Abstract
In multiple sclerosis (MS), a subset of chronic active white matter lesions are identifiable on MRI by their paramagnetic rims, and increasing evidence supports their association with clinical disease severity. Here we study their potential role in differential diagnosis, screening an international multicenter clinical research-based sample of 438 individuals affected by different neurological conditions (MS, other inflammatory, infectious, and non-inflammatory). Paramagnetic rim lesion detection, rarely occurring in other neurological conditions (52% of MS vs 7% of non-MS cases), yielded high specificity (93%) in differentiating MS from non-MS. Future prospective multicenter studies should further validate its role as a diagnostic biomarker.
Keywords: susceptibility-based MRI, paramagnetic rims, multiple sclerosis, differential diagnosis
Introduction
Improving diagnostic accuracy is a major clinical need in multiple sclerosis (MS).1 This is especially critical in the current therapeutic era, when some treatments are highly immunosuppressive and potentially associated with substantial morbidity. In an effort to reduce misdiagnosis, which may be on the order of 20%,2, 3 new and pathologically specific imaging biomarker discovery has garnered renewed attention. Two such markers have been identified on susceptibility-based MRI at 7- and 3-tesla in recent years: the central vein sign (CVS) and paramagnetic rim lesions (PRL) [Figure 1A]. The specificity of the CVS4–7 arises as a result of tissue remodeling following disruption of the blood-brain barrier in post-capillary venules at the time of lesion onset,8 whereas PRL reflect perilesional chronic inflammation, in particular residual and detrimental iron-laden microglia/macrophage accumulation at the lesion edge after acute inflammation subsides (Figure 1B).9, 10 Consistent with neuropathological findings,11, 12 we recently reported a high prevalence of PRL in MS (>50% of MS cases harbor at least one PRL), as well as an association with clinical disability.13 However, unlike the CVS, little is known about whether the PRL is specific to MS.
Figure 1. (A) Typical susceptibility-based MRI features of MS vs non-MS lesions.
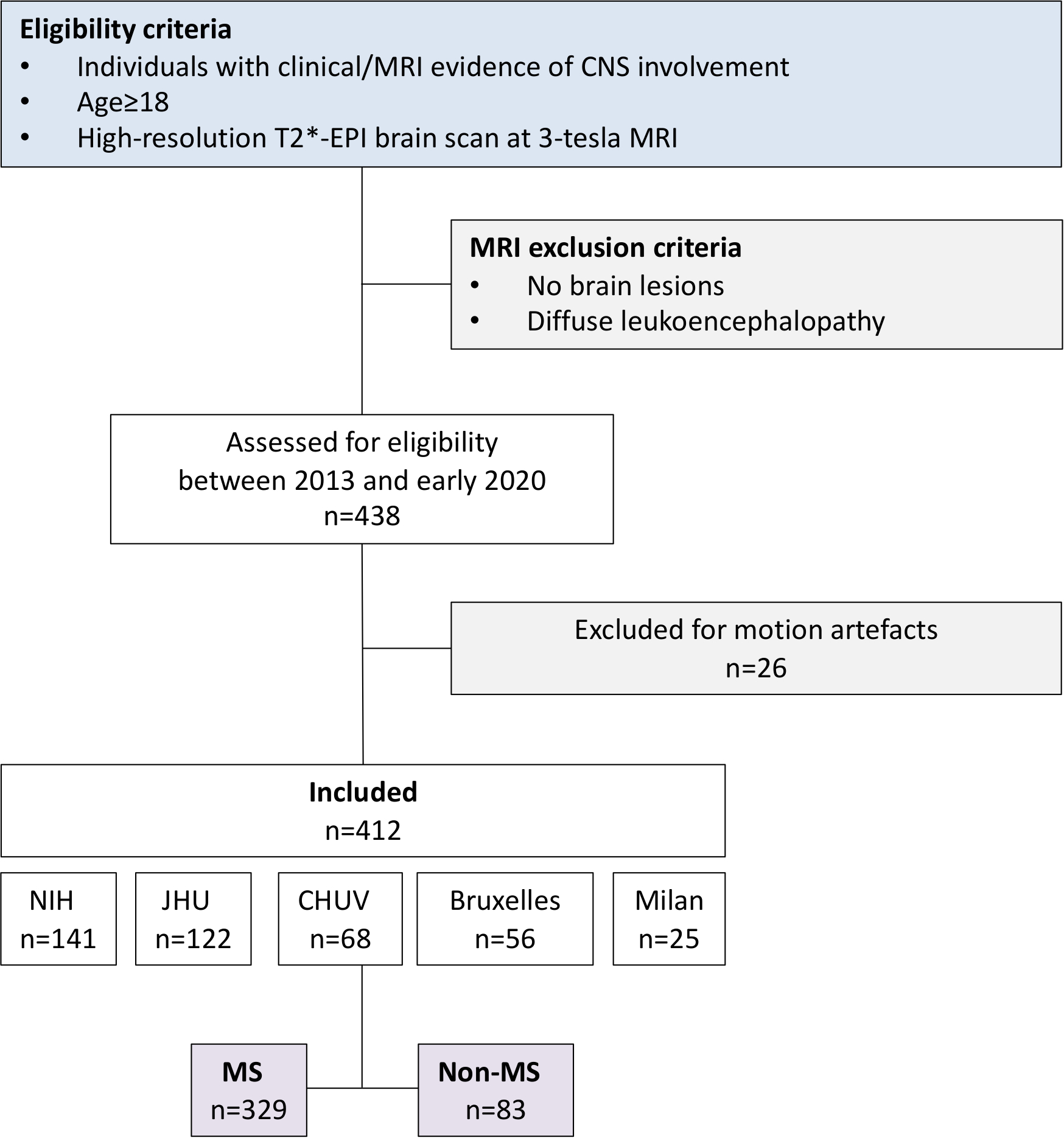
Representative axial T2* magnitude and phase 3T MR images from individuals with relapsing-remitting multiple sclerosis and sarcoidosis. Whereas MS lesions are generally centered around veins (arrows) and have a hypointense paramagnetic rim (arrowhead), these same features are not typical of non-MS lesions.
(B) Representative histopathology of a chronic active rim MS lesion
Fluorescent immunostaining of a chronic active lesion in a 58-year old man with secondary progressive MS (31 years of disease; autopsy performed as part of a research program). A dense inflammatory infiltrate of iron-laden (FTL) microglia/macrophages (CD68) characterizes the edge of chronic active/rim lesions in MS and is visible as a paramagnetic rim on susceptibility-based MRI. Chronic active/rim lesions are completely demyelinated without signs of remyelination, as seen with both MBP and PLP myelin staining. Bar scale= 50 μm.
(C-G) Frequency of paramagnetic rim lesions and perivenular lesions in non-MS cases
(C, D) Distribution of the frequency of PRL (C) and perivenular lesions (D) in the 83 non-MS cases enrolled in the study. Overlapping colored regions refer to the frequency of the same MRI variable in the MS cohort.
(E) Identification of cases with at least one PRL and ≥40% perivenular lesions. Only a single case of HAM/TSP satisfied both criteria [MRI images are shown in (F)], and this may be an individual with both diagnoses.
(F) Susceptibility-based MRI images of a 49-year-old woman with HAM/TSP, disease duration 10 years, who required two walking aids to walk ~20m without resting. In the inset, 3 of her 4 suprantentorial lesions show some MS-like features including the central vein sign on T2* magnitude images and/or a PRL on phase images.
(G) Axial MRI images of a 36-year-old man with Susac disease [cases highlighted in (C)] showing a lesion within the corpus callosum with a rim on T2* images.
Abbreviations: MS: multiple sclerosis; FTL: ferritin; MBP: myelin basic protein; PLP: myelin proteolipid protein; PRL: paramagnetic rim lesions; NMOSD: neuromyelitis optica spectrum disorder; SLE: systemic lupus erythematosus; PACNS: primary angiitis of the central nervous system; APS: antiphospholipid syndrome; HIV: human immunodeficiency virus; HAM/TSP: HTLV associated myelopathy/ tropical spastic paraparesis; NIND: non-inflammatory neurological diseases.
Here, we retrospectively evaluated the prevalence and MS-specificity of PRL on 3-tesla susceptibility-based MR images in MS (n=329) vs non-MS cases (n=83) in a multicenter sample drawn from 5 academic research hospitals at sites in Europe and the United States. We report the prevalence of PRL in the largest multicenter MS cohort to date, as well as the diagnostic specificity of PRL (alone and in combination with the CVS) in MS vs non-MS neurological diseases (both inflammatory and non-inflammatory) and neurotropic viral infections.
Patients and Methods
From 2013 to early 2020, imaging, laboratory, and clinical data were prospectively collected under institutional review board-approved protocols in 438 adult individuals with clinical/MRI evidence of CNS involvement from 5 academic research hospitals: the NIH Clinical Center (Bethesda, MD, USA), Johns Hopkins University Hospital (Baltimore, MD, USA), San Raffaele Hospital (Milan, Italy), Erasme University Hospital and Saint Luc University Hospital (Brussels, Belgium), and Lausanne University Hospital (Lausanne, Switzerland). The study received approval from an ethical standards committee on human experimentation in each of the five academic research hospitals, and written informed consent was obtained from all participants.
Patient eligibility criteria encompassed: 1) age ≥18 years, 2) clinical/MRI evidence of CNS involvement and availability of a clinical diagnosis, and 3) availability of 3-tesla 3D segmented T2*-weighted echo-planar-imaging (EPI) brain images. Cases with diffuse leukoencephalopathy, or with no discrete brain lesions, were excluded from the analysis a priori. The CONSORT flowchart is shown in Figure 2.
Figure 2.
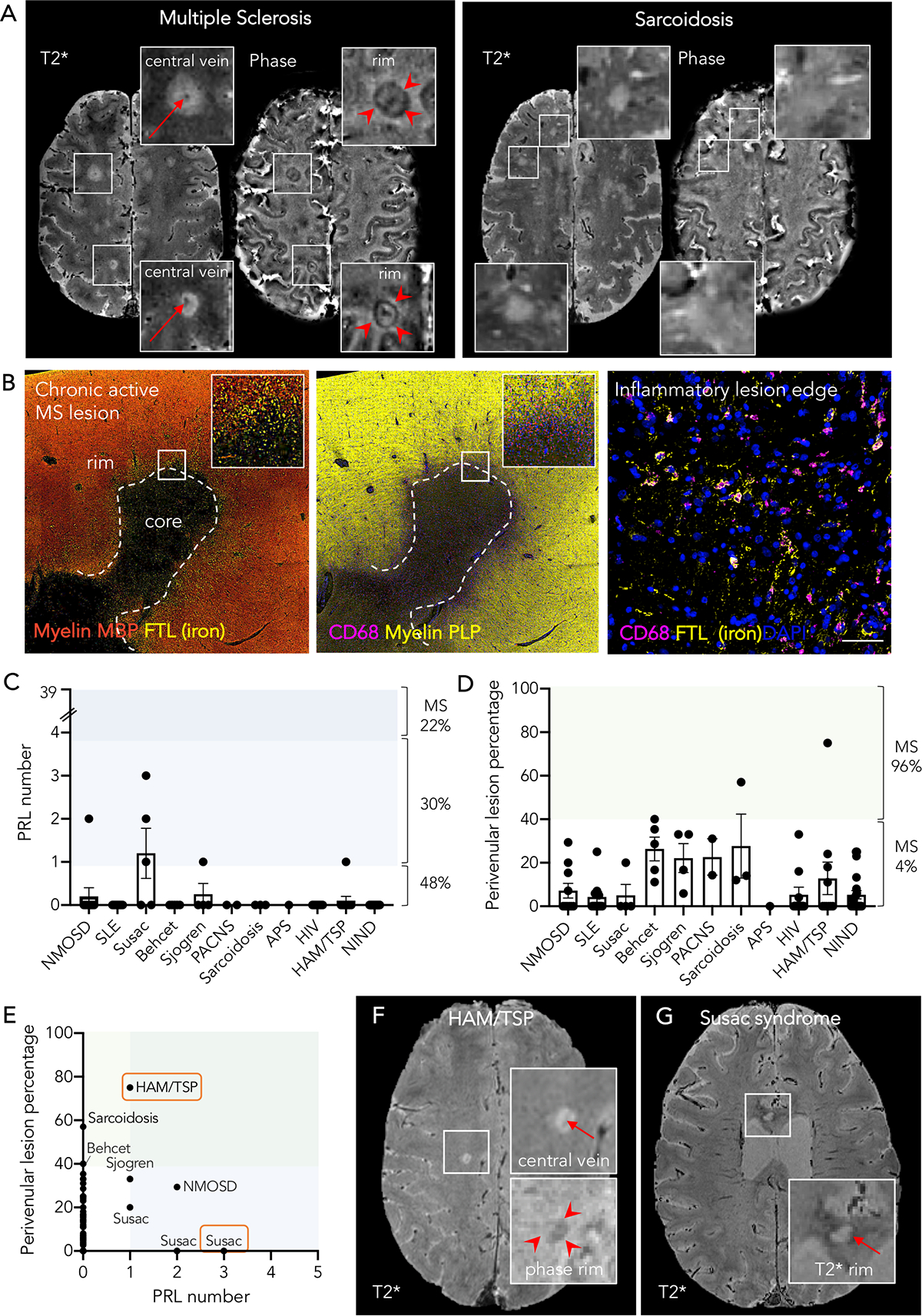
Flow chart summarizing patients’ progress through the study
Site abbreviations:
NIH: National Institutes of Health, Clinical Center (Bethesda, MD, USA)
JHU: Johns Hopkins University Hospital (Baltimore, MD, USA)
CHUV: Lausanne University Hospital (Lausanne, Switzerland)
Bruxelles: Erasme University Hospital and Saint Luc University Hospital (Brussels, Belgium)
Milan: San Raffaele University Hospital (Milan, Italy)
MRI acquisition.
MRI studies were performed on 7 MRI 3-tesla scanners: 4 Philips Intera or Ingenia scanners (Philips Medical Systems, The Netherlands), and 3 Siemens Skyra or Prisma scanners (Siemens AG, Germany). In all scans, 3D T2-FLAIR images and submillimeter isotropic 3D segmented T2*-weighted EPI,14–16 providing both magnitude and phase images, were acquired before or during intravenous injection of a single dose (0.1 mmol/kg) of gadolinium-based contrast material. The same 3D T2*-EPI sequence14–16 was adapted and optimized to the different MRI scanners with minimal parameter modification (Supplementary table). Additional routine MRI images were acquired for clinical use, including postcontrast T1-weighted sequences.
PRL and CVS assessment.
Since raters were partially involved in the recruitment of cases (PM of the European cases; MA and DSR of the US cases), all images were de-identified before analysis, including the final diagnosis. In each individual, the number of supratentorial chronic non-enhancing PRL on unwrapped phase images was determined by consensus of 2 raters (PM and MA). Interrater reliability was tested for all non-MS cases and an equal number of MS cases. All non-MS cases with PRL were further reviewed by a third rater (DSR) for final adjudication. A chronic lesion was defined as a PRL when it showed a hypointense rim on phase images and internal isointensity to extralesional white matter.17 In order to compare the results of this large multicenter MS cohort with our previously published work (single-center study),13 MS cases were further classified based on the number of PRL into 3 groups (0, 1–3, and ≥4 PRL, respectively), as previously described.13
For all non-MS cases and an equal number of randomly selected MS cases, the percentage of CVS lesions was also assessed by consensus of 2 raters (PM and MA), according to the NAIMS guidelines,18 as previously described.6 In details, all cases were equally divided among the two raters (PM and MA) for initial CVS assessment, then each rater reviewed the CVS annotations of the other rater for consensus and final agreement. Interrater reliability was assessed based on the previously described “40%-rule”.4, 6, 19
Statistical analysis.
Demographic, clinical, and MRI differences were assessed with ANOVA and Tukey’s post-hoc multiple-comparison test, with Fisher’s exact or chi-square test, when appropriate. The interrater reliability for PRL and CVS assessment were computed using the Cohen’s κ. The association of PRL number with disability and disease severity scores (Expanded Disability Status Scale [EDSS],20 MS severity score [MSSS],21 global age-related MS severity score [ARMSS]22) was evaluated using Spearman correlation coefficients, since PRL number had a highly skewed distribution (skewness=4.4) and sex, age, and disease duration had no significant effect on PRL number. Clinical phenotype was not considered as a covariate because it was significantly correlated (p<0.0001) with EDSS, MSSS, and ARMSS.
SAS procedure LOGISTIC was used to produce receiver operating characteristic (ROC) curves. In the logistic regression model, diagnosis (MS vs. non-MS) was a dependent variable and PRL (or CVS) an independent variable. The ROC curve is a plot of sensitivity against 1–specificity. The sensitivity and specificity values were obtained by varying the cut-off to dichotomize PRL (or CVS). Youden’s index method was used to obtain the optimal cutoff value for each biomarker.
Results
Of 438 consecutive eligible cases, data from 412 were included in this analysis (26 were excluded for motion-related MRI artifacts) and grouped according to their clinical diagnosis (based on international published diagnostic criteria), as follows:
329 individuals with MS diagnosed according to the 2017 McDonald criteria23, 24 (7 with clinically isolated syndrome, 226 relapsing-remitting, 96 progressive);
- 41 individuals with other inflammatory neurological diseases (OIND), including:
- 11 cases of neuromyelitis optica spectrum disorder (NMOSD):25 9 of them were AQP4 antibody-positive, one MOG antibody-positive, and one seronegative;
- 10 cases of systemic lupus erythematosus (SLE);26
- 5 cases of Susac syndrome;27
- 5 cases of Behçet disease;28
- 4 cases of Sjögren Disease;29
- 3 cases of sarcoidosis;30
- 2 case of primary angiitis of the central nervous system (PACNS, imaging or biopsy-proven);31
- 1 case of antiphospholipid syndrome (APS).32
- 20 individuals infected by neurotropic viruses, randomly selected from larger datasets, including:
- 10 individuals with HTLV-associated myelopathy/tropical spastic paraparesis (HAM/TSP).
- 10 individuals with HIV infection, without other comorbidities, on antiretroviral therapy.
22 individuals affected by non-inflammatory neurological diseases (NIND), including small vessel disease and migraine.
Demographic and clinical data are reported in Table 1. The interrater reliability for PRL and CVS assessment were “substantial/good” with Cohen’s k of 0.79 and 0.86, respectively.
Table 1. Cohort characteristics.
| OIND | NIND | Neurotropic viruses | MS | ||
|---|---|---|---|---|---|
| HTLV-infected HAM/TSP | HIV-infected | ||||
| # of participants | 41 | 22 | 10 | 10 | 329 |
| # of women (%) | 30 (73%) | 16 (73%) | 7 (70%) | 1 (10%) | 198 (60%) |
| Mean age (range) [years] | 45 (21–74) | 51 (26–75) | 57 (37–75) | 56 (45–62) | 45 (20–76) |
| OCB positive (# and %) of cases with CSF available for review | 10/32 (31%) | 0/5 (0%) | 9/9 (100%) | - | 121/151 (80%) |
| Fulfillment of dissemination in space MRI criteria according to McDonald revised MS criteria 2017 | |||||
| # cases (%) | 26/41 (63%) | 6/22 (27%) | 2/10 (20%) | 0/10 (0%) | 329/329 (100%) |
| Paramagnetic rim lesion detection | |||||
| # cases with at least one PRL (%) | 5 (12%) | 0 | 1 (10%) | 0 | all: 172/329 (52%) CIS/RR: 116/233 (50%) Progressive: 56/96 (58%) |
| Perivenular lesion detection | |||||
| Mean perivenular lesion frequency (range) | 12.4% (0–57%) | 5.3% (0–25%) | 12.8% (0–75%) | 5.3% (0–33%) | 75.2% (35–100%) |
| # cases with ≥ 40% perivenular lesions* (%) | 2 (5%) | 0 | 1 (10%) | 0 | 80/83 (96%) |
Cases were dichotomized as overall CVS+ vs CVS- based on the previously described “40% rule”.4, 6, 19
Abbreviations: OIND: other inflammatory/infectious neurological diseases; HTLV: human T-lymphotropic virus; HAM/TSP: HTLV associated myelopathy/ tropical spastic paraparesis; HIV: human immunodeficiency virus; NIND: non-inflammatory neurological diseases; MS: multiple sclerosis; CIS=clinically isolated syndrome; RR=relapsing remitting; OCB: oligoclonal bands; PRL: paramagnetic rim lesion.
In the 412 analyzed scans, PRL were detected in 172/329 (52%) of MS cases vs 6/83 non-MS cases (7%). In MS, 58% of progressive cases had at least one PRL, compared to 50% of relapsing cases. PRL number was positively correlated with all disability and severity scores (EDSS Spearman r=0.17, p=0.002; MSSS r=0.18, p=0.001; global ARMSS r=0.22, p<0.0001, respectively). When previously published PRL categories were implemented,13 cases with ≥4 PRL had higher disability and severity scores, but not significantly longer disease duration or older age (Table 2). In addition, they were 1.8-fold more frequently treated with second-line antibody-based therapies than those without PRL (chi-square p=0.0002).
Table 2. MS cohort characteristics by paramagnetic rim lesion (PRL) category.
| PRL category | No detected PRL | 1–3 PRL | ≥ 4 PRL | Statistical analysis |
|---|---|---|---|---|
| # | 157 (48%) | 99 (30%) | 73 (22%) | - |
| Demographic and clinical characteristics | ||||
| Clinical phenotype | CIS/RR 117 (74%) SP 28 (18%) PP 12 (8%) |
CIS/RR 72 (73%) SP 19 (19%) PP 8 (8%) |
RR 44 (60%) SP 13 (18%) PP 16 (22%) |
Fisher 2×3 p=0.057 n.s. |
| Sex (female, %) | 101 (64%) | 57 (58%) | 40 (55%) | Fisher 2×3 p=0.4 n.s. |
| Mean age, years (SD) | 46.3 (13.0) | 44.1 (1124) | 44.0 (12.6) | ANOVA p=0.3 n.s. |
| Mean disease duration, years (SD) | 12.1 (10) | 11.1 (10) | 11.2 (8) | ANOVA p=0.6 n.s. |
| Median EDSS (range) | 2 (0–8)* | 2 (0–8) | 3 (0–7.5)* | ANOVA p=0.006 |
| Mean MSSS (SD) | 3.3 (2.5)* | 3.8 (2.5) | 4.7 (3.0)* | ANOVA p=0.001 |
| Mean global ARMSS (SD) | 3.7 (2.4)# | 4.3 (2.6)* | 5.3 (2.5)*# | ANOVA p<0.0001 |
| - Antibody-based | 22% | 13% | 40% | |
| Perivenular lesion detection$ | ||||
| Mean perivenular lesion percentage (range) | 75% (35–100%) | 77% (38–100%) | 74% (57–92%) | ANOVA p=0.7 n.s. |
| # of cases with < 40% perivenular lesions (%) | 2/33 (6%) | 1/30 (3%) | 0/20 (0%) | - |
p<0.05, Tukey’s post-hoc multiple-comparison test
p<0.0001, Tukey’s post-hoc multiple-comparison test
Type of treatments: first line-injectables (interferon beta, glatiramer acetate); oral (fingolimod, dimethyl fumarate, teriflunomide), antibody-based (natalizumab, rituximab, ocrelizumab, alemtuzumab).
refers to antibody-based vs all other types of treatment (table 2×3, df=2).
The perivenular lesion detection was assessed in 83 randomly selected MS cases with at least 3 qualifying brain lesions according to the NAIMS CVS detection guidelines.18
Abbreviations: PRL: paramagnetic rim lesion; CIS=clinically isolated syndrome; RR=relapsing remitting; SP= secondary progressive; PP=primary progressive; n.s.: not significant; SD: standard deviation; EDSS: Expanded Disability Status Scale;20 MSSS: MS severity score;21 ARMSS: Age Related Multiple Sclerosis Severity.22
In non-MS cases, PRL were seen in only a few other inflammatory/infectious neurological conditions (Table 1, Figure 1C–G). The highest prevalence of PRL was detected in Susac syndrome (3/5 cases, 60%). Of these, one case had 3 PRL, one had 2 PRL, and one had a single PRL. These were located within the corpus callosum and had signal intensity isointense to cerebrospinal fluid on T1-weighted images, suggesting severe tissue damage (Figure 1G). Sporadically, PRL were seen also in NMOSD (1 AQP4-positive case with 2 PRL), Sjögren disease (1 case with 1 PRL), and HAM/TSP (1 case with 1 PRL; Figure 1F). None of the NIND (including small vessel disease and migraine) or HIV cases had PRL.
Consistent with previous data in literature,4, 6, 7 the frequency of CVS lesions was remarkably high in our MS cohort (mean 75%, median 75%, range 35–100%) and was unrelated to the number of PRL (Table 1). On the other hand, non-MS cases showed very low CVS lesion frequency (mean 9%, median 0%, range 0–75%). Among non-MS, HAM/TSP and OIND showed the highest mean frequency of CVS lesions (13% and 12%, respectively), followed by HIV (5%), and NIND (5%) (Table 1 and Figure 1D).
Of relevance, 34 of 83 (41%) non-MS cases fulfilled the dissemination in space (DIS) MRI criteria for MS, according to the 2017 revised McDonald criteria.24 In this context, the identification of ≥1 PRL (optimal cutoff) was associated with high diagnostic specificity (93%, 95% CI=85%−87%), but relatively low sensitivity (52%, 95% CI=47%−58%) and overall accuracy (area under ROC curve=0.77, 95% CI=0.71–0.83), whereas CVS detection alone (optimal cutoff 35.5–38%) could better discriminate MS from non-MS cases with high specificity (96%, 95% CI=89%−99%), sensitivity (99%, 95% CI=93%−100%), and accuracy (area under ROC curve=0.99, 95% CI=0.98–1). The optimal cutoff for CVS (35.5–38%) is in line with the previously reported “40%-rule”.4,6 The combination of the two biomarkers (fulfillment of both ≥1 PRL and ≥40% CVS) further improved the specificity (99%), but sensitivity remained low (59%). In the subset of non-MS cases with OCB available to review (n=46), no case fully satisfied all 4 criteria (DIS, OCB positivity, CVS >35.5–38%, and PRL ≥1).
Discussion
In this cross-sectional study, we evaluated the prevalence and specificity of PRL on 3-tesla susceptibility-based images, screening a multicenter academic clinical research-based sample of individuals affected by a variety of neurological conditions. Unlike in MS, we found that PRL were only rarely observed in inflammatory/infectious neurological conditions. Furthermore, the absence of PRL in individuals without underlying inflammatory neurological disease provides additional support to the recent notion that PRL in MS indicate perilesional chronic inflammation.9, 10
This 3-tesla study expands on preliminary results from two previous 7-tesla33, 34 and one 3-tesla35 studies in limited non-MS cohorts. In OIND, the highest prevalence of PRL was observed in Susac syndrome, a rare autoimmune disease causing the occlusion of small vessels of the brain, retina, and inner ear.36 In line with its etiopathogenesis, MRI features of corpus callosum lesions in Susac syndrome — CSF-like T1 signal, T2* rim, and no central veins — resemble those seen in lacunar ischemic lesions.33 We can speculate that the origin of the iron in Susac syndrome’s PRL might not be related to perilesional chronic inflammation (accumulation of iron-laden microglia/macrophages), as occurs in MS, but rather to sequelae of the hemoglobin extravasation (heme-iron bioproducts) at the edge of the post-ischemic cavitation. In addition, we had one HAM/TSP case that was an outlier, containing both a high proportion (75%) of CVS lesions and a PRL indistinguishable from those seen in the MS cohort; this individual also met 2017 McDonald Criteria for dissemination in space, and we speculate that both MS and HAM/TSP were present simultaneously (Figure 1F). Aside from these cases, very few non-MS cases, including the relatively frequent NMOSD, had PRL.
Thus, PRL detection might prove a valuable tool in the differential diagnostic work-up, especially for improving diagnostic specificity. Although the overall diagnostic performance of PRL detection alone is inferior to the CVS particularly with respect to sensitivity, our data suggest that the combination of these two imaging biomarkers may help in the diagnostic work-up in two specific scenarios: (1) cases with suspected MS but low frequency of CVS (e.g. due to small vessel disease comorbidity);4, 37, 38 and (2) cases with suspected non-MS but high frequency of CVS lesions.6, 19
The results of this study also strengthen our recently published single-center MRI data on the frequency of PRL and their association with disability in MS,13 expanding the results to what is to date the largest multicenter cohort of MS cases acquired with the same 3T susceptibility-based sequence. We confirm that more than half of MS cases (52%) harbor chronically inflamed lesions despite receiving approved disease-modifying treatments specifically designed to be highly effective in reducing T and/or B lymphocyte-driven inflammation. We also replicated that higher PRL burden can stratify MS cases with significantly higher disability (as assessed by the EDSS)20 and MS severity (accounting for the disease duration [MSSS]21 or for patient age [global ARMSS]22). Since some PRL might fade over time,39 this further corroborates the role of PRL accrual in potentially driving clinical progression at young age and/or relatively short disease duration.13, 40
A major technical advantage of this large multicenter study (438 eligible individuals) is the use of the same 3-tesla EPI MRI sequence14, 15 with minimal parameter adjustments across MS centers and centralized analysis. The high-resolution susceptibility-based sequence implemented here has been shown to provide T2*/FLAIR*14, 15 and phase images for reliable assessment of both CVS6, 19 and PRL.16 The short acquisition time (~5 minutes) makes this sequence compatible with routine clinical scanning.
This work has some limitations, including its retrospective nature and use of a convenience sample (with a relatively low number of non-MS cases in comparison to MS cases and skewness toward relapsing cases [70% of all MS]). Also, it might not reflect real-world diagnosis in a newly presenting population. Although clinical implementation of these diagnostic biomarkers might be limited by the availability of 3-tesla MR scanners, the preference for gadolinium injection to increase the sensitivity of CVS detection (but not necessarily of PRL detection), the current need for manual assessment of CVS and PRL (though automatic detection algorithms41, 42 are being developed), and the lack of a product 3D T2*-EPI sequence on several scanner platforms, it is not difficult to envision these barriers overcome.
In conclusion, PRL yielded high specificity for MS lesions. Future prospective multicenter studies should further validate its role as a diagnostic biomarker.
Supplementary Material
Acknowledgments
The study was partially supported by Intramural Research Program of the NIH/NINDS (to DSR), by NIH/NINDS (grant R01NS082347 to PAC), and by the Conrad N. Hilton Foundation (grant 17313 to MA).
The authors thank the study participants; the neuroimmunology clinics in each center for recruiting, evaluating the patients and for coordinating the scans; and Blake Dewey (Johns Hopkins University), Antonella Iadanza (Milan), Tobias Kober (Advanced Clinical Imaging Technology, Siemens Healthineers, Lausanne), Julie Absil (Erasme University Hospital, Bruxelles), and Jean-Baptiste Ledoux (Lausanne University Hospital) for assistance with 3T MRI scan acquisition. We thank Dragan Maric for helping with the fluorescent multiplex immunostaining of brain MS autopsy tissue (shown in Figure 1).
Footnotes
Potential Conflicts of Interest
Nothing to report.
References
- 1.Solomon AJ, Corboy JR. The tension between early diagnosis and misdiagnosis of multiple sclerosis. Nat Rev Neurol 2017;13:567–572. [DOI] [PubMed] [Google Scholar]
- 2.Solomon AJ, Bourdette DN, Cross AH, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: A multicenter study. Neurology 2016;87:1393–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaisey M, Solomon AJ, Luu M, Giesser BS, Sicotte NL. Incidence of multiple sclerosis misdiagnosis in referrals to two academic centers. Mult Scler Relat Disord 2019;30:51–56. [DOI] [PubMed] [Google Scholar]
- 4.Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology 2011;76:534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mistry N, Dixon J, Tallantyre E, et al. Central veins in brain lesions visualized with high-field magnetic resonance imaging: a pathologically specific diagnostic biomarker for inflammatory demyelination in the brain. JAMA Neurol 2013;70:623–628. [DOI] [PubMed] [Google Scholar]
- 6.Maggi P, Absinta M, Grammatico M, et al. Central vein sign differentiates Multiple Sclerosis from central nervous system inflammatory vasculopathies. Ann Neurol 2018;83:283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinnecker T, Clarke MA, Meier D, et al. Evaluation of the Central Vein Sign as a Diagnostic Imaging Biomarker in Multiple Sclerosis. JAMA Neurol 2019;76(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Absinta M, Nair G, Monaco MCG, et al. The “central vein sign” in inflammatory demyelination: The role of fibrillar collagen type I. Ann Neurol 2019;85:934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest 2016;126:2597–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol 2017;133:25–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frischer JM, Weigand SD, Guo Y, et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann Neurol 2015;78:710–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luchetti S, Fransen NL, van Eden CG, Ramaglia V, Mason M, Huitinga I. Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: a retrospective autopsy cohort analysis. Acta Neuropathol 2018;135:511–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Absinta M, Sati P, Masuzzo F, et al. Association of Chronic Active Multiple Sclerosis Lesions With Disability In Vivo. JAMA Neurol 2019;76:1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sati P, George IC, Shea CD, Gaitan MI, Reich DS. FLAIR*: a combined MR contrast technique for visualizing white matter lesions and parenchymal veins. Radiology 2012;265:926–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sati P, Thomasson DM, Li N, et al. Rapid, high-resolution, whole-brain, susceptibility-based MRI of multiple sclerosis. Mult Scler 2014;20:1464–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Absinta M, Sati P, Fechner A, Schindler MK, Nair G, Reich DS. Identification of Chronic Active Multiple Sclerosis Lesions on 3T MRI. AJNR Am J Neuroradiol 2018;39:1233–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao B, Bagnato F, Matsuura E, et al. Chronic multiple sclerosis lesions: characterization with high-field-strength MR imaging. Radiology 2012;262:206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol 2016;12:714–722. [DOI] [PubMed] [Google Scholar]
- 19.Maggi P, Absinta M, Sati P, et al. The “central vein sign” in patients with diagnostic “red flags” for multiple sclerosis: A prospective multicenter 3T study. Mult Scler 2020;26:421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–1452. [DOI] [PubMed] [Google Scholar]
- 21.Roxburgh RH, Seaman SR, Masterman T, et al. Multiple Sclerosis Severity Score: using disability and disease duration to rate disease severity. Neurology 2005;64:1144–1151. [DOI] [PubMed] [Google Scholar]
- 22.Manouchehrinia A, Westerlind H, Kingwell E, et al. Age Related Multiple Sclerosis Severity Score: Disability ranked by age. Mult Scler 2017;23:1938–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018;17:162–173. [DOI] [PubMed] [Google Scholar]
- 25.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petri M, Orbai AM, Alarcon GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleffner I, Dorr J, Ringelstein M, et al. Diagnostic criteria for Susac syndrome. J Neurol Neurosurg Psychiatry 2016;87:1287–1295. [DOI] [PubMed] [Google Scholar]
- 28.Disease ISGfBs. Criteria for diagnosis of Behcet’s disease. Lancet 1990;335:1078–1080. [PubMed] [Google Scholar]
- 29.Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjogren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol 2017;69:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stern BJ, Royal W 3rd, Gelfand JM, et al. Definition and Consensus Diagnostic Criteria for Neurosarcoidosis: From the Neurosarcoidosis Consortium Consensus Group. JAMA Neurol 2018;75:1546–1553. [DOI] [PubMed] [Google Scholar]
- 31.Schuster S, Bachmann H, Thom V, et al. Subtypes of primary angiitis of the CNS identified by MRI patterns reflect the size of affected vessels. J Neurol Neurosurg Psychiatry 2017;88:749–755. [DOI] [PubMed] [Google Scholar]
- 32.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306. [DOI] [PubMed] [Google Scholar]
- 33.Wuerfel J, Sinnecker T, Ringelstein EB, et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler 2012;18:1592–1599. [DOI] [PubMed] [Google Scholar]
- 34.Sinnecker T, Schumacher S, Mueller K, et al. MRI phase changes in multiple sclerosis vs neuromyelitis optica lesions at 7T. Neurol Neuroimmunol Neuroinflamm 2016;3:e259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clarke MA, Pareto D, Pessini-Ferreira L, et al. Value of 3T Susceptibility-Weighted Imaging in the Diagnosis of Multiple Sclerosis. AJNR Am J Neuroradiol 2020;41(6):1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dorr J, Krautwald S, Wildemann B, et al. Characteristics of Susac syndrome: a review of all reported cases. Nat Rev Neurol 2013;9:307–316. [DOI] [PubMed] [Google Scholar]
- 37.Mistry N, Abdel-Fahim R, Samaraweera A, et al. Imaging central veins in brain lesions with 3-T T2*-weighted magnetic resonance imaging differentiates multiple sclerosis from microangiopathic brain lesions. Mult Scler 2016;22:1289–1296. [DOI] [PubMed] [Google Scholar]
- 38.Guisset F, Lolli V, Bugli C, et al. The central vein sign in MS patients with vascular comorbidities. Mult Scler 2020, Aug 4. 10.1177/1352458520943785 [DOI] [PubMed] [Google Scholar]
- 39.Chen W, Gauthier SA, Gupta A, et al. Quantitative susceptibility mapping of multiple sclerosis lesions at various ages. Radiology 2014;271:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bozin I, Ge Y, Kuchling J, et al. Magnetic Resonance Phase Alterations in Multiple Sclerosis Patients with Short and Long Disease Duration. PLoS One 2015;10:e0128386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dworkin JD, Sati P, Solomon A, et al. Automated Integration of Multimodal MRI for the Probabilistic Detection of the Central Vein Sign in White Matter Lesions. AJNR Am J Neuroradiol 2018;39:1806–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maggi P, Fartaria MJ, Jorge J, et al. CVSnet: A machine learning approach for automated central vein sign assessment in multiple sclerosis. NMR Biomed 2020;33:e4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


