Abstract
Adverse pregnancy outcomes occur frequently in women with sickle cell disease (SCD) across the globe. In the United States, Black women experience disproportionately worse maternal health outcomes than all other racial groups. To better understand how social determinants of health impact SCD maternal morbidity, we used California’s Department of Health Care Access and Information data (1991–2019) to estimate the cumulative incidence of pregnancy outcomes in Black women with and without SCD–adjusted for age, insurance status, and Distressed Community Index (DCI) scores. Black pregnant women with SCD were more likely to deliver at a younger age, use government insurance, and live in at-risk or distressed neighborhoods, compared to those without SCD. They also experienced higher stillbirths (26.8, 95% confidence interval [CI]: 17.5–36.1 vs. 12.4 [CI: 12.1–12.7], per 1,000 births) and inpatient maternal mortality (344.5 [CI: 337.6–682.2] vs. 6.1 [CI: 2.3–8.4], per 100,000 live births). Multivariate logistic regression models showed Black pregnant women with SCD had significantly higher odds ratios (OR) for sepsis (OR 14.89, CI: 10.81, 20.52), venous thromboembolism (OR 13.60, CI: 9.16, 20.20), and postpartum hemorrhage (OR 2.25, CI 1,79–2.82), with peak onset in the 2nd trimester, 3rd trimester, and 6 weeks postpartum, respectively. Despite adjusting for sociodemographic factors, Black women with SCD still experienced significantly worse pregnancy outcomes than those without SCD. We need additional studies to determine if early introduction to reproductive health education, continuation of SCD-modifying therapies during pregnancy, and increasing access to multidisciplinary perinatal care can reduce morbidity in pregnant women with SCD.
Keywords: sickle cell disease, sickle cell anemia, pregnancy, maternal health, maternal morbidity
Introduction
Public health efforts and therapeutic advancements have transformed sickle cell disease (SCD) from an inherited hemoglobin disorder with high childhood mortality to a chronic illness characterized by episodic acute complications and cumulative morbidity across the lifespan.1 Many Americans with SCD, most of whom are of African or Hispanic ancestry,2 are living well into their 40s and 50s,3,4 which encompasses most women’s reproductive years.5 Pregnancy-associated endothelial activation and cytokine-mediated inflammation exacerbate the chronic hemolysis, microvascular occlusion, and hypercoagulability of SCD; when combined with the physiologic demands of pregnancy, these metabolic stressors significantly increase risks for acute disease-related and obstetrical complications in pregnant women with SCD. 6–10 In both low and high healthcare resource settings, maternal-fetal complications such as cesarean sections (C-sections), preeclampsia/eclampsia, venous thromboembolism (VTE), cardiomyopathy, sepsis, postpartum hemorrhage, intrauterine growth restriction, small-for-gestational-age or low birthweight infants, and preterm deliveries occur more frequently in women with SCD, compared with those without SCD.8,11–16
Using cross-sectional data from the Healthcare Cost and Utilization Project Nationwide Inpatient Sample (HCUP NIS, 1999–2008), investigators showed that pregnant SCD women who developed vasoocclusive crises (VOCs) at the time of delivery (82% Black) experienced higher rates of preeclampsia, eclampsia, and cardiomyopathy, compared to those without peripartum VOCs.14 Others used more recent NIS data (2012–2018) to show that pregnant women with SCD, also predominantly Black, were more likely to have severe maternal morbidity than women in the general US population or Black women without SCD.17 To create a more comprehensive picture of, and determine factors associated with, pregnancy outcomes for women with SCD, we analyzed administrative data from California over the last 30 years. While race is a social construct and not a biological risk factor for adverse health outcomes,18,19 we hypothesized that comparing pregnancy outcomes in Black women with and without SCD could account for any unmeasured social determinants of health associated with disparately poor maternal outcomes in Black women in the US.20–22 Compared to previous cross-sectional SCD pregnancy studies using NIS data, our longitudinal administrative dataset allows us to describe the cumulative incidence and time-course of common maternal complications in women with SCD.
Methods
Databases
We conducted this retrospective observational cohort study using de-identified patient records from the Patient Discharge Data (PDD) and the Emergency Department Utilization (EDU) databases, both obtained from the California Department of Health Care Access and Information (CA HCAI). Since July 1990, the State of California has required that all non-federal inpatient facilities record up to 25 diagnoses and 21 procedures for each hospital encounter using codes from the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM). CA HCAI adopted the 10th revision of these diagnostic and procedural codes (ICD-10-CM) on 10/1/2015. Similarly, all emergency departments (ED) associated with non-federal hospitals have reported encounter diagnoses into the EDU database since 2005. In addition to patient characteristics such as age, sex, race/ethnicity, residential zip-code, and type of health insurance recorded with each encounter, CA HCAI uses a record linkage number (RLN)—an encrypted form of the social security number— that allows serial tracking of an individual over multiple encounters. We included CA HCAI data from 1991–2019 in this study.
Patient Selection Criteria
We identified SCD cases using SCD-specific ICD-9/10-CM codes (Supplemental Table 1) from hospitalizations and ED visits recorded in the California PDD and EDU databases. Cases met at least 1 of the following criteria: (i) 2 different encounters with a SCD code in the principal position/diagnosis, (ii) 1 encounter with SCD as principal diagnosis and 2 more encounters with SCD as a secondary diagnosis, or (iii) 3 separate encounters with SCD in any secondary position. This algorithm combines stringent case definitions from our previous work23–27 and broader criteria used by our colleagues28–30 to arrive at similar number of SCD cases from hospital and ED discharge data sources reported in a recent external validation study.31 Further, we only included SCD females of reproductive potential, which we defined as those between ages 10–45 years. We chose this age range because it (i) encompasses the average ages at menarche and menopause in the US,5,32,33 and (ii) excludes expectant females at extremes of age, who may experience more pregnancy-related complications.34–37
We identified all SCD females with at least 1 in-hospital delivery in the PDD or EDU using viable and non-viable pregnancy-specific ICD-9/10-CM codes from 1991–2019 (Supplemental Table 1). Per the American College of Obstetricians and Gynecologists (ACOG) criteria, we defined non-viable pregnancies as those with gestational age ≤ 22 weeks, while viable pregnancies exceeded 22 weeks gestational age.38 Although the CA HCAI dataset allows us to follow health outcomes in the same person over time, we analyzed peripartum outcomes during the first delivery only to eliminate repeated measures from multiparous women and the potential for increased maternal morbidity with higher parity.39,40 Of the group of SCD women with at least 1 in-hospital delivery, 88% self-identified as Black (Supplemental Figure 1). We compared peripartum outcomes between this cohort of Black pregnant women with SCD and all Black women without SCD, ages 10–45 years, with at least 1 in-hospital delivery over the 30-year study period. This study was approved by the California Health and Human Services Agency’s Committee for the Protection of Human Subjects and the University of California, Davis, Human Research Protections Program.
Exposure/Outcomes
Our primary study endpoints were peripartum outcomes from the first hospitalization with an ICD-9/10-CM delivery code (Supplemental Table 1). A delivery code captures the end of a pregnancy, while pregnancy-codes denote any time during gestation. Peripartum outcomes of interest included non-viable vs viable pregnancy, stillbirth vs livebirth, mode of delivery (C-section vs vaginal), and inpatient maternal mortality. We defined inpatient maternal mortality as any woman’s death following a livebirth within the same hospital encounter. To further identify obstetric and SCD complications occurring during a woman’s first viable pregnancy, we ascertained ICD-9/10-CM codes for pregnancy-related outcomes that occurred in the 40 weeks prior to, and up to 6 weeks after, the delivery discharge date. Pregnancy-associated outcomes of interest included pre-eclampsia/eclampsia, preterm delivery, gestational diabetes, C-section, post-partum hemorrhage, sepsis, and venous thromboembolism (deep vein thrombosis and/or pulmonary embolism). Hospital admissions or ED visits for VOCs or pneumonia/acute chest syndrome (PNA/ACS) (defined by specific ICD-9/10-CM codes) were also ascertained during that same period. VOCs were defined as any admission with a code for “SCD with crisis.” PNA/ACS was defined as any of the following: (i) admission with pneumonia in the principal diagnostic position, (ii) SCD code in the principal position with pneumonia in the second position, or (iii) ACS in any diagnostic position of the hospital encounter from 2003–2019 ( of note, specific ACS codes were only available in the PDD/EDU after 2003).
Covariate Descriptions
We defined the “first pregnancy” as the first encounter in the PDD/EDU with a viable or non-viable delivery code. Age at first delivery was the age at discharge for the patients’ first delivery encounter. We used the Elixhauser comorbidity index during the delivery encounter to determine the number of comorbidities for each patient.41 We excluded “Blood loss anemia” from the comorbidities list as we were concerned about its validity in SCD patients. We categorized the number of comorbidities into 0, 1–2, and ≥3 and the delivery era into 3 decades (1991–1999, 2000–2009, 2010–2019). Health insurance type at delivery is available in the PDD and EDU, and labeled as Medicare, Medi-Cal, other government, self-pay, private insurance, and unknown. Lastly, we included the Distressed Communities Index (DCI), which compares the economic well-being of communities across the US using a weighted average of 7 populations-based metrics derived from zip code level data, then normalizes that value into a single summary statistic (or DCI score) ranging from 0–100.42 We assigned each woman’s DCI score based on her residential zip code at time of delivery as reported in the PDD or EDU, then categorized them into 1 of 5 predefined economic groups: prosperous (0–20), comfortable (21–40), mid-tier (41–60), at-risk (61–80), and distressed (81–100).
Statistical analysis
For pregnant women with SCD, we used paired t-tests to compare the number of PDD/EDU encounters for VOCs or PNA/ACS before and during their first pregnancy, over an equal duration of observation. We used χ2 tests to compare the characteristics of Black pregnant women with and without SCD; and unpaired t-tests to compare their mean DCI scores. We also calculated rates of maternal mortality per 100,000 livebirths and stillbirths per 1,000 births. We then used multivariable logistic regression models to analyze risk factors associated with each peripartum outcome—adjusted for age, comorbidities, era, type of health insurance, and DCI score—at the time of delivery. Since C-sections are often indicated for obstetrical complications, we further adjusted the C-section logistic regression model for all peripartum complications. Postpartum hemorrhage models were adjusted for VTE, pre-eclampsia/eclampsia, and C-section delivery because concurrent anticoagulation (typically initiated for VTE treatment), placental vasculopathy associated with pre-eclampsia/eclampsia,7 and C-sections could all be associated with higher bleeding rates. We used the cumulative incidence function to determine the timing of 3 common peripartum outcomes: sepsis, VTE, and postpartum hemorrhage. Pregnancy-related sepsis and VTE were ascertained from the assumed conception date of 40 weeks prior to the delivery discharge date. Postpartum hemorrhage was evaluated from delivery date up to time of postpartum hemorrhage diagnosis codes or 6 weeks post-delivery discharge, which ever occurred first. We performed all analyses using SAS 9.4 software and all between-group differences to be significant if p-values < 0.05.
Results
We identified 4,972 women with SCD from the PDD/ED databases from 1991–2019, with 4,174 women between the ages of 10–45 years. Of these, 1,656 women accounted for a total of 3,764 pregnancies (median=2, range=1–15), which we further categorized into viable and non-viable births, per ACOG criteria.38 Non-viable pregnancies had the following outcomes: 41.7% spontaneous abortion, 20.6% elective termination, 16.5% missed abortion, 11.5% ectopic pregnancies, 7.3% unspecified abortion, and 2.3% other (Supplemental Figure 2). Women with SCD were hospitalized for acute VOCs or ACS for a mean frequency of 1.31 times during pregnancy compared with 0.88 times in the 46-week period preceding their first pregnancy, for a difference in means of 0.43 (p-value <0.00001) and 95% confidence interval (CI) (0.33, 0.53). Women with SCD had a progressively increasing risk of acute VOCs throughout their pregnancy with cumulative incidence rate of 14.3% (95% CI: 12.5, 16.1) at the end of the 1st trimester rising to 35.4% (95% CI: 33.0, 37.9) at delivery (Supplemental Figure 3A). PNA/ACS cumulative incidence rates increased from 1.7% (95% CI: 1.2, 2.5) at the end of the 2nd trimester/beginning of the 3rd trimester to 4.2% (95% CI: 3.2, 5.3) at delivery (Supplemental Figure 3B).
Of the 1,438 women with SCD who had at least 1 delivery during the study period, 1260 (88%) self-identified as Black. We compared their pregnancy outcomes with 469,018 Black women without SCD of similar age, who also had at least 1 delivery. A significantly higher proportion of Black women with SCD delivered at younger age, compared with Black women without SCD (Supplemental Figure 4). Most pregnant Black women in this study did not have comorbidities at the time of their first delivery (comorbidity score = 0 in 65.8% with SCD vs. 80.2% without SCD). While most Black women had Medi-Cal insurance at delivery, a higher proportion of women with SCD had Medicare (Table 1). A significantly higher proportion of Black women with SCD lived in distressed neighborhoods (18.3% vs. 16.1%, p-value=0.0316), compared with those without SCD (Table 1).
Table 1:
Baseline characteristics of Black women in California, with and without sickle cell disease, during their 1st pregnancy (1991–2019)
| With SCD | Without SCD | p-value* | |||
|---|---|---|---|---|---|
| Variables | N | % | N | % | |
| All | 1,260 | 100.0% | 469,018 | 100.0% | |
| Comorbidities at delivery | |||||
| 0 | 829 | 65.8% | 376,034 | 80.2% | <.0001 |
| 1–2 | 300 | 23.8% | 88,108 | 18.8% | <.0001 |
| ≥3 | 32 | 2.5% | 4,626 | 1.0% | <.0001 |
| Delivery Era | |||||
| 1991–1999 | 612 | 48.6% | 233,346 | 49.8% | 0.4026 |
| 2000–2009 | 359 | 28.5% | 136,059 | 29.0% | 0.6862 |
| 2010–2019 | 289 | 22.9% | 99,613 | 21.2% | 0.1412 |
| Health Insurance | |||||
| Medicare | 73 | 5.8% | 2,149 | 0.5% | <.0001 |
| Medi-Cal | 798 | 63.3% | 245,825 | 52.4% | <.0001 |
| Other Government | 19 | 1.5% | 9,892 | 2.1% | 0.1379 |
| Self-Pay | 16 | 1.3% | 8,814 | 1.9% | 0.1115 |
| Private | 350 | 27.8% | 201,171 | 42.9% | <.0001 |
| Other | 4 | 0.3% | 1,080 | 0.2% | 0.5192 |
| Unknown | . | . | 48 | 0.0% | 0.7195 |
| DCI Quintile | |||||
| 1-Prosperous | 105 | 8.3% | 48,213 | 10.3% | 0.0231 |
| 2-Comfortable | 164 | 13.0% | 73,061 | 15.6% | 0.0123 |
| 3-Mid-Tier | 302 | 24.0% | 111,115 | 23.7% | 0.8172 |
| 4-At Risk | 434 | 34.4% | 152,099 | 32.4% | 0.127 |
| 5-Distressed | 231 | 18.3% | 75,534 | 16.1% | 0.0316 |
| Unknown Zip code | 24 | 1.9% | 8,996 | 1.9% | 0.9726 |
| DCI Distress Score | |||||
| Mean (std) | 58.88 (22.82) | 56.23 (23.68) | |||
| Median (q1, q3) | 63.02 (44.11, 75.25) | 59.32 (38.70, 74.55) | |||
SCD-Sickle cell disease; DCI-Distressed Community Index; std-standard deviation; q1-quartile 1; q3-quartile 3
χ2 test to compare distribution of SCD vs without SCD
Black women with SCD were more likely to undergo a C-section during their first delivery, compared to those without SCD (40% vs. 29%, p-value<0.0001) (Table 2). While the median length of stay after a C-section was similar between Black women with and without SCD, the interquartile ranges were wider for the SCD subgroup. Univariate analyses showed Black women with SCD had higher rates of stillbirth, inpatient maternal mortality, and adverse peripartum outcomes (except gestational diabetes), compared to those without SCD (Table 2). In the multivariate logistic regression models, Black women with SCD had higher odds of experiencing adverse peripartum outcomes compared to those without SCD, most strikingly for sepsis and VTE (Figure 1). The cumulative incidence of sepsis increased from 0.5% (95% CI: 0.2, 1.0) by the end of the 1st trimester to 3.4% (95% CI: 2.5, 4.5) at the end of the postpartum period, while the cumulative incidence of VTE increased from 0.3% (95% CI: 0.1, 0.8) at the end of the 2nd trimester to 2.1% (95% CI: 1.5, 3.1) at the end of the postpartum period in Black women with SCD (Figure 2). In contrast, Black women without SCD had significantly low risk for sepsis (0.2%; 95% CI: 0.2, 0.2) and VTE (0.15%; 95% CI: 0.1, 0.1) throughout their pregnancy. Postpartum hemorrhage was twice as likely in women with SCD (5.6%; 95% CI: 4.5–7.0), compared to those without SCD (2.6%; 95% CI: 2.5–2.6). Using ICD-10-CM codes (available from 10/1/2015 and onwards), we determined that women with SCD delivered at a median gestational age of 38 weeks, compared with 39 weeks for women without SCD. In a sensitivity analysis, we regenerated cumulative incidence curves for sepsis and VTE based on these revised gestational periods, with similar results to our prior analysis using 40 weeks gestational age (Supplemental Figure 5).
Table 2.
Peripartum outcomes among Black women in California, with and without sickle cell disease, during their 1st pregnancy (1991–2019)
| With SCD | Without SCD | p-value* | |||
|---|---|---|---|---|---|
| Variables | N | % | N | % | |
| All | 1,260 | 100.0% | 469,018 | 100.0% | |
| Mode of Delivery | |||||
| Vaginal | 757 | 60.1% | 333,453 | 71.1% | <.0001 |
| C-Section | 503 | 39.9% | 135,565 | 28.9% | <.0001 |
| Length of Delivery Admission, days median (q1, q3) | |||||
| Overall | 3 | (2, 5) | 2 | (2, 3) | <.0001 |
| Vaginal Delivery | 2 | (1, 2) | 2 | (2, 3) | <.0001 |
| C-Section Delivery | 4 | (3, 7) | 4 | (3, 4) | <.0001 |
| Birth Outcome | |||||
| Live Birth | 1,161 | 92.1% | 443,081 | 94.5% | 0.0003 |
| Still Birth | 32 | 2.5% | 5,574 | 1.2% | <.0001 |
| Maternal Complication | |||||
| Inpatient Mortality | 4 | 0.3% | 27 | 0.0% | <.0001 |
| Venous Thromboembolism | 27 | 2.1% | 695 | 0.1% | <.0001 |
| Sepsis | 43 | 3.4% | 917 | 0.2% | <.0001 |
| Postpartum Hemorrhage | 83 | 6.6% | 13,216 | 2.8% | <.0001 |
| Preeclampsia | 139 | 11.0% | 21,557 | 4.6% | <.0001 |
| Preterm Delivery | 205 | 16.3% | 37,975 | 8.1% | <.0001 |
| Gestational Diabetes | 30 | 2.4% | 15,672 | 3.3% | 0.0581 |
SCD- Sickle cell disease; C-section-Cesarean section
χ2 test to compare distribution of SCD vs without SCD
Figure 1. Peripartum outcomes adjusted for complications, comorbidities, and sociodemographic factors in Black pregnant women with SCD (compared with those without SCD) in California, during their 1st pregnancy (1991–2019).
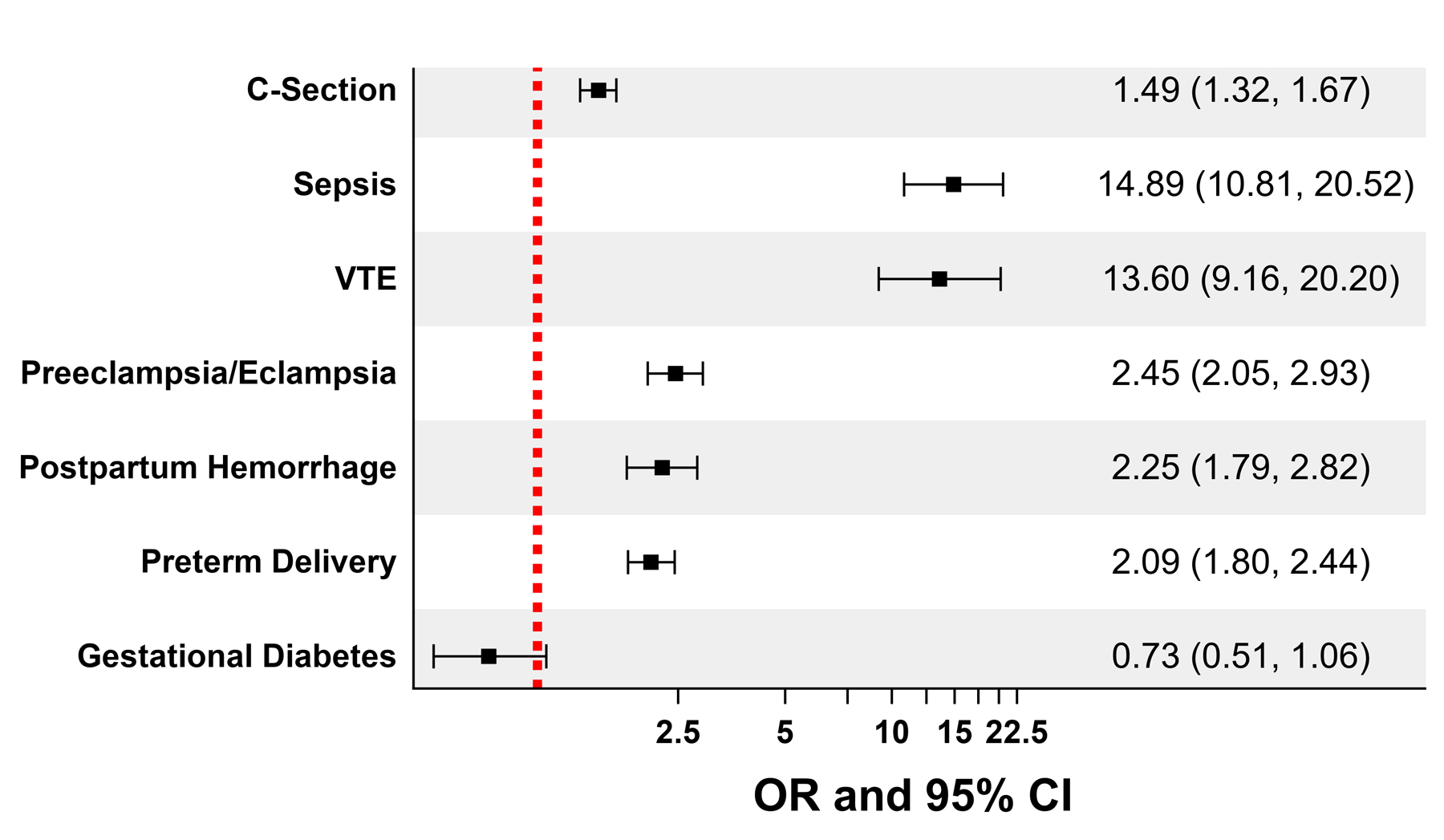
Footnote:
OR- Odds Ratio; CI- Confidence Interval; SCD- Sickle cell disease; C-section-Cesarean section; VTE- Venous thromboembolism
All models were adjusted for age, era, comorbidities, health insurance and Distressed Communities Index scores at the time of delivery
C-Section model was further adjusted for all peripartum complications
Postpartum hemorrhage model was further adjusted for C-section, VTE, and preeclampsia/eclampsia
Figure 2. Cumulative incidence of sepsis(A), VTE (B), and Postpartum hemorrhage (C) among Black women with and without SCD in California, during their 1st pregnancy (1991–2019).
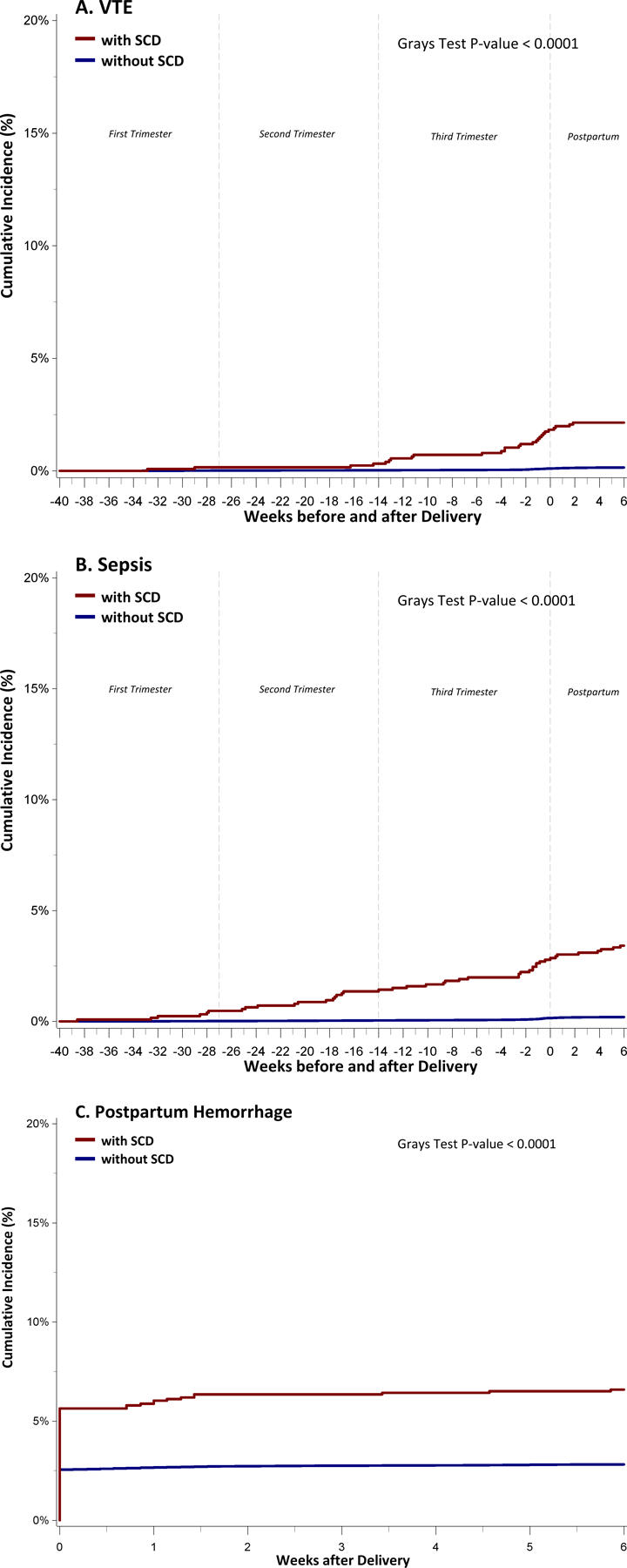
Footnote:
We assumed that all women had a gestational period of 40 weeks prior to their delivery date.
SCD- Sickle cell disease; VTE- Venous thromboembolism
Discussion
In this study, we described pregnancy-related outcomes for women with SCD across the socio-economically diverse state of CA from 1991–2019. We used longitudinal data to determine the cumulative risks of common peripartum outcomes in Black women with and without SCD, after adjusting for clinical risk factors, type of health insurance, and DCI scores at the time of delivery. Our main study findings were (i) frequency of acute care visits for VOCs and PNA/ACS increased during pregnancy for all women with SCD, compared to their pre-pregnant baseline; (ii) Black pregnant women with SCD experienced higher rates of peripartum complications, including C-sections, stillbirth, and maternal mortality, compared with Black women without SCD; and (iii) cumulative incidence rates for sepsis, VTE, and postpartum hemorrhage significantly differed between Black pregnant women with and without SCD. Despite advances in SCD and obstetrical care in the US, these results show worse peripartum outcomes in Black women with SCD, even after accounting for relevant clinical and sociodemographic factors.
There was a statistically significant difference in mean rates of acute care visits for VOCs and PNA/ACS in primigravid women with SCD, compared to their immediate non-pregnant baseline. The cumulative incidence rates for VOCs increased as the pregnancy progressed, while PNA/ACS risk rose during the 3rd trimester up to 6 weeks postpartum. Investigators from the Cooperative Study of Sickle Cell Disease (CSSCD) studied maternal-fetal outcomes in a prospective cohort of 297 women with 445 pregnancies, recruited from SCD centers across the US between 1979–1981.16 They found that women with sickle cell anemia (HbSS genotype) enrolled in CSSCD experienced similar VOC rates before, during, and after the first and subsequent pregnancies. Differences between our results and these CSSCD findings likely stem from our study cohort comprising a sizeable proportion of compound heterozygote SCD women with varying susceptibility to complications, though SCD genotype is inaccurately coded in our administrative dataset.43 Our findings of increased VOCs and PNA/ACS during pregnancy are more consistent with a contemporary prospective cohort study of 99 pregnant women with SCD (HbSS 41%, HbSC 59%) followed at a tertiary center in Accra, Ghana.44 The investigators compared the frequency of acute SCD complications during pregnancy to the postnatal period (> 6 weeks through 52 weeks after delivery),44 while we chose the time prior to the first pregnancy for comparison. It is also possible that current SCD-modifying therapies have improved survival for women with severe SCD, who may then experience more disease-related complications during pregnancy in our contemporary study cohort, compared to women with homozygous SCD enrolled in the CSSCD over 40 years ago. Whether acute care utilization in women with SCD returns to their baseline prenatal frequency after delivery remains unknown; further, caring for a newborn likely confounds health care utilization rates after delivery.
Although maternal age at first delivery has trended upwards in the general US population over time,45 we found that Black women with SCD were younger than those without SCD at their first delivery. This could be due to higher rates of unplanned pregnancies in adolescents with SCD, which highlights the importance of incorporating reproductive health education and genetic counseling into comprehensive care for all pre-pubertal children living with SCD.46–49 Subfertility and premature ovarian failure in women with SCD could also account for lower pregnancy rates with older age; thus, studies on fertility preservation and non-gonadotoxic therapies for SCD should remain a high research priority.10,46,50 Lastly, cumulative end-organ damage and decreased median survival may prematurely truncate the reproductive lifespan of women living with SCD.3,4 Of note, we studied the interaction between SCD and delivery era (in decades) for all peripartum outcomes. Preterm delivery rates significantly differed between Black women with and without SCD from 1991–1999 to 2000–2009, but not in 2010–2019. All other peripartum complications did not significantly change over the 3 decades included in this study duration.
Primary C-section rates increased in Black women with SCD across each 10-year era of our study, though this increase did not significantly differ from that of primigravid Black women without SCD. Since 1966, C-section rates have steadily risen in women of all ages and from all racial/ethnic groups across the US, but remain highest in non-Hispanic Black women older than 35 years of age.51,52 Outside of medical necessity, higher C-section rates may also be attributed to expectant women’s preferences due to their perceived innocuity of the procedure, short-term postpartum benefits, or obstetricians’ recommendations due to their concerns over litigations for adverse maternal-infant outcomes.51,53 We suspect that higher C-sections in Black pregnant women with SCD, compared with those without SCD of similar age, health insurance status, and DCI scores, likely reflects medical necessity given their increased peripartum morbidity.
The overall number of in-hospital maternal deaths in our study cohort was low, similar to findings from the more recent NIS study.17 Despite these small absolute events, we found a striking difference in inpatient maternal mortality rates between Black pregnant women with and without SCD during their first deliveries. Maternal mortality rates have steadily risen in the US since 2005 and it remains highest for non-Hispanic Black women at 49 per 100,000 livebirths, compared with overall mortality of 29 per 100,000 livebirths for all women. 22,54,55 Black pregnant women in the US disproportionally experience adverse peripartum outcomes even after adjusting for maternal age, socioeconomic status, educational attainment, comorbidities, gestational age, or prenatal care, which suggests other factors such as systemic racism and limited access to maternal-fetal specialists contribute to this disparity.56–58 Others showed similar maternal mortality rates in women with and without SCD, after adjusting for healthcare resource settings13 and instituting multidisciplinary perinatal care.59 Investigating how predictors for adverse maternal morbidity and mortality in the general population,22,60 frequency of SCD-related complications, or higher obstetrical morbidity44,59,61 contribute to increased mortality in pregnant women with SCD remains an important area for research.
Multiple studies show increased maternal morbidity in SCD,11,12,62 yet few have investigated the timing of these peripartum complications to aid with appropriate monitoring or interventions.44 We found that in Black women with SCD, sepsis risk steadily increased throughout pregnancy into the postpartum period. The significantly higher rates of sepsis in Black pregnant women with SCD compared to those without SCD may be due, in part, to additive immunocompromise from their underlying SCD and physiologic changes of pregnancy.63 VTE risk for Black women with SCD mainly increased in the 3rd trimester up to 6-weeks postpartum. Our group and others previously showed increased VTE risks in SCD in general,25,26,64 which is likely further exacerbated by the hypercoagulable state of pregnancy. Postpartum hemorrhage remained twice as high in Black women with SCD compared to those without SCD up to 6 weeks post-delivery, even after adjusting for VTE during pregnancy (a proxy for likely anticoagulation use), preeclampsia/eclampsia (possible underlying vasculopathy and/or aspirin use), and C-section history. The etiology for increased postpartum hemorrhage in women with SCD remains unclear. Additional studies are required to determine if targeted interventions like prophylactic antimicrobials, anticoagulation, or antifibrinolytics can reduce these common complications in pregnant women with SCD.
An expert panel of multidisciplinary clinical investigators, health analysts, epidemiologists, research biologists, policy makers, and community-based advocates convened by the Centers for Disease Control and Prevention (CDC) and the Foundation for Women and Girls with Blood Disorders, laid out a framework for future research priorities, funding mechanisms, and healthcare policies geared towards improving female reproductive health across the SCD lifespan.46 Their broad review of complications spanning menarche to menopause highlighted the need for better understanding of timely interventions that can mitigate adverse maternal-fetal outcomes in SCD. Others have demonstrated that implementing cost-effective multidisciplinary interventions early in antenatal care can significantly reduce maternal mortality rates in pregnant women with SCD.59,61,65 Our study partly address this knowledge gap by highlighting when sepsis, VTE, and postpartum hemorrhage are most likely to occur in pregnant women with SCD.
There are limitations to this study. The CA HCAI dataset only includes emergency department and inpatient discharge diagnostic and procedural codes, so women who solely received antenatal care as an outpatient or delivered outside of California were not included. However, we have no reason to suspect these detection and migration biases would significantly differ between pregnant women with and without SCD. We lacked accurate laboratory data on SCD genotype,16 pharmacy records on antimicrobial and anticoagulation prescriptions and refills, and use of disease-modifying interventions during pregnancy; e.g., hydroxyurea66 or prophylactic red blood cell transfusions,67 which may have modified peripartum outcomes in our study cohort. Since women with SCD have potentially higher mortality risks from disease-related complications, we limited our maternal mortality analyses to inpatient deaths only. Thus, our maternal mortality rates cannot be directly compared with CDC data, which include all-cause mortality in pregnant women from the time of delivery through their first postpartum year. Study strengths include the large sample size, longitudinal follow-up for each woman, ability to ascertain the time-course of complications, and comparison to a large, contemporaneous population-based group of Black pregnant women without SCD. Since 99% of US births occur in a hospital setting,45 our findings are likely generalizable to most women living with SCD in California and across the country.
Conclusion
In this large retrospective cohort study of pregnant women with their first deliveries occurring in the hospital, we found higher rates for maternal morbidity and inpatient maternal mortality in Black women with SCD, compared to those without SCD, despite adjusting for age, insurance status, and other sociodemographic factors at the time of delivery. Our results should not dissuade women with SCD who desire parenthood; rather, it is important to note that most pregnancies in women with SCD resulted in live births. Our data emphasizes the need for integrating reproductive health counseling into the care of girls and women with SCD, so they can make informed reproductive decisions. Our results highlight the importance of multidisciplinary perinatal care, ongoing studies to target SCD modification, and increased public health efforts to reduce disparities in pregnancy-related outcomes for women with SCD.
Supplementary Material
Funding statement:
This work was supported by the American Society of Hematology HONORS (Hematology Opportunities for the Next Generation of Research Scientists) award to S.C.F., as well as federal funding from the National Heart, Lung, and Blood Institute (5K23HL148310) to O.O.A., the Eunice Kennedy Shriver National Institute of Child Health and Human Development (5R21HD103034) to O.O. A. and B.Y., and the National Center for Advancing Translational Science (UL10001860) to T.W.
Footnotes
Conflict of Interest Disclosures: The authors declare no competing financial interests.
References
- 1.Chaturvedi S, DeBaun MR. Evolution of sickle cell disease from a life-threatening disease of children to a chronic disease of adults: The last 40 years. American journal of hematology 2016;91(1):5–14. [DOI] [PubMed] [Google Scholar]
- 2.Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med 2010;38(4 Suppl):S512–521. [DOI] [PubMed] [Google Scholar]
- 3.Johnston EE, Adesina OO, Alvarez E, et al. Acute Care Utilization at End of Life in Sickle Cell Disease: Highlighting the Need for a Palliative Approach. J Palliat Med 2020;23(1):24–32. [DOI] [PubMed] [Google Scholar]
- 4.DeBaun MR, Ghafuri DL, Rodeghier M, et al. Decreased median survival of adults with sickle cell disease after adjusting for left truncation bias: a pooled analysis. Blood 2019;133(6):615–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appiah D, Nwabuo CC, Ebong IA, Wellons MF, Winters SJ. Trends in Age at Natural Menopause and Reproductive Life Span Among US Women, 1959–2018. Jama 2021;325(13):1328–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C, Grewal J, Betran AP, Vogel JP, Souza JP, Zhang J. Severe anemia, sickle cell disease, and thalassemia as risk factors for hypertensive disorders in pregnancy in developing countries. Pregnancy Hypertens 2018;13:141–147. [DOI] [PubMed] [Google Scholar]
- 7.Malinowski AK, Dziegielewski C, Keating S, et al. Placental histopathology in sickle cell disease: A descriptive and hypothesis-generating study. Placenta 2020;95:9–17. [DOI] [PubMed] [Google Scholar]
- 8.Smith-Whitley K Complications in pregnant women with sickle cell disease. Hematology Am Soc Hematol Educ Program 2019;2019(1):359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boga C, Ozdogu H. Pregnancy and sickle cell disease: A review of the current literature. Crit Rev Oncol Hematol 2016;98:364–374. [DOI] [PubMed] [Google Scholar]
- 10.Ghafuri DL, Stimpson SJ, Day ME, James A, DeBaun MR, Sharma D. Fertility challenges for women with sickle cell disease. Expert review of hematology 2017;10(10):891–901. [DOI] [PubMed] [Google Scholar]
- 11.Oteng-Ntim E, Ayensah B, Knight M, Howard J. Pregnancy outcome in patients with sickle cell disease in the UK – a national cohort study comparing sickle cell anaemia (HbSS) with HbSC disease. British journal of haematology 2015;169(1):129–137. [DOI] [PubMed] [Google Scholar]
- 12.Oteng-Ntim E, Meeks D, Seed PT, et al. Adverse maternal and perinatal outcomes in pregnant women with sickle cell disease: systematic review and meta-analysis. Blood 2015;125(21):3316–3325. [DOI] [PubMed] [Google Scholar]
- 13.Boafor TK, Olayemi E, Galadanci N, et al. Pregnancy outcomes in women with sickle-cell disease in low and high income countries: a systematic review and meta-analysis. BJOG 2015. [DOI] [PubMed]
- 14.Alayed N, Kezouh A, Oddy L, Abenhaim HA. Sickle cell disease and pregnancy outcomes: population-based study on 8.8 million births. J Perinat Med 2014;42(4):487–492. [DOI] [PubMed] [Google Scholar]
- 15.Daigavane MM, Jena RK, Kar TJ. Perinatal outcome in sickle cell anemia: a prospective study from India. Hemoglobin 2013;37(6):507–515. [DOI] [PubMed] [Google Scholar]
- 16.Smith JA, Espeland M, Bellevue R, Bonds D, Brown AK, Koshy M. Pregnancy in sickle cell disease: experience of the Cooperative Study of Sickle Cell Disease. Obstet Gynecol 1996;87(2):199–204. [DOI] [PubMed] [Google Scholar]
- 17.Early M, Eke A, Lanzkron S, Pecker L. Low Mortality and High Severe Maternal Morbidity in Pregnancy Among People With Sickle Cell Disease [A174]. Obstetrics & Gynecology 2022;139:50S–51S. [Google Scholar]
- 18.Vyas DA, Eisenstein LG, Jones DS. Hidden in Plain Sight — Reconsidering the Use of Race Correction in Clinical Algorithms. New England Journal of Medicine 2020;383(9):874–882. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan JB, Bennett T. Use of Race and Ethnicity in Biomedical Publication. Jama 2003;289(20):2709–2716. [DOI] [PubMed] [Google Scholar]
- 20.Power-Hays A, Patterson A, Sobota A. Household material hardships impact emergency department reliance in pediatric patients with sickle cell disease. Pediatric blood & cancer 2020;67(10):e28587. [DOI] [PubMed] [Google Scholar]
- 21.Power-Hays A, Li S, Mensah A, Sobota A. Universal screening for social determinants of health in pediatric sickle cell disease: A quality-improvement initiative. Pediatric blood & cancer 2020;67(1):e28006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang E, Glazer KB, Howell EA, Janevic TM. Social Determinants of Pregnancy-Related Mortality and Morbidity in the United States: A Systematic Review. Obstet Gynecol 2020;135(4):896–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adesina O, Brunson A, Keegan THM, Wun T. Osteonecrosis of the femoral head in sickle cell disease: prevalence, comorbidities, and surgical outcomes in California. Blood Adv 2017;1(16):1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hariharan N, Brunson A, Mahajan A, Keegan THM, Wun T. Bleeding in patients with sickle cell disease: a population-based study. Blood Adv 2020;4(5):793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunson A, Keegan T, Mahajan A, White R, Wun T. High incidence of venous thromboembolism recurrence in patients with sickle cell disease. American journal of hematology 2019;94(8):862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brunson A, Lei A, Rosenberg AS, White RH, Keegan T, Wun T. Increased incidence of VTE in sickle cell disease patients: risk factors, recurrence and impact on mortality. British journal of haematology 2017. [DOI] [PubMed]
- 27.Brunson A, Keegan THM, Bang H, Mahajan A, Paulukonis S, Wun T. Increased risk of leukemia among sickle cell disease patients in California. Blood 2017;130(13):1597–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paulukonis ST, Feuchtbaum LB, Coates TD, et al. Emergency department utilization by Californians with sickle cell disease, 2005–2014. Pediatric blood & cancer 2017;64(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paulukonis ST, Eckman JR, Snyder AB, et al. Defining Sickle Cell Disease Mortality Using a Population-Based Surveillance System, 2004 through 2008. Public Health Rep 2016;131(2):367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paulukonis ST, Harris WT, Coates TD, et al. Population based surveillance in sickle cell disease: methods, findings and implications from the California registry and surveillance system in hemoglobinopathies project (RuSH). Pediatric blood & cancer 2014;61(12):2271–2276. [DOI] [PubMed] [Google Scholar]
- 31.Snyder AB, Lakshmanan S, Hulihan MM, et al. Surveillance for Sickle Cell Disease - Sickle Cell Data Collection Program, Two States, 2004–2018. MMWR Surveill Summ 2022;71(9):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee PA, Guo SS, Kulin HE. Age of puberty: data from the United States of America. APMIS 2001;109(2):81–88. [DOI] [PubMed] [Google Scholar]
- 33.Chumlea WC, Schubert CM, Roche AF, et al. Age at menarche and racial comparisons in US girls. Pediatrics 2003;111(1):110–113. [DOI] [PubMed] [Google Scholar]
- 34.de la Calle M, Bartha JL, Lopez CM, et al. Younger Age in Adolescent Pregnancies Is Associated with Higher Risk of Adverse Outcomes. International Journal of Environmental Research and Public Health 2021;18(16):8514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malabarey OT, Balayla J, Klam SL, Shrim A, Abenhaim HA. Pregnancies in young adolescent mothers: a population-based study on 37 million births. J Pediatr Adolesc Gynecol 2012;25(2):98–102. [DOI] [PubMed] [Google Scholar]
- 36.Kenny LC, Lavender T, McNamee R, O’Neill SM, Mills T, Khashan AS. Advanced maternal age and adverse pregnancy outcome: evidence from a large contemporary cohort. PloS one 2013;8(2):e56583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salem Yaniv S, Levy A, Wiznitzer A, Holcberg G, Mazor M, Sheiner E. A significant linear association exists between advanced maternal age and adverse perinatal outcome. Arch Gynecol Obstet 2011;283(4):755–759. [DOI] [PubMed] [Google Scholar]
- 38.American College of O, Gynecologists, Society for Maternal-Fetal M. Obstetric Care consensus No. 6: Periviable Birth. Obstet Gynecol 2017;130(4):e187–e199. [DOI] [PubMed] [Google Scholar]
- 39.Bai J, Wong FW, Bauman A, Mohsin M. Parity and pregnancy outcomes. American journal of obstetrics and gynecology 2002;186(2):274–278. [DOI] [PubMed] [Google Scholar]
- 40.Luo J, Fan C, Luo M, Fang J, Zhou S, Zhang F. Pregnancy complications among nulliparous and multiparous women with advanced maternal age: a community-based prospective cohort study in China. BMC pregnancy and childbirth 2020;20(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schoenman JA, Sutton JP, Elixhauser A, Love D. Understanding and enhancing the value of hospital discharge data. Med Care Res Rev 2007;64(4):449–468. [DOI] [PubMed] [Google Scholar]
- 42.Distressed Communities Index Report 2020.
- 43.Snyder AB, Lane PA, Zhou M, Paulukonis ST, Hulihan MM. The accuracy of hospital ICD-9-CM codes for determining Sickle Cell Disease genotype. J Rare Dis Res Treat 2017;2(4):39–45. [PMC free article] [PubMed] [Google Scholar]
- 44.Asare EV, Olayemi E, Boafor T, et al. Third trimester and early postpartum period of pregnancy have the greatest risk for ACS in women with SCD. American journal of hematology 2019;94(12):E328–E331. [DOI] [PubMed] [Google Scholar]
- 45.Osterman M, Hamilton B, Martin JA, Driscoll AK, Valenzuela CP. Births: Final Data for 2020. Natl Vital Stat Rep 2021;70(17):1–50. [PubMed] [Google Scholar]
- 46.Pecker LH, Sharma D, Nero A, et al. Knowledge gaps in reproductive and sexual health in girls and women with sickle cell disease. British journal of haematology 2021;194(6):970–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pecker LH, Hussain S, Lanzkron S, et al. Women with sickle cell disease report low knowledge and use of long acting reversible contraception. Journal of the National Medical Association 2021;113(5):552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith-Whitley K Reproductive issues in sickle cell disease. Blood 2014;124(24):3538–3543. [DOI] [PubMed] [Google Scholar]
- 49.Early ML, Strodel RJ, Lake IV, et al. Acceptable, hopeful, and useful: development and mixed-method evaluation of an educational tool about reproductive options for people with sickle cell disease or trait. Journal of Assisted Reproduction and Genetics 2022;39(1):183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pecker L, Hussain S, Mahesh J, Varadhan R, Christianson MS, Lanzkron S. Diminished ovarian reserve in young women with sickle cell anemia. Blood 2021. [DOI] [PMC free article] [PubMed]
- 51.Antoine C, Young BK. Cesarean section one hundred years 1920–2020: the Good, the Bad and the Ugly. J Perinat Med 2020;49(1):5–16. [DOI] [PubMed] [Google Scholar]
- 52.MacDorman MF, Menacker F, Declercq E. Cesarean birth in the United States: epidemiology, trends, and outcomes. Clin Perinatol 2008;35(2):293–307, v. [DOI] [PubMed] [Google Scholar]
- 53.Declercq E, Menacker F, Macdorman M. Maternal risk profiles and the primary cesarean rate in the United States, 1991–2002. Am J Public Health 2006;96(5):867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moaddab A, Dildy GA, Brown HL, et al. Health Care Disparity and Pregnancy-Related Mortality in the United States, 2005–2014. Obstet Gynecol 2018;131(4):707–712. [DOI] [PubMed] [Google Scholar]
- 55.Kramer MR, Strahan AE, Preslar J, et al. Changing the conversation: applying a health equity framework to maternal mortality reviews. Am J Obstet Gynecol 2019;221(6):609.e601–609.e609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Howell EA, Brown H, Brumley J, et al. Reduction of Peripartum Racial and Ethnic Disparities: A Conceptual Framework and Maternal Safety Consensus Bundle. Obstet Gynecol 2018;131(5):770–782. [DOI] [PubMed] [Google Scholar]
- 57.Howell EA. Reducing Disparities in Severe Maternal Morbidity and Mortality. Clin Obstet Gynecol 2018;61(2):387–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Villarosa L Why America’s black mothers and babies are in a life-or-death crisis. The New York Times Magazine 2018;11. [Google Scholar]
- 59.Oppong SA, Asare EV, Olayemi E, et al. Multidisciplinary care results in similar maternal and perinatal mortality rates for women with and without SCD in a low-resource setting. American journal of hematology 2019;94(2):223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lubeck D, Agodoa I, Bhakta N, et al. Estimated Life Expectancy and Income of Patients With Sickle Cell Disease Compared With Those Without Sickle Cell Disease. JAMA Netw Open 2019;2(11):e1915374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Asare EV, Olayemi E, Boafor T, et al. Implementation of multidisciplinary care reduces maternal mortality in women with sickle cell disease living in low-resource setting. American journal of hematology 2017;92(9):872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oakley LL, Mitchell S, von Rege I, et al. Perinatal outcomes in women with sickle cell disease: a matched cohort study from London, UK. British journal of haematology 2022;196(4):1069–1075. [DOI] [PubMed] [Google Scholar]
- 63.Jain D, Atmapoojya P, Colah R, Lodha P. Sickle Cell Disease and Pregnancy. Mediterr J Hematol Infect Dis 2019;11(1):e2019040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wun T, Brunson A. Sickle cell disease: an inherited thrombophilia. Hematology Am Soc Hematol Educ Program 2016;2016(1):640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swarray-Deen A, Asare EV, Ayettey Brew R, et al. Sustainability of low maternal mortality in pregnant women with SCD in a low-resource setting. Blood Adv 2022. [DOI] [PMC free article] [PubMed]
- 66.Kroner BL, Hankins JS, Pugh N, et al. Pregnancy outcomes with hydroxyurea use in women with sickle cell disease. American journal of hematology 2022;97(5):603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malinowski AK, Shehata N, D’Souza R, et al. Prophylactic transfusion for pregnant women with sickle cell disease: a systematic review and meta-analysis. Blood 2015;126(21):2424–2435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


