Abstract
Background
Sepsis-associated encephalopathy (SAE) is characterized by a diffuse cerebral dysfunction that accompanies sepsis in the absence of direct central nervous system infection. The endothelial glycocalyx is a dynamic mesh containing heparan sulfate linked to proteoglycans and glycoproteins, including selectins and vascular/intercellular adhesion molecules (V/I-CAMs), which protects the endothelium while mediating mechano-signal transduction between the blood and vascular wall. During severe inflammatory states, components of the glycocalyx are shed into the circulation and can be detected in soluble forms. Currently, SAE remains a diagnosis of exclusion and limited information is available on the utility of glycocalyx-associated molecules as biomarkers for SAE. We set out to synthesize all available evidence on the association between circulating molecules released from the endothelial glycocalyx surface during sepsis and sepsis-associated encephalopathy.
Methods
MEDLINE (PubMed) and EMBASE were searched since inception until May 2, 2022 to identify eligible studies. Any comparative observational study: i) evaluating the association between sepsis and cognitive decline and ii) providing information on level of circulating glycocalyx-associated molecules was eligible for inclusion.
Results
Four case-control studies with 160 patients met the inclusion criteria. Meta-analysis of biomarkers ICAM-1 (SMD 0.41; 95% CI 0.05–0.76; p = 0.03; I2 = 50%) and VCAM-1 (SMD 0.55; 95% CI 0.12–0.98; p = 0.01; I2 = 82%) revealed higher pooled mean concentration in patients with SAE compared to the patients with sepsis alone. Single studies reported elevated levels of P-selectin (MD 0.80; 95% CI -17.77–19.37), E-selectin (MD 96.40; 95% Cl 37.90–154.90), heparan sulfate NS2S (MD 19.41; 95% CI 13.37–25.46), and heparan sulfate NS+NS2S+NS6S (MD 67.00; 95% CI 31.00–103.00) in patients with SAE compared to the patients with sepsis alone.
Conclusion
Plasma glycocalyx-associated molecules are elevated in SAE and may be useful for early identification of cognitive decline in sepsis patients.
1. Background
Sepsis is defined as a life-threatening organ dysfunction resulting from a dysregulated host response to infection [1–3]. Sepsis is the leading cause of non-cardiac deaths in intensive care units [4]. It is a major public health concern accounting for $32, 421 in hospitalization per patient [5] and an estimated $20 billion in total annual US hospital costs [6, 7].
Sepsis-associated encephalopathy (SAE) is characterized by a diffuse cerebral dysfunction that accompanies sepsis in the absence of direct central nervous system (CNS) infection [8–11]. Clinical presentation of SAE ranges from cognitive decline and mild delirium to deep coma [8, 12]. Differential diagnosis of SAE is based on neurological exam of mental status, neck stiffness, motor responses, muscular strength, plantar and deep tendon reflexes, and cranial nerves as well as imaging, electroencephalogram (EEG), and cerebrospinal fluid (CSF) analysis [13, 14]. However, bedside detection of cognitive performance is often limited by patient sedation [15].
SAE affects up to 76% of sepsis survivors [16]. Patients with sepsis with SAE exhibit significantly higher APACHE II and SOFA scores as well as 28- and 180-day mortality compared to patients with sepsis without SAE [17]. Thus, SAE is independently associated with increased mortality and poor outcomes such as long-lasting neurocognitive deficits [18, 19]. Despite increase in recognition, however, SAE has been understudied and its pathophysiology remains incompletely understood. Suggested mechanisms involve blood-brain barrier (BBB) breakdown, impairment of endothelial function, compromised cerebral autoregulation, and altered neurotransmission that ultimately results in neuronal dysfunction and cell death [20–25].
The vascular endothelium covering the luminal surface of blood vessels serves as a semi-permeable barrier that restricts the passage of circulating substances and cells from the blood to the tissues and is key in the regulation of vascular tone [26–28]. Protecting the apical surface of endothelial cells is the glycocalyx, a dynamic mesh comprised of membrane-attached proteoglycans and glycoproteins [29–31]. Proteoglycans, such as syndecans and glypicans, have a protein core and are covalently attached to one or more negatively charged glycosaminoglycans (GAGs) such as heparan, chondroitin, dermatan, and keratan sulfates [31, 32]. GAGs are long linear polymers composed of amino sugars and other sugars such as uronic acid and galactose [33]. Glycoproteins are short carbohydrate chains capped with sialic acid that mainly function as endothelial adhesion molecules such as selectins (E- and P-selectin), integrins, and immunoglobulins (ICAM and VCAM) [34].
Under inflammatory conditions, the endothelial cells become activated in response to proinflammatory stimuli (cytokines such as TNF-alpha) and increase the surface expression of E- and P- selectins, which facilitate leukocyte-endothelial interactions promoting rolling and initial attachment to the wall of postcapillary venules [35–37]. Subsequently, ICAM-1 and VCAM-1 mediate the firm adhesion of leukocytes to the endothelium followed by transmigration [36, 38]. ICAM-1 and VCAM-1 also play an active role in inflammation by increasing vascular permeability to permit solute and fluid exchange across inflamed microvessels [39]. These adhesion glycoproteins are upregulated in endothelial cells in response to cytokines or endotoxins [38, 40]. This sequence of events leads to leukocyte diapedesis into the inflamed tissues where they undergo chemotaxis towards invading pathogens or injured cells [36, 41].
During severe inflammatory states, proteoglycans, glycoproteins and GAGs, are shed into the circulation, some of which can be detected in soluble forms or as low molecular weight fragments [42]. The degree of glycocalyx damage and concentration of circulating glycocalyx-degradation products in the blood have been recognized as a marker of endothelial dysfunction following sepsis [29, 43–45]. Moreover, the injury to the glycocalyx and the release of inflammatory mediators contribute to a number of clinical manifestations of sepsis, including acute kidney injury, respiratory failure, coagulopathy and septic cardiomyopathy [46].
It has been shown that certain glycoprotein or proteoglycan fragments, including the extracellular domains of CD44 and syndecans, are highly capable of targeting endothelial junctions and promoting microvascular leakage [47]. Importantly, circulating glycocalyx products as a consequence of lung injury have been found to penetrate the hippocampal BBB during sepsis leading to cognitive dysfunction [48]. Additionally, increased concentration of sICAM-1 has been associated with vascular cognitive impairment in older adults [49]. The glycocalyx of the specialized brain endothelial cells acts as an additional layer together with other components of the BBB, synergistically regulating the trafficking of potentially harmful substances triggered by the inflammatory response from entering the brain [50].
Currently, there is no hallmark biomarker for SAE, and SAE remains a diagnosis of exclusion [51]. Although previous studies and ongoing clinical trials on SAE examine biomarkers associated with neuronal cell death such as neuron specific enolase (NSE), central nervous system specific protein (S100β), glial fibrillary acidic protein (GFAP), and Tau protein [52–55], there is a lack of information on whether endothelium damage and specifically glycocalyx-associated degradation products are useful for early diagnosis of SAE.
The aim of this systematic review is to synthesize all available evidence on the association between glycocalyx-associated molecules and SAE in patients with sepsis.
2. Materials and methods
This systematic review was conducted following a prespecified protocol and is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline (S1 Table).
2.1 Patient and public involvement
There is no patient or public involvement in this study.
2.2 Criteria for study selection
Any observational comparative study which: i) evaluated the association between sepsis and cognitive decline and ii) provided information on level of glycocalyx-associated products were eligible for inclusion.
2.3 Search and study selection
We searched MEDLINE (PubMed) and EMBASE databases on 05/02/2022 using the search strategy (S1 File). We did not limit the search by date or language. The title and abstract of each reference were reviewed for initial screening. References were excluded if they did not include patients with SAE, did not evaluate glycocalyx-associated biomarkers, were not performed in humans, did not describe a clinical study (review, editorial, etc.), or were not in English. Reference lists were searched. Two authors (SB and TR) independently conducted the search and selected studies. Any disagreement was resolved by a third author (NV).
2.4 Data collection and management
Two authors (SB and TR) performed the data extraction using a standardized data extraction form. Any disagreement between the two authors was resolved by a third author (NV). We collected data on: study characteristics (study type, arms, location, funding, objectives, and patient enrollment), patient characteristics (age, gender, setting, etiology of sepsis, disease severity, admission characteristics, underlying disease, neurological characteristics), and levels of glycocalyx-associated products. When data were not extractable from the included reference, an attempt was made to contact the corresponding author.
2.5 Risk of bias assessment
Risk of bias was assessed using the Newcastle Ottawa Tool for Case-Control Studies. The assessment included the following three domains: selection, comparability, and exposure. For the selection domain, we examined if the case definition was adequate, representativeness of the cases, selection of controls, and definition of controls. For the comparability domain, we examined comparison of cases and controls. For the exposure domain, we examined the ascertainment of exposure, the method of ascertainment for cases and controls, and the nonresponse rate. For each criterion, the risk of bias was judged as low, high, or unclear.
2.6 Data analysis
When data were available from studies with similar populations and methods of measurement for exposures and outcomes, they were included in a meta-analysis. For continuous outcomes, mean and standard deviation were used to compute the mean difference for each study. We pooled the mean differences from individual studies using the random-effects model and reported the pooled mean difference, 95% CIs, and p-values. In the case where there was heterogeneity in the method of measurement but the population and outcome remained the same, we used the standardized mean difference to compute the pooled effect. Heterogeneity of pooled studies was evaluated using the I2 statistic. An I2<30% was considered low heterogeneity, I2<60% moderate heterogeneity, and I2≥60% high heterogeneity. To explore possible sources of heterogeneity of pooled studies and to test the robustness of the results, we performed a subgroup analysis according to definition of carcinoid crisis used by the authors.
Additionally, we performed a sensitivity analysis according to the timing of prophylaxis treatment.
All meta-analyses were conducted using STATA 16 [cite: StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC].
3. Results
3.1 Results of search and study selection
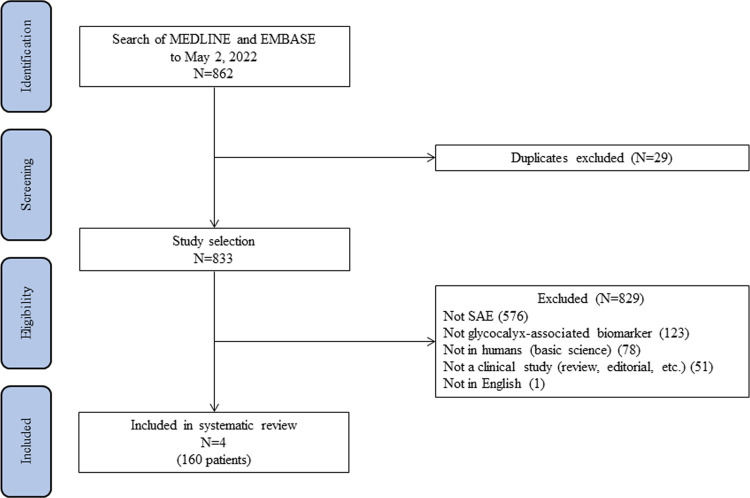
Our initial search yielded 862 citations, of which 29 duplicates were excluded (Fig 1). After a further review of the abstracts of the remaining 833 citations, 829 were excluded for various reasons: 576 citations were not about SAE, 123 citations were not about glycocalyx-associated biomarker, 78 citations were not in humans, 51 citations were not clinical studies, and 1 citation was not in English. Interestingly, 2 citations examined chondroitin sulfate. However, only the abstracts were available, and the results were pending publication. Ultimately, 4 studies met the inclusion criteria and were included in this analysis.
Fig 1. The PRISMA flow diagram.
Our initial search yielded 862 citations, of which 29 duplicated were excluded. The abstracts of the remaining 833 citations were carefully checked and 829 were excluded. Ultimately, 4 studies were included in this analysis.
3.2 Characteristics of included studies
All included studies utilized a case-control study design and were published between 2009 and 2019. A total of 160 patients were included in these studies, of whom 105 and 55 patients had sepsis and SAE, respectively. SAE was diagnosed with Pediatric Glasgow Coma Scale (PGCS), Montreal Cognitive Assessment (MoCA), 2 or more presenting symptoms, or sepsis patients who later developed delirium. Three studies had adult patients while one study had pediatric patients (mean 4.31 years). In total, there were 97 males and 63 females. Disease severity was classified as sepsis or severe sepsis, with APACHE score, SOFA score, and/or CURB-65 score. The four studies were conducted in Egypt, Taiwan, Brazil, and United States with higher mortality in SAE group compared to sepsis group. See Table 1 for characteristics of included studies.
Table 1. Characteristics of included studies.
| Study | N | Method of diagnosis for SAE | Age | Sex | Disease severity | Setting | Mortality |
|---|---|---|---|---|---|---|---|
| Hamed et al. 2009 | Sepsis only (N = 24) | Pediatric Glasgow Coma Scale < 12 | 4.31 years | Male (N = 24) | Sepsis or severe sepsis | Pediatric Department, Egypt | N = 2, 5% |
| SAE (N = 16) | Female (N = 16) | ||||||
| Hippensteel et al. 2019 | Sepsis only (N = 14) | Montreal Cognitive Assessment < 21 | 58 years | Male (N = 12) | APACHE III score (100) | ICU, University of Pennsylvania | --- |
| SAE (N = 6) | Female (N = 8) | ||||||
| Su et al. 2014 | Sepsis only (N = 47) | 2 or more symptoms: somnolence, stupor, coma, confusion, disorientation, agitation, irritability, and decreased level of GCS. | Sepsis (62.6 years) | Male (N = 48) | • Severe sepsis and septic shock | Emergency Room, Taiwan | Sepsis only (N = 5, 11%) |
| SAE (N = 23) | SAE (68.0 years) | Female (N = 22) | • APACHE II score: Sepsis only (17.5) and SAE (21.3) | SAE (N = 9, 40%) | |||
| • SOFA score: Sepsis only (5.4) and SAE (8.2) | |||||||
| Tomasi et al. 2017 | Sepsis only (N = 20) | Sepsis patients who develop delirium within 3 days of hospital admission | Sepsis only (N = 20) | Male (N = 13) | CURB-65 score (2) | Respiratory Care Unit, Brazil | Sepsis only (N = 0, 0%) |
| SAE (N = 10) | SAE (N = 10) | Female (N = 17) | SAE (N = 1, 10%) |
All included studies utilized a case-control study design. A total of 160 patients were included in these studies, of whom 105 and 55 patients had sepsis and SAE, respectively. Three studies had adult patients while one study had pediatric patients (mean 4.31 years). There was higher mortality in SAE group compared to sepsis group.
3.3 Risk of bias in included studies
The overall risk of bias in included studies was judged as ‘low risk’ for most elements of the risk of bias assessment (Table 2). Two studies were judged to be at a high risk for bias regarding comparability of cases and controls since the studies do not distinguish the patient characteristics between each group [48, 56].
Table 2. Risk of bias assessment.
| Hamed et al. 2009 | Hippensteel et al. 2019 | Su et al. 2014 | Tomasi et al. 2017 | |
|---|---|---|---|---|
| Is the case definition adequate? | Low risk | Low risk | Low risk | Low risk |
| Representative of the cases | Low risk | Low risk | Low risk | Low risk |
| Selection of controls | Low risk | Low risk | Low risk | Low risk |
| Definition of controls | Low risk | Low risk | Low risk | Low risk |
| Comparability of cases and controls on the basis of the design or analysis | High risk | High risk | Low risk | Low risk |
| Ascertainment of exposure | Low risk | Low risk | Low risk | Low risk |
| Same method of ascertainment for cases and controls | Low risk | Low risk | Low risk | Low risk |
| Nonresponse rate | Low risk | Low risk | Low risk | Low risk |
The overall risk of bias was judged as ‘low risk’ for most elements of the assessment. Two studies were judged to be at a high risk for bias regarding comparability of cases and controls since the studies do not distinguish the patient characteristics between each group.
3.4 Outcomes
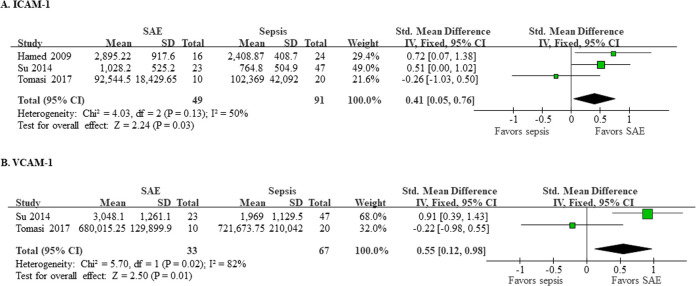
Three studies (140 patients) reported data for ICAM-1 (Fig 2A). The pooled mean concentration of ICAM-1 was significantly higher in patients with SAE compared to the patients with sepsis alone (SMD 0.41; 95% CI 0.05–0.76; p = 0.03). The heterogeneity between studies was moderate (I2 = 50%). Regarding VCAM-1, two studies (100 patients) reported data (Fig 2B). The pooled mean concentration of VCAM-1 was significantly higher in patients with SAE compared to the patients with sepsis alone (SMD 0.55; 95% CI 0.12–0.98; p = 0.01). The heterogeneity between studies was high (I2 = 82%).
Fig 2. Levels of ICAM-1 and VCAM-1.
Three studies (140 patients) reported data for ICAM-1 (A), and two studies (100 patients) reported data for VCAM-1 (B). The pooled mean concentration of ICAM-1 was higher in patients with SAE compared to the patients with sepsis alone (SMD 0.41; 95% CI 0.05–0.76; p = 0.03), and there was moderate heterogeneity (I2 = 50%) between studies. The pooled mean concentration of VCAM-1 was higher in patients with SAE compared to the patients with sepsis alone (SMD 0.55; 95% CI 0.12–0.98; p = 0.01), and the heterogeneity between studies was high (I2 = 82%).
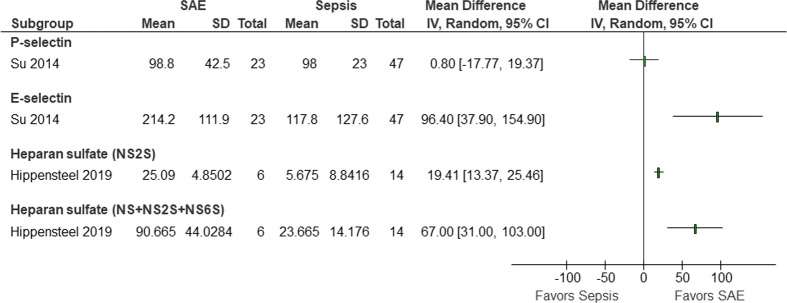
Other glycocalyx-associated biomarkers were reported by single studies (Fig 3), and therefore meta-analysis was not possible. Nevertheless, these studies reported mean concentrations higher in patients with SAE compared to the patients with sepsis alone. The mean difference of P-selectin, E-selectin, heparan sulfate (NS2S), and heparan sulfate (NS+NS2S+NS6S) were 0.80 (95% CI -17.77–19.37), 96.40 (95% Cl 37.90–154.90), 19.41 (95% CI 13.37–25.46), and 67.00 (95% CI 31.00–103.00), respectively.
Fig 3. Levels of other biomarkers reported only by single studies.
Single studies reported higher mean concentrations of P-selectin, E-selectin, and heparan sulfates in patients with SAE compared to patients with sepsis alone.
4. Discussion
The findings from this systematic review and meta-analysis with 4 studies and enrolling a combined total of 160 patients show significantly increased levels of circulating heparan sulfate fragments and glycoproteins (ICAM-1, VCAM-1, P-selectin, and E-selectin) in sepsis patients with SAE compared to sepsis patients without SAE. A recent study by Hippensteel et al. [48] included 20 patients and proposed that circulating heparan sulfate fragments, as a consequence of glycocalyx degradation, accumulate in the hippocampus and inhibit brain-derived neurotrophic factor (BDNF) leading to interruption of long-term potentiation, a process responsible for spatial memory formation. This might be one of the most meaningful studies since it showed for the first time that the presence of sulfated heparan fragments in the plasma of septic patients correlated with cognitive impairment.
We also included three additional studies with a total of 140 patients that reported plasma values of glycoproteins ICAM-1, VCAM-1, P-selectin, and E-selectin. Specifically, Hamed et al. [56] and Su et al. [57] found increased levels of ICAM-1, VCAM-1, P-selectin, and E-selectin in SAE patients compared to sepsis patients. In contrast, one study, Tomasi et al. [58], showed no difference in ICAM-1 and VCAM-1 levels in SAE and sepsis patients.
Our analysis suggests that the circulating glycocalyx-associated products are elevated in sepsis patients with cognitive decline, and the extent of such marker production seems to be higher than that in sepsis patients without cognitive impairment. However, we acknowledge several limitations of the current meta-analysis. First, there is a limited number of studies addressing our research question. While our initial hypothesis involved proteoglycans, glycoproteins and glycosaminoglycans at equal importance, our search failed to identify sufficient studies specifically measuring proteoglycans; thus, we included studies that evaluated glycoproteins such as selectins and immunoglobulins. In addition, we suspect reporting bias, but we could not formally assess it due to the limited number of studies in this systematic review. Second, the differences in circulating levels among studies might be due to variations in disease severity, method of diagnosing SAE patients (GCS vs MoCA vs symptoms), and age of participants which may all have contributed to increased heterogeneity of the data. For example, Hamed et al. [56] evaluated pediatric patients with mean age of 4.31 years compared to adult patients in the other three studies [48, 57, 58]. Yet another reason for variations of the results may be due to timing of biomarker measurements. All studies report measuring biomarker levels during initial admission. However, the exact timing is not reported in any of the studies for comparison. Due to these limitations, it is difficult to conclude a threshold value for diagnosis of SAE. Specifically, one of the studies, Tomasi et al. [58], did not provide units for the reported ICAM-1 and VCAM-1 values, affecting the conclusions of our current analysis. Thirdly, the diagnostic specificity of these markers, especially in the case of adhesion glycoproteins such as ICAM-1, VCAM-1, E-selectin, and P-selectin, may be limited due to their shedding and production in other disease conditions such as diabetes mellitus, atherosclerosis, breast cancer, and Sjögren’s syndrome [59–63].
A significant strength of the current study is the identification of circulating glycocalyx products as a novel and potential serological biomarker for SAE independent of neuronal injury markers or cognitive assessments. Currently, neuronal biomarkers are being examined for the diagnosis of SAE. For example, Erikson et al. [64] found that delirium in septic shock patients was associated with an elevated S-100β, a cytoplasmic low molecular weight calcium-binding protein, when using a laboratory cutoff value of 0.15 μg/L. Another study determined that a neuron specific enolase concentration >12.5 μg/L was independently associated with a 23.3% (95% CI 6.7–39.9, P = .006) increased risk of 30-day mortality and a 29.3% (95% CI 8.8–49.8, P = .005) increased risk of delirium [65]. Other studies [54, 55] reported elevated glial fibrillary acidic protein and tau protein levels in SAE patients and proposed a serum cutoff value of 0.532 ng/ml and 75.92 pg/mL, respectively. However, most of these circulating neuronal degradation biomarkers are found to be elevated after cerebral injury has occurred, while glycocalyx degradation products may serve as early phase SAE biomarkers allowing time for clinical intervention.
5. Conclusion
To the best of our knowledge, this is the first systematic review to address the value of endothelial glycocalyx molecules for the diagnosis of SAE. Our study suggests that the glycosaminoglycan heparan sulfate and the glycoproteins ICAM-1, VCAM-1, and E-selectin are significantly increased in the plasma of sepsis patients with cognitive impairment. However, additional research is required to assess sensitivity, specificity, threshold values, and diagnostic utility of glycocalyx biomarkers. Future research on other glycocalyx components such as syndecans and chondroitin sulfate, as well as BBB-specific glycocalyx biomarkers, may further elucidate the role of glycocalyx degradation products in the diagnosis of SAE.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the USF Health Research, Innovation & Scholarly Endeavors grant (to Sheon Baby) and the National Institutes of Health grants HL150732, GM097270, and GM142110 (to Sarah Yuan). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama 2016;315(8):801–10. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrier KM. Summary of the 2016 International Surviving Sepsis Campaign: A Clinician’s Guide. Crit Care Nurs Clin North Am 2018;30(3):311–21. doi: 10.1016/j.cnc.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 3.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992;20(6):864–74. [PubMed] [Google Scholar]
- 4.Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive care medicine 2010;36(2):222–31. doi: 10.1007/s00134-009-1738-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arefian H, Heublein S, Scherag A, Brunkhorst FM, Younis MZ, Moerer O, et al. Hospital-related cost of sepsis: A systematic review. J Infect 2017;74(2):107–17. doi: 10.1016/j.jinf.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 6.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29(7):1303–10. doi: 10.1097/00003246-200107000-00002 [DOI] [PubMed] [Google Scholar]
- 7.Torio CM, Andrews RM. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2011: Statistical Brief #160. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD); 2006. [PubMed] [Google Scholar]
- 8.Gofton TE, Young GB. Sepsis-associated encephalopathy. Nature Reviews Neurology 2012;8(10):557. doi: 10.1038/nrneurol.2012.183 [DOI] [PubMed] [Google Scholar]
- 9.Chaudhry N, Duggal AK. Sepsis Associated Encephalopathy. Adv Med 2014;2014:762320. doi: 10.1155/2014/762320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung HY, Wickel J, Brunkhorst FM, Geis C. Sepsis-Associated Encephalopathy: From Delirium to Dementia? J Clin Med 2020;9(3). doi: 10.3390/jcm9030703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papadopoulos MC, Davies DC, Moss RF, Tighe D, Bennett ED. Pathophysiology of septic encephalopathy: a review. Crit Care Med 2000;28(8):3019–24. doi: 10.1097/00003246-200008000-00057 [DOI] [PubMed] [Google Scholar]
- 12.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010;304(16):1787–94. doi: 10.1001/jama.2010.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iacobone E, Bailly-Salin J, Polito A, Friedman D, Stevens RD, Sharshar T. Sepsis-associated encephalopathy and its differential diagnosis. Critical care medicine 2009;37(10):S331–S6. doi: 10.1097/CCM.0b013e3181b6ed58 [DOI] [PubMed] [Google Scholar]
- 14.Hosokawa K, Gaspard N, Su F, Oddo M, Vincent JL, Taccone FS. Clinical neurophysiological assessment of sepsis-associated brain dysfunction: a systematic review. Crit Care 2014;18(6):674. doi: 10.1186/s13054-014-0674-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zampieri FG, Park M, Machado FS, Azevedo LCP. Sepsis-associated encephalopathy: not just delirium. Clinics 2011;66(10):1825–31. doi: 10.1590/s1807-59322011001000024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rivera-Lara L. The role of impaired brain perfusion in septic encephalopathy. BioMed Central; 2019. doi: 10.1186/s13054-018-2299-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng Q, Ai Y-H, Gong H, Wu L, Ai M-L, Deng S-Y, et al. Characterization of sepsis and sepsis-associated encephalopathy. Journal of intensive care medicine 2019;34(11–12):938–45. doi: 10.1177/0885066617719750 [DOI] [PubMed] [Google Scholar]
- 18.Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. Bmj 2016;353:i1585. doi: 10.1136/bmj.i1585 [DOI] [PubMed] [Google Scholar]
- 19.Sonneville R, de Montmollin E, Poujade J, Garrouste-Orgeas M, Souweine B, Darmon M, et al. Potentially modifiable factors contributing to sepsis-associated encephalopathy. Intensive Care Med 2017;43(8):1075–84. doi: 10.1007/s00134-017-4807-z [DOI] [PubMed] [Google Scholar]
- 20.Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-brain barrier: from physiology to disease and back. Physiological reviews 2019;99(1):21–78. doi: 10.1152/physrev.00050.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haileselassie B, Joshi AU, Minhas PS, Mukherjee R, Andreasson KI, Mochly-Rosen D. Mitochondrial dysfunction mediated through dynamin-related protein 1 (Drp1) propagates impairment in blood brain barrier in septic encephalopathy. J Neuroinflammation 2020;17(1):36. doi: 10.1186/s12974-019-1689-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nwafor DC, Brichacek AL, Mohammad AS, Griffith J, Lucke-Wold BP, Benkovic SA, et al. Targeting the Blood-Brain Barrier to Prevent Sepsis-Associated Cognitive Impairment. J Cent Nerv Syst Dis 2019;11:1179573519840652. doi: 10.1177/1179573519840652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schramm P, Klein KU, Falkenberg L, Berres M, Closhen D, Werhahn KJ, et al. Impaired cerebrovascular autoregulation in patients with severe sepsis and sepsis-associated delirium. Crit Care 2012;16(5):R181. doi: 10.1186/cc11665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharshar T, Polito A, Checinski A, Stevens RD. Septic-associated encephalopathy—everything starts at a microlevel. Crit Care 2010;14(5):199. doi: 10.1186/cc9254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Gool WA, van de Beek D, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet 2010;375(9716):773–5. doi: 10.1016/S0140-6736(09)61158-2 [DOI] [PubMed] [Google Scholar]
- 26.Sturtzel C. Endothelial Cells. Adv Exp Med Biol 2017;1003:71–91. doi: 10.1007/978-3-319-57613-8_4 [DOI] [PubMed] [Google Scholar]
- 27.Vanhoutte PM. Endothelial control of vasomotor function: from health to coronary disease. Circ J 2003;67(7):572–5. doi: 10.1253/circj.67.572 [DOI] [PubMed] [Google Scholar]
- 28.Vanhoutte PM, Shimokawa H, Feletou M, Tang EH. Endothelial dysfunction and vascular disease—a 30th anniversary update. Acta Physiol (Oxf) 2017;219(1):22–96. doi: 10.1111/apha.12646 [DOI] [PubMed] [Google Scholar]
- 29.Uchimido R, Schmidt EP, Shapiro NI. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Critical Care 2019;23(1):16. doi: 10.1186/s13054-018-2292-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch 2007;454(3):345–59. doi: 10.1007/s00424-007-0212-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng 2007;9:121–67. doi: 10.1146/annurev.bioeng.9.060906.151959 [DOI] [PubMed] [Google Scholar]
- 32.Li L, Ly M, Linhardt RJ. Proteoglycan sequence. Mol Biosyst 2012;8(6):1613–25. doi: 10.1039/c2mb25021g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esko JD, Kimata K, Lindahl U. Proteoglycans and Sulfated Glycosaminoglycans. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, et al., editors. Essentials of Glycobiology. Cold Spring Harbor (NY); 2009. [PubMed] [Google Scholar]
- 34.Alphonsus C, Rodseth R. The endothelial glycocalyx: a review of the vascular barrier. Anaesthesia 2014;69(7):777–84. doi: 10.1111/anae.12661 [DOI] [PubMed] [Google Scholar]
- 35.Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell 1991;65(5):859–73. doi: 10.1016/0092-8674(91)90393-d [DOI] [PubMed] [Google Scholar]
- 36.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 2007;7(9):678–89. doi: 10.1038/nri2156 [DOI] [PubMed] [Google Scholar]
- 37.Selectins Sperandio M. and glycosyltransferases in leukocyte rolling in vivo. FEBS J 2006;273(19):4377–89. [DOI] [PubMed] [Google Scholar]
- 38.Albelda SM, Smith CW, Ward PA. Adhesion molecules and inflammatory injury. FASEB J 1994;8(8):504–12. [PubMed] [Google Scholar]
- 39.Sumagin R, Lomakina E, Sarelius IH. Leukocyte-endothelial cell interactions are linked to vascular permeability via ICAM-1-mediated signaling. Am J Physiol Heart Circ Physiol 2008;295(3):H969–H77. doi: 10.1152/ajpheart.00400.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawson C, Wolf S. ICAM-1 signaling in endothelial cells. Pharmacol Rep 2009;61(1):22–32. doi: 10.1016/s1734-1140(09)70004-0 [DOI] [PubMed] [Google Scholar]
- 41.Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol 2003;24(6):327–34. doi: 10.1016/s1471-4906(03)00117-0 [DOI] [PubMed] [Google Scholar]
- 42.Goligorsky MS, Sun D. Glycocalyx in Endotoxemia and Sepsis. Am J Pathol 2020;190(4):791–8. doi: 10.1016/j.ajpath.2019.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colbert JF, Schmidt EP. Endothelial and Microcirculatory Function and Dysfunction in Sepsis. Clin Chest Med 2016;37(2):263–75. doi: 10.1016/j.ccm.2016.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nelson A, Berkestedt I, Schmidtchen A, Ljunggren L, Bodelsson M. Increased levels of glycosaminoglycans during septic shock: relation to mortality and the antibacterial actions of plasma. Shock 2008;30(6):623–7. doi: 10.1097/SHK.0b013e3181777da3 [DOI] [PubMed] [Google Scholar]
- 45.Steppan J, Hofer S, Funke B, Brenner T, Henrich M, Martin E, et al. Sepsis and major abdominal surgery lead to flaking of the endothelial glycocalix. J Surg Res 2011;165(1):136–41. doi: 10.1016/j.jss.2009.04.034 [DOI] [PubMed] [Google Scholar]
- 46.Martin L, Koczera P, Zechendorf E, Schuerholz T. The Endothelial Glycocalyx: New Diagnostic and Therapeutic Approaches in Sepsis. Biomed Res Int 2016;2016:3758278. doi: 10.1155/2016/3758278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang X, Meegan JE, Jannaway M, Coleman DC, Yuan SY. A disintegrin and metalloproteinase 15-mediated glycocalyx shedding contributes to vascular leakage during inflammation. Cardiovasc Res 2018;114(13):1752–63. doi: 10.1093/cvr/cvy167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hippensteel JA, Anderson BJ, Orfila JE, McMurtry SA, Dietz RM, Su G, et al. Circulating heparan sulfate fragments mediate septic cognitive dysfunction. J Clin Invest 2019;129(4):1779–84. doi: 10.1172/JCI124485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gregory MA, Manuel-Apolinar L, Sanchez-Garcia S, Villa Romero AR, de Jesus Iuit Rivera J, Basurto Acevedo L, et al. Soluble Intercellular Adhesion Molecule-1 (sICAM-1) as a Biomarker of Vascular Cognitive Impairment in Older Adults. Dement Geriatr Cogn Disord 2019;47(4–6):243–53. doi: 10.1159/000500068 [DOI] [PubMed] [Google Scholar]
- 50.Kutuzov N, Flyvbjerg H, Lauritzen M. Contributions of the glycocalyx, endothelium, and extravascular compartment to the blood-brain barrier. Proc Natl Acad Sci U S A 2018;115(40):E9429–E38. doi: 10.1073/pnas.1802155115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heming N, Mazeraud A, Verdonk F, Bozza FA, Chrétien F, Sharshar T. Neuroanatomy of sepsis-associated encephalopathy. Critical Care 2017;21(1):65. doi: 10.1186/s13054-017-1643-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yao B, Zhang LN, Ai YH, Liu ZY, Huang L. Serum S100β is a better biomarker than neuron-specific enolase for sepsis-associated encephalopathy and determining its prognosis: a prospective and observational study. Neurochem Res 2014;39(7):1263–9. [DOI] [PubMed] [Google Scholar]
- 53.Zenaide PV, Gusmao-Flores D. Biomarkers in septic encephalopathy: a systematic review of clinical studies. Rev Bras Ter Intensiva 2013;25(1):56–62. doi: 10.1590/s0103-507x2013000100011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu L, Ai ML, Feng Q, Deng S, Liu ZY, Zhang LN, et al. Serum glial fibrillary acidic protein and ubiquitin C-terminal hydrolase-L1 for diagnosis of sepsis-associated encephalopathy and outcome prognostication. J Crit Care 2019;52:172–9. [DOI] [PubMed] [Google Scholar]
- 55.Zhao T, Xia Y, Wang D, Pang L. Association between Elevated Serum Tau Protein Level and Sepsis-Associated Encephalopathy in Patients with Severe Sepsis. Can J Infect Dis Med Microbiol 2019;2019:1876174. doi: 10.1155/2019/1876174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamed SA, Hamed EA, Abdella MM. Septic encephalopathy: relationship to serum and cerebrospinal fluid levels of adhesion molecules, lipid peroxides and S-100B protein. Neuropediatrics 2009;40(2):66–72. doi: 10.1055/s-0029-1231054 [DOI] [PubMed] [Google Scholar]
- 57.Su CM, Cheng HH, Tsai TC, Hsiao SY, Tsai NW, Chang WN, et al. Elevated serum vascular cell adhesion molecule-1 is associated with septic encephalopathy in adult community-onset severe sepsis patients. Biomed Res Int 2014;2014:598762. doi: 10.1155/2014/598762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tomasi CD, Vuolo F, Generoso J, Soares M, Barichello T, Quevedo J, et al. Biomarkers of Delirium in a Low-Risk Community-Acquired Pneumonia-Induced Sepsis. Mol Neurobiol 2017;54(1):722–6. doi: 10.1007/s12035-016-9708-6 [DOI] [PubMed] [Google Scholar]
- 59.Siddiqui K, George TP, Nawaz SS, Joy SS. VCAM-1, ICAM-1 and selectins in gestational diabetes mellitus and the risk for vascular disorders. Future Cardiol 2019;15(5):339–46. doi: 10.2217/fca-2018-0042 [DOI] [PubMed] [Google Scholar]
- 60.Al-Rubeaan K, Nawaz SS, Youssef AM, Al Ghonaim M, Siddiqui K. IL-18, VCAM-1 and P-selectin as early biomarkers in normoalbuminuric Type 2 diabetes patients. Biomark Med 2019;13(6):467–78. doi: 10.2217/bmm-2018-0359 [DOI] [PubMed] [Google Scholar]
- 61.Hwang SJ, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM, Jr., et al. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation 1997;96(12):4219–25. doi: 10.1161/01.cir.96.12.4219 [DOI] [PubMed] [Google Scholar]
- 62.Silva HC, Garcao F, Coutinho EC, De Oliveira CF, Regateiro FJ. Soluble VCAM-1 and E-selectin in breast cancer: relationship with staging and with the detection of circulating cancer cells. Neoplasma 2006;53(6):538–43. [PubMed] [Google Scholar]
- 63.Błochowiak KJ, Olewicz-Gawlik A, Trzybulska D, Nowak-Gabryel M, Kocięcki J, Witmanowski H, et al. Serum ICAM-1, VCAM-1 and E-selectin levels in patients with primary and secondary Sjögren’s syndrome. Adv Clin Exp Med 2017;26(5):835–42. [DOI] [PubMed] [Google Scholar]
- 64.Erikson K, Ala-Kokko TI, Koskenkari J, Liisanantti JH, Kamakura R, Herzig KH, et al. Elevated serum S-100β in patients with septic shock is associated with delirium. Acta Anaesthesiol Scand 2019;63(1):69–73. [DOI] [PubMed] [Google Scholar]
- 65.Anderson BJ, Reilly JP, Shashaty MGS, Palakshappa JA, Wysoczanski A, Dunn TG, et al. Admission plasma levels of the neuronal injury marker neuron-specific enolase are associated with mortality and delirium in sepsis. J Crit Care 2016;36:18–23. doi: 10.1016/j.jcrc.2016.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.