Abstract
Cardiovascular disease (CVD) comprises a broad spectrum of pathological conditions that affect the heart or blood vessels, including sequelae that arise from damaged vasculature in other organs of the body, such as the brain, kidneys or eyes. Atherosclerosis is a chronic inflammatory disease of the arterial intima and is the primary cause of coronary artery disease, peripheral vascular disease, heart attack, stroke and renal pathology. It represents a leading cause of mortality worldwide and the loss of human productivity that is marked by an altered immune response. Endometriosis is a heritable, heterogeneous, common gynecological condition influenced by multiple genetic, epigenetic and environmental factors, affecting up to 10% of the female population of childbearing age, causing pain and infertility; it is characterized by the ectopic growth of endometrial tissue outside the uterine cavity. Of note, epidemiological data obtained thus far have suggested a link between endometriosis and the risk of developing CVD. The similarities observed in specific molecular and cellular pathways of endometriosis and CVD may be partially explained by a shared genetic background. The present review presents and discusses the shared genetic factors which have been reported to be associated with the development of both disorders.
Keywords: cardiovascular disease, endometriosis, inflammation, angiogenesis, genetics, gene polymorphisms
1. Introduction
The cardiovascular system consists of the heart and its blood vessels. Cardiovascular disease (CVD) is a general term that describes a type of disease that affects any of these components. The majority of the anatomical structures that are related to the cardiovascular system display a common tissue organization, with endothelial cells (ECs), smooth muscle cells (SMCs) and myocardial cells being three of the major cell types at the cellular level. In this context, several types of CVD exist, affecting only the blood vessels or the heart, but most commonly, both of these, either simultaneously as result of a common cause or as a sequalae arising, for example, from one condition that leads to other diseases within the group (e.g., coronary vessel disease that leads to ischemic cardiac disease or arrhythmias) (1).
Endometriosis is a common, benign, estrogen-dependent gynecological disease, associated with chronic pelvic pain and subfertility, defined by the presence of ectopic endometrial glands and stroma outside of the uterine cavity on other organs (2,3). It affects 10 to 15% of women of reproductive age, exhibiting varying symptoms such as severe pelvic pain, irregular menstrual bleeding, heavy menstrual pain, urinary tract and gastrointestinal symptoms and pain during intercourse, and can significantly affect the quality of life of patients (4-6). Notably, endometriosis possesses numerous features that are reminiscent of a benign neoplastic process, which has the potential for malignant transformation. Genetic factors contribute to the development of this condition, in combination with environmental ones, such as toxins and pollution agents (3,7). Although the exact molecular and pathophysiological pathways leading to endometriosis remain unclear, various hypotheses have been suggested thus far, and all cases are not able to be explained by one theory alone (8). There is strong evidence to suggest the contribution of not only hormonal aspects, but also of multiple immune-mediated processes related to chronic inflammation, increased oxidative stress and an atherogenic lipid profile (9,10).
Women with endometriosis, which is a heterogeneous condition, are at a high risk of developing several other chronic diseases, including cancer (11) and various autoimmune diseases, such as systemic lupus erythematosus (SLE), multiple sclerosis, rheumatoid arthritis (RA), Crohn's disease, scleroderma, ulcerative colitis, autoimmune thyroid disorder, Sjögren's syndrome, coeliac disease and ankylosing spondylitis (12-14). Of note, previous findings have suggested that endometriosis can increase the susceptibility for cardiovascular disorders in these women, considering that both conditions share pathogenic mechanisms based on hormonal deviations, as well as aberrant immunology and genetic profiles (15-17). Moreover, studies have reported a similar potential association between endometriosis and atherosclerotic CVD, underlying similar pathogenic mechanisms including coronary microvascular dysfunction, endothelial dysfunction and atherogenic lipid profile (18,19). There is an extended amount of literature focusing on the increased risk that women with endometriosis have for developing myocardial infarction, ischemic heart disease, hypertension, atherosclerosis requiring bypass and angioplasty or stenting procedures (16,20-24). However, CVD in women with endometriosis remains underdiagnosed and, therefore, detailed studies are required to fully understand the clinical relevance, as well as the underlying pathophysiological mechanisms of the interactions between these conditions.
The present review discusses and summarizes the observed increased risk of CVD among women with endometriosis from the genetic point of view, focusing on the potential underlying shared genetic factors that warrant further study. Understanding these associations in depth requires mechanistic and functional genomics research in an effort to determine the causal shared risk factors.
2. Genetics of CVD and endometriosis
Recent advances in human genetics, mainly due to the rapid development of genome-wide association studies (GWAS), polygenic risk score and next-generation sequencing (NGS) technologies, have revealed thousands of genetic associations between common DNA sequence variants [e.g., single nucleotide polymorphisms (SNPs)] and complex human diseases or traits, thus leading to a marked increase in the number of risk alleles identified in patients with complex diseases. CVDs are the leading public health problem worldwide (25). Among these, atherosclerosis of the coronary circulation along with its complications of acute coronary syndromes, and ischemic heart failure are the principal entities. Genetic studies have led to a more in-depth knowledge of the pathophysiological processes in coronary artery disease (CAD) and the identification of novel treatment targets (e.g., in lipid metabolism).
The first GWAS for CAD were published in 2007 (26-28). Since then, the appearance of high-throughput genotyping arrays along with advances in statistical methods and large data sets have revolutionized the identification of thousands of disease-associated variants. It is currently well-established that CVDs are characterized by a strong genetic background. This genetic component includes in its minority, genes that are involved in pathways of traditional risk factors. Of note, experimental research has revealed novel, and, in some circumstances surprisingly, non-predictable mechanisms. Three independent pioneer studies in CVD research almost synchronously identified the chromosome 9p21 locus as a risk factor for CVD (26-29). Together with the lipoprotein(a) (LPA) locus, they exert the most prominent effect on the risk of developing CAD (30). The 9p21 locus was initially designated as a 'gene desert' as no known genes could be recognized within it. Although the coding genes of the cyclin-dependent kinase (CDK) inhibitors 2A/2B (CDKN2A/CDKN2B) reside in close proximity, extensive research could not provide evidence for the involvement of these genes (31). On the other hand, long non-coding RNA (lncRNA) ANRIL was identified in the same genetic region and genetic variants in that region proved to affect its expression. The increased expression of linear ANRIL was found to enhance atherosclerosis (32), in contrast to circular ANRIL, which was protective (33). Genetic engineering in induced pluripotent stem cells identified that the risk genotype accompanied by an increased ANRIL expression, also induced a pro-atherogenic phenotype in vascular SMCs (VSMCs) (34).
Currently, known CAD genes that target the vessel wall and the process of atherosclerosis independently of the genes involved in the regulation of risk factors, can be classified as genes affecting plaque progression and platelet function, although they are not limited to this classification. Genes involved in the pathophysiological process include genes affecting the vascular remodeling (VSMCs), the proliferation/mitosis of angiogenic cells, transcriptional regulation, inflammation, angiogenesis and nitric oxide signaling (35).
Endometriosis is a complex disease and genetic, epigenetic and environmental influences interact with each other, thus leading to the disease phenotype. Apart from the aberrant immunological responses, angiogenesis processes and biochemical alterations leading to the growth of endometrial tissue outside the uterus, which impair fertility, the disease has a strong genetic component, as firstly shown by monozygotic twin-based and family studies performed (36,37). Considering that the association between genetic polymorphisms and clinical disease has long been recognized, 'candidate gene' studies have greatly assisted investigators in identifying causal genetic variants underlying endometriosis over the past decades, taking into account hundreds of genes and SNPs (38,39), while GWAS have succeeded in identifying common genetic variants of moderate effects for various complex diseases (40-44). Currently, the number of novel endometriosis-associated loci is increased due to the developments in NGS techniques, which result in the detection of common or rarer variants conferring a high risk of disease. However, apart from the progress made in the identification and confirmation of novel SNPs associated with endometriosis, many studies have obtained controversial results, mainly due to the enrolment of small populations and the unclear definition of race and/or ethnicity (38). Thus, some of these endometriosis-associated loci were confirmed in other populations and/or replication studies, while further new loci were also identified through meta-analyses, as presented in a comprehensive analysis by Sapkota et al (45). In a meta-analysis conducted by Rahmioglu et al (39), involving four GWAS and four replication datasets, a genome-wide level association of SNPs in six out of nine genetic loci was detected for rs12700667 on 7p15.2, rs7521902 near Wingless and Int-1 (Wnt) family member 4 (WNT4), rs10859871 near vezatin adherens junctions transmembrane protein (VEZT) gene, rs1537377 near CDKN2B antisense RNA 1 (CDKN2B-AS1), rs7739264 near inhibitor of DNA binding 4 (ID4) gene and rs13394619 in growth regulating estrogen receptor binding 1 (GREB1) gene with all these loci; however, VEZT SNP exhibited a stronger association with stage III/IV of endometriosis. A further meta-analysis by Sapkota et al (44) identified five novel loci significantly (at a genome-wide level) associated with the risk of developing endometriosis, with these genes being involved in sex steroid hormone pathways [fibronectin 1, coiled-coil domain containing 170, estrogen receptor 1 (ESR1), spectrin repeat containing nuclear envelope protein 1 and follicle stimulating hormone subunit beta]. Since then, in the largest GWAS and replication meta-analysis of endometriosis that was performed to date, 42 loci (31 novel) were identified. According to that study, these loci explained 5.5% of disease variance with roles in progesterone resistance, cell cycle regulation, oncogenesis and ovarian tissue enhancers (46). Of note, whole exome sequencing (WES) that was used for the first time in family studies allowed for the detection of two genes, UDP glucuronosyltransferase family 2 member B28 (UGT2B28) and ubiquitin specific peptidase 17 like family member 2 (USP17L2), which are novel endometriosis-associated genes, by investigating a three-generation family from Crete, Greece (47).
3. Influence of the immune system and inflammation on CVD and endometriosis
Several well-conducted basic science studies on atherosclerosis and CVD over the past years have undoubtedly established the concept that the deregulation of the immune system plays a major role in its pathophysiology (48-51). The translational confirmation of this concept was provided by the Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS study) (52,53) and studies using colchicine in patients with CAD (54,55). These pioneer studies proved that independently of the control of dyslipidemia as a traditional risk factor, targeting inflammatory cytokines reduced cardiovascular complications in high-risk subjects. Data from these studies definitively established the role of inflammation in CVD and several additive experimental evidence [summarized in (56)], is now supported by genetic data. Of particular importance, genetic data from GWAS assigned to the inflammation pathways associated with CAD, do not contain the expected and easily predictable molecules, such as interleukin (IL)-1β (57) or components of the NOD-like receptor protein 3 inflammasome (58) or classic pro-atherogenic chemokines [e.g., chemokine (C-X-C motif) ligand (CXCL)1] (59). Instead of usual suspects, a member of the CXCL family, CXCL12 and IL-6 receptor were identified as CAD genetic risk loci (26,60). As is known, CXCL12 is the ligand for C-X-C motif chemokine receptor 4. Signaling pathways via this receptor play a complex role (61); however, generally, in ECs, it appears to have pro-atherosclerotic properties (62).
Immune and inflammatory cells are not the only players in the inflammation 'game' of atherosclerosis. Although it is well known that subsets of ECs in different tissues and organs have functions, such as alloimmunity, immune cell recruitment, immune tolerance and vascular inflammation (63-65), additive immune functions, typical of immune cells, have been recognized. Co-stimulation and co-inhibition, (66), the induction of apoptosis in other cells (67) and roles as semi-professional-antigen-presenting cells, have all been assigned to ECs.
Of utmost importance, within the past 20 years, new data have confirmed that atherosclerosis is an inflammatory and immune-mediated disease, reducing the rank of traditional risk factors. There is accumulating evidence to suggest that ~40% of individuals with confirmed CVD were never exposed to smoking or diagnosed with diabetes mellitus. On the other hand, it is well established that atherogenesis is accelerated by autoimmune diseases, such as SLE, antiphospholipid syndrome, RA and vasculitis (68). In this context, it is not surprising that a marked improvement in the understanding of the mechanisms regulating the engagement and activation of various immune cells in atherosclerosis and the production of several chemo- and cytokines that regulate these recruitment and process, may serve as a common molecular mechanism explaining the co-occurrence of atherosclerosis in several other diseases with strong inflammatory and immune components, independently of the male sex, older age or other classic risk factors such as endometriosis, known to affect young women without traditional risk factors for CVD (as exactly is the point in SLE).
Taking the aforementioned data into account, endometriosis and CVD may be considered as inflammatory diseases. Inflammatory cytokines, chemokines and growth factors that are involved in the generation of localized inflammation have been found in atherosclerotic plaque and the peritoneal fluid of female patients with endometriosis (10). For example, the deregulated production of interferon-γ has been reported as an underlying mechanism between endometriosis and atherosclerosis, indicating that female patients with endometriosis are at an increased risk of developing microvascular dysfunction and atherosclerosis (69).
Detailed studies have been conducted in an attempt to elucidate the influence of inflammation in endometriosis, thus shedding light on the pathogenetic mechanisms leading to this disorder. The association between inflammation and endometriosis was initially suggested in infertile women, characterized by an extended intra-peritoneal inflammation (70). Endometriosis, highlighted by systemic inflammation in affected organs throughout the body, is caused by a variety of inflammatory factors, such as cytokines, prostaglandins, macrophages, and tumor necrosis factor (TNF). Inflammation is part of the non-specific immune response, playing a key role in the pathophysiology of the disease, by altering the function of immune cells and increasing the levels of pro-inflammatory mediators in the peritoneal cavity, endometrium and blood. In particular, this marked elevation refers to pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α), while JNK in eutopic endometrial cells from women with endometriosis upregulates the expression of inflammatory cytokines (71). Furthermore, levels of various inflammatory factors, such as intracellular adhesion molecule 1 (ICAM-1), C-reactive protein, IL-1, IL-6, TNF-α and vascular endothelial growth factor (VEGF) have been found to be elevated in the peritoneal fluid and peripheral blood of women with endometriosis (72). In addition, endometriosis has been shown to be accompanied by alterations in the levels and function of inflammatory products, including macrophage migration inhibitory factor, C-C chemokine monocyte chemoattractant protein-1, serum amyloid A and chemokine (C-C motif) receptor (CCR)1 (73).
Of note, Monsanto et al (74) reported that the inflammatory profile was significantly altered, both locally and systemically, in women with endometriosis upon surgical lesion removal, thus suggesting that ectopic lesions may drive systemic inflammation in endometriosis. Indeed, endometriosis lesions have been found to produce estrogen themselves, which stimulates mast cells to support the inflammatory process (75). Considering that degranulating mast cells have been found in high quantities in endometriotic lesions, pain and hyperalgesia in endometriosis may be attributed to inflammation due to mast cells (76). Various data have demonstrated that hormonal alterations in endometriosis are related to the inflammatory unbalance characterizing this disease, with steroid hormones promoting the expression and release of pro-inflammatory factors, with inflammation altering hormonal regulation by modulating sex steroid receptors expression (77). Additionally, the deregulation of the inflammasome pathway has been suggested to participate in disease progression through promoting adhesion, invasion and cell proliferation and by preventing cell death (78). In a recent study by Rafi et al (17), it was shown that in women with endometriosis, inflammation causes a hypercoagulable status, which leads to the development of atherosclerosis and other cardiac complications. Thus, it can be hypothesized that endometriosis may be a risk factor of cardiovascular disorders in these patients. Furthermore, Bao et al (79) demonstrated that multi-layered genomic datasets, based on GWAS data in endometriosis, regulatory genomics and protein interactome, led to the generation of an atlas of genetic target prioritizations in endometriosis, which enhanced the understanding of endometriosis as an inflammatory systemic disease.
4. Influence of angiogenesis on CVD and endometriosis
Various studies have significantly associated endometriosis with angiogenesis, lymphangiogenesis and neurogenesis, which may be mediated by the activation of inflammatory cells, thus leading to the ectopic growth of endometrial tissue. Angiogenesis is significantly involved in endometriosis in general. A continuum vascular development results in the development of endometriosis, through the induction of angiogenesis and lymphangiogenesis, while it is well known that endometriotic lesions have lymphangiogenic properties (80,81). Angiogenesis and neurogenesis are activated coordinately, with angiogenesis allowing the maintenance of lesions, supplying them with functional blood vessels, thus forming a dense vascularization (82). Taking into account that pelvic pain represents a usual manifestation of endometriosis, the endometriosis-associated increase in neurogenesis is likely to be a consequence of the observed proximity between mast cells and nerve fibers and, therefore, mast cells may contribute to pain (83). In this framework, it has been suggested that neurogenesis contributes to the growth of nerve fibers, the subsequent peripheral neuroinflammation and the generation of chronic pain (84). Notably, the identification of novel molecular factors associated with angiogenesis and lymphangiogenesis may facilitate the development of novel therapeutic strategies for this disorder. Moreover, in a recent study focusing on the measurement and validation of circulating proteins in predominantly adolescents and young adult women with endometriosis, a significant enrichment and increased activation of proteins that are related to angiogenesis and cell migration pathway was observed (85).
Although in adults the majority of ECs remain quiescent, postnatal angiogenesis can take place under both physiological and pathological stimuli, including reproduction, inflammation, tissue regeneration and tumor growth. In line with this phenomenon, pathological angiogenesis has been implicated in the pathogenesis of numerous diseases, such as cancer, atherosclerosis, ischemic heart disease, hypertension and vascular retinopathies. The mechanisms of angiogenesis are numerous, with angiogenic factors and their corresponding signaling pathways playing a key role in blood vessel growth and morphogenesis (86). In the cascade of angiogenesis and vascular diseases, important functions for ncRNAs have been assigned. ncRNAs can be classified as microRNAs (miRNAs or miRs), circular RNAs, other small RNAs (including tRNAs, 5S and 5.8S ribosomal RNA, small nuclear RNAs and piRNAs) and lncRNAs. Currently, although the functions and mechanisms of miRs in angiogenesis and vascular diseases are well established (87,88), the perspective role of lncRNAs remains unknown. What definitely be concluded about the functional mechanisms of lncRNAs regarding their expression, regulation and roles in angiogenesis and vascular disease, is that central mechanisms predicted from shared genetic background between endometriosis and CVD, are cross-linked in several molecular pathways that are significantly modified by lncRNAs, thus emphasizing the rationale for a detailed description of these mechanisms [reviews on lncRNAs in CVD and therapeutic angiogenesis have been previously provided (89,90)].
The standard sequence of pathogenic events involved in the progression of atherosclerosis has been described, including endothelial dysfunction and endothelial activation as initial events, followed by monocyte/macrophage adhesion, activation and migration, lipid deposition, extracellular matrix synthesis, SMC migration and proliferation and finally, plaque neovascularization (48,91). Specific environments in atherosclerotic areas (relative anoxia, inflammation, oxidative stress) induce angiogenic factors that primarily promote sprouting angiogenesis from pre-existing vasa vasorum (92-97). Neovascularization acts by supplying nutrients and O2 to the local microenvironment; however, the incomplete maturation and the fragility of neocapillaries promote intra-plaque hemorrhages that may lead to plaque instability and rupture (98). Indeed, the presence of neocapillaries in atherosclerotic plaques play a role in their progression and complications (93), with the adventitial delivery of VEGF promoting neoangiogenesis and intimal hyperplasia (99), whereas inhibitors of angiogenesis attenuate plaque growth (100).
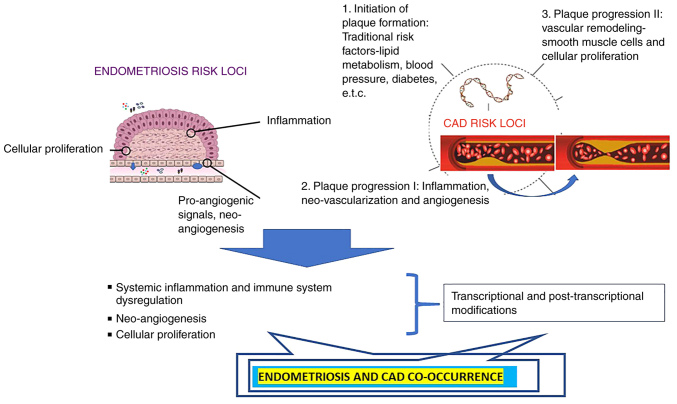
It appears reasonable that the vascular wall where resident, recruited and circulating cells and lipids interact for the initial formation and proceeding plaque progression, along with increasing evidence of the involvement of immune cells, inflammation [for a detailed overview please see (101), and for an overview of platelets please see (102)] in atherosclerosis, has been subjected to detailed investigations. VSMCs have been already described to influence atherosclerosis via vascular tone and via local processes, including inflammation andvascular remodeling, leading either to the stabilization or destabilization of the plaque. Significant genetic overlap exists between these two functions. The nitric oxide signaling pathway induces vasodilatation via the second messenger cyclic guanosine monophosphate (103) and simultaneously inhibits the migration of VSMCs (104), which itself is a hallmark of athero-progression (105). Another genetic locus affecting VSMC biology is the 9p21 locus (33,34), which will be described in the following sections of the present review. A schematic diagram of the shared pathogenesis of CVDs and endometriosis is presented in Fig. 1.
Figure 1.
Proposed mechanisms of the endometriosis-cardiovascular interaction. Genetic risk loci associated with coronary artery disease, are classified into major distinct mechanistic pathways, assigned to initiation and plaque progression Major mechanisms include pro-inflammatory, pro-angiogenic, and aberrant immune functions. Common genetic risk loci influence the initiation and progression of endometriosis and are classified in the same three distinct molecular pathways. All these pathways predicted from genetic data, are influenced at the transcriptional and post-transcriptional level, being the cross-link mechanism of the shared genetic background for both diseases. CAD, coronary artery disease.
5. Shared susceptibility loci between CVD and endometriosis
Previous findings have suggested an association between endometriosis and an increased risk of developing CVD in various populations (15,17-23), with this observation posing the interesting question regarding the potential role of a shared genetic background on the co-occurrence of endometriosis and CVD. Considering that various genes involved in inflammation are significantly associated with both endometriosis and CVD, it appears reasonable that shared genetic components between these diseases exist. Indeed, recent findings have linked endometriosis to several pathological mechanisms, ranging from systemic inflammation and enhanced oxidative stress to endothelial dysfunction (106). Given the fact that CVD is also a systemic and multifactorial condition, it may be intriguing to further explore the shared mechanisms underlying endometriosis and CVD, by highlighting key clinical evidence that links endometriosis with adverse cardiovascular events. Considering that the aforementioned mechanisms of systemic inflammation and endothelial dysfunction are considered the key mechanisms in the development of atherosclerosis/CVD and that both endometriosis and CVD are characterized by a strong genetic component, as described above, the present review presents current knowledge regarding the potential shared genetic background of these diseases by performing an extensive search of the current literature.
The results of the literature research revealed that the VEGF rs1570360 and rs3025039 SNPs (107-111), ESR1 (or ER-alpha) rs9340799 (112,113), IL-6 rs1800796 (114,115), IL-10 rs1800871 (116-118), IL-16 rs11556213 (9,16,119), 5,10 methylenetetrahydrofolate reductase (MTHFR) rs1801133 (Ala222Val) (120,121), C-C motif chemokine ligand 21 (CCL21) rs2812378 (122,123), rs507666 at ABO/9q34.2 (124,125) and tumor protein p53 (TP53) rs1042522 (126,127) are associated with both diseases under investigation (Table I).
Table I.
Overview of genetic polymorphisms related to the development of both endometriosis and CVD, as confirmed by gene association studies and/or GWAS.
| dbSNP ID | Endometriosis and CVD-associated gene | Function | (Refs.) |
|---|---|---|---|
| rs1570360 | VEGF | A signaling protein involved in both vasculogenesis and angiogenesis | (108,109) |
| rs3025039 | VEGF | A signaling protein involved in both vascularization and angiogenesis | (108,111) |
| rs1800796 | IL-6 | A pro-inflammatory cytokine; stimulator of osteoclast formation | (114,115) |
| rs1800871 | IL-10 | An anti-inflammatory cytokine; inhibitor of Th1 differentiation | (116,118) |
| rs11556213 | IL-16 | A T-cell proinflammatory cytokine; a chemoattractant factor | (9,119) |
| rs9340799 | ESR1 | A nuclear receptor activated by the sex hormone estrogen | (112,113) |
| rs1801133 | MTHFR | A key regulatory enzyme in folate and homocysteine metabolism | (120,121) |
| rs507666 | ABO | Blood group determined by the presence of A and B antigens on the surface of the red blood cells | (124,125) |
| rs1042522 | TP53 | A nuclear phosphoprotein; a tumor suppressor factor | (126,180) |
| rs2812378 | CCL21 | A cytokine that mediating homing of lymphocytes to secondary lymphoid organs | (122,123) |
CVD, cardiovascular disease; VEGF, vascular endothelial growth factor; IL, interleukin; ESR1, estrogen receptor 1; MTHFR, methylenetetrahydrofolate reductase; CCL21, C-C motif chemokine ligand 21.
Of note, rs10757272 of CDKN2B and rs1333049 of ANRIL (128,129), rs10965235 in CDKN2BAS (130,131) and rs7865618 in CDKN2B-AS1 (41,132,133) have also been reported to be associated with both diseases. Moreover, various miRs have been found to be associated with the development of both conditions, including miR-146a (rs2920164) and miR-499 (rs3746444) (134,135), miR-27a (rs895819) (136,137), miR-331 located on 12q22 (44,138), miR-214 located on 1q24.3 (44,139), miR-3120 located also on 1q24.3 (44,140), miR-143 (rs41291957) (135,141), miR-143 (rs4705342) (142,143), miR-145 (141), miR-23B and miR-27B probably located on 9q22.32, miR-423 (rs6505162) (137,144) and miR-196a2 (rs11614913) (145,146) (Table II).
Table II.
Overview of genetic polymorphisms in non-coding RNAs related to the development of both endometriosis and CVD.
| SNP | Annotation | Chromosome | (Refs.) |
|---|---|---|---|
| rs2910164 | miR-146A | 5 | (22,135) |
| rs3746444 | miR-499A | 20 | (22,135) |
| rs895819 | miR-27A | 19 | (136,137) |
| rs41291957 | carmn miR-143, miR-145 | 5 | (135,201) |
| rs4705342 | carmn miR-143 | 5 | (135,142,143) |
| rs6505162 | miR-423 | 17 | (136,137,144) |
| rs11614913 | miR-196A2 | 12 | (145,146) |
|
miR-214
miR-3120 |
1 | (44,139,140,224) | |
| miR-331 | 12q22 | (44,138) | |
| rs10757272 | cdkn2b | 9p21 | (129,190) |
| rs1333049 | cdkn2b | 9p21 | (128,129) |
| rs10965235 | cdkn2bas | 9p21 | (131,191) |
| rs7865618 | cdkn2bas | 9p21 | (41,132) |
CVD, cardiovascular disease; SNP, single nucleotide polymorphism.
With a closer look at these common genetic findings, one can easily conclude that they can be grouped into three major gene groups, representing inflammation and immunity, angiogenesis and cell proliferation and, finally, the group of ncRNAs (either miRs or lncRNAs) that influence several biological pathways by transcriptional and post-transcriptional regulation, cross-linking all the above pathways in one way or another (9,147-150). As it is widely acknowledged that endometriosis is a multifactorial condition involving hormonal, pro-inflammatory, pro-angiogenic, immunological and genetic processes that have been previously extensively reviewed (8,9,106), in the following section, the present review describes in further detail the influence of this common genetic background in the context of possible risk prediction for both diseases and putative implications in therapeutic management, which is the ultimate task in every genetic analysis.
6. Current knowledge of biological mechanisms involving the shared genetic loci
VEGF is encoded by the human VEGF gene located on chromosome 6p21.3 and it serves as a powerful regulator of angiogenesis, while is also associated with endothelial cell dysfunction. The rs1570360 (-1154G/A) SNP in the promoter region, which is located within a transcription factor binding site (TFBS), has been shown to be associated with both coronary heart disease (CHD) (109,151) and endometriosis (152). The rs1570360 SNP in the promoter region, located at -1,154 bp from the transcriptional start site of the VEGF gene, has been found to be associated with a number of disease conditions in humans. Of note, the G allele creates four unique potential TFBS, while the A allele creates six potential TFBS (153). VEGF functions as a pro-inflammatory cytokine, strongly induced by hypoxia, representing a potent endothelial cell-specific angiogenic factor (154). Several polymorphisms within the VEGF gene have been found to be associated with alterations in the production of VEGF protein and an increased susceptibility to several disorders, where angiogenesis plays a critical role in their pathogenesis (155,156). Considering that angiogenesis is involved in the development of endometriosis, VEGF substantially promotes neovascularization, which contributes to the implantation of endometrial cells in ectopic sites (157); thus, it can be hypothesized that alterations in VEGF production may be crucial regarding the initiation of endometriosis. Furthermore, both rs1570360 and rs3025039 SNPs have been shown to be associated with altered expression levels of VEGF in CHD, and for this reason, they were considered to be ideal candidate genetic biomarkers of this disease (158).
Cytokines modify immune responses and contribute to the maintenance of the balance between pro-inflammatory and anti-inflammatory stimuli in the process of CVD. The IL-6 gene, located on chromosome 7p21, encodes a multifunctional pro-inflammatory cytokine and represents a physiological link between the endocrine and the immune systems. An association of the IL-6 promoter region rs1800796 (-634C/G) SNP with the increased susceptibility to both CAD and endometriosis has been reported (114,115). This SNP influences IL-6 transcription rates in vitro and basal IL-6 levels in vivo (159), while elevated levels of IL-6 were assessed in both peritoneal fluid and serum of women with endometriosis (130). Similarly, Lu et al (115) reported that the G allele of this SNP may influence the expression of the IL-6 gene, given that bioinformatics analysis revealed that it is located in a potential transcription factor binding site. It was suggested that this SNP predisposes to CAD, considering that IL-6 is expressed at relatively high levels in human atherosclerotic plaques (160).
IL-10, produced by Th2 cells and macrophages, is an immunomodulatory, anti-inflammatory cytokine, involved in the ongoing coronary inflammation and may inhibit the activation and function of T-cells, macrophages and monocytes (161). The human IL-10 gene maps to chromosome 1q31-32. It has been demonstrated that IL-10 promoter polymorphisms are associated with the production of anti-CA II antibody in patients with endometriosis (162). Previous research on women with endometriosis has demonstrated that allele C of rs1800871 SNP is associated with a 2-fold reduced risk of developing the disease compared with the common TT genotype, while the C allele is associated with higher levels of IL-10 compared with the T allele in women suffering from this condition (163). Furthermore, a significant increase in the IL-10 serum level in patients with CVD has been previously reported (164).
The IL-16 gene, located at chromosome 15q26.3, is translated into a 631 amino acid precursor protein (165). The IL-16 protein is a T-cell proinflammatory and immunoregulatory cytokine, a chemoattractant factor that is secreted by CD8+ T-lymphocytes, mast cells and B-cells (166). The binding of IL-16 has been found to stimulate monocytes to secrete a series of cytokines, including IL-1β, IL-6 and TNF-α (166). The IL-16 rs11556218 G/T SNP is located in the exon 6 region of the IL-16 gene and corresponds to a missense mutation, wherein asparagine is substituted by lysine. This SNP is significantly associated with the risk of both CAD and endometriosis (9,119). The G allele has been found to be strongly associated with the risk of developing CAD in the Chinese population (119). Although IL-16 is closely associated with CD4+ lymphocyte-mediated immunity, with CD4+ T-lymphocytes being closely associated with atherosclerosis, the functions of the IL-16 gene polymorphism and rs11556218 warrant further clarification regarding their involvement in atherosclerosis and CAD (119). In a recent study conducted by the authors, it was found that rs11556218 was associated with endometriosis in Greek women (9). In particular, allele 'G' of rs11556218 was found to be associated with an increased susceptibility of developing endometriosis. In an attempt to clarify the functional role of this SNP, the authors previously performed a bioinformatics analysis by constructing 3D models and it w concluded that this SNP may result in the aberrant expression of IL-16, thus indicating a possible association between the mutation and functional modification of Pro-IL-16 (9). Of note, a previous study demonstrated that the level of IL-16 was found to be elevated in the peritoneal fluid and serum of women with endometriosis but, due to the small number of subjects analyzed, this finding has to be validated in larger studies (167).
The MTHFR gene encodes a key regulatory enzyme in folate and homocysteine metabolism, which catalyzes the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate (168). The rs1801133 (Ala222Val) SNP, located in exon 4 of the MTHFR gene, has been shown to be significantly associated with endometriosis-associated infertility (121). Thus, it was hypothesized that the dietary folate requirements in women can be modulated by estrogen levels, thus modulating the production of MTHFR. Of note, the variant T allele of the rs1801133 SNP (677C>T polymorphism) has been found to be associated with an increased risk of developing CAD and abnormal lipid levels (120). In particular, this allele was shown to be associated with elevated levels of total cholesterol and low-density lipoprotein cholesterol (LDL-C). Furthermore, it has been widely reported that the rs1801133 SNP 'T' allele results in elevated plasma homocysteine levels, which has been considered as a CAD risk factor with oxidative stress, endothelial injury and vascular inflammation being involved in the underlying mechanisms in which homocysteine causes CAD (120,169).
The CCL21 gene, one of several Cys-Cys cytokine genes located on the short arm of chromosome 9, encodes a chemokine that is involved in homing lymphocytes to secondary lymphoid organs. Its function refers to the recruitment of CCR7-expressing lymphocytes and dendritic cells to secondary lymphoid tissues (170). Notably, both CVD and endometriosis are associated with variants of the CCL21 gene (117,122,123). Angiogenesis, which is dependent on endothelial cell activation, migration and proliferation, is involved in the development of CVD and CCL21 appears to play a pivotal role in the pathophysiological mechanism of these diseases (171). Notably, it has been suggested that CCL21, as well as its receptor, CCR7, may possibly play a role in the development of atherosclerosis through the recruitment of T-cells and macrophages to the atherosclerotic lesions, by mediating inflammatory responses in these cells (172). The role of CCL21 protein in the development of endometriosis remains unknown, although a shift toward a Th1 response may contribute to the increased cytokine/inflammatory profile observed in this condition (173).
Estrogen exerts its effects on tissues upon binding (with high affinity) to and activating estrogen receptors (ERs). ERs are steroid nuclear ligands that are involved in the regulation of the transcription of the estrogen-responsive genes (113). Apart from the initial thoughts that estrogens are hormones involved in female reproduction, it has been found that they are also involved in lipid homeostasis, vascular tissue repair, insulin signaling and CVD (174,175). The eESR1 gene, considered the primary receptor for estrogen, is located on chromosome 6q25.1 and consists of eight exons separated by seven introns. The rs9340799 SNP, which is an XbaI restriction site polymorphism involving an A-to-G transition in intron-1, has been found in previous studies to increase the risk of developing both CVD and endometriosis (112,113). It has been suggested that the A to G transition of rs9340799 may alter transcription, thus exerting its effect on the gene expression levels as well as ER-related molecular mechanisms (176). In particular, a decreased number of ERs may result in less effective estrogen signaling and, as a consequence, may indirectly influence molecular signaling mechanisms that are related to risk factors for CVD (113). Moreover, numerous studies have shown an association between G allele of rs9340799 SNP and at least one risk factor for CVD, while the GG genotype was reported to increase the risk of developing endometriosis 4-fold (177,178).
The TP53 gene at 17p13.1 codes for the TP53 nuclear phosphoprotein, a transcription factor that is involved in programmed cell death (apoptosis), cell cycle regulation, angiogenesis and DNA repair, thus playing a crucial role as a tumor suppressor that protects the genome from damage (179). rs1042522 is a functional polymorphism of the TP53 gene, located within exon 4 (codon 72), leading to the substitution of proline (CCC) to arginine (CGC); it has been reported to be implicated in susceptibility to several clinical diseases, including CAD and endometriosis (126,180). Of note, the arginine allele has been shown to be associated with a greater apoptotic function than proline, which itself appears to be associated more with inflammation and activation of the transcription process (181). In accordance with this finding, it was shown that arginine stimulates apoptosis at least 5-fold more effectively than proline through its better localization of the mitochondria (182).
The ABO blood group is determined by the presence of A and B antigens on the surface of the red blood cells with the frequency of the common ABO phenotypes varying among different populations, probably due to evolutionary selection (183). ABO blood groups have been shown to be associated with various disease phenotypes, including cardiometabolic diseases (184). rs507666 at ABO/9q34.2, located within intron 1, has been previously shown to be associated with CVD and, recently, with endometriosis as well (124,141). Various studies have shown that the ABO glycotransferase may result in the development of atherosclerotic CVD by a broader mechanism than simply modulating thrombosis (141). The study by Pare et al (185) demonstrated that rs507666, which represents a perfect tag for ABO blood group A1, was associated with decreased levels of circulating soluble ICAM-1 (sICAM-1) when compared to the O allele. Moreover, in a previous meta-analysis, it was found that heterozygotes, as well homozygotes for the minor allele of rs507666 had lower plasma levels of sICAM-1, soluble P-selectin (sP-selectin) and soluble E-selectin (sE-selectin) (186).
Since the first GWAS on CAD, the chr9p21 risk locus has emerged as a top signal in GWAS of atherosclerotic CVD and was shown to increase susceptibility to CAD in carriers of certain alleles of SNPs located within this locus (26,187). This locus codes for an antisense RNA (CDKN2B-AS1 or ANRIL), which is located close to the CDKN2A-CDKN2B gene cluster (188). Both the CDKN2A and CDKN2B genes have been reported to be significantly associated with an increased risk of CAD (26). Thus, rs10757272 of CDKN2B, as well as the rs10965235 of ANRIL were found to be associated with CAD (189), while rs1333049 of ANRIL has been found to be associated with myocardial infarction (128,131,190). Notably, all the aforementioned SNPs have been associated with endometriosis as well (129,191). It has been reported that the majority of genetic variants associated with CAD are located within intronic and flanking sequences of ANRIL, which is involved in the regulation of both CDKN2A and CDKN2B genes (189). This locus mediates its risk at the vessel wall, given that the repression of these genes causes SMC proliferation, which appears at the coronary artery wall during the initial stages of atherosclerosis (192). In this framework, it has also been reported that the genotype of individuals determines the production of atherogenic (linear) over anti-atherogenic ANRIL RNA species (circular), thus pointing to the functional role of ANRIL regulation in CAD (131). However, the molecular mechanisms through which the genotype of this locus controls the ratio of linear and circular ANRIL are currently unclear (131).
A wide repertoire of miRs have been implicated in vascular pathologies (174). The bicistronic miR-143/145 cluster has been reported to play a role in vessel development and vascular diseases, including atherosclerosis and pulmonary hypertension, mainly affecting the proliferation of VSMCs and ECs (193-200). Along with these biological properties, a prognostic value in human CVD, of a miR-143/145 SNP was recently identified as significant, namely, rs41291957. This VSMC-specific miR-SNP-rs41291957 affects miR-143 and miR-145 expression and is linked to disease progression in patients with stable CAD (201).
Nimi-Hoveidi et al (202) genotyped miR-143 rs41291957 and rs4705342 SNPs in infertile women with endometriosis and matched healthy subjects. They reported an association between the C allele of rs4705342 and an increased risk of endometriosis. In addition, the A allele of rs41291957 polymorphism was found to be associated with susceptibility to endometriosis (202); the expression level of miR-145 has also been found to be significantly upregulated in stage I or II of endometriosis (203). Although the exact mechanisms through which miR-143/miR-145 affect endometrial tissue have not yet been fully elucidated, it can hypothesized that their regulatory role in VSMCs and cell proliferation/differentiation is a key mechanism, thus making sense of the common molecular pathways of angiogenesis/cell proliferation between endometriosis and CVD. Cardiac mesoderm enhancer-associated non-coding RNA (CARMN) is a lncRNA implicated in cardiac differentiation (204,205). The initial transcript contains miR-143/145 cluster, which are considered miRs as regulators of SMC differentiation (86,193,194,197,206,207). In this manner, CARMN maintains VSMC contractile phenotype by directly binding to myocardin (MYOCD) and potentiating MYOCD functions, representing a potential therapeutic target for diseases encompass SMC proliferation (208).
The miR-146 family has been implicated by recent studies in the innate immune response and immune-mediated diseases like infection (209,210), CVD (211,212) and cancer (213-215). miR-146b regulates critical cellular functions, such as cell death (216) and autophagy (217) and its expression significantly upregulated in peritoneal fluid and serum samples of patients with endometriosis. The majority of miR-146b targets that have been identified are key regulators of macrophage functions. miR-146b inhibits IL-1 receptor-associated kinase 1 (IRAK1) and TNF receptor-associated factor 6 (TRAF6) expression by binding to the 3΄UTR of IRAK1 or TRAF6, suppressing the TLR-mediated pathway (218).
rs11614913 in miR-196A2 has been shown to be associated with the development and progression of endometriosis. Genetic variations with a role in ribosome biogenesis that have been assigned to the risk allele, appear to be mediated by regulating the expression of multiple small nucleolar RNAs (snoRNAs) and ribosomal proteins (RPs) and these snoRNAs and RPs are upregulated in endometriotic lesions and thus active ribosome biogenesis in cell nucleoli drives endometriosis (145). The same SNP, rs11614913, in miR-196α2 has been reported as genetic risk factor of newly developed CAD, but not with its complications such as in-stent coronary restenosis (146). Taking account that stent restenosis involves VSMC proliferation, it can be hypothesized that the miR-196α2 C/T rs11614913 SNP interferes with cell cycle progression and the proliferation of cell types differential to VSMCs, such as ECs (promoting neo-angiogenesis) and/or immune cells.
The significant associations of rs895819 in miR-27A and rs6505162 in miR-423 have been recently reported in endometriosis (137). Of note, the pre-miR-27a rs895819 polymorphism is associated with myocardial infarction susceptibility in the Chinese Han population (136) with a possible mechanism being its unique role in EC dysfunction (219) and the promotion of angiogenesis (220). On the other hand, the polymorphism rs6505162 C>A is located in pre-miR-423 region, and it has been reported to promote the expression of mature mir-423 (221). The miR-423 C>A gene variation has been reported to be associated with susceptibility to CAD (144) and miR-423 heterozygosity or A/C genotype have been shown to be associated with an increased susceptibility to CAD in the Indian population (143). When miR-423 is overexpressed, it inhibits the translation of O-GlcNAc transferase (OGT) and catalyzes the addition of O-GlcNAc to proteins and is required for cell division and embryogenesis in cardiomyocyte (222) mRNA, which promotes the apoptosis of cardiomyocytes (223).
Diverse and complex roles of miR-214 have been described, such as an oncogene, tumor suppressor, protector against Ca2+ overload and oxidative damage, mediator of angiogenesis and pathological fibrosis and, as a regulator of osteogenesis, myogenesis and cellular immunity through a vast repertoire of targets, influencing multiple cellular functions and oftentimes contrasting roles, are attributed to particular targets (224). Nevertheless, it is evident that miR-214 can regulate several aspects of major signaling pathways via the post-transcriptional modifications of critical genes in the immunity/inflammation pathway, cell survival and cell proliferation pathway, including angiogenesis. The implication of this locus in endometriosis, along with the reported functions in CVD (139), may aid in the understanding of how CVD and endometriosis share common genetic pathways that can be translated in common molecular pathways in the pathogenesis of both diseases.
7. Conclusions and future perspectives
Based on the aforementioned data, a clear identification of a link between endometriosis and lifelong CVD would necessitate a radical shift in public health management. Although endometriosis remains an enigmatic disease and little is understood about the main causes of the disease, it has been associated with various diseases, including CVD, thus suggesting that women with endometriosis may represent a high-risk group for CVD. Of note, Taskin et al (225) pointed out that endometriosis should be considered as a risk factor for CVD, thus requiring specific counseling and prevention. Key questions that arise from these studies refer to whether gynecologists have to recommend women with endometriosis for a cardiology assessment, as well as the possibility of these findings leading to substantial changes in treatment options. However, it should not be under-recognized that other known risk factors for CVD in women, such as diabetes, LDL-C levels, obesity and hypertension, may have a larger combined effect on the risk of CVD compared to endometriosis alone. Evidently, an in-depth understanding of the association between these conditions may enrich the existing knowledge and may provide new insight into the chronic consequences of endometriosis. Furthermore, given the high prevalence of endometriosis, the development of preventive and early detection guidelines for CVD for women with endometriosis may prove beneficial for public health (226).
Advances in cardiovascular genetics prompted the American Heart Association (AHA) to publish a very recent Scientific Statement for the incorporation of polygenic risk scores to the management of five cardiometabolic diseases (coronary artery disease, hypercholesterolemia, type 2 diabetes, atrial fibrillation, and venous thromboembolic disease) (227). This report summarizes the dogma for the CVD as a multifactorial and complex one. Despite advancements being made, risk prediction remains imprecise with persistently high rates of incident CVD. Currently, such scores are often used in research, one field being the study the inter-relationship between the risk of CAD and other phenotypes. In an attempt to unravel the mechanisms underlying the risk association between endometriosis and CVD, various possible explanations can be suggested. Thus, it can be hypothesized that endometriosis may cause chronic inflammation, considering that inflammation is the precursor to a number of different disease pathologies, including CVD. To this end, various markers of endothelial function (including common carotid intima-media thickness) can be useful for an evaluation of a preclinical and subclinical risk of atherosclerosis in women with endometriosis, not only in the peritoneal cavity, but also at a systemic level (10). Notably, a higher risk of CHD has been observed among women who have had a hysterectomy/oophorectomy than in those who have not undergone this surgical procedure and, as a consequence, this fact has to be taken into account before a decision for this invasive treatment (15). The data presented herein provide evidence of various genetic factors that are shared between endometriosis and CVD, thus demonstrating apparent genetic links between these conditions. Noteworthy, it is beneficial for clinicians to be aware of the possibility of a co-occurrence of these diseases in order to provide suitable medication to women with endometriosis. Given the existing link between endometriosis and early menopause (228), hormone replacement therapy during perimenopause has shown that estrogen therapy is cardioprotective (229). However, although estrogens have been found to be generally cardioprotective in women with early atherogenesis, it has been reported that it is potentially harmful in women with established atherosclerosis (230). In addition, the common treatment of endometriosis by the inhibition of the production of prostaglandins (using drugs such as ibuprofen or naproxen) in an attempt to significantly reduce the symptoms of disease should be avoided, considering that these can lead to an increased cardiovascular risk as an undesirable secondary effect (231).
Future research should attempt to fully unravel the shared molecular pathways underpinning the association between endometriosis and CVD, thus allowing physicians to develop and customize novel therapeutic interventions based on an individual's molecular and clinical profiles.
Acknowledgments
Not applicable.
Funding Statement
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
VMV, MIZ, EE and GNG designed the present study and drafted the manuscript. GNG, MIZ, DV, LP, DAS and DC searched the literature. DC, EE, DV, DC, LP, MIZ and VMV analyzed and organized the data to be included in the review. DAS, DV, LP and DC critically revised the manuscript. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article. The other authors declare that they have no competing interests.
References
- 1.Organization WH . Global status report on noncommunicable diseases 2014. World Health Organization; 2014. [Google Scholar]
- 2.Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308:1587–1589. doi: 10.1126/science.1111445. [DOI] [PubMed] [Google Scholar]
- 3.Symons LK, Miller JE, Kay VR, Marks RM, Liblik K, Koti M, Tayade C. The immunopathophysiology of endometriosis. Trends Mol Med. 2018;24:748–762. doi: 10.1016/j.molmed.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Simpson JL, Elias S, Malinak LR, Buttram VC., Jr Heritable aspects of endometriosis. I. Genetic studies. Am J Obstet Gynecol. 1980;137:327–331. doi: 10.1016/0002-9378(80)90917-5. [DOI] [PubMed] [Google Scholar]
- 5.Maroun P, Cooper MJ, Reid GD, Keirse MJ. Relevance of gastrointestinal symptoms in endometriosis. Aust N Z J Obstet Gynaecol. 2009;49:411–414. doi: 10.1111/j.1479-828X.2009.01030.x. [DOI] [PubMed] [Google Scholar]
- 6.De Graaff AA, D'Hooghe TM, Dunselman GA, Dirksen CD, Hummelshoj L, WERF EndoCost Consortium and Simoens S The significant effect of endometriosis on physical, mental and social wellbeing: Results from an international cross-sectional survey. Human Reprod. 2013;28:2677–2685. doi: 10.1093/humrep/det284. [DOI] [PubMed] [Google Scholar]
- 7.Vassilopoulou L, Matalliotakis M, Zervou MI, Matalliotaki C, Krithinakis K, Matalliotakis I, Spandidos DA, Goulielmos GN. Defining the genetic profile of endometriosis. Exp Ther Med. 2019;17:3267–3281. doi: 10.3892/etm.2019.7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vercellini P, Vigano P, Somigliana E, Fedele L. Endometriosis: Pathogenesis and treatment. Nat Rev Endocrinol. 2014;10:261–275. doi: 10.1038/nrendo.2013.255. [DOI] [PubMed] [Google Scholar]
- 9.Matalliotakis M, Zervou MI, Eliopoulos E, Matalliotaki C, Rahmioglu N, Kalogiannidis I, Zondervan K, Spandidos DA, Matalliotakis I, Goulielmos GN. The role of IL16 gene polymorphisms in endometriosis. Int J Mol Med. 2018;41:1469–1476. doi: 10.3892/ijmm.2018.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santanam N, Song M, Rong R, Murphy AA, Parthasarathy S. Atherosclerosis, oxidation and endometriosis. Free Radical Res. 2002;36:1315–1321. doi: 10.1080/1071576021000049908. [DOI] [PubMed] [Google Scholar]
- 11.Kvaskoff M, Mu F, Terry KL, Harris HR, Poole EM, Farland L, Missmer SA. Endometriosis: A high-risk population for major chronic diseases? Hum Reprod Update. 2015;21:500–516. doi: 10.1093/humupd/dmv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shigesi N, Kvaskoff M, Kirtley S, Feng Q, Fang H, Knight JC, Missmer SA, Rahmioglu N, Zondervan KT, Becker CM. The association between endometriosis and autoimmune diseases: A systematic review and meta-analysis. Hum Reprod Update. 2019;25:486–503. doi: 10.1093/humupd/dmz014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zervou MI, Vlachakis D, Papageorgiou L, Eliopoulos E, Goulielmos GN. Increased risk of rheumatoid arthritis in patients with endometriosis: Genetic aspects. Rheumatology (Oxford) 2022;61:4252–4262. doi: 10.1093/rheumatology/keac143. [DOI] [PubMed] [Google Scholar]
- 14.Yin Z, Low HY, Chen BS, Huang KS, Zhang Y, Wang YH, Ye Z, Wei JC. Risk of ankylosing spondylitis in patients with endometriosis: A population-based retrospective cohort study. Front Immunol. 2022;13:877942. doi: 10.3389/fimmu.2022.877942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn SH, Khalaj K, Young SL, Lessey BA, Koti M, Tayade C. Immune-inflammation gene signatures in endometriosis patients. Fertility Sterility. 2016;106:1420–1431.e7. doi: 10.1016/j.fertnstert.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hai-Feng T, Wei W, Yuan-Yuan Y, Jun Z, Su-Ping G, Hui-Ming L. Association between polymorphisms in IL-16 genes and coronary heart disease risk. Pakistan J Med Sci. 2013;29:1033–1037. doi: 10.12669/pjms.294.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rafi U, Ahmad S, Bokhari SS, Iqbal MA, Zia A, Khan MA, Roohi N. Association of Inflammatory Markers/Cytokines with cardiovascular risk manifestation in patients with endometriosis. Mediators Inflamm. 2021;2021:3425560. doi: 10.1155/2021/3425560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinugasa S, Shinohara K, Wakatsuki A. Increased asymmetric dimethylarginine and enhanced inflammation are associated with impaired vascular reactivity in women with endometriosis. Atherosclerosis. 2011;219:784–788. doi: 10.1016/j.atherosclerosis.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Melo AS, Rosa-e-Silva JC, Rosa-e-Silva AC, Poli-Neto OB, Ferriani RA, Vieira CS. Unfavorable lipid profile in women with endometriosis. Fertil Steril. 2010;93:2433–2436. doi: 10.1016/j.fertnstert.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 20.Mu F, Rich-Edwards J, Rimm EB, Spiegelman D, Missmer SA. Endometriosis and risk of coronary heart disease. Circ Cardiovasc Qual Outcomes. 2016;9:257–264. doi: 10.1161/CIRCOUTCOMES.115.002224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akinjero A, Adegbala O, Akinyemiju T. Abstract P320: Is Co-occurring endometriosis among women with myocardial infarction associated with worse In-hospital outcomes? Findings from the nationwide inpatient sample. Circulation. 2017;135:AP320–AP320. doi: 10.1161/circ.135.suppl_1.p320. [DOI] [Google Scholar]
- 22.Chiang HJ, Lan KC, Yang YH, Chiang JY, Kung FT, Huang FJ, Lin YJ, Su YT, Sung PH. Risk of major adverse cardiovascular and cerebrovascular events in Taiwanese women with endometriosis. J Formos Med Assoc. 2021;120:327–336. doi: 10.1016/j.jfma.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Okoth K, Wang J, Zemedikun D, Thomas GN, Nirantharakumar K, Adderley NJ. Risk of cardiovascular outcomes among women with endometriosis in the United Kingdom: A retrospective matched cohort study. BJOG. 2021;128:1598–1609. doi: 10.1111/1471-0528.16692. [DOI] [PubMed] [Google Scholar]
- 24.Marchandot B, Curtiaud A, Matsushita K, Trimaille A, Host A, Faller E, Garbin O, Akladios C, Jesel L, Morel O. Endometriosis and cardiovascular disease. Eur Heart J Open. 2022;2:oeac001. doi: 10.1093/ehjopen/oeac001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. Heart disease and stroke statistics-2019 Update: A report from the american heart association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 26.Samani NJ, Erdmann J, Hall AS, Hengstenberg C, Mangino M, Mayer B, Dixon RJ, Meitinger T, Braund P, Wichmann HE, et al. Genomewide association analysis of coronary artery disease. N Engl J Med. 2007;357:443–453. doi: 10.1056/NEJMoa072366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McPherson R, Pertsemlidis A, Kavaslar N, Stewart A, Roberts R, Cox DR, Hinds DA, Pennacchio LA, Tybjaerg-Hansen A, Folsom AR, et al. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316:1488–1491. doi: 10.1126/science.1142447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helgadottir A, Thorleifsson G, Manolescu A, Gretarsdottir S, Blondal T, Jonasdottir A, Jonasdottir A, Sigurdsson A, Baker A, Palsson A, et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316:1491–1493. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- 29.Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, Parish S, Barlera S, Franzosi MG, Rust S, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 31.Visel A, Zhu Y, May D, Afzal V, Gong E, Attanasio C, Blow MJ, Cohen JC, Rubin EM, Pennacchio LA. Targeted deletion of the 9p21 non-coding coronary artery disease risk interval in mice. Nature. 2010;464:409–412. doi: 10.1038/nature08801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holdt LM, Teupser D. Recent studies of the human chromosome 9p21 locus, which is associated with atherosclerosis in human populations. Arterioscler Thromb Vasc Biol. 2012;32:196–206. doi: 10.1161/ATVBAHA.111.232678. [DOI] [PubMed] [Google Scholar]
- 33.Holdt LM, Stahringer A, Sass K, Kulak NA, Wilfert W, Kohlmaier A, Herbst A, Northoff BH, Nicolaou A, Gäbel G, et al. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat Commun. 2016;7:12429. doi: 10.1038/ncomms12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lo Sardo V, Chubukov P, Ferguson W, Kumar A, Teng EL, Duran M, Zhang L, Cost G, Engler AJ, Urnov F, et al. Unveiling the role of the most impactful cardiovascular risk locus through haplotype editing. Cell. 2018;175:1796–1810.20. doi: 10.1016/j.cell.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kessler T, Schunkert H. Coronary artery disease genetics enlightened by genome-wide association studies. JACC Basic Transl Sci. 2021;6:610–623. doi: 10.1016/j.jacbts.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stortoni P, Cecati M, Giannubilo SR, Sartini D, Turi A, Emanuelli M, Tranquilli AL. Placental thrombomodulin expression in recurrent miscarriage. Reprod Biol Endocrinol. 2010;8:1. doi: 10.1186/1477-7827-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saha R, Pettersson HJ, Svedberg P, Olovsson M, Bergqvist A, Marions L, Tornvall P, Kuja-Halkola R. Heritability of endometriosis. Fertil Steril. 2015;104:947–952. doi: 10.1016/j.fertnstert.2015.06.035. [DOI] [PubMed] [Google Scholar]
- 38.Falconer H, D'Hooghe T, Fried G. Endometriosis and genetic polymorphisms. Obstet Gynecol Surv. 2007;62:616–628. doi: 10.1097/01.ogx.0000279293.60436.60. [DOI] [PubMed] [Google Scholar]
- 39.Rahmioglu N, Montgomery GW, Zondervan KT. Genetics of endometriosis. Women's Health. 2015;11:577–586. doi: 10.2217/whe.15.41. [DOI] [PubMed] [Google Scholar]
- 40.Nyholt DR, Low SK, Anderson CA, Painter JN, Uno S, Morris AP, MacGregor S, Gordon SD, Henders AK, Martin NG, et al. Genome-wide association meta-analysis identifies new endometriosis risk loci. Nat Genet. 2012;44:1355–1359. doi: 10.1038/ng.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rahmioglu N, Nyholt DR, Morris AP, Missmer SA, Montgomery GW, Zondervan KT. Genetic variants underlying risk of endometriosis: Insights from meta-analysis of eight genome-wide association and replication datasets. Hum Reprod Update. 2014;20:702–716. doi: 10.1093/humupd/dmu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uimari O, Rahmioglu N, Nyholt DR, Vincent K, Missmer SA, Becker C, Morris AP, Montgomery GW, Zondervan KT. Genome-wide genetic analyses highlight mitogen-activated protein kinase (MAPK) signaling in the pathogenesis of endometriosis. Hum Reprod. 2017;32:780–793. doi: 10.1093/humrep/dex024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe K, Taskesen E, van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat Commun. 2017;8:1826. doi: 10.1038/s41467-017-01261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sapkota Y, Steinthorsdottir V, Morris AP, Fassbender A, Rahmioglu N, De Vivo I, Buring JE, Zhang F, Edwards TL, Jones S, et al. Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nat Commun. 2017;8:15539. doi: 10.1038/ncomms15539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sapkota Y, Fassbender A, Bowdler L, Fung JN, Peterse D, O D, Montgomery GW, Nyholt DR, D'Hooghe TM. Independent replication and meta-analysis for endometriosis risk loci. Twin Res Hum Genet. 2015;18:518–525. doi: 10.1017/thg.2015.61. [DOI] [PubMed] [Google Scholar]
- 46.Rahmioglu N, Mortlock S, Ghiasi M, Møller PL, Stefansdottir L, Galarneau G, Turman C, Danning R, Law MH, Sapkota Y, et al. The genetic basis of endometriosis and comorbidity with other pain and inflammatory conditions. Nat Genet. doi: 10.1038/s41588-023-01323-z. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Albertsen HM, Matalliotaki C, Matalliotakis M, Zervou MI, Matalliotakis I, Spandidos DA, Chettier R, Ward K, Goulielmos GN. Whole exome sequencing identifies hemizygous deletions in the UGT2B28 and USP17L2 genes in a threegeneration family with endometriosis. Mol Med Rep. 2019;19:1716–1720. doi: 10.3892/mmr.2019.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 49.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Randolph GJ. The fate of monocytes in atherosclerosis. J Thromb Haemost. 2009;7(Suppl 1):S28–S30. doi: 10.1111/j.1538-7836.2009.03423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–166. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ridker PM. Residual inflammatory risk: Addressing the obverse side of the atherosclerosis prevention coin. Eur Heart J. 2016;37:1720–1722. doi: 10.1093/eurheartj/ehw024. [DOI] [PubMed] [Google Scholar]
- 53.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 54.Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 55.Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, The SHK, Xu XF, Ireland MA, Lenderink T, et al. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383:1838–1847. doi: 10.1056/NEJMoa2021372. [DOI] [PubMed] [Google Scholar]
- 56.Schloss MJ, Swirski FK, Nahrendorf M. Modifiable cardiovascular risk, hematopoiesis, and innate immunity. Circ Res. 2020;126:1242–1259. doi: 10.1161/CIRCRESAHA.120.315936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vromman A, Ruvkun V, Shvartz E, Wojtkiewicz G, Santos Masson G, Tesmenitsky Y, Folco E, Gram H, Nahrendorf M, Swirski FK, et al. Stage-dependent differential effects of interleukin-1 isoforms on experimental atherosclerosis. Eur Heart J. 2019;40:2482–2491. doi: 10.1093/eurheartj/ehz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grebe A, Hoss F, Latz E. NLRP3 inflammasome and the IL-1 pathway in atherosclerosis. Circ Res. 2018;122:1722–1740. doi: 10.1161/CIRCRESAHA.118.311362. [DOI] [PubMed] [Google Scholar]
- 59.Zernecke A, Shagdarsuren E, Weber C. Chemokines in atherosclerosis: An update. Arterioscler Thromb Vasc Biol. 2008;28:1897–1908. doi: 10.1161/ATVBAHA.107.161174. [DOI] [PubMed] [Google Scholar]
- 60.Schunkert H, Konig IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, Preuss M, Stewart AF, Barbalic M, Gieger C, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43:333–338. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doring Y, Noels H, van der Vorst EPC, Neideck C, Egea V, Drechsler M, Mandl M, Pawig L, Jansen Y, Schröder K, et al. Vascular CXCR4 limits atherosclerosis by maintaining arterial integrity: Evidence from mouse and human studies. Circulation. 2017;136:388–403. doi: 10.1161/CIRCULATIONAHA.117.027646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doring Y, van der Vorst EPC, Duchene J, Jansen Y, Gencer S, Bidzhekov K, Atzler D, Santovito D, Rader DJ, Saleheen D, Weber C. CXCL12 derived from endothelial cells promotes atherosclerosis to drive coronary artery disease. Circulation. 2019;139:1338–1340. doi: 10.1161/CIRCULATIONAHA.118.037953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 64.Lohse AW, Knolle PA, Bilo K, Uhrig A, Waldmann C, Ibe M, Schmitt E, Gerken G, Meyer Zum, Büschenfelde KH. Antigen-presenting function and B7 expression of murine sinusoidal endothelial cells and Kupffer cells. Gastroenterology. 1996;110:1175–1181. doi: 10.1053/gast.1996.v110.pm8613007. [DOI] [PubMed] [Google Scholar]
- 65.Wedgwood JF, Hatam L, Bonagura VR. Effect of interferon-gamma and tumor necrosis factor on the expression of class I and class II major histocompatibility molecules by cultured human umbilical vein endothelial cells. Cell Immunol. 1988;111:1–9. doi: 10.1016/0008-8749(88)90046-9. [DOI] [PubMed] [Google Scholar]
- 66.Carman CV, Martinelli R. T Lymphocyte-endothelial interactions: Emerging understanding of trafficking and antigen-specific immunity. Front Immunol. 2015;6:603. doi: 10.3389/fimmu.2015.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Motz GT, Santoro SP, Wang LP, Garrabrant T, Lastra RR, Hagemann IS, Lal P, Feldman MD, Benencia F, Coukos G. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med. 2014;20:607–615. doi: 10.1038/nm.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Full LE, Ruisanchez C, Monaco C. The inextricable link between atherosclerosis and prototypical inflammatory diseases rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res Ther. 2009;11:217. doi: 10.1186/ar2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Glavind MT, Forman A, Arendt LH, Nielsen K, Henriksen TB. Endometriosis and pregnancy complications: A Danish cohort study. Fertil Steril. 2017;107:160–166. doi: 10.1016/j.fertnstert.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 70.Haney AF, Jenkins S, Weinberg JB. The stimulus responsible for the peritoneal fluid inflammation observed in infertile women with endometriosis. Fertil Steril. 1991;56:408–413. doi: 10.1016/S0015-0282(16)54532-4. [DOI] [PubMed] [Google Scholar]
- 71.Uz YH, Murk W, Bozkurt I, Kizilay G, Arici A, Kayisli UA. Increased c-Jun N-terminal kinase activation in human endometriotic endothelial cells. Histochem Cell Biol. 2011;135:83–91. doi: 10.1007/s00418-010-0770-2. [DOI] [PubMed] [Google Scholar]
- 72.Bedaiwy MA, Falcone T. Peritoneal fluid environment in endometriosis. Clinicopathological implications. Minerva Ginecol. 2003;55:333–345. [PubMed] [Google Scholar]
- 73.Agic A, Xu H, Finas D, Banz C, Diedrich K, Hornung D. Is endometriosis associated with systemic subclinical inflammation? Gynecol Obstet Invest. 2006;62:139–147. doi: 10.1159/000093121. [DOI] [PubMed] [Google Scholar]
- 74.Monsanto SP, Edwards AK, Zhou J, Nagarkatti P, Nagarkatti M, Young SL, Lessey BA, Tayade C. Surgical removal of endometriotic lesions alters local and systemic proinflammatory cytokines in endometriosis patients. Fertil Steril. 2016;105:968–977.e965. doi: 10.1016/j.fertnstert.2015.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Graziottin A, Skaper SD, Fusco M. Mast cells in chronic inflammation, pelvic pain and depression in women. Gynecol Endocrinol. 2014;30:472–477. doi: 10.3109/09513590.2014.911280. [DOI] [PubMed] [Google Scholar]
- 76.Indraccolo U, Barbieri F. Effect of palmitoylethanolamide-polydatin combination on chronic pelvic pain associated with endometriosis: Preliminary observations. Eur J Obstet Gynecol Reprod Biol. 2010;150:76–79. doi: 10.1016/j.ejogrb.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 77.Grandi G, Mueller MD, Papadia A, Kocbek V, Bersinger NA, Petraglia F, Cagnacci A, McKinnon B. Inflammation influences steroid hormone receptors targeted by progestins in endometrial stromal cells from women with endometriosis. J Reprod Immunol. 2016;117:30–38. doi: 10.1016/j.jri.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 78.Garcia-Gomez E, Vazquez-Martinez ER, Reyes-Mayoral C, Cruz-Orozco OP, Camacho-Arroyo I, Cerbon M. Regulation of Inflammation Pathways and Inflammasome by Sex Steroid Hormones in Endometriosis. Front Endocrinol. 2019;10:935. doi: 10.3389/fendo.2019.00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bao C, Wang H, Fang H. Genomic evidence supports the recognition of endometriosis as an inflammatory systemic disease and reveals disease-specific therapeutic potentials of targeting neutrophil degranulation. Front Immunol. 2022;13:758440. doi: 10.3389/fimmu.2022.758440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Malhotra N, Karmakar D, Tripathi V, Luthra K, Kumar S. Correlation of angiogenic cytokines-leptin and IL-8 in stage, type and presentation of endometriosis. Gynecol Endocrinol. 2012;28:224–227. doi: 10.3109/09513590.2011.593664. [DOI] [PubMed] [Google Scholar]
- 81.Kobayashi H, Higashiura Y, Shigetomi H, Kajihara H. Pathogenesis of endometriosis: The role of initial infection and subsequent sterile inflammation (Review) Mol Med Rep. 2014;9:9–15. doi: 10.3892/mmr.2013.1755. [DOI] [PubMed] [Google Scholar]
- 82.Shifren JL, Tseng JF, Zaloudek CJ, Ryan IP, Meng YG, Ferrara N, Jaffe RB, Taylor RN. Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. J Clin Endocrinol Metab. 1996;81:3112–3118. doi: 10.1210/jcem.81.8.8768883. [DOI] [PubMed] [Google Scholar]
- 83.Kirchhoff D, Kaulfuss S, Fuhrmann U, Maurer M, Zollner TM. Mast cells in endometriosis: Guilty or innocent bystanders? Expert Opin Ther Targets. 2012;16:237–241. doi: 10.1517/14728222.2012.661415. [DOI] [PubMed] [Google Scholar]
- 84.Wu J, Xie H, Yao S, Liang Y. Macrophage and nerve interaction in endometriosis. J Neuroinflammation. 2017;14:53. doi: 10.1186/s12974-017-0828-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sasamoto N, Ngo L, Vitonis AF, Dillon ST, Missmer SA, Libermann TA, Terry KL. Circulating proteomic profiles associated with endometriosis in adolescents and young adults. Hum Reprod. 2022;37:2042–2053. doi: 10.1093/humrep/deac146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ahmed Z, Bicknell R. Angiogenic signalling pathways. Methods Mol Biol. 2009;467:3–24. doi: 10.1007/978-1-59745-241-0_1. [DOI] [PubMed] [Google Scholar]
- 87.Landskroner-Eiger S, Moneke I, Sessa WC. miRNAs as modulators of angiogenesis. Cold Spring Harb Perspect Med. 2013;3:a006643. doi: 10.1101/cshperspect.a006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang S, Olson EN. AngiomiRs-key regulators of angiogenesis. Curr Opin Genet Dev. 2009;19:205–211. doi: 10.1016/j.gde.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Uchida S, Dimmeler S. Long noncoding RNAs in cardiovascular diseases. Circ Res. 2015;116:737–750. doi: 10.1161/CIRCRESAHA.116.302521. [DOI] [PubMed] [Google Scholar]
- 90.Kumar MM, Goyal R. LncRNA as a therapeutic target for angiogenesis. Curr Top Med Chem. 2017;17:1750–1757. doi: 10.2174/1568026617666161116144744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Michel JB, Thaunat O, Houard X, Meilhac O, Caligiuri G, Nicoletti A. Topological determinants and consequences of adventitial responses to arterial wall injury. Arterioscler Thromb Vasc Biol. 2007;27:1259–1268. doi: 10.1161/ATVBAHA.106.137851. [DOI] [PubMed] [Google Scholar]
- 93.Moreno PR, Purushothaman KR, Sirol M, Levy AP, Fuster V. Neovascularization in human atherosclerosis. Circulation. 2006;113:2245–2252. doi: 10.1161/CIRCULATIONAHA.105.578955. [DOI] [PubMed] [Google Scholar]
- 94.Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res. 2001;49:507–521. doi: 10.1016/S0008-6363(00)00281-9. [DOI] [PubMed] [Google Scholar]
- 95.Simons M. Angiogenesis: Where do we stand now? Circulation. 2005;111:1556–1566. doi: 10.1161/01.CIR.0000159345.00591.8F. [DOI] [PubMed] [Google Scholar]
- 96.Tanaka K, Nagata D, Hirata Y, Tabata Y, Nagai R, Sata M. Augmented angiogenesis in adventitia promotes growth of atherosclerotic plaque in apolipoprotein E-deficient mice. Atherosclerosis. 2011;215:366–373. doi: 10.1016/j.atherosclerosis.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 97.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 98.Michel JB, Martin-Ventura JL, Nicoletti A, Ho-Tin-Noe B. Pathology of human plaque vulnerability: Mechanisms and consequences of intraplaque haemorrhages. Atherosclerosis. 2014;234:311–319. doi: 10.1016/j.atherosclerosis.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 99.Bhardwaj S, Roy H, Heikura T, Yla-Herttuala S. VEGF-A, VEGF-D and VEGF-D(DeltaNDeltaC) induced intimal hyperplasia in carotid arteries. Eur J Clin Invest. 2005;35:669–676. doi: 10.1111/j.1365-2362.2005.01555.x. [DOI] [PubMed] [Google Scholar]
- 100.Moulton KS, Heller E, Konerding MA, Flynn E, Palinski W, Folkman J. Angiogenesis inhibitors endostatin or TNP-470 reduce intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Circulation. 1999;99:1726–1732. doi: 10.1161/01.CIR.99.13.1726. [DOI] [PubMed] [Google Scholar]
- 101.Swirski FK, Nahrendorf M. Cardioimmunology: The immune system in cardiac homeostasis and disease. Nat Rev Immunolo. 2018;18:733–744. doi: 10.1038/s41577-018-0065-8. [DOI] [PubMed] [Google Scholar]
- 102.Dang TA, Schunkert H, Kessler T. cGMP signaling in cardiovascular diseases: Linking genotype and phenotype. J Cardiovasc Pharmacol. 2020;75:516–525. doi: 10.1097/FJC.0000000000000744. [DOI] [PubMed] [Google Scholar]
- 103.Feil R, Kemp-Harper B. cGMP signalling: From bench to bedside. Conference on cGMP generators, effectors and therapeutic implications. EMBO Rep. 2006;7:149–153. doi: 10.1038/sj.embor.7400627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dubey RK, Jackson EK, Luscher TF. Nitric oxide inhibits angiotensin II-induced migration of rat aortic smooth muscle cell. Role of cyclic-nucleotides and angiotensin1 receptors. J Clin Invest. 1995;96:141–149. doi: 10.1172/JCI118014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 106.Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet. 2021;397:839–852. doi: 10.1016/S0140-6736(21)00389-5. [DOI] [PubMed] [Google Scholar]
- 107.Bacci M, Capobianco A, Monno A, Cottone L, Di Puppo F, Camisa B, Mariani M, Brignole C, Ponzoni M, Ferrari S, et al. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am J Pathol. 2009;175:547–556. doi: 10.2353/ajpath.2009.081011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bianco B, André GM, Vilarino FL, Peluso C, Mafra FA, Christofolini DM, Barbosa CP. The possible role of genetic variants in autoimmune-related genes in the development of endometriosis. Hum Immunol. 2012;73:306–315. doi: 10.1016/j.humimm.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 109.Liu D, Song J, Ji X, Liu Z, Cong M, Hu B. Association of genetic polymorphisms on VEGFA and VEGFR2 with risk of coronary heart disease. Medicine. 2016;95:e3413. doi: 10.1097/MD.0000000000003413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ma H, Marti-Gutierrez N, Park SW, Wu J, Hayama T, Darby H, Van Dyken C, Li Y, Koski A, Liang D, Ma, et al. reply. Nature. 2018;560:E10–E23. doi: 10.1038/s41586-018-0381-y. [DOI] [PubMed] [Google Scholar]
- 111.Shimizu Y, Arima K, Noguchi Y, Yamanashi H, Kawashiri SY, Nobusue K, Nonaka F, Aoyagi K, Nagata Y, Maeda T. Vascular endothelial growth factor (VEGF) polymorphism rs3025039 and atherosclerosis among older with hypertension. Sci Rep. 2022;12:5564. doi: 10.1038/s41598-022-09486-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eldafira E, Prasasty VD, Abinawanto A, Syahfirdi L, Pujianto DA. Polymorphisms of estrogen receptor-α and estrogen receptor-β genes and its expression in endometriosis. Turk J Pharm Sci. 2021;18:91–95. doi: 10.4274/tjps.galenos.2019.94914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Casazza K, Page GP, Fernandez JR. The association between the rs2234693 and rs9340799 estrogen receptor alpha gene polymorphisms and risk factors for cardiovascular disease: A review. Biol Res Nurs. 2010;12:84–97. doi: 10.1177/1099800410371118. [DOI] [PubMed] [Google Scholar]
- 114.Mao T, Zong LL, Wang YF, Zhao X, Fu YG, Zeng J, Rao XQ. Association of the IL-6 gene 634C/G polymorphism with susceptibility to endometriosis. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2011;28:555–558. doi: 10.3760/cma.j.issn.1003-9406.2011.05.019. In Chinese. [DOI] [PubMed] [Google Scholar]
- 115.Lu S, Wang Y, Wang Y, Hu J, Di W, Liu S, Zeng X, Yu G, Wang Y, Wang Z. The IL-6 rs1800795 and rs1800796 polymorphisms are associated with coronary artery disease risk. J Cell Mol Med. 2020;24:6191–6207. doi: 10.1111/jcmm.15246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kumari R, Kumar S, Ahmad MK, Singh R, Kant Kumar S, Pradhan A, Chandra S, Kumar S. Promoter variants of TNF-α rs1800629 and IL-10 rs1800871 are independently associated with the susceptibility of coronary artery disease in north Indian. Cytokine. 2018;110:131–136. doi: 10.1016/j.cyto.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 117.Juo SH, Wu R, Lin CS, Wu MT, Lee JN, Tsai EM. A functional promoter polymorphism in interleukin-10 gene influences susceptibility to endometriosis. Fertil Steril. 2009;92:1228–1233. doi: 10.1016/j.fertnstert.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 118.Zheng J, Chen T, Lin H. IL-10, IL-18 gene polymorphisms might influence predisposition to coronary artery disease in east asians: A meta-analysis. Immunol Invest. 2021;50:37–46. doi: 10.1080/08820139.2020.1726382. [DOI] [PubMed] [Google Scholar]
- 119.Chen Y, Huang H, Liu S, Pan LA, Zhou B, Zhang L, Zeng Z. IL-16 rs11556218 gene polymorphism is associated with coronary artery disease in the Chinese Han population. Clin Biochem. 2011;44:1041–1044. doi: 10.1016/j.clinbiochem.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 120.Luo Z, Lu Z, Muhammad I, Chen Y, Chen Q, Zhang J, Song Y. Associations of the MTHFR rs1801133 polymorphism with coronary artery disease and lipid levels: A systematic review and updated meta-analysis. Lipids Health Dis. 2018;17:191. doi: 10.1186/s12944-018-0837-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Szczepanska M, Mostowska A, Wirstlein P, Lianeri M, Marianowski P, Skrzypczak J, Jagodziński PP. Polymorphic variants of folate and choline metabolism genes and the risk of endometriosis-associated infertility. Eur J Obstet Gynecol Reprod Biol. 2011;157:67–72. doi: 10.1016/j.ejogrb.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 122.Rodriguez-Rodriguez L, Lopez-Mejias R, Garcia-Bermudez M, Gonzalez-Juanatey C, Gonzalez-Gay MA, Martin J. Genetic markers of cardiovascular disease in rheumatoid arthritis. Mediators Inflamm. 2012;2012:574817. doi: 10.1155/2012/574817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sundqvist J, Falconer H, Seddighzadeh M, Vodolazkaia A, Fassbender A, Kyama C, Bokor A, Stephansson O, Padyukov L, Gemzell-Danielsson K, D'Hooghe TM. Endometriosis and autoimmune disease: Association of susceptibility to moderate/severe endometriosis with CCL21 and HLA-DRB1. Fertil Steril. 2011;95:437–440. doi: 10.1016/j.fertnstert.2010.07.1060. [DOI] [PubMed] [Google Scholar]
- 124.Nilufer R, Karina B, Paraskevi C, Rebecca D, Genevieve G, Ayush G, Stuar M, Sally M, Yadav S, Andrew SJ, et al. Large-scale genome-wide association meta-analysis of endometriosis reveals 13 novel loci and genetically-associated comorbidity with other pain conditions. bio Rxiv. 2018;406967 doi: 10.1101/406967. [DOI] [Google Scholar]
- 125.Zhang H, Mooney CJ, Reilly MP. ABO blood groups and cardiovascular diseases. Int J Vasc Med. 2012;2012:641917. doi: 10.1155/2012/641917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Omrani-Nava V, Hedayatizadeh-Omran A, Alizadeh-Navaei R, Mokhberi V, Jalalian R, Janbabaei G, Amjadi O, Rahmatpour G, Mozaffari A. TP53 single nucleotide polymorphism (rs1042522) in Iranian patients with coronary artery disease. Biomed Rep. 2018;9:259–265. doi: 10.3892/br.2018.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li J, Chen Y, Mo Z, Li L. TP53 Arg72Pro polymorphism (rs1042522) and risk of endometriosis among Asian and Caucasian populations. Eur J Obstet Gynecol Reprod Biol. 2015;189:73–78. doi: 10.1016/j.ejogrb.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 128.Shakhtshneider E, Orlov P, Semaev S, Ivanoshchuk D, Malyutina S, Gafarov V, Ragino Y, Voevoda M. Analysis of polymorphism rs1333049 (Located at 9P21.3) in the white population of western siberia and associations with clinical and biochemical markers. Biomolecules. 2019;9:290. doi: 10.3390/biom9070290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lalami I, Abo C, Borghese B, Chapron C, Vaiman D. Genomics of endometriosis: From genome wide association studies to exome sequencing. Int J Mol Sci. 2021;22:7297. doi: 10.3390/ijms22147297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kang YJ, Jeung IC, Park A, Park YJ, Jung H, Kim TD, Lee HG, Choi I, Yoon SR. An increased level of IL-6 suppresses NK cell activity in peritoneal fluid of patients with endometriosis via regulation of SHP-2 expression. Hum Reprod. 2014;29:2176–2189. doi: 10.1093/humrep/deu172. [DOI] [PubMed] [Google Scholar]
- 131.Holdt LM, Teupser D. Long noncoding RNA ANRIL: Lnc-ing genetic variation at the chromosome 9p21 locus to molecular mechanisms of atherosclerosis. Front Cardiovasc Med. 2018;5:145. doi: 10.3389/fcvm.2018.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Matoo S, Fallah MS, Daneshpour MS, Mousavi R, Sedaghati Khayat B, Hasanzad M, Azizi F. Increased risk of CHD in the presence of rs7865618 (A allele): Tehran lipid and glucose study. Arch Iran Med. 2017;20:153–157. [PubMed] [Google Scholar]
- 133.Manjula G, Pranavchand R, Kumuda I, Reddy BS, Reddy BM. The SNP rs7865618 of 9p213 locus emerges as the most promising marker of coronary artery disease in the southern Indian population. Sci Rep. 2020;10:21511. doi: 10.1038/s41598-020-77080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang Y, Shi X, Du Z, Zhou G, Zhang X. Associations between genetic variations in microRNA and myocardial infarction susceptibility: A meta-analysis and systematic review. Herz. 2022;47:524–535. doi: 10.1007/s00059-021-05086-3. [DOI] [PubMed] [Google Scholar]
- 135.Farsimadan M, Ismail Haje M, Khudhur Mawlood C, Arabipour I, Emamvirdizadeh A, Takamoli S, Masumi M, Vaziri H. MicroRNA variants in endometriosis and its severity. Br J Biomed Sci. 2021;78:206–210. doi: 10.1080/09674845.2021.1889157. [DOI] [PubMed] [Google Scholar]
- 136.Cai MY, Cheng J, Zhou MY, Liang LL, Lian SM, Xie XS, Xu S, Liu X, Xiong XD. The associationbetween pre-miR-27a rs 895819 polymorphism and myocardial infarction risk in a Chinese Han population. Lipids Health Dis. 2018;17:7. doi: 10.1186/s12944-017-0652-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jaafar SO, Jaffar JO, Ibrahim SA, Jarjees KK. MicroRNA Variants miR-27a rs895819 and miR-423 rs6505162, but not miR-124-1 rs531564, are Linked to endometriosis and its severity. Br J Biomed Sci. 2022;79:10207. doi: 10.3389/bjbs.2021.10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang Z, Li H, Zhao Z, Gao B, Meng L, Feng X. miR-146b level and variants is associated with endometriosis related macrophages phenotype and plays a pivotal role in the endometriotic pain symptom. Taiwan J Obstet Gynecol. 2019;58:401–408. doi: 10.1016/j.tjog.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 139.Zhao Y, Ponnusamy M, Zhang L, Zhang Y, Liu C, Yu W, Wang K, Li P. The role of miR-214 in cardiovascular diseases. Eur J Pharmacol. 2017;816:138–145. doi: 10.1016/j.ejphar.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 140.Jones Buie JN, Goodwin AJ, Cook JA, Halushka PV, Fan H. The role of miRNAs in cardiovascular disease risk factors. Atherosclerosis. 2016;254:271–281. doi: 10.1016/j.atherosclerosis.2016.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.CARDIoGRAMplusC4D Consortium. Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR, Ingelsson E, Saleheen D, Erdmann J, et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45:25–33. doi: 10.1038/ng.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ghafouri-Fard S, Shoorei H, Taheri M. Role of Non-coding RNAs in the pathogenesis of endometriosis. Front Oncol. 2020;10:1370. doi: 10.3389/fonc.2020.01370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Besseling J, Hovingh GK, Huijgen R, Kastelein JJP, Hutten BA. Statins in familial hypercholesterolemia: Consequences for coronary artery disease and All-cause mortality. J Am Coll Cardiol. 2016;68:252–260. doi: 10.1016/j.jacc.2016.04.054. [DOI] [PubMed] [Google Scholar]
- 144.Jha CK, Mir R, Elfaki I, Khullar N, Rehman S, Javid J, Banu S, Chahal SMS. Potential impact of MicroRNA-423 gene variability in coronary artery disease. Endocr Metab Immune Disord Drug Targets. 2019;19:67–74. doi: 10.2174/1871530318666181005095724. [DOI] [PubMed] [Google Scholar]
- 145.Chang CY, Lai MT, Chen Y, Yang CW, Chang HW, Lu CC, Chen CM, Chan C, Chung C, Tseng CC, et al. Up-regulation of ribosome biogenesis by MIR196A2 genetic variation promotes endometriosis development and progression. Oncotarget. 2016;7:76713–76725. doi: 10.18632/oncotarget.11536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fragoso JM, Ramirez-Bello J, Martinez-Rios MA, Peña-Duque MA, Posadas-Sánchez R, Delgadillo-Rodríguez H, Jiménez-Morales M, Posadas-Romero C, Vargas-Alarcón G. miR-196a2 (rs11614913) polymorphism is associated with coronary artery disease, but not with in-stent coronary restenosis. Inflamm Res. 2019;68:215–221. doi: 10.1007/s00011-018-1206-z. [DOI] [PubMed] [Google Scholar]
- 147.Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109(23 Suppl 1):III39–II43. doi: 10.1161/01.CIR.0000131517.20177.5a. [DOI] [PubMed] [Google Scholar]
- 148.Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209:123–137. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hoyer FF, Naxerova K, Schloss MJ, Hulsmans M, Nair AV, Dutta P, Calcagno DM, Herisson F, Anzai A, Sun Y, et al. Tissue-specific macrophage responses to remote injury impact the outcome of subsequent local immune challenge. Immunity. 2019;51:899–914 e897. doi: 10.1016/j.immuni.2019.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511–519. doi: 10.1016/j.fertnstert.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhao N, Zhang J. Role of alternative splicing of VEGF-A in the development of atherosclerosis. Aging. 2018;10:2695–2708. doi: 10.18632/aging.101580. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 152.Zhao W, Li Y, Zhao J, Kang S. A functional promoter polymorphism in interleukin 12B gene is associated with an increased risk of ovarian endometriosis. Gene. 2018;666:27–31. doi: 10.1016/j.gene.2018.04.082. [DOI] [PubMed] [Google Scholar]
- 153.Buroker NE. SNP (rs1570360) in transcriptional factor binding sites of the VEGFA promoter is associated with hypertensive nephropathy and diabetic retinopathy. Austin J Endocrinol Diabetes. 2015;2:1–5. [Google Scholar]
- 154.Kim I, Moon SO, Kim SH, Kim HJ, Koh YS, Koh GY. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J Biol Chem. 2001;276:7614–7620. doi: 10.1074/jbc.M009705200. [DOI] [PubMed] [Google Scholar]
- 155.McCarron SL, Edwards S, Evans PR, Gibbs R, Dearnaley DP, Dowe A, Southgate C, Easton DF, Eeles RA, Howell WM. Influence of cytokine gene polymorphisms on the development of prostate cancer. Cancer Res. 2002;62:3369–3372. [PubMed] [Google Scholar]
- 156.Howell WM, Bateman AC, Turner SJ, Collins A, Theaker JM. Influence of vascular endothelial growth factor single nucleotide polymorphisms on tumour development in cutaneous malignant melanoma. Genes Immunity. 2002;3:229–232. doi: 10.1038/sj.gene.6363851. [DOI] [PubMed] [Google Scholar]
- 157.Vigano P, Parazzini F, Somigliana E, Vercellini P. Endometriosis: Epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynaecol. 2004;18:177–200. doi: 10.1016/j.bpobgyn.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 158.Wang W, Xu A, Xu H. The roles of vascular endothelial growth factor gene polymorphisms in congenital heart diseases: A meta-analysis. Growth Factors. 2018;36:232–238. doi: 10.1080/08977194.2018.1513505. [DOI] [PubMed] [Google Scholar]
- 159.Wieser F, Fabjani G, Tempfer C, Schneeberger C, Sator M, Huber J, Wenzl R. Analysis of an interleukin-6 gene promoter polymorphism in women with endometriosis by pyrosequencing. J Soc Gynecol Investig. 2003;10:32–36. [PubMed] [Google Scholar]
- 160.Schieffer B, Schieffer E, Hilfiker-Kleiner D, Hilfiker A, Kovanen PT, Kaartinen M, Nussberger J, Harringer W, Drexler H. Expression of angiotensin II and interleukin 6 in human coronary atherosclerotic plaques: Potential implications for inflammation and plaque instability. Circulation. 2000;101:1372–1378. doi: 10.1161/01.CIR.101.12.1372. [DOI] [PubMed] [Google Scholar]
- 161.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 162.Kitawaki J, Obayashi H, Ohta M, Kado N, Ishihara H, Koshiba H, Kusuki I, Tsukamoto K, Hasegawa G, Nakamura N, et al. Genetic contribution of the interleukin-10 promoter polymorphism in endometriosis susceptibility. Am J Reprod Immunol. 2002;47:12–18. doi: 10.1034/j.1600-0897.2002.1o029.x. [DOI] [PubMed] [Google Scholar]
- 163.Zhang X, Hei P, Deng L, Lin J. Interleukin-10 gene promoter polymorphisms and their protein production in peritoneal fluid in patients with endometriosis. Mol Hum Reprod. 2007;13:135–140. doi: 10.1093/molehr/gal106. [DOI] [PubMed] [Google Scholar]
- 164.Tabrez S, Ali M, Jabir NR, Firoz CK, Ashraf GM, Hindawi S, Damanhouri GA, Nabil Alama M. A putative association of interleukin-10 promoter polymorphisms with cardiovascular disease. IUBMB Life. 2017;69:522–527. doi: 10.1002/iub.1637. [DOI] [PubMed] [Google Scholar]
- 165.Center DM, Kornfeld H, Cruikshank WW. Interleukin-16. Int J Biochem Cell Biol. 1997;29:1231–1234. doi: 10.1016/S1357-2725(97)00053-8. [DOI] [PubMed] [Google Scholar]
- 166.Mathy NL, Scheuer W, Lanzendörfer M, Honold K, Ambrosius D, Norley S, Kurth R, et al. Interleukin-16 stimulates the expression and production of pro-inflammatory cytokines by human monocytes. Immunology. 2000;100:63–69. doi: 10.1046/j.1365-2567.2000.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cheong YC, Shelton JB, Laird SM, Richmond M, Kudesia G, Li TC, Ledger WL. IL-1, IL-6 and TNF-alpha concentrations in the peritoneal fluid of women with pelvic adhesions. Hum Reprod. 2002;17:69–75. doi: 10.1093/humrep/17.1.69. [DOI] [PubMed] [Google Scholar]
- 168.Watkins D, Rosenblatt DS. Inherited disorders of folate and cobalamin transport and Metabolism. In: Valle DL, Antonarakis S, Ballabio A, Beaudet AL, Mitchell GA, editors. The Online Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill Education; New York, NY: 2019. [Google Scholar]
- 169.Lian Z, Lv FF, Yu J, Wang JW. The anti-inflammatory effect of microRNA-383-3p interacting with IL1R2 against homocysteine-induced endothelial injury in rat coronary arteries. J Cell Biochem. 2018;119:6684–6694. doi: 10.1002/jcb.26854. [DOI] [PubMed] [Google Scholar]
- 170.Cyster JG. Leukocyte migration: Scent of the T zone. Curr Biol. 2000;10:R30–R33. doi: 10.1016/S0960-9822(99)00253-5. [DOI] [PubMed] [Google Scholar]
- 171.Li G, Zhao J, Li B, Ma J, Zhao Q, Wang X, Lv Z, Li K, Du Z, Ma X, Liu J. Associations between CCL21 gene polymorphisms and susceptibility to rheumatoid arthritis: A meta-analysis. Rheumatol Int. 2017;37:1673–1681. doi: 10.1007/s00296-017-3784-4. [DOI] [PubMed] [Google Scholar]
- 172.Damas JK, Smith C, Øie E, Fevang B, Halvorsen B, Waehre T, Boullier A, Breland U, Yndestad A, Ovchinnikova O, et al. Enhanced expression of the homeostatic chemokines CCL19 and CCL21 in clinical and experimental atherosclerosis: Possible pathogenic role in plaque destabilization. Arterioscler Thromb Vasc Biol. 2007;27:614–620. doi: 10.1161/01.ATV.0000255581.38523.7c. [DOI] [PubMed] [Google Scholar]
- 173.Nakayama T, Kitaya K, Okubo T, Kuroboshi H, Daikoku N, Fushiki S, Honjo H. Fluctuation of 6Ckine expression in human endometrium during the menstrual cycle. Fertil Steril. 2003;80:1461–1465. doi: 10.1016/j.fertnstert.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 174.Nilsson S, Gustafsson JA. Biological role of estrogen and estrogen receptors. Crit Rev Biochem Mol Biol. 2002;37:1–28. doi: 10.1080/10409230290771438. [DOI] [PubMed] [Google Scholar]
- 175.Howard BV, Criqui MH, Curb JD, Rodabough R, Safford MM, Santoro N, Wilson AC, Wylie-Rosett J. Risk factor clustering in the insulin resistance syndrome and its relationship to cardiovascular disease in postmenopausal white, black, hispanic, and Asian/Pacific Islander women. Metabolism. 2003;52:362–371. doi: 10.1053/meta.2003.50057. [DOI] [PubMed] [Google Scholar]
- 176.Huang Q, Wang TH, Lu WS, Mu PW, Yang YF, Liang WW, Li CX, Lin GP. Estrogen receptor alpha gene polymorphism associated with type 2 diabetes mellitus and the serum lipid concentration in Chinese women in Guangzhou. Chin Med J (Engl) 2006;119:1794–1801. doi: 10.1097/00029330-200611010-00006. [DOI] [PubMed] [Google Scholar]
- 177.Lawlor DA, Timpson N, Ebrahim S, Day IN, Smith GD. The association of oestrogen receptor alpha-haplotypes with cardiovascular risk factors in the British Women's Heart and Health Study. Eur Heart J. 2006;27:1597–1604. doi: 10.1093/eurheartj/ehi833. [DOI] [PubMed] [Google Scholar]
- 178.Almeida S, Hutz MH. Genetic variation of estrogen metabolism and the risks of cardiovascular disease. Curr Opin Investig Drugs. 2007;8:814–820. [PubMed] [Google Scholar]
- 179.Naccarati A, Polakova V, Pardini B, Vodickova L, Hemminki K, Kumar R, Vodicka P. Mutations and polymorphisms in TP53 gene-an overview on the role in colorectal cancer. Mutagenesis. 2012;27:211–218. doi: 10.1093/mutage/ger067. [DOI] [PubMed] [Google Scholar]
- 180.Bennett BJ, Davis RC, Civelek M, Orozco L, Wu J, Qi H, Pan C, Packard RR, Eskin E, Yan M, et al. Genetic Architecture of atherosclerosis in mice: A systems genetics analysis of common inbred strains. PLoS Genet. 2015;11:e1005711. doi: 10.1371/journal.pgen.1005711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Frank AK, Leu JI, Zhou Y, Devarajan K, Nedelko T, Klein-Szanto A, Hollstein M, Murphy ME. The codon 72 polymorphism of p53 regulates interaction with NF-{kappa}B and transactivation of genes involved in immunity and inflammation. Mol Cell Biol. 2011;31:1201–1213. doi: 10.1128/MCB.01136-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dumont C, Corsoni-Tadrzak A, Ruf S, de Boer J, Williams A, Turner M, Kioussis D, Tybulewicz VL. Rac GTPases play critical roles in early T-cell development. Blood. 2009;113:3990–3998. doi: 10.1182/blood-2008-09-181180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Storry JR, Olsson ML. The ABO blood group system revisited: A review and update. Immunohematology. 2009;25:48–59. doi: 10.21307/immunohematology-2019-231. [DOI] [PubMed] [Google Scholar]
- 184.Reilly MP, Li M, He J, Ferguson JF, Stylianou IM, Mehta NN, Burnett MS, Devaney JM, Knouff CW, Thompson JR, et al. Identification of ADAMTS7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: Two genome-wide association studies. Lancet. 2011;377:383–392. doi: 10.1016/S0140-6736(10)61996-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pare G, Ridker PM, Rose L, Barbalic M, Dupuis J, Dehghan A, Bis JC, Benjamin EJ, Shiffman D, Parker AN, Chasman DI. Genome-wide association analysis of soluble ICAM-1 concentration reveals novel associations at the NFKBIK, PNPLA3, RELA, and SH2B3 loci. PLoS Genet. 2011;7:e1001374. doi: 10.1371/journal.pgen.1001374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kiechl S, Pare G, Barbalic M, Qi L, Dupuis J, Dehghan A, Bis JC, Laxton RC, Xiao Q, Bonora E, et al. Association of variation at the ABO locus with circulating levels of soluble intercellular adhesion molecule-1, soluble P-selectin, and soluble E-selectin: A meta-analysis. Circ Cardiovasc Genet. 2011;4:681–686. doi: 10.1161/CIRCGENETICS.111.960682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Roberts R, Stewart AF. 9p21 and the genetic revolution for coronary artery disease. Clin Chem. 2012;58:104–112. doi: 10.1373/clinchem.2011.172759. [DOI] [PubMed] [Google Scholar]
- 188.Broadbent HM, Peden JF, Lorkowski S, Goel A, Ongen H, Green F, Clarke R, Collins R, Franzosi MG, Tognoni G, et al. Susceptibility to coronary artery disease and diabetes is encoded by distinct, tightly linked SNPs in the ANRIL locus on chromosome 9p. Hum Mol Genet. 2008;17:806–814. doi: 10.1093/hmg/ddm352. [DOI] [PubMed] [Google Scholar]
- 189.Cunnington MS, Santibanez Koref M, Mayosi BM, Burn J, Keavney B. Chromosome 9p21 SNPs associated with multiple disease phenotypes correlate with ANRIL expression. PLoS Genet. 2010;6:e1000899. doi: 10.1371/journal.pgen.1000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kalpana B, Murthy DK, Balakrishna N. 9p213 coronary artery disease risk locus and interferon alpha 21: Association study in an Asian Indian population. Indian Heart J. 2019;71:476–480. doi: 10.1016/j.ihj.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lee GH, Choi YM, Hong MA, Yoon SH, Kim JJ, Hwang K, Chae SJ. Association of CDKN2B-AS and WNT4 genetic polymorphisms in Korean patients with endometriosis. Fertil Steril. 2014;102:1393–1397. doi: 10.1016/j.fertnstert.2014.07.1237. [DOI] [PubMed] [Google Scholar]
- 192.Motterle A, Pu X, Wood H, Xiao Q, Gor S, Ng FL, Chan K, Cross F, Shohreh B, Poston RN, et al. Functional analyses of coronary artery disease associated variation on chromosome 9p21 in vascular smooth muscle cells. Hum Mol Genet. 2012;21:4021–4029. doi: 10.1093/hmg/dds224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Boettger T, Beetz N, Kostin S, Schneider J, Krüger M, Hein L, Braun T. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest. 2009;119:2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Elia L, Quintavalle M, Zhang J, Contu R, Cossu L, Latronico MV, Peterson KL, Indolfi C, Catalucci D, Chen J, et al. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: Correlates with human disease. Cell Death Differ. 2009;16:1590–1598. doi: 10.1038/cdd.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Elia L, Kunderfranco P, Carullo P, Vacchiano M, Farina FM, Hall IF, Mantero S, Panico C, Papait R, Condorelli G, Quintavalle M. UHRF1 epigenetically orchestrates smooth muscle cell plasticity in arterial disease. J Clin Invest. 2018;128:2473–2486. doi: 10.1172/JCI96121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Xin M, Small EM, Sutherland LB, Qi X, McAnally J, Plato CF, Richardson JA, Bassel-Duby R, Olson EN. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009;23:2166–2178. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Quintavalle M, Elia L, Condorelli G, Courtneidge SA. MicroRNA control of podosome formation in vascular smooth muscle cells in vivo and in vitro. J Cell Biol. 2010;189:13–22. doi: 10.1083/jcb.200912096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Climent M, Quintavalle M, Miragoli M, Chen J, Condorelli G, Elia L. TGFβ Triggers miR-143/145 transfer from smooth muscle cells to endothelial cells, thereby modulating vessel stabilization. Circ Res. 2015;116:1753–1764. doi: 10.1161/CIRCRESAHA.116.305178. [DOI] [PubMed] [Google Scholar]
- 200.Faccini J, Ruidavets JB, Cordelier P, Martins F, Maoret JJ, Bongard V, Ferrières J, Roncalli J, Elbaz M, Vindis C. Circulating miR-155, miR-145 and let-7c as diagnostic biomarkers of the coronary artery disease. Sci Rep. 2017;7:42916. doi: 10.1038/srep42916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hall IF, Climent M, Viviani Anselmi C, Papa L, Tragante V, Lambroia L, Farina FM, Kleber ME, März W, Biguori C, et al. rs41291957 controls miR-143 and miR-145 expression and impacts coronary artery disease risk. EMBO Mol Med. 2021;13:e14060. doi: 10.15252/emmm.202114060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nimi-Hoveidi E, Kohan L, Hashemi SS. Association of miR-143 rs41291957 and rs4705342 genetic variants with endometriosis risk in infertile women. Feyz J Kashan Univ Med Sci. 2016;20:441–446. [Google Scholar]
- 203.Bashti O, Noruzinia M, Garshasbi M, Abtahi M. miR-31 and miR-145 as potential non-invasive regulatory biomarkers in patients with endometriosis. Cell J. 2018;20:84–89. doi: 10.22074/cellj.2018.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ounzain S, Micheletti R, Arnan C, Plaisance I, Cecchi D, Schroen B, Reverter F, Alexanian M, Gonzales C, Ng SY, et al. CARMEN, a human super enhancer-associated long noncoding RNA controlling cardiac specification, differentiation and homeostasis. J Mol Cell Cardiol. 2015;89:98–112. doi: 10.1016/j.yjmcc.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 205.Plaisance I, Perruchoud S, Fernandez-Tenorio M, Gonzales C, Ounzain S, Ruchat P, Nemir M, Niggli E, Pedrazzini T. Cardiomyocyte lineage specification in adult human cardiac precursor cells via modulation of enhancer-associated long noncoding RNA expression. JACC Basic Transl Sci. 2016;1:472–493. doi: 10.1016/j.jacbts.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, Deitch EA, Huo Y, Delphin ES, Zhang C. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res. 2009;105:158–166. doi: 10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang X, Hu G, Zhou J. Repression of versican expression by microRNA-143. J Biol Chem. 2010;285:23241–23250. doi: 10.1074/jbc.M109.084673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dong K, Shen J, He X, Hu G, Wang L, Osman I, Bunting KM, Dixon-Melvin R, Zheng Z, Xin H, et al. CARMN is an evolutionarily conserved smooth muscle cell-specific LncRNA that maintains contractile phenotype by binding myocardin. Circulation. 2021;144:1856–1875. doi: 10.1161/CIRCULATIONAHA.121.055949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Li M, Wang J, Fang Y, Gong S, Li M, Wu M, Lai X, Zeng G, Wang Y, Yang K, Huang X. microRNA-146a promotes mycobacterial survival in macrophages through suppressing nitric oxide production. Sci Rep. 2016;6:23351. doi: 10.1038/srep23351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sidorkiewicz M, Grek M, Jozwiak B, Krol A, Piekarska A. The impact of chronic hepatitis C infection on cholesterol metabolism in PBMCs is associated with microRNA-146a expression. Eur J Clin Microbiol Infect Dis. 2017;36:697–702. doi: 10.1007/s10096-016-2851-1. [DOI] [PubMed] [Google Scholar]
- 211.Takahashi Y, Satoh M, Minami Y, Tabuchi T, Itoh T, Nakamura M. Expression of miR-146a/b is associated with the Toll-like receptor 4 signal in coronary artery disease: Effect of renin-angiotensin system blockade and statins on miRNA-146a/b and Toll-like receptor 4 levels. Clin Sci. 2010;119:395–405. doi: 10.1042/CS20100003. [DOI] [PubMed] [Google Scholar]
- 212.Gangwar RS, Rajagopalan S, Natarajan R, Deiuliis JA. Noncoding RNAs in cardiovascular disease: Pathological relevance and emerging role as biomarkers and therapeutics. Am J Hypertens. 2018;31:150–165. doi: 10.1093/ajh/hpx197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhou L, Zhao X, Han Y, Lu Y, Shang Y, Liu C, Li T, Jin Z, Fan D, Wu K. Regulation of UHRF1 by miR-146a/b modulates gastric cancer invasion and metastasis. FASEB J. 2013;27:4929–4939. doi: 10.1096/fj.13-233387. [DOI] [PubMed] [Google Scholar]
- 214.Ramirez-Moya J, Wert-Lamas L, Santisteban P. MicroRNA-146b promotes PI3K/AKT pathway hyperactivation and thyroid cancer progression by targeting PTEN. Oncogene. 2018;37:3369–3383. doi: 10.1038/s41388-017-0088-9. [DOI] [PubMed] [Google Scholar]
- 215.Zhu Y, Wu G, Yan W, Zhan H, Sun P. miR-146b-5p regulates cell growth, invasion, and metabolism by targeting PDHB in colorectal cancer. Am J Cancer Res. 2017;7:1136–1150. [PMC free article] [PubMed] [Google Scholar]
- 216.Jin H, Zhang H, Ma T, Lan H, Feng S, Zhu H, Ji Y. Resveratrol protects murine chondrogenic ATDC5 cells against LPS-induced inflammatory injury through Up-regulating MiR-146b. Cell Physiol Biochem. 2018;47:972–980. doi: 10.1159/000490141. [DOI] [PubMed] [Google Scholar]
- 217.Gao S, Zhao Z, Wu R, Wu L, Tian X, Zhang Z. MiR-146b inhibits autophagy in prostate cancer by targeting the PTEN/Akt/mTOR signaling pathway. Aging. 2018;10:2113–2121. doi: 10.18632/aging.101534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Curtale G, Mirolo M, Renzi TA, Rossato M, Bazzoni F, Locati M. Negative regulation of Toll-like receptor 4 signaling by IL-10-dependent microRNA-146b. Proc Natl Acad Sci USA. 2013;110:11499–11504. doi: 10.1073/pnas.1219852110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Chistiakov DA, Orekhov AN, Bobryshev YV. The role of miR-126 in embryonic angiogenesis, adult vascular homeostasis, and vascular repair and its alterations in atherosclerotic disease. J Mol Cell Cardiol. 2016;97:47–55. doi: 10.1016/j.yjmcc.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 220.Urbich C, Kaluza D, Frömel T, Knau A, Bennewitz K, Boon RA, Bonauer A, Doebele C, Boeckel JN, Hergenreider E, et al. MicroRNA-27a/b controls endothelial cell repulsion and angiogenesis by targeting semaphorin 6A. Blood. 2012;119:1607–1616. doi: 10.1182/blood-2011-08-373886. [DOI] [PubMed] [Google Scholar]
- 221.Su X, Hu Y, Li Y, Cao JL, Wang XQ, Ma X, Xia HF. The polymorphism of rs6505162 in the MIR423 coding region and recurrent pregnancy loss. Reproduction. 2015;150:65–76. doi: 10.1530/REP-15-0007. [DOI] [PubMed] [Google Scholar]
- 222.Medford HM, Marsh SA. The role of O-GlcNAc transferase in regulating the gene transcription of developing and failing hearts. Future Cardiol. 2014;10:801–812. doi: 10.2217/fca.14.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Luo P, He T, Jiang R, Li G. MicroRNA-423-5p targets O-GlcNAc transferase to induce apoptosis in cardiomyocytes. Mol Med Rep. 2015;12:1163–1168. doi: 10.3892/mmr.2015.3491. [DOI] [PubMed] [Google Scholar]
- 224.Amin MMJ, Trevelyan CJ, Turner NA. MicroRNA-214 in health and disease. Cells. 2021;10:3274. doi: 10.3390/cells10123274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Taskin O, Rikhraj K, Tan J, Sedlak T, Rowe TC, Bedaiwy MA. Link between Endometriosis, Atherosclerotic Cardiovascular Disease, and the Health of Women Midlife. J Minim Invasive Gynecol. 2019;26:781–784. doi: 10.1016/j.jmig.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 226.Missmer SA. Commentary: Endometriosis-epidemiologic considerations for a potentially 'high-risk' population. Int J Epidemiol. 2009;38:1154–1155. doi: 10.1093/ije/dyp249. [DOI] [PubMed] [Google Scholar]
- 227.O'Sullivan JW, Raghavan S, Marquez-Luna C, Luzum JA, Damrauer SM, Ashley EA, O'Donnell CJ, Willer CJ, Natarajan P American Heart Association Council on Genomic and Precision Medicine et al. Polygenic risk scores for cardiovascular disease: A scientific statement from the American Heart Association. Circulation. 2022;146:e93–e118. doi: 10.1161/CIR.0000000000001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Bulun SE, Monsavais D, Pavone ME, Dyson M, Xue Q, Attar E, Tokunaga H, Su EJ. Role of estrogen receptor-β in endometriosis. Semin Reprod Med. 2012;30:39–45. doi: 10.1055/s-0031-1299596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Stevenson JC. Long-term benefits and risks of HRT (Section 11): Cardiovascular disease. Post Reprod Health. 2016;22:80–82. doi: 10.1177/2053369116648856. [DOI] [PubMed] [Google Scholar]
- 230.Newson L. Menopause and cardiovascular disease. Post Reprod Health. 2018;24:44–49. doi: 10.1177/2053369117749675. [DOI] [PubMed] [Google Scholar]
- 231.Patel BG, Lenk EE, Lebovic DI, Shu Y, Yu J, Taylor RN. Pathogenesis of endometriosis: Interaction between Endocrine and inflammatory pathways. Best Pract Res Clin Obstet Gynaecol. 2018;50:50–60. doi: 10.1016/j.bpobgyn.2018.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.