Abstract
Objectives:
Accumulating evidence suggests that hearing loss (HL) treatment may benefit depressive symptoms among older adults with Major Depressive Disorder (MDD), but the specific individual characteristics of those who stand to improve most are unknown.
Methods:
N=37 patients ≥60 years with HL and MDD received either active or sham hearing aids in this 12-week double-blind randomized controlled trial. A combined moderator approach was utilized in the analysis in order to examine multiple different pretreatment individual characteristics to determine the specific qualities that predicted the best depressive symptom response to hearing aids. Pretreatment characteristics included: Hearing Handicap Inventory for the Elderly (HHIE-S), pure tone average (PTA), speech reception threshold (SRT), Short Physical Performance Battery (SPPB), cognition (Repeatable Battery for the Assessment of Neuropsychological Status).
Results:
The analysis revealed a combined moderator, predicting greater improvement with active versus sham hearing aids, that had a larger effect size than any individual moderator (combined effect size [ES]=0.49 [95% CI: 0.36, 0.76]). Individuals with worse hearing-related disability (HHIE-S: individual ES=−0.16), speech recognition (SRT: individual ES=−0.14), physical performance (SPPB: individual ES=0.41), and language functioning (individual ES=0.19) but with relatively less severe audiometric thresholds (PTA: individual ES=0.17) experienced greater depressive symptom improvement with active hearing aids.
Conclusions:
Older adults with relatively worse HL-related, physical, and cognitive functioning may stand to benefit most from hearing aids. Given the large number of older adults experiencing HL and MDD, a non-invasive and scalable means of targeting those most likely to respond to interventions would be valuable.
Keywords: late life depression, hearing loss, hearing aids, treatment moderators, personalized medicine
INTRODUCTION
Age-related hearing loss (HL) is a common health condition affecting older adults,1 and it has been estimated that by 2050 nearly one in four individuals worldwide will experience HL.2 HL can be a significant risk factor for neuropsychiatric dysfunction in older adults, including cognitive decline3–5 and major depressive disorder (MDD)6. Risk posed by HL for MDD has been identified in multiple independent datasets,4,7 in individuals of different ethnicities,8 and even in older adults with subclinical HL (i.e., at a level still considered “normal”).9 Unfortunately, it has been estimated that >85% of Americans with HL are untreated, as over 22.9 million older Americans with HL do not use hearing aids.10
Recent evidence suggests that hearing rehabilitation may not only improve hearing ability and quality of life but can also have beneficial effects on depression. For example, naturalistic studies of neuropsychiatric status have shown depressive symptom improvement after treatment with both hearing aids and cochlear implants.11–13 One study comparing hearing treatment to a wait list control group observed increased self-reported quality-of-life and decreased depressive symptoms post hearing aid prescription,14 but wait list groups are in general weak controls that may result in an overestimation of treatment effects. A recent randomized controlled trial found improvement in depressive symptoms after 4-weeks of aural rehabilitation,15 but did not select participants with clinically significant depressive symptoms, thus limiting the generalizability of such results to a population with MDD.
Our research group examined the effects of hearing aids in HL and MDD in two rigorously controlled pilot studies that included comprehensive audiologic, psychiatric, and cognitive assessments as well as double-blind randomization to either hearing aids or sham hearing aids.16,17 In the first study of N=13 participants with MDD and HL, we found that treatment with hearing aids (versus sham) was associated with greater improvement in depressive symptoms and immediate recall on a measure of episodic memory functioning.16 In a subsequent trial of N=25 participants, we found that hearing aids were associated with improvements in executive and episodic memory functioning.17 While hearing aid treatment was effective in improving hearing functioning in this study, no significant effect of treatment on the primary depression outcome was observed.17
Due to the relatively small sample size of these studies, it is possible that they were underpowered to detect the effects of hearing aids on depressive symptoms. One may also hypothesize that we failed to observe an effect of hearing aids on depressive symptoms due to the heterogeneity of the sample, as participants varied widely in their hearing, cognitive, and physical characteristics. Despite the high prevalence of HL (40 million Americans), little is known about the clinical characteristics of individuals with HL and MDD whose depression may respond best to hearing aids. Given the vast underutilization of hearing aids, a better understanding of these patient-related factors may allow the implementation of a precision medicine model, ensuring that individuals with the factors most associated with treatment response are maximizing health benefits from hearing aids. For example, as speech recognition can vary among individuals with the same degree of HL, poor speech recognition may identify individuals with communication difficulties who may be at highest risk for depression and thus benefit most from hearing rehabilitation. In addition, HL has been associated with decreased physical functioning18 and frailty;19 as frailty is highly comorbid in older adults with MDD and is associated with worse antidepressant treatment response,20 it would be instructive to know whether poor physical functioning may affect an individual’s depressive symptom response to hearing aids.
In the present analysis, we sought to identify characteristics of older adults with comorbid HL and MDD whose depressive symptoms were most likely to benefit from treatment with hearing aids. To do this we aggregated data from our pilot studies, which largely shared selection criteria, randomized controlled trial methodology, and assessments, and applied a combined moderator statistical approach. This approach was chosen because of its ability to use multiple different baseline characteristics (or moderators) to determine which treatment may be optimally suited for the individual patient, as it has been proposed that the strongest determinations for personalized treatment selection may require simultaneous consideration of multiple moderators.21,22 It has been successfully utilized in other randomized controlled trials in MDD to indicate which treatment is preferable for an individual based on specific clinical characteristics.21,23,24 This statistical design can account for interactions between variables, which we felt was helpful given the significant inter-relationships between an individual’s hearing capacities, physical functioning, cognition, and psychiatric status. As such, we hypothesized that a combined moderator approach accounting for multiple interacting variables would show an improved ability to differentiate response to hearing aids vs. sham over any individual factor it comprises. Given the vast numbers of older adults experiencing HL and depressive symptoms as well as the continuing high expense of hearing aids, a means of predicting which individuals are most likely to benefit using easily obtainable and non-invasive assessments would be highly valuable.
METHODS
Participants
Data from two 12-week pilot randomized controlled trials of hearing aids for HL and MDD with similar methodology were aggregated for the purpose of this analysis.16,17 Study methodology for both studies was previously published.16,17 All participants were adults aged ≥60 years who met Diagnostic and Statistical Manual 5 (DSM-V)25 criteria for MDD or persistent depressive disorder of at least six months’ duration and had a 24-item Hamilton Rating Scale for Depression (HRSD)26 score ≥16. Participants were excluded for history of psychosis or bipolar disorder, recent hearing aid use, substance abuse/dependence within the past year, probable dementia, Mini-Mental State Examination (MMSE) score ≤24, significant suicidality, current treatment with antipsychotics or mood stabilizers, contraindication to hearing aid placement, and acute/unstable medical illness.
Between the two studies, eligibility criteria varied only by HL severity. In the first study, all participants had mild to severe HL with a pure-tone average (PTA) ≥30dB HL (mean hearing threshold at 0.5, 1, 2, and 4 kHz in the better hearing ear).16 In the subsequent study, all participants had moderate to profound HL with a PTA ≥50dB HL (mean hearing threshold at 2 and 3 kHz in the better hearing ear).17
Study Assessments
Study assessments were identical in the two 12-week studies included in this analysis and were described elsewhere.16,17 A Structured Clinical Interview Diagnostic for DSM-V (SCID)27 was performed at baseline to confirm participant eligibility. Depressive symptoms were assessed using the 24-item HRSD and was performed at Weeks 0, 2, 4, 6, and 12. For audiological assessment, participants were seated in a double walled sound-attenuated booth, and pure tone testing was performed using insert earphones and bone conducted stimuli. Pure tone average (PTA) was measured as the average hearing threshold at 500, 1000, 2000, and 4000Hz in the better hearing ear. Speech reception thresholds (SRT) were obtained in each ear using standard spondee words. The Hearing Handicap Inventory for the Elderly Screening Version (HHIE-S)28 was used to assess the social/emotional effects of HL. The Short Physical Performance Battery (SPPB)29 provided measures of gait, balance, and lower extremity strength. The Repeatable Battery for the Assessment of Neuropsychological Status for Hearing Impaired Individuals (RBANS-H)30,31 evaluated attention, memory, language, and visuospatial/constructional ability.
Hearing aid intervention and psychiatric treatment as usual (TAU)
In both studies, active and sham hearing aids were Audeo B-R 90 devices manufactured by Phonak (Aurora, IL) and were added to psychiatric TAU in a naturalistic study design. Active and sham hearing aids were identical in appearance, battery use, and data logging capability. Real ear measures were performed to verify fitting, education and counseling was provided regarding the use of the hearing aid, and participants were informed that a minimum of 8 hours/day hearing aid usage was required to stay in the study. After hearing aid fitting, individuals in the active and sham hearing aid groups underwent the exact same audiological procedures and all participants had follow-up audiology appointments at Weeks 2, 6, and 12, which served to verify fitting and provide counseling. Further details regarding hearing aid procedures were previously published.16,17
In the initial pilot study of N=13 participants, active hearing aids were programmed to 100% gain targets, while sham hearing aids were programmed to flat hearing threshold of 10 dB HL across all frequencies.16 Active hearing aids were programmed to 100% prescribed (NAL-2) target gain (amplification) and verified within the test box (Verifit) for the HL that was entered. The sham hearing threshold was chosen to be a small but noticeable volume increase without substantively improving the ability to discriminate speech. Based on a discussion of the clinical options and their preference, participants could continue their antidepressant medication if they were taking one, start a new medication, or participate in the study while off medications.
In the subsequent study of N=25 participants, hearing aids were programmed to the same 100% gain targets, but sham hearing aids were programmed to a flat hearing threshold of 30 dB HL.17 A 30 dB HL gain was chosen based on results from the earlier pilot study that revealed inadequate concealment of treatment allocation when the devices were programmed to a smaller gain.16 All participants were on antidepressant medications through the study and decided to either start a study medication (escitalopram or duloxetine) or be continued on the medications they were already taking prior to study enrollment. For participants who chose to start a study medication, medication was titrated up to a therapeutic dose as clinically indicated (escitalopram up to 20 mg, duloxetine up to 90 mg).
Data Analysis
Baseline characteristics were assessed using means and standard deviations for continuous measures and percentages for categorical measures, and were compared between the hearing aid groups using two-sample t-tests (continuous measures) and Chi-squared tests (categorical measures). Analysis of covariance (ANCOVA) was used to analyze the between-group differences (i.e., active-sham effect) on HRSD outcomes. HRSD was modeled as the change from baseline (Week 0) to the end of study (Week 12) with predictors including baseline HRSD, group (active vs. sham), and covariates of age and education.
In the combined moderator analysis, all moderators were standardized. Two individuals with missing observations for HRSD post-treatment. Missing observations were imputed using a linear regression model with random effect for individual and slope. There were 20 missing observations for the moderators across different variables, which were imputed using the Mice procedure in R. We first created a new data set from all possible pairs of an individual in the active h (Condition 1) and sham hearing aid (Condition 2) conditions (n1*n2 pairs, where ni is the number of individuals in condition I, i=1,2).32 For each pair, we calculated the average moderator value (for each moderator) and HRSD change from pre-treatment to post-treatment. For each potential moderator, we computed the non-parametric Spearman correlations in the new data set between the differences in HRSD changes and each moderator average across all pairs. Non-parametric Spearman correlation was used to allow for non-normally distributed moderators and to reduce the potential influence of outliers in the data.
Second, we created the combined moderator, which is an optimally weighted combination of individual moderators. Individual moderators that were considered in the combined model included pre-treatment assessments of hearing, cognitive, and physical functioning. When considering all 15 variables, the resulting effect size (ES) was 0.63 [95% CI: 0.64, 0.97]), where the point estimate of the combined moderator ES was not included in its own CI. To reduce the CI length, we decided to include fewer moderators to create the combined moderator and selected the five moderators that had the largest association with the outcome as estimated above. Next, the weight assigned to each individual moderator selected in the preliminary step was estimated using a LASSO regression with the glmnet package in R. The dependent variable in the LASSO regression was the difference in HRSD change of each pair, and the predictors were the averages of the potential moderators. The LASSO regression is a linear regression, but it uses a penalty on coefficient absolute values to avoid overfitting. Unimportant variables (e.g., unrelated to the outcome and/or highly correlated with other variables) are expected to shrink to zero. The estimated standardized coefficients were used as weights for calculating the combined moderator score of each individual, and the combined moderator represented an optimally weighted linear combination of the individual moderator scores. The resulting moderation ES is measured by the correlation of the averages of the combined moderator (M) of each pair and their differences in HRSD change (in the paired data), denoted by Cor(M). We used bootstrapping to obtain a confidence interval. We resampled with replacement individuals, separately from each condition, resulting in 1000 estimates of the correlation. The 2.5 and 97.5 quantiles of the estimates served as confidence intervals. Finally, we assessed the discriminative utility of the combined moderator, using a linear regression of hearing aid condition, the combined moderator, and the combined moderator by condition interaction on treatment outcome.
RESULTS
Participant Disposition and Characteristics
One participant of the second pilot study was not included in these analyses as they dropped out immediately after randomization, resulting in a total sample of N=37. Among the sample of N=37 individuals, N=18 participants received hearing aids and N=19 received sham. Only N=2 participants in the hearing aid group and N=3 participants in the sham group were not taking antidepressant medications. Of the N=32 participants who were taking antidepressant medications, N=24 participants were started on study medications and N=8 participants continued the antidepressants they were taking prior to study enrollment. Demographics and baseline characteristics of the study participants are provided in Table 1. The active and sham hearing aid groups differed only in that the active hearing aid group was younger (active 70.1 vs. sham 76.2, df=35, t=−2.47, p=0.0187) and had better baseline delayed memory scores (active 107.9 vs. sham 94.6, df=33, t=2.83, p=0.0078). Between individuals participating in the two studies included in this analysis,16,17 there were no statistically significant baseline differences in age, gender, race, education years, PTA, SRT, HHIE-S, SPPB, or cognition, except for language functioning (Language Index; first study 95.3 vs. second study 107.3, df=33, t=−2.33, p=0.0262) and MMSE scores (first study 27.7 vs. second study 29.0, df=35, t=−2.91, p=0.0062).
Table 1.
Characteristics of study participants.
| Sham (n=19) | Active (n=18) | Statistics | |||
|---|---|---|---|---|---|
| n | Mean (SD) or % | n | Mean (SD) or % | t-value or χ2 (df), p-value | |
| Sex Male Female |
7 12 |
28.6 71.4 |
8 10 |
44.4 63.6 |
χ2(1) = 0.22, p = 0.637 |
| Race Asian Black/African-American White Other |
1 2 16 0 |
5.3% 10.5% 84.2% 0% |
1 1 15 1 |
5.6% 5.6% 83.3% 5.6% |
χ2(3) =1.33, p = 0.720 |
| Ethnicity Hispanic/Latino Not Hispanic/Latino |
0 19 |
0% 100% |
1 17 |
5.6% 94.4% |
χ2(1) =1.08, p = 0.298 |
| Age | 19 | 76.2 (6.8) | 18 | 70.1 (8.1) | t (35) =−2.47, p = 0.0187 |
| Education (years) | 12 | 16.4 (2.2) | 14 | 17.1 (4.08) | t (24) = 0.50, p = 0.6242 |
| Depression | |||||
| Hamilton Rating Scale for Depression (HRSD) | 19 | 19.2 (4.2) | 18 | 23.6 (4.7) | t (35) = 2.99, p = 0.0051 |
| Hearing | |||||
| Pure Tone Average (PTA) | 19 | 44.4 (7.7) | 18 | 41.1 (8.1) | t (35) = −1.30, p = 0.203 |
| Speech Reception Threshold (SRT) | 19 | 35.5 (12.8) | 17 | 32.9 (10.2) | t (34) = −0.67, p = 0.510 |
| Hearing Handicap Inventory for the Elderly Screening Version (HHIE-S) | 19 | 30.6 (7.4) | 11 | 30.1 (7.6) | t (35)= −0.21, p = 0.834 |
| Cognition | |||||
| Mini Mental State Examination | 19 | 28.2 (1.3) | 18 | 28.9 (1.4) | t (35) = 1.64, p = 0.110 |
| Immediate Memory | 19 | 99.1 (16.8) | 16 | 105.3 (16.3) | t (33) = 1.09, p = 0.282 |
| Delayed Memory | 19 | 94.6 (17.0) | 16 | 107.9 (8.4) | t (33) = 2.83, p = 0.0078 |
| Attention | 19 | 103.2 (18.9) | 16 | 109.0 (12.5) | t (33) = 1.06, p = 0.299 |
| Language | 19 | 102.4 (13.6) | 16 | 104.3 (17.7) | t (33) = 0.36, p = 0.725 |
| Visuospatial/Constructional | 19 | 89.9 (16.6) | 16 | 95.8 (7.9) | t (33) = 1.29, p = 0.205 |
| Physical | |||||
| Short Physical Performance Battery (SPPB) | 19 | 8.6 (3.0) | 18 | 9.8 (2.2) | t (35) = 1.39, p = 0.174 |
Hearing Aid Treatment Effects on Depression
Effects of hearing aid treatment on depressive and cognitive outcomes in both studies were previously published.16,17 For the combined sample of N=37 participants, hearing aid treatment as opposed to sham was associated with a 3.98-point improvement on HRSD (active −9.72 vs. sham −5.74, t=1.92, df=205, p=0.0562).
Hearing Aid Combined Moderator Effect
The combined moderator had a larger effect size (ES) than any individual moderator (ES=0.49 [95% CI: 0.34, 0.77]) as shown in Table 2. The linear interaction of the combined moderator and hearing aid group is illustrated in Figure 1. A negative HRSD change reflects an improvement in depressive symptoms, and a positive HRSD change reflects a worsening in depressive symptoms. When the combined moderator is lower than the cross-point (i.e., to the left of the cross-point in Figure 1, N=25), the hearing aid condition showed more symptom reduction than the sham condition (hearing aid −12.3 vs. sham −5.44 HRSD points, Cohen’s d=−0.75 [95% CI: −2.11, −0.16]). When the combined moderator is higher than the cross-point (N=12), the sham condition showed more symptom reduction than the hearing aid condition (hearing aid −6.5 vs. sham −9.0 HRSD points, Cohen’s d=0.32 [95% CI: −0.67, 3.96]). However, as it is unlikely that sham hearing aids ever work better than active hearing aids in this population, a more reasonable interpretation of these findings is that there may be specific patient subgroups for whom hearing aids are most effective. According to the model, a positive association indicates that lower scores of PTA (less severe HL), SPPB (worse physical functioning), and language index (worse language functioning) were associated with higher depressive symptom improvement for the active hearing aid vs. sham group through the 12-week study. A negative association indicates that higher scores of HHIE-S (more hearing-related disability) and SRT (worse speech recognition) were associated with higher depressive symptom improvement for the active hearing aid vs. sham group.
Table 2.
Individual moderator effect sizes and their weights in the combined moderator.
| Correlation | Weight | 95% CI | |
|---|---|---|---|
| HHIE-S | −0.16 | −0.02 | −0.17, 0.50 |
| PTA | 0.17 | 0.00 | −0.50, 0.22 |
| SRT | −0.14 | −0.23 | −0.49, 0.26 |
| SPPB | 0.41 | 0.56 | 0.12, 0.67 |
| Language | 0.19 | 0.20 | −0.17, 0.54 |
|
| |||
| Combined moderator | 0.49 | 0.34, 0.77 | |
Notes: HHIE-S = Hearing Handicap Inventory for the Elderly – Screening Version; PTA = Pure Tone Average; SRT = Speech Reception Threshold; SPPB = Short Physical Performance Battery; Language = Language Index score on the Repeatable Battery for the Assessment of Neuropsychological Status.
Figure 1.
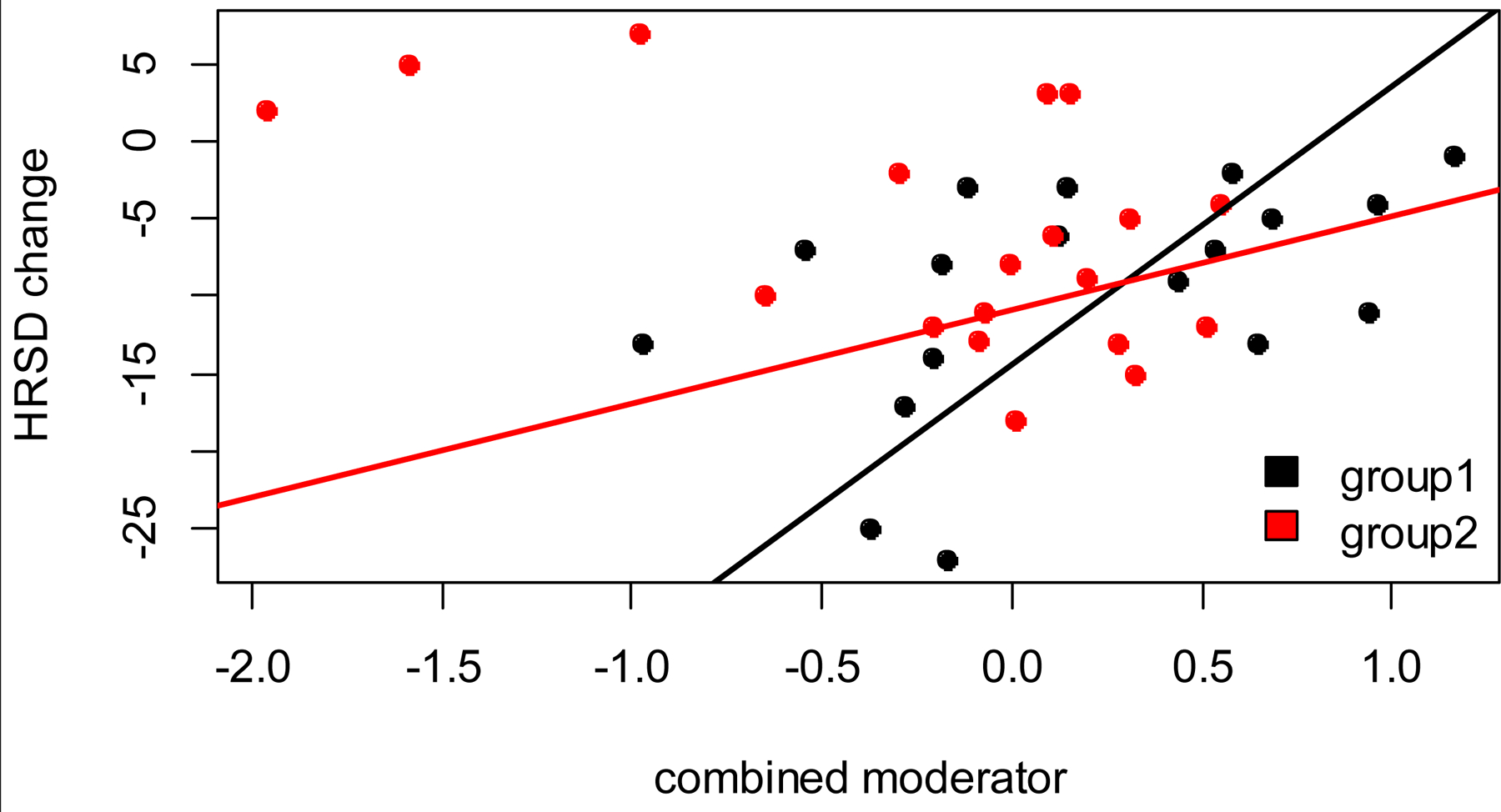
Interaction of the combined moderator and hearing aid condition in predicting the slope of depressive symptom reduction.
Group 1 (black) = active hearing aid group. Group 2 (red) = sham hearing aid group. HRSD change = Change in 24-item HRSD from Week 0 to Week 12. Negative HRSD change = more depressive symptom improvement. When the combined moderator is lower than the cross-point, the active hearing aid group showed more symptom reduction than the sham hearing aid group.
DISCUSSION
Using a combined moderator approach to these clinical trial data, we were able to identify pre-treatment characteristics of older adults with comorbid HL and MDD who were most likely to experience depressive symptom improvement with active versus sham hearing aids. Our data-driven approach characterized a set of moderators that together resulted in a moderate effect size of 0.49. This effect size was much larger than what is typically seen for both individual and combined moderator studies in MDD21,23 and was larger than the effect sizes we found for each separate moderator (ranging from −0.16 ≤ R ≤ 0.41). Our findings suggest that individuals who had a larger depressive symptom improvement with hearing aids had more impairment in lower extremity physical functioning, hearing handicap, speech recognition, and language functioning, but less severe HL on pure tone testing. Among the individual moderators, lower extremity physical functioning was found to have the highest weight and appeared to be a strong driver of the combined moderator effect.
Existing literature has identified specific pre-treatment factors that are associated with improved satisfaction with hearing aid treatment among the general population. For example, more severe HL,33 better word recognition scores,33 higher perceived social support,34 and the absence of tinnitus35 have been associated with improved perceived satisfaction and effectiveness of hearing aids. However, to our knowledge this is the first study to evaluate individual factors related to improved depressive symptom response to hearing aids. In addition, while the above reviewed studies solely evaluate individual factors related to hearing functioning, we extend the existing literature by investigating the effects of baseline physical and cognitive functioning on response to hearing aids.
Due to the exploratory nature of this combined moderator approach and the potential for interactions between individual moderators, one must be wary of interpreting the effects of each individual moderator on predicting response to hearing aid treatment. For example, moderators used in these analyses may be highly correlated to one another (e.g., PTA, SRT, and HHIE-S), which can influence the specific direction and weight of each individual moderator. However, it was interesting to find that for all measures, except for PTA, worse baseline scores predicted better depressive symptom response to hearing aids. As HL and MDD are both associated with impairment in physical, cognitive, and social functioning, it is intuitive to hypothesize that depressive symptoms may improve by addressing these factors with hearing aids. Consistent with this hypothesis, hearing aids have been proposed to improve physical functioning and postural instability,36 reduce risk of falls,37 and improve loneliness.38 Likewise, naturalistic studies have observed improvement in executive functioning with hearing aid treatment.39
Our observed association between worse language functioning and improved response to hearing aids is consistent with previous studies that have demonstrated expressive language deficits among older adults with HL. For example, we have found in a previous analysis of the National Alzheimer’s Coordinating Center dataset that older adults with more severe and longer lasting HL were more likely to have language impairment compared to those without HL.5 Likewise, our finding of an association between higher HHIE-S scores and improved response to hearing aids is consistent with existing studies examining effectiveness of hearing aids on hearing-related outcomes. While the literature is mixed,40 analyses have in general observed a positive correlation between self-perceived hearing disability and post-fitting hearing aid satisfaction.41 The HHIE-S is a self-reported screening tool that can be easily incorporated into a busy clinical practice to identify individuals with hearing-related handicap who may benefit from audiometric assessment and HL treatment. However, even when hearing-related handicap is identified, many older adults are unable to obtain treatment for HL due to issues related to accessibility, cost, and stigma. Fortunately, there has been a national effort to increase accessibility of HL treatment, leading to the subsequent passage of the Over-the-Counter Hearing Aid Act of 2017.42
The one discrepant result among the factors making up the combined moderator was that a lower degree of HL (as measured by PTA) was associated with a better depressive symptom response to hearing aids in these data. For example, most previous analyses have observed a positive association between HL severity and hearing aid effectiveness on hearing-related outcomes.33 However, PTA had the lowest weight in our model, suggesting that it was the least influential variable on predicting depressive symptom response to hearing aids. It may also be the case that the close relationships between individual hearing-related moderators led to interactions in the model, and one must be wary of interpreting the direction of each individual moderator on predicting response to treatment. Another possible interpretation of this finding is that higher PTA is associated with a longer duration, more chronic type of HL, which may be less responsive to hearing aid treatment. One may speculate that the effects of untreated HL on an individual’s social, cognitive, physical, and brain health may be less recoverable as HL persists and becomes more severe.
These findings, while suggestive of potential mediators of a depressive symptom response to hearing aids, are perhaps most useful clinically in identifying characteristics of those whose depression is most likely to respond to hearing aids. To demonstrate the potential clinical utility of this approach, consider the examples of two participants (not included in the analyzed studies) who presented to our clinic. Patient 1 presents with mild HL but markedly impaired mobility, significant self-reported hearing-related disability, and mildly impaired language functioning (SPPB=4, SRT=35, HHIE-S=14, PTA=29, Language=96), while Patient 2 presents with moderate HL but relatively preserved functioning, mobility, and language (SPPB=12, SRT=35, HHIE-S=0, PTA=40, Language=105). Using the same combined moderator model generated in this study, Patient 1 is predicted to improve 18.0 HRSD points, while Patient 2 is predicted to only improve 7.3 HRSD points after 12-weeks of hearing aids and psychiatric TAU. To be most clinically useful, future analyses may consider how a clinician may predict hearing aid response using incomplete data (i.e., 3 out of the 5 characteristics). However, the higher weights of SPPB, SRT, and language in our combined moderator (Table 2) indicate that these variables contributed most to the model and may be important characteristics to collect. As all patients with HL and MDD would likely benefit from hearing treatment, future studies may investigate how best to treat those individuals who, according to our model, are predicted to demonstrate less depressive symptom response to hearing aids. Such studies may consider evaluating depression and hearing treatment based on individual characteristics that extend beyond the specific hearing, physical, and cognitive assessments evaluated our analysis.
These findings must be considered in light of several limitations. While the two samples utilized in this analysis underwent similar study procedures, the combined moderator did not account for differences in antidepressant medication treatments between the two studies. The fact that some patients started new AD medications, others were continued on their current AD medications, and a few were not taking any AD medications may have affected our observed outcomes. In addition, the improved response to treatment in the hearing aid (vs. sham) group may have been influenced by their younger age and better pre-treatment delayed memory scores. However, while the two randomized controlled trials aggregated for this analysis varied in hearing eligibility criteria, there were no significant differences in hearing functioning between the samples. The sample size in the study was small, and one must be careful not to overinterpret the results of the combined moderator effects until they can be independently validated and replicated in external larger samples. Furthermore, there other unmeasured aspects which can motivate hearing aid use and influence hearing aid success (i.e., spousal motivation, access to care, listening demands) and may have potentially biased comparison arms within the trial. Lastly, our results have limited generalizability to other forms of HL treatment such as cochlear implants and aural rehabilitation, which may also be therapeutic for mood and cognition.
By using a combined moderator approach, the present study is the first to evaluate the specific clinical characteristics of those with comorbid HL and MDD whose depressive symptoms were most likely to benefit from hearing aids. Given the links between age-related biological processes and later-life neuropsychiatric disorders, the psychiatric evaluation of an older adult may be an important opportunity to assess hearing, cognitive, and physical functioning by either performing a brief screening tool (e.g., HHIE-S, MMSE, gait assessment) or a referring the patient for specialized testing. While the model needs to be further refined and rigorously evaluated in multiple independent samples before it can be used in clinical practice, this approach may represent a promising precision medicine tool based on inexpensive and scalable measures that may be incorporated into clinical practice. Identifying specific patient-related factors associated with hearing aid effectiveness may allow for optimization of depression treatment among individuals with HL.
Key Points:
Using a combined moderator approach, we identified pre-treatment characteristics of older adults with hearing loss whose depression was more likely to improve with active vs. sham hearing aids.
Older adults with relatively worse hearing loss-related, physical, and cognitive functioning were observed to benefit most from hearing aids in depression.
Given the large number of older adults with hearing loss and depression and the underutilization of hearing aids, these results may represent a non-invasive and scalable means of targeting those most likely to respond to treatment.
Acknowledgements:
This study was funded by the National Institute on Aging R21 AG059130 (Rutherford), the Irving Institute at Columbia University as a Phase I and Phase II Collaborative and Multidisciplinary Pilot Research Planning Grant (Rutherford), and the National Institute of Mental Health T32 MH020004–22 (Roose). Dr. Rutherford and Dr. Kim report receiving support in the form of hearing aids for this study from Phonak Ltd.
Sponsor’s Role:
Hearing aids for this study were provided by Phonak Ltd. However, the sponsor played no role in the design, methods, subject requirement, data collections, analysis, or preparation of this paper.
Footnotes
Conflicts of Interest: Drs. Brewster, Zilcha-Mano, Wallace, Brown, Roose, Kuhlmey and Galatioto have no disclosures or conflicts of interest to report. Dr. Rutherford and Dr. Kim report receiving support in the form of hearing aids for this study from Phonak Ltd. Dr. Kim receives research support from Advanced Bionics. Dr. Golub received travel expenses for industry-sponsored meetings (Cochlear, Advanced Bionics, Oticon Medical), consulting fees or honoraria (Oticon Medical, Auditory Insight, Optinose, Abbott, Decibel Therapeutics), and department received unrestricted educational grants (Storz, Stryker, Acclarent, 3NT, Decibel Therapeutics).
Previous Presentation: This paper has not been previously presented anywhere else.
Contributor Information
Katharine K. Brewster, Columbia University Vagelos College of Physicians and Surgeons, New York State Psychiatric Institute, 1051 Riverside Drive, Box 92, New York, NY 10032.
Sigal Zilcha-Mano, Department of Psychology, University of Haifa, Israel.
Meredith L. Wallace, University of Pittsburgh, Department of Psychiatry.
Ana H. Kim, Columbia University Vagelos College of Physicians and Surgeons, Columbia University, Department of Otolaryngology—Head and Neck Surgery.
Patrick J. Brown, Columbia University Vagelos College of Physicians and Surgeons, New York State Psychiatric Institute.
Steven P. Roose, Columbia University Vagelos College of Physicians and Surgeons, New York State Psychiatric Institute.
Justin S. Golub, Columbia University Vagelos College of Physicians and Surgeons, Columbia University, Department of Otolaryngology—Head and Neck Surgery.
Jessica Galatioto, Columbia University Vagelos College of Physicians and Surgeons, Columbia University, Department of Otolaryngology—Head and Neck Surgery.
Megan Kuhlmey, Columbia University Vagelos College of Physicians and Surgeons, Columbia University Department of Otolaryngology—Head and Neck Surgery.
Bret R. Rutherford, Columbia University Vagelos College of Physicians and Surgeons, New York State Psychiatric Institute.
REFERENCES
- 1.Collins JG. Prevalence of selected chronic conditions: United States, 1990–1992. Vital Health Stat 10. Jan 1997;(194):1–89. [PubMed] [Google Scholar]
- 2.World report on hearing Geneva: World Health Organization; 2021. Licence: CCBY-NC-SA 3.0 IGO. [Google Scholar]
- 3.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet Aug 8 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brewster KK, Hu MC, Zilcha-Mano S, et al. Age-Related Hearing Loss, Late-Life Depression, and Risk for Incident Dementia in Older Adults. J Gerontol A Biol Sci Med Sci Apr 30 2021;76(5):827–834. doi: 10.1093/gerona/glaa242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brewster KK, Hu MC, Wall MM, et al. Age-Related Hearing Loss, Neuropsychological Performance, and Incident Dementia in Older Adults. J Alzheimers Dis 2021;80(2):855–864. doi: 10.3233/JAD-200908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawrence BJ, Jayakody DMP, Bennett RJ, Eikelboom RH, Gasson N, Friedland PL. Hearing Loss and Depression in Older Adults: A Systematic Review and Meta-analysis. Gerontologist Apr 2 2020;60(3):e137–e154. doi: 10.1093/geront/gnz009 [DOI] [PubMed] [Google Scholar]
- 7.Brewster KK, Ciarleglio A, Brown PJ, et al. Age-Related Hearing Loss and Its Association with Depression in Later Life. Am J Geriatr Psychiatry Jul 2018;26(7):788–796. doi: 10.1016/j.jagp.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golub JS, Brewster KK, Brickman AM, et al. Association of Audiometric Age-Related Hearing Loss With Depressive Symptoms Among Hispanic Individuals. JAMA Otolaryngol Head Neck Surg Feb 1 2019;145(2):132–139. doi: 10.1001/jamaoto.2018.3270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golub JS, Brewster KK, Brickman AM, et al. Subclinical Hearing Loss is Associated With Depressive Symptoms. Am J Geriatr Psychiatry May 2020;28(5):545–556. doi: 10.1016/j.jagp.2019.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chien W, Lin FR. Prevalence of hearing aid use among older adults in the United States. Arch Intern Med Feb 13 2012;172(3):292–3. doi: 10.1001/archinternmed.2011.1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castiglione A, Benatti A, Velardita C, et al. Aging, Cognitive Decline and Hearing Loss: Effects of Auditory Rehabilitation and Training with Hearing Aids and Cochlear Implants on Cognitive Function and Depression among Older Adults. Audiol Neurootol 2016;21 Suppl 1:21–28. doi: 10.1159/000448350 [DOI] [PubMed] [Google Scholar]
- 12.Mener DJ, Betz J, Genther DJ, Chen D, Lin FR. Hearing loss and depression in older adults. J Am Geriatr Soc Sep 2013;61(9):1627–9. doi: 10.1111/jgs.12429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsimpida D, Kontopantelis E, Ashcroft DM, Panagioti M. The dynamic relationship between hearing loss, quality of life, socioeconomic position and depression and the impact of hearing aids: answers from the English Longitudinal Study of Ageing (ELSA). Soc Psychiatry Psychiatr Epidemiol Feb 2022;57(2):353–362. doi: 10.1007/s00127-021-02155-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mulrow CD, Aguilar C, Endicott JE, et al. Quality-of-life changes and hearing impairment. A randomized trial. Ann Intern Med Aug 1 1990;113(3):188–94. doi: 10.7326/0003-4819-113-3-188 [DOI] [PubMed] [Google Scholar]
- 15.Marques T, Marques FD, Migueis A. Age-related hearing loss, depression and auditory amplification: a randomized clinical trial. Eur Arch Otorhinolaryngol Mar 2022;279(3):1317–1321. doi: 10.1007/s00405-021-06805-6 [DOI] [PubMed] [Google Scholar]
- 16.Brewster KK, Pavlicova M, Stein A, et al. A pilot randomized controlled trial of hearing aids to improve mood and cognition in older adults. Int J Geriatr Psychiatry Aug 2020;35(8):842–850. doi: 10.1002/gps.5311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brewster K, Choi CJ, He X, et al. Hearing Rehabilitative Treatment for Older Adults With Comorbid Hearing Loss and Depression: Effects on Depressive Symptoms and Executive Function. Am J Geriatr Psychiatry Aug 14 2021;doi: 10.1016/j.jagp.2021.08.006 [DOI] [PMC free article] [PubMed]
- 18.Martinez-Amezcua P, Powell D, Kuo PL, et al. Association of Age-Related Hearing Impairment With Physical Functioning Among Community-Dwelling Older Adults in the US. JAMA Netw Open Jun 1 2021;4(6):e2113742. doi: 10.1001/jamanetworkopen.2021.13742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamil RJ, Betz J, Powers BB, et al. Association of Hearing Impairment With Incident Frailty and Falls in Older Adults. J Aging Health Jun 2016;28(4):644–60. doi: 10.1177/0898264315608730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown PJ, Ciarleglio A, Roose SP, et al. Frailty Worsens Antidepressant Treatment Outcomes in Late Life Depression. Am J Geriatr Psychiatry Sep 2021;29(9):944–955. doi: 10.1016/j.jagp.2020.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallace ML, Frank E, Kraemer HC. A novel approach for developing and interpreting treatment moderator profiles in randomized clinical trials. JAMA Psychiatry Nov 2013;70(11):1241–7. doi: 10.1001/jamapsychiatry.2013.1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraemer HC. Discovering, comparing, and combining moderators of treatment on outcome after randomized clinical trials: a parametric approach. Stat Med May 20 2013;32(11):1964–73. doi: 10.1002/sim.5734 [DOI] [PubMed] [Google Scholar]
- 23.Zilcha-Mano S, Roose SP, Brown PJ, Rutherford BR. A Machine Learning Approach to Identifying Placebo Responders in Late-Life Depression Trials. Am J Geriatr Psychiatry Jun 2018;26(6):669–677. doi: 10.1016/j.jagp.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smagula SF, Wallace ML, Anderson SJ, et al. Combining moderators to identify clinical profiles of patients who will, and will not, benefit from aripiprazole augmentation for treatment resistant late-life major depressive disorder. J Psychiatr Res Oct 2016;81:112–8. doi: 10.1016/j.jpsychires.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Psychiatric A, American Psychiatric A, Force DSMT. Diagnostic and statistical manual of mental disorders : DSM-5 American Psychiatric Association; 2017. [Google Scholar]
- 26.Hamilton M A rating scale for depression. J Neurol Neurosurg Psychiatry Feb 1960;23:56–62. doi: 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.First MB, Williams JBW. SCID-5-CV : structured clinical interview for DSM-5 disorders : clinician version 2016.
- 28.Ventry IM, Weinstein BE. The hearing handicap inventory for the elderly: a new tool. Ear Hear May-Jun 1982;3(3):128–34. doi: 10.1097/00003446-198205000-00006 [DOI] [PubMed] [Google Scholar]
- 29.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol Mar 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85 [DOI] [PubMed] [Google Scholar]
- 30.Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol Jun 1998;20(3):310–9. doi: 10.1076/jcen.20.3.310.823 [DOI] [PubMed] [Google Scholar]
- 31.Garcia C, Leahy B, Corradi K, Forchetti C. Component structure of the Repeatable Battery for the Assessment of Neuropsychological Status in dementia. Arch Clin Neuropsychol Jan 2008;23(1):63–72. doi: 10.1016/j.acn.2007.08.008 [DOI] [PubMed] [Google Scholar]
- 32.Zilcha-Mano S, Wallace ML, Brown PJ, Sneed J, Roose SP, Rutherford BR. Who benefits most from expectancy effects? A combined neuroimaging and antidepressant trial in depressed older adults. Transl Psychiatry Sep 15 2021;11(1):475. doi: 10.1038/s41398-021-01606-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houmøller SS, Wolff A, Möller S, et al. Prediction of successful hearing aid treatment in first-time and experienced hearing aid users: Using the International Outcome Inventory for Hearing Aids. International Journal of Audiology 2021:1–11. doi: 10.1080/14992027.2021.1916632 [DOI] [PubMed]
- 34.Singh G, Lau ST, Pichora-Fuller MK. Social Support Predicts Hearing Aid Satisfaction. Ear Hear Nov-Dec 2015;36(6):664–76. doi: 10.1097/AUD.0000000000000182 [DOI] [PubMed] [Google Scholar]
- 35.Andersson G, Keshishi A, Baguley DM. Benefit from hearing aids in users with and without tinnitus. Audiological Medicine 2011/06/01 2011;9(2):73–78. doi: 10.3109/1651386X.2011.570914 [DOI] [Google Scholar]
- 36.Rumalla K, Karim AM, Hullar TE. The effect of hearing aids on postural stability. Laryngoscope Mar 2015;125(3):720–3. doi: 10.1002/lary.24974 [DOI] [PubMed] [Google Scholar]
- 37.Mahmoudi E, Basu T, Langa K, et al. Can Hearing Aids Delay Time to Diagnosis of Dementia, Depression, or Falls in Older Adults? J Am Geriatr Soc Nov 2019;67(11):2362–2369. doi: 10.1111/jgs.16109 [DOI] [PubMed] [Google Scholar]
- 38.Weinstein BE, Sirow LW, Moser S. Relating Hearing Aid Use to Social and Emotional Loneliness in Older Adults. Am J Audiol Mar 2016;25(1):54–61. doi: 10.1044/2015_AJA-15-0055 [DOI] [PubMed] [Google Scholar]
- 39.Sarant J, Harris D, Busby P, et al. The Effect of Hearing Aid Use on Cognition in Older Adults: Can We Delay Decline or Even Improve Cognitive Function? J Clin Med Jan 17 2020;9(1)doi: 10.3390/jcm9010254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong LL, Hickson L, McPherson B. Hearing aid satisfaction: what does research from the past 20 years say? Trends Amplif 2003;7(4):117–61. doi: 10.1177/108471380300700402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knudsen LV, Oberg M, Nielsen C, Naylor G, Kramer SE. Factors influencing help seeking, hearing aid uptake, hearing aid use and satisfaction with hearing aids: a review of the literature. Trends Amplif Sep 2010;14(3):127–54. doi: 10.1177/1084713810385712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.S.670, Over-the-Counter Hearing Aid Act of 2017. https://www.congress.gov/bill/115th-congress/senate-bill/670.


