Abstract
Determining the degree to which humans relied on coastal resources in the past is key for understanding long-term social and economic development, as well as for assessing human health and anthropogenic impacts on the environment. Prehistoric hunter–gatherers are often assumed to have heavily exploited aquatic resources, especially those living in regions of high marine productivity. For the Mediterranean, this view has been challenged, partly by the application of stable isotope analysis of skeletal remains which has shown more varied coastal hunter–gatherer diets than in other regions, perhaps due to its lower productivity. By undertaking a more specific analysis of amino acids from bone collagen of 11 individuals from one of the oldest and best-known Mesolithic cemeteries in the Mediterranean, at El Collado, Valencia, we show that high levels of aquatic protein consumption were achieved. By measuring both carbon and nitrogen in amino acids, we conclude that some of the El Collado humans relied heavily on local lagoonal fish and possibly shellfish, rather than open marine species. By contrast to previous suggestions, this study demonstrates that the north-western coast of the Mediterranean basin could support maritime-oriented economies during the Early Holocene.
Keywords: compound-specific isotope analysis, mediterranean, mesolithic, Iberian peninsula, Bayesian modelling, palaeodiet
1. Introduction
Once considered marginal to prehistoric human societies, coastal environments have become increasingly central in archaeological theories [1,2], with studies demonstrating their role in early human dispersal around the continents [3,4], in fuelling population growth, complex forms of social organization [5–7], the emergence and spread of early food production systems [8–10]. Nevertheless, the degree to which coastal Mediterranean hunter–gatherers relied on aquatic resources remains contentious. It is often thought that exploitation of marine taxa broadened and intensified in the Holocene through the development of specialized fishing and shellfish gathering and new technologies [11,12] thereby encouraging sedentism and surplus production. The counterargument is that coastal environments were heavily exploited well before the Holocene, but that much of the evidence has been lost due to rising sea levels [3]. The extent to which regional variation in marine productivity and thus the relative availability of coastal resources influenced the development of hunter–gatherer subsistence economies is also unclear. It is often assumed that the Mediterranean basin, due to its relatively low primary productivity, low tidal amplitude and diminished intertidal zone [13], led to a different dietary adaptation compared to the maritime-focused hunter–gatherer–fisher economies that developed along the Atlantic and Baltic coasts. Certainly, the frequency of coastal shell middens is lower. Yet, the remains of marine mammals, fish and molluscs are frequently found on Mediterranean Mesolithic coastal sites, sometimes dominating the assemblages (e.g. [14,15]), suggesting, at the very least, they were a key strategic supplement to terrestrial-based subsistence [16–19].
Taphonomy biases adversely affecting the preservation and recovery of marine fish bone and plant remains prevents a more precise assessment of the relative contribution of marine and terrestrial foods. For this reason, stable carbon (δ13Ccoll) and nitrogen isotopes (δ15Ncoll) of human bone collagen have provided a useful tool for directly investigating spatial and temporal dietary variation among coastal hunter–gatherers. Indeed, such analysis supports a much greater reliance on marine resources by Late Mesolithic Atlantic and Baltic hunter–gatherers compared to those from the Mediterranean. For example, in Atlantic contexts from Britain, France and Portugal, analysis of Late Mesolithic skeletal remains show a sustained and heavy reliance on marine foods, particularly fish, estimated to contribute at least half their total dietary protein and up to 90% [20]. By contrast, analyses of Upper Palaeolithic and Mesolithic skeletal remains from the Mediterranean reveal diets where the majority of protein is derived from the terrestrial hunted game [15,17,18,21–30], even at sites where fish remains dominate the faunal assemblage [15,19,28,29,31]. From these data it is argued that the availability of marine foods from the Mediterranean may have been, in general, too low to sustain human populations, leading to the development of a broader, mixed marine-terrestrial strategy and perhaps greater terrestrial mobility [29].
Nevertheless, the accuracy and confidence for quantifying the marine contribution to diet using stable isotopes of bone collagen have been questioned. Concerns have centred around the ability to detect low but significant marine consumption (e.g. <20% by dry weight [32]). As collagen is synthesized from different dietary macronutrients [33] (i.e. protein, carbohydrate and lipids), it is proposed that a mixed diet derived from different types of marine and terrestrial foods is not always easily decipherable from bulk collagen stable isotope values. Also, without knowledge of the isotope values of baseline primary producers, which may vary spatially, it is difficult to make accurate comparisons between regions. This is particularly relevant to the Mediterranean region where the range of collagen δ15N values (δ15Ncoll) of marine fish from archaeological sites is typically lower than those from Atlantic sites [22,34] and often overlaps with those of terrestrial mammals [35]. Carbon and nitrogen compound-specific isotope analysis (CSIA) of individual collagen amino acids (CSIA-AA) is being increasingly applied to circumvent these issues, as amino acids can be traced to dietary sources with more certainty and independently assess baseline δ15N values and trophic position (e.g. [36–39]). This approach has been used to investigate intra-site dietary differences in marine consumption with more accuracy and precision than bulk isotope analysis [40].
Here, we apply CSIA-AA to one of the largest and most important Mesolithic human bone assemblages from the Mediterranean coast; the site of El Collado (Oliva, Valencia, Spain) [41,42]. Previous bulk collagen isotope analyses of nine individuals from this site, showed variability in the extent of marine consumption; some individuals had entirely terrestrial diets whereas it was suggested that others obtained approximately 25% of total dietary protein from marine sources [29]. This assessment was based on the linear interpolation between endpoints estimated from terrestrial and marine fauna. Subsequent to this important finding, it was shown that although δ13Ccoll values clearly discriminate between marine and terrestrial Mediterranean fauna, δ15Ncoll are more difficult to interpret since the marine and terrestrial endpoints for the Mediterranean are more similar [34]. The objective of this study was therefore to extend the sampling of human remains from El Collado and to establish whether the CSIA can more accurately quantify the marine contribution by considering the δ15N and δ13C of individual amino acids obtained from bone collagen. As one of only very few Late Mesolithic burial grounds in the Western Mediterranean, furthermore detailed dietary analysis has the potential to provide an almost unique insight into food consumption practices during a key period in Mediterranean prehistory and one that dates immediately prior to the arrival of farmers, with further implications for agricultural adoption.
(a) . The El Collado site
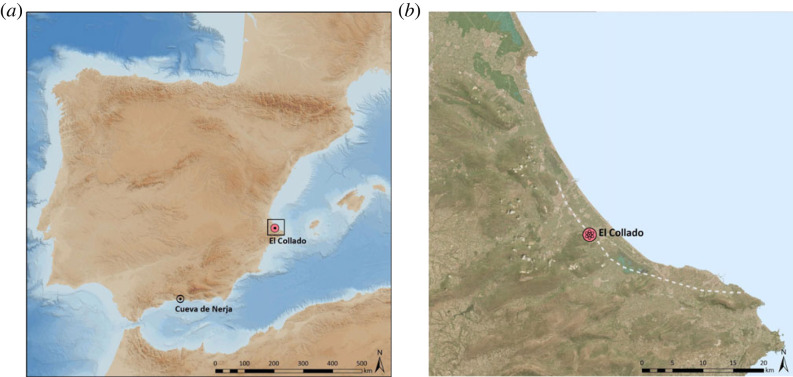
El Collado is located in Oliva about 7 km from the modern Mediterranean coastline in the Gulf of Valencia, Spain (figure 1). It is an open-air site on a hillside, sheltered by a limestone cliff, about 10 m above sea level. During the Mesolithic, the lowland around the site consisted of coastal freshwater swamps and marshes, and brackish lagoon biotopes [43], following a rise in sea level from about 8300 BP reaching a maximum at 6130 BP. Excavations in the late 1980s revealed a total of 14 graves (COLL 1–14), 13 of which were primary burials attributed to the Mesolithic period. A secondary burial (COLL 9) contained only a skull and an isolated skull was also recovered close to burial 12 (COLL 12). AMS dates of 10 of the individuals showed that the site was occupied, possibly in phases, from ca 9500 cal BP to 8500 cal BP making it one of the oldest known cemeteries on the Iberian Peninsula [42]. Furthermore, the cemetery is clearly connected to a habitation site consisting of a substantial mixed marine-terrestrial shell midden, with lithic tools and other faunal remains, including a fish assemblage dominated by the gilthead sea bream (Sparus aurata) [17].
Figure 1.
(a) Map of the Iberian Peninsula showing the location of the sites of El Collado (red) and Cueva de Nerja; and (b) the location of the El Collado site in the Southern Sector of the Valencian Gulf, Spain, with the representation of the position of the Flandrian coastline 6130 ± 100 BP (dashed line), showing a sea level 2 m higher and about 3 km inwards from the current position of the coastline, based on [17].
2. Material and methods
(a) . Sampling
In this study, 11 individuals from the 1987–1988 excavations were analysed with permission provided by the Museu de Prehistòria de València (Valencia, Spain). The original osteological report [44] was reassessed during the sampling procedure, and the sex and age-at-death of the 11 individuals were refined including updated ostoarchaeological methods [45–47] (electronic supplementary material, table S1). The sampling process was performed carefully by selecting, whenever possible, fragments of long bone diaphysis from the lower limbs. In conjunction with the analysis of human remains, seven faunal remains from El Collado (four terrestrial herbivores and three marine fish) were analysed (electronic supplementary material, table S1). To increase the faunal reference further, collagen extraction was attempted from 13 marine fish bones from Mesolithic (n = 7) and Early Neolithic (n = 6) layers at the sites of Cueva de Nerja (Malaga, Spain), chosen for its proximity in time and space to El Collado.
(b) . Collagen extraction
Collagen was extracted and prepared for the stable isotope analysis at the BioArCh laboratories in the Department of Archaeology, University of York (UK), using previously published approaches [48]. Briefly, the bone samples were cleaned using a sandblaster (80 060 Master Problast 3) to remove potential post-depositional contamination. Shards of bones (approx. 200 to 300 mg) were demineralized using 0.6 M HCl at 4°C for several days, then rinsed with ultrapure H2O (milli-Q) and gelatinized with 0.001 M HCl at 80°C for 48 h. The supernatant containing the collagen was filtered using polyethylene Ezee filters (Elkay Laboratories, 9 ml, pore size 60–90 µm) and then 30 kDa Amicon Ultra-4 centrifugal filter units (Millipore, MA, USA). Samples were then frozen for 24–48 h at −20°C, lyophilized, and weighed into tin capsules (0.5 mg per duplicate) for stable isotope analysis.
(c) . Bulk EA-IRMS
The measurement of the stable carbon and nitrogen isotope ratios was performed in duplicate in the BioArCh laboratories, Department of Archaeology at the University of York, using a Sercon EA-GSL elemental analyser coupled to a Sercon 20–22 continuous flow isotope ratio mass spectrometer (Sercon, Crewe, UK). This measures the ratios of 13C:12C and 15N:14N relative to a standard (V-PDB for carbon and AIR for nitrogen) and expresses the stable isotope values in delta notation (δ) in parts per mil (‰). The accuracy of the measurements was determined according to Kragten [49] by combining uncertainties in the values of standard reference materials and sample replicates within each analytical run. These were international standards IAEA 600 (caffeine: δ13Craw = − 27.59 ± 0.08‰, δ13Ctrue = − 27.77 ± 0.043‰, δ15Nraw = 1.02 ± 0.08‰, δ15Ntrue = 1 ± 0.2‰), IA CANE (sugar cane: δ13Craw = − 11.64 ± 0.13‰, δ13Ctrue = − 11.64 ± 0.03‰), IAEA N2 (ammonium sulfate: δ15Nraw = 20.17 ± 0.06‰, δ15Ntrue = 20.3 ± 0.2‰) and an internal standard (fish gelatine: δ13Craw = −15.43 ± 0.14‰, δ13Ctrue = −15.32 ± 0.03‰, δ15Nraw = 15.01 ± 0.09‰, δ15Ntrue = 15.2 ± 0.12‰).
In addition, a bovine bone control was extracted and analysed within the same batch, producing the following average values (δ13C = − 22.97 ± 0.10‰, δ15N = 5.85 ± 0.17‰). This was within the overall mean value from 50 separate extracts of this bone sample, which produced values of δ13C = − 22.93 ± 0.20‰ and δ15N = 6.15 ± 0.29‰.
(d) . Compound-specific stable isotope measurements of amino acids
The isotopic composition of amino acids was analysed in 11 human collagen samples and five faunal collagen samples (two bovids, two cervids and one fish) from El Collado, and five fish collagen samples from Cueva de Nerja. Collagen extracts were hydrolysed (6 M HCl, 200 µl, 110°C, 24 h) after addition of 50 µl of an internal norleucine standard (Sigma-Aldrich) of known isotopic composition. The hydrolysates were centrifuged (11 000g, 1 min) using Pall Nanosep filtres (0.45 µm) to remove the remaining insoluble material. The hydrolysates were gently dried at room temperature under N2, redissolved in 0.1 M HCl (100 µl), and stored at −20°C until required for analysis.
Amino acids were then derivatized to form N-acetyl-i-propyl (NAIP) esters [50]. Briefly, isopropanol and acetyl chloride (1 ml; 4 : 1 v/v) were added to the dried samples, and the tubes were sealed and heated at 100°C (1 h). After 1 hour, sample mixtures were cooled (at −20°C), and the solution was dried under a gentle stream of N2. Dichloromethane (DCM) was added (2 × 0.5 ml) and blown down under a gentle stream of N2 to remove excess reagents. Next, a mixture of acetic anhydride, triethylamine and acetone (1 ml; 1 : 2:5, v/v/v) was added to the tubes and heated at 60°C (10 min). The mixture was cooled and evaporated to dryness under a gentle stream of N2. NAIP esters were then dissolved in ethyl acetate (EtAc; 2 ml). A saturated NaCl solution (1 ml) was added to separate polar and/or inorganic components, and the organic phase was transferred into a new culture tube. The phase separation was repeated with additional EtAc (1 ml). Trace amounts of water were removed with molecular sieves (sodium aluminium silicate, 0.3 nm: Merck KGaA, Darmstadt, Germany). The EtAc containing the NAIP esters was blown down under a gentle stream of N2, and then DCM (1 ml) was added and dried to remove excess water. Samples were redissolved in known quantities of EtAc and stored at −20°C until required for analysis by GC-C-IRMS. The same derivatization procedure was used for preparing mixtures of international reference standards (Indiana, USA and SHOKO Science, Japan) and standards purchased from Sigma-Aldrich (Sigma-Aldrich Company, UK).
The GC-C-IRMS measurements of the amino acids were conducted using a Delta V Plus IRMS (Thermo Fisher Scientific, Bremen, Germany) linked to a Trace Ultra gas chromatograph (Thermo Fisher Scientific, Bremen, Germany) with a GC IsoLink II interface fitted with a Cu/Ni combustion reactor maintained at 1000°C. Ultrahigh-purity-grade helium with a flow rate of 1.4 ml min−1 was used as the carrier gas, and parallel acquisition of flame ionization data were achieved by diverting a small part of the flow to an integrated flame ionization detector (Thermo Fisher Scientific). Ethyl acetate was used to dilute the samples, and 1 µl of each sample and 2 µl of each standard were injected at 240°C with a 3.5 s pre-injection dwell time onto a custom DB-35 fused silica column (60 m × 0.32 mm × 0.50 µm; Agilent J&W Scientific Technologies, Folsom, CA, USA). All samples were injected in triplicate. The oven temperature program used for samples and standards was as follows: 40°C (hold 5 min) and then increasing by 15°C min−1 up to 120°C, then by 3°C min−1 up to 180°C, then by 1.5°C min−1 up to 210°C, then by 5°C min−1 up to 280°C (hold 8 min). A Nafion membrane removed water, and a cryogenic trap was used to remove CO2 from the oxidized and reduced sample when operated in nitrogen mode. In carbon mode, eluted products were combusted to CO2 and ionized in a mass spectrometer by electron impact. Ion intensities of mass/charge ratio (m/z) 44, 45 and 46 were monitored to automatically compute the 13C:12C ratio of each peak in the samples. In nitrogen mode, ion intensities of m/z 28, 29 and 30 were monitored to automatically compute the 15N:14N ratio of each peak in the samples. Computations were made with Isodat (v. 3.0; Thermo Fisher Scientific) and were based on comparisons with a repeatedly measured high-purity standard reference gas (CO2 or N2). The results from the analysis are reported in parts per mil (‰) relative to international standards using the δ notation.
(e) . δ15N measurements of amino acids
The δ15N values reported in the present are the mean of triplicate δ15N measurements. An amino acid international standard mixture of known isotopic composition was run after every three sample injections to monitor instrument performance and drift. The amino acid standard mixture used for δ15N determinations comprised eight international standards (Indiana and SHOKO Science) and L-norleucine (Sigma-Aldrich). δ15N true values of L-norleucine were determined in-house by EA-IRMS. International standard average raw values and s.d. across the runs were as follows: Ala, 44.46 ± 1.66‰ (true: + 43.25 ± 0.07‰); Gly, 3.44 ± 1.49‰ (true: + 1.76 ± 0.06‰); Val, −4.30 ± 0.98‰ (true: −5.21 ± 0.05‰); Leu, 7.83 ± 1.29‰ (true: 6.22‰); Nle, + 15.68 ± 0.78‰ (true: + 14.55 ± 0.23‰); Asp, 35.03 ± 0.16‰ (true: 35.2‰); Glu, −3.76 ± 0.43‰ (true: −4.52 ± 0.06‰); Hyp, −8.73 ± 0.44‰ (true: −9.17‰); Phe, 1.82 ± 0.32‰ (true: 1.70 ± 0.06‰). Sample δ15N raw values were corrected by the calibration curve and the L-norleucine internal standard true value.
(f) . δ13C measurements of amino acids
The δ13C values reported are a mean of triplicate δ13C measurements. Amino acids in the samples were first corrected for the isotopic difference between L-norleucine in the standard mixture and L-norleucine in the sample. δ13C amino acid measurements were then corrected by specific correction factors to account for the derivatizing carbon and the kinetic isotope effect [51]. A standard amino acid mixture was run after every three sample injections, and the average correction factors from the standard mixture were used for the correction of the samples (Sigma-Aldrich, UK). True δ13C values of standards were measured by EA-IRMS. The standard δ13C average correction factor values and SD across the runs were as follows: Ala, −30.15 ± 1.28‰ (true: −19.31 ± 0.02‰); Gly, −35.02 ± 1.65‰ (true: −33.31 ± 0.02‰); Val, −25.35 ± 1.83‰ (true: −10.89 ± 0.02‰); Leu, −24.67 ± 1.64‰ (true: −13.78 ± 0.06‰); Ile, −31.57 ± 1.73‰ (true: 24.89 ± 0.07‰); Nle, −31.81 ± 1.85‰ (true: −27.59 ± 0.02‰); Thr, −31.40 ± 1.67‰ (true: −10.46 ± 0.01‰); Ser, −33.54 ± 1.50‰ (true: −12.54 ± 0.09‰); Pro, −23.72 ± 1.66‰ (true: −12.33 ± 0.02‰); Asp, −30.60 ± 1.71‰ (true: −27.52 ± 0.12‰); Met, −33.98 ± 2.12‰ (true: −29.88 ± 0.14‰); Glu, −30.17 ± 1.67‰ (true: −28.57 ± 0.09‰); Hyp, −29.79 ± 1.56‰ (true: ); Phe, −20.21 ± 1.43‰ (true: −11.52 ± 0.05‰); Lys, −28.80 ± 1.88‰ (true: −13.7 ± 0.11‰); Tyr, −32.19 ± 1.30‰ (true: −24.85 ± 0.02‰). Correction factors induce a new source of error; therefore, the error propagated for each amino acid was calculated according [51].
(g) . CSIA-AA quality control criteria
We calculated the theoretical collagen δ13C and δ15N values based on their amino acid values. The amino acids measured by GC-C-IRMS represent 90.4% and 80.69% of total carbon and nitrogen, respectively in mammalian collagen. We calculated the estimated bulk collagen δ13C and δ15N values by mass balance equations considering the relative contribution of each amino acid to collagen and compared the obtained values with those measured via EA-IRMS as previously detailed [40]. Generally, we excluded samples where the estimated observed offset was greater than 2σ of the mean value which included all the δ13C values measured on the terrestrial fauna and two of the humans (Coll 5 and Coll 8). We observed a consistent Δ13Cest-obs offset for the archaeological fish remains from Cueva de Nerja (3.9 ± 0.7‰) which might relate to substantial amounts of lipid co-extracted in these extracts that are reducing the bulk collagen δ13C value (80). We have therefore included these data in figure 3 where they plot in the range of other marine fish samples. The mean Δ15Nest-obs of all the samples used in this study was 0.22‰ (±0.81‰). We also monitored the relationship between proline and hydroxyproline stable isotope values which should be highly correlated due to similarities in the way these amino acids are biosynthesized [52]. The Pearson's product-moment correlation between the δ13C and δ15N values of proline and hydroxyproline from this study were R = 0.988 for δ15N (t = 28.563, d.f. = 19, p-value < 2.2 × 10−16) and R = 0.961 of C (t = 15.254, d.f. = 19, p-value = 4.094 × 10−12).
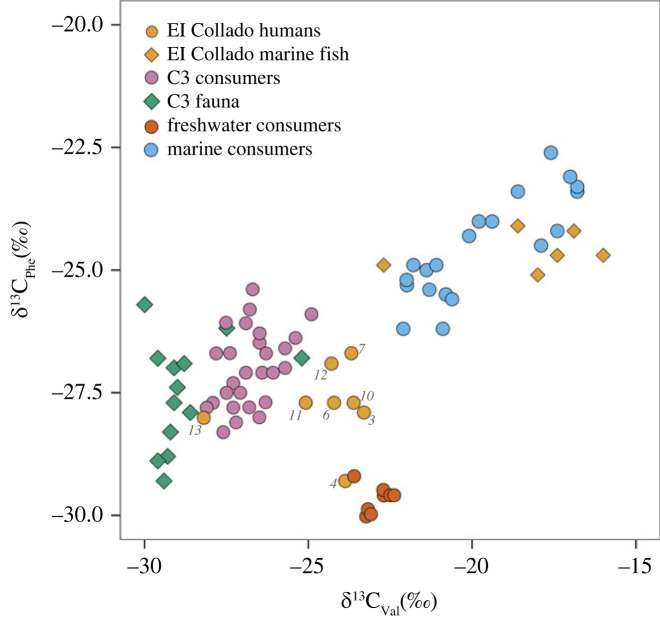
Figure 3.
Plot of δ13C of valine against the δ13C of phenylalanine from collagen extracted from marine fish and humans from El Collado compared with previously published values [52].
(h) . Bayesian mixing model
FRUITS (v. 3.0 beta) was used to generate the Bayesian mixing model (available at http://sourceforge.net/projects/fruits/). The model was adapted from Model 2 reported by Soncin et al. [40], a concentration-dependent model that uses δ15N values of source (Phe) and trophic (Glx) amino acids to estimate protein contribution. The δ15N values of Phe and Glx of the human individuals from El Collado (i.e. targets) were analysed against the two fractions ‘Phe’ and ‘WholeN’ of animal products and marine and freshwater fish, respectively. Human δ15N values of phenylalanine are directly linked, with negligible isotopic fractionation, to the δ15N values of phenylalanine of the protein sources, fraction ‘Phe’, which corresponds to the source δ15N values of phenylalanine. While δ15N values of glutamic acid are the results of the transamination reactions of the nitrogen metabolic pool; fraction ‘Whole N’, which corresponds to the source bulk δ15N values [53]. The offsets from diet to consumer were obtained from multiple feeding experiments studies reported by Soncin et al. [40] and these are: Δ15NPhe-Phe = + 0.1 ± 0.2 ‰ and Δ15NGlx-wholeN = + 9.7 ± 2.5 ‰. As sources, we used terrestrial herbivores from El Collado representing ‘animal products’ and marine fish from El Collado and Cueva de Nerja (electronic supplementary material, table S1) and freshwater fish from Naito et al. [39] representing ‘fish’. The concentrations of the two fractions ‘Phe’ and ‘WholeN’ were derived from the USDA National Nutrient Database for Standard Reference (available at https://fdc.nal.usda.gov/) and expressed as dry weight (%). The items used from the USDA database are the same reported by Soncin et al. [40]. The associated uncertainties of all the reported measurements are standard errors of the mean. Model estimates are reported in electronic supplementary material, table S3.
3. Results and discussion
The criteria for assessing the suitability of collagen followed those reported in [40]. In total, collagen of sufficient quality for bulk elemental analysis-isotope ratio mass spectrometry (EA-IRMS), and determination of both δ13C and δ15N of at least 13 amino acids by gas chromatography combustion-IRMS (GC-C-IRMS), was obtained from all 11 human individuals, all 4 terrestrial fauna, but only 6 out of 16 of the marine fish bones (electronic supplementary material, table S1). When compared to bulk stable isotope data [29] the differences are typically ±1‰, although here we provide additional stable isotope data on four additional individuals (COLL 8, 10, 11 and 14) whereas three individuals (COLL 1, 2 and 9) were not available for further analysis. This dataset was compared with previously published bulk and single compound stable isotope data from freshwater fish recovered from the Epipalaeolithic rockshelter of Pont d'Ambon located in Western France dating from between (13 000 to 9500 cal BP [39]).
(a) . Bulk collagen results
The bulk stable isotope ratios obtained for the faunal skeletal material from El Collado and Cueva de Nerja (electronic supplementary material, table S1, figure 2a) confirms previous studies [35] that show a lack of clear distinction in δ15Ncoll between marine and terrestrial fauna; although the mean values for the terrestrial species are lower than the marine, the ranges partially overlap (figure 2a). The δ13Ccoll clearly discriminates marine from terrestrial and freshwater organisms but not terrestrial from freshwater fauna. One of the Bos primigenius samples had a δ13Ccoll value of −15.71‰, falling outside the range for the consumption of terrestrial C3 plants suggesting consumption of wild C4 plants or marine macroalgae, as has been previously suggested for wild fauna foraging along coastal regions [56,57]. Overall, the variability in the bulk isotope values of both marine and terrestrial fauna introduces more uncertainty when determining the contribution of these sources to human diets as is apparent in figure 2a, which also includes freshwater fish from the Pont d'Ambon. The bulk collagen values of the humans from El Collado measured in this study range from −19.2‰ to −17.4‰ for δ13Ccoll and, 8.86‰ to 13.44‰ for δ15Ncoll (electronic supplementary material, table S1) and are in-line with previous measurements of this assemblage [29].
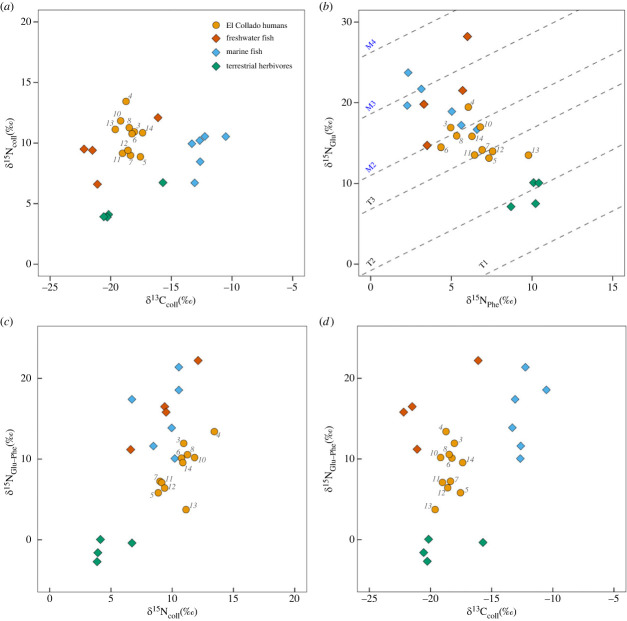
Figure 2.
Plot of a) Bulk collagen δ13C and δ15N values of humans and fauna from El Collado, Cueva de Nerja compared with freshwater fish from Pont d'Ambon [39]; (b) δ15N of glutamic acid against the δ15N of phenylalanine from collagen; the difference between δ15NGlu and δ15Nphe values from collagen against (c) bulk collagen δ15N and D) bulk collagen δ13C. The trophic position lines (T = terrestrial TP, M = marine TP) shown in B are from Naito [54] for reference only (see [55]).
(b) . Estimation of trophic position using compound-specific isotope analysis of amino acids (CSIA-AA)
Compound-specific isotope analysis of amino acids is finding increased application in ecology to estimate a consumer trophic position [38,58]. The benefit of this approach is that it allows simultaneous estimation of the baseline δ15N value by considering the values of source amino acids, as well as those fractionated through trophic transfer (i.e. trophic amino acids). The approach has been applied to archaeological human remains, for example, to investigate the degree of aquatic food consumption in hunter–gatherers in central France and coastal Japan [39,58]. While the precise estimation of trophic position and dietary quantification using this approach is complicated by variability in the values of the source and trophic amino acids in primary produces at the base of the food web (β value) [38,58]) and the trophic enrichment factor (TEF), further exacerbated for human with diets consisting of mixed terrestrial and marine foods [40], such comparisons nonetheless provide a useful indicator. A comparison of the δ15N values of glutamic acid (Glu; a trophic amino acid derived from both glutamic acid and glutamine residues in bone collagen) and Phe (a source amino acid) is presented in figure 2 with the bulk collagen data (δ13Ccoll and δ15Ncoll). The Glu-Phe Δ15N values for the terrestrial herbivores (figure 2b) range from 0–3‰ and are in line with other measurements made on southern European prehistoric and historic terrestrial animals [39,40]. Interestingly six out of the 11 human individuals have Δ15NGlu-Phe values placing them at one trophic position above these terrestrial animals (figure 2b). Instead, these values are within the range of contemporaneous marine fish measured in this study and marine and freshwater fish measured elsewhere [39,40].
Considering the new data presented here, it is evident that aquatic products were much more prominent in the diet than originally postulated using bulk isotope analysis [29]. This is explained by only a moderate correlation (Pearson's; R = 0.62; 0.30 to 0.82 (95% confidence), p-value = 0.0008) between bulk δ15Ncoll and Δ15NGlu-Phe (figure 2c), if all the human and faunal samples are considered. The Δ15NGlu-Phe values result in much greater discrimination between aquatic and terrestrial fauna compared to δ15Ncoll, as noted by Soncin et al. [40]. Therefore, even high trophic level Mediterranean fish have δ15Ncoll that cannot be easily discriminated from terrestrial fauna due to their relatively low δ15N values of source amino acids (e.g. Phe) (figure 2b). This observation also seems to be true of freshwater fish from the Epipalaeolithic site of Pont d'Ambon in Southwestern France [39] but could indeed vary spatially and temporally due to differences in nitrogen assimilation by primary producers. However, Garcia-Guixé et al. 2006 [29]) also drew on the bulk collagen δ13C values to make their interpretation. By plotting Δ15NGlu-Phe against δ13Ccoll (figure 2d), the marine fauna is clearly enriched in δ13C compared to the humans despite the overlap in the range of Δ15NGlx-Phe values. One reason for this is that El Collado fish assemblages were dominated by gilthead bream, Sparus aurata [17]) a euryhaline marine teleost [59] that is likely to have resided in nearby brackish lagoons or estuaries adjacent to the site. Unfortunately, no sea bream was available for analysis from El Collado and the single individual measured from Cueva de Nerja may have resided in more saline, open sea environments given the site's location.
To investigate further, we examined the δ13C of amino acids from the El Collado humans and faunal samples. Unfortunately, only 8 of the human samples and the marine fish samples passed the stringent quality control criteria (see Materials and Methods) implemented by Soncin et al. [40]. It has been shown that the δ13CAA values of essential amino acids such as valine, phenylalanine and leucine can adequately distinguish marine, freshwater and terrestrial consumers [60,61]. In figure 3, we have plotted the δ13C of valine against phenylalanine and compared them with global references for C3, terrestrial and marine consumers reported by Shulting [52]. These comparative data are drawn from Europe and East Asia as none are available for the Mediterranean region. Firstly, the marine fish from the Mediterranean analysed here plot broadly within the range of marine protein consumers from elsewhere, which include marine species and coastal hunter–gatherers from Japan and Greenland (electronic supplementary material, table S2). Secondly, the analysis of humans from El Collado δ13CAA broadly corresponds with the δ15NAA identifying Coll 4 as a high trophic level ‘aquatic’ consumer and Coll 13 as the lowest trophic level ‘terrestrial’ consumer. However, with the δ13CAA analysis, we were able to confirm that freshwater or brackish fish was an important dietary source at El Collado for most of the individuals, as evident in figure 3.
(c) . Multivariate analysis
Consideration of a greater range of amino acid stable isotope measurements can provide further insights into the human diet at El Collado than that obtained using only the proxies considered above. To do this, we conducted principal components analysis (PCA) [62] on a wider range of amino acids to distinguish the different taxonomic groups and individuals and second by using a Bayesian mixing model to quantify individual human diets based on prior assumptions of amino acid metabolism [40]. Given that the δ13C measurements were not available for terrestrial fauna and are probably an inappropriate dietary endpoint, as discussed above, they are omitted from this analysis.
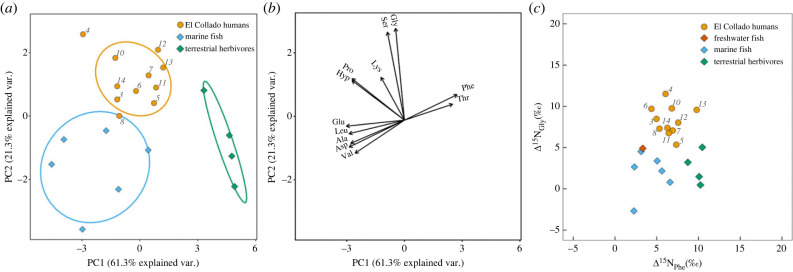
The PCA analysis conducted on all the available amino acid δ15N measurements (12 in total; electronic supplementary electronic data; figure 4) shows that the specimens cluster according to their taxonomic groupings (humans, marine fauna, terrestrial herbivores). Both PCA 1 (61.3% of the variation) and PCA 2 (21.3% of the variation) effectively distinguish these different groups. The humans plot intermediary between marine and terrestrial baselines on component one, implying that at least some of the amino acid nitrogen was obtained from a marine-brackish source. As may be expected for higher trophic level marine organisms, most amino acids are enriched in 15N in the Mediterranean fish samples. Conversely, as noted in §3b, phenylalanine is enriched in 15N in the terrestrial herbivores compared to the marine fish and provides an effective means of discrimination in this context (figure 4c). Threonine is also relatively enriched in the herbivores compared to humans and marine fish. Threonine is atypical in that with increasing trophic level its δ15N values will decrease due to preferential enzymatic catabolism of 15N-Thr to alpha-ketobutyrate and ammonia leading to 15N depletion relative to the dietary source, with the magnitude of the effect influenced by the amount of dietary protein and/or gluconeogenesis [63]. The El Collado human δ15Nthr values for the most extreme marine consumers are within the range of the fish (electronic supplementary material, table S1) but the degree to which this is due to protein intake and dietary quality is difficult to assess.
Figure 4.
Principal component analysis of δ15N values of 12 amino acids measured in human, terrestrial animal and marine fish bone collagen (a). To facilitate the visualization of the data, the vectors have been plotted in a separate graph (b). The δ15N values of phenylalanine against glycine are plotted in figure 4c.
Interestingly, PC2 distinguishes humans from both terrestrial and marine fauna (figure 3) and is correlated with the two biosynthetically related glucogenic amino acids, serine and glycine, and to a lesser degree lysine, proline and hydroxyproline. The relative enrichment in δ15Ngly from the human samples compared to the marine and terrestrial faunal samples are shown in figure 3c. One explanation is that for humans, the nitrogen atom in glycine (and serine) was preferentially obtained through extensive transamination and therefore derived to a greater extent from the metabolic nitrogen pool [64]. Whereas a greater proportion of glycine is directly routed from dietary sources in the terrestrial and marine fauna. The metabolic and dietary conditions to explain this effect are unclear and potentially multifactorial [63], one of which is the amount of protein in the diet. As can be seen in figure 4a, the correlation between different human individuals and component 2 is variable which might be related to the degree of routing versus synthesis of glycine or related to the δ15N values of nitrogen in the metabolic pool. The relative enrichment in glycine in humans partly explains their higher than predicted δ15Ncoll values when the Glu-Phe proxy is considered (figure 2c), as glycine is the most dominant amino acid in bone collagen.
(d) . Quantifying protein consumption using a mixing model
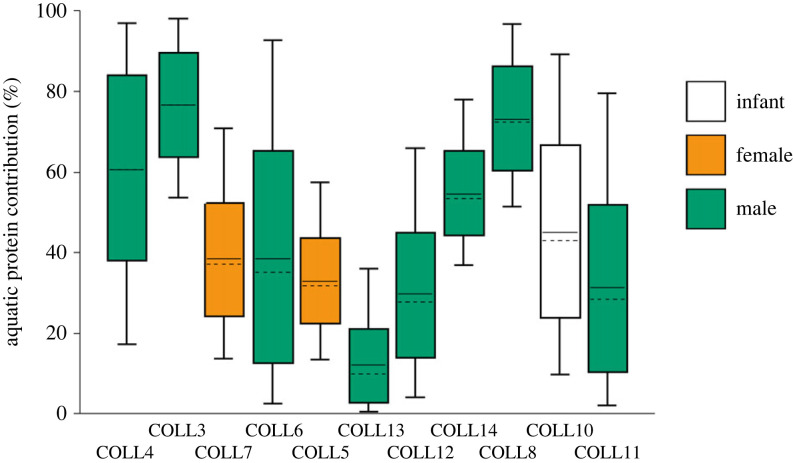
To quantitatively contextualize and evaluate dietary variability at El Collado, we developed a mixing model adapted from Soncin et al. [40]. This model (figure 5) considers nitrogen-stable isotope values from one source amino acid, phenylalanine and one trophic amino acid, glutamic acid (derived from glutamic acid and glutamine residues). The data are obtained directly from terrestrial fauna and aquatic organisms, the latter comprising marine fish from El Collado and Cueva de Nerja, and freshwater fish previously published from Pont d'Ambon [39]. The advantage of this model is that it excludes any variability in the δ13C between marine and freshwater fish. This is particularly relevant here considering we know from the δ13CAA measurements that freshwater or brackish fish were likely to have been an important source of dietary protein. Using this model (figure 5), we estimate that the aquatic protein contribution to total dietary protein could have been as high as 80% for some of the individuals at El Collado (electronic supplementary material, table S3). The estimates provided by the mixing model show relatively high uncertainty compared to previous studies [40] since the model only relies on only δ15N values of just two amino acids. Nevertheless, the compound-specific approach used here provides greater accuracy, as a result of the limited number of assumptions made, and overall brings dietary variability across the assemblage into much sharper relief compared to previous studies that considered only the bulk stable isotope values (figure 2a) [29].
Figure 5.
Estimated aquatic protein contribution in the individuals from El Collado using a Bayesian mixing model applied to δ15Nphe and δ15Nglu values. Individuals are organized chronologically from early to late phases of the Mesolithic sequence (9475–8408 cal BP) following [41]. Boxes represent a 68% credible interval (corresponding to the 16th and 84th percentiles) while the whiskers represent a 95% credible interval (corresponding to the 2.5th and 97.5th percentiles). The horizontal continuous line represents the mean while the horizontal discontinuous line represents the median (50th percentile).
(e) . Dietary variability at El Collado
Using either multivariate analysis of the amino acids nitrogen values or by observing the different proxies, there seems to be high variability in consumption of marine and terrestrial protein across the El Collado burial ground, from diets clearly dominated by aquatic foods to those where it was conceivably absent. The collagen signal represents the averaging of diet over many years prior to death although weighted to periods of rapid bone growth during adolescence [65]. The results, therefore, reflect enduring practices related to access to foodstuffs or dietary preferences. Most significantly, the dietary reconstruction using CSIA on El Collado calls for the re-evaluation of the importance of aquatic resources to pre-agricultural diets in the Western Mediterranean Basin. Faunal and bulk collagen stable isotope analysis picture the role of marine resources as occasional and sporadic food supplements to subsistence systems dominated by terrestrial mammals and plants [15–18,21,23,25]. Our study challenges this conventional view by showing that at least in shelf waters of the north-western Mediterranean, brackish coastal environments could have supported subsistence systems strongly dependent on fishing and shellfish exploitation, notably in times of increased resource pressure on land and rapid human population growth of the Late Pleistocene and Early Holocene [66,67].
It is tempting to link this variability to the different tasks performed by individuals within the community during their lifetime and is less consistent with the hypothesis that foods were shared equally across the group, as sometimes envisaged in models of egalitarian societies which have been often drawing on contemporary or historical accounts of hunter–gatherers [53,68]. While such assumptions are highly questionable anyway [69], we also need to consider that El Collado burial ground represents individuals buried over a long period of time, perhaps up to a millennium and at the very least seven centuries [41], providing scope for considerable change in food procurement strategies. There are no clear patterns between diet and burial phase (figure 5; electronic supplementary material, table S1); there is considerable dietary variation both within the earliest group of burials (Coll 4, 3 and 7) and the later ones, perhaps pointing to dynamic food procurement strategies even over relatively short periods. The degree of residential mobility among the group is also unclear but perhaps the cemetery served more than one community primarily located in different areas of the landscape (e.g. coastal lagoon, littoral, forest). Similarly, there are not enough data to suggest sex-based dietary differences, with only two females and perhaps an additional maternal signal partially represented through the diet of the 9-month-old infant (Coll 10), who was probably breastfeeding at the time of death [70,71].
4. Conclusion
The overall aim of this study was to clarify the relative contribution of aquatic resources to the diets of the Mesolithic coastal population at El Collado. Using a CSIA approach applied to collagen amino acids we have highlighted lagoonal aquatic resources, such as demersal fish, were a major source of dietary protein to 4 out of the 11 individuals investigated, and this component was likely to have been non-negligible in all but two individuals. These new findings reflect a strong coastal-oriented economy which included individuals who were routinely involved in the capture and processing of fish and presumably shellfish, and who may have possessed the knowledge and the technology to occasionally pursue fishing in open marine environments in other locations. We argue against the notion that aquatic foods served only as a fall-back or seasonal resource, although this may have been the case in some instances. For some individuals, the contribution of marine resources to diet approaches the level observed among Mesolithic hunter–gatherers of the Atlantic coast, implying that any differences in productivity or inter-tidal harvestable biomass did not have a major impact on diet, although specific fishing and harvesting practices might have varied between regions. This study rekindles a longstanding debate regarding the origin and changing nature of coastal subsistence strategies in the Mediterranean. Our data show that the region was, at least, capable of supporting ‘marine’ adapted coastal groups in the Mesolithic period with similar diets to those described for the European Atlantic façade [72–74].
The study also highlights the advantage of deploying compound-specific isotope analysis in Mediterranean contexts, especially when the bulk collagen δ15N fails to adequately discriminate between aquatic and terrestrial consumers. By using other proxies from single amino acids, we were able to circumvent this problem, identify additional dietary sources and more effectively quantify the degree of aquatic intake. Missing sources, such as terrestrial plants and shellfish, prevent accurate quantification, but by relying on source amino acids that only minimally fractionate from through the food chain, this issue is partially mitigated. The data presented here will also be of importance when considering amino acid isotope measurements of humans from the earliest agricultural societies in the Western Mediterranean, to assess the degree of dietary change associated with the introduction of domesticated plants and animals.
Acknowledgements
We thank the Museu de Prehistòria de València, especially their curator Alfred Sanchis, for providing access to El Collado human remains; the Fundación Cueva de Nerja and their curator Luis-Efrén Fernández for access to the fish remains from Cueva de Nerja. We thank Maite García-Collado for the accurate map. Thanks to Geoff Bailey and Nik Haussman for their comments on a draft of this manuscript. We also thank the three referees for their comments that helped improve this article.
Data accessibility
All data generated by the study are present in the paper and reported in the electronic supplementary material [75].
Authors' contributions
M.F.-C.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, validation, visualization, writing—original draft, writing—review and editing; S.S.: data curation, formal analysis, investigation, methodology, writing—original draft, writing—review and editing; H.M.T.: data curation, formal analysis, writing—review and editing; M.v.T.: data curation, formal analysis, writing—review and editing; J.F.G.: investigation, writing—review and editing; A.C.C.: investigation, writing—review and editing; O.E.C.: conceptualization, data curation, formal analysis, supervision, validation, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This project was funded by the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement NEOMEDIS: No. 792130. Additional support was provided by: 'Tools, Techniques and Specialists: the keys to understanding the Mesolithic-Neolithic transition in Mediterranean Europe (Tool & Tech)' (PID2020-112513RB-I00); by the European Research Advanced Grant ANCESTORS (ERC-2019-ADG Grant agreement ID 885137); the 'María de Maetzu' Programme for Units of Excellence of the Spanish Ministry of Science and Innovation (CEX2019-000940-M); and the Ministry of Science and Innovation and 'Agencia Estatal de Investigación' of Spain: PID2020-112513RB-I00.
References
- 1.Erlandson JM. 2001. The archaeology of aquatic adaptations: paradigms for a new millennium. J. Archaeol. Res. 9, 287-350. ( 10.1023/a:1013062712695) [DOI] [Google Scholar]
- 2.Bailey G. 2004. World prehistory from the margins: the role of coastlines in human evolution. J. Interdiscip. Hist. Archaeol. 1, 39-50. [Google Scholar]
- 3.Bailey GN, Flemming NC. 2008. Archaeology of the continental shelf: marine resources, submerged landscapes and underwater archaeology. Quat. Sci. Rev. 27, 2153-2165. ( 10.1016/j.quascirev.2008.08.012) [DOI] [Google Scholar]
- 4.Erlandson JM, Braje TJ. 2011. From Asia to the Americas by boat? Paleogeography, paleoecology, and stemmed points of the northwest Pacific. Quat. Int. 239, 28-37. ( 10.1016/J.QUAINT.2011.02.030) [DOI] [Google Scholar]
- 5.Toso A, et al. 2021. Fishing intensification as response to Late Holocene socio-ecological instability in southeastern South America. Sci. Rep. 11, 23506. ( 10.1038/s41598-021-02888-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis JP, et al. 2020. Marine resource abundance drove pre-agricultural population increase in Stone Age Scandinavia. Nat. Commun. 11, 2006. ( 10.1038/s41467-020-15621-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moseley ME. 1974. The maritime foundations of andean civilization. San Francisco, CA: Benjamin-Cummings Publishing Company. [Google Scholar]
- 8.Zilhão J. 2001. Radiocarbon evidence for maritime pioneer colonization at the origins of farming in west Mediterranean Europe. Proc. Natl Acad. Sci. USA 98, 14 180-14 185. ( 10.1073/pnas.241522898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma T, Rolett BV, Zheng Z, Zong Y. 2020. Holocene coastal evolution preceded the expansion of paddy field rice farming. Proc. Natl Acad. Sci. USA 117, 24 138-24 143. ( 10.1073/pnas.1919217117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stothert KE, Piperno DR, Andres TC. 2002. New evidence of early holocene agriculture from the Coast of Ecuador: a multidisciplinary approach. Cult. Agric. 24, 31-41. ( 10.1525/cag.2002.24.2.31) [DOI] [Google Scholar]
- 11.Stiner MC, Munro ND. 2011. On the evolution of diet and landscape during the Upper Paleolithic through Mesolithic at Franchthi Cave (Peloponnese, Greece). J. Hum. Evol. 60, 618-636. ( 10.1016/j.jhevol.2010.12.005) [DOI] [PubMed] [Google Scholar]
- 12.Enghoff IB. 1994. Fishing in Denmark during the Ertebølle periodxs. Int. J. Osteoarchaeol. 4, 65-96. ( 10.1002/oa.1390040203) [DOI] [Google Scholar]
- 13.Fa D. 2008. Effects of tidal amplitude on intertidal resource availability and dispersal pressure in prehistoric human coastal populations: the Mediterranean–Atlantic transition. Quat. Sci. Rev. 27, 2194-2209. ( 10.1016/j.quascirev.2008.07.015) [DOI] [Google Scholar]
- 14.Rainsford C, O'Connor T, Miracle P. 2014. Fishing in the Adriatic at the Mesolithic–Neolithic transition: evidence from Vela Spila, Croatia. Environ. Archaeol. 19, 311-320. ( 10.1179/1749631414Y.0000000018) [DOI] [Google Scholar]
- 15.Colonese AC, et al. 2018. Late Pleistocene-Holocene coastal adaptation in central Mediterranean: snapshots from Grotta d'Oriente (NW Sicily). Quat. Int. 493, 114-126. ( 10.1016/j.quaint.2018.06.018) [DOI] [Google Scholar]
- 16.Colonese AC, Mannino MA, Bar-Yosef Mayer DE, Fa DA, Finlayson JC, Lubell D, Stiner MC. 2011. Marine mollusc exploitation in Mediterranean prehistory: an overview. Quat. Int. 239, 86-103. ( 10.1016/j.quaint.2010.09.001) [DOI] [Google Scholar]
- 17.Fernández-López de Pablo J, Gabriel S. 2016. El Collado shell midden and the exploitation patterns of littoral resources during the Mesolithic in the Eastern Iberian Peninsula. Quat. Int. 407, 106-117. ( 10.1016/j.quaint.2015.11.100) [DOI] [Google Scholar]
- 18.Mannino MA, Thomas KD, Leng MJ, Di Salvo R, Richards MP. 2011. Stuck to the shore? Investigating prehistoric hunter-gatherer subsistence, mobility and territoriality in a Mediterranean coastal landscape through isotope analyses on marine mollusc shell carbonates and human bone collagen. Quat. Int. 244, 88-104. ( 10.1016/j.quaint.2011.05.044) [DOI] [Google Scholar]
- 19.Mannino MA, et al. 2012. Origin and diet of the prehistoric hunter-gatherers on the mediterranean island of Favignana (Ègadi Islands, Sicily). PLoS ONE 7, e49802. ( 10.1371/journal.pone.0049802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richards MP, Hedges REM. 1999. Stable isotope evidence for similarities in the types of marine foods used by Late Mesolithic humans at sites along the Atlantic Coast of Europe. J. Archaeol. Sci. 26, 717-722. ( 10.1006/jasc.1998.0387) [DOI] [Google Scholar]
- 21.Salazar-García DC, Aura JE, Olària CR, Talamo S, Morales JV, Richards MP. 2014. Isotope evidence for the use of marine resources in the Eastern Iberian Mesolithic. J. Archaeol. Sci. 42, 231-240. ( 10.1016/j.jas.2013.11.006) [DOI] [Google Scholar]
- 22.Mannino MA, et al. 2015. Climate-driven environmental changes around 8,200 years ago favoured increases in cetacean strandings and Mediterranean hunter-gatherers exploited them. Sci. Rep. 5, 1-12. ( 10.1038/srep16288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cristiani E, et al. 2018. Dental calculus and isotopes provide direct evidence of fish and plant consumption in Mesolithic Mediterranean. Sci. Rep. 8, 8147. ( 10.1038/s41598-018-26045-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bocherens H. 1999. Etude biochimique du squelette prénéolithique de l'abri d'Araguina-Sennola (Corse): Résultats préliminaires sur la conservation du collagène et première estimation de la position trophique de l'individu. SRA de Corse, p. 5. [Google Scholar]
- 25.Francalacci P. 1988. Comparison of archaeological, trace element, and stable isotope data from two Italian coastal sites. Riv. Antropol. 66, 239-250. [Google Scholar]
- 26.Paine C, O'Connell T, Miracle PT. 2009. Stable isotopic reconstruction of Early Mesolithic diet at Pupićina Cave. In Mesolithic horizons (eds McCartan S, Schulting R, Warren G, Woodman R), pp. 210-216. Oxford, UK: Oxbow Books. [Google Scholar]
- 27.Lightfoot E, Boneva B, Miracle PT, Šlaus M, O'Connell TC. 2011. Exploring the Mesolithic and Neolithic transition in Croatia through isotopic investigations. Antiquity 85, 73-86. ( 10.1017/S0003598X00067442) [DOI] [Google Scholar]
- 28.Goude G, Willmes M, Wood R, Courtaud P, Leandri F, Cesari J, Grün R. 2017. New insights into Mesolithic human diet in the Mediterranean from stable isotope analysis: the sites of campu stefanu and Torre d'Aquila. Corsica. Int. J. Osteoarchaeol. 27, 707-714. ( 10.1002/oa.2578) [DOI] [Google Scholar]
- 29.Garcia-Guixé E, Richards MP, Subirà ME. 2006. Palaeodiets of humans and fauna at the Spanish Mesolithic site of El Collado. Curr. Anthropol. 47, 549-557. ( 10.1086/504170) [DOI] [Google Scholar]
- 30.Pettitt PB, Richards M, Maggi R, Formicola V. 2003. The Gravettian burial known as the Prince (Il Principe): new evidence for his age and diet. Antiquity 77, 15-19. ( 10.1017/S0003598X00061305) [DOI] [Google Scholar]
- 31.Lightfoot E, Miracle PT, Radić D, Šlaus M, O'Connell TC. 2010. Stable isotope analysis of human diets during the mesolithic and neolithic periods at Vela Spila Cave, Korčula. Izdanja HAD 26, 19-25. [Google Scholar]
- 32.Milner N, Craig OE, Bailey GN, Pedersen K, Andersen SH. 2004. Something fishy in the Neolithic? A re-evaluation of stable isotope analysis of Mesolithic and Neolithic coastal populations. Antiquity 78, 9-22. ( 10.1017/S0003598X00092887) [DOI] [Google Scholar]
- 33.Jim S, Jones V, Ambrose SH, Evershed RP. 2006. Quantifying dietary macronutrient sources of carbon for bone collagen biosynthesis using natural abundance stable carbon isotope analysis. Br. J. Nutr. 95, 1055-1062. ( 10.1079/bjn20051685) [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Guixé E, Subirà ME, Marlasca R, Richards MP. 2010. δ13C and δ15N in ancient and recent fish bones from the Mediterranean Sea. J. Nordic Archaeol. Sci. 17, 83-92. [Google Scholar]
- 35.Lelli R, et al. 2012. Examining dietary variability of the earliest farmers of South-Eastern Italy. Am. J. Phys. Anthropol. 149, 380-390. ( 10.1002/ajpa.22134) [DOI] [PubMed] [Google Scholar]
- 36.Jaouen K, Richards MP, Le Cabec A, Welker F, Rendu W, Hublin J-J, Soressi M, Talamo S. 2019. Exceptionally high δ 15 N values in collagen single amino acids confirm Neandertals as high-trophic level carnivores. Proc. Natl Acad. Sci. USA 116, 4928-4933. ( 10.1073/pnas.1814087116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Commendador AS, Finney BP, Fuller BT, Tromp M, Dudgeon JV. 2019. Multiproxy isotopic analyses of human skeletal material from Rapa Nui: evaluating the evidence from carbonates, bulk collagen, and amino acids. Am. J. Phys. Anth. 169, 714-729. ( 10.1002/ajpa.23851) [DOI] [PubMed] [Google Scholar]
- 38.Ohkouchi N, et al. 2017. Advances in the application of amino acid nitrogen isotopic analysis in ecological and biogeochemical studies. Org. Geochem. 113, 150-174. ( 10.1016/j.orggeochem.2017.07.009) [DOI] [Google Scholar]
- 39.Naito YI, Chikaraishi Y, Ohkouchi N, Drucker DG, Bocherens H. 2013. Nitrogen isotopic composition of collagen amino acids as an indicator of aquatic resource consumption: insights from Mesolithic and Epipalaeolithic archaeological sites in France. World Archaeol. 45, 338-359. ( 10.1080/00438243.2013.820650) [DOI] [Google Scholar]
- 40.Soncin S, et al. 2021. High-resolution dietary reconstruction of victims of the 79 CE Vesuvius eruption at Herculaneum by compound-specific isotope analysis. Sci. Adv. 7, 1-9. ( 10.1126/sciadv.abg5791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gibaja JF, Eulàlia Subirà M, Terradas X, Javier Santos F, Agulló L, Gómez-Martínez I, Allièse F, Fernández-López de Pablo J. 2015. The Emergence of Mesolithic Cemeteries in SW Europe: insights from the El Collado (Oliva, Valencia, Spain) Radiocarbon Record. PLoS ONE 10, 1-18. ( 10.1371/journal.pone.0115505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aparicio J. 2008. La necrópolis mesolítica de El collado (oliva-valencia). varia VIII. València, Spain: Diputación provincial de Valencia. [Google Scholar]
- 43.Viñals MJ, Fumanal MP. 1995. Quaternary development and evolution of the sedimentary environments in the Central Mediterranean Spanish coast. Quat. Int. 29–30, 119-128. ( 10.1016/1040-6182(95)00014-A) [DOI] [Google Scholar]
- 44.Pérez-Pérez A, Chimenos E, Lalueza C, Mercadal O. 1995. Human remains from the mesolithic site of El Collado (Oliva, Valencia, Spain). HOMO- J. Comp. Hum. Biol. 45, 243-256. [Google Scholar]
- 45.Mays S, Cox M. 2000. Sex determination in skeletal remains. In Human osteology in archaeology and forensic science (eds Cox M, Mays S), pp. 117-130. London, UK: Greenwich Medical Media. [Google Scholar]
- 46.Scheuer L, Black S. 2000. Development and ageing of the juvenile skeleton. In Human osteology: In archaeology and forensic science (eds Cox M, Mays S), pp. 9-22. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 47.Cox M. 2000. Ageing adults from the skeleton. In Human osteology in archaeology and forensic science (eds Cox M, Mays S), pp. 61-82. London, UK: Greenwich Medical Media. [Google Scholar]
- 48.Brown TA, Nelson DE, Vogel JS, Southon JR. 1988. Improved collagen extraction by modified Longin method. Radiocarbon 30, 171-177. ( 10.1017/s0033822200044118) [DOI] [Google Scholar]
- 49.Kragten J. 1994. Tutorial review. Calculating standard deviations and confidence intervals with a universally applicable spreadsheet technique. Analyst. 119, 2161. ( 10.1039/an9941902161) [DOI] [Google Scholar]
- 50.Philben M, Billings SA, Edwards KA, Podrebarac FA, van Biesen G, Ziegler SE. 2018. Amino acid δ15N indicates lack of N isotope fractionation during soil organic nitrogen decomposition. Biogeochemistry 138, 69-83. ( 10.1007/s10533-018-0429-y) [DOI] [Google Scholar]
- 51.Docherty G, Jones V, Evershed RP. 2001. Practical and theoretical considerations in the gas chromatography/combustion/isotope ratio mass spectrometry δ13 C analysis of small polyfunctional compounds. Rapid Commun. Mass Spectrom. 15, 730-738. ( 10.1002/rcm.270) [DOI] [PubMed] [Google Scholar]
- 52.Schulting RJ, MacDonald R, Richards MP. In press. FRUITS of the sea? A cautionary tale regarding Bayesian modelling of palaeodiets using stable isotope data. Quat. Int. ( 10.1016/j.quaint.2022.02.012) [DOI] [Google Scholar]
- 53.Kelly RL. 2013. The lifeways of hunter-gatherers: The foraging spectrum, 382 p. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 54.Naito YI, Honch NV, Chikaraishi Y, Ohkouchi N, Yoneda M. 2010. Quantitative evaluation of marine protein contribution in ancient diets based on nitrogen isotope ratios of individual amino acids in bone collagen: an investigation at the Kitakogane Jomon site. Am. J. Phys. Anthropol. 143, 31-40. ( 10.1002/ajpa.21287) [DOI] [PubMed] [Google Scholar]
- 55.O'Connell TC, Collins MJ. 2018. Comment on ‘Ecological niche of Neanderthals from Spy Cave revealed by nitrogen isotopes of individual amino acids in collagen’ [J. Hum. Evol. 93 (2016) 82–90]. J. Hum. Evol. 117, 53-55. ( 10.1016/j.jhevol.2017.05.006) [DOI] [PubMed] [Google Scholar]
- 56.Mulville J, Madgwick R, Stevens R, O'Connell T, Craig O, Powell A, Sharples N, Pearson MP. 2009. Isotopic analysis of faunal material from South Uist, Western Isles, Scotland. J. N. Atl. 2, 51-59. ( 10.3721/037.002.0106) [DOI] [Google Scholar]
- 57.Maida GD, Di Maida G, Mannino MA, Krause-Kyora B, Jensen TZT, Talamo S. 2019. Radiocarbon dating and isotope analysis on the purported Aurignacian skeletal remains from Fontana Nuova (Ragusa, Italy). PLoS ONE 14, e0213173. ( 10.1371/journal.pone.0213173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramirez MD, Besser AC, Newsome SD, McMahon KW. 2021. Meta-analysis of primary producer amino acid δ 15 N values and their influence on trophic position estimation. Methods Ecol. Evol. 12, 1750-1767. ( 10.1111/2041-210x.13678) [DOI] [Google Scholar]
- 59.Bodinier C, Sucré E, Lecurieux-Belfond L, Blondeau-Bidet E, Charmantier G. 2010. Ontogeny of osmoregulation and salinity tolerance in the gilthead sea bream Sparus aurata. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 157, 220-228. ( 10.1016/j.cbpa.2010.06.185) [DOI] [PubMed] [Google Scholar]
- 60.Honch NV, McCullagh JSO, Hedges REM. 2012. Variation of bone collagen amino acid δ13c values in archaeological humans and fauna with different dietary regimes: developing frameworks of dietary discrimination. Am. J. Phys. Anthropol. 148, 495-511. ( 10.1002/ajpa.22065) [DOI] [PubMed] [Google Scholar]
- 61.Webb EC, Honch NV, Dunn PJH, Linderholm A, Eriksson G, Lidén K, Evershed RP. 2018. Compound-specific amino acid isotopic proxies for distinguishing between terrestrial and aquatic resource consumption. Archaeol. Anthropol. Sci. 10, 1-18. ( 10.1007/s12520-015-0309-5) [DOI] [Google Scholar]
- 62.Larsen T, Ventura M, Andersen N, O'Brien DM, Piatkowski U, McCarthy MD. 2013. Tracing carbon sources through aquatic and terrestrial food webs using amino acid stable isotope fingerprinting. PLoS ONE 8, e73441. ( 10.1371/journal.pone.0073441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fuller BT, Petzke KJ. 2017. The dietary protein paradox and threonine15N-depletion: Pyridoxal-5′-phosphate enzyme activity as a mechanism for the δ15N trophic level effect. Rapid Commun. Mass Spectrom. 31, 705-718. ( 10.1002/rcm.7835) [DOI] [PubMed] [Google Scholar]
- 64.O'Connell TC. 2017. ‘Trophic’ and ‘source’ amino acids in trophic estimation: a likely metabolic explanation. Oecologia 184, 317-326. ( 10.1007/s00442-017-3881-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hedges REM, Clement JG, Thomas CDL, O'Connell TC. 2007. Collagen turnover in the adult femoral mid-shaft: Modelled from anthropogenic radiocarbon tracer measurements. Am. J. Phys. Anthropol. 133, 808-816. ( 10.1002/ajpa.20598) [DOI] [PubMed] [Google Scholar]
- 66.Fernández-López de Pablo J, Gutiérrez-Roig M, Gómez-Puche M, McLaughlin R, Silva F, Lozano S. 2019. Palaeodemographic modelling supports a population bottleneck during the Pleistocene-Holocene transition in Iberia. Nat. Commun. 10, 1872. ( 10.1038/s41467-019-09833-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dias R, Detry C, Bicho N. 2016. Changes in the exploitation dynamics of small terrestrial vertebrates and fish during the Pleistocene–Holocene transition in the SW Iberian Peninsula: a review. Holocene 26, 964-984. ( 10.1177/0959683615622547) [DOI] [Google Scholar]
- 68.Lee RB, DeVore I.. 2017. Man the hunter. Abingdon, UK: Routledge. [Google Scholar]
- 69.Speth JD. 1990. Seasonality, resource stress, and food sharing in so-called ‘egalitarian’ foraging societies. J. Anthropol. Archaeol. 9, 148-188. ( 10.1016/0278-4165(90)90002-u) [DOI] [Google Scholar]
- 70.Katzenberg MA, Herring DA. 1996. Weaning and infant mortality: evaluating the skeletal evidence. Am. J. Phys. Anthropol. 39, 177-199. [Google Scholar]
- 71.Balasse M, Bocherens H, Mariotti A, Ambrose SH. 2001. Detection of dietary changes by intra-tooth carbon and nitrogen isotopic analysis: an experimental study of dentine collagen of cattle (Bos taurus). J. Archaeol. Sci. 28, 235-245. [Google Scholar]
- 72.Richards MP, Mellars PA. 1998. Stable isotopes and the seasonality of the Oronsay middens. Antiquity 72, 178-184. ( 10.1017/S0003598X00086373) [DOI] [Google Scholar]
- 73.Schulting RJ, Richards MP. 2001. Dating women and becoming farmers: new palaeodietary and AMS dating evidence from the breton mesolithic cemeteries of Téviec and Hoëdic. J. Anthropol. Archaeol. 20, 314-344. ( 10.1006/jaar.2000.0370) [DOI] [Google Scholar]
- 74.Fontanals-Coll M, Subirà ME, Marín-Moratalla N, Ruiz J, Gibaja JF. 2014. From Sado Valley to Europe: Mesolithic dietary practices through different geographic distributions. J. Archaeol. Sci. 50, 539-550. ( 10.1016/j.jas.2014.07.028) [DOI] [Google Scholar]
- 75.Fontanals-Coll M, Soncin S, Talbot HM, von Tersch M, Gibaja JF, Colonese AC, Craig OE. 2023. Stable isotope analyses of amino acids reveal the importance of aquatic resources to Mediterranean coastal hunter-gatherers. Figshare. ( 10.6084/m9.figshare.c.6414092) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Fontanals-Coll M, Soncin S, Talbot HM, von Tersch M, Gibaja JF, Colonese AC, Craig OE. 2023. Stable isotope analyses of amino acids reveal the importance of aquatic resources to Mediterranean coastal hunter-gatherers. Figshare. ( 10.6084/m9.figshare.c.6414092) [DOI] [PMC free article] [PubMed]
Data Availability Statement
All data generated by the study are present in the paper and reported in the electronic supplementary material [75].