Abstract
During cardiac development and morphogenesis cardiac progenitor cells differentiate into cardiomyocytes (CMs) that expand in number and size to generate the fully formed heart. Much is known about the factors that regulate initial differentiation of CMs, and there is ongoing research to identify how these fetal and immature CMs develop into fully functioning, mature cells. Accumulating evidence indicates that maturation limits proliferation and conversely proliferation occurs rarely in CMs of the adult myocardium. We term this oppositional interplay the proliferation-maturation dichotomy (PMD). Here we review the factors that are involved in this interplay and discuss how a better understanding of the PMD could advance the utility of human induced pluripotent stem cell-derived CMs for modeling in 3D engineered cardiac tissues to obtain truly adult-level function.
Keywords: Cardiomyocyte, Proliferation, Maturation, Cardiac Organoid, Tissue Engineering
Introduction
The Roman god of doorways, Janus, depicted with two faces – one looking forward, the other backward – represented transition across a metaphorical threshold dividing two incompatible ways of being1. He thus makes an apt representation for our emerging understanding of the incompatibility of two key modes of activity of cardiomyocytes (CMs), both essential for different reasons to cardiac regeneration: proliferation and maturation. Proliferation is always associated with an immature state, or with de-differentiation, while maturation invariably precludes proliferation2–5. Understanding this dichotomy and its mechanistic underpinnings allows us to better understand the challenges facing cardiac tissue engineering, and also opens opportunities for managing or directing the transition across the threshold that divides proliferation and maturation. We review below key findings demonstrating the proliferation-maturation dichotomy (PMD) and suggest engineering approaches that take advantage of it.
During embryonic and early postnatal development, the mammalian heart grows in size due to proliferation of CMs and subsequent increase in CM cell volume (hypertrophy)4–8 (Figure 1). However, CMs quickly lose their ability to proliferate soon after birth (within days in mice)7, 9, 10. During the progressive loss of cell cycle activity, CMs undergo terminal differentiation to attain a completely mature phenotype4, 7, 11, 12 (Figure 1). Proliferative CMs are immature with disorganized sarcomeric structures, small size, slow calcium (Ca2+) shuttling, short action potential duration and are dependent on the glycolytic pathway for ATP generation (Figure 1). During the maturation process CMs gradually become rod-shaped cells with aligned sarcomeres, transverse tubules (T-tubules) invaginate into the cells allowing for propagation of electrical activity into the CMs, and intercalated discs connect CMs to neighboring CMs to allow simultaneous contraction (Figure 1). To date, the cellular components and processes found to change most dynamically with the transition between proliferative and mature CM are: sarcomeric structure, Ca2+ handling, ploidy, metabolism, and transcription factor activation. We begin the review with a dissection of each component or process.
Figure 1. Proliferation-maturation dichotomy in CMs.

The mammalian heart grows in size due to CM proliferation and hypertrophy. Proliferative CMs are immature with disorganized sarcomeric structures, smaller size, slow Ca2+ shuttling, low AP duration and dependent on the glycolytic pathway for ATP generation. In maturation, CMs become rod-shaped cells with aligned sarcomeres with well-arranged mitochondria, transverse tubules (T-tubules) invaginate into the cells to propagate electrical activity into the CMs, and intercalated discs connect CMs to neighboring CMs. Transition between proliferative and mature states involves changes in sarcomeric structure, calcium handling, metabolism, and transcription factor activation.
Key Cellular Characteristics in the PMD
Sarcomere structure
Sarcomeres are the collection of proteins that confer CM shape and that are responsible for generating power stroke in CMs. At early developmental stages, when CM are proliferative, CMs contain fewer and less-organized sarcomeres4, 13, 14. During CM maturation, new sarcomeres are added to the preexisting myofibrillar network and collectively, sarcomeres undergo structural changes as adult isoforms of sarcomeric proteins replace the fetal isoforms. For example, mammalian cardiac myosin heavy chain proteins have two isoforms: α-isoform (MYH6) and β-isoform (MYH7) encoded by two distinct genes15. In human hearts, the β-isoform (MYH7) is predominant in the adult human ventricular myocardium (~95%)15, 16. The mammalian MYH6 is expressed in the developing (i.e., immature) human ventricle and adult atrium and is associated with higher actomyosin ATPase activity than the MYH7 isoform. Therefore, hearts expressing MYH6 possess more rapid contractile function than hearts expressing MYH716. As another example, Troponin I has three isoforms namely, ssTNI, fast skeletal TNI (fsTNI) and cTNI isoform, that are encoded by TNNI1, TNNI2 and TNNI3 respectively17, 18. With development, the ratio of the ssTNI to cTNI decreases with no detectable levels of ssTNI in adult heart18. These changes ultimately contribute to changes in cell shape, increased stroke volume and cardiac output during embryonic and early postnatal development19.
Although it is still unclear how the changes in the cytoskeletal and sarcomeric structure contribute to CM cell cycle arrest, the evidence collected during the last two decades suggest that the two processes indeed influence one another20–23. One of the earliest indications showed that proliferating CMs undergo sarcomere disassembly in vitro and in vivo prior to cytokinesis4, 23, 24. In addition, genetic mouse models with enhanced CM cell cycle activity such as cardiac specific overexpression of active-Yap1 (YAP5SA) or the pluripotency reprogramming factors (OCT4, SOX2, KLF4 and c-MYC, also known as OSKM)25, resulted in a breakdown of the sarcomere network, especially within the proliferating CM population20, 23, 26, 27 (Figure 1). Similar observations were made in regenerative zebrafish hearts, where AP-1 was identified as an important regulator of sarcomere breakdown28, 29, which is likely to occur in two sequential steps. First, Z-disk and thin filaments are disassembled, followed by the breakdown of the M-band (myomesin and obscurins) and myosin-rich thick filaments30, 31. Although this process has been shown to be proteasome-dependent, certain signaling pathways such as Notch and Hippo also regulate mRNA expressions of sarcomeric proteins such as Myh6 and troponin T (Tnnt1), indicating that sarcomere density can also be regulated via transcriptional mechanisms. In addition, some of these pathways rely on epigenetic modifications to influence the transcriptome. In particular, the RNA expression of DNA methylation profiles of dedifferentiating, cycling CMs is drastically different from the adult CM32,33. These studies indicate the importance of sarcomere assembly for the function of mature CMs and suggest that sarcomere disassembly is equally important for CM proliferation (Figure 1). Indeed, recent findings show that CRISPR-Cas9-mediated Troponin I knockout CMs more readily engraft in the infarcted rat heart with enhanced proliferation and no observed apoptosis relative to wild type controls34. One could therefore imagine transient modulation of the sarcomeric status of CM to encourage expansion of CMs as a means to generate cardiac tissue models with increased muscle mass. Modulation could include overexpression or suppression of isoforms of sarcomeric protein associated with early (proliferative) or late (maturation) phenotypes.
Ca2+ Dynamics
Calcium (Ca2+) plays a pivotal role in CM contraction and homeostasis. During CM maturation, the amount of available Ca2+ in the sarcoplasmic reticulum (SR) increases significantly with expansion of the SR and the myofibrillar network35. Increase in intracellular Ca2+ levels ultimately lead to activation of several proteins including calcineurin (CnA), a Ca2+ dependent serine/threonine phosphatase. Recent work shows that CnA mediates Hoxb13 translocation – possibly via dephosphorylation at Ser204 – to the CM nuclei, where Hoxb13 acts as a cofactor for Meis1 and inhibits CM cell cycle activity36. It was previously documented that the transcription factor Meis1 mediates CM cell cycle arrest postnatally37. Later, the same group identified cooperative interactions between transcription factor Hoxb13 and Meis1 in this process using co-immunoprecipitation and proximity ligation assays. Constitutive Hoxb13 deletion resulted in decreased CM cross-sectional area and increased cTnT+pHH3+ and cTnT+AuroraB kinase+ CMs at P14 with similar findings noted using an inducible system in adult hearts. Double knockout of Meis1-Hoxb13 in murine CMs had similar and more pronounced findings relative to the single Meis1 and Hoxb13 mutants with reduced CM size, increased proliferation (cTnT+pHH3+ and cTnT+Aurora B kinase+), increased CM number, decreased multinucleation, and sarcomere disorganization36. Further, it has been shown that deletion or inhibition of CnA enhances CM proliferation and causes CM dedifferentiation and sarcomere disassembly36. In addition, another study showed that the electrical coupling of NRVMs with adult CMs can induce dedifferentiation and proliferation of adult CMs in a co-culture setting38. Importantly, the increase in intracellular Ca2+ levels or the activation of calcineurin can block this dedifferentiation process, highlighting the direct role of intracellular Ca2+ in mediating CM maturation. Moreover, it is possible that the dedifferentiation of adult CMs in this co-culture setting also relies on the removal of intracellular Ca2+ and subsequent inhibition of calcineurin. Similarly, interruption of this coupling process or increasing Ca2+ levels of NRVMs prevents or reverses the dedifferentiation of adult CMs, indicating removal of available of Ca2+ can reverse the maturation process38. Collectively, these results demonstrate that changes in calcium dynamics can modulate shifts between proliferative and mature CM states.
Metabolic Substrates
The adult human heart consumes the highest amount of energy per organ weight, largely due to high ATP demand of myosin and Serca2a39, 40. The ATP used during these processes is mostly produced by oxidative phosphorylation of fatty acids in the adult heart41, 42. During fetal development, CMs reside in a hypoxic environment and mostly generate ATP through the glycolytic pathway4, 43–45. After blood circulation is established and especially after birth when circulating oxygen levels rise, CMs switch to aerobic respiration and begin using fatty acids as an energy source39. At the same time, mitochondria undergo morphological and functional changes. In fetal CMs, mitochondria are large, and rod shaped with low abundance and disorganized cristae46, 47. As CMs mature, mitochondria become small and spherical with more abundant and organized cristae47. During CM maturation, the mitochondrial networks starts to align with the sarcomeres, presumably to effectively shuttle ATP to myosins47. Whether this is accomplished by tethering mitochondria to the SR or through other means is not clear.
In normoxic conditions, >95% of ATP is derived from mitochondrial oxidative phosphorylation, whereas ~5% comes from glycolytic pathway41, 42. As CMs mature, they switch from glycolytic processes to fatty acid oxidation processes for the generation of ATP48, 49. Importantly, this switch is necessary to sustain the increase in cardiac output during development. The gradual increase in oxygen levels immediately after birth is directly responsible for the switch from glucose to fatty acid metabolism and the inhibition of CM cell cycle activity through DNA damage in CMs50–53. Moreover, hypoxemic CMs in the adult mammalian heart, characterized by low capillary density surrounding them, are capable of proliferating even in adulthood33 (Figure 1). Likewise, preserving hypoxic conditions after birth prolongs the proliferative state of CMs33, 50. At the molecular level, hypoxia leads to the activation and stabilization of proliferative proteins such as Fam64 and Hif1a while suppressing the activity of anti-proliferative factors like Meis137, 54. Analogous to increased circulating oxygen levels, enhanced fatty acid utilization during CM maturation eventually leads to the inhibition of cell cycle activity55, 56. Furthermore, if the rate of glycolysis is increased relative to fatty acid oxidation, CMs regain the ability to reenter the cell cycle during adulthood55, 57 (Figure 1). Collectively, these results suggest that the PMD is inextricably linked via hypoxia- and substrate-dependent metabolic shifts58. Metabolic pathway-mediated CM maturation has recently been explored59 and increased our understanding of the role of metabolic process and energy demands in the mature CMs.
Polyploidy
During maturation, mammalian CMs exit the cell cycle during mitosis or cytokinesis and become one of the few polyploid cell types under homeostatic conditions21 (Figure 1). Currently, it is unknown whether CM polyploidy occurs as an adaptation mechanism during maturation, although evolutionary evidence suggests that polyploidy allows for increased cell size and resistance to stress60, 61. However, recent evidence showed that in adult hearts, mono and multinucleated CMs are comparable in their molecular and cellular profiles, suggesting that ploidy per se does not play a significant role in mature CMs62. Furthermore, ploidy status does not alter CM remodeling in response to injury during adulthood63. In contrast, polyploidy is a determinant for proliferative potential of CMs in immature hearts. Based on previous studies, mononucleated immature CMs better preserve their proliferative potential during the postnatal period compared to binucleated counterparts64, 65. Moreover, very rare CM cell cycle events in adult hearts following injury are likely to originate from mononucleated CM65. Similarly, inhibition of CM cytokinesis and the subsequent increase in number of binucleated CMs diminishes the proliferative potential of zebrafish and neonatal mouse hearts after apical resection66, 67. By and large, but not unanimously, these studies suggest that polyploidy limits the regenerative potential of CMs during maturation.
Modulation of Cellular Characteristics in the PMD
Although multiple aspects of CM maturation can influence PMD, their efficacy and indispensability in regulating CM cell cycle vary greatly. Among these, solely altering metabolism or oxygen consumption seems to be sufficient to drive CM cell cycle activation51, 68. However, recent studies have shown that this activation leads to CM polyploidy as it primarily induces the expression of G1-S phase related genes and not regulators of cytokinesis68. On the other hand, sarcomere disassembly is a marker and most likely an essential step for CM cytokinesis2, 20, 23, 69. It is currently unknown whether sarcomere disassembly by itself can promote cell cycle activation or cytokinesis. However, if the latter is correct, inducing sarcomere disassembly can be potential strategy to drive cytokinesis and mitigate CM polyploidy after hypoxia-mediated cell cycle activation50, 68. However, viability of sarcomere disassembly as a treatment is questionable at best since it would lead to cardiac dysfunction and possibly heart failure. Similarly, targeting calcineurin or modulating Ca2+ dynamics can lead to detrimental consequences for cardiac function. More importantly, it is currently unknown whether calcineurin inhibition can lead to CM proliferation in adult hearts. Therefore, it is unlikely that modulating these aspects alone or even in combination will serve as a safe and sufficient treatment for inducing CM proliferation as a therapeutic to regenerate the adult heart. However, in the case of engineered tissues, where the maintenance of cardiac function is not paramount at all points in time, these approaches may be more viable. We explore these possibilities in the engineered tissues section.
Currently, only modulating molecular pathways that are robust enough to revert all aspects of CM maturation seem to achieve effective proliferative outcome in adult hearts26, 70. These pathways are promising targets for achieving CM proliferation in disease states such as heart failure. However, the question remains whether CMs are re-differentiated to attain mature phenotype after the withdrawal of manipulations/treatments and needs to be investigated. In previous findings, persistent immature state of CMs led to tumor formation and ventricular hyperplasia20. Therefore, more studies need to be performed to understand how to reinduce maturation in CMs.
Transcription Factors and Epigenetic Changes Linked to Cellular Characteristics of the PMD
It has previously been shown that GATA family members 4/5/6 mediate CM proliferation during fetal development71–73. Interestingly, genetic deletion of Gata4/6 prevented an increase in CM size during postnatal development and caused a switch from Myh6 (adult, murine) to Myh7 (fetal, murine) isoform, suggesting a possible role of Gata proteins in the regulation of CM maturation74 (Figure 2). A similar trend was observed in murine models involving the transcription factor Yap1 and its co-factor Transcriptional enhancer factor Tef-1 (Tead1)75. CM-specific deletion of Yap1 inhibited proliferation and caused cardiac dysfunction while activation of Yap1 or forced expression of YAP5SA led to CM cell cycle reentry during adulthood27 (Figure 2). Moreover, based on pull-down studies, Yap1 directly interacts with β-catenin to promote CM cell cycling75 (Figure 2). From the maturation standpoint, Yap1 overactivation led to decreased CM size, sarcomere disassembly and decreased fatty acid utilization, indicating Yap1 activity induces CM proliferation through dedifferentiation76. Further, Yap1 selectively represses the expression of Sox17 to disrupt the differentiation of CMs. Transcription factors belonging to the T-box gene family of transcription factors are also essential for cardiac development and mutations in these genes often lead to congenital defects77, 78. Notably, overexpression of the regulator of fetal cardiac development, Tbx20 in adult mice led to dedifferentiation and proliferation of CMs78. Similarly, Hif1a has been shown as an important regulator of CM proliferation, possibly via CM dedifferentiation leading to generation of immature CM following hypoxic stimuli 33, 50. Whether these transcription factors converge to a common regulatory node to control the PMD is unclear and needs further investigation. Identification of a single regulatory node could provide methodologies to induce CM proliferation or maturation for regenerative medicine and drug discovery respectively79.
Figure 2. Signaling pathways at the interplay between CM proliferation and maturation.

CM schematic showing the activation of signaling pathway from extracellular signals to cytoplasmic intermediates and nuclear effectors, including the canonical Wnt/β-catenin signaling pathway, noncanonical Wnt-YAP1 signaling pathway, crosstalk between PI3K/AKT and Wnt signaling pathway, interplay between MAPK and PI3K/AKT signaling pathways, Hoxb13-Meis1-calcineurin signaling, and thyroid hormone (T3) signaling with noncanonical MAPK activation. Abbreviations: Low-density lipoprotein receptor-related protein-5/6 (LRP-5/6), Adenomatosis Polyposis Coli (APC), Frizzled (Fz), Dishevelled (DVL), Casein Kinase-1 (CK1), Glycogen synthase kinase-3β (GSK3β), T-cell factor/lymphoid enhancer factor (TCF/LEF), Yes1 associated transcriptional regulator (YAP1), Transcriptional enhanced associate domain (TEAD), Receptor Tyrosine Kinase (RTKs), Phosphatidylinositol 3-kinase (PI3K), 3-phosphoinositide-dependent protein kinase 1 (PDK1), Cyclin Dependent Kinase-4/6 (CDK4/6), Mitogen Activated Protein kinase kinase kinase (MAP3K), Dual specificity mitogen-activated protein kinase kinase 3/6 (MKK3/6), Rat Sarcoma Virus (RAS), Rapidly Accelerated Fibrosarcoma (RAF), Dual specificity mitogen-activated protein kinase kinase 1/2 (MEK1/2), Extracellular Signal-regulated kinase 1/2 (ERK1/2), Transcription Factor (TF), L-Type Calcium Channel (LTCC), Calmodulin (CaM), Calcineurin A (CnA), Calcineurin B (CnB), Homeobox protein Hox-B13 (Hoxb13), Homeobox protein Meis1 (Meis1), Monocarboxylate transporter 8/10 (MCT8/10), Thyroid hormone, triiodothyronine (T3), Thyroid hormone receptor α (TRα), Retinoid X Receptor (RXR), hydrogen peroxide (H2O2), Peroxiredoxin 1 (Prx1), c-Jun N-terminal kinase-2 (JNK2α2).
Studies related to epigenetic regulation of CM proliferation and maturation have shown that genetic ablation of histone demethylases Kdm6ba and Kdm6bb in zebrafish leads to decreased ventricular chamber trabeculation without impacting the chamber formation80. Further, a ~50% reduction in CM proliferation was observed based on EdU incorporation and proliferating cell nuclear antigen (PCNA) expression studies at 5 days post fertilization (5dpf). More recently, transcriptional, and post-transcriptional regulatory mechanisms of ventricular trabeculation and compaction were identified in murine hearts81. Using the CM-specific deletion of G-quadruplex (G4) resolvase RNA helicase associated with AU-rich element (RHAU) in mice, the authors showed reduced CM proliferation, defective trabeculation and compaction at embryonic (E) day 12.5 and E14.5. Electron microscopic studies showed an increase in sarcomere length, a maturation marker, in both the compact and trabecular zones. RNA-seq analysis revealed that RHAU regulates post-transcriptional expression of several genes involved in ventricular trabeculation, such as Nkx2-5 and Hey2, as well as sarcomeric genes such as Myh7. Epigenomic profiling revealed that RHAU’s DNA resolvase activity also facilitates expression of Myh7 in these murine models81. These studies revealed new insights of the regulation of PMD at the transcriptional and epigenetic level.
Altogether, the cellular characteristics of CMs described above show the greatest evidence of change as part of the PMD. In the following section, we expand upon the transcriptional and signaling landscape governing the PMD, as well as the relative contribution of both CM and non-CMs to this process.
Signaling Pathways of Cardiac Cells Governing the PMD
Decades of research have led to a deep understanding of cell-intrinsic factors (such as signaling pathways) and extrinsic factors (including non-CMs) in PMD. That non-CMs, such as epicardium, endocardium, and cardiac fibroblasts, and other cell-extrinsic factors play an important role in the modulation of PMD is well documented. In this section, we focus on intrinsic (Figure 2) and extrinsic factors (Figure 3) involved in the PMD in CMs.
Figure 3. Cellular milieu contributes to CM proliferation and maturation.

(A) Schematic diagram showing the interplay between the three cardiac layers and their effects on CM proliferation and maturation. (B) HAPLN1+ epicardial cells promote CM proliferation and chamber maturation through hyaluronan deposition and direct co-culture of epicardial cells with CMs induce bi-directional transcriptional changes resulting in decreased CM proliferation and increased maturation. (C) Angiocrines, signaling molecules secreted by endothelial cells lining the heart vessels as well as endocardium, contribute to CM proliferation and maturation, and fibronectin-integrin α5β1 interactions between endocardium and CM promote proliferation and maturation. (D) Adult cardiac fibroblasts, through the chemokine signaling pathway, decrease CM proliferation and promote CM maturation, and POSTN+ neonatal cardiac fibroblasts stimulate CM proliferation and maturation during the first 7 days of postnatal development in murine hearts.
CM Intrinsic Factors involved in the PMD
Canonical Wnt/β-catenin signaling pathway
The canonical Wnt/β-catenin signaling pathway is critical for cardiogenesis and in the regulation of CM proliferation and differentiation82–84. Recent studies demonstrated that modulation of Wnt signaling not only affected CM proliferation but also CM maturation profiles in vitro84, 85. Recently, it was shown that Wnt-activation via glycogen synthase kinase3-β (GSK3-β) inhibition promotes sustained proliferation of day-12 hiPSC-CMs in a cell-density dependent manner85. Assessment of cTnT+Ki-67+ and cTnT+pHH3+ cells showed that sparse seeding condition promotes the greatest increase in Wnt-signaling mediated stimulation of CM proliferation85. Further, these conditions resulted in downregulation of maturation markers: sarcomere organization, contractility, multinucleation, and expression of sarcomeric (MYL2, TNNI3, and MYOM2), excitation (GJA1), and metabolism (COX6A2 and CKMT) related genes85 (Figure 2). Interestingly, there were no differences in Ca2+ transients and spontaneous action potential (AP) at the single-cell resolution between treatment groups and controls. scRNA-seq analysis revealed the presence of proliferating and non-proliferating atrial and ventricular-like CM populations with an overrepresentation of proliferating CMs in the CHIR99201 (Wnt activator)-treated group85. Notably, GSK3-β inhibition (Wnt activation) did not alter the expression of cardiac progenitor genes, such as ISL1 or MESP1/2, and other cardiac transcription factor, such as GATA4, TBX5, MEF2C, and NKX2-585. Mechanistically, activation of Wnt signaling resulted in activation of PI3K/AKT signaling via phosphorylation of AKT at Thr30885. Together, activated β-catenin and pAKT induce CM proliferation via Cyclin D2, and interfere with CM maturation by downregulating sarcomere gene expression. It was also shown that Wnt-mediated induction of CM proliferation was independent of yes-associated protein-1 (YAP) activity (key effector of the Hippo signaling pathway), a known regulator of density-dependent cell cycle activity85, 86. Importantly, removal of GSK-3β inhibition did not impair the ability of CMs to terminally differentiate and upregulate maturation networks as assessed by gene expression and contractility assays85. Lastly, β-Catenin increases the expression of cytokinesis regulators such as epithelial cell transforming 2 (Ect2) to induce CM cytokinesis in immature hearts87. These studies highlight the importance of Wnt signaling in regulating CM proliferation and maturation, as well as unveil new understanding of contact-mediated feedback on CMs regulating this transition.
Hippo-YAP signaling pathway
In addition to the crosstalk between Hippo and Wnt/β-catenin signaling pathway75, 86, the Hippo-YAP signaling pathway is critical for proper heart development88, 89. Briefly, the Hippo-signaling cascade involves sequential phosphorylation of the mammalian STE20-like protein kinase 1/2 (MST1/2)–Salvador family protein 1 (SAV1) complex, which then phosphorylates the large tumor suppressor 1 and 2 (LATS1/2)–Mps One binder 1 (MOB1) complex (Figure 2). These then phosphorylate the effector molecule, YAP1 and the transcriptional co-activator PDZ-binding motif (TAZ/WWTR1) to prevent nuclear localization, which gets ubiquitinated and targeted for degradation. In the absence of activated Hippo signaling, YAP1/TAZ translocate to the nucleus, where they bind to other transcription factors promoting cell proliferation and inhibiting differentiation90 (Figure 2).
Another post-translational regulatory mechanism of Hippo signaling occurs via NEDDylation during heart development89. NEDDylation involves neural precursor cell expressed developmentally down-regulated protein 8 (NEDD8)-specific E1, E2, and E3 enzymes to conjugate NEDD8 to target proteins for degradation. CM-specific (αMHCCre) constitutive deletion of NEDD8-activating enzyme E1 subunit 1 (NAE1CKO), to inhibit NEDDylation, resulted in perinatal lethality with hypertrophic features and reduced ejection fraction at E18.589. In these settings, the authors found reduced myocardial compaction and thicker trabecular layer localized to the LV and not the right ventricle (RV) at E16.5 prior to their lethality89. Further analysis of the cardiac hypoplasia revealed reduced CM proliferation as assessed by cTnT+Edu+ and cTnT+pHH3+ expression, mainly localized to the compact layer, and significantly upregulated gene expression of negative cell cycle regulators89. Treatment with MLN4924 (a specific NAE1 inhibitor) in isolated rat ventricular CMs (NRVCs) resulted in abolished NEDDylation, and more profound changes in the expression of cell cycle regulator genes, many of which are direct targets of YAP189. Indeed, the total protein and phospho-protein expression levels of the Hippo kinases (MST1/2, LATS1/2, MOB1), including p-YAP1 at Ser127, were found to be upregulated in both NAE1CKO hearts and MLN4924-treated NRVCs compared to controls, suggesting upregulation of the Hippo signaling pathway89. Using a series of biochemical assays, it was shown that NEDDylated Cullin 7, a ubiquitin ligase, targets MST1 and LATS2 for ubiquitination and subsequent degradation, resulting in YAP1-mediated upregulation of CM proliferation. These studies underscore an important post-translational modification in the regulation of Hippo signaling during heart development and its involvement in CM proliferation and ventricular chamber maturation (Figure 2). It should be noted that ventricular chamber maturation is distinct from cellular-scale CM maturation. Ventricular chamber maturation involves trabeculation and compaction, which are both highly dependent on extensive CM proliferation. Confusingly, especially in the context of this review, immature ventricular chambers are often denoted as a thin-walled chamber due to suboptimal CM proliferation whereas mature ventricular chambers are often denoted as a thick-walled chamber due to appropriate CM proliferation. Indeed, Yap1 overactivation leads to decreased CM size, sarcomere disassembly and decreased fatty acid utilization, indicating Yap1 activity drives CM dedifferentiation and limits CM maturation76.
Transforming Growth Factor-β (TGFβ) signaling pathway
Activation of the TGFβ signaling pathway through Activin A/bone morphogenetic protein 4 (BMP4) ligands results in efficient differentiation of embryoid bodies (EBs) into cardiac progenitors91. Further, the role of TGFβ signaling is well document in cardiac repair and remodeling in the adult heart92. In 2018, a new role of myocardial Bmp2 in regulating CM proliferation and maturation was revealed93. Ectopic expression of Bmp2 in murine myocardium (Nkx2.5Cre) is embryonic lethal by E15.5, with resulting dilated ventricles by E14.5 under histological inspection. Electron microscopy analysis of Nkx2.5Cre/+;Bmp2Tg/+ mice revealed poorly defined sarcomeric structures with undefined Z-bands and densely packed CMs within the trabecular layer, suggesting impaired CM maturation. Indeed, the trabecular myocardium of Nkx2.5Cre/+;Bmp2Tg/+ mice was larger and contained more CMs compared to controls as well as a higher number of proliferating CMs as assessed by 5-bromodeoxyuridine (BrdU) incorporation assays. Gene expression by in situ hybridization (ISH) showed dysregulated expression of Hey2, Hand1, n-Myc, Cx43, Bmp10, and Smad6, indicating upregulation of CM proliferation and impaired CM differentiation. The expression patterns revealed by ISH were later confirmed by RNA-seq and qPCR of the transgenic hearts as well as murine in vitro assays with EBs where Bmp2 was overexpressed and inhibited. In their in vitro assays, the group found increased proliferation of EBs and decreased CM differentiation, which was attenuated when treating the cells with Noggin, a TGFβ antagonist. These studies shed new light on the effects of TGFβ signaling on CM proliferation and differentiation and further substantiate the PMD.
More recently, the role of TGFβ signaling has been documented in neonatal CM proliferation and maturation through the action of misoprostol, a prostaglandin E1 (PGE1) analog94. Previous studies using a murine model of neonatal hypoxia had shown that misoprostol is cardioprotective by inducing alternative splicing of BCL2/adenovirus E1B 19-kDa protein-interacting protein 3 (BNIP3), resulting in a variant lacking exon 3 (murine) or exon 2 (humans; sNip)95. Misoprostol-induced sNip expression counteracted hypoxia-induced proliferation of NRVCs cultured at 10% O2 as assessed by live cell imaging, cTnT+Aurora B kinase+, Cyclin-D1 expression and increased CM surface area under both normoxic and hypoxic conditions. Further, sNip also reduced expression of three key signaling molecules namely: myocyte enhancer factor 2C (MEF2C), myocardin (Myoc), and Bmp10. Treatment of NRVCs exposed to hypoxia with misoprostol reduced their glycolytic flux activity through downregulation of lactate dehydrogenase A. Decreased CM proliferation with sNip was coupled to metabolic changes suggestive of a more mature phenotype with significant increase in nuclear Ca2+ signaling and the expression of the calcineurin-regulated nuclear factor of activated T-cells, cytoplasmic 3 (NFATc3) in the nucleus. In agreement with NFATc3 nuclear accumulation, the group observed an ~800% increase in Bmp2 expression when sNip is transduced in NRVCs94. Lastly, the group found that oral ingestion of misoprostol for 5 days in a rat neonatal hypoxia model reduced hypoxia-induced CM proliferation in vivo and confirmed increased expression of sNip. The contrasting effects of Bmp2 signaling on CM proliferation and maturation between these two studies could be, in part, the result of initial CM maturation state, wherein Bmp2 promotes proliferation and opposes maturation in nascent CMs in the myocardium, it appears to have the opposite effect in neonatal CMs even under normoxic conditions.
MAPK and PI3K/AKT signaling pathway
The role of thyroid hormone, triiodothyronine (T3) on neonatal CM proliferation and maturation is documented96, 97. Although T3 is considered a pro-maturation molecule, in their studies98, the group found a proliferative response to T3 treatment in neonatal CMs through mitochondrial hydrogen peroxide (mH2O2)-mediated activation of a non-canonical mitogen-activated protein kinase (MAPK) pathway. Increase in mitochondrial biogenesis results in more reactive oxygen species (ROS), activating the MAPK signaling pathway leading to increased cell cycling. A 5-hour 10 nmol/L T3 treatment of isolated mouse neonatal CMs resulted in robust gene expression changes of many CM maturation markers such as mitochondrial biogenesis (NRF1, PGC1α, TFAM), oxidative phosphorylation (COX5B, CYCT, SDHA) and lipid biosynthesis (FASN, ATP5A1). Mechanistically, the authors found that T3, through the thyroid hormone receptor-α (TR-α), induces transcription of mitochondrial biogenesis genes, a key step in the metabolic maturation transition from glycolysis to oxidative phosphorylation, which results in an increase in H2O2. The H2O2 produced by the mitochondria then reacts with sensitive thiol peroxidase (peroxiredoxin-1), which leads to phosphorylation of c-Jun N-terminal kinase-2 (JNK2α2), a non-canonical MAPK, which subsequently phosphorylates c-Jun (Figure 2). Phosphorylated c-Jun binds at the promoter region of Igf-1 leading to Igf-1-mediated activation of Erk1/2 signaling and cell cycle progression. These studies unveiled a novel mechanism for T3-mediated CM metabolic maturation and proliferation in murine neonatal CMs via mH2O2 production and activation of non-canonical MAPK signaling with subsequent upregulation of IGF-1 signaling and cell cycle progression (Figure 2).
The MAPK and PI3K/AKT signaling pathways have been implicated in CM hypertrophy in both normal99 and pathological100 conditions with extensive crosstalk between them101–103. Recently, our group showed that the MAPK and PI3K/AKT signaling pathways are key regulatory modulators of CM maturation and proliferation during development104. We combined RNA-seq and ChIP-seq analysis of human cardiac tissue across the lifespan, and showed that the Hippo signaling, Wnt signaling and MAPK/PI3K/AKT signaling pathways were downregulated postnatally (Figure 2). Several other studies using mammalian systems have also underscored the downregulation of PI3K/AKT signaling with increasing CM maturation as well as its role in promoting CM proliferation105.
In our studies, we showed that treatment of day-25 hiPSC-CMs with a cocktail of small molecules targeting both MAPK and PI3K/AKT signaling pathways for 5 days significantly enhanced the maturation status with concomitant downregulation of proliferation104. After the 5-day treatment, day-30 hiPSC-CMs have comparable or better maturation levels as untreated day-60 hiPSC-CMs in several domains: adult isoform sarcomeric protein expression, multinucleation, cell volume, T-tubule density, sarcomere length, metabolism, calcium transients, AP conduction velocity, and field potential duration. CM Proliferation, as assessed by MF20+Ki-67+ and cTnT+pHH3+, was significantly reduced like late-stage untreated day-60 hiPSC-CMs. This was further verified in our transcriptional analysis showing that treated hiPSC-CMs display a shift in gene expression away from human fetal stage and closer to the adult stage.
Other signaling pathways such as Hedgehog signaling, Notch signaling, and FGF signaling have been shown to be involved in heart development4, 106–109. However, the roles of these pathways in PMD are not very well-described and represent a fertile ground for further exploration. As we have described here, canonical and non-canonical signaling pathways that traditionally have been associated with either CM proliferation or maturation are now being found to have a more nuanced molecular regulatory role in the PMD. These studies suggest that targeting either side of the dichotomy is sufficient to affect the other. For example, activation of Wnt signaling, which promotes CM proliferation, temporarily reverses CM maturation at the transcriptional and structural level. On the other hand, inhibition of proliferation through dual inhibition of MAPK and PI3K/AKT signaling pathways results in enhancement of CM maturation along several domains. The evidence thus far suggests that the PMD in the CM is under a multifactorial layer of regulation with multiple and overlapping signaling pathways at play. More studies dissecting the temporal relationships of these signaling pathways on the PMD will significantly enhance efforts to design more robust and mature 3D engineered cardiac tissues. For example, an outstanding question in the field is whether there is an optimal sequence of signaling pathway activation and inhibition that would promote the most mature 3D engineered cardiac tissue, and if there is, which are the most necessary signaling pathways to modulate. Similarly, an understanding of sequential modulation of CM proliferation from a mature state and then back to a mature state would be beneficial for regenerative therapies.
Non-CM Cardiac Cell Types influencing PMD (Cell Extrinsic Factors)
Epicardial cells
The human epicardium is a serous membrane of simple squamous mesothelium and is considered part of the heart wall as it covers the outer surface of the heart. Studies related to epicardial cell development were first reported during the early to mid-1980s in several species including mouse, chick, and axolotl, among others110–113 but only recently has attention focused on how epicardial cells contribute to CM proliferation and maturation.
Recently, a new epicardial cell subpopulation was defined by paralogs of hyaluronan and proteoglycan link protein 1 (hapln1) expression that contributes to CM proliferation and regeneration in zebrafish114 (Figure 3). Genetic ablation of hapln1a or inactivation of hapln1b resulted in defective heart wall regeneration and reduced injury-induced gata4+ CM proliferation (Figure 3). Mechanistically, the authors suggest that decreased hyaluronan deposition at the injury site could result in impaired epithelial-mesenchymal transition and migration, thus reducing CM proliferation (Figure 3). From a development perspective, the authors show that juvenile and early adult zebrafish require hapln1a+ epicardial cells are needed for proper ventricular chamber formation and myocardium compaction. These results underscore the critical importance of the extracellular environment, through hyaluronan organization, for proper CM proliferation, regeneration and ventricular chamber maturation. However, it is still unknown whether the loss of hapln1 after sufficient CM proliferation actually aids in cellular-scale maturation of CM.
Direct co-culture of hiPSC-derived cardiac progenitors with epicardial cells can modulate CM proliferation and maturation115. After 2 weeks of direct co-culture in the presence of TGFβ inhibitor, A83-01, used to maintain epicardial cell identity, day-20 hiPSC-CMs proliferation increased to 20%. Additionally, direct-contact co-culture conditions accelerated the sarcomeric isoform switch expression from the atrial isoform of myosin light chain (MLC-2a) to the ventricular isoform (MLC-2v) in day-20 hiPSC-CMs. Here, they found that co-culture conditions using a 3:1 ratio of epicardial cells to cardiac progenitors resulted in up to ~80% cTnT+ MLC-2v+ expression. Nonetheless, other relevant sarcomeric and morphological features of CM maturation were negatively affected. For example, there was a significant reduction in myosin heavy chain (MHC) β-to-α ratio, sarcomere organization and cell size. These investigators also found that indirect transwell co-culture or the use of epicardial cell conditioned medium failed to reproduce the results that direct co-culture yielded. Interestingly, scRNA-seq studies revealed bidirectional effects of direct co-culture whereby epicardial cell identity shifted from a dominant secreted frizzled related protein 2 (SFRP2) population, a Wnt signaling ligand, to a delta like non-canonical notch ligand 1 (DLK1) or complement component 3 (C3) population. These studies reveal an extensive crosstalk between epicardial cells and CMs, likely via cell-ECM or cell-cell interactions that could impact the maturation and proliferation profile, and thus influence potential future cell therapies. It should be noted that a direct link between proliferation and maturation in this context has not been established.
Endothelial cells and endocardium
Endocardial cells are specialized endothelial cells present at the innermost layer of the heart. Recent studies showed that these specialized cells, as well as endothelial cells lining the major heart vessels, may play a role in CM proliferation and maturation often through cell-cell contacts and through secretion of signaling molecules116. In the context of hypoplastic left heart syndrome (HLHS), a congenital heart disease that presents with improper development of the LV chamber as well as the heart valves within that chamber and the aorta, it was found that endocardial cells contributed to proper CM proliferation and maturation117. After validating a previously reported118 compendium of de novo mutations associated with HLHS at single-cell resolution in a developing human fetal heart, the group found highly enriched expression of those genes in the endocardial and endothelial cell populations. In HLHS, endocardial and endothelial cells were shown to have a developmentally impaired transcriptional profile with reduced expression of cadherin-11 (CDH11) and downregulation of several signaling pathways including NOTCH (Figure 3). Transwell and direct-contact co-cultures of day-15 and day-30 hiPSC-CMs with unaffected or HLHS iPSC-endocardial endothelial cells (iEECs) revealed a reduction in CM proliferation, as shown by decreased cTnT+Ki67+ CM and reduced gene expression of cell cycling genes (PCNA, CCNA2, CCNB1 and CCND2) in the context of HLHS. In addition to reduced proliferation, the authors found a significant impairment in sarcomeric organization and reduced expression of CM maturation markers (TTN-N2BA and TNNI3), which were validated by bulk RNA-seq analysis. Further, they showed impaired expression of fibronectin-1 (FN1) in endothelial cells results in reduced CM proliferation and maturation as assessed by cell cycle transcripts, adult-isoform sarcomeric genes, ion channels and contractility assays. Although it is possible that these could be due to promotion of CM survival rather than their involvement in CM proliferation-maturation transition, these findings unveiled a new role of endocardial cells in CM proliferation and maturation where ETS1 and CDH7 regulate endocardial FN1 expression (Figure 3). Unraveling the molecular mechanisms leading to CM proliferation and maturation downstream of integrin signaling is an active area of investigation.
In contrast to contact-mediated cues, in vivo studies in a murine model of ventricular non-compaction revealed that angiocrines, signaling molecules secreted by endothelial/endocardial cells, regulate CM proliferation and maturation116 (Figure 3). scRNA-seq analysis of embryonic murine hearts from endothelial cell-specific deletion of Ino80 (Tie2Cre;Ino80fl/fl), which exhibit ventricular non-compaction, and relevant controls revealed an increase in the proliferative profile of CMs that were later confirmed to localize to the non-compacted myocardium116. Leveraging their murine dataset in combination with scRNA-seq from human heart tissue of patients with LVNC, the group identified putative endothelial and endocardial secreted factors that could influence CM proliferation and maturation. They found that Col15a1, which is expressed by coronary vessel endothelial cells, directly stimulated CM proliferation in ex vivo and in vitro studies of murine and human CMs, respectively. Further, secreted factors by endothelial and endocardial cells including, Tgfb1, Isg15, Igfbp3, and Adm (termed 4F) led to reduced CM proliferation following treatment of ex vivo embryonic murine CMs and in vitro hiPSC-CMs. These 4F also led to increased CM maturation such as increased expression of myomesin, more organized sarcomeres, mature electrophysiological profile with increased maximum upstroke velocity of ~30 V/s, AP amplitude of ~95mV, and a more hyperpolarized resting membrane potential of −70mV.
Cardiac fibroblasts
Cardiac fibroblasts contribute to extracellular matrix (ECM) homeostasis, cell signaling, tissue repair, and cardiac remodeling in health and disease119. Seminal studies from the early 1980s and 1990s indicated that this cell population, by number, constituted the majority of all the cells in the adult mammalian heart120, 121. However, recent studies using single-cell and single-nucleus RNA-seq of adult non-ischemic human hearts suggest a more modest cellular composition of cardiac fibroblasts ranging from 14–32%122, 123.
It has been shown that a specialized population of highly proliferative periostin-expressing (Postn+) cardiac fibroblasts in the early postnatal heart express proliferation and neuronal-related genes, whereas Tcf21+ CFs preferentially express extracellular matrix-related genes. Further, ablation of Postn+ cells leads to reduced CM growth, mitotic activity, and binucleation with increase sympathetic nerve area124. To better understand how the cellular microenvironment affects CM maturation, scRNA-seq studies of murine hearts from postnatal day 1 (P1) and P56 have been conducted125. Globally, they observed that with increasing CM maturation status, there is progressive downregulation of the phosphoinositide-3 kinase (PI3K)-Akt signaling pathway in CMs, involved in cellular proliferation and growth, and upregulation of signaling pathways involved in cytokine-cytokine receptor interactions (Figure 3). Given the upregulation of extracellular signaling with CM maturation, the group found that cardiac fibroblasts contributed significantly as a source of secreted proteins promoting CM maturation. To test the hypothesis that adult cardiac fibroblasts promote maturation of neonatal CMs, the authors conducted direct co-culture studies of reisolated neonatal CMs with either neonatal or adult fibroblasts for 3.5 days. Here they found significantly decreased CM proliferation based on α-actinin+ pHH3+/Ki-67+/Aurora kinase B+ expression, and significant improvements in other maturation domains: increased T-tubule density, Ca2+ handling, sodium currents, maximum upstroke velocity, as well as transcriptional changes. However, not all of the electrophysiology parameters tested in this in vitro system resulted in a more mature profile. For example, there was not a significant decrease in the resting membrane potential or an increase in AP duration/amplitude, as would be expected. Using Plerixafor (an inhibitor of chemokine signaling pathway via CXCR4 and CXCL12 antagonism), and BP-1-102 (an ECM inhibitor via STAT3 suppression), this group showed compromised in vitro sarcomeric alignment of murine CMs. Furthermore, direct injection of these compounds into the hearts of P1 mice resulted in increased in vivo proliferation relative to DMSO controls at P14 as well as reduced gap junction (Cx43+) formation at P14 and P21, suggesting a reduced maturation status. Interestingly, when administered immediately after myocardial infarction surgery in 2-month-old mice, these two compounds promoted CM proliferation in the border zone of the infarcted areas and improved heart function. These investigators also co-cultured hiPSC-CMs with primary human adult fibroblasts for 7 days at a ratio of 1:0.33 and found a significant decrease of proliferation markers and improved electrophysiology status in beating frequency and AP duration, but no changes in AP amplitude, resting membrane potential or upstroke velocity. These studies revealed a critical function for fibroblasts in CM proliferation and maturation in both murine and human models.
The contribution of cardiac fibroblast in controlling CM proliferation and maturation has also been verified in genetic mouse models. For example, genetic ablation of periostin+ cardiac fibroblasts from P0 to P7 resulted in a two-fold reduction in desmin+pHH3+ cells but no change in cytokinesis as assessed by Aurora B kinase expression124 (Figure 3). In addition to changes in CM proliferation, genetic ablation of periostin+ cardiac fibroblasts resulted in reduced maturation parameters: ~20% decrease in CM binucleation levels (from 70% to 50%), reduction in CM size, and an increase in fetal isoform troponin I (Tnni1) expression. Here, similar to the studies of endocardial cells in HLHS, further work is needed to determine whether loss of cardiac fibroblasts and associated ECM production simply limit cell viability or if they specifically impact the PMD. Additionally, more work is needed to further elucidate the crosstalk between CM and non-CMs populations, and to decipher their effect on the PMD (Figure 3). For instance, it is unclear how are angiocrines from endothelial and endocardial cells affecting epicardial cell proliferation/maturation status, since epicardial cell signaling is also affecting the PMD within CMs. The evidence from cell extrinsic factors (non-CMs) discussed thus far suggests that, for complex multi-cellular tissues, it will be critical to maximize the maturation status of these cell populations in order to generate more relevant models (Figure 3). Elucidation of these interactions have already facilitated the generation of more robust three-dimensional models for disease modeling and drug discovery. In the next section, we review the studies implementing these intrinsic and extrinsic factors to develop more advanced and physiologically faithful engineered heart tissues.
Harnessing the PMD to Advance Cardiac Tissue Engineering
The ability to generate cardiac cell types from induced pluripotent stem cells (iPSCs) has revolutionized the field of cardiac tissue engineering, the process of generating 3D tissues for development and disease modeling, drug testing, and regenerative medicine126–129. An extensive literature exists on cardiac development and regeneration in various model systems including amphibians, fish, rodent and non-human primates (extensively reviewed elsewhere)67, 130–132. However, translating this information from animal to human has been challenging due to differences in molecular and functional parameters between animal and humans133–135. Human 3D-engineered models may be able to address these issues. However, cardiac tissue engineering approaches have been limited by 1) an inability to obtain highly dense cellular architecture, 2) cellular discontinuity, and 3) incomplete maturation. Understanding the pathways and barriers to promote a proliferative environment for CMs followed by advanced maturation would augment the utility of cardiac tissue engineering. Future studies in this area and the field would also benefit from standardized measures of proliferation and CM functional maturation as some current reports of improved maturation are difficult to interpret as comparative culture conditions were suboptimal. Even with these drawbacks and struggles, engineered cardiac tissues have already been used to great advantage126, 136–141. For example, engineered heart tissues (EHT) have been used to successfully predict efficacy and cardiac toxicity in humans, to mimic human cardiac pathology, and to improve systolic and diastolic function in infarcted rodent hearts126, 138, 142. In recent years, tremendous emphasis has been placed on driving functional maturation of the CMs of engineered tissues to better approximate adult human cardiac function8, 59, 70, 143. Most previous work has focused either on proliferation processes or maturation aspects to generate 3D-tissues (Table 1). Furthermore, emphasis on the contribution of the non-CMs in attaining 3D-engineered tissue models are understudied. Although many years of research have led to a common understanding that “maturation” is indicative of terminally differentiated cells including CMs (i.e., quiescent CM)4, 48, 70, elucidation of PMD in these model systems is insufficient, and limited effort aimed at controlling these processes in tandem to improve the utility of engineered cardiac tissue (Table 1). Similarly, regulatory mechanisms operational in these 3D-tissue models needs to be investigated in depth to decipher the similarities or differences between in vitro and in vivo systems.
Table 1:
Comparison of CM functional parameters in various engineered tissue types with fetal and adult human hearts
| Assessment | 2D CM | EHT | hChaMP | Fetal Human Heart | Adult Human Heart |
|---|---|---|---|---|---|
| Proliferation Index | ~8–20% (5, 97, 85, 151) | ~5–17% (147, 151) | ~25% (166) | NA | ~0.009–0.3% (8, 174) |
| Maturation Index | Fetal-like (5, 18) | Fetal/Neonatal/Adult-like (143, 150,151) | Fetal/Neonatal-like (166) | Fetal (7, 174) | Mature (7, 8, 127, 173) |
| Cell size/Shape | Circular Rod-shape on patterned surface (5, 97, 85, 139–141) | Rod-like (143, 151) | NA | Rod-like (12) | Rod-shape (11) |
| APD | ~200 ms(APD50) (152) | ~390ms (APD50) ~440ms (APD90) (151) | ~499 ms(APD80) (166) | ~200 ms(APD50) (173) | ~400 ms(APD80) (173) |
| Sarcomere length | 1.8–1.9 μm (5, 17, 18) | 1.9–2.2 μm (142–144, 151) | 1.8 μm (166) | 2.1–2.3 μm (12) | 1.9–2.1 μm (12) |
| Ejection fraction (EF) | NA | NA | 25% (EFmax) (166) | 61.5–67.9% (172) | 65.6–67.7% (171) |
| Major Energy source | Glycolysis (5, 59) | Glycolysis/Fatty acid oxidation (59, 143, 149) | NA | Glycolysis (41, 42) | Fatty acid oxidation (40–42) |
Different methodologies have been used to generate engineered cardiac tissue including 1) casting of cells, scaffolds and/or soluble cues, 2) organoid culture, and 3) bioprinting methodologies (Figure 4). The casting and bioprinting approaches typically include mixtures of scaffolds with differentiated cardiac cell types but can include stem cells that are later stimulated to differentiate and mature. The organoid approach is typically initiated by aggregating suspensions of singularized stem cells or differentiated cells to generate spheroid-like structures that are exposed to differentiation and/or maturation stimuli. In the next section, we review existing technologies to generate cardiac tissues and provide key insights and considerations for the precise control of proliferation and maturation to advance outcomes for each tissue type (Figure 4).
Figure 4. PMD in engineered cardiac tissues.
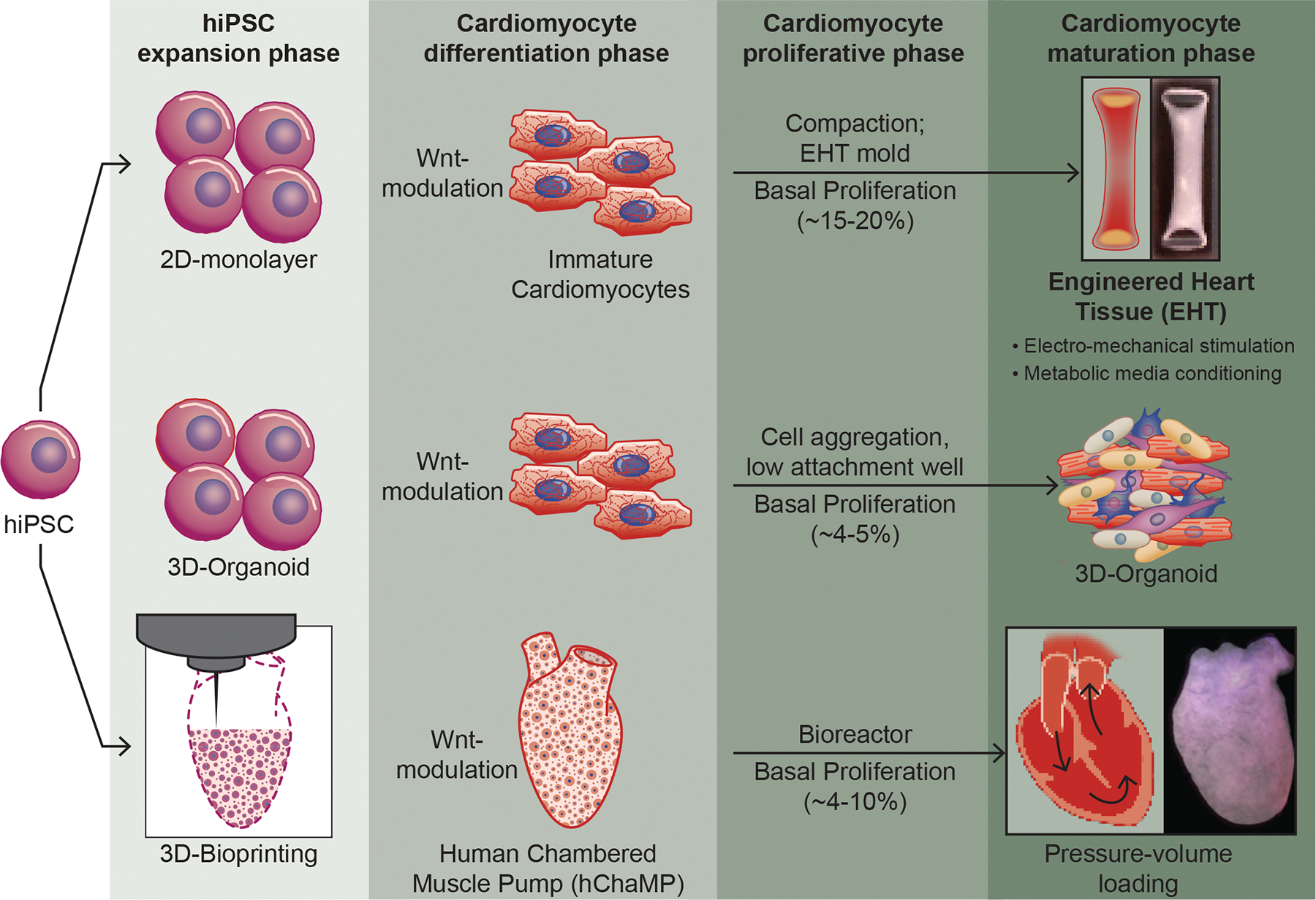
Top panel: 3D-engineered heart tissues (EHTs) are generated using differentiated hiPSC-CM. Each of these steps involves expansion, differentiation, proliferation and maturation phases. A number of strategies have been developed to obtain EHTs with varying degrees of success. Recently several modifications have been made including mechanical loading, metabolic changes, and electrical stimulation to further mature them to attain an adult-like phenotype. EHTs are millimeter scale tissue strips suspended between posts that impose passive resistance akin to cardiac afterload. Studies have shown that induction of maturation leads to reduced proliferation (Ki67+ CMs) as well as a reduction in phospho-histone (pHH3+) CMs with increased maturation. These have been successfully used for drug discovery, disease modeling and functional assessment. Middle panel: 3D-organoids are aggregated 3D-microtissues that arise from stem cell or progenitor cell aggregates conditioned to progress down a certain lineage with external cues. These aggregated 3D-microtissue exhibit low level of Ki67+ CM (proliferation marker) with induction of maturation markers including higher expression of myosins and aligned sarcomeres. Bottom panel: 3D-bioprinting methodology, including, Freeform Reversible Embedding of Suspended Hydrogels (FRESH), Sacrificial Writing In to Functional Tissue (SWIFT) have been used to generate 3D, high-resolution, structures with geometric complexity. Our laboratory has pioneered the development of a 3D-construct, termed human chambered muscle pump (hChaMP), a centimeter-scale 3D cardiac pump model consisting of hiPSC-CMs in a perfusable, chambered organoid. These constructs are generated using in situ expansion of hiPSCs with subsequent CM differentiation to yield electromechanically coupled human cardiac muscle with high cell density without the requirement of tissue compaction. Future work to enhance the pumping capacity of the hChaMP could include the inhibition of maturation to allow for maximum hiPSC-CM expansion.
Engineered heart tissue (EHT) and the PMD
Engineered heart tissues (EHTs) are millimeter scale tissue strips suspended between posts that impose passive resistance akin to cardiac afterload144 (Figure 4). Engineered tissues were first developed by mixing isolated rat CM and fibroblasts populations with collagen type I extracellular matrix proteins and then casting into cylindrical molds144. Implantation of these ring-shaped constructs in a rat model of myocardial infarction showed electrical coupling with the host tissue with improved cardiac function compared to the sham-operated rats142, 145. This pioneering work clearly demonstrated the utility of EHTs for the functional improvement of a damaged heart128, 142, 146. In recent years, EHTs are generated using hiPSC-CM and several modifications have been made to further mature the CM of EHTs toward an adult-like phenotype (Figure 4) (Table 1). Although less well documented, a few of these studies have demonstrated that inducing CM maturation leads to reduced proliferation. For example, EHTs typically grown in glucose-containing media, will undergo enhanced maturation when cultured in medium with low carbohydrates, low insulin, and palmitate without serum59, 147. This forced shift in metabolic substrate mimics the change that occurs with development59. Several signaling pathways involved in CM proliferation including Wnt, YAP, and Notch signaling were reduced in these maturation conditions, suggesting the antagonistic nature of CM proliferation and maturation processes persists in engineered tissues147, 148.
Electromechanical stimulation has also been used to stimulate the maturation of CM of EHTs 143 (Figure 4). In these conditions, CM of EHTs have been shown to convert from glycolytic to oxidative phosphorylation-based ATP production 149. In addition, it was possible to attain mature CM with organized ultrastructure, physiologic sarcomere length (2.2 μm), high density of mitochondria (30%), presence of t-tubules, oxidative metabolism, a positive force-frequency relationship, and efficient Ca2+ handling143. 150. Although several studies have documented the usage of electrical stimulation to induce CM maturation, it is not absolutely necessary151. In these studies, EHTs were generated by casting in a mold followed by their maturation for 7 days under mechanical loading. These EHTs demonstrated structural and functional properties of postnatal myocardium, such as rod-shaped CM with M bands, a positive force-frequency response151. The force–frequency relationship is a valuable parameter to assess EHT maturity as it integrates multiple elements of excitation–contraction coupling such as communication between sarcolemma, t-tubules, SR, and myofilaments137, 150, 152. Generally, this relationship is negative in embryonic hearts or immature EHTs, flat in neonatal hearts (aged <2 weeks) and positive in hearts 3 months after birth153. Several studies have shown that electromechanical loading leads to a switch in the force–frequency relationship from negative to flat in EHTs, indicating progressive maturation of CMs of EHTs143, 150. It was also found that progressive electrical stimulation of EHTs – from 2 to 6 Hz over 2 weeks at a frequency increasing by 0.33 Hz/day – leads to increased CM maturation with a dense cellular network and a well-organized ultrastructure including M-bands and connexins143, 154, 155. Of note, none of the studies with imposed electromechanical stimulation carefully examined CM proliferation, though it was noted that greatest success in maturation outcome, came with electromechanical induction at early time points following hiPSC-CM differentiation using differentiated hiPSC-CM at Day 12 when levels of proliferation are still relatively high143, 155. This observation supports the possibility that the PMD might be most effective with the slow imposition of maturation stimuli starting at an early developmental and proliferative state143. Further, it is important to note that generation of EHT relies on supporting mesenchymal cell types (e.g., fibroblasts, mesenchymal stromal cells) to productively remodel the scaffold to promote electromechanical coupling and likely also soluble cues that promote CM health and function155. Studies using murine model have shown that adult cardiac fibroblasts promote maturation of neonatal CMs, suggesting the importance of non-CMs interaction with CM populations to induce maturation156. Future studies could consider the modulation of proliferation of supporting cell types in addition to CM in an effort to promote productive matrix remodeling, cellular crosstalk, and ultimately functional maturation.
It is important to note that very few studies have evaluated the PMD in EHTs models, possibly due to the focused goal of inducing maturation to obtain adult-like tissues143, 154, 155. A recent study using the Heart Dyno model (a miniaturized EHT set up in 96-well plate) showed that induction of maturation via metabolic media supplements results in reduced Ki67+ CMs as well as a reduction in phospho-histone (pHH3+) CMs147. This group screened ~5000 compounds to find activators of CM proliferation and identified the mevalonate pathway as a new avenue to reenter the cell cycle, both in vivo and in vitro147, 148. Increased proliferation of CM of EHTs was also demonstrated following transplantation at a site of cardiac injury. In these studies, the authors proposed that the final graft size depends not purely on cell survival, but also on proliferation157. Another approach to activating CM proliferation in EHTs has been to add a Wnt activator. In these studies, the Wnt activator was added in concert with a metabolic maturation media and no proliferative effect was observed147. This outcome suggests that in this case maturation outcompetes proliferation. Future approaches might benefit from a graded and progressive transition from conditions that support proliferation to those that support maturation and vice versa.
PMD in 3D-organoid tissue
3D-organoids arise from stem cell or progenitor cell aggregates conditioned to progress down a certain lineage with external cues148, 158 (Figure 4). The end result is a miniaturized version of multicellular tissues which hold promise for studying organ function in an in vitro setting. Recent success in the formation of cardiac-organoids has led to generation of distinct atrium- and ventricle-like tissue types in vitro159. These 3D-cardioids revealed self-organizing principles of human cardiogenesis and were able to form two heart fields via modulation of Bmp/Wnt signaling160. Notably, these aggregated 3D-microtissue showed low level of Ki67+ CM (proliferation marker) with induction of maturation markers, including higher expression of myosins and aligned sarcomere suggesting a fetal or early neonatal developmental stage160 (Figure 4).
Comprehensive analysis of the maturation indices revealed induction of mitochondrial activity and corresponding metabolic maturation. Recent studies have shown that the LEFTY-PITX2 signaling pathway plays critical roles in the maturation process of cardiac organoids derived from hiPSC-cardiac progenitors161. Transcriptional profiling from these organoids revealed that extracellular matrix-integrin, focal adhesion, and LEFTY-PITX2 signaling pathways are upregulated in these conditions. Further, they showed that LEFTY knockdown affected extracellular matrix (ECM)-integrin-focal adhesion (FA) signaling pathways and demonstrated that LEFTY-PITX2 signaling plays key roles for CM maturation and specification into the ventricular-like CM subtype in cardiac organoids161. Maturation of cardiac organoids was also induced by a metabolic analog, 2-Cl-C.OXT-A (COA-Cl) in co-culture of human dermal fibroblasts, hiPSC- endothelial cells and hiPSC-CMs162. These studies support the importance of metabolites and paracrine factors in inducing CM maturation. Recently, it was shown that mature cardiac organoids were achieved via the contribution of paracrine factors from gut tissue to cardiac tissue within 3D-organoids163. Further, the role of cardiac fibroblasts in the maturation of 3D cardiac microtissues is well documented in promoting sarcomere alignment, associated T-tubules, enhanced contractility, and mitochondrial respiration and mature electrophysiology70, 143. Also in the context of EHTs, a recent co-culture study demonstrated that switching fibroblast subtypes from a neonatal to adult state promotes maturation of neonatal CMs, suggesting the importance of non-CMs interaction with CM populations to induce maturationn156. Taken together, these studies demonstrate development of powerful 3D cardiac organoid technology with utility in both biological studies and for clinical applications. Missing in these studies is a clear understanding of the optimum duration for proliferative expansion of the cells of the aggregate (hiPSCs or CMs) prior to specification and maturation (Figure 4). Indeed, the rationale for reported time intervals is not delineated, but could dramatically impact on the reproducibility of organoid composition and the functional maturation of said tissues. Furthermore, these engineered tissue models cannot easily be mechanically loaded either via the passive resistance of a mechanical restraints or with pressure-driven loading, suggesting the need to develop a chamber model to mimic native myocardium.
PMD in 3D-bioprinted chambered cardiac tissue
In recent years, tissue engineering approaches have been used to generate cardiac tissues that mimic the chambers of the heart164–166. For example, a pouch-like heart muscle construct was generated using a novel casting technology and applied to the heart as an “embracing” cardiac graft in vivo167. Similarly, a three-dimensional (3D) electro-mechanically coupled, fluid-ejecting miniature human ventricle-like cardiac organoid chamber (hvCOC) was generated using hiPSC-derived ventricular CMs165. These chambered cardiac structures contained CM with organized sarcomeres, myofibrillar microstructures, and upregulation of key Ca2+ handling ion channel transcripts. Another study reported engineering of a human LV mimic using nanofibrous scaffolds and ventricular CMs to obtain chambered heart models. These models showed native-like anisotropic myocardial tissue formations and chamber-level contractile function. In the recent years, 3D bioprinting technology has been applied to regenerative medicine to address the need for tissues and organs suitable for transplantation168. 3D-bioprinting is a bio-fabrication technology involving layer-by-layer deposition of biomaterials (ECM proteins) together with living cells according to a digital template. The biomaterial(s) plus cells are commonly referred to as bioinks as these materials are able to support cellular survival and proliferation. Precise bioprinting is achieved using methods such as extrusion, inkjet, laser-assisted, or stereolithography to generate a 3D tissue construct based on a digital template. For example, Freeform Reversible Embedding of Suspended Hydrogels (FRESH) is an extrusion-based printing method that has been used to generate 3D, high-resolution structures with geometric complexity169. 3D printing with FRESH was used to generate thick cardiac patches and ventricles by embedding the printed hydrogel within a secondary hydrogel that serves as a temporary, thermoreversible, and biocompatible support. This enables 3D printing of hydrated materials with an elastic modulus <500 kPa including alginate, collagen and fibrin. In these studies, the authors utilized either neonatal cardiac cells or early-stage hiPSC-CMs to print the engineered cardiac tissue, possibly relying on the proliferative capacity of these immature CMs169. However, they did not evaluate the proliferative or maturation indices in these conditions. Recently, the 3D-bioprinting method, Sacrificial Writing In to Functional Tissue (SWIFT) was used to create a 3D-cardiac tissue akin to an open ventricular chamber170. This method relies on assembling ~400,000 spheroids/organoids as a tissue matrix together with extrusion of a sacrificial ink to form a structure with an open lumen, resulting in formation of a cardiac tissue with ~240,000 million cells which can beat synchronously over 7 days in culture. Using this protocol, the authors were able to generate up to 6 ml (upon compaction) of beating cardiac tissue containing 79 ± 6% CMs (cardiac troponin T-positive, cTnT+) and 19 ± 6% stromal cells (cTnT−, Vimentin+). Importantly, during this period (8 days), the overall tissue contractility was increased by > 20 times and beating capability was enhanced by ~40%. Further, coordinated contraction patterns were observed and the tissue responded to paced stimulation. All these parameters suggest CM maturation occurred in the 3D bioprinted constructs and that proliferation occurred in the spheroids prior to printing. Based on the studies summarized and reevaluated in the context of this review, we predict that the extent to which maturation can be further enhanced relies in part on the proliferative potential of the spheroids as they enter the engineered construct and the extent to which proliferation can be quelled as the bioprinted structure begins to mature and obtain adult function11, 171–174.
Our laboratory has pioneered the development of a 3D-construct, termed human chambered muscle pumps (hChaMPs), a novel centimeter-scale 3D cardiac pump model consisting of hiPSC-CMs in a perfusable, chambered organoid166 (Figure 4). These constructs are generated using in situ expansion of hiPSCs with subsequent CM differentiation to yield electromechanically coupled human cardiac muscle with high cell density without the requirement of tissue compaction. Realizing that both hiPSC and hiPSC-CM expansion were vital for generating muscle mass, we utilized a gene-edited hiPSC line expressing cyclin D2 under the myosin heavy chain promoter (MHC-CCND2). Previous work showed that CCND2 overexpression enhances the regenerative potency of hiPSC-CMs and leads to remuscularization of the injured ventricle175. In fact, we did find Ki67+ CM in the hChaMPs cultured for at least 60 days, albeit at a frequency lower than that reported in EHTs (~4% vs ~15%). Consistent with the lower proliferation rate at the 60-day time point, hChaMPs expressed several maturation markers including SERCA, Kir2.1, Bin1, RyR2 with interconnected CMs mediated via CX43 expression. Further, the resulting hChaMP demonstrate continuous action potential propagation along with synchronous macroscale contraction and associated pump function166. Future work to enhance the pumping capacity of the hChaMP could include the inhibition of maturation to allow for maximum hiPSC-CM expansion. Once a physiologic density of CMs has been obtained, progressive blockade of proliferation pathways could accompany multiple avenues to promote CM maturation. PMD modulation of this type could be easily undertaken in in vitro engineered 3D-constructs where organismal survival is not dependent on continuous cardiac function. Manipulations of the same type in vivo will take considerably more precision to maintain continuous cardiac function during treatment.
Conclusion
Janus, for whom the month of January is named, was often invoked at the beginning of a pursuit or quest – Roman armies would march through a Janus archway prior to departing to war – as he embodied the liminal space between the past and the future. The revealing of the mechanistic basis for the opposition of CM proliferation and maturation similarly opens up the opportunity for a new beginning in cardiac tissue engineering: the rational development of regenerative approaches that take advantage of the inherent dichotomy of proliferation and maturation.
Acknowledgments:
We would like to acknowledge Cynthia Faraday for her support with graphical design included in this work.
Sources of Funding:
This project was supported by NIH grants NIAMS R01 AR078571 (R.C.R.P.), NIAMS R21 AR079236 (R.C.R.P.), NHLBI R01 HL155993 (J.H.v.B.), NHLBI R01HL160665 (J.H.v.B.), NHLBI R01 HL137204 (B.M.O.), NHLBI R01 HL160779 (B.M.O.) and NIAMS R01 AR055685 (M.K.). J.H.v.B. was supported by a Summer’s Wish Pediatric Cardiology Innovators Fund and Regenerative Medicine Minnesota. B.I.G. was supported by NHLBI F30 HL151138 and NIGMS T32 GM008244. This project also received seed funds from the University of Minnesota Lillehei Heart Institute (R.C.R.P.) and the Institute for Engineering in Medicine (IEM) Group Grant (B.M.O.)
Nonstandard Abbreviations and Acronyms
- APD
Action Potential Duration
- AP
Action Potential
- ATP
Adenosine Triphosphate
- BMP
Bone Morphogenic Protein
- BrdU
Bromo-deoxy-Uridine
- CKO
Conditional Knockout
- CM
Cardiomyocyte
- CnA
Calcineurin
- C3
Complement Component 3
- CDH
Cadherin
- CX43
Connexin 43
- DPF
Days Post Fertilization
- EB
Embryoid Bodies
- ECT 2
Epithelial Cell Transforming 2
- ECM
Extra Cellular Matrix
- EHT
Engineered Heart Tissue
- FN
Fibronectin
- FRESH
Freeform Reversible Embedding of Suspended Hydrogel
- GSK3-β
Glycogen Synthase Kinase 3-β
- HIF1a
Hypoxia Inducible Factor 1a
- HLHS
Hypoplastic Left Heart Syndrome
- hChaMP
Human Chambered Muscle Pump
- hiPSC
Human Induced Pluripotent Stem Cell
- hvCOC
Human Ventricle-like Cardiac Organoid Chamber
- iEECs
iPSC-Endocardial Endothelial Cells
- LV
Left Ventricle
- LVNC
Left Ventricle Non-Compaction
- MAPK
Mitogen Activated Protein Kinase
- MOB1
MPS One Binder 1
- MYH
Myosin Heavy Chain
- NRVM
Neonatal Rat Ventricular Myocyte
- PCNA
Proliferating Cell Nuclear Antigen
- PMD
Proliferation-Maturation Dichotomy
- PSC
Pluripotent Stem Cell
- RHAU
RNA Helicase Associated with AU-rich Element
- ROS
Reactive Oxygen Species
- RV
Right Ventricle
- RyR2
Ryanodine Receptor 2
- SAV1
Salvador Family Protein 1
- SFRP2
Secreted Frizzled Related Protein 2
- SR
Sarcoplasmic Reticulum
- SWIFT
Sacrificial Writing Into Functional Tissue
- TGFβ
Transforming Growth Factor-β
- TNI
Troponin
- YAP
Yes-Associated Protein
Footnotes
Disclosures: The authors declare no conflict of interest.
References
- 1.Szemler GJ. Festivals and Ceremonies of the Roman-Republic - Scullard,Hh. Am Hist Rev. 1984;89:739–740. [Google Scholar]
- 2.Yao Y and Wang C. Dedifferentiation: inspiration for devising engineering strategies for regenerative medicine. NPJ Regen Med. 2020;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rylski M, Welch JJ, Chen YY, Letting DL, Diehl JA, Chodosh LA, Blobel GA and Weiss MJ. GATA-1-mediated proliferation arrest during erythroid maturation. Mol Cell Biol. 2003;23:5031–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo Y and Pu WT. Cardiomyocyte Maturation: New Phase in Development. Circ Res. 2020;126:1086–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X, Rodriguez M, Pabon L, Fischer KA, Reinecke H, Regnier M, Sniadecki NJ, Ruohola-Baker H and Murry CE. Tri-iodo-l-thyronine promotes the maturation of human cardiomyocytes-derived from induced pluripotent stem cells. J Mol Cell Cardiol. 2014;72:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naqvi N, Li M, Calvert JW, Tejada T, Lambert JP, Wu J, Kesteven SH, Holman SR, Matsuda T, Lovelock JD, Howard WW, Iismaa SE, Chan AY, Crawford BH, Wagner MB, Martin DI, Lefer DJ, Graham RM and Husain A. A proliferative burst during preadolescence establishes the final cardiomyocyte number. Cell. 2014;157:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bishop SP, Zhou Y, Nakada Y and Zhang J. Changes in Cardiomyocyte Cell Cycle and Hypertrophic Growth During Fetal to Adult in Mammals. J Am Heart Assoc. 2021;10:e017839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mollova M, Bersell K, Walsh S, Savla J, Das LT, Park SY, Silberstein LE, Dos Remedios CG, Graham D, Colan S and Kuhn B. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci U S A. 2013;110:1446–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN and Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashimoto H, Yuasa S, Tabata H, Tohyama S, Hayashiji N, Hattori F, Muraoka N, Egashira T, Okata S, Yae K, Seki T, Nishiyama T, Nakajima K, Sakaue-Sawano A, Miyawaki A and Fukuda K. Time-lapse imaging of cell cycle dynamics during development in living cardiomyocyte. J Mol Cell Cardiol. 2014;72:241–9. [DOI] [PubMed] [Google Scholar]
- 11.Bird SD, Doevendans PA, van Rooijen MA, Brutel de la Riviere A, Hassink RJ, Passier R and Mummery CL. The human adult cardiomyocyte phenotype. Cardiovasc Res. 2003;58:423–34. [DOI] [PubMed] [Google Scholar]
- 12.Ball AJ and Levine F. Telomere-independent cellular senescence in human fetal cardiomyocytes. Aging Cell. 2005;4:21–30. [DOI] [PubMed] [Google Scholar]
- 13.Bishop SP, Zhang J and Ye L. Cardiomyocyte Proliferation from Fetal- to Adult- and from Normal- to Hypertrophy and Failing Hearts. Biology (Basel). 2022;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed RE, Tokuyama T, Anzai T, Chanthra N and Uosaki H. Sarcomere maturation: function acquisition, molecular mechanism, and interplay with other organelles. Philos Trans R Soc Lond B Biol Sci. 2022;377:20210325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss A, Schiaffino S and Leinwand LA. Comparative sequence analysis of the complete human sarcomeric myosin heavy chain family: implications for functional diversity. J Mol Biol. 1999;290:61–75. [DOI] [PubMed] [Google Scholar]
- 16.Miyata S, Minobe W, Bristow MR and Leinwand LA. Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circ Res. 2000;86:386–90. [DOI] [PubMed] [Google Scholar]
- 17.Sheng JJ and Jin JP. TNNI1, TNNI2 and TNNI3: Evolution, regulation, and protein structure-function relationships. Gene. 2016;576:385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wheelwright M, Mikkila J, Bedada FB, Mandegar MA, Thompson BR and Metzger JM. Advancing physiological maturation in human induced pluripotent stem cell-derived cardiac muscle by gene editing an inducible adult troponin isoform switch. Stem Cells. 2020;38:1254–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiesmann F, Ruff J, Hiller KH, Rommel E, Haase A and Neubauer S. Developmental changes of cardiac function and mass assessed with MRI in neonatal, juvenile, and adult mice. Am J Physiol Heart Circ Physiol. 2000;278:H652–7. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Luttmann FF, Schoger E, Scholer HR, Zelarayan LC, Kim KP, Haigh JJ, Kim J and Braun T. Reversible reprogramming of cardiomyocytes to a fetal state drives heart regeneration in mice. Science. 2021;373:1537–1540. [DOI] [PubMed] [Google Scholar]
- 21.Walsh S, Ponten A, Fleischmann BK and Jovinge S. Cardiomyocyte cell cycle control and growth estimation in vivo--an analysis based on cardiomyocyte nuclei. Cardiovasc Res. 2010;86:365–73. [DOI] [PubMed] [Google Scholar]
- 22.Guo Y, Cao Y, Jardin BD, Sethi I, Ma Q, Moghadaszadeh B, Troiano EC, Mazumdar N, Trembley MA, Small EM, Yuan GC, Beggs AH and Pu WT. Sarcomeres regulate murine cardiomyocyte maturation through MRTF-SRF signaling. Proc Natl Acad Sci U S A. 2021;118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Uva G, Aharonov A, Lauriola M, Kain D, Yahalom-Ronen Y, Carvalho S, Weisinger K, Bassat E, Rajchman D, Yifa O, Lysenko M, Konfino T, Hegesh J, Brenner O, Neeman M, Yarden Y, Leor J, Sarig R, Harvey RP and Tzahor E. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat Cell Biol. 2015;17:627–38. [DOI] [PubMed] [Google Scholar]
- 24.Kubin T, Poling J, Kostin S, Gajawada P, Hein S, Rees W, Wietelmann A, Tanaka M, Lorchner H, Schimanski S, Szibor M, Warnecke H and Braun T. Oncostatin M is a major mediator of cardiomyocyte dedifferentiation and remodeling. Cell Stem Cell. 2011;9:420–32. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi K and Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. [DOI] [PubMed] [Google Scholar]
- 26.Bersell K, Arab S, Haring B and Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–70. [DOI] [PubMed] [Google Scholar]
- 27.Monroe TO, Hill MC, Morikawa Y, Leach JP, Heallen T, Cao S, Krijger PHL, de Laat W, Wehrens XHT, Rodney GG and Martin JF. YAP Partially Reprograms Chromatin Accessibility to Directly Induce Adult Cardiogenesis In Vivo. Dev Cell. 2019;48:765–779 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honkoop H, Nguyen PD, van der Velden VEM, Sonnen KF and Bakkers J. Live imaging of adult zebrafish cardiomyocyte proliferation ex vivo. Development. 2021;148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beisaw A, Kuenne C, Guenther S, Dallmann J, Wu CC, Bentsen M, Looso M and Stainier DYR. AP-1 Contributes to Chromatin Accessibility to Promote Sarcomere Disassembly and Cardiomyocyte Protrusion During Zebrafish Heart Regeneration. Circ Res. 2020;126:1760–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahuja P, Perriard E, Perriard JC and Ehler E. Sequential myofibrillar breakdown accompanies mitotic division of mammalian cardiomyocytes. J Cell Sci. 2004;117:3295–306. [DOI] [PubMed] [Google Scholar]
- 31.Fan X, Hughes BG, Ali MA, Cho WJ, Lopez W and Schulz R. Dynamic Alterations to alpha-Actinin Accompanying Sarcomere Disassembly and Reassembly during Cardiomyocyte Mitosis. PLoS One. 2015;10:e0129176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Zhong JF, Qiu H, MacLellan WR, Marban E and Wang C. Epigenomic Reprogramming of Adult Cardiomyocyte-Derived Cardiac Progenitor Cells. Sci Rep. 2015;5:17686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura W, Xiao F, Canseco DC, Muralidhar S, Thet S, Zhang HM, Abderrahman Y, Chen R, Garcia JA, Shelton JM, Richardson JA, Ashour AM, Asaithamby A, Liang H, Xing C, Lu Z, Zhang CC and Sadek HA. Hypoxia fate mapping identifies cycling cardiomyocytes in the adult heart. Nature. 2015;523:226–30. [DOI] [PubMed] [Google Scholar]
- 34.Pettinato AM, Yoo D, VanOudenhove J, Chen YS, Cohn R, Ladha FA, Yang X, Thakar K, Romano R, Legere N, Meredith E, Robson P, Regnier M, Cotney JL, Murry CE and Hinson JT. Sarcomere function activates a p53-dependent DNA damage response that promotes polyploidization and limits in vivo cell engraftment. Cell Rep. 2021;35:109088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seki S, Nagashima M, Yamada Y, Tsutsuura M, Kobayashi T, Namiki A and Tohse N. Fetal and postnatal development of Ca2+ transients and Ca2+ sparks in rat cardiomyocytes. Cardiovasc Res. 2003;58:535–48. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen NUN, Canseco DC, Xiao F, Nakada Y, Li S, Lam NT, Muralidhar SA, Savla JJ, Hill JA, Le V, Zidan KA, El-Feky HW, Wang Z, Ahmed MS, Hubbi ME, Menendez-Montes I, Moon J, Ali SR, Le V, Villalobos E, Mohamed MS, Elhelaly WM, Thet S, Anene-Nzelu CG, Tan WLW, Foo RS, Meng X, Kanchwala M, Xing C, Roy J, Cyert MS, Rothermel BA and Sadek HA. A calcineurin-Hoxb13 axis regulates growth mode of mammalian cardiomyocytes. Nature. 2020;582:271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahmoud AI, Kocabas F, Muralidhar SA, Kimura W, Koura AS, Thet S, Porrello ER and Sadek HA. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature. 2013;497:249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang WE, Li L, Xia X, Fu W, Liao Q, Lan C, Yang D, Chen H, Yue R, Zeng C, Zhou L, Zhou B, Duan DD, Chen X and Houser SR. Dedifferentiation, Proliferation, and Redifferentiation of Adult Mammalian Cardiomyocytes After Ischemic Injury. Circulation. 2017;136:834–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piquereau J and Ventura-Clapier R. Maturation of Cardiac Energy Metabolism During Perinatal Development. Front Physiol. 2018;9:959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibbs CL. Cardiac energetics. Physiol Rev. 1978;58:174–254. [DOI] [PubMed] [Google Scholar]
- 41.Mommaerts WF and Langer GA. Fundamental concepts of cardiac dynamics and energetics. Annu Rev Med. 1963;14:261–96. [DOI] [PubMed] [Google Scholar]
- 42.Beer M, Seyfarth T, Sandstede J, Landschutz W, Lipke C, Kostler H, von Kienlin M, Harre K, Hahn D and Neubauer S. Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with (31)P-SLOOP magnetic resonance spectroscopy. J Am Coll Cardiol. 2002;40:1267–74. [DOI] [PubMed] [Google Scholar]
- 43.Gaspar JA, Doss MX, Hengstler JG, Cadenas C, Hescheler J and Sachinidis A. Unique metabolic features of stem cells, cardiomyocytes, and their progenitors. Circ Res. 2014;114:1346–60. [DOI] [PubMed] [Google Scholar]
- 44.Calmettes G, John SA, Weiss JN and Ribalet B. Hexokinase-mitochondrial interactions regulate glucose metabolism differentially in adult and neonatal cardiac myocytes. J Gen Physiol. 2013;142:425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ascuitto RJ and Ross-Ascuitto NT. Substrate metabolism in the developing heart. Semin Perinatol. 1996;20:542–63. [DOI] [PubMed] [Google Scholar]
- 46.Vasquez-Trincado C, Garcia-Carvajal I, Pennanen C, Parra V, Hill JA, Rothermel BA and Lavandero S. Mitochondrial dynamics, mitophagy and cardiovascular disease. J Physiol. 2016;594:509–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dorn GW 2nd, Vega RB and Kelly DP. Mitochondrial biogenesis and dynamics in the developing and diseased heart. Genes Dev. 2015;29:1981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uosaki H, Cahan P, Lee DI, Wang S, Miyamoto M, Fernandez L, Kass DA and Kwon C. Transcriptional Landscape of Cardiomyocyte Maturation. Cell Rep. 2015;13:1705–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopaschuk GD, Collins-Nakai RL and Itoi T. Developmental changes in energy substrate use by the heart. Cardiovasc Res. 1992;26:1172–80. [DOI] [PubMed] [Google Scholar]
- 50.Nakada Y, Canseco DC, Thet S, Abdisalaam S, Asaithamby A, Santos CX, Shah AM, Zhang H, Faber JE, Kinter MT, Szweda LI, Xing C, Hu Z, Deberardinis RJ, Schiattarella G, Hill JA, Oz O, Lu Z, Zhang CC, Kimura W and Sadek HA. Hypoxia induces heart regeneration in adult mice. Nature. 2017;541:222–227. [DOI] [PubMed] [Google Scholar]
- 51.Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, Santos CX, Thet S, Mori E, Kinter MT, Rindler PM, Zacchigna S, Mukherjee S, Chen DJ, Mahmoud AI, Giacca M, Rabinovitch PS, Aroumougame A, Shah AM, Szweda LI and Sadek HA. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell. 2014;157:565–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang D, Li Y, Heims-Waldron D, Bezzerides V, Guatimosim S, Guo Y, Gu F, Zhou P, Lin Z, Ma Q, Liu J, Wang DZ and Pu WT. Mitochondrial Cardiomyopathy Caused by Elevated Reactive Oxygen Species and Impaired Cardiomyocyte Proliferation. Circ Res. 2018;122:74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Menendez-Montes I, Abdisalaam S, Xiao F, Lam NT, Mukherjee S, Szweda LI, Asaithamby A and Sadek HA. Mitochondrial fatty acid utilization increases chromatin oxidative stress in cardiomyocytes. Proc Natl Acad Sci U S A. 2021;118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hashimoto K, Kodama A, Honda T, Hanashima A, Ujihara Y, Murayama T, Nishimatsu SI and Mohri S. Fam64a is a novel cell cycle promoter of hypoxic fetal cardiomyocytes in mice. Sci Rep. 2017;7:4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cardoso AC, Lam NT, Savla JJ, Nakada Y, Pereira AHM, Elnwasany A, Menendez-Montes I, Ensley EL, Petric UB, Sharma G, Sherry AD, Malloy CR, Khemtong C, Kinter MT, Tan WLW, Anene-Nzelu CG, Foo RS, Nguyen NUN, Li S, Ahmed MS, Elhelaly WM, Abdisalaam S, Asaithamby A, Xing C, Kanchwala M, Vale G, Eckert KM, Mitsche MA, McDonald JG, Hill JA, Huang L, Shaul PW, Szweda LI and Sadek HA. Mitochondrial Substrate Utilization Regulates Cardiomyocyte Cell Cycle Progression. Nat Metab. 2020;2:167–178. [PMC free article] [PubMed] [Google Scholar]
- 56.Cao T, Liccardo D, LaCanna R, Zhang X, Lu R, Finck BN, Leigh T, Chen X, Drosatos K and Tian Y. Fatty Acid Oxidation Promotes Cardiomyocyte Proliferation Rate but Does Not Change Cardiomyocyte Number in Infant Mice. Front Cell Dev Biol. 2019;7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Magadum A, Singh N, Kurian AA, Munir I, Mehmood T, Brown K, Sharkar MTK, Chepurko E, Sassi Y, Oh JG, Lee P, Santos CXC, Gaziel-Sovran A, Zhang G, Cai CL, Kho C, Mayr M, Shah AM, Hajjar RJ and Zangi L. Pkm2 Regulates Cardiomyocyte Cell Cycle and Promotes Cardiac Regeneration. Circulation. 2020;141:1249–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jopling C, Suñé G, Faucherre A, Fabregat C and Izpisua Belmonte JC. Hypoxia induces myocardial regeneration in zebrafish. Circulation. 2012;126:3017–27. [DOI] [PubMed] [Google Scholar]
- 59.Feyen DAM, McKeithan WL, Bruyneel AAN, Spiering S, Hormann L, Ulmer B, Zhang H, Briganti F, Schweizer M, Hegyi B, Liao Z, Polonen RP, Ginsburg KS, Lam CK, Serrano R, Wahlquist C, Kreymerman A, Vu M, Amatya PL, Behrens CS, Ranjbarvaziri S, Maas RGC, Greenhaw M, Bernstein D, Wu JC, Bers DM, Eschenhagen T, Metallo CM and Mercola M. Metabolic Maturation Media Improve Physiological Function of Human iPSC-Derived Cardiomyocytes. Cell Rep. 2020;32:107925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ovrebo JI and Edgar BA. Polyploidy in tissue homeostasis and regeneration. Development. 2018;145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Britton JS and Edgar BA. Environmental control of the cell cycle in Drosophila: nutrition activates mitotic and endoreplicative cells by distinct mechanisms. Development. 1998;125:2149–58. [DOI] [PubMed] [Google Scholar]
- 62.Yekelchyk M, Guenther S, Preussner J and Braun T. Mono- and multi-nucleated ventricular cardiomyocytes constitute a transcriptionally homogenous cell population. Basic Res Cardiol. 2019;114:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hesse M, Bednarz R, Carls E, Becker C, Bondareva O, Lother A, Geisen C, Dressen M, Krane M, Roell W, Hein L, Fleischmann BK and Gilsbach R. Proximity to injury, but neither number of nuclei nor ploidy define pathological adaptation and plasticity in cardiomyocytes. J Mol Cell Cardiol. 2021;152:95–104. [DOI] [PubMed] [Google Scholar]
- 64.Windmueller R, Leach JP, Babu A, Zhou S, Morley MP, Wakabayashi A, Petrenko NB, Viatour P and Morrisey EE. Direct Comparison of Mononucleated and Binucleated Cardiomyocytes Reveals Molecular Mechanisms Underlying Distinct Proliferative Competencies. Cell Rep. 2020;30:3105–3116 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Becker C and Hesse M. Role of Mononuclear Cardiomyocytes in Cardiac Turnover and Regeneration. Curr Cardiol Rep. 2020;22:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gonzalez-Rosa JM, Sharpe M, Field D, Soonpaa MH, Field LJ, Burns CE and Burns CG. Myocardial Polyploidization Creates a Barrier to Heart Regeneration in Zebrafish. Dev Cell. 2018;44:433–446 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gan P, Patterson M and Sucov HM. Cardiomyocyte Polyploidy and Implications for Heart Regeneration. Annu Rev Physiol. 2020;82:45–61. [DOI] [PubMed] [Google Scholar]
- 68.Jiang YH, Zhu Y, Chen S, Wang HL, Zhou Y, Tang FQ, Jian Z and Xiao YB. Re-enforcing hypoxia-induced polyploid cardiomyocytes enter cytokinesis through activation of beta-catenin. Sci Rep. 2019;9:17865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang WE, Li L, Xia X, Fu W, Liao Q, Lan C, Yang D, Chen H, Yue R, Zeng C, Zhou L, Zhou B, Duan DD, Chen X, Houser SR and Zeng C. Dedifferentiation, Proliferation, and Redifferentiation of Adult Mammalian Cardiomyocytes After Ischemic Injury. Circulation. 2017;136:834–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karbassi E, Fenix A, Marchiano S, Muraoka N, Nakamura K, Yang X and Murry CE. Cardiomyocyte maturation: advances in knowledge and implications for regenerative medicine. Nat Rev Cardiol. 2020;17:341–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zeisberg EM, Ma Q, Juraszek AL, Moses K, Schwartz RJ, Izumo S and Pu WT. Morphogenesis of the right ventricle requires myocardial expression of Gata4. J Clin Invest. 2005;115:1522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Berlo JH, Elrod JW, van den Hoogenhof MM, York AJ, Aronow BJ, Duncan SA and Molkentin JD. The transcription factor GATA-6 regulates pathological cardiac hypertrophy. Circ Res. 2010;107:1032–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singh MK, Li Y, Li S, Cobb RM, Zhou D, Lu MM, Epstein JA, Morrisey EE and Gruber PJ. Gata4 and Gata5 cooperatively regulate cardiac myocyte proliferation in mice. J Biol Chem. 2010;285:1765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prendiville TW, Guo H, Lin Z, Zhou P, Stevens SM, He A, VanDusen N, Chen J, Zhong L, Wang DZ, Gao G and Pu WT. Novel Roles of GATA4/6 in the Postnatal Heart Identified through Temporally Controlled, Cardiomyocyte-Specific Gene Inactivation by Adeno-Associated Virus Delivery of Cre Recombinase. PLoS One. 2015;10:e0128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL and Martin JF. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin Z, von Gise A, Zhou P, Gu F, Ma Q, Jiang J, Yau AL, Buck JN, Gouin KA, van Gorp PR, Zhou B, Chen J, Seidman JG, Wang DZ and Pu WT. Cardiac-specific YAP activation improves cardiac function and survival in an experimental murine MI model. Circ Res. 2014;115:354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Greulich F, Rudat C and Kispert A. Mechanisms of T-box gene function in the developing heart. Cardiovasc Res. 2011;91:212–22. [DOI] [PubMed] [Google Scholar]
- 78.Xiang FL, Guo M and Yutzey KE. Overexpression of Tbx20 in Adult Cardiomyocytes Promotes Proliferation and Improves Cardiac Function After Myocardial Infarction. Circulation. 2016;133:1081–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.da Rocha AM, Campbell K, Mironov S, Jiang J, Mundada L, Guerrero-Serna G, Jalife J and Herron TJ. hiPSC-CM Monolayer Maturation State Determines Drug Responsiveness in High Throughput Pro-Arrhythmia Screen. Sci Rep. 2017;7:13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Akerberg AA, Henner A, Stewart S and Stankunas K. Histone demethylases Kdm6ba and Kdm6bb redundantly promote cardiomyocyte proliferation during zebrafish heart ventricle maturation. Dev Biol. 2017;426:84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang X, Zhao K, Jiang M, Qiu D, Zhou J and Yang Z. The G4 resolvase RHAU regulates ventricular trabeculation and compaction through transcriptional and post-transcriptional mechanisms. J Biol Chem. 2022;298:101449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li D, Sun J and Zhong TP. Wnt Signaling in Heart Development and Regeneration. Curr Cardiol Rep. 2022. [DOI] [PubMed] [Google Scholar]
- 83.Bruneau BG. Signaling and transcriptional networks in heart development and regeneration. Cold Spring Harb Perspect Biol. 2013;5:a008292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ and Palecek SP. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci U S A. 2012;109:E1848–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Buikema JW, Lee S, Goodyer WR, Maas RG, Chirikian O, Li G, Miao Y, Paige SL, Lee D, Wu H, Paik DT, Rhee S, Tian L, Galdos FX, Puluca N, Beyersdorf B, Hu J, Beck A, Venkamatran S, Swami S, Wijnker P, Schuldt M, Dorsch LM, van Mil A, Red-Horse K, Wu JY, Geisen C, Hesse M, Serpooshan V, Jovinge S, Fleischmann BK, Doevendans PA, van der Velden J, Garcia KC, Wu JC, Sluijter JPG and Wu SM. Wnt Activation and Reduced Cell-Cell Contact Synergistically Induce Massive Expansion of Functional Human iPSC-Derived Cardiomyocytes. Cell Stem Cell. 2020;27:50–63 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen X, Yuan W, Li Y, Luo J and Hou N. Role of Hippo-YAP1/TAZ pathway and its crosstalk in cardiac biology. Int J Biol Sci. 2020;16:2454–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu H, Zhang CH, Ammanamanchi N, Suresh S, Lewarchik C, Rao K, Uys GM, Han L, Abrial M, Yimlamai D, Ganapathy B, Guillermier C, Chen N, Khaladkar M, Spaethling J, Eberwine JH, Kim J, Walsh S, Choudhury S, Little K, Francis K, Sharma M, Viegas M, Bais A, Kostka D, Ding J, Bar-Joseph Z, Wu Y, Yechoor V, Moulik M, Johnson J, Weinberg J, Reyes-Mugica M, Steinhauser ML and Kuhn B. Control of cytokinesis by beta-adrenergic receptors indicates an approach for regulating cardiomyocyte endowment. Sci Transl Med. 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen X, Li Y, Luo J and Hou N. Molecular Mechanism of Hippo-YAP1/TAZ Pathway in Heart Development, Disease, and Regeneration. Front Physiol. 2020;11:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zou J, Ma W, Li J, Littlejohn R, Zhou H, Kim IM, Fulton DJR, Chen W, Weintraub NL, Zhou J and Su H. Neddylation mediates ventricular chamber maturation through repression of Hippo signaling. Proc Natl Acad Sci U S A. 2018;115:E4101–E4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mia MM and Singh MK. The Hippo Signaling Pathway in Cardiac Development and Diseases. Front Cell Dev Biol. 2019;7:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J and Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–40. [DOI] [PubMed] [Google Scholar]
- 92.Frangogiannis NG. Transforming growth factor-β in myocardial disease. Nat Rev Cardiol. 2022;19:435–455. [DOI] [PubMed] [Google Scholar]
- 93.Prados B, Gómez-Apiñániz P, Papoutsi T, Luxán G, Zaffran S, Pérez-Pomares JM and de la Pompa JL. Myocardial Bmp2 gain causes ectopic EMT and promotes cardiomyocyte proliferation and immaturity. Cell Death Dis. 2018;9:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martens MD, Field JT, Seshadri N, Day C, Chapman D, Keijzer R, Doucette CA, Hatch GM, West AR, Ivanco TL and Gordon JW. Misoprostol attenuates neonatal cardiomyocyte proliferation through Bnip3, perinuclear calcium signaling, and inhibition of glycolysis. J Mol Cell Cardiol. 2020;146:19–31. [DOI] [PubMed] [Google Scholar]
- 95.Field JT, Martens MD, Mughal W, Hai Y, Chapman D, Hatch GM, Ivanco TL, Diehl-Jones W and Gordon JW. Misoprostol regulates Bnip3 repression and alternative splicing to control cellular calcium homeostasis during hypoxic stress. Cell Death Discov. 2018;4:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chattergoon NN, Giraud GD, Louey S, Stork P, Fowden AL and Thornburg KL. Thyroid hormone drives fetal cardiomyocyte maturation. FASEB J. 2012;26:397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Woo LA, Tkachenko S, Ding M, Plowright AT, Engkvist O, Andersson H, Drowley L, Barrett I, Firth M, Akerblad P, Wolf MJ, Bekiranov S, Brautigan DL, Wang QD and Saucerman JJ. High-content phenotypic assay for proliferation of human iPSC-derived cardiomyocytes identifies L-type calcium channels as targets. J Mol Cell Cardiol. 2019;127:204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tan L, Bogush N, Naib H, Perry J, Calvert JW, Martin DIK, Graham RM, Naqvi N and Husain A. Redox activation of JNK2α2 mediates thyroid hormone-stimulated proliferation of neonatal murine cardiomyocytes. Sci Rep. 2019;9:17731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Purcell NH, Wilkins BJ, York A, Saba-El-Leil MK, Meloche S, Robbins J and Molkentin JD. Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo. Proc Natl Acad Sci U S A. 2007;104:14074–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Taniyama Y, Ito M, Sato K, Kuester C, Veit K, Tremp G, Liao R, Colucci WS, Ivashchenko Y, Walsh K and Shiojima I. Akt3 overexpression in the heart results in progression from adaptive to maladaptive hypertrophy. J Mol Cell Cardiol. 2005;38:375–85. [DOI] [PubMed] [Google Scholar]
- 101.Fey D, Croucher DR, Kolch W and Kholodenko BN. Crosstalk and signaling switches in mitogen-activated protein kinase cascades. Front Physiol. 2012;3:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hong CC, Kume T and Peterson RT. Role of crosstalk between phosphatidylinositol 3-kinase and extracellular signal-regulated kinase/mitogen-activated protein kinase pathways in artery-vein specification. Circ Res. 2008;103:573–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang W, Yano N, Deng M, Mao Q, Shaw SK and Tseng YT. β-Adrenergic receptor-PI3K signaling crosstalk in mouse heart: elucidation of immediate downstream signaling cascades. PLoS One. 2011;6:e26581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Garay BI, Givens S, Abreu P, Liu M, Yücel D, Baik J, Stanis N, Rothermel TM, Magli A, Abrahante JE, Goloviznina NA, Soliman HAN, Dhoke NR, Kyba M, Alford PW, Dudley SC, van Berlo JH, Ogle B and Perlingeiro RRC. Dual inhibition of MAPK and PI3K/AKT pathways enhances maturation of human iPSC-derived cardiomyocytes. Stem Cell Reports. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Buikema JW, Lee S, Goodyer WR, Maas RG, Chirikian O, Li G, Miao Y, Paige SL, Lee D, Wu H, Paik DT, Rhee S, Tian L, Galdos FX, Puluca N, Beyersdorf B, Hu J, Beck A, Venkamatran S, Swami S, Wijnker P, Schuldt M, Dorsch LM, van Mil A, Red-Horse K, Wu JY, Geisen C, Hesse M, Serpooshan V, Jovinge S, Fleischmann BK, Doevendans PA, van der Velden J, Garcia KC, Wu JC, Sluijter JPG and Wu SM. Wnt Activation and Reduced Cell-Cell Contact Synergistically Induce Massive Expansion of Functional Human iPSC-Derived Cardiomyocytes. Cell Stem Cell. 2020;27:50–63.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Washington Smoak I, Byrd NA, Abu-Issa R, Goddeeris MM, Anderson R, Morris J, Yamamura K, Klingensmith J and Meyers EN. Sonic hedgehog is required for cardiac outflow tract and neural crest cell development. Dev Biol. 2005;283:357–72. [DOI] [PubMed] [Google Scholar]
- 107.Singh BN, Koyano-Nakagawa N, Gong W, Moskowitz IP, Weaver CV, Braunlin E, Das S, van Berlo JH, Garry MG and Garry DJ. A conserved HH-Gli1-Mycn network regulates heart regeneration from newt to human. Nat Commun. 2018;9:4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu X, Sasse J, McAllister D and Lough J. Evidence that fibroblast growth factors 1 and 4 participate in regulation of cardiogenesis. Dev Dyn. 1996;207:429–38. [DOI] [PubMed] [Google Scholar]
- 109.Niessen K and Karsan A. Notch signaling in cardiac development. Circ Res. 2008;102:1169–81. [DOI] [PubMed] [Google Scholar]
- 110.Virágh S and Challice CE. The origin of the epicardium and the embryonic myocardial circulation in the mouse. Anat Rec. 1981;201:157–68. [DOI] [PubMed] [Google Scholar]
- 111.Kuhn HJ and Liebherr G. The early development of the epicardium in Tupaia belangeri. Anat Embryol (Berl). 1988;177:225–34. [DOI] [PubMed] [Google Scholar]
- 112.Fransen ME and Lemanski LF. Epicardial development in the axolotl, Ambystoma mexicanum. Anat Rec. 1990;226:228–36. [DOI] [PubMed] [Google Scholar]
- 113.Shimada Y, Ho E and Toyota N. Epicardial covering over myocardial wall in the chicken embryo as seen with the scanning electron microscope. Scan Electron Microsc. 1981:275–80. [PubMed] [Google Scholar]
- 114.Sun J, Peterson EA, Wang AZ, Ou J, Smith KE, Poss KD and Wang J. Defines an Epicardial Cell Subpopulation Required for Cardiomyocyte Expansion During Heart Morphogenesis and Regeneration. Circulation. 2022;146:48–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Floy ME, Dunn KK, Mateyka TD, Reichardt IM, Steinberg AB and Palecek SP. Direct coculture of human pluripotent stem cell-derived cardiac progenitor cells with epicardial cells induces cardiomyocyte proliferation and reduces sarcomere organization. J Mol Cell Cardiol. 2022;162:144–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rhee S, Paik DT, Yang JY, Nagelberg D, Williams I, Tian L, Roth R, Chandy M, Ban J, Belbachir N, Kim S, Zhang H, Phansalkar R, Wong KM, King DA, Valdez C, Winn VD, Morrison AJ, Wu JC and Red-Horse K. Endocardial/endothelial angiocrines regulate cardiomyocyte development and maturation and induce features of ventricular non-compaction. Eur Heart J. 2021;42:4264–4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miao Y, Tian L, Martin M, Paige SL, Galdos FX, Li J, Klein A, Zhang H, Ma N, Wei Y, Stewart M, Lee S, Moonen JR, Zhang B, Grossfeld P, Mital S, Chitayat D, Wu JC, Rabinovitch M, Nelson TJ, Nie S, Wu SM and Gu M. Intrinsic Endocardial Defects Contribute to Hypoplastic Left Heart Syndrome. Cell Stem Cell. 2020;27:574–589.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jin SC, Homsy J, Zaidi S, Lu Q, Morton S, DePalma SR, Zeng X, Qi H, Chang W, Sierant MC, Hung WC, Haider S, Zhang J, Knight J, Bjornson RD, Castaldi C, Tikhonoa IR, Bilguvar K, Mane SM, Sanders SJ, Mital S, Russell MW, Gaynor JW, Deanfield J, Giardini A, Porter GA, Srivastava D, Lo CW, Shen Y, Watkins WS, Yandell M, Yost HJ, Tristani-Firouzi M, Newburger JW, Roberts AE, Kim R, Zhao H, Kaltman JR, Goldmuntz E, Chung WK, Seidman JG, Gelb BD, Seidman CE, Lifton RP and Brueckner M. Contribution of rare inherited and de novo variants in 2,871 congenital heart disease probands. Nat Genet. 2017;49:1593–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ivey MJ and Tallquist MD. Defining the Cardiac Fibroblast. Circ J 2016;80:2269–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vliegen HW, van der Laarse A, Cornelisse CJ and Eulderink F. Myocardial changes in pressure overload-induced left ventricular hypertrophy. A study on tissue composition, polyploidization and multinucleation. Eur Heart J. 1991;12:488–94. [DOI] [PubMed] [Google Scholar]
- 121.Nag AC. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios. 1980;28:41–61. [PubMed] [Google Scholar]
- 122.Litviňuková M, Talavera-López C, Maatz H, Reichart D, Worth CL, Lindberg EL, Kanda M, Polanski K, Heinig M, Lee M, Nadelmann ER, Roberts K, Tuck L, Fasouli ES, DeLaughter DM, McDonough B, Wakimoto H, Gorham JM, Samari S, Mahbubani KT, Saeb-Parsy K, Patone G, Boyle JJ, Zhang H, Viveiros A, Oudit GY, Bayraktar OA, Seidman JG, Seidman CE, Noseda M, Hubner N and Teichmann SA. Cells of the adult human heart. Nature. 2020;588:466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tucker NR, Chaffin M, Fleming SJ, Hall AW, Parsons VA, Bedi KC, Akkad AD, Herndon CN, Arduini A, Papangeli I, Roselli C, Aguet F, Choi SH, Ardlie KG, Babadi M, Margulies KB, Stegmann CM and Ellinor PT. Transcriptional and Cellular Diversity of the Human Heart. Circulation. 2020;142:466–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hortells L, Valiente-Alandi I, Thomas ZM, Agnew EJ, Schnell DJ, York AJ, Vagnozzi RJ, Meyer EC, Molkentin JD and Yutzey KE. A specialized population of Periostin-expressing cardiac fibroblasts contributes to postnatal cardiomyocyte maturation and innervation. Proc Natl Acad Sci U S A. 2020;117:21469–21479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang Y, Yao F, Wang L, Li Z, Ren Z, Li D, Zhang M, Han L, Wang SQ and Zhou B. Single-cell analysis of murine fibroblasts identifies neonatal to adult switching that regulates cardiomyocyte maturation. Nat Commun. 2020;11:2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Eder A, Vollert I, Hansen A and Eschenhagen T. Human engineered heart tissue as a model system for drug testing. Adv Drug Deliv Rev. 2016;96:214–24. [DOI] [PubMed] [Google Scholar]
- 127.von Bibra C, Shibamiya A, Geertz B, Querdel E, Kohne M, Studemann T, Starbatty J, Schmidt FN, Hansen A, Hiebl B, Eschenhagen T and Weinberger F. Human engineered heart tissue transplantation in a guinea pig chronic injury model. J Mol Cell Cardiol. 2022;166:1–10. [DOI] [PubMed] [Google Scholar]
- 128.Weinberger F, Breckwoldt K, Pecha S, Kelly A, Geertz B, Starbatty J, Yorgan T, Cheng KH, Lessmann K, Stolen T, Scherrer-Crosbie M, Smith G, Reichenspurner H, Hansen A and Eschenhagen T. Cardiac repair in guinea pigs with human engineered heart tissue from induced pluripotent stem cells. Sci Transl Med. 2016;8:363ra148. [DOI] [PubMed] [Google Scholar]
- 129.Ribeiro AJS, Schwab O, Mandegar MA, Ang YS, Conklin BR, Srivastava D and Pruitt BL. Multi-Imaging Method to Assay the Contractile Mechanical Output of Micropatterned Human iPSC-Derived Cardiac Myocytes. Circ Res. 2017;120:1572–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Laflamme MA and Murry CE. Heart regeneration. Nature. 2011;473:326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Steinhauser ML and Lee RT. Regeneration of the heart. EMBO Mol Med. 2011;3:701–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Garbern JC and Lee RT. Heart regeneration: 20 years of progress and renewed optimism. Dev Cell. 2022;57:424–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yamauchi-Takihara K, Sole MJ, Liew J, Ing D and Liew CC. Characterization of human cardiac myosin heavy chain genes. Proc Natl Acad Sci U S A. 1989;86:3504–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gulick J, Subramaniam A, Neumann J and Robbins J. Isolation and characterization of the mouse cardiac myosin heavy chain genes. J Biol Chem. 1991;266:9180–5. [PubMed] [Google Scholar]
- 135.Kass DA, Hare JM and Georgakopoulos D. Murine cardiac function: a cautionary tail. Circ Res. 1998;82:519–22. [DOI] [PubMed] [Google Scholar]
- 136.Eschenhagen T and Zimmermann WH. Engineering myocardial tissue. Circ Res. 2005;97:1220–31. [DOI] [PubMed] [Google Scholar]
- 137.Tzatzalos E, Abilez OJ, Shukla P and Wu JC. Engineered heart tissues and induced pluripotent stem cells: Macro- and microstructures for disease modeling, drug screening, and translational studies. Adv Drug Deliv Rev. 2016;96:234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hansen A, Eder A, Bonstrup M, Flato M, Mewe M, Schaaf S, Aksehirlioglu B, Schwoerer AP, Uebeler J and Eschenhagen T. Development of a drug screening platform based on engineered heart tissue. Circ Res. 2010;107:35–44. [DOI] [PubMed] [Google Scholar]
- 139.McDevitt TC, Angello JC, Whitney ML, Reinecke H, Hauschka SD, Murry CE and Stayton PS. In vitro generation of differentiated cardiac myofibers on micropatterned laminin surfaces. J Biomed Mater Res. 2002;60:472–9. [DOI] [PubMed] [Google Scholar]
- 140.Bursac N, Parker KK, Iravanian S and Tung L. Cardiomyocyte cultures with controlled macroscopic anisotropy: a model for functional electrophysiological studies of cardiac muscle. Circ Res. 2002;91:e45–54. [DOI] [PubMed] [Google Scholar]
- 141.Ribeiro AJ, Ang YS, Fu JD, Rivas RN, Mohamed TM, Higgs GC, Srivastava D and Pruitt BL. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc Natl Acad Sci U S A. 2015;112:12705–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H and Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–8. [DOI] [PubMed] [Google Scholar]
- 143.Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, Morikawa K, Teles D, Yazawa M and Vunjak-Novakovic G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 2018;556:239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zimmermann WH, Schneiderbanger K, Schubert P, Didie M, Munzel F, Heubach JF, Kostin S, Neuhuber WL and Eschenhagen T. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90:223–30. [DOI] [PubMed] [Google Scholar]
- 145.Wendel JS, Ye L, Zhang P, Tranquillo RT and Zhang JJ. Functional consequences of a tissue-engineered myocardial patch for cardiac repair in a rat infarct model. Tissue Eng Part A. 2014;20:1325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zimmermann WH, Didie M, Wasmeier GH, Nixdorff U, Hess A, Melnychenko I, Boy O, Neuhuber WL, Weyand M and Eschenhagen T. Cardiac grafting of engineered heart tissue in syngenic rats. Circulation. 2002;106:I151–7. [PubMed] [Google Scholar]
- 147.Mills RJ, Titmarsh DM, Koenig X, Parker BL, Ryall JG, Quaife-Ryan GA, Voges HK, Hodson MP, Ferguson C, Drowley L, Plowright AT, Needham EJ, Wang QD, Gregorevic P, Xin M, Thomas WG, Parton RG, Nielsen LK, Launikonis BS, James DE, Elliott DA, Porrello ER and Hudson JE. Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc Natl Acad Sci U S A. 2017;114:E8372–E8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mills RJ, Parker BL, Quaife-Ryan GA, Voges HK, Needham EJ, Bornot A, Ding M, Andersson H, Polla M, Elliott DA, Drowley L, Clausen M, Plowright AT, Barrett IP, Wang QD, James DE, Porrello ER and Hudson JE. Drug Screening in Human PSC-Cardiac Organoids Identifies Pro-proliferative Compounds Acting via the Mevalonate Pathway. Cell Stem Cell. 2019;24:895–907 e6. [DOI] [PubMed] [Google Scholar]
- 149.Ulmer BM, Stoehr A, Schulze ML, Patel S, Gucek M, Mannhardt I, Funcke S, Murphy E, Eschenhagen T and Hansen A. Contractile Work Contributes to Maturation of Energy Metabolism in hiPSC-Derived Cardiomyocytes. Stem Cell Reports. 2018;10:834–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Godier-Furnemont AF, Tiburcy M, Wagner E, Dewenter M, Lammle S, El-Armouche A, Lehnart SE, Vunjak-Novakovic G and Zimmermann WH. Physiologic force-frequency response in engineered heart muscle by electromechanical stimulation. Biomaterials. 2015;60:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tiburcy M, Hudson JE, Balfanz P, Schlick S, Meyer T, Chang Liao ML, Levent E, Raad F, Zeidler S, Wingender E, Riegler J, Wang M, Gold JD, Kehat I, Wettwer E, Ravens U, Dierickx P, van Laake LW, Goumans MJ, Khadjeh S, Toischer K, Hasenfuss G, Couture LA, Unger A, Linke WA, Araki T, Neel B, Keller G, Gepstein L, Wu JC and Zimmermann WH. Defined Engineered Human Myocardium With Advanced Maturation for Applications in Heart Failure Modeling and Repair. Circulation. 2017;135:1832–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ma J, Guo L, Fiene SJ, Anson BD, Thomson JA, Kamp TJ, Kolaja KL, Swanson BJ and January CT. High purity human-induced pluripotent stem cell-derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents. Am J Physiol Heart Circ Physiol. 2011;301:H2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wiegerinck RF, Cojoc A, Zeidenweber CM, Ding G, Shen M, Joyner RW, Fernandez JD, Kanter KR, Kirshbom PM, Kogon BE and Wagner MB. Force frequency relationship of the human ventricle increases during early postnatal development. Pediatr Res. 2009;65:414–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hirt MN, Hansen A and Eschenhagen T. Cardiac tissue engineering: state of the art. Circ Res. 2014;114:354–67. [DOI] [PubMed] [Google Scholar]
- 155.Ronaldson-Bouchard K, Yeager K, Teles D, Chen T, Ma S, Song L, Morikawa K, Wobma HM, Vasciaveo A, Ruiz EC, Yazawa M and Vunjak-Novakovic G. Engineering of human cardiac muscle electromechanically matured to an adult-like phenotype. Nat Protoc. 2019;14:2781–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang Y, Yao F, Wang L, Li Z, Ren Z, Li D, Zhang M, Han L, Wang SQ, Zhou B and Wang L. Single-cell analysis of murine fibroblasts identifies neonatal to adult switching that regulates cardiomyocyte maturation. Nat Commun. 2020;11:2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Querdel E, Reinsch M, Castro L, Kose D, Bahr A, Reich S, Geertz B, Ulmer B, Schulze M, Lemoine MD, Krause T, Lemme M, Sani J, Shibamiya A, Studemann T, Kohne M, Bibra CV, Hornaschewitz N, Pecha S, Nejahsie Y, Mannhardt I, Christ T, Reichenspurner H, Hansen A, Klymiuk N, Krane M, Kupatt C, Eschenhagen T and Weinberger F. Human Engineered Heart Tissue Patches Remuscularize the Injured Heart in a Dose-Dependent Manner. Circulation. 2021;143:1991–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Voges HK, Mills RJ, Elliott DA, Parton RG, Porrello ER and Hudson JE. Development of a human cardiac organoid injury model reveals innate regenerative potential. Development. 2017;144:1118–1127. [DOI] [PubMed] [Google Scholar]
- 159.Lee J, Sutani A, Kaneko R, Takeuchi J, Sasano T, Kohda T, Ihara K, Takahashi K, Yamazoe M, Morio T, Furukawa T and Ishino F. In vitro generation of functional murine heart organoids via FGF4 and extracellular matrix. Nat Commun. 2020;11:4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hofbauer P, Jahnel SM, Papai N, Giesshammer M, Deyett A, Schmidt C, Penc M, Tavernini K, Grdseloff N, Meledeth C, Ginistrelli LC, Ctortecka C, Salic S, Novatchkova M and Mendjan S. Cardioids reveal self-organizing principles of human cardiogenesis. Cell. 2021;184:3299–3317 e22. [DOI] [PubMed] [Google Scholar]
- 161.Song MH, Choi SC, Noh JM, Joo HJ, Park CY, Cha JJ, Ahn TH, Ko TH, Choi JI, Na JE, Rhyu IJ, Jang Y, Park Y, Gim JA, Kim JH and Lim DS. LEFTY-PITX2 signaling pathway is critical for generation of mature and ventricular cardiac organoids in human pluripotent stem cell-derived cardiac mesoderm cells. Biomaterials. 2021;278:121133. [DOI] [PubMed] [Google Scholar]
- 162.Kitsuka T, Itoh M, Amamoto S, Arai KI, Oyama J, Node K, Toda S, Morita S, Nishida T and Nakayama K. 2-Cl-C.OXT-A stimulates contraction through the suppression of phosphodiesterase activity in human induced pluripotent stem cell-derived cardiac organoids. PLoS One. 2019;14:e0213114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Silva AC, Matthys OB, Joy DA, Kauss MA, Natarajan V, Lai MH, Turaga D, Blair AP, Alexanian M, Bruneau BG and McDevitt TC. Co-emergence of cardiac and gut tissues promotes cardiomyocyte maturation within human iPSC-derived organoids. Cell Stem Cell. 2021;28:2137–2152 e6. [DOI] [PubMed] [Google Scholar]
- 164.Noor N, Shapira A, Edri R, Gal I, Wertheim L and Dvir T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv Sci (Weinh). 2019;6:1900344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li RA, Keung W, Cashman TJ, Backeris PC, Johnson BV, Bardot ES, Wong AOT, Chan PKW, Chan CWY and Costa KD. Bioengineering an electro-mechanically functional miniature ventricular heart chamber from human pluripotent stem cells. Biomaterials. 2018;163:116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kupfer ME, Lin WH, Ravikumar V, Qiu K, Wang L, Gao L, Bhuiyan DB, Lenz M, Ai J, Mahutga RR, Townsend D, Zhang J, McAlpine MC, Tolkacheva EG and Ogle BM. In Situ Expansion, Differentiation, and Electromechanical Coupling of Human Cardiac Muscle in a 3D Bioprinted, Chambered Organoid. Circ Res. 2020;127:207–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yildirim Y, Naito H, Didie M, Karikkineth BC, Biermann D, Eschenhagen T and Zimmermann WH. Development of a biological ventricular assist device: preliminary data from a small animal model. Circulation. 2007;116:I16–23. [DOI] [PubMed] [Google Scholar]
- 168.Liu N, Ye X, Yao B, Zhao M, Wu P, Liu G, Zhuang D, Jiang H, Chen X, He Y, Huang S and Zhu P. Advances in 3D bioprinting technology for cardiac tissue engineering and regeneration. Bioact Mater. 2021;6:1388–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue HJ, Ramadan MH, Hudson AR and Feinberg AW. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci Adv. 2015;1:e1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Skylar-Scott MA, Uzel SGM, Nam LL, Ahrens JH, Truby RL, Damaraju S and Lewis JA. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci Adv. 2019;5:eaaw2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.King J and Lowery DR. Physiology, Cardiac Output StatPearls; Treasure Island (FL); 2022. [PubMed] [Google Scholar]
- 172.Crispi F, Valenzuela-Alcaraz B, Cruz-Lemini M and Gratacos E. Ultrasound assessment of fetal cardiac function. Australas J Ultrasound Med. 2013;16:158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shih HT. Anatomy of the action potential in the heart. Tex Heart Inst J. 1994;21:30–41. [PMC free article] [PubMed] [Google Scholar]
- 174.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S and Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhao M, Nakada Y, Wei Y, Bian W, Chu Y, Borovjagin AV, Xie M, Zhu W, Nguyen T, Zhou Y, Serpooshan V, Walcott GP and Zhang J. Cyclin D2 Overexpression Enhances the Efficacy of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes for Myocardial Repair in a Swine Model of Myocardial Infarction. Circulation. 2021;144:210–228. [DOI] [PMC free article] [PubMed] [Google Scholar]


