Summary
Spinal muscular atrophy, a leading cause of early infant death, is caused by bi-allelic mutations of SMN1. Sequence analysis of SMN1 is challenging due to high sequence similarity with its paralog SMN2. Both genes have variable copy numbers across populations. Furthermore, without pedigree information, it is currently not possible to identify silent carriers (2+0) with two copies of SMN1 on one chromosome and zero copies on the other. We developed Paraphase, an informatics method that identifies full-length SMN1 and SMN2 haplotypes, determines the gene copy numbers, and calls phased variants using long-read PacBio HiFi data. The SMN1 and SMN2 copy-number calls by Paraphase are highly concordant with orthogonal methods (99.2% for SMN1 and 100% for SMN2). We applied Paraphase to 438 samples across 5 ethnic populations to conduct a population-wide haplotype analysis of these highly homologous genes. We identified major SMN1 and SMN2 haplogroups and characterized their co-segregation through pedigree-based analyses. We identified two SMN1 haplotypes that form a common two-copy SMN1 allele in African populations. Testing positive for these two haplotypes in an individual with two copies of SMN1 gives a silent carrier risk of 88.5%, which is significantly higher than the currently used marker (1.7%–3.0%). Extending beyond simple copy-number testing, Paraphase can detect pathogenic variants and enable potential haplotype-based screening of silent carriers through statistical phasing of haplotypes into alleles. Future analysis of larger population data will allow identification of more diverse haplotypes and genetic markers for silent carriers.
Keywords: spinal muscular atrophy, long-read sequencing, genomics, bioinformatics, carrier screening, segmental duplications, genetic diseases, PacBio HiFi, copy number variations, homologous genes
We developed Paraphase, an informatics method that, combined with highly accurate long reads, can resolve the highly homologous SMN1/SMN2 genes involved in spinal muscular atrophy. We characterized SMN1/SMN2 haplotypes across populations and identified new genetic markers for silent carriers (2+0) with both copies of SMN1 on the same chromosome.
Introduction
Spinal muscular atrophy (SMA) is a neuromuscular disease caused in most cases by bi-allelic mutations of SMN1 (MIM: 600354).1,2,3 SMA is a leading cause of early infant death with an incidence of 1 in 6,000–10,000 live births and a carrier frequency of 1 in 40–80 across ethnic groups.4,5,6,7,8 SMA can be classified into four clinical types (types I–IV [MIM: 253300, 253550, 253400, 271150]) that differ in age of onset and disease severity.1
SMN1 and its paralog SMN2 (MIM: 601627) reside in a highly complex genomic region on chromosomal band 5q13 that is frequently subject to unequal crossing over and gene conversion, resulting in variable copy numbers (CNs) of SMN1 and SMN2.7,9 SMN1 and SMN2 are nearly identical in sequence with just one functionally different base (GenBank: NM_000344.3; c.840C>T). In SMN2, c.840T disrupts a splicing enhancer leading to skipping of exon 710 and, as a result, most SMN2 transcripts are unstable and almost nonfunctional. Since SMN2 can produce a small amount of functional protein, the CN of SMN2 is a modifier of the SMA disease severity.11 The majority (∼96%) of 5q-linked SMA cases are caused by bi-allelic absence of SMN1 c.840C through either large deletions or gene conversion to c.840T, while a smaller percentage (∼4%) are caused by other small pathogenic variants in SMN1 in trans with c.840C loss.8,12,13,14
Because of the high carrier frequency and severity of SMA, the American College of Medical Genetics and Genomics recommends population-wide SMA screening.15 Conventional SMA screening tests use PCR-based methods, such as multiplex ligation-dependent probe amplification (MLPA)16,17 and qPCR,18 to determine the SMN1 dosage (copy number) in exon 7, mostly targeting c.840C>T. To date, a few next-generation sequencing (NGS)-based SMN1 callers have been reported.19,20,21,22 These callers rely on short reads to identify copy-number variations and distinguish SMN1 and SMN2 based on a limited number of differentiating bases centered around c.840C>T. However, dosage testing fails to identify carriers with pathogenic variants other than c.840C>T, which represent ∼1%–2% of all carriers.5 In addition, detecting SMN2 variants in individuals with SMA is also important for understanding the disease-modifying effect.23 Both SMN1 and SMN2 are ∼28 kb long, and detailed sequence analysis of the complete genes is labor intensive for traditional Sanger sequencing and impossible for conventional short-read NGS methods due to the high sequence similarity between the two genes.
Furthermore, current tests (i.e., dosage testing) are unable to accurately phase alleles. Phasing is important to distinguish between individuals carrying the normal SMN1 genes on both alleles (1+1) versus silent carriers (2+0) with two copies of SMN1 on one chromosome and zero copies on the other. Silent carriers account for approximately 3%–9% of carriers in non-African populations and 27% of carriers in African populations.5,6,21 Throughout this paper, we use the term “singleton SMN1 allele” to refer to chromosomes with a single copy of SMN1, and “two-copy SMN1 alleles” to refer to alleles with two copies of SMN1 occurring on the same chromosome. Previous studies have identified the g.27134T>G SNP (GenBank: NG_008691.1; g.32134T>G; rs143838139; GenBank: NM_000344.3; c.∗3+80T>G) as a marker of the two-copy SMN1 allele24 and this SNP is now commonly tested to modify the residual carrier risk, i.e., the probability that an individual with two copies of SMN1 is a carrier. However, this SNP is rare and has low sensitivity in non-African populations. In Africans it is common but it is also present on almost 20% of singleton SMN1 alleles,21 so it does not have a high positive predictive value (PPV). When an African individual with two copies of SMN1 tests positive for g.27134T>G, the residual risk of being a carrier, which is largely the silent carrier risk, is estimated to be just 1.7%–3.0%.20,21,24 More population studies are needed to identify better markers to detect two-copy SMN1 alleles, but again, short-read based methods suffer from the difficulty to differentiate SMN1 from SMN2 due to the high sequence similarity and thus are not ideal methods for identifying these markers.
To better facilitate SMA screening, there is an urgent need for a method that performs comprehensive full-gene SMN1 and SMN2 profiling. This method should ideally be able to (1) identify the CN of intact SMN1 and SMN2 based on c.840, (2) identify pathogenic variants in SMN1 other than loss of c.840C, and (3) identify silent carriers. Accurate long-read sequencing is ideal for resolving regions with high sequence homology and the utility of long-read PacBio HiFi sequencing in SMN1 was previously demonstrated in an amplicon-based study for a Chinese population,25 though informatics methods are still lacking for shotgun HiFi sequencing, where high sequence homology results in ambiguous alignments. Here we describe a method, Paraphase, that accurately detects the CN, as well as variants throughout SMN1 and SMN2 using PacBio HiFi sequencing. We applied Paraphase to population samples from five ethnicities and performed a population-wide haplotype analysis of these genes. We identified major haplogroups for SMN1 and SMN2 and quantified their co-segregation patterns. Furthermore, we identified specific haplotypes forming two-copy SMN1 alleles which could greatly improve the accuracy of silent carrier detection.
Material and methods
Paraphase: HiFi-based SMN1 and SMN2 caller
Paraphase extracts HiFi reads aligned to either SMN1 or SMN2 and realigns them to the SMN1 region. It then identifies variant positions throughout the 44 kb long region of interest (chr5: 70,917,100–70,961,220, GRCh38), which includes the SMN1 gene body plus upstream/downstream regions. Paraphase then assembles haplotypes by linking the phases of each variant site (Figure 1). Haplotypes are assigned to SMN1 or SMN2 based on the sequence at the c.840 site, i.e., C is SMN1 and T is SMN2. In addition, Paraphase identifies the common truncated form, SMNΔ7–8, that has a 6.3 kb deletion of exons 7–8. Generally, the number of unique SMN1 and SMN2 haplotypes reflects SMN1 and SMN2 CNs. For samples with only one SMN1 or SMN2 haplotype identified, to rule out possible rare cases where two identical haplotypes exist, we calculate whether the depth at the c.840C (T) site is consistent with one or two copies of SMN1 (SMN2). A no-call is reported when the read depth could not reliably distinguish CN1 vs. CN2. CN calls are also adjusted when the number of supporting reads of one haplotype suggests twice the CN of the other haplotypes. With the complete haplotypes resolved, Paraphase makes phased variant calls throughout the genes by calling differences from the reference. Paraphase also assigns haplotypes to haplogroups (see “assigning haplotypes to haplogroups” section below) to enable further haplotype-based analysis for identifying genetic markers. Paraphase works on both whole-genome sequencing (WGS) and hybrid capture-based enrichment data.
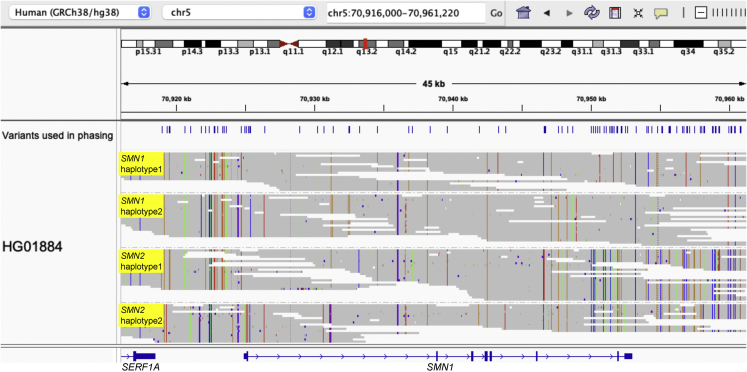
Figure 1.
Visualization of phased SMN1 and SMN2 haplotypes, using HG01884 as an example
Paraphase produces haplotagged bamlets to facilitate examination of haplotypes with all relevant reads realigned to SMN1. Variant positions used in phasing are shown in the top panel and reads are grouped by their assigned haplotypes (IGV option: group by HP tag).
Validation of CN calls
To verify the accuracy of our CN calls, we included 107 Coriell samples, 7 from Genome in a Bottle (GIAB),26 and 100 from the Human Pangenome Reference Center (HPRC).27 For these samples, SMN1 and SMN2 CNs were previously called by a short-read WGS-based method21 which has been shown to have 99.7% concordance against MLPA and digital PCR. Three of the 107 samples had MLPA calls that agree with short-read based calls.28 We also included 9 carrier (1+0) samples from Genomic Answers for Kids (GA4K) at Children’s Mercy Kansas City with MLPA results (SALSA MLPA P060 SMA Carrier probemix, MRC-Holland). Finally, we included an SMA trio from the 100,000 Genomes Project, where the SMN1 CN of both parents is one and the proband has zero copies of SMN1 (the SMN2 CNs for these three samples are unknown). In total, we had 119 samples with SMN1 CN information and 116 samples with SMN2 CN information. Detailed validation sample information is summarized in Table S1.
Population samples
We included 341 pedigrees (26 duos, 308 trios and 7 quartets) from five ethnic populations to study co-segregation of SMN1 and SMN2 alleles (Tables S2 and S3). We collected these data from GIAB,26 the Chinese Quartet project,29 HPRC,27 1000 Genomes Project,30 the 100,000 Genomes Project, Radboud University Medical Center, and GA4K. Among these pedigrees, 198 are of European (EUR) origin, 37 African (AFR), 35 admixed American (AMR), 26 South Asian (SAS), and 18 East Asian (EAS); 18 are of mixed ancestry and 9 are of unknown ethnicity. In addition, we included 67 samples without pedigree information from GA4K for other frequency calculations (Table S3).
Assigning haplotypes to haplogroups
Multiple sequence alignment and a neighbor-joining tree for the SMN1 and SMN2 haplotypes identified across populations were produced by Mafft server31 (v.7) with default parameters (https://mafft.cbrc.jp/alignment/server/). Haplogroups were identified by manually examining the tree for monophyletic groups. In Paraphase, a new haplotype is assigned a haplogroup by comparing the sequence similarity with representative sequences from each haplogroup and selecting the most similar haplogroup. A small number of haplotypes from each haplogroup were used to produce trees in Figure 2 and Figure S1, visualized with FigTree v.1.4.4 (https://github.com/rambaut/figtree/). Sequences of the same set of haplotypes were visualized in IGV in Figure 3.
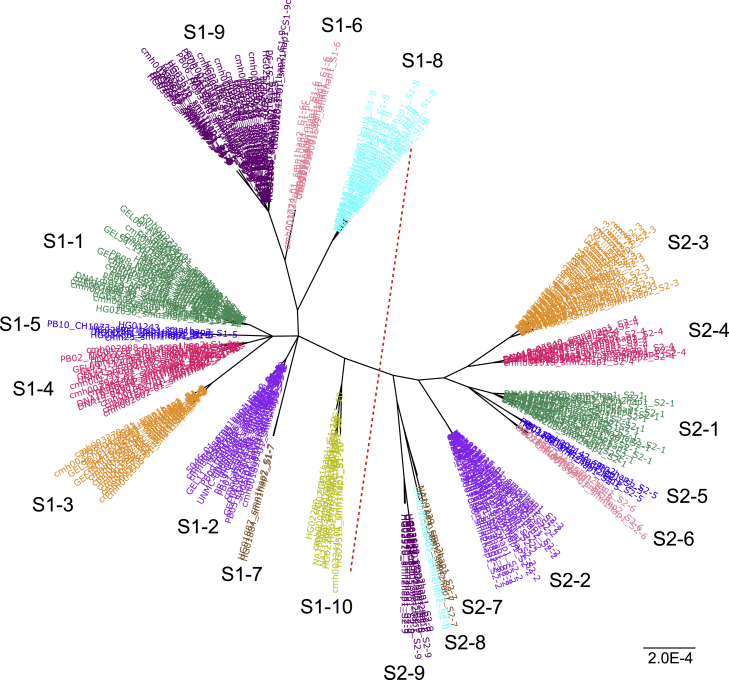
Figure 2.
Population-wide haplotype analysis identified major SMN1 and SMN2 haplogroups
Representative haplotype sequences of the gene region from each SMN1 and SMN2 haplogroup were used to create an unrooted tree. The red dotted line in the middle separates SMN1 (left) and SMN2 (right). Figure S1 shows a tree of the same haplotypes created using the gene plus upstream/downstream regions, and a tree of the same haplotypes created using sequences of exons 1–6. The scale bar indicates the number of substitutions per site.
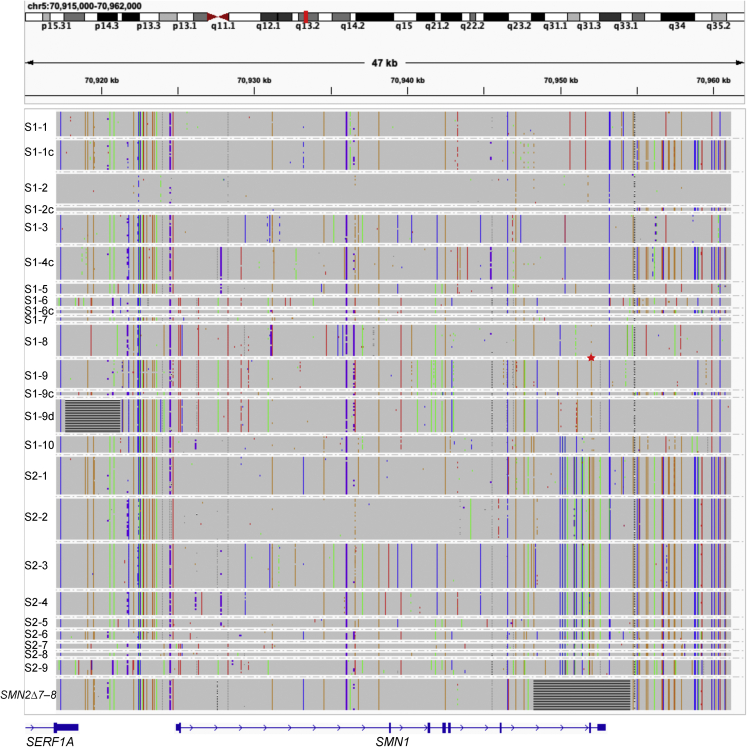
Figure 3.
Representative haplotype sequences from each SMN1 and SMN2 haplogroup as well as SMN2Δ7–8
IGV snapshot showing the haplotype sequences used in the phylogenetic analysis in Figure 2. Sequences of the gene region plus upstream and downstream regions were included. SMN2Δ7–8 has the 6.3 kb deletion of exons 7–8. S1-9d has a 3.6 kb deletion upstream of SMN1. The SNP g.27134T>G, commonly used in silent carrier screening, is marked with a red star symbol between S1-8 and S1-9.
Pedigree-based phasing of haplotypes into alleles
For this study, we use the term “haplotype” to refer to a set of phased variants (SNPs or indels) in one copy of a gene (SMN1 or SMN2). Conversely, we use the term “allele” to refer to one or several haplotypes that are inherited on the same chromosome, e.g., co-segregation of two SMN1 haplotypes or one SMN1 and one SMN2 haplotype. Phasing of haplotypes into alleles was done by comparing the haplotypes/haplogroups in parents and probands. Haplotypes were directly assigned haplogroups by Paraphase in samples with >20X HiFi WGS coverage. For parents with either Illumina short read data or low coverage HiFi data (Table S2), i.e., where phasing is not possible or accurate, representative variants for each haplogroup were queried in the parent data to identify the haplogroups in the parent. Haplogroups carried by the parents and the proband were compared to identify which haplotype(s) is inherited on each allele. In ambiguous cases, i.e., both parents have haplotypes of the same haplogroup, manual examination of data in IGV was conducted to find unique SNPs that distinguish these haplotypes and phase them into alleles.
Results
Validation of Paraphase copy-number calling
The SMN1 and SMN2 CN calls made by Paraphase were compared against orthogonal methods including short-read WGS-based CN calls, MLPA calls, and SMA trio-based inference (see material and methods). The CN call concordance is 99.2% for SMN1 and 100% for SMN2 (Table 1). We correctly called all SMA-affected individuals and carriers and did not make any false positive case or carrier calls. The SMNΔ7–8 calls are also concordant with orthogonal methods.
Table 1.
Validation against samples with known SMN1/SMN2 copy numbers (CNs)
| CN by orthogonal methods | Total | Concordant | Discordant | No-call | Agreement (excluding no-calls) |
|---|---|---|---|---|---|
| SMN1 | |||||
| 0 | 1 | 1 | 0 | 0 | 100% |
| 1 | 12 | 12 | 0 | 0 | 100% |
| 2 | 79 | 79 | 0 | 0 | 100% |
| >2 | 27 | 26 | 1a | 0 | 96.3% |
| Total | 119 | 118 | 1 | 0 | 99.2% |
| SMN2 | |||||
| 0 | 8 | 8 | 0 | 0 | 100% |
| 1 | 43 | 42 | 0 | 1b | 100% |
| 2 | 63 | 63 | 0 | 0 | 100% |
| >2 | 2 | 2 | 0 | 0 | 100% |
| Total | 116 | 115 | 0 | 1 | 100% |
| SMNΔ7–8 | |||||
| 0 | 104 | 104 | 0 | 0 | 100% |
| 1 | 3 | 3 | 0 | 0 | 100% |
| Total | 107 | 107 | 0 | 0 | 100% |
The discordant call was a CN3 miscalled as CN2, due to two of the three haplotypes being identical in sequence.
The no-call was due to an ambiguous read depth that could not reliably distinguish CN1 vs. CN2 when only one haplotype was found.
We next applied Paraphase to our collection of population samples (see material and methods). While the sample sizes for non-European populations are small, among 259 unrelated European individuals, there are 6 (2.32%, all validated with MLPA) with one copy of SMN1 (SMA 1+0 carriers), and 61 (23.6%) samples have SMNΔ7–8, agreeing with previous studies.5,6,21
SMN1 and SMN2 haplotypes across populations
We performed a population-wide haplotype analysis of 925 SMN1 haplotypes and 645 SMN2 haplotypes (excluding SMNΔ7–8) and identified ten and nine major SMN1 and SMN2 haplogroups, respectively (Figure 2). Representative haplotype sequences from each haplogroup are shown in Figure 3, together with SMNΔ7–8 sequences. Through pedigree-based analysis (see material and methods), we phased SMN1 haplotypes into alleles and summarized their population frequencies (Table 2, SMN2 allele frequencies are listed in Table S4). A few SMN1 haplotypes are labeled with suffix “c” to indicate that the downstream region of SMN1 is similar to that of SMN2 (Figure 3). For example, S1-1c is similar to its corresponding haplotype without the suffix, S1-1, in the gene body and is similar to SMN2 downstream of the gene. These haplotypes form separate clades and group with SMN2 haplotypes when sequences of the upstream and downstream regions are included in the phylogenetic analysis (Figure S1A). These haplotypes could have arisen through gene conversion.32,33
Table 2.
SMN1 allele frequencies across five ethnic populations
| SMN1 Alleles | European | East Asian | South Asian | Admix American | African | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Zero-copy (no SMN1) | 5 | 1.2% | 1 | 2.4% | 1 | 1.9% | 0 | 0.0% | 0 | 0.0% |
| Singleton SMN1 alleles | ||||||||||
| S1-1 | 233 | 55.9% | 35 | 83.3% | 27 | 51.9% | 47 | 67.1% | 26 | 29.9% |
| S1-1c | 16 | 3.8% | 1 | 2.4% | 2 | 3.8% | 2 | 2.9% | 2 | 2.3% |
| S1-2 | 80 | 19.2% | 2 | 4.8% | 7 | 13.5% | 6 | 8.6% | 1 | 1.1% |
| S1-2c | 0 | 0.0% | 1 | 2.4% | 1 | 1.9% | 0 | 0.0% | 0 | 0.0% |
| S1-3 | 65 | 15.6% | 0 | 0.0% | 7 | 13.5% | 8 | 11.4% | 1 | 1.1% |
| S1-4c | 7 | 1.7% | 0 | 0.0% | 1 | 1.9% | 1 | 1.4% | 0 | 0.0% |
| S1-5 | 1 | 0.2% | 0 | 0.0% | 0 | 0.0% | 1 | 1.4% | 1 | 1.1% |
| S1-6 | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 1 | 1.4% | 3 | 3.4% |
| S1-6c | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 1 | 1.4% | 1 | 1.1% |
| S1-7 | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 2 | 2.3% |
| S1-8 | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 1 | 1.1% |
| S1-9d | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 1 | 1.1% |
| S1-9 | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 9 | 10.3% |
| S1-10 | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 8 | 9.2% |
| Singleton total | 402 | 96.4% | 39 | 92.9% | 45 | 86.5% | 67 | 95.7% | 56 | 64.4% |
| Two-copy SMN1 alleles | ||||||||||
| S1-1+S1-1 | 3 | 0.7% | 0 | 0.0% | 2 | 3.8% | 0 | 0.0% | 0 | 0.0% |
| S1-1+S1-2 | 1 | 0.2% | 1 | 2.4% | 1 | 1.9% | 0 | 0.0% | 0 | 0.0% |
| S1-1+S1-3 | 4 | 1.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| S1-1+S1-1c | 0 | 0.0% | 1 | 2.4% | 3 | 5.8% | 1 | 1.4% | 0 | 0.0% |
| S1-2+S1-2c | 1 | 0.2% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| S1-4c+S1-4c | 1 | 0.2% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| S1-5+S1-5 | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 1 | 1.4% | 0 | 0.0% |
| S1-6+S1-6c | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 1 | 1.4% | 1 | 1.1% |
| S1-8+S1-9d | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 21 | 24.1% |
| S1-8+S1-9c | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 2 | 2.3% |
| S1-9+S1-9 | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 2 | 2.3% |
| S1-10+S1-10 | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 2 | 2.3% |
| S1-1+S1-9d | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 2 | 2.3% |
| S1-1+S1-8 | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 1 | 1.1% |
| Two-copy total | 10 | 2.4% | 2 | 4.8% | 6 | 11.5% | 3 | 4.3% | 31 | 35.6% |
| Total alleles | 417 | 42 | 52 | 70 | 87 | |||||
For single-copy SMN1 alleles, S1-1 is the most common haplotype across all ethnicities, with a frequency ranging from 29.9% in Africans to 83.3% in East Asians. S1-2 and S1-3 are also common (10%–20%) in Europeans, South Asians, and Admixed Americans, while they are less common (<3%) in Africans and East Asians. Notably, it is not the most common haplotype, S1-1, but S1-2 that is represented by the reference genome (GRCh38). Additionally, we observed several African-specific haplogroups (S1-7, S1-8, S1-9, S1-9d, and S1-10). Out of all SMN1 haplogroups, S1-10 is closest in sequence to SMN2 (Figures 2, 3 and S2).
The sequence differences between SMN1 and SMN2 are mainly located in exon 7 and exon 8, as well as the downstream region (Figure 3). SMNΔ7–8 is a truncated form with a 6.3 kb deletion of exons 7–821,23 (Figure 3). For all the SMNΔ7–8 haplotypes found in our data, the downstream region is highly similar to that of SMN2 (thus labeled as SMN2Δ7–8), confirming previous findings that this common truncated form likely derives from SMN2.21 Note that as the sequence flanking the deletion breakpoint is identical between SMN1 and SMN2, this deletion can possibly occur in SMN123 (a rare haplotype that we have not seen in our data), and the nonfunctional status would be the same as when it occurs in SMN2. Conversely, the upstream region and exons 1–6 are highly similar between SMN1 and SMN2 and there is not a single SNP that could distinguish SMN1 from SMN2 reliably in this region, i.e., there is not any SNP that is present in <10% of SMN1 haplotypes and >90% of SMN2 haplotypes, or vice versa. SMN1 and SMN2 haplotypes do not separate when only exons 1–6 sequences are included in the phylogenetic analysis (Figure S1B). As a result of the high similarity, read alignments are often ambiguous in this region, even for long reads.
In addition to small variants and the 6.3 kb known deletion in SMN2, we also found a previously unknown common structural variant in this region. A 3.6 kb (chr5: 70,917,700–70,921,260, GRCh38) deletion occurs upstream of SMN1 in S1-9d, which is otherwise similar to S1-9.
Two-copy SMN1 alleles
African individuals have more copies of SMN1 than other populations, with about 45%–50% of the population carrying >2 copies of SMN1 indicating the presence of two-copy SMN1 alleles.5,6,21 The higher frequency of two-copy SMN1 alleles leads to a higher frequency (estimated at ∼27% of all carriers) of 2+0 silent carriers where an individual has two copies of SMN1 but both occur on the same chromosome. Without pedigree information, two-copy SMN1 alleles are impossible to detect directly with current technologies. Through pedigree-based phasing of haplotypes into alleles, we studied two-copy SMN1 alleles and their frequencies. In African individuals, there exist a few haplotypes (S1-8, S1-9c, and S1-9d) that are commonly found in two-copy SMN1 alleles but not singleton SMN1 alleles (Table 2) and these could serve as potential markers for two-copy SMN1 alleles. In particular, we identified a common two-copy SMN1 allele, S1-8+S1-9d, that comprises two-thirds (21 out of 31) of African two-copy SMN1 alleles and 24.1% of total African alleles. These two SMN1 haplotypes, S1-8 and S1-9d, are rarely present as singletons (both at 1.1%, Table 2). Taking the previous estimate of zero-copy SMN1 allele frequency in Africans (0.68%6), if an African individual has two copies of SMN1, S1-8 and S1-9d, the likelihood of the two haplotypes being on the same chromosome, i.e., a silent carrier (2+0), is 7.7 times higher than the two haplotypes being present on different chromosomes, and thus the probability of being a silent carrier is 88.5%.
The SNP g.27134T>G in intron 7 of SMN1 is commonly used as a marker of two-copy SMN1 alleles.24 In our data, this SNP is only found in haplogroups S1-8 (21.9%), S1-9 (100%), S1-9c (100%), and S1-9d (96.3%). Samples positive for g.27134T>G are mainly those carrying the two-copy alleles S1-8+S1-9d, S1-8+S1-9c, and S1-9 singletons. S1-9 is commonly found as singleton SMN1 alleles in Africans (10.3% of all African alleles and 16.1% of singleton African alleles) and it differs from S1-9d only by the 3.6 kb deletion upstream of SMN1 and differs from S1-9c only in the downstream region. Therefore, g.27134T>G is expected to be present on a high percentage of singleton SMN1 alleles (16.1% in our data), consistent with previous maximum-likelihood estimates (18.4%),21 and thus not an accurate marker for two-copy SMN1 alleles. Conversely, using HiFi reads, Paraphase can accurately distinguish S1-9d or S1-9c from S1-9. In addition, being able to identify the other haplotype of the pair, S1-8, further improves Paraphase’s accuracy of detecting the two-copy SMN1 alleles.
For non-African populations, 57.1% (12 of 21) of two-copy SMN1 alleles involve combinations of common SMN1 haplotypes, i.e., S1-1+S1-1, S1-1+S1-2, and S1-1+S1-3 (Table 2). We also observed four two-copy SMN1 alleles where one of the copies of SMN1 includes the SMN2 sequence in the downstream region (flagged with the “c” suffix), i.e., S1-1+S1-1c, S2-2+S2-2c, S1-4c+S1-4c, and S1-6+S1-6c. It is possible that gene conversion from SMN2 to SMN1 in exons 7–8 resulted in these two-copy SMN1 alleles. Taking all non-African samples together, this pattern explains 8 out of 21 (38.1%) two-copy SMN1 alleles, or 4 out of 8 (50%) distinct two-copy SMN1 alleles. This is in line with the previous finding that paralog-specific variants (PSVs) between SMN1 and SMN2 downstream of the genes are overrepresented in signature variants enriched in two-copy SMN1 alleles in a Chinese population.25 However, as these “c” haplotypes are also present as singleton SMN1 alleles (6.0% of all non-African singleton alleles) and the other haplotype of the pair is often a highly common singleton allele such as S1-1 and S1-2, these haplotypes will frequently occur on two different chromosomes, so this “c” haplotype pattern as a marker does not have a high PPV as was observed for the S1-8+S1-9d allele in Africans.
Co-segregation of SMN1 and SMN2 haplotypes
We next investigated the co-segregation of SMN1 and SMN2 haplotypes. Our results show that SMN2 (including SMN2Δ7–8) is present on 85.3% of singleton SMN1 alleles but only 26.9% of two-copy SMN1 alleles. This indicates that gains of SMN1 are often accompanied with losses of SMN221 and it is possible that many two-copy SMN1 alleles were generated through gene conversion of SMN2 into SMN1.32
For standard alleles with one copy of SMN1 and one copy of full-length SMN2, i.e., excluding SMN2Δ7–8, we examined the types of SMN1 and SMN2 haplotypes on the same allele. We found that an SMN1 haplogroup is usually segregated with a specific SMN2 haplogroup (Table 3). This suggests that it is possible to probabilistically phase SMN1 and SMN2 together. For simplicity we named the SMN2 haplogroups to match the corresponding SMN1 haplogroups that they usually co-segregate with (e.g., S1-1 and S2-1 usually co-segregate). Interestingly, when we queried the sequence similarity between SMN1 and SMN2 haplogroups in exons 1–6 (exons 7–8 are not included as they are differentiated between SMN1 and SMN2), SMN1 haplogroups usually share the highest similarity with the co-segregating SMN2 haplogroups (Figure S3A). This is true for the three most common haplogroups (S1-1, S1-2, and S1-3), as well as three out of the six less common haplogroups (S1-4 through S1-9; S1-10 is not included as none of S1-10 haplotypes occurs on the same allele as SMN2, see below). As a result, some of the co-segregating SMN1 and SMN2 haplogroups group together when exons 1–6 sequences were used to create the phylogeny (Figure S1B). For less common alleles, a larger sample size is needed to further confirm the co-segregation pattern and the sequence similarity, especially for S1-7 (n = 2) and S1-8 (n = 1).
Table 3.
SMN1-SMN2 haplogroup co-segregation on alleles with one copy of full-length SMN1 and one copy of full-length SMN2
| SMN1 haplogroup | SMN2 haplogroup | # co-segregated alleles | # SMN1 haplogroups segregated with other SMN2 haplogroups | # SMN2 haplogroups segregated with other SMN1 haplogroups | % co-segregation |
|---|---|---|---|---|---|
| S1-1/S1-1c | S2-1 | 297 | 8a | 8b | 94.9% |
| S1-2/S1-2c | S2-2 | 101 | 0 | 2 | 98.1% |
| S1-3 | S2-3 | 70 | 5b | 6a | 86.4% |
| S1-4c | S2-4 | 8 | 2 | 0 | 80.0% |
| S1-5 | S2-5 | 3 | 0 | 0 | 100.0% |
| S1-6/S1-6c | S2-6 | 4 | 1 | 0 | 80.0% |
| S1-7 | S2-7 | 2 | 0 | 0 | 100.0% |
| S1-8 | S2-8 | 1 | 0 | 0 | 100.0% |
| S1-9/S1-9d | S2-9 | 8 | 0 | 0 | 100.0% |
Among these alleles, 6 are S1-1 co-segregated with S2-3.
Among these alleles, 5 are S1-3 co-segregated with S2-1.
We also examined co-segregation of alleles other than one copy of SMN1 and one copy of full-length SMN2. First, S1-10 alleles always contain zero copy of SMN2 (8 out of 8 alleles). Since S1-10 is closest in sequence to SMN2 among all SMN1 haplogroups (Figure 2) and S1-10 alleles never contain SMN2, S1-10 could be a hybrid gene between SMN1 and SMN2 created by a fusion deletion. Next, SMN2Δ7–8 alleles segregate with S1-1 in 98% (51 out of 52) of cases. SMN2Δ7–8 is most similar in sequence in exons 1–6 to S1-1 and S2-1 (Figure S3B). Both the co-segregation and the sequence similarity suggest that SMN2Δ7–8 is most likely derived from S2-1. Finally, we summarized the frequency of SMN1 (SMN2) haplotypes on alleles without SMN2 (SMN1) (Table S5). Among our limited sample of four alleles without SMN1 (zero-copy SMN1 alleles), four contain more than one copy of SMN2. Among these four alleles, two of them carry an SMN2 haplotype with the downstream region similar to SMN1 (Figure S4), suggesting possible loss of SMN1 through gene conversion from SMN1 to SMN2.
Discussion
Here we provide the most comprehensive analysis of variation in one of the most difficult, clinically important regions of the human genome. Extending beyond copy-number testing based primarily on c.840C>T as is often done, Paraphase phases the region to provide a much richer level of information. Using the phasing information, Paraphase can detect other pathogenic variants and enable haplotype-based screening of silent carriers. Since Paraphase works mainly by phasing variant positions from long reads, it works for both WGS and hybrid capture-based enrichment data and can be adapted to work with amplicon sequencing data, when the full SMN1/SMN2 regions are captured or amplified. Compared with short-read based methods, highly accurate HiFi reads can provide long-range haplotype information through entire genes and easily pick up large structural variants such as the 6.3 kb deletion in SMNΔ7–8 and the 3.6 kb deletion in the SMN1 haplotype S1-9d.
In this study we conducted a population-wide full-gene haplotype analysis of SMN1 and SMN2. Combining our gene-level phasing with pedigree information, we identified haplotypes that form two-copy SMN1 alleles. Most importantly, we identified a common two-copy SMN1 allele that comprises 67.7% of two-copy SMN1 alleles in Africans. The two individual haplotypes on this allele each occur very rarely as singleton SMN1 alleles in the population. Based on our limited sample of 87 African alleles, we estimate that testing positive for these two haplotypes in an individual with two copies of SMN1 gives a silent carrier risk of 88.5%, which is significantly higher than the previously found marker SNP g.27134T>G (1.7%–3.0%).20,21,24
In addition, we found co-segregation patterns between SMN1 and SMN2 haplotypes. An SMN1 haplogroup often co-segregates with the SMN2 haplogroup that is most similar in sequence, suggesting that intrachromosomal gene conversion between SMN1 and SMN2 plays a significant role in the evolution of this region. With larger sample datasets enabling more accurate allele frequency calculations, it should be possible to build a probabilistic model to predict the most likely allele/genotype configurations based on the haplotypes seen in an individual. This would be very helpful for silent carrier detection. For example, an individual with S1-8, S1-9d, and S2-1 is very likely a silent carrier, as S1-8 and S1-9d rarely exist as singleton SMN1 alleles and S2-1 rarely segregates with S1-8 or S1-9d. For an individual with these haplotypes, the most likely alleles are two copies of SMN1 (S1-8+S1-9d) with no SMN2 on one allele and one copy of SMN2 (S2-1) with no SMN1 on the other allele.
One limitation in this study is the relatively small number of samples (438) studied. To make more statistically powered findings out of the haplotype analysis, it is desirable to increase the sample size, particularly for non-European populations. Future analysis of large population data with Paraphase, using either HiFi WGS or possibly a hybrid capture based or other targeted long-read approaches, will allow a better characterization of variants in both genes, identification of more diverse haplotypes, analysis of alleles carrying two or even more copies of SMN1 or SMN2, and discovery of more genetic markers for silent carrier detection.
Paraphase is designed to resolve single copies of SMN1 or SMN2 from both WGS and targeted sequence data and our study points to the utility of haplotype-based statistical phasing in predicting phasing information between multiple copies of SMN1/SMN2. While it would be useful to assemble and phase the complete region covering SMN1 and SMN2 (a 2–4 Mb highly complex region with many segmental duplications), we are limited by the high sequence homology throughout the region. The SMN1/SMN2 region has long been known to have a high degree of variability in the population.34 Recently, Vollger et al. investigated this region in CHM13 and a few more near-complete de novo assemblies and showed that there is a high amount of variation among different alleles and that this region could not be consistently resolved across samples.35 Therefore, while we attempted to provide a preliminary analysis of the flanking genes to study the structure of the region (see Supplemental Note, Figures S5 and S6), complete resolution of the entire region will require a future study that utilizes carefully designed de novo assembly methods and high-quality pedigree data to QC assemblies.
The method employed in Paraphase can be applied to other segmental duplication regions with extremely high sequence similarity and frequent copy-number variations. We are currently extending this method to solve similar gene paralog problems such as CYP21A2, and we will apply this method to more clinically relevant genes in the future. The development of more targeted informatics solutions for difficult regions with HiFi data will bring us one step closer to consolidating the numerous genetic tests that are currently offered into a single test.
Acknowledgments
We thank the Human Pangenome Reference Center (HPRC) for generating and releasing the HiFi WGS data. This research was made possible through access to the data and findings generated by the 100,000 Genomes Project. The 100,000 Genomes Project is managed by Genomics England Limited (a wholly owned company of the Department of Health and Social Care). The 100,000 Genomes Project is funded by the National Institute for Health Research and NHS England. The Wellcome Trust, Cancer Research UK, and the Medical Research Council have also funded research infrastructure. The 100,000 Genomes Project uses data provided by patients and collected by the National Health Service as part of their care and support. The GA4K HiFi sequencing data was made possible by the generous gifts to Children’s Mercy Research Institute and the Genomic Answers for Kids program at Children’s Mercy Kansas City.
Declaration of interests
X.C., J.H., and M.A.E. are employees of Pacific Biosciences.
Published: January 19, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ajhg.2023.01.001.
Web resources
OMIM, https://www.omim.org/
Supplemental information
Data and code availability
Paraphase can be downloaded from https://github.com/PacificBiosciences/paraphase.
Bamlets for visualizing SMN1 and SMN2 haplotypes of GIAB and HPRC samples can be downloaded from https://github.com/xiao-chen-xc/SMN_phased_data.
References
- 1.Lunn M.R., Wang C.H. Spinal muscular atrophy. Lancet. 2008;371:2120–2133. doi: 10.1016/S0140-6736(08)60921-6. [DOI] [PubMed] [Google Scholar]
- 2.Mercuri E., Bertini E., Iannaccone S.T. Childhood spinal muscular atrophy: controversies and challenges. Lancet Neurol. 2012;11:443–452. doi: 10.1016/S1474-4422(12)70061-3. [DOI] [PubMed] [Google Scholar]
- 3.Prior T.W. Perspectives and diagnostic considerations in spinal muscular atrophy. Genet. Med. 2010;12:145–152. doi: 10.1097/GIM.0b013e3181c5e713. [DOI] [PubMed] [Google Scholar]
- 4.Ogino S., Leonard D.G.B., Rennert H., Ewens W.J., Wilson R.B. Genetic risk assessment in carrier testing for spinal muscular atrophy. Am. J. Med. Genet. 2002;110:301–307. doi: 10.1002/ajmg.10425. [DOI] [PubMed] [Google Scholar]
- 5.Hendrickson B.C., Donohoe C., Akmaev V.R., Sugarman E.A., Labrousse P., Boguslavskiy L., Flynn K., Rohlfs E.M., Walker A., Allitto B., et al. Differences in SMN1 allele frequencies among ethnic groups within North America. J. Med. Genet. 2009;46:641–644. doi: 10.1136/jmg.2009.066969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugarman E.A., Nagan N., Zhu H., Akmaev V.R., Zhou Z., Rohlfs E.M., Flynn K., Hendrickson B.C., Scholl T., Sirko-Osadsa D.A., Allitto B.A. Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: clinical laboratory analysis of >72 400 specimens. Eur. J. Hum. Genet. 2012;20:27–32. doi: 10.1038/ejhg.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacDonald W.K., Hamilton D., Kuhle S. SMA carrier testing: a meta-analysis of differences in test performance by ethnic group. Prenat. Diagn. 2014;34:1219–1226. doi: 10.1002/pd.4459. [DOI] [PubMed] [Google Scholar]
- 8.Verhaart I.E.C., Robertson A., Wilson I.J., Aartsma-Rus A., Cameron S., Jones C.C., Cook S.F., Lochmüller H. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy - a literature review. Orphanet J. Rare Dis. 2017;12:124. doi: 10.1186/s13023-017-0671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stabley D.L., Harris A.W., Holbrook J., Chubbs N.J., Lozo K.W., Crawford T.O., Swoboda K.J., Funanage V.L., Wang W., Mackenzie W., et al. SMN1 and SMN2 copy numbers in cell lines derived from patients with spinal muscular atrophy as measured by array digital PCR. Mol. Genet. Genomic Med. 2015;3:248–257. doi: 10.1002/mgg3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lorson C.L., Hahnen E., Androphy E.J., Wirth B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc. Natl. Acad. Sci. USA. 1999;96:6307–6311. doi: 10.1073/pnas.96.11.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butchbach M.E.R. Copy number variations in the survival motor neuron genes: implications for spinal muscular atrophy and other neurodegenerative diseases. Front. Mol. Biosci. 2016;3:7. doi: 10.3389/fmolb.2016.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wirth B., Herz M., Wetter A., Moskau S., Hahnen E., Rudnik-Schöneborn S., Wienker T., Zerres K. Quantitative analysis of survival motor neuron copies: identification of subtle SMN1 mutations in patients with spinal muscular atrophy, genotype-phenotype correlation, and implications for genetic counseling. Am. J. Hum. Genet. 1999;64:1340–1356. doi: 10.1086/302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wirth B. An update of the mutation spectrum of the survival motor neuron gene (SMN1) in autosomal recessive spinal muscular atrophy (SMA) Hum. Mutat. 2000;15:228–237. doi: 10.1002/(SICI)1098-1004(200003)15:3<228::AID-HUMU3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Alías L., Bernal S., Fuentes-Prior P., Barceló M.J., Also E., Martínez-Hernández R., Rodríguez-Alvarez F.J., Martín Y., Aller E., Grau E., et al. Mutation update of spinal muscular atrophy in Spain: molecular characterization of 745 unrelated patients and identification of four novel mutations in the SMN1 gene. Hum. Genet. 2009;125:29–39. doi: 10.1007/s00439-008-0598-1. [DOI] [PubMed] [Google Scholar]
- 15.Prior T.W., Professional Practice and Guidelines Committee Carrier screening for spinal muscular atrophy. Genet. Med. 2008;10:840–842. doi: 10.1097/GIM.0b013e318188d069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arkblad E.L., Darin N., Berg K., Kimber E., Brandberg G., Lindberg C., Holmberg E., Tulinius M., Nordling M. Multiplex ligation-dependent probe amplification improves diagnostics in spinal muscular atrophy. Neuromuscul. Disord. 2006;16:830–838. doi: 10.1016/j.nmd.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Scarciolla O., Stuppia L., De Angelis M.V., Murru S., Palka C., Giuliani R., Pace M., Di Muzio A., Torrente I., Morella A., et al. Spinal muscular atrophy genotyping by gene dosage using multiple ligation-dependent probe amplification. Neurogenetics. 2006;7:269–276. doi: 10.1007/s10048-006-0051-3. [DOI] [PubMed] [Google Scholar]
- 18.Feldkötter M., Schwarzer V., Wirth R., Wienker T.F., Wirth B. Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am. J. Hum. Genet. 2002;70:358–368. doi: 10.1086/338627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larson J.L., Silver A.J., Chan D., Borroto C., Spurrier B., Silver L.M. Validation of a high resolution NGS method for detecting spinal muscular atrophy carriers among phase 3 participants in the 1000 Genomes Project. BMC Med. Genet. 2015;16:100. doi: 10.1186/s12881-015-0246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng Y., Ge X., Meng L., Scull J., Li J., Tian X., Zhang T., Jin W., Cheng H., Wang X., et al. The next generation of population-based spinal muscular atrophy carrier screening: comprehensive pan-ethnic SMN1 copy-number and sequence variant analysis by massively parallel sequencing. Genet. Med. 2017;19:936–944. doi: 10.1038/gim.2016.215. [DOI] [PubMed] [Google Scholar]
- 21.Chen X., Sanchis-Juan A., French C.E., Connell A.J., Delon I., Kingsbury Z., Chawla A., Halpern A.L., Taft R.J., et al. NIHR BioResource Spinal muscular atrophy diagnosis and carrier screening from genome sequencing data. Genet. Med. 2020;22:945–953. doi: 10.1038/s41436-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez-Lopez D., Loucera C., Carmona R., Aquino V., Salgado J., Pasalodos S., Miranda M., Alonso Á., Dopazo J. SMN1 copy-number and sequence variant analysis from next-generation sequencing data. Hum. Mutat. 2020;41:2073–2077. doi: 10.1002/humu.24120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruhno C., McGovern V.L., Avenarius M.R., Snyder P.J., Prior T.W., Nery F.C., Muhtaseb A., Roggenbuck J.S., Kissel J.T., Sansone V.A., et al. Complete sequencing of the SMN2 gene in SMA patients detects SMN gene deletion junctions and variants in SMN2 that modify the SMA phenotype. Hum. Genet. 2019;138:241–256. doi: 10.1007/s00439-019-01983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo M., Liu L., Peter I., Zhu J., Scott S.A., Zhao G., Eversley C., Kornreich R., Desnick R.J., Edelmann L. An Ashkenazi Jewish SMN1 haplotype specific to duplication alleles improves pan-ethnic carrier screening for spinal muscular atrophy. Genet. Med. 2014;16:149–156. doi: 10.1038/gim.2013.84. [DOI] [PubMed] [Google Scholar]
- 25.Li S., Han X., Xu Y., Chang C., Gao L., Li J., Lu Y., Mao A., Wang Y. Comprehensive analysis of spinal muscular atrophy: SMN1 copy number, intragenic mutation, and 2 + 0 carrier analysis by third-generation sequencing. J. Mol. Diagn. 2022;24:1009–1020. doi: 10.1016/j.jmoldx.2022.05.001. Published online May 31, 2022:S1525-1578. [DOI] [PubMed] [Google Scholar]
- 26.Zook J.M., McDaniel J., Olson N.D., Wagner J., Parikh H., Heaton H., Irvine S.A., Trigg L., Truty R., McLean C.Y., et al. An open resource for accurately benchmarking small variant and reference calls. Nat. Biotechnol. 2019;37:561–566. doi: 10.1038/s41587-019-0074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang T., Antonacci-Fulton L., Howe K., Lawson H.A., Lucas J.K., Phillippy A.M., Popejoy A.B., Asri M., Carson C., Chaisson M.J.P., et al. The Human Pangenome Project: a global resource to map genomic diversity. Nature. 2022;604:437–446. doi: 10.1038/s41586-022-04601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vijzelaar R., Snetselaar R., Clausen M., Mason A.G., Rinsma M., Zegers M., Molleman N., Boschloo R., Yilmaz R., Kuilboer R., et al. The frequency of SMN gene variants lacking exon 7 and 8 is highly population dependent. PLoS One. 2019;14:e0220211. doi: 10.1371/journal.pone.0220211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren L, Duan X, Dong L, et al. Quartet DNA reference materials and datasets for comprehensively evaluating germline variants calling performance. Published online September 28, 2022:2022.09.28.509844. doi:10.1101/2022.09.28.509844 [DOI] [PMC free article] [PubMed]
- 30.1000 Genomes Project Consortium. Auton A., Brooks L.D., Durbin R.M., Garrison E.P., Kang H.M., Korbel J.O., Marchini J.L., McCarthy S., McVean G.A., Abecasis G.R. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katoh K., Rozewicki J., Yamada K.D. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019;20:1160–1166. doi: 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen T.H., Tzeng C.C., Wang C.C., Wu S.M., Chang J.G., Yang S.N., Hung C.H., Jong Y.J. Identification of bidirectional gene conversion between SMN1 and SMN2 by simultaneous analysis of SMN dosage and hybrid genes in a Chinese population. J. Neurol. Sci. 2011;308:83–87. doi: 10.1016/j.jns.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Vollger MR, DeWitt WS, Dishuck PC, Harvey, WT, Guitart, X, Goldberg, ME, Rozanski, A, Lucas, J, Asri, M, Munson, KM and Lewis, AP Increased mutation rate and interlocus gene conversion within human segmental duplications.Preprint at bioRxiv, Published online July 7, 2022:2022.07.06.498021. doi:10.1101/2022.07.06.498021
- 34.Campbell L., Potter A., Ignatius J., Dubowitz V., Davies K. Genomic variation and gene conversion in spinal muscular atrophy: implications for disease process and clinical phenotype. Am. J. Hum. Genet. 1997;61:40–50. doi: 10.1086/513886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vollger M.R., Guitart X., Dishuck P.C., Mercuri L., Harvey W.T., Gershman A., Diekhans M., Sulovari A., Munson K.M., Lewis A.P., et al. Segmental duplications and their variation in a complete human genome. Science. 2022;376:eabj6965. doi: 10.1126/science.abj6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Paraphase can be downloaded from https://github.com/PacificBiosciences/paraphase.
Bamlets for visualizing SMN1 and SMN2 haplotypes of GIAB and HPRC samples can be downloaded from https://github.com/xiao-chen-xc/SMN_phased_data.