room temperature. Basal fluorescence was monitored for 20 s (1 read/s) and then throughout ligand stimulation for an additional 250 s. Peak fluorescence in each well was normalized as a maximum fold increase over the baseline. Changes in fluorescence amplitude were analyzed with the sigmoidal curve fitting function in GraphPad Prism (version 9.0.2; Dotmatics) to generate concentration-response curves and derive EC50 values. Results represent a minimum of three independent experiments for each construct.
Bioluminescence Resonance Energy Transfer Assays
HEK293T cells were maintained and passaged in DMEM medium (10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin, 37°C and 5% CO2). Cells were cotransfected with a 1:2:2:2 ratio of 5-HT2BR:Gα-Rluc8 (Renilla luciferase):Gβ:Gγ-GFP2 with a 1:3 DNA-to-TransIT-2020 (Mirus catalog no. 5405) ratio in Opti-MEM (Thermo-Fisher catalog no. 31985070). After 48 h, cells were harvested and plated in 200 µL of DMEM containing 1% dialyzed FBS at a density of ∼50,000 cells/well into poly-d-lysine-coated white, clear-bottomed 96-well assay plates (Greiner Bio-One). The next day, medium was gently decanted and cells washed twice with assay buffer (60 μL, 1× HBSS, 20 mM HEPES, pH 7.4). Assay buffer (60 μL) was replenished in each well, and cells were placed in a 37°C incubator to equilibrate before experimentation. The assay was initiated by drug addition (30 μL, 3× stock per well in assay buffer containing 0.3% BSA and 0.03% ascorbic acid) followed by incubation for 60 min. At a time point 15 min before reading, 10 μL of Renilla luciferase (RLuc) substrate (5 μM final concentration coelenterazine 400a; Nanolight Technologies) was added per well. Plates were read in a CLARIOstar Plus microplate reader (BMG LabTech) for at least three cycles after drug addition, with measurements from the sixth read used for analysis. Both luminescence values (λ = 400 nm) and green fluorescent protein (GFP) emission (λ = 515 nm) were measured for 1 s per well. The net BRET, the ratio GFP/RLuc, was calculated per well and plotted as a function of drug concentration with GraphPad Prism (v.9.0.2). Results represent a minimum of three independent experiments for each construct.
Cell Surface Expression Assay
An ELISA assay was used to quantify cell surface expression of each 5-HT2BR mutant. Individual Flp-In-293 monoclonal stable cell lines were induced with tetracycline (2 µg/mL) and plated on poly-lysine-coated white 384-well plates at a density of 15,0000 cells/well in plating medium (DMEM containing 1% dialyzed FBS). The next day, medium was decanted and cells were fixed in paraformaldehyde (PFA, 4%) in PBS (15 min at room temperature). Cells were then washed three times with PBS and incubated with 5% BSA (in PBS) for 30 min to block nonspecific antibody binding. Next, cells were incubated with a mouse monoclonal anti-FLAG HRP-conjugated antibody (1:10,000 dilution in PBS with 0.5% BSA; Sigma A8592) for 1 h at room temperature. To detect cell surface expression of each 5-HT2BR mutant, cells were washed three times with PBS (80 µL/well) and SuperSignal Pico substrate (prepared by combining enhancer and stable peroxide solutions at 1:1) was added to each plate (20 µL/well) and incubated in the dark at room temperature for 15 min. Luminescence values were measured over 20 read cycles on a MicroBeta TriLux and values expressed as counts per second (CPS) and then normalized to that of the wild-type 5-HT2B receptor control. Results represent a minimum of three independent experiments for each construct.
Measurements of Vascular Tone by Wire Myography
Responses to (±)-PZQ were measured in mouse mesenteric vessels isolated from female Swiss Webster mice (aged 10–13 wk; Charles River Laboratories) by wire myography. Female mice were used to enable future comparison to schistosome-infected mice, which are sourced as sole-sex infections. Second-order mesenteric vessel strips were isolated and equilibrated for ≥30 min in gassed (95% O2, 5% CO2) physiological saline solution (PSS, in mM: 130 NaCl, 4.7 KCl, 1.18 KH2PO4, 1.17 MgSO4, 14.9 NaHCO3, 5.5 dextrose, 0.026 EDTA, 1.6 CaCl2, pH 7.4 at 37°C) before experimentation. Four vessel strips were randomly selected and mounted on a four-channel myography system (DMT; Aarhus, Denmark), and after vessel equilibration as previously described (7) reactivity was measured under isometric conditions in response to high-potassium PSS (KPSS, in mM: 74.7 NaCl, 60 KCl, 1.18 KH2PO4, 1.17 MgSO4, 14.9 NaHCO3, 5.5 dextrose, 0.02 EDTA, 1.6 CaCl2, pH 7.4 at 37°C) or indicated ligands. If vessels did not show a robust contraction to KPSS, they were excluded from subsequent experimentation. Such attrition of nonresponsive strips was rarely observed (<5% samples). No comparative experiments were performed between cohorts, so blinding interventions were not relevant to the experimental design. All animal experiments followed ethical regulations approved by the University of Minnesota Institutional Animal Care and Use Committee (IACUC). Mice were euthanized with carbon dioxide, following American Veterinary Medical Association (AVMA) guidelines.
Confocal Ca2+ Imaging
Primary mesenteric artery smooth muscle cells (MASMCs) were isolated from wild-type female Swiss Webster mice (aged 10–13 wk; Charles River Laboratories), following an established protocol (17). Dissociated cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with high glucose, supplemented with 10% dialyzed fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 μg/mL), as a subconfluent culture and used for experiments within 10 days. The purity of the arterial smooth muscle cultures was verified by staining for α-actin (17) and through comparison of yield of fluorescent cells from a transgenic mouse expressing a red fluorescent protein under the control of the smooth muscle actin promoter (18). For live cell Ca2+ imaging, primary cultured MASMCs were seeded 1 day before imaging onto an eight-chambered coverglass slide (ThermoFisher, 155411PK) at a density of 2 × 104 cells per well. The following day, cells were washed with HBSS, incubated with Fluo-4 NW and probenecid (30 min, 37°C), and kept at room temperature for 30 min. Slides were mounted onto an Olympus IX81 microscope, and changes in fluorescence (λex = 488 nm; λem = 513 ± 15 nm band-pass filter) were monitored with a Yogokawa spinning disk confocal (CSU-X-M1N) and an Andor iXon Ultra 888 EMCCD camera. Results were expressed as a ratio (F/F0) of fluorescence at any given time (F) relative to fluorescence at time 0 before the addition of the drug (F0). Data represent mean ± standard error from n ≥ 3 independent experiments.
Two-Photon Imaging in Mesenteric Arteries
Naive mice (aged 12–20 wk; Charles River Laboratories) were used to isolate intact mesenteric arteries. Arteries were manually dissected in an ice-cold low-Ca2+ physiological saline solution (PSS, in mM: 140 NaCl, 0.1 CaCl2, 1.2 MgCl2, 4.5 KCl, 6 d-glucose, 10 HEPES; pH 7.4) and washed to remove excess tissue. Dissected mesenteric vessels were loaded with the high-affinity Ca2+ indicator Fluo-8H dye (AAT Bioquest; Kd for Ca2+ = 232 nM) supplemented with 0.05% Pluronic F-127. During loading, cells were incubated in the dark at room temperature for 1 h on a rotating shaker. After incubation, vessels were washed two or three times to remove extracellular dye, and all subsequent procedures were performed in PSS with 2 mM Ca2+ as previously described (19). Fluorescence from vessels was identified by two-photon laser-scanning microscopy (Upright Olympus Fluoview FV1000 equipped with Ti:sapphire laser tuned to 820 nm) and intracellular Ca2+ dynamics imaged with a ×25 (NA 1.05 and working distance 2 mm) water-immersion objective lens (XLPL25XWMP; Olympus). After collection of baseline fluorescence, (±)-PZQ (100 µM) was applied to the bath solution and fluorescence within different regions of interest monitored and analyzed as arbitrary units of fluorescence intensity (20).
Statistical Analyses
Statistical comparisons were performed with the Student’s t test, with details of significance levels and replication provided in individual figure legends. All data were analyzed with GraphPad Prism (version 9.0.2; Dotmatics), with criteria described previously for the different reported assays. Data are presented as means with standard deviation, unless specified otherwise.
RESULTS
Praziquantel ((±)-PZQ) was first identified as a human 5-HT2B receptor ligand from a virtual docking screen, with the interaction with 5-HT2B receptor validated by radioligand binding and functional assays (7). This result was surprising for two reasons. First, the ability of (±)-PZQ as a ligand that lacks a free amine group to bind a bioaminergic receptor was unexpected. Unlike 5-HT, PZQ does not contain a basic amine (Fig. 1, A and B). Second, although PZQ is a nonobvious activator of 5-HT2B receptors, it also displayed selectivity for engaging 5-HT2B receptors over the other closely related 5-HT family members, 5-HT2A and 5-HT2C receptors (7).
To understand the molecular basis for these observations, we investigated the binding pose of (R)-PZQ within the human 5-HT2B receptor. With available crystal structures of the human 5-HT2B receptor (21–24), the most likely binding pose of (R)-PZQ in the 5-HT2B receptor was defined by induced-fit docking. The originally predicted docking pose for (R)-PZQ (7) aligned well with the induced-fit docking of (R)-PZQ into the 5-HT2B receptor crystal structure. In comparison to binding of 5-HT (Fig. 1C), (R)-PZQ occupied a shallower position within the orthosteric binding pocket (Fig. 1D), engaging in a distinct binding pose compared with the binding pose of 5-HT (Fig. 1, E and F). The predicted 5-HT and (R)-PZQ docking poses are compared in Supplemental Fig. S1 (available at https://doi.org/10.6084/m9.figshare.21534708).
To interrogate the proposed (R)-PZQ binding pose, we generated a suite of point mutants within the 5-HT2B receptor binding pocket and examined their effect on (R)-PZQ activity with a Ca2+ mobilization assay. The Ca2+ assay provided a facile, high-signal to noise assay in which 5-HT elevated cytoplasmic Ca2+ with high sensitivity [EC50 for 5-HT at wild-type 5-HT2B receptor = 0.167 nM (pEC50 = 9.78 ± 0.08)] and robust signal (peak ΔF/F = 6.19 ± 0.49). The choice of mutations to be profiled was guided by the docking poses for 5-HT2B receptor activators (21–24) compared with (R)-PZQ (Fig. 1D). The expression of each binding pocket mutant was evaluated with an ELISA-based cell surface expression assay (Supplemental Fig. S2, available at https://doi.org/10.6084/m9.figshare.21534759). Data for the suite of mutants are aggregated in Table 1.
Table 1.
Functional profiling of 5-HT2B receptor mutants
| Mutant | Agonist | Assay | WT EC50 (pEC50 ± SE) | Mutant EC50 (pEC50 ± SE) | Δlog(Emax/EC50) |
|---|---|---|---|---|---|
| D135L | 5-HT | Ca2+ | 0.167 nM | no activity | n/a |
| (9.78 ± 0.08) | |||||
| (R)-PZQ | Ca2+ | 2,000 nM | 5,200 nM | −0.47 | |
| (5.70 ± 0.05) | (5.28 ± 0.11) | ||||
| 5-HT | BRET | 0.97 nM | no activity | n/a | |
| (9.01 ± 0.22) | |||||
| (R)-PZQ | BRET | 12,100 nM | 14,300 nM | −0.24 | |
| (4.92 ± 0.21) | (4.84 ± 0.20) | ||||
| D135N | 5-HT | Ca2+ | 0.167 nM | 754 nM | −3.68 |
| (9.78 ± 0.08) | (6.12 ± 0.04) | ||||
| (R)-PZQ | Ca2+ | 2,000 nM | 11,000 nM | −0.83 | |
| (5.70 ± 0.05) | (4.95 ± 0.04) | ||||
| 5-HT | BRET | 0.97 nM | 13,800 nM | −4.17 | |
| (9.01 ± 0.22) | (4.86 ± 0.10) | ||||
| (R)-PZQ | BRET | 12,100 nM | 26,500 nM | −0.88 | |
| (4.92 ± 0.21) | (4.58 ± 0.57) | ||||
| D135A | 5-HT | Ca2+ | 0.167 nM | 95,300 nM | −5.75 |
| (9.78 ± 0.08) | (4.02 ± 0.13) | ||||
| (R)-PZQ | Ca2+ | 2,000 nM | 141 nM | 1.12 | |
| (5.70 ± 0.05) | (6.85 ± 0.10) | ||||
| 5-HT | BRET | 0.97 nM | no activity | n/a | |
| (9.01 ± 0.22) | |||||
| (R)-PZQ | BRET | 12,100 nM | 374 nM | 1.32 | |
| (4.92 ± 0.21) | (6.43 ± 0.22) | ||||
| S139A | 5-HT | Ca2+ | 0.278 nM | 0.978 nM | −0.54 |
| (9.56 ± 0.06) | (9.01 ± 0.04) | ||||
| (R)-PZQ | Ca2+ | 1,720 nM | 247 nM | 0.91 | |
| (5.77 ± 0.08) | (6.61 ± 0.03) | ||||
| 5-HT | BRET | 0.97 nM | 35.0 nM | −1.32 | |
| (9.01 ± 0.22) | (7.46 ± 0.13) | ||||
| (R)-PZQ | BRET | 15,400 nM | 13,900 nM | 0.36 | |
| (4.81 ± 0.23) | (4.86 ± 0.15) | ||||
| S139C | 5-HT | Ca2+ | 0.278 nM | 1.85 nM | −0.89 |
| (9.56 ± 0.06) | (8.70 ± 0.18) | ||||
| (R)-PZQ | Ca2+ | 1,720 nM | 1,030 nM | 0.28 | |
| (5.77 ± 0.08) | (5.99 ± 0.06) | ||||
| 5-HT | BRET | 0.97 nM | 10.6 nM | −1.05 | |
| (9.01 ± 0.22) | (7.97 ± 0.19) | ||||
| (R)-PZQ | BRET | 15,400 nM | 11,100 nM | 0.32 | |
| (4.81 ± 0.23) | (4.95 ± 0.19) | ||||
| S139V | 5-HT | Ca2+ | 0.278 nM | 38.9 nM | −1.46 |
| (9.56 ± 0.06) | (7.40 ± 0.56) | ||||
| (R)-PZQ | Ca2+ | 1,720 nM | 1,530 nM | 0.13 | |
| (5.77 ± 0.08) | (5.82 ± 0.12) | ||||
| 5-HT | BRET | 0.97 nM | no activity | n/a | |
| (9.01 ± 0.22) | |||||
| (R)-PZQ | BRET | 15,400 nM | 6,600 nM | 0.59 | |
| (4.81 ± 0.23) | (5.18 ± 0.28) | ||||
| F340L | 5-HT | Ca2+ | 0.77 nM | 1,087 nM | −3.43 |
| (9.11 ± 0.14) | (5.96 ± 0.02) | ||||
| (R)-PZQ | Ca2+ | 3,310 nm | no activity | n/a | |
| (5.48 ± 0.11) | |||||
| F341L | 5-HT | Ca2+ | 0.77 nM | no activity | n/a |
| (9.11 ± 0.14) | |||||
| (R)-PZQ | Ca2+ | 3,310 nM | no activity | n/a | |
| (5.48 ± 0.11) | |||||
| L209A | 5-HT | Ca2+ | 0.278 nM | 0.441 nM | −0.24 |
| (9.56 ± 0.06) | (9.36 ± 0.08) | ||||
| (R)-PZQ | Ca2+ | 1,720 nM | >150 µM | < −2.00 | |
| (5.77 ± 0.08) | (<4.00) | ||||
| 5-HT | BRET | 0.972 nM | 2.09 nM | −0.34 | |
| (9.01 ± 0.22) | (8.68 ± 0.18) | ||||
| (R)-PZQ | BRET | 15,400 nM | no activity | n/a | |
| (4.81 ± 0.23) | |||||
| N344A | 5-HT | Ca2+ | 0.278 nM | 14.2 nM | −0.80 |
| (9.56 ± 0.06) | (7.85 ± 0.09) | ||||
| (R)-PZQ | Ca2+ | 1,720 nM | 2,480 nM | −0.29 | |
| (5.77 ± 0.08) | (5.61 ± 0.07) | ||||
| 5-HT | BRET | 0.972 nM | 378 nM | −2.56 | |
| (9.01 ± 0.22) | (6.42 ± 0.14) | ||||
| (R)-PZQ | BRET | 15,400 nM | 11,100 nM | 0.13 | |
| (4.81 ± 0.23) | (4.95 ± 0.14) |
EC50 values reported as mean (pEC50 ± SE) for the wild-type and mutant 5-HT2B receptor in response to 5-hydroxytryptamine (5-HT) and (R)-praziquantel [(R)-PZQ] are shown. Δlog(Emax/EC50) (where Emax is maximum effect) calculated as log(Emax/EC50)mutant − log(Emax/EC50)WT. Data represent n ≥ 3 independent experiments for all assays. BRET, bioluminescence resonance energy transfer; n/a, not available.
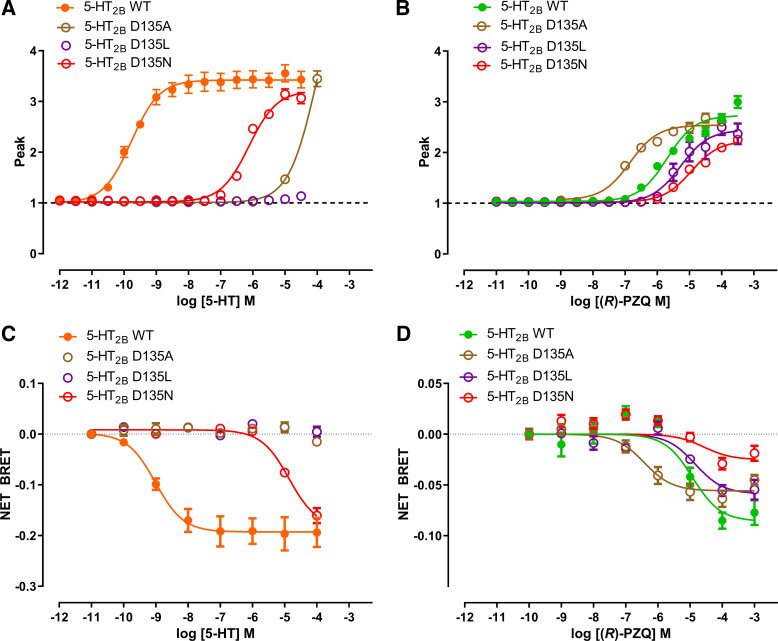
First, we focused on the highly conserved aspartic acid residue D1353.32 found at the base of the orthosteric binding pocket (Fig. 1, C and E). The engagement of this residue by the ionized amine of serotonin is critical to the binding of all serotonergic GPCRs by 5-HT (21–24). (R)-PZQ, which lacks an ionizable amine, was not predicted to engage D3.32 (Fig. 1, D and F). Mutation of D3.32 strongly impaired the action of 5-HT but not (R)-PZQ (Fig. 2). For example, D135L3.32 caused a complete loss in responsiveness to 5-HT, but potency of (R)-PZQ decreased only by approximately twofold (Fig. 2, A and B; Table 1). D135N3.32 decreased 5-HT potency by ∼5,000-fold but only an ∼4-fold shift in (R)-PZQ potency (Fig. 2, A and B; Table 1). Similarly, D135A3.32 strongly impaired 5-HT action but resulted in an increased potency for (R)-PZQ (Fig. 2, A and B; Table 1). The higher expression levels of the D135A3.32 construct (Supplemental Fig. S2) likely contribute to the increased potency of (R)-PZQ.
Figure 2.
Functional profiling of D135 mutants. A and B: effect of D3.32 (D135) point mutants on Ca2+ mobilization evoked by various concentrations of 5-hydroxytryptamine (5-HT; A) or (R)-praziquantel [(R)-PZQ; B]. C and D: effect of D3.32 (D135) point mutants on bioluminescence resonance energy transfer (BRET) evoked by various concentrations of 5-HT (C) or (R)-PZQ (D). Data points represent mean ± SE from n = 3 independent assays, except for wild type (WT) in C (n = 4) and wild type in D (n = 5).
To confirm the second messenger assay results, which are sensitive to receptor expression and reserve issues, we performed bioluminescence resonance energy transfer (BRET) assays to directly measure Gq protein dissociation from the wild-type 5-HT2B receptor and associated mutants (21, 25). Profiling the D3.32 mutants in the BRET assay (Fig. 2, C and D) yielded results similar to those seen in the Ca2+ assay (Fig. 2, A and B; Table 1). Whereas mutation of D3.32 strongly impaired 5-HT signaling (Fig. 2C), responses to (R)-PZQ were less impacted (Fig. 2D). This is consistent with the prediction that (R)-PZQ minimally interacts with D3.32, as it does not contain an amine group.
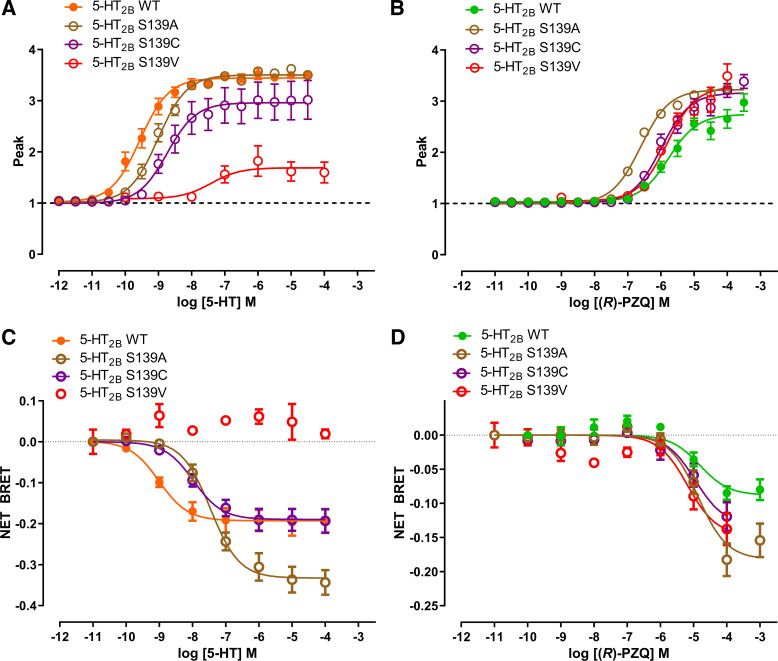
Next, we analyzed S1393.36, a residue that interacts with 5-HT at the base of the binding pocket. In the Ca2+ mobilization assay, mutants (S139A, S139C, and S139V) at this residue impaired 5-HT but not (R)-PZQ signaling (Fig. 3, A and B; Table 1). Similar results were also observed in the BRET assay with a rightward shift in potency of 5-HT but not (R)-PZQ (Fig. 3, C and D; Table 1). Again, interactions important for coordinating 5-HT deep in the binding pocket showed that S1393.36 is minimally involved with engagement of (R)-PZQ.
Figure 3.
Functional profiling of S139 mutants. A and B: effect of S3.36 (S139) point mutants on Ca2+ mobilization evoked by various concentrations of 5-hydroxytryptamine (5-HT; A) or (R)-praziquantel [(R)-PZQ; B]. C and D: effect of S3.36 (S139) point mutants on bioluminescence resonance energy transfer (BRET) evoked by various concentrations of 5-HT (C) or (R)-PZQ (D). Data points represent mean ± SE from n = 3 independent assays, except for wild type (WT) in C and D (n = 4) and S139V (n = 2).
The docking model for (R)-PZQ identified several interactions with the 5-HT2B receptor that are predicted to be important for PZQ molecular recognition (Fig. 1F). These comprised hydrophobic interactions with dual phenylalanine residues (F3406.51 and F3416.52) in transmembrane helix 6 (TM6) that establish π-π stacking with the core ring structures of PZQ, as also evident for other orthosteric 5-HT2B ligands (23, 26). In addition, the “external” carbonyl of (R)-PZQ formed a hydrogen bond with the backbone N-H of a leucine residue in the extracellular loop 2 (L209EL2). The importance of these interactions was assessed by mutagenesis. Mutation of either phenylalanine residue (F340L, F341L) inhibited 5-HT2B receptor activation in response to both 5-HT and (R)-PZQ (Table 1). This was unsurprising, as it is well established that ligands that activate the 5-HT2B receptor (e.g., LY266097, methylergonovine, methysergide, LSD) interact with F6.51 and F6.52 (21). In contrast, mutation of L209A inhibited (R)-PZQ action in both the Ca2+ reporter and BRET assays, but there was little effect of this mutation on the activity of 5-HT (Fig. 4; Table 1). Finally, no obvious residue interacted with the “internal” carbonyl of (R)-PZQ in the docking pose (Fig. 1). The nearest candidate residue was an asparagine in TM6 (N3446.55). However functional assessment of the N344A mutant revealed weak impact on (R)-PZQ potency in the Gq-mediated calcium mobilization assay, whereas 5-HT potency was substantially decreased by this mutation (Fig. 4; Table 1). In summary, these data confirm the importance of residues L209, F3406.51, and F3416.52 for (R)-PZQ engagement, consistent with both the induced-fit docking (Fig. 1) and a prior modeling study (7).
Selectivity of 5-HT2B toward (R)-PZQ
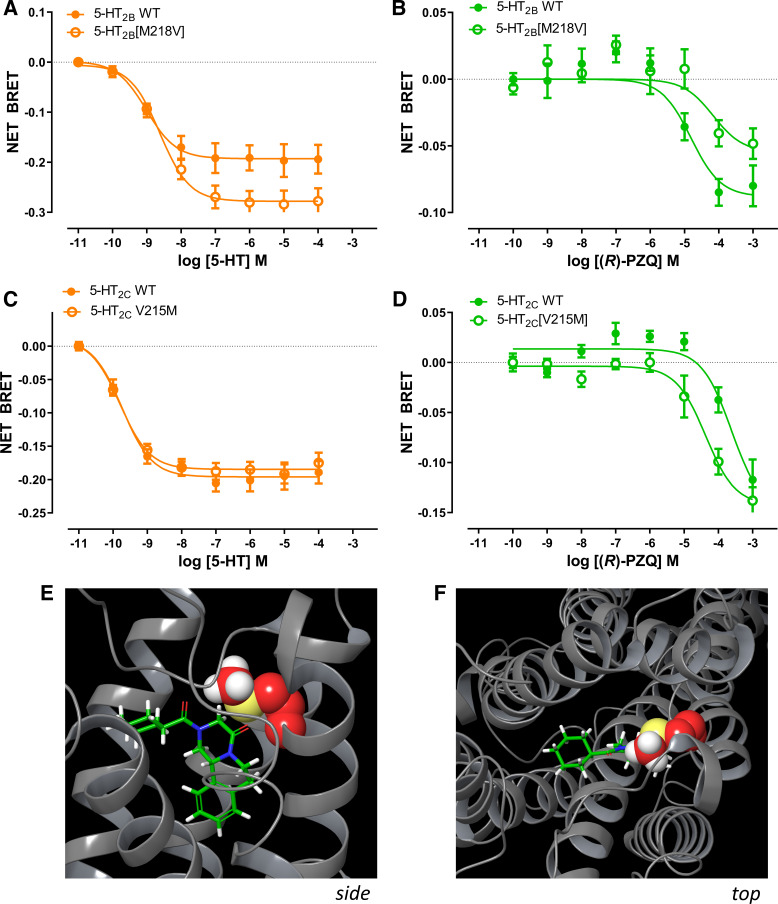
Why does (R)-PZQ display selectivity for the human 5-HT2B receptor? Based on the docking model (Fig. 1), we speculated that (R)-PZQ might be constrained within the peripheral binding pocket of 5-HT2B by a hydrophobic lid formed from two residues: M2185.39 and L2195.40 (in TM5). The methionine residue at position 5.39 is unique to the 5-HT2B receptor and is not conserved across the different human 5-HT2 receptor isoforms, being represented by a valine residue in 5-HT2A and 5-HT2C. By contrast, an aliphatic residue in TM5 (e.g., L219) is conserved across the 5-HT2 subtypes.
Consequently, we examined the effects of reciprocal mutations of 5-HT2B[M218V] and 5-HT2C[V215M] on both 5-HT and (R)-PZQ activity. The 5-HT2B[M218V] mutation caused a more pronounced decrease in (R)-PZQ activity compared with 5-HT, which still retained nanomolar potency (Fig. 5, A and B). In contrast, the 5-HT2C[V215M] mutant caused an increase in (R)-PZQ activity, with negligible effect on 5-HT potency (Fig. 5, C and D). These data suggest that the bulky methionine residue, unique to the 5-HT2B receptor, forms a hydrophobic cap over the binding pocket trapping (R)-PZQ within a shallow binding pose in the 5-HT2B receptor orthosteric pocket (Fig. 5, E and F). The smaller valine residue, present in 5-HT2A and 5-HT2C, fails to form a tight seal over (R)-PZQ in the binding pocket, facilitating ligand dissociation and poor affinity at these 5-HT receptor subtypes. This interaction is less important for 5-HT, as 5-HT binds deeper within the binding pocket. These data provide an explanation for the selectivity of (R)-PZQ toward the human 5-HT2B receptor.
Figure 5.
A–D: selectivity of (R)-praziquantel [(R)-PZQ] at the human 5-HT2B receptor is determined by M5.39 bioluminescence resonance energy transfer (BRET) assay for 5-hydroxytryptamine (5-HT; A and C) and (R)-PZQ (B and D) profiled against the human 5-HT2B receptor and the mutant 5-HT2B[M218V] (A and B) and the human 5-HT2C receptor and the mutant 5-HT2C[V215M] (C and D). Data points represent mean ± SE from n = 3 independent assays, except for wild type (WT; n = 4), M218V (n = 5) in A. E and F: side-on (E) and top-down (F) views of space-filling models depicting the “hydrophobic cap” over (R)-PZQ provided by M218 (M5.39) in the human 5-HT2B receptor as generated by induced-fit docking. M218 side chain carbons are depicted in red, sulfur in yellow, and hydrogens in white.
Overall, assays with the various 5-HT2B receptor point mutants were consistent with the (R)-PZQ binding pose within the human 5-HT2B receptor coordinated by L209 in EL2 and aromatic interactions with F6.51 and F6.52, but not requiring residues (D3.32, S3.36) deeper in the orthosteric binding pocket that mediate high-affinity binding of 5-HT.
Cellular Basis of PZQ Action in the Mesenteric Vasculature
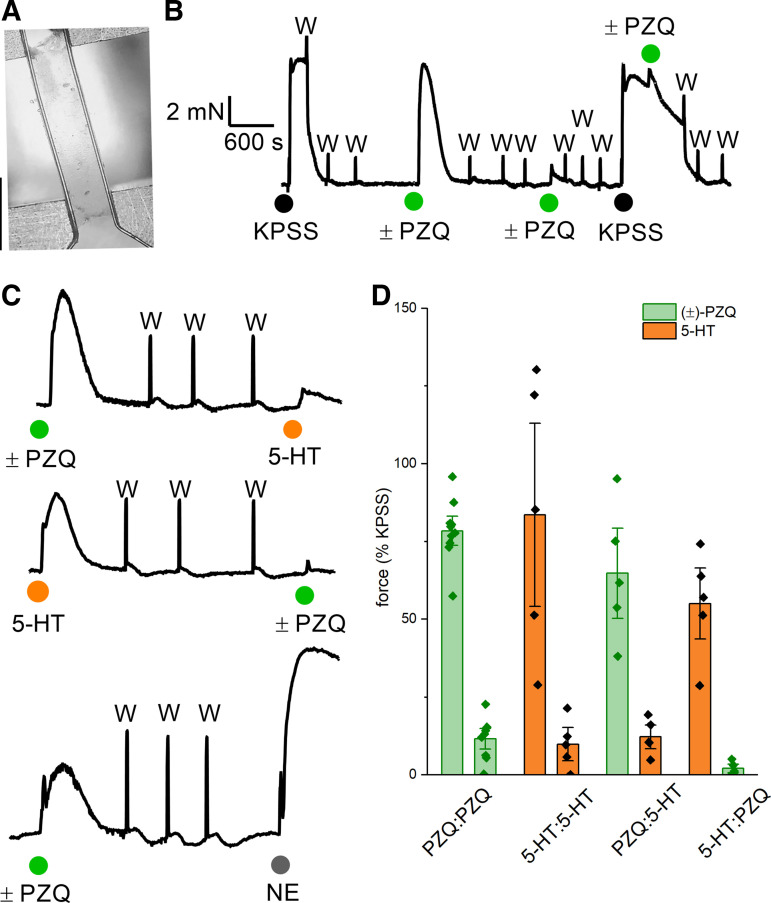
What cell type(s) are targeted by PZQ in the host mesenteric vasculature, where the adult blood flukes reside? Responses to (±)-PZQ and 5-HT were assessed by wire myography in mouse mesenteric arteries (Fig. 6A). In isolated vessels, the addition of (±)-PZQ caused a rapid increase in contractile tension. Responses to (±)-PZQ displayed kinetics similar to responses observed with 5-HT, which also elicited vasoconstriction (7). Application of a high concentration of (±)-PZQ desensitized subsequent responses to (±)-PZQ (Fig. 6B) as well as 5-HT (Fig. 6C), where the application of either ligand attenuated responses to subsequent application of the other. This behavior was not observed in the case of challenge with norepinephrine (Fig. 6C). Cumulative measurements from these dual-challenge experiments are shown in Fig. 6D.
Figure 6.
Contractile responses of mouse mesenteric artery strips measured by wire myography. A: representative image of a surgically isolated piece of mouse mesenteric artery held under tension between 2 clamped wires. Scale bar, 1 mm. B: representative trace of contractile responses of mouse mesenteric artery to repeated challenges with high-potassium solution (KPSS) and racemic praziquantel [(±)-PZQ, 100 µM; green]. Application of KPSS served as a normalization control defining the maximum active tension attainable in each vessel strip. C: examples of vessel tension in dual-challenge experiments where the initial response to (±)-PZQ (100 µM, green) or 5-hydroxytryptamine (5-HT, 10 µM; orange) was compared to subsequent challenges with 5-HT (top), (±)-PZQ (middle), or norepinephrine (NE, 1 µM; bottom). D: cumulative data set reporting peak tension in response to primary and secondary challenges of (±)-PZQ (100 µM; green) or 5-HT (10 µM; orange). Data points show mean ± SD from measurements on different vessel strips.
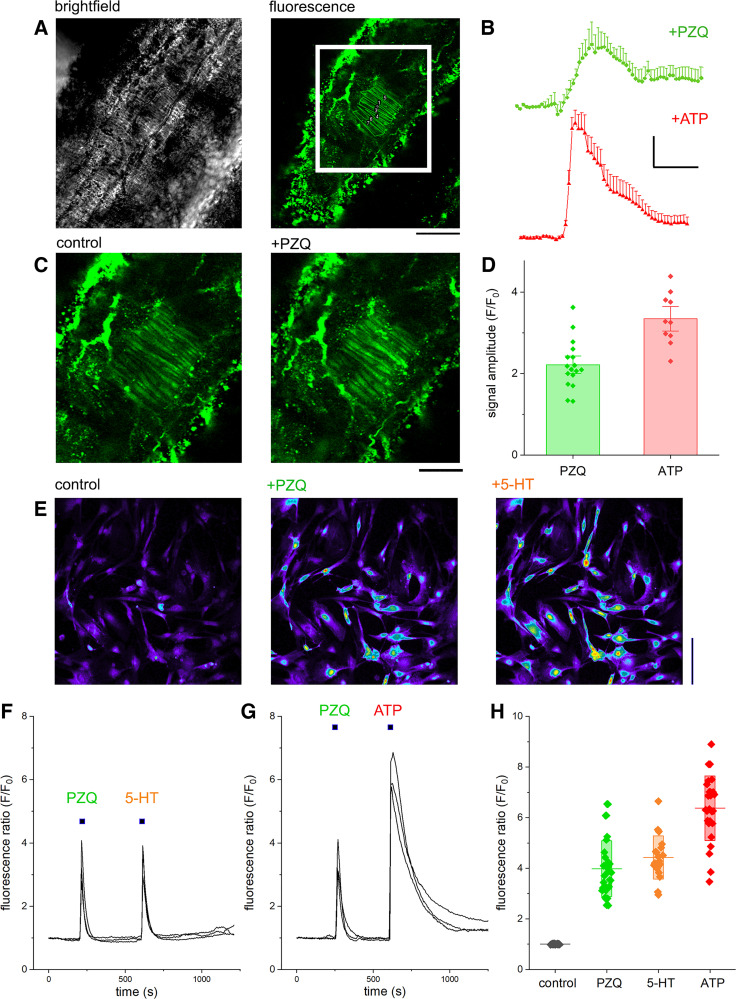
To identify the cellular basis for (±)-PZQ action, isolated vessel strips were imaged by two-photon microscopy. After incubation of vessel strips with a high-affinity Ca2+ indicator (Fluo-8H), fluorescence from a layer of tissue comprising mesenteric artery smooth muscle cells (MASMCs), endothelial cells, and fibroblasts was visualized. Representative bright-field and fluorescence images from an isolated mesenteric artery are shown in Fig. 7A. The addition of (±)-PZQ (100 µM) to the bath solution evoked a Ca2+ signal in multiple MASMCs as indicated by a rapid increase in cytoplasmic fluorescence (Fig. 7B), whereas (±)-PZQ did not evoke any signals in the endothelial or fibroblast sublayers of the vessel. Vessel viability was confirmed by the application of ATP (10 µM), which caused an increased in MASMC fluorescence following activation of endogenous purinoreceptors (Fig. 7C). Endogenous Ca2+ signals evoked by ATP were significantly higher in amplitude than those observed with (±)-PZQ (Fig. 7D). These assays demonstrated that (±)-PZQ caused Ca2+ transients within the vascular smooth muscle cells of mouse mesenteric arteries.
Figure 7.
Ca2+ imaging of praziquantel (PZQ) action in mesenteric artery smooth muscle cells (MASMCs). A: ex vivo analysis of intracellular Ca2+ dynamics in mesenteric vessels by 2-photon microscopy. Left: bright-field image of isolated mouse mesenteric artery strip, together with baseline fluorescence (Fluo-8H) at chosen 2-photon imaging plane (right). Boxed region containing smooth muscle cells is enlarged in B. Scale bar, 150 μm. B: an example of fluorescence signals recorded in smooth muscle cells during addition of racemic PZQ [(±)-PZQ, 100 µM; black] or ATP (10 µM; red). Scale bar, 20 s (horizontal), F/F0 = 1 (fluorescence/fluorescence at time = 0, F/F0, vertical). C: representative images of intracellular Ca2+ dynamics in smooth muscle cells before (left) and after (right) addition of (±)-PZQ (100 µM). Scale bar, 85 μm. D: statistical analyses summarizing differences in peak amplitude of fluorescent transient in the individual SMCs (n ≥ 10 cells from at least 3 different vessels. E: representative images of isolated primary mouse MASMCs loaded with the fluorescent Ca2+ indicator Fluo-4 NW before and after treatment with (±)-PZQ (100 µM) or 5-hydroxytryptamine (5-HT; 1 µM). Scale bar, 100 μm. F and G: fluorescence traces over time detailing responses to (±)-PZQ (100 µM), 5-HT (1 µM), and ATP (100 µM). H: cumulative measurements of peak fluorescence ratio (F/F0) from Ca2+ imaging experiments from primary MASMCs. Plot shows individual data points, together with mean (line) and SD (shaded bar) for PZQ (green), 5-HT (orange), and ATP (red) from 3 independent experiments.
To confirm the action of (±)-PZQ on vascular smooth muscle cells, we performed a series of experiments using isolated, cultured primary MASMCs. With an established protocol for isolation and short-term culture of MASMCs (17), subconfluent primary cultures of vascular smooth muscle cells were prepared and loaded with fluo-4. Cultures were challenged with various agonists while fluorescence was monitored by confocal microscopy (Fig. 7E). Addition of (±)-PZQ evoked cytoplasmic Ca2+ signals in isolated MASMCs (Fig. 7E), with the cells that responded to (±)-PZQ also responding to 5-HT (Fig. 7F). Just as observed during intact tissue imaging, the peak amplitude of Ca2+ signals evoked by ATP was higher than those evoked by (±)-PZQ (Fig. 7G). Based on these data, we conclude that (±)-PZQ-evoked Ca2+ signals in mouse mesenteric vessels are mediated through direct activation of MASMCs. This conclusion was also consistent with results from other cell types. Application of (R)-PZQ failed to elicit Ca2+ signals in human prostatic carcinoma cells (PC-3), human umbilical vein endothelial cells (HUVECs), or human dermal microvascular endothelial cells (HMEC-1) (Supplemental Fig. S3, available at https://doi.org/10.6084/m9.figshare.21717917). However, (R)-PZQ-evoked Ca2+ signals were resolved in rat renal artery smooth muscle cells, and these signals were blocked by preincubation with a 5-HT2B receptor antagonist, SB204741 (Supplemental Fig. S3), just as observed in mesenteric vessels (7).
On the basis of these different approaches, we conclude that vasoconstrictive action of (±)-PZQ on mesenteric vasculature is mediated by a direct action of the antiparasitic drug on host 5-HT receptors localized within smooth muscle cells.
DISCUSSION
Here we interrogated the molecular basis for (R)-PZQ association with, and selectivity toward, the human 5-HT2B receptor. As (R)-PZQ lacks a free amine, the association of (R)-PZQ with a bioaminergic receptor was unexpected. Therefore, the definition of the binding mode of (R)-PZQ and the basis underpinning selectivity between these highly homologous GPCRs is of interest. This situation is somewhat reminiscent of the discovery of salvinorin A, a nonnitrogenous ligand that displayed selective agonism at the κ-opioid receptor over all other opioid GPCR subtypes (27) as well as adenosine-based analogs that act as 5-HT2B receptor antagonists (28, 29). To our knowledge, (R)-PZQ is the first 5-HT2B receptor agonist that lacks a basic amine.
(R)-PZQ was shown to occupy an extended binding pocket within the 5-HT2B receptor (Supplemental Fig. S1), which was distinct from the key interactions of 5-HT that confer recognition for GPCR activation (Fig. 1). These include a lack of interaction with residues at the base of the binding pocket, specifically the charged residue D3.32 (which interacts with the amino group of 5-HT) and the polar residue S3.36 (which docking suggests may interact with the indole of 5-HT). Instead, (R)-PZQ was coordinated via a hydrogen bond interaction between the external carbonyl of (R)-PZQ and the backbone of L209 (in extracellular loop 2, EL2) and π-π interactions between the core rings of PZQ and residues F3406.51 and F3416.52. L209 has been identified as a key residue for binding the psychedelic LSD, acting as a “latch” to regulate the dynamics of EL2 residues that function as a hydrophobic lid regulating ligand association and dissociation from the binding cavity (24). Our data implicate M2185.39 as a component of this lid, with this bulky residue forming a tight seal that constrains (R)-PZQ within the orthosteric pocket. In other 5-HT2 receptors, residue 5.39 is represented by a smaller valine residue (V235 in 5-HT2A, V215 in 5-HT2C), such that (R)-PZQ has better opportunity to dissociate from the binding pocket. These data provide a molecular explanation for the observed selectivity of (R)-PZQ toward the human 5-HT2B receptor.
Compared with the interactions that mediate high-affinity (R)-PZQ engagement of its binding site in the parasite ion channel TRPMPZQ (5), the interactions of (R)-PZQ with the human 5-HT2B receptor differ in key respects, lacking the required hydrophobic environment around both the cyclohexyl ring and core ring constituents of (R)-PZQ, as well as an identified hydrogen bonding interaction that coordinates the internal carbonyl group. This suite of interactions underpins the high potency of (R)-PZQ action at TRPMPZQ. Furthermore, the projections of polar (S3.36) and charged (D3.32) groups into the 5-HT2B receptor binding pocket are likely detrimental to PZQ association, as supported by recent molecular dynamic simulations exploring how the orientation of acidic residues at the base of the TRPMPZQ binding pocket control (R)-PZQ sensitivity. Specifically, the projection of a charged residue into the binding pocket decreased (R)-PZQ occupancy (30). This is consistent with the observation here that neutralization of these charges increased (R)-PZQ potency (Fig. 2 and Fig. 3). If a parasite GPCR were to serve as a high-affinity target for (R)-PZQ, one would predict that either the ligand binding pocket would need to be considerably more lipophilic or the ligand binding pose avoid interaction with charged binding pocket residues. For high-affinity binding, the binding pocket must also present correctly oriented hydrogen bonding partners to coordinate both the carbonyl groups of (R)-PZQ. The nonoptimal nature of these interactions in the human 5-HT2B receptor underpins the >10-fold lower potency of (R)-PZQ at this human GPCR (low micromolar) compared with Sm.TRPMPZQ. Systemic 5-HT2B receptors will not be activated by circulating (R)-PZQ owing to the low affinity of this interaction relative to plasma concentrations of (R)-PZQ after first-pass metabolism. This is consistent with the excellent safety profile of this antiparasitic drug. (R)-PZQ concentrations in the mesenteric vasculature are, however, sufficient to activate endogenous 5-HT2B receptors (Fig. 6), before first-pass metabolism in the liver.
Having established the molecular basis for (R)-PZQ engagement of 5-HT2B receptors, we addressed the cellular locale of PZQ action on mesenteric arteries. This was demonstrated to be vascular smooth muscle by imaging (±)-PZQ-evoked responses in primary arterial smooth muscle cultures isolated from mouse mesenteric arteries as well as by direct visualization of (±)-PZQ-evoked Ca2+ transients in freshly isolated vessels (Fig. 7, A–D). We contend that (±)-PZQ-evoked vasoconstriction is mediated via (±)-PZQ engagement of vascular myocyte 5-HT2B receptors. The activation of 5-HT2B receptors in mesenteric smooth muscle by (±)-PZQ is consistent with the known contractile function of 5-HT2B receptors in other types of muscle (31–33). Likely (±)-PZQ-engagement of 5-HT2B receptors in different types of smooth muscles and cardiac muscle underpins the previously observed effects of (±)-PZQ in various isolated tissues (9, 10). The selectivity of (±)-PZQ toward 5-HT2B receptors may prove to be a valuable tool for assessing the relative contribution of different 5-HT receptor subtypes to these physiological responses. These data are also consistent with prior observations of 5-HT2B receptor expression in mesenteric vessels (34, 35) and a role for 5-HT2B receptor-mediated contraction of mesenteric arteries (36–40). As 5-HT2B receptor expression also depends on the state of progression of different diseases, including hypertension (36–40), the effects of (±)-PZQ on blood vessels may increase throughout the progression of chronic schistosomiasis. This would be consistent with observations that mice infected with Schistosoma mansoni exhibit a higher vascular reactivity to 5-HT (41, 42). Furthermore, given the role played by 5-HT2B receptors in hepatic fibrosis and liver regeneration (43–46), it will be of interest to probe whether any antifibrotic action of (±)-PZQ during treatment of chronic infections is mediated via engagement of the host serotonergic signaling pathways. This would represent a tractable pathway for therapeutic intervention to combat the chronic sequelae of schistosomiasis resulting from fibrotic responses to worm eggs deposited in host tissues.
In conclusion, this work has demonstrated that the host vasoactivity of (±)-PZQ is mediated by a direct action of this antiparasitic ligand on mesenteric artery vascular smooth muscle cells. This action depends on the ability of (R)-PZQ to engage host 5-HT2B receptors, acting as a noncanonical ligand that elevates myocyte cytoplasmic [Ca2+] to cause vasoconstriction.
DATA AVAILABILITY
Data will be made available upon reasonable request.
SUPPLEMENTAL MATERIAL
Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.21534708.
Supplemental Fig. S2: https://doi.org/10.6084/m9.figshare.21534759.
Supplemental Fig. S3: https://doi.org/10.6084/m9.figshare.21717917.
GRANTS
Work was supported by the National Institutes of Health (R01-AI145871 to J.S.M. and R35-GM133421 to J.D.M). D.J.S. acknowledges support from the NIH (T32 HL134643) and the MCW Cardiovascular Center’s A.O. Smith Fellowship Scholars Program. N.A.Y. was supported by NIH F31‐AI145091. O.P. acknowledges endowed funds from the South Carolina SmartState Centers of Excellence.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.D.M. and J.S.M. conceived and designed research; N.A.Y., J.K.L., D.J.S., and O.P. performed experiments; N.A.Y., J.K.L., D.J.S., and O.P. analyzed data; N.A.Y., J.K.L., D.J.S., O.P., and J.D.M. interpreted results of experiments; N.A.Y., J.K.L., D.J.S., O.P., and J.S.M. prepared figures; J.S.M. drafted manuscript; N.A.Y., J.K.L., D.J.S., O.P., J.D.M., and J.S.M. edited and revised manuscript; N.A.Y., J.K.L., D.J.S., O.P., J.D.M., and J.S.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank neighboring laboratories at the Medical College of Wisconsin (MCW) (Stucky, Beyer, and Sorokin laboratories) for providing samples of various cell lines for Ca2+ imaging experiments and acknowledge the computational resources and technical support provided by the Research Computing Center at MCW.
The graphical abstract was created with BioRender.com and published with permission.
REFERENCES
- 1. Andrews P, Thomas H, Pohlke R, Seubert J. Praziquantel. Med Res Rev 3: 147–200, 1983. doi: 10.1002/med.2610030204. [DOI] [PubMed] [Google Scholar]
- 2. McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ, Zhou XN. Schistosomiasis. Nat Rev Dis Primers 4: 13, 2018. doi: 10.1038/s41572-018-0013-8. [DOI] [PubMed] [Google Scholar]
- 3. Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet 383: 2253–2264, 2014. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Park SK, Gunaratne GS, Chulkov EG, Moehring F, McCusker P, Dosa PI, Chan JD, Stucky CL, Marchant JS. The anthelmintic drug praziquantel activates a schistosome transient receptor potential channel. J Biol Chem 294: 18873–18880, 2019. doi: 10.1074/jbc.AC119.011093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park SK, Friedrich L, Yahya NA, Rohr CM, Chulkov EG, Maillard D, Rippmann F, Spangenberg T, Marchant JS. Mechanism of praziquantel action at a parasitic flatworm ion channel. Sci Transl Med 13: eabj5832, 2021. doi: 10.1126/scitranslmed.abj5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park SK, Marchant JS. The journey to discovering a flatworm target of praziquantel: a long TRP. Trends Parasitol 36: 182–194, 2020. doi: 10.1016/j.pt.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chan JD, Cupit PM, Gunaratne GS, McCorvy JD, Yang Y, Stoltz K, Webb TR, Dosa PI, Roth BL, Abagyan R, Cunningham C, Marchant JS. The anthelmintic praziquantel is a human serotoninergic G-protein-coupled receptor ligand. Nat Commun 8: 1910, 2017. doi: 10.1038/s41467-017-02084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chan JD, Day TA, Marchant JS. Coalescing beneficial host and deleterious antiparasitic actions as an antischistosomal strategy. eLife 7: e35755, 2018. doi: 10.7554/eLife.35755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chubb JM, Bennett JL, Akera T, Brody TM. Effects of praziquantel, a new anthelmintic, on electromechanical properties of isolated rat atria. J Pharmacol Exp Ther 207: 284–293, 1978. [PubMed] [Google Scholar]
- 10. Jim K, Triggle DJ. Actions of praziquantel and 1-methyladenine in guinea-pig ileal longitudinal muscle. Can J Physiol Pharmacol 57: 1460–1462, 1979. doi: 10.1139/y79-217. [DOI] [Google Scholar]
- 11. Liu J, Kong D, Qiu J, Xie Y, Lu Z, Zhou C, Liu X, Zhang R, Wang Y. Praziquantel ameliorates CCl4-induced liver fibrosis in mice by inhibiting TGF-beta/Smad signalling via upregulating Smad7 in hepatic stellate cells. Br J Pharmacol 176: 4666–4680, 2019. doi: 10.1111/bph.14831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pinlaor S, Hiraku Y, Yongvanit P, Tada-Oikawa S, Ma N, Pinlaor P, Sithithaworn P, Sripa B, Murata M, Oikawa S, Kawanishi S. iNOS-dependent DNA damage via NF-kappaB expression in hamsters infected with Opisthorchis viverrini and its suppression by the antihelminthic drug praziquantel. Int J Cancer 119: 1067–1072, 2006. doi: 10.1002/ijc.21893. [DOI] [PubMed] [Google Scholar]
- 13. Buttle GA, Khayyal MT. Rapid hepatic shift of worms in mice infected with Schistosoma mansoni after a single injection of tartar emetic. Nature 194: 780–781, 1962. doi: 10.1038/194780b0. [DOI] [PubMed] [Google Scholar]
- 14. Woelfle M, Seerden JP, de Gooijer J, Pouwer K, Olliaro P, Todd MH. Resolution of praziquantel. PLoS Negl Trop Dis 5: e1260, 2011. doi: 10.1371/journal.pntd.0001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kroeze WK, Sassano MF, Huang XP, Lansu K, McCorvy JD, Giguère PM, Sciaky N, Roth BL. PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat Struct Mol Biol 22: 362–369, 2015. doi: 10.1038/nsmb.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Olsen RH, DiBerto JF, English JG, Glaudin AM, Krumm BE, Slocum ST, Che T, Gavin AC, McCorvy JD, Roth BL, Strachan RT. TRUPATH, an open-source biosensor platform for interrogating the GPCR transducerome. Nat Chem Biol 16: 841–849, 2020. doi: 10.1038/s41589-020-0535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Golovina VA, Blaustein MP. Preparation of primary cultured mesenteric artery smooth muscle cells for fluorescent imaging and physiological studies. Nat Protoc 1: 2681–2687, 2006. doi: 10.1038/nprot.2006.425. [DOI] [PubMed] [Google Scholar]
- 18. Shui B, Lee JC, Reining S, Lee FK, Kotlikoff MI. Optogenetic sensors and effectors: CHROMus—the Cornell Heart Lung Blood Institute Resource for Optogenetic Mouse Signaling. Front Physiol 5: 428, 2014. doi: 10.3389/fphys.2014.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klemens CA, Chulkov EG, Wu J, Hye Khan MA, Levchenko V, Flister MJ, Imig JD, Kriegel AJ, Palygin O, Staruschenko A. Loss of chloride channel 6 (CLC-6) affects vascular smooth muscle contractility and arterial stiffness via alterations to Golgi calcium stores. Hypertension 77: 582–593, 2021. doi: 10.1161/HYPERTENSIONAHA.120.16589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Palygin O, Miller B, Ilatovskaya DV, Sorokin A, Staruschenko A. Two-photon imaging of endothelin-1-mediated intracellular Ca2+ handling in smooth muscle cells of rat renal resistance arteries. Life Sci 159: 140–143, 2016. doi: 10.1016/j.lfs.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McCorvy JD, Wacker D, Wang S, Agegnehu B, Liu J, Lansu K, Tribo AR, Olsen RH, Che T, Jin J, Roth BL. Structural determinants of 5-HT2B receptor activation and biased agonism. Nat Struct Mol Biol 25: 787–796, 2018. doi: 10.1038/s41594-018-0116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wacker D, Wang C, Katritch V, Han GW, Huang XP, Vardy E, McCorvy JD, Jiang Y, Chu M, Siu FY, Liu W, Xu HE, Cherezov V, Roth BL, Stevens RC. Structural features for functional selectivity at serotonin receptors. Science 340: 615–619, 2013. doi: 10.1126/science.1232808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang C, Jiang Y, Ma J, Wu H, Wacker D, Katritch V, Han GW, Liu W, Huang XP, Vardy E, McCorvy JD, Gao X, Zhou XE, Melcher K, Zhang C, Bai F, Yang H, Yang L, Jiang H, Roth BL, Cherezov V, Stevens RC, Xu HE. Structural basis for molecular recognition at serotonin receptors. Science 340: 610–614, 2013. doi: 10.1126/science.1232807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wacker D, Wang S, McCorvy JD, Betz RM, Venkatakrishnan AJ, Levit A, Lansu K, Schools ZL, Che T, Nichols DE, Shoichet BK, Dror RO, Roth BL. Crystal structure of an LSD-bound human serotonin receptor. Cell 168: 377–389.e12, 2017. doi: 10.1016/j.cell.2016.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaczor AA, Makarska-Bialokoz M, Selent J, de la Fuente RA, Martí-Solano M, Castro M. Application of BRET for studying G protein-coupled receptors. Mini Rev Med Chem 14: 411–425, 2014. doi: 10.2174/1389557514666140428113708. [DOI] [PubMed] [Google Scholar]
- 26. McCorvy JD, Roth BL. Structure and function of serotonin G protein-coupled receptors. Pharmacol Ther 150: 129–142, 2015. doi: 10.1016/j.pharmthera.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, Rothman RB. Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc Natl Acad Sci USA 99: 11934–11939, 2002. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tosh DK, Ciancetta A, Warnick E, Crane S, Gao ZG, Jacobson KA. Structure-based scaffold repurposing for G protein-coupled receptors: transformation of adenosine derivatives into 5HT2B/5HT2C serotonin receptor antagonists. J Med Chem 59: 11006–11026, 2016. doi: 10.1021/acs.jmedchem.6b01183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Paoletta S, Tosh DK, Salvemini D, Jacobson KA. Structural probing of off-target G protein-coupled receptor activities within a series of adenosine/adenine congeners. PLoS One 9: e97858, 2014. doi: 10.1371/journal.pone.0097858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rohr CM, Sprague DJ, Park SK, Malcolm NJ, Marchant JS. Natural variation in the binding pocket of a parasitic flatworm TRPM channel resolves the basis for praziquantel sensitivity. Proc Natl Acad Sci USA 120: e2217732120, 2023. doi: 10.1073/pnas.2217732120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Borman RA, Tilford NS, Harmer DW, Day N, Ellis ES, Sheldrick RL, Carey J, Coleman RA, Baxter GS. 5-HT2B receptors play a key role in mediating the excitatory effects of 5-HT in human colon in vitro. Br J Pharmacol 135: 1144–1151, 2002. doi: 10.1038/sj.bjp.0704571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baxter GS, Murphy OE, Blackburn TP. Further characterization of 5-hydroxytryptamine receptors (putative 5-HT2B) in rat stomach fundus longitudinal muscle. Br J Pharmacol 112: 323–331, 1994. doi: 10.1111/j.1476-5381.1994.tb13072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jahnel U, Rupp J, Ertl R, Nawrath H. Positive inotropic response to 5-HT in human atrial but not in ventricular heart muscle. Naunyn Schmiedebergs Arch Pharmacol 346: 482–485, 1992. doi: 10.1007/BF00169000. [DOI] [PubMed] [Google Scholar]
- 34. Davis RP, Pattison J, Thompson JM, Tiniakov R, Scrogin KE, Watts SW. 5-Hydroxytryptamine (5-HT) reduces total peripheral resistance during chronic infusion: direct arterial mesenteric relaxation is not involved. BMC Pharmacol 12: 4, 2012. doi: 10.1186/1471-2210-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Watts SW, Darios ES, Seitz BM, Thompson JM. 5-HT is a potent relaxant in rat superior mesenteric veins. Pharmacol Res Perspect 3: e00103, 2015. doi: 10.1002/prp2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Watts SW, Fink GD. 5-HT2B-receptor antagonist LY-272015 is antihypertensive in DOCA-salt-hypertensive rats. Am J Physiol Heart Circ Physiol 276: H944–H952, 1999. doi: 10.1152/ajpheart.1999.276.3.H944. [DOI] [PubMed] [Google Scholar]
- 37. Banes AK, Watts SW. Upregulation of arterial serotonin 1B and 2B receptors in deoxycorticosterone acetate-salt hypertension. Hypertension 39: 394–398, 2002. doi: 10.1161/hy02t2.102793. [DOI] [PubMed] [Google Scholar]
- 38. Banes AK, Watts SW. Arterial expression of 5-HT2B and 5-HT1B receptors during development of DOCA-salt hypertension. BMC Pharmacol 3: 12, 2003. doi: 10.1186/1471-2210-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Russell A, Banes A, Berlin H, Fink GD, Watts SW. 5-Hydroxytryptamine2B receptor function is enhanced in the Nomega-nitro-L-arginine hypertensive rat. J Pharmacol Exp Ther 303: 179–187, 2002. doi: 10.1124/jpet.102.037390. [DOI] [PubMed] [Google Scholar]
- 40. Watts SW, Gilbert L, Webb RC. 5-Hydroxytryptamine2B receptor mediates contraction in the mesenteric artery of mineralocorticoid hypertensive rats. Hypertension 26: 1056–1059, 1995. doi: 10.1161/01.hyp.26.6.1056. [DOI] [PubMed] [Google Scholar]
- 41. Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron 2: 1313–1323, 1989. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- 42. Oliveira SD, Silva CL. Schistosomiasis differentially affects vasoconstrictor responses: up-regulation of 5-HT receptor-mediated aorta contraction. Mem Inst Oswaldo Cruz 106: 456–460, 2011. doi: 10.1590/s0074-02762011000400012. [DOI] [PubMed] [Google Scholar]
- 43. Ebrahimkhani MR, Oakley F, Murphy LB, Mann J, Moles A, Perugorria MJ, Ellis E, Lakey AF, Burt AD, Douglass A, Wright MC, White SA, Jaffré F, Maroteaux L, Mann DA. Stimulating healthy tissue regeneration by targeting the 5-HT2B receptor in chronic liver disease. Nat Med 17: 1668–1673, 2011. doi: 10.1038/nm.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ruddell RG, Oakley F, Hussain Z, Yeung I, Bryan-Lluka LJ, Ramm GA, Mann DA. A role for serotonin (5-HT) in hepatic stellate cell function and liver fibrosis. Am J Pathol 169: 861–876, 2006. doi: 10.2353/ajpath.2006.050767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mann DA, Oakley F. Serotonin paracrine signaling in tissue fibrosis. Biochim Biophys Acta 1832: 905–910, 2013. doi: 10.1016/j.bbadis.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA. Platelet-derived serotonin mediates liver regeneration. Science 312: 104–107, 2006. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.21534708.
Supplemental Fig. S2: https://doi.org/10.6084/m9.figshare.21534759.
Supplemental Fig. S3: https://doi.org/10.6084/m9.figshare.21717917.
Data Availability Statement
Data will be made available upon reasonable request.