Abstract
The unique combination of physical and chemical properties of MXenes has propelled a growing number of applications in biomedicine and healthcare. The expanding library of MXenes with tunable properties is paving the way for high-performance, application-specific MXene-based sensing and therapeutic platforms. In this article, we highlight the emerging biomedical applications of MXenes with specific emphasis on bioelectronics, biosensors, tissue engineering, and therapeutics. We present examples of MXenes and their composites enabling novel technological platforms and therapeutic strategies, and elucidate potential avenues for further developments. Finally, we discuss the materials, manufacturing, and regulatory challenges that need to be synergistically addressed for the clinical translation of MXene-based biomedical technologies.
Graphical abstract
Keywords: MXene, Bioelectronics, Biosensing, Tissue engineering, Therapeutics, Clinical translation
Introduction
MXenes are a rapidly growing family of two-dimensional (2D) nanomaterials that are finding applications in energy storage and conversion, optics, electronics, catalysis, and medicine.1,2 Since 2017, at least 100 individual structural compositions of MXenes have been theoretically predicted, and >40 have already been synthesized.2,3 In parallel, a deeper understanding of the relationships between molecular structure and application-tunable properties and functionalities has been established. The overall result of these efforts are MXenes with high electrical conductivity,4 tunable optical absorption,4 high fracture toughness,5 excellent electrochemical charge storage,6 solution-based processibility,7 and ease of functionalization.8 These developments in material synthesis and processing have opened up new and exciting opportunities for biomedical technologies relying on MXenes and their composites.
In 2018, we published one of the first topical reviews on biomedical applications and biocompatibility of MXenes.9 At that time, the vast majority of the studies relied on Ti3C2Tx and Nb2C for electrochemical biosensing,10 diagnostic imaging,11 antibacterial,12 drug delivery,11 and photothermal therapy, either assembled in 2D films or directly injected in the bloodstream.13 The broad biocompatibility of MXenes at cell, tissue, organ, and whole-body level had also been established.9,14–17 Since then, more than 450 new scientific articles have been published,18 expanding on previously proposed technologies or opening up entire new fields. Such exponential growth demonstrates how the rise of MXenes is profoundly impacting biomedical research and health care.
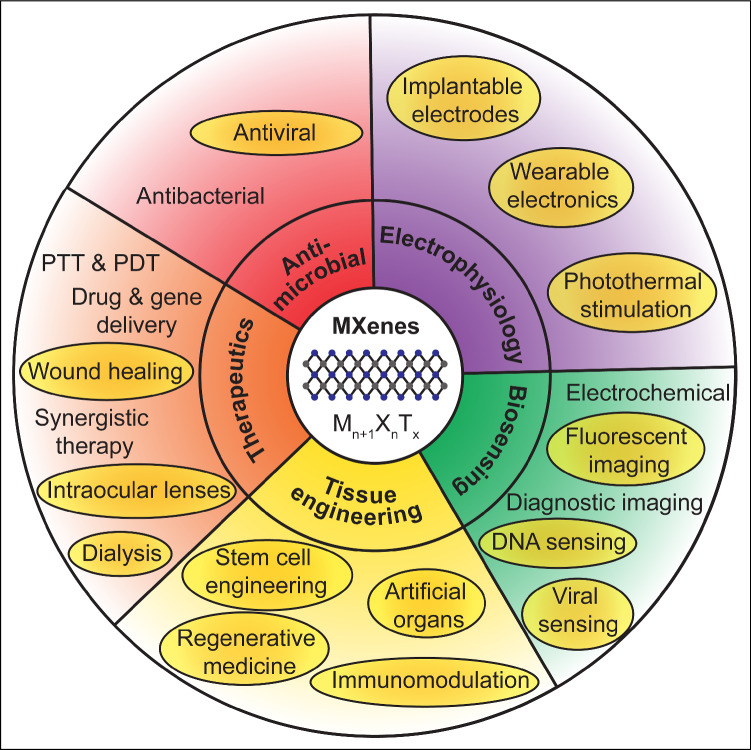
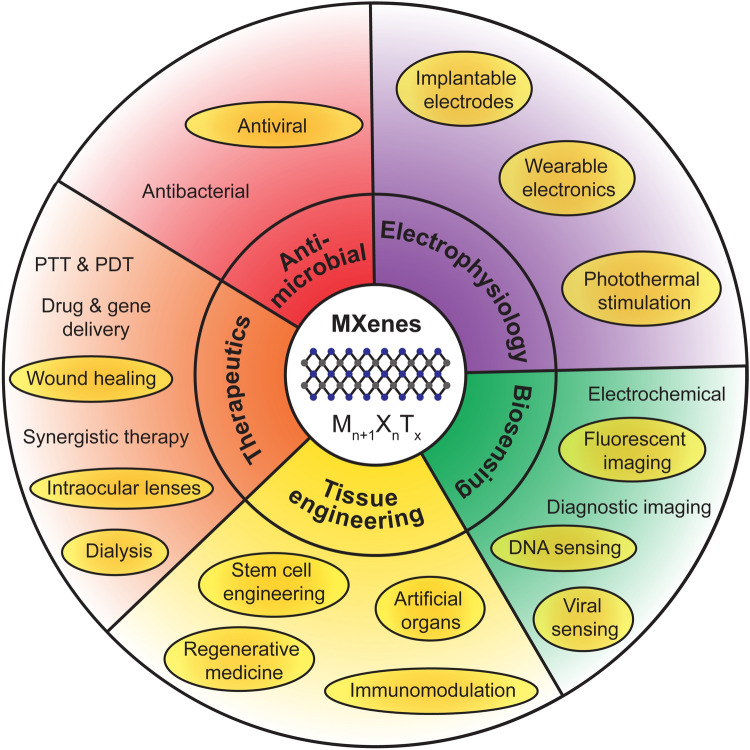
In this article, we highlight recent works published after 2018 focusing on emerging and novel areas of applications of MXenes, specifically in tissue engineering, bioelectronics, therapeutics, immunotherapy, and blood purification (Figure 1). In each section, we discuss the underlying physical mechanisms behind each application, the enabling material structure, compositions, and functions, the current status of technology development, as well as potential avenues for further improvements. Finally, we discuss existing challenges that need to be synergistically addressed for supporting the human translation of MXene-based medical technologies.
Figure 1.
Biomedical applications of MXenes. Shaded ovals highlight emerging applications and areas of research from 2019 to date. PTT, photothermal therapy; PDT, photodynamic therapy.
Bioelectronics with MXenes
The ability to record and modulate the electrical activity of cells and organs is critical for scientific investigations on functions and disease, and for the design of effective therapeutic interventions.19,20 Input/output bioelectronics allow recording electrophysiological signals and delivering electrical stimulation to control the functions of target cells and tissues.
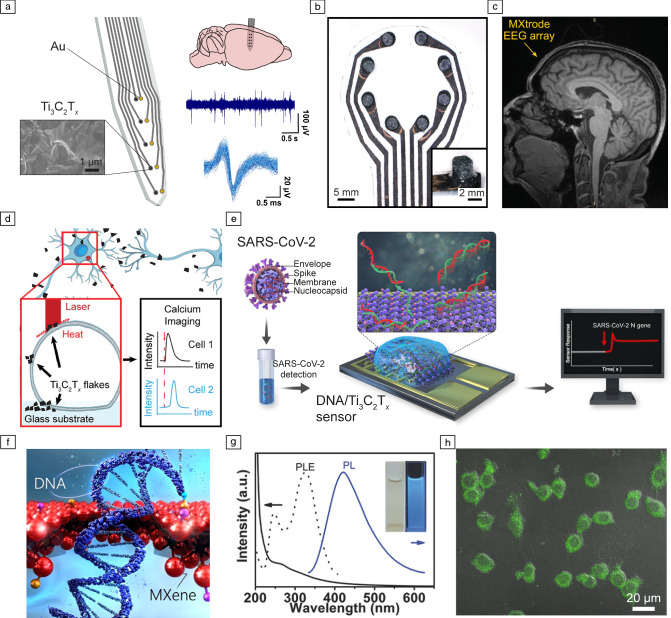
MXenes, and in particular Ti3C2Tx, are attractive materials for bioelectronics, due to their high conductivity4 high specific-surface area,6 ease of processability and scalability.1 Ti3C2Tx microelectrode arrays (MEAs) fabricated through standard microfabrication21,22 show a fourfold reduction in electrochemical impedance compared to Au MEAs with similar dimensions (Figure 2a).21 This allows Ti3C2Tx MEAs to be deployed as probes for cortical surface and intracortical in vivo recording of neural activity (Figure 2a). In the brain, the enhanced interfacial properties of Ti3C2Tx MEAs result in higher sensitivity and signal-to-noise ratio (SNR) compared to size-matched Au devices.21 Ti3C2Tx has also been recently used in large-scale arrays for epidermal sensing.23,24 Rapid prototyping of large-area (~1–102 cm2), high channel count (>40) arrays of Ti3C2Tx printed on cellulose-polyester substrates has been demonstrated for noninvasively monitoring muscle (electromyography, EMG), cardiac (electrocardiography, ECG), and brain (electroencephalography, EEG) activity (Figure 2b).23 These electrode arrays are also more compatible with clinical imaging techniques such as magnetic resonance imaging (MRI) and computed tomography (CT) than metals such as Pt. This is due to Ti3C2Tx weak paramagnetic behavior, low susceptibility difference with human tissues (tissue: −9.04 × 10–6, Ti3C2Tx: 2.08 × 10–7, Pt: 1.0 × 10–4), and low density (Ti3C2Tx: 3.7 × 103 kg m−3, Pt: 3.7 × 103 kg m−3, Figure 2c).23 The superior imaging compatibility of Ti3C2Tx opens opportunities for simultaneous functional MRI and EEG mapping, which is particularly challenging with conventional metal electrodes.23
Figure 2.
Bioelectronics and multimodal biosensing with MXenes. (a) Schematics of an intracortical array patterned with 25 µm Ti3C2Tx and adjacent Au contacts for recording neural activity in vivo. The close-up view shows a scanning electron microscopy image of the surface of one Ti3C2Tx contact. The arrays can record in vivo intracortical neural activity with higher signal-to-noise ratio and reduced susceptibility to 60 Hz interference than Au (reproduced with permission from Reference 21). (b) Photograph of Ti3C2Tx-infused electrode arrays for electroencephalography (EEG). (c) MXene EEG electrode array placed on human forehead and imaged in 3T clinical MRI scanner using T1-weighted magnetization prepared rapid acquisition gradient echo. (b, c) Reproduced with permission from Reference 23. (d) Schematics of the photothermal stimulation of a dorsal root ganglion (DRG) neuron network with Ti3C2Tx flakes (reproduced with permission from Reference 30). (e) Schematics illustrating the operation of ssDNA/Ti3C2Tx sensors for the detection of SARS-CoV-2 nucleocapsid (N) gene (reproduced with permission from Reference 39). (f) Schematic illustrating DNA translocation through a MXene nanopore sensor (reproduced with permission from Reference 44). (g) Photoluminescent Ti3C2Tx MXene quantum dots (MQDs) for multicolor cellular imaging. UV–vis spectra (solid line), photoluminescence excitation (PLE) (dashed line), and photoluminescence (PL) spectra (solid line, Ex = 320 nm) of MQD-100 in aqueous solutions. (h) Merged bright-field and confocal images (Ex = 488 nm) of MQD-100 interfaced with RAW 264.7 cells. (g, h) Reproduced with permission from Reference 48.
Additional approaches for realizing MXene-based wearable bioelectronics include stretchable conductive hydrogels,25 microneedle arrays,26 and sweat-stable epidermal electrodes.27 Ti3C2Tx catalyzed poly(acrylic acid) (PAA) hydrogels exhibit high electrical conductivity, which allows ECG, EMG, and electrooculography (EOG) monitoring with high SNR even after repeated cycles of adhesion and removal.25 The presence of carboxyl groups on the PAA chains leads to electrostatic interactions, which allow the hydrogel electrodes to self-adhere with various surfaces, increasing usability.25,28 Multifunctional MXene-microneedle electrodes can be fabricated by drop-casting Ti3C2Tx onto prefabricated PLA microneedles.26 These microelectrodes penetrate through the outermost epidermis layer of the skin to establish a low-impedance interface compared to conventional gel patch electrodes.26 To increase stability and prevent damage from sweat accumulation, Ti3C2Tx-infused porous cellulose electrodes have been developed.27 Ionic cross-linking of the dangling bonds of Ti3C2Tx in saline solution causes the electrodes to become hydrophobic, which increases resistance to degradation in wet environments.27 These electrodes demonstrate high air permeability and conductivity, which allows ECG, EMG, and EOG measurements.27 Engineering mesoscopic arrangement of Ti3C2Tx flakes in 2D MXene films is an effective method for controlling crack propagation in stretchable sensors.29 Such material structures can be used as strain sensors, and synergistic integration of machine learning (ML) algorithms with these piezoresistive sensors can be used to track continuous full-body motions.29
Modulation of neuronal activity through direct current injection as well as remotely via nongenetic photothermal stimulation has also been achieved with Ti3C2Tx. The enhanced charge injection and charge-storage capacity of Ti3C2Tx-based electrodes enable safe electrical stimulation as demonstrated in vivo in rodent brains.23 The high photothermal energy-conversion efficiency of Ti3C2Tx allows remote stimulation of neurons through photothermal mechanisms.30 Here, the localized heat dissipation by individual MXene flakes, upon illumination with light pulses, results in a change in the membrane capacitance of neurons and subsequent depolarization (Figure 2d).30 The subcellular dimensions of Ti3C2Tx flakes enable photothermal stimulation of networks of connected neurons with high spatiotemporal resolution.30 Remote photothermal stimulation of neurons is also effective when delivered through Ti3C2Tx films.
Multimodal biosensing with MXenes
Sensitive and selective detection of biomarkers, DNA, drugs, and pathogens has deepened our understanding of disease etiologies and their progression.31 Biosensing encompasses the various approaches for the detection of such species through electrochemical, optical, or acoustic techniques.32
Owing to the high surface area, metallic conductivity, and tunable functionalization, MXenes are efficient transducers that can be functionalized with specific biological receptors for electrochemical sensing of different biomolecules.33 For example, Ti3C2Tx electrodes modified with Prussian blue allow real-time glucose detection.34 On the other hand, the functional groups on Ti3C2Tx form hydrogen bonds with target molecules such as urea and Cu2+-chelated creatinine, which increases sensitivity of electrochemical sensors.35 The recent SARS-CoV-2 (COVID-19) pandemic has led to a demand for low-cost, real-time virus detection systems.36 Electrochemical sensing through DNA hybridization at the electrode offers high specificity (comparable to reverse transcriptase polymerase chain reaction, RT-PCR) without the need for specialized equipment.37,38 Single-strand DNA (ssDNA) functionalized Ti3C2Tx electrodes have been developed for real-time sensing of SARS-CoV-2N gene (Figure 2e).39 The sensors exhibit low limit of detection (< 105 copies/mL) and a linear response in the clinically relevant range of 105–109 copies/mL of the virus.39 Alternatively, MXene-based active electrodes can be functionalized with specific antibodies for real-time virus detection.40
Detection and sequencing of individual molecules (e.g., DNA) have been demonstrated at commercial and industrial scales using nanopore-based technologies.41,42 The atomically thin structure of 2D materials makes them ideal candidates for nanopore membranes, as it enables higher spatial resolution than conventional solid-state membranes (e.g., SiNx).43 Recently, Ti2CTx and Ti3C2Tx nanopore membranes have been demonstrated for selective and sensitive DNA detection with high yield (Figure 2f).44 The passage of individual molecules through the nanopore is detected as a change in ionic current across the membrane.44 By tuning the surface chemistry and electrical conductivity of MXenes, a vast library of nanopore membranes with high selectivity and specificity can be developed.45
Live cell imaging is a technique for direct visualization of cells and biological processes.46,47 Quantum dots (QDs) are efficient contrast agents for live cell imaging due to their tunable optical properties and surface functionalities.46,47 Recently, Ti3C2Tx QDs have been obtained through facile hydrothermal synthesis.48 Increasing the synthesis temperature results in larger MXene QDs, which directly influence the photoluminescence (PL) excitation and the subsequent PL response (Figure 2g).48 When incubated with macrophages (RAW 264.7 cells) for 4 h, the Ti3C2Tx QDs are endocytosed.48 Illumination with different wavelengths leads to PL response of specific MXene QDs, facilitating multimodal bioimaging (Figure 2h).48 Optical sensing of intracellular pH has been demonstrated using polyethylenimine (PEI)-passivated Ti3C2Tx QDs. Here, variations in local pH cause protonation and deprotonation at the surface of the PEI-Ti3C2Tx QDs, which localizes valence electrons resulting in reduced photoabsorption.49 A similar approach has been used for optical sensing of glutathione (GSH), where molecular bonding between Ti3C2Tx QDs and GSH generates a QD-GSH complex.50 Electron transfer from Ti3C2Tx to GSH quenches the PL response of Ti3C2Tx QDs.50 Quenching of the fluorescence response of heteroatom (S and N) doped Nb2C QDs by Cu2+ ions has also been used for optical sensing of Cu2+ ions and live cellular imaging.51
Tissue engineering and immunomodulation with MXenes
Tissue engineering involves the design and engineering of biological tissues for restoring or regenerating lost functions due to disease or injury.52 In this context, MXenes have attracted increasing attention in stem cell engineering,53–55 regenerative medicine,56–59 artificial organs,56 and immunomodulation.60–62
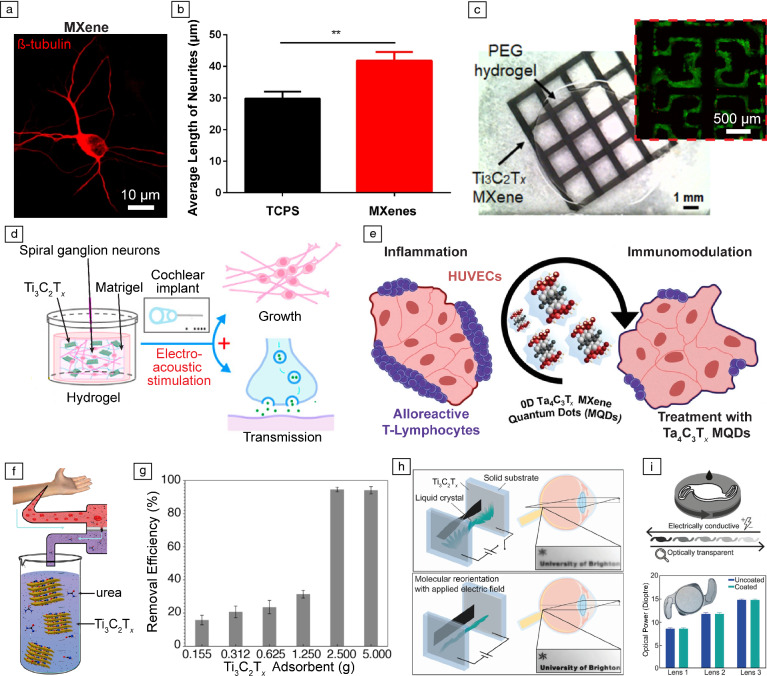
Guided differentiation of stem cells through external physical and chemical cues is a promising approach for tissue regeneration and function recovery.63 Ti3C2Tx has been demonstrated to promote differentiation of stem cells into neural,53 cardiac,54 and osteogenic phenotypes.59 Electrically conductive Ti3C2Tx films promote neurite growth and significantly enhance neuronal spiking, which is indicative of cell maturation (Figure 3a–b).53 Osteogenic differentiation of human mesenchymal stem cells and pre-osteoblasts on Ti3C2Tx films and composites has also been achieved.54,55 The ultimate goal of these MXene-based tissue engineering platforms is the development of novel approaches for regenerative medicine and artificial organ growth.56–59 For example, 3D printed Ti3C2Tx conductive scaffolds lead to phenotypical and electrophysiological maturation of connected networks of cardiomyocytes, resulting in synchronous beating (Figure 3c).56 Recently, dual functionality of Ti3C2Tx and bioactive glass scaffolds for bone tumor ablation and regeneration of bone tissue has been demonstrated, enabled by the high photothermal energy-conversion efficiency and cellular adhesion of the scaffold.58 Implantation of Ti3C2Tx scaffolds into artificially created cranial defects in the frontal-parietal bone of rats results in accelerated bone regeneration compared to control scaffolds without Ti3C2Tx.58 Furthermore, Ti3C2Tx and Matrigel hydrogels have been demonstrated to promote the development of growth cones and neurites in spiral ganglion neurons under electrical stimulation (Figure 3d).57 This approach can be leveraged for restoring impaired cochlear and auditory functions.57
Figure 3.
Tissue engineering and therapeutic technologies enabled by MXenes. (a) Representative newly generated neurons cultured on Ti3C2Tx flakes. (b) Average length of neurites for new neurons cultured on tissue culture polystyrene (TCPS) and Ti3C2Tx. ** p < 0.01. (a, b) Adapted with permission from Reference 53. (c) Printed Ti3C2Tx on PEG hydrogel with a square (81 mm2) pattern. The inset presents patterned-induced pluripotent stem cell derived cardiomyocytes on 3D printed Ti3C2Tx-PEG hydrogel after seven days in culture (reproduced with permission from Reference 56). (d) Schematic illustrating 3D Ti3C2Tx MXene–Matrigel with electroacoustic stimulation for enhanced growth of spiral ganglion neurons (reproduced with permission from Reference 57). (e) Schematic representation of in vitro immunomodulatory model (reproduced with permission from Reference 61). (f) Schematics of Ti3C2Tx flakes as adsorbents for urea in dialysate. (g) Urea removal efficiency (%) from dialysate using different mass loadings of Ti3C2Tx. (f, g) Reproduced with permission from Reference 68. (h) Schematics of a proof-of-concept design for Ti3C2Tx adjustable focus lens. (i) (Top) Schematic of Ti3C2Tx spin-cast onto an acrylate intraocular lens (IOL); (bottom) optical power measurement on three different lens powers before and after coating with Ti3C2Tx (n = 5). Inset is an optical image of spin-coated IOL. (h, i) Reproduced with permission from Reference 69.
The immune response plays a critical role in governing the success of regenerative medicine and tissue-engineering strategies in vivo. Thus, understanding and modulating the immune response toward the implanted biomaterials is of paramount importance.64 The broad biocompatibility of Ti3C2Tx has been extensively investigated and established.21,48 Recently, high-dimensional immune and functional profiling of Nb4C3, Mo2Ti2C3, and Ta4C3Tx has revealed that these forms of MXenes do not affect immune cell functionality and have excellent biocompatibility.65 Furthermore, these MXenes possess intrinsic immunomodulatory properties that facilitate engineering the immune and inflammatory response of different cells and tissues (Figure 3e).60,61 For example, treating activated cocultures of human umbilical vein endothelial cells (HUVECs) and interferon-gamma (IFN-γ) expressing T-lymphocytes with Ta4C3Tx QDs significantly reduced the percentage of IFN-γ+ T-lymphocytes, indicating successful immunomodulation.61
MXene-based therapeutic technologies
Therapeutic technologies aim at curing or alleviating pathologies, thereby impacting patient health directly. Since 2016, MXenes have been highly investigated for cancer therapeutics and drug delivery.9,13,66 Today, there is a surge in novel therapeutic platforms such as wound dressings,67 dialysis membranes,68 and intraocular lenses.69
Ti3C2Tx-based multifunctional hydrogels have been developed as wound healing dressings and benchmarked against commercial wound dressings.67 Ti3C2Tx hydrogels exhibit high electrical conductivity, mechanical compliance with the wound bed, and biodegradability.67 Application of an external electric field via the conductive Ti3C2Tx scaffolds results in a significant reduction of the wound area and accelerated healing.67 The antimicrobial properties of MXenes represent an added benefit to support safe wound healing platforms.70
MXene materials are also being researched in clinical therapeutics. The 2D layered structure can be easily intercalated by H2O and organic molecules and leveraged to capture metabolic byproducts and toxins, such as urea (Figure 3f).68 By engineering the distribution of the functional groups (–OH, –O, and –F) on the surface of Ti3C2Tx, the affinity of Ti3C2Tx membranes toward urea can be fine-tuned.68 Ti3C2Tx membranes show high efficiency toward urea removal from dialysate of patients with chronic kidney disease (Figure 3g).68 Engineering the composition and chemistry of MXene membranes can further boost the filtering efficiency for application in artificial kidneys. Physisorption and reduction of heavy metal ions and contaminants using MXene-based membrane is also gaining traction for water purification.71,72 Ti3C2Tx thin films have also been applied on conductive coatings in accommodative intraocular lenses (Figure 3h).69,73 Under applied electric fields, Ti3C2Tx transparent thin films enable molecular reorientation of the sandwiched liquid crystal for tuning the refractive index and optical power of the lens architecture (Figure 3i).69 Such accommodative intraocular lenses can mimic the function of a natural lens and be deployed as replacement lens to restore visual accommodation.
Conclusions and future outlook
The exponential growth of MXenes in biomedical research, and particularly in the fields of bioelectronics, biosensing, tissue engineering, and therapeutics, is fueled by material synthesis and processing advances. The synthesis of MXenes with varying chemical compositions (e.g., Ti3C2Tx, Nb4C3, and Ta4C3Tx) along with the growing understanding of their fundamental properties have led to the development of application-tailored structures. For example, the high electrical conductivity, surface area, and ease of functionalization of Ti3C2Tx has enabled bioelectronics and biosensing applications.9 Other forms of MXenes, such as Nb4C3, Mo2Ti2C3, and Ta4C3Tx are gaining traction for immunomodulation due to their excellent biocompatibility and intrinsic immunomodulatory properties.65
We note that, in the recent past, the focus was primarily on MXene biosensing and theranostics. Today, wearable bioelectronics and tissue-engineering applications of MXenes show high potential for further expansion and translation. In the field of wearables, with improvements in environmental stability and production scale-up, MXenes can potentially replace high-cost noble metals (such as gold and platinum) and support high-throughput device manufacturing approaches. In tissue engineering, the ease of fabricating functionalized MXene composites can support the realization of multimodal platforms for regenerative medicine, artificial organ engineering, and immunomodulation.
Pushing MXene-based biomedical technologies through the translational pipeline for eventual human use will necessitate a thorough evaluation of the biosafety, toxicity, and long-term stability of MXenes. Although detailed investigations of the biosafety of Nb4C3, Mo2Ti2C3, and Ta4C3 were recently demonstrated,65 similar analysis needs to be performed for other MXenes and in larger animal models over chronic time scales, in relevant use-case and form factor scenarios, and according to established standards.74 Implantable bioelectronic devices and chronic biosensors will require strategies for minimizing the host immune response and to extend the material lifetime to the required time scales. Scaling-up of materials synthesis and processing is also of paramount importance for the commercialization of any MXene-based biomedical technology. Finally, the regulatory processes for evaluating the efficacy of each clinical application of MXenes need to be well defined to facilitate and support commercialization. Synergistic developments addressing these upcoming challenges will be key to bringing a new generation of MXene-based diagnostic and therapeutic technologies to the clinic.
Acknowledgments
We would like to acknowledge funding support from the National Institutes of Health (Award Nos. R01AR081062, K12HD073945, and R01NS121219-01).
Biographies
Raghav Garg
is a postdoctoral researcher in the Department of Neurology and in the Center for Neuroengineering and Therapeutics at the University of Pennsylvania. He obtained his BTech degree in biological sciences and bioengineering from the Indian Institute of Technology-Kanpur, India, in 2015, MSc degree in biomedical engineering from Carnegie Mellon University (CMU) in 2016, and a PhD degree in materials science and engineering from CMU in 2021. For his doctoral research, he received the Bradford and Diane Smith Graduate Fellowship (2019) and the Paxton Award for Best Doctoral Dissertation in Materials Science and Engineering (2021). Garg can be reached by email at raghav.garg@pennmedicine.upenn.edu. 
Flavia Vitale
is an assistant professor of neurology and Bioengineering at the University of Pennsylvania. She received her PhD degree in chemical engineering from the Università di Roma "La Sapienza," Italy, then completed her training in chemical and biomolecular engineering at Rice University in 2015, where she was a Welch Foundation Postdoctoral Fellow. She is the recipient of the National Institutes of Health Interdisciplinary Rehabilitation Engineering Career Development Award (IREK12), the CURE Taking Flight, the McCabe Fellow, and the iCANX Global Young Scientist Awards. Her research focuses on novel bioelectronic technologies based on MXenes and other nanomaterials. Vitale can be reached by email at vitalef@pennmedicine.upenn.edu. 
Conflict of interest
F.V. is a co-inventor on two following pending international patent applications related to MXene bioelectronics: PCT/US2020/055147; PCT/US2018/051084.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Raghav Garg, Email: raghav.garg@pennmedicine.upenn.edu.
Flavia Vitale, Email: vitalef@pennmedicine.upenn.edu.
References
- 1.VahidMohammadi A, Rosen J, Gogotsi Y. Science. 2021;372(6547):abf1581. doi: 10.1126/science.abf1581. [DOI] [PubMed] [Google Scholar]
- 2.Gogotsi Y, Anasori B. ACS Nano. 2019;13:8491. doi: 10.1021/acsnano.9b06394. [DOI] [PubMed] [Google Scholar]
- 3.Rajan AC, Mishra A, Satsangi S, Vaish R, Mizuseki H, Lee K-R, Singh AK. Chem. Mater. 2018;30:4031. doi: 10.1021/acs.chemmater.8b00686. [DOI] [Google Scholar]
- 4.Hantanasirisakul K, Gogotsi Y. Adv. Mater. 2018;30:1804779. doi: 10.1002/adma.201804779. [DOI] [PubMed] [Google Scholar]
- 5.Lipatov A, Lu H, Alhabeb M, Anasori B, Gruverman A, Gogotsi Y, Sinitskii A. Sci. Adv. 2018;4:eaat0491. doi: 10.1126/sciadv.aat0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Zhang Z, Zhou Z. J. Energy Chem. 2018;27:73. doi: 10.1016/j.jechem.2017.08.004. [DOI] [Google Scholar]
- 7.Shuck CE, Sarycheva A, Anayee M, Levitt A, Zhu Y, Uzun S, Balitskiy V, Zahorodna V, Gogotsi O, Gogotsi Y. Adv. Eng. Mater. 2020;22:1901241. doi: 10.1002/adem.201901241. [DOI] [Google Scholar]
- 8.Peng J, Chen X, Ong W-J, Zhao X, Li N. Chemistry. 2019;5:18. doi: 10.1016/j.chempr.2018.08.037. [DOI] [Google Scholar]
- 9.F. Vitale, N. Driscoll, B. Murphy, “Biomedical Applications of MXenes,” in 2D Metal Carbides and Nitrides (MXenes) (Springer, New York, 2019), pp. 503–524
- 10.Xu B, Zhu M, Zhang W, Zhen X, Pei Z, Xue Q, Zhi C, Shi P. Adv. Mater. 2016;28:3333. doi: 10.1002/adma.201504657. [DOI] [PubMed] [Google Scholar]
- 11.Han X, Huang J, Lin H, Wang Z, Li P, Chen Y. Adv. Healthc. Mater. 2018;7:1701394. doi: 10.1002/adhm.201701394. [DOI] [PubMed] [Google Scholar]
- 12.Rasool K, Helal M, Ali A, Ren CE, Gogotsi Y, Mahmoud KA. ACS Nano. 2016;10:3674. doi: 10.1021/acsnano.6b00181. [DOI] [PubMed] [Google Scholar]
- 13.Lin H, Gao S, Dai C, Chen Y, Shi J. J. Am. Chem. Soc. 2017;139:16235. doi: 10.1021/jacs.7b07818. [DOI] [PubMed] [Google Scholar]
- 14.Lim GP, Soon CF, Ma NL, Morsin M, Nayan N, Ahmad MK, Tee KS. Environ. Res. 2021;201:111592. doi: 10.1016/j.envres.2021.111592. [DOI] [PubMed] [Google Scholar]
- 15.Dai C, Lin H, Xu G, Liu Z, Wu R, Chen Y. Chem. Mater. 2017;29:8637. doi: 10.1021/acs.chemmater.7b02441. [DOI] [Google Scholar]
- 16.Jastrzębska A, Szuplewska A, Wojciechowski T, Chudy M, Ziemkowska W, Chlubny L, Rozmysłowska A, Olszyna A. J. Hazard. Mater. 2017;339:1. doi: 10.1016/j.jhazmat.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Huang Q, Huang H, Mao L, Liu M, Zhang X, Wei Y. Nanoscale. 2020;12:3574. doi: 10.1039/C9NR08542D. [DOI] [PubMed] [Google Scholar]
- 18.Lens.org Database, Keywords: MXene, Biomedical, Bioelectronic, Biosensor, Tissue engineering, Cancer, DNA, Immune, Implant, Wound, Therapeutic, Antimicrobial, Range: 2019–2022. https://link.lens.org/BaEmcCzSO2. Accessed 11 Nov 2022
- 19.Garg R, Roman DS, Wang Y, Cohen-Karni D, Cohen-Karni T. Biophysics Reviews. 2021;2:041304. doi: 10.1063/5.0073870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.San Roman D, Garg R, Cohen-Karni T. APL Mater. 2020;8:100906. doi: 10.1063/5.0020455. [DOI] [Google Scholar]
- 21.Driscoll N, Richardson AG, Maleski K, Anasori B, Adewole O, Lelyukh P, Escobedo L, Cullen DK, Lucas TH, Gogotsi Y. ACS Nano. 2018;12:10419. doi: 10.1021/acsnano.8b06014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.N. Driscoll, K. Maleski, A.G. Richardson, B. Murphy, B. Anasori, T.H. Lucas, Y. Gogotsi, F. Vitale, J. Vis. Exp. (156), e60741 (2020) [DOI] [PubMed]
- 23.Driscoll N, Erickson B, Murphy BB, Richardson AG, Robbins G, Apollo NV, Mentzelopoulos G, Mathis T, Hantanasirisakul K, Bagga P. Sci. Transl. Med. 2021;13:e8629. doi: 10.1126/scitranslmed.abf8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy BB, Mulcahey PJ, Driscoll N, Richardson AG, Robbins GT, Apollo NV, Maleski K, Lucas TH, Gogotsi Y, Dillingham T. Adv. Mater. Technol. 2020;5:2000325. doi: 10.1002/admt.202000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Q, Pan X, Lin C, Gao H, Cao S, Ni Y, Ma X. Chem. Eng. J. 2020;401:126129. doi: 10.1016/j.cej.2020.126129. [DOI] [Google Scholar]
- 26.Yang Y-C, Lin Y-T, Yu J, Chang H-T, Lu T-Y, Huang T-Y, Preet A, Hsu Y-J, Wang L, Lin T-E. ACS Appl. Nano Mater. 2021;4:7917. doi: 10.1021/acsanm.1c01237. [DOI] [Google Scholar]
- 27.Song D, Ye G, Zhao Y, Zhang Y, Hou X, Liu N. ACS Nano. 2022;16(10):17168. doi: 10.1021/acsnano.2c07646. [DOI] [PubMed] [Google Scholar]
- 28.Gan D, Xing W, Jiang L, Fang J, Zhao C, Ren F, Fang L, Wang K, Lu X. Nat. Commun. 2019;10(1):1487. doi: 10.1038/s41467-019-09351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang H, Li J, Xiao X, Wang J, Li Y, Li K, Li Z, Yang H, Wang Q, Yang J. Nat. Commun. 2022;13:1. [Google Scholar]
- 30.Wang Y, Garg R, Hartung JE, Goad A, Patel DA, Vitale F, Gold MS, Gogotsi Y, Cohen-Karni T. ACS Nano. 2021;15:14662. doi: 10.1021/acsnano.1c04431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu R, Ye X, Cui T. Research. 2020;2020:7949037. doi: 10.34133/2020/7949037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhalla N, Jolly P, Formisano N, Estrela P. Essays Biochem. 2016;60(1):1. doi: 10.1042/EBC20150001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinha A, Dhanjai, Zhao H, Huang Y, Lu X, Chen J, Jain R. Trends Analyt. Chem. 2018;105:424. doi: 10.1016/j.trac.2018.05.021. [DOI] [Google Scholar]
- 34.Lei Y, Zhao W, Zhang Y, Jiang Q, He JH, Baeumner AJ, Wolfbeis OS, Wang ZL, Salama KN, Alshareef HN. Small. 2019;15:1901190. doi: 10.1002/smll.201901190. [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Jiang X, Zhang R, Zhang Y, Wu L, Lu W, Li J, Li Y, Zhang H. Adv. Func. Mater. 2019;29:1807326. doi: 10.1002/adfm.201807326. [DOI] [Google Scholar]
- 36.Morales-Narváez E, Dincer C. Biosens. Bioelectron. 2020;163:112274. doi: 10.1016/j.bios.2020.112274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loan PTK, Zhang W, Lin CT, Wei KH, Li LJ, Chen CH. Adv. Mater. 2014;26:4838. doi: 10.1002/adma.201401084. [DOI] [PubMed] [Google Scholar]
- 38.Vermisoglou E, Panáček D, Jayaramulu K, Pykal M, Frébort I, Kolář M, Hajdúch M, Zbořil R, Otyepka M. Biosens. Bioelectron. 2020;166:112436. doi: 10.1016/j.bios.2020.112436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen WY, Lin H, Barui AK, Gomez AMU, Wendt MK, Stanciu LA. ACS Appl. Nano Mater. 2021;5:1902. doi: 10.1021/acsanm.1c03520. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Peng Z, Holl NJ, Hassan MR, Pappas JM, Wei C, Izadi OH, Wang Y, Dong X, Wang C. ACS Omega. 2021;6:6643. doi: 10.1021/acsomega.0c05421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jain M, Olsen HE, Paten B, Akeson M. Genome Biol. 2016;17:239. doi: 10.1186/s13059-016-1103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Z, Jiang Y, Dunphy DR, Adams DP, Hodges C, Liu N, Zhang N, Xomeritakis G, Jin X, Aluru N. Nat. Mater. 2010;9:667. doi: 10.1038/nmat2805. [DOI] [PubMed] [Google Scholar]
- 43.Qiu H, Zhou W, Guo W. ACS Nano. 2021;15:18848. doi: 10.1021/acsnano.1c07960. [DOI] [PubMed] [Google Scholar]
- 44.Mojtabavi M, VahidMohammadi A, Liang W, Beidaghi M, Wanunu M. ACS Nano. 2019;13:3042. doi: 10.1021/acsnano.8b08017. [DOI] [PubMed] [Google Scholar]
- 45.Shi W, Friedman AK, Baker LA. Anal. Chem. 2017;89:157. doi: 10.1021/acs.analchem.6b04260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma P, Brown S, Walter G, Santra S, Moudgil B. Adv. Colloid Interface. Sci. 2006;123:471. doi: 10.1016/j.cis.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 47.Kairdolf BA, Smith AM, Stokes TH, Wang MD, Young AN, Nie S. Annu. Rev. Anal. Chem. 2013;6:143. doi: 10.1146/annurev-anchem-060908-155136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xue Q, Zhang H, Zhu M, Pei Z, Li H, Wang Z, Huang Y, Huang Y, Deng Q, Zhou J. Adv. Mater. 2017;29:1604847. doi: 10.1002/adma.201604847. [DOI] [PubMed] [Google Scholar]
- 49.Chen X, Sun X, Xu W, Pan G, Zhou D, Zhu J, Wang H, Bai X, Dong B, Song H. Nanoscale. 2018;10:1111. doi: 10.1039/C7NR06958H. [DOI] [PubMed] [Google Scholar]
- 50.Xu X, Zhang H, Diao Q, Zhu Y, Yang G, Ma B. J. Mater. Sci. Mater. Electron. 2020;31:175. doi: 10.1007/s10854-019-02682-2. [DOI] [Google Scholar]
- 51.Yan X, Ma J, Yu K, Li J, Yang L, Liu J, Wang J, Cai L. Chin. Chem. Lett. 2020;31:3173. doi: 10.1016/j.cclet.2020.05.020. [DOI] [Google Scholar]
- 52.Langer R, Vacanti JP. Science. 1993;260:920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 53.Li Y, Hu Y, Wei H, Cao W, Qi Y, Zhou S, Zhang P, Li H, Li G-L, Chai R. J. Nanobiotechnol. 2022;20:1. doi: 10.1186/s12951-021-01184-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang R, Chen X, Dong Y, Zhang X, Wei Y, Yang Z, Li W, Guo Y, Liu J, Yang Z. ACS Appl. Bio Mater. 2020;3:2125. doi: 10.1021/acsabm.0c00007. [DOI] [PubMed] [Google Scholar]
- 55.Jang J-H, Lee E-J. Materials. 2021;14:4453. doi: 10.3390/ma14164453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Basara G, Saeidi-Javash M, Ren X, Bahcecioglu G, Wyatt BC, Anasori B, Zhang Y, Zorlutuna P. Acta Biomater. 2022;139:179. doi: 10.1016/j.actbio.2020.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liao M, Hu Y, Zhang Y, Wang K, Fang Q, Qi Y, Shen Y, Cheng H, Fu X, Tang M. ACS Nano. 2022;16:16744. doi: 10.1021/acsnano.2c06306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pan S, Yin J, Yu L, Zhang C, Zhu Y, Gao Y, Chen Y. Advanced Science. 2020;7:1901511. doi: 10.1002/advs.201901511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen K, Chen Y, Deng Q, Jeong S-H, Jang T-S, Du S, Kim H-E, Huang Q, Han C-M. Mater. Lett. 2018;229:114. doi: 10.1016/j.matlet.2018.06.063. [DOI] [Google Scholar]
- 60.Rafieerad A, Yan W, Sequiera GL, Sareen N, Abu-El-Rub E, Moudgil M, Dhingra S. Adv. Healthc. Mater. 2019;8:1900569. doi: 10.1002/adhm.201900569. [DOI] [PubMed] [Google Scholar]
- 61.Rafieerad A, Yan W, Alagarsamy KN, Srivastava A, Sareen N, Arora RC, Dhingra S. Adv. Funct. Mater. 2021;31:2106786. doi: 10.1002/adfm.202106786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang X, Wu Q, Wu Y, Xi X, Cao J, Chu H, Liu Q, Li Y, Wu W, Fang X. Nano Lett. 2022;22:8321. doi: 10.1021/acs.nanolett.2c03260. [DOI] [PubMed] [Google Scholar]
- 63.Bianco P, Robey PG. Nature. 2001;414:118. doi: 10.1038/35102181. [DOI] [PubMed] [Google Scholar]
- 64.Kelly SH, Shores LS, Votaw NL, Collier JH. Adv. Drug Deliv. Rev. 2017;114:3. doi: 10.1016/j.addr.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fusco L, Gazzi A, Shuck CE, Orecchioni M, Alberti D, D'Almeida SM, Rinchai D, Ahmed E, Elhanani O, Rauner M. Adv. Mater. 2022;34:2205154. doi: 10.1002/adma.202205154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu D, Shou X, Yu Y, Wang X, Chen G, Zhao Y, Sun L. Adv. Funct. Mater. 2022;32:2205847. doi: 10.1002/adfm.202205847. [DOI] [Google Scholar]
- 67.Mao L, Hu S, Gao Y, Wang L, Zhao W, Fu L, Cheng H, Xia L, Xie S, Ye W. Adv. Healthc. Mater. 2020;9:2000872. doi: 10.1002/adhm.202000872. [DOI] [PubMed] [Google Scholar]
- 68.Meng F, Seredych M, Chen C, Gura V, Mikhalovsky S, Sandeman S, Ingavle G, Ozulumba T, Miao L, Anasori B. ACS Nano. 2018;12:10518. doi: 10.1021/acsnano.8b06494. [DOI] [PubMed] [Google Scholar]
- 69.Ward EJ, Lacey J, Crua C, Dymond MK, Maleski K, Hantanasirisakul K, Gogotsi Y, Sandeman S. Adv. Funct. Mater. 2020;30:2000841. doi: 10.1002/adfm.202000841. [DOI] [Google Scholar]
- 70.Zhou L, Zheng H, Liu Z, Wang S, Liu Z, Chen F, Zhang H, Kong J, Zhou F, Zhang Q. ACS Nano. 2021;15:2468. doi: 10.1021/acsnano.0c06287. [DOI] [PubMed] [Google Scholar]
- 71.Xie X, Chen C, Zhang N, Tang Z-R, Jiang J, Xu Y-J. Nature Sustainability. 2019;2:856. doi: 10.1038/s41893-019-0373-4. [DOI] [Google Scholar]
- 72.Ihsanullah I. Chem. Eng. J. 2020;388:124340. doi: 10.1016/j.cej.2020.124340. [DOI] [Google Scholar]
- 73.Cooksley G, Dymond M, Stewart N, Bucca G, Hesketh A, Lacey J, Gogotsi Y, Sandeman SR. 2D Mater. 2022;10:014003. doi: 10.1088/2053-1583/ac95a7. [DOI] [Google Scholar]
- 74.International Organization for Standardization (ISO), Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing within a Risk Management Process (ISO 10993-1, 2018)