Highlights:
-
•
SAEW treatment ameliorated the quality and storage properties of carambola fruit.
-
•
SAEW treatment reduced respiration rate and cell membrane permeability of carambola.
-
•
SAEW treatment maintained higher bioactive and nutritional compounds of carambola.
-
•
SAEW treatment improved apparent color and commercial acceptability rate of carambola.
-
•
SAEW displayed a great potential application for harvested carambola fruit.
Keywords: Carambola fruit, Postharvest physiology, Quality attributes, Nutritive properties, Slightly acidic electrolyzed water
Abstract
This study aimed to explore the impacts of slightly acidic electrolyzed water (SAEW) treatment on the physiology, quality, and storage properties of postharvest carambola. The carambolas were immersed in SAEW with a pH value of 6.0, ORP of 1340 mV and ACC of 80 mg/L. Results demonstrated that SAEW could significantly reduce the respiration rate, inhibit the increase in cell membrane permeability, and delay apparent color change. Relatively higher contents of bioactive compounds and nutritional components, such as flavonoids, polyphenols, reducing sugars, sucrose, vitamin C, total soluble sugar, and total soluble solid, as well as higher titratable acidity were maintained in SAEW-treated carambola. In addition, SAEW-treated carambola exhibited a higher commercial acceptability rate and a higher firmness, but lower weight loss and peel browning index than control fruits. Our results indicated that SAEW treatment achieved high fruit quality and nutritional values, potentially contributing to improve storage properties of harvested carambola.
Introduction
Carambola (Averrhoa carambola Linn.), commonly known as “star fruit”, is a tropical and subtropical non-climacteric fruit of the Oxalidaceae family (Duan et al., 2022, Shui and Leong, 2006, Zainudin et al., 2014). Carambola is an economically important and popular fruit in the global market because of its thin skin, thick pulp, rich juice, and good taste (Nimitkeatkai et al., 2022, Zhu et al., 2021). Carambola fruit possesses high nutritional and medicinal properties as it is rich in nutrients and bioactive ingredients, such as carotenoids, flavonoids, polyphenols, polysaccharides, sucrose, fructose, glucose, and vitamin C (Ahmad and Ali, 2019, Mekontso et al., 2021, Mustafa et al., 2016). However, the thin peel and juicy properties of carambola fruits make them prone to postharvest skin dehydration, shrinkage, yellowing, browning, and fruit softening, which seriously decrease their quality and market value (Gol et al., 2013, Imahori et al., 2021). The application of γ-aminobutyric acid improves the quality of postharvest carambola fruit by enhancing antioxidant capacity and delaying lipid peroxidation and electrolyte leakage (Mekontso et al., 2021). According to a previous study, brassinolide effectively enhanced flesh firmness and decreased percentage weight loss and chilling injury index of carambola fruits (Duan et al., 2022). Other chemical compounds, including methyl jasmonate (Mustafa et al., 2016), polyamines (Ahmad & Ali, 2019), 1-methylcyclopropene (Zhou, et al., 2012), chitosan, gumarabic, and alginate coatings (Gol et al., 2013), have also been proven to delay quality deterioration and extend the shelf life of postharvest carambola fruit. However, the abovementioned methods are limited by chemical residues, food contamination, and environmental pollution, warranting the development of a novel, environmentally friendly, and safe approach for preserving carambola fruit.
Slightly acidic electrolyzed water (SAEW) refers to electrolyzed oxidizing water with a pH value of 5.0–6.5 and oxidation–reduction potential (ORP) of ≥ 600 mV. In general, SAEW is produced by electrolyzing the aqueous solution of sodium chloride or hydrochloric acid in a non-membrane electrolytic chamber (Roy et al., 2021, Song et al., 2021, Wang et al., 2022, Zhang et al., 2021, Zhao et al., 2021). In particular, SAEW exhibits strong bactericidal activity in a specific pH range (close to neutral pH) in the presence of a high concentration of hypochlorous acid (Tango et al., 2017, Wang et al., 2022, Zhang et al., 2021). Compared with strong acidic electrolyzed water (2.0 ≤ pH ≤ 3.0), SAEW exhibits significant antibacterial activity and less negative effects on human health and the environment. Additionally, SAEW is a low-cost and safe postharvest approach for fresh produces (Song et al., 2021, Sun et al., 2022). Thus, it has been widely used as a preservative in food industries (Huang et al., 2008, Rahman et al., 2016, Yoon et al., 2021). Studies have shown that SAEW treatment significantly decreases the amount of foodborne pathogenic bacteria, viruses, and mold in fresh fruits and vegetables (Ding et al., 2015; Mansur & Oh, 2015; Saravanakumar et al., 2021, Zhang et al., 2019, Zhang et al., 2021, Zhang et al., 2016). For example, in cherry tomatoes and strawberries, a 10-min SAEW treatment resulted in a 3.29 and 3.59 log decrease in total aerobic bacteria as well as a 2.32 and 3.01 log decrease in yeast and mold, respectively (Ding et al., 2015). In addition, SAEW treatment with an available chlorine concentration (ACC) of 22 mg/L for 3 min could effectively inactivate Pectobacterium carotovorum subsp. carotovorum in fresh-cut cabbage (Song et al., 2021). However, few studies have explored the effects of SAEW on the quality and storage properties of fruits. To date, the effects of SAEW on the postharvest physiology, quality, and storage properties of carambola fruit remain unknown. Therefore, this study aimed to clarify the role of SAEW in the preservation of postharvest carambola fruit with regard to its physiology, quality, and storage properties.
Materials and methods
Carambola sample preparation
‘Xiangmi’ Carambola (Averrhoa carambola Linn. cv. Xiangmi) fruits (approximately 60 days after full bloom stage, roughly 80 % maturity, total soluble solids about 6–9 %) were freshly handpicked from a carambola orchard (Yunxiao, Fujian, China). Fruits were cautiously packed and delivered to the laboratory on the same day. Later, the fruits were grouped by maturity and size, and only those without damage and with uniform quality were used for subsequent analyses.
SAEW preparation
SAEW was produced by electrolyzing dilute hydrochloric acid solution in a SAEW generator (BD-600L, Shanghai Fu-Qiang-Wang Sanitary Products Co., ltd. Shanghai, China). The ORP of SAEW was determined using an ORP meter (A57-B, JIA-BEI Water Treatment Co., ltd., Guangdong, China). The ACC of SAEW was quantified using a high density chlorine meter (RC-3F, Kasahara Chemical Instruments Corp, Saitama, Japan).
Treatment of carambola fruits
For preliminary analyses, SAEW at a pH of 6.0, ORP of 1340 mV, and ACCs of 0, 40, 60, 80, and 100 mg/L was utilized to treat carambola fruits for 10 min. After treatment, the samples were air-dried and stored at 15 °C ± 1 °C under 85 % relative humidity. Fruit storage after SAEW treatment with an ACC of 80 mg/L displayed the lowest fruit yellowing index. Among different treatment groups, the value and rank of fruit yellowing index at day 18 was in the order of 4.65 (80 mg/L ACC) < 4.78 (60 mg/L ACC) < 4.86 (100 mg/L ACC) < 4.98 (40 mg/L ACC) < 5.12 (control). Accordingly, SAEW with an ACC of 80 mg/L was employed for subsequent analysis.
The initial biochemical and physiological properties of 80 carambola fruits were quantified on the day of harvest (day 0). Another 820 carambola fruits were randomly selected and equally categorized into two groups. The first group was submerged in SAEW (pH = 6.0, ORP = 1340 mV and ACC = 80 mg/L) for 10 min, whereas the second group was immersed in sterile distilled water for 10 min and used as the control. All fruits were air-dried under ambient condition after soaking, and each fruit was then sealed in a polyethylene bag. The packed carambola fruits were subsequently stored for 24 days at 15 °C ± 1 °C under 85 % relative humidity. During storage, 80 fruits were randomly selected from each group at an interval of 6 days to analyze their postharvest physiology, quality, and storage properties. In addition, 90 fruits from each group were selected and used to determine the commercial acceptability rate, peel browning index and fruit yellowing index at 6-day intervals during storage.
Assessment of fruit respiration rate and peel cell membrane permeability
The respiration rate of 10 selected carambola fruits was measured using an infrared CO2 analyzer (GXH-3051H, JUN-FANG-LI-HUA Technology Research Institute, Beijing, China) and was expressed as mg CO2 kg−1h−1. Samples were prepared according to the method described by Chen et al. (2015). Briefly, peel disks were obtained from the equatorial region of fruits using a cork borer (diameter = 1 cm). After washing, 5 g of disks was immersed in 30 mL of distilled water for 2 h at 28 °C. The electrical conductivity (P1) of extraction was measured using a conductivity meter (Model 3173, Shanghai Electronics Co., ltd, China). Subsequently, 5 g of disks was boiled for 30 min in 30 mL of distilled water. The total electrical conductivity (P2) was evaluated after cooling the extracted solution to 28 °C. The percentage cell membrane permeability (%) was calculated using the following formula: (P1/P2) × 100 %.
Measurement of the apparent color characteristics of fruit peels
The chromaticity of carambola peel was evaluated as described by Jiang et al. (2018) using a Chroma meter (Minolta CR 400, Konica Minolta Sensing, Inc., Osaka, Japan). In total, 25 edges of 5 carambola fruits were sliced into small pieces (2 cm × 2 cm) at the equator for color determination. L* (brightness level), a* (red to green), b* (yellow to blue), and h° (hue angle) values were determined for two opposing sites of the sliced pieces.
Determination of peel chlorophyll and carotenoid contents and the fruit yellowing index
Overall, 2 g of peel sample was obtained from the equatorial region of 10 carambola fruits. The contents of carotenoids and chlorophyll were estimated based on the methods described by Chen et al. (2015).
Thirty carambola fruits were used to compute the fruit yellowing index, according to the methods described by Ali et al., 2004, Omar et al., 2012, Chen et al., 2015. According to the degree of yellowing of carambola fruit, the degree of yellowing of carambola is divided into the following 6 grades: 1, 80 % fullness, fruit color is green; 2, the pericarp changes from green to white; 3, fruit starts to turn yellow from the middle, green more than yellow; 4, the pericarp is yellow and green, more yellow than green, emitting the fragrance of carambola; 5, the pericarp is basically turning yellow, only the edges are green; 6, the pericarp is completely yellow, the peel is thin and easy to separate. The fruit yellowing index was calculated using the following formula: ∑ (yellowing scale × proportion of corresponding fruit within each class).
Measurement of fruit pulp flavonoid and total phenolic contents
The flavonoid and total phenolic contents of fruit pulp were determined using a 2 g fresh pulp tissue obtained from 10 different carambola fruits, following a previously described method (Lin et al. 2020). The flavonoid and total phenolic contents were expressed as g catechin equivalents (CE) kg−1 and g gallic acid (GA) kg−1 of fresh carambola pulp, respectively.
Analysis of the contents of reducing sugar, sucrose, vitamin C, total soluble sugar, total soluble solid (TSS), and titratable acidity (TA) in carambola pulp
For the measurement of nutritional properties, we used 10 g of pulp samples from 10 different carambola fruits. The contents of reducing sugar, sucrose, vitamin C, total soluble sugar, TSS, and TA were quantified according to previously described methods by Chen et al., 2017, Duan et al., 2022; and Lin et al. (2020).
Assessment of the commercial acceptability rate, percentage weight loss, firmness, and peel browning index of fruits
The commercial acceptability rate of 30 carambola fruits was assessed according to the method described by Chen, Zhang, Lin, Lin, & Lin (2013). The percentage weight loss during storage in 10 fruits was evaluated based on the approach described by Chen et al., 2014, Mekontso et al., 2021. The percentage weight loss was computed by determining the weight of carambola fruits before and after storage.
Fruit firmness was quantified according to the procedure described by Duan et al. (2022) with few modifications using a texture analyzer (TA. XT Plus, Stable Micro Systems, Godalming, Surrey, UK) with a cylindrical probe (diameter = 5 mm) at a speed of 1.5 mm s−1 and insertion depth of 10 mm. Five carambola fruits were randomly selected, and the equatorial region of each fruit was sliced into 2 cm thick sections. Fruit firmness was estimated in three regions, and each treatment group was measured 15 times. Firmness was expressed in N.
The peel browning index of 30 different fruits was determined using the methods reported by Chen et al. (2020a) with few modifications. The extent of total peel browning area was determined using the apparent visual scale as follows: 1, no browning; 2, browning area < 1/20; 3, 1/20 ≤ browning area < 1/10; 4, 1/10 ≤ browning area < 1/4; 5, 1/4 ≤ browning area < 1/2; 6, 1/2 ≤ browning area < 3/4; 7, browning area ≥ 3/4; and 8, complete browning.
Statistical analysis
Measurement of each property was conducted in triplicates. The values are expressed as the mean ± standard error (n = 3). Statistical analysis was conducted using analysis of variance (ANOVA) with t-test of SPSS version 22 (SPSS Inc., Chicago, IL, USA). The P values of < 0.05 and < 0.01 indicated statistical significance.
Results and discussion
SAEW treatment decreases the respiration rate and cell membrane permeability of carambola fruit
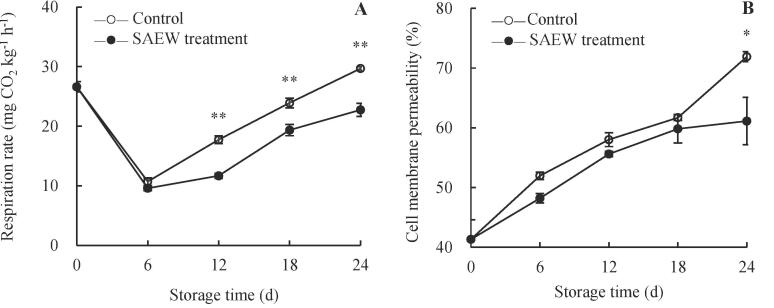
After harvest, carambola fruits continue to function as a living organism and require energy and intermediate substances for life activities that are produced through respiratory metabolism (Chen et al., 2020a). However, respiratory metabolism disorders can decrease the disease resistance of fruits and lead to infection of pathogenic microbes, which accelerate respiratory metabolism and nutrient consumption, ultimately leading to the decline in fruit quality (Chen et al., 2020a, Chen et al., 2020b, Lin et al., 2016). Therefore, the respiration rate of postharvest fruits is affected by the growth of microorganisms to a certain extent. In this study, the respiration rate of the control fruits dramatically declined during the first six days of storage and then markedly increased (Fig. 1A). Although similar rates of respiration were observed in the SAEW-treated and control fruits, the increase in fruit respiration rate was significantly inhibited by SAEW during 12–24 d (P < 0.01). The respiration rate of SAEW-treated fruits was 65.8 %, 80.9 %, and 76.6 % of that of the control fruits on days 12, 18, and 24, respectively. This result suggests that SAEW can reduce the respiration rate of carambola fruits within 12–24 d, likely by inhibiting fruit microbial growth.
Fig. 1.
Effects of SAEW treatment on the respiration rate (A) and peel cell membrane permeability (B) of postharvest carambola. Values are expressed as the mean ± standard error (n = 3). Differences in samples were based on one-way ANOVA with t-test. * and ** represents statistical significance at P < 0.05 and P < 0.01, respectively.
The cell membrane permeability of harvested fruit peels may reflect its structural integrity, extent of cell senescence, and degree of cell damage (Chen et al., 2015, Chen et al., 2020a). The cell membrane permeability of carambola fruit peels increased with storage period (Fig. 1B). Over the same storage period, the cell membrane permeability of SAEW-treated peels was lower than that of the control peels. Notably, at the end of storage period, the cell membrane permeability of SAEW-treated peels was lower than that of the control peels by 14.98 %. This result is consistent with the findings of previous studies showing that electrolyzed water treatment effectively decreased the cell membrane permeability of postharvest longan fruit and fresh-cut kiwifruit, thereby maintaining the cell membrane integrity of fruits during storage (Zhao et al., 2021, Chen et al., 2020a). Additionally, the cell membrane permeability (Fig. 1B) of the control and SAEW-treated fruits had positive correlations (P < 0.05, Supplementary Fig. S1) with fruit yellowing index (Fig. 3C), weight loss percentage (Fig. 6B), and peel browning index (Fig. 6D), revealing the vital role of cell membrane permeability in the process of fruit quality deterioration.
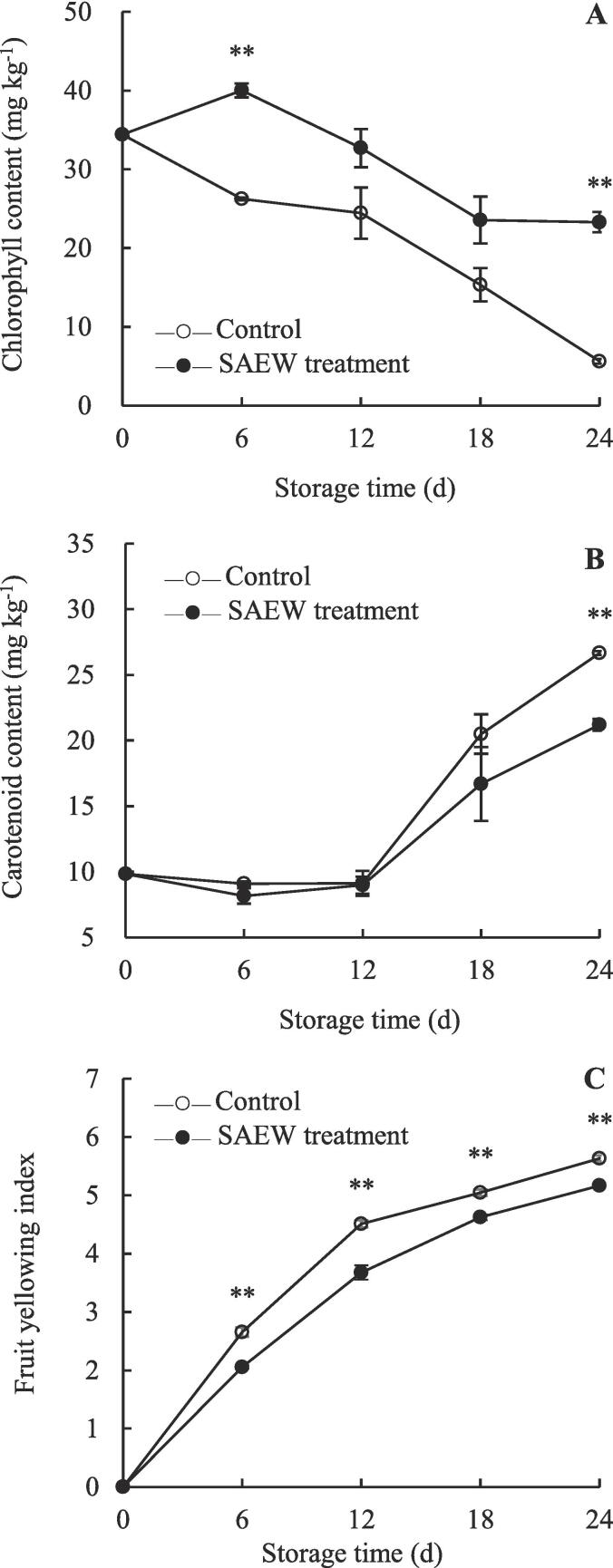
Fig. 3.
Variation in the contents of peel chlorophyll (A) and carotenoids (B) as well as fruit yellowing index (C) of postharvest SAEW-treated and control carambola fruits. Values are expressed as the mean ± standard error (n = 3). Differences in samples were based on one-way ANOVA with t-test. * and ** represents statistical significance at P < 0.05 and P < 0.01, respectively.
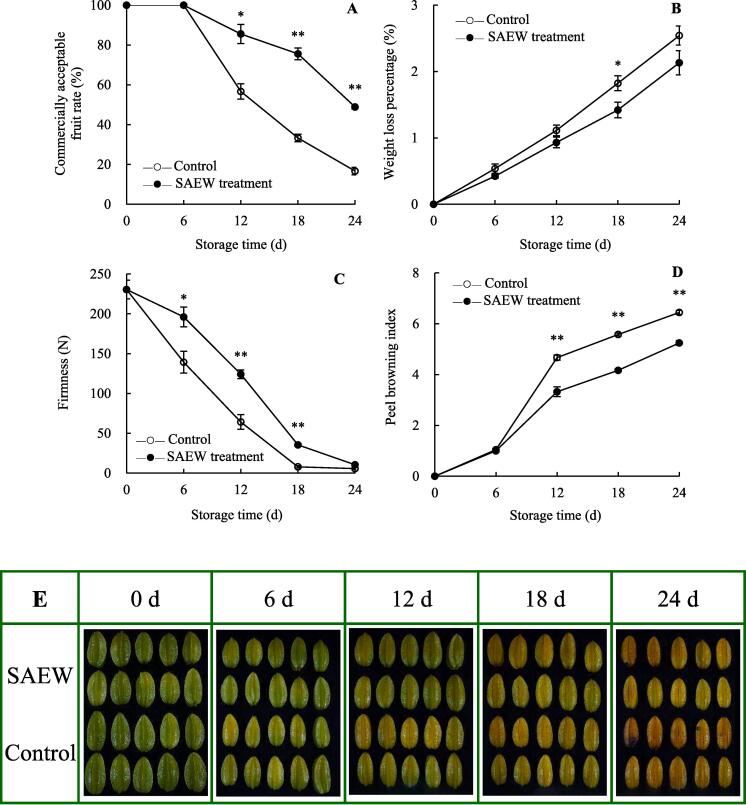
Fig. 6.
Effects of SAEW on the commercial acceptability rate (A), percentage weight loss (B), firmness (C), peel browning index (D), and appearance images (E) of postharvest carambola fruits. Values are expressed as the mean ± standard error (n = 3). Differences in samples were based on one-way ANOVA with t-test. * and ** represents statistical significance at P < 0.05 and P < 0.01, respectively.
Effects of SAEW on the apparent color characteristics of carambola fruits
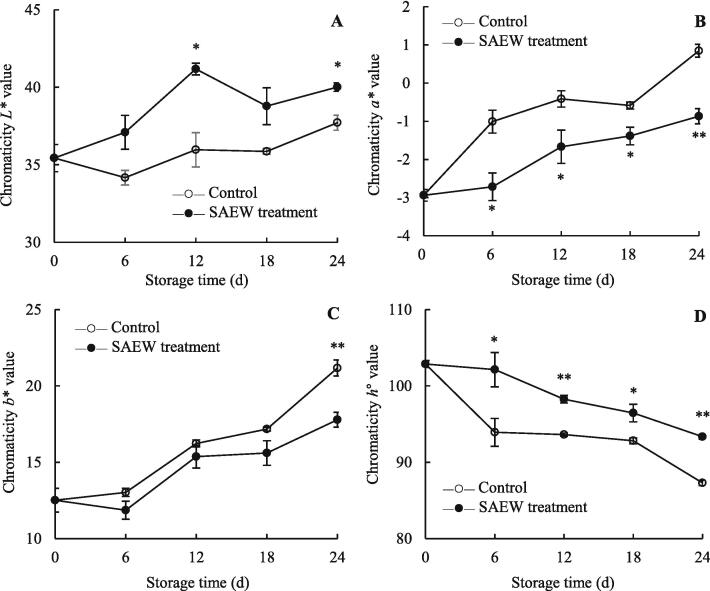
The apparent color characteristics of fruits are essential visual indices for evaluating fruit quality and are normally expressed using L*, a*, b*, and h° values (Jiang et al., 2018, Wrolstad et al., 2005, Zhu et al., 2021). The pericarp color of carambola fruit showed a change from green to yellow during the storage (Fig. 6E). On day 6, The color of control fruit had started to turn from green to yellow, while the SAEW-treated fruit remained green. Especially, On day 12, SAEW was effective in preventing the fruit from turning yellow, while the control group had mostly turned yellow (Fig. 6E). The chromaticity L* value of the control and SAEW-treated groups increased with storage period (Fig. 2A). Over the same storage period, SAEW-treated fruits exhibited higher chromaticity L* values than the control fruits. The chromaticity a* value of the control fruits rapidly increased with storage period (Fig. 2B). Compared with the control fruits, the chromaticity a* value of SAEW-treated fruits slightly increased within 0–6 d and then increased steadily. Statistical analysis revealed that the chromaticity a* value of SAEW-treated fruits was significantly lower than that of the control fruits during 6–24 d (P < 0.05). Additionally, the chromaticity b* value of the control fruits tended to increase during the storage period (Fig. 2C). However, at the end of the storage period, SAEW treatment delayed the increase in chromaticity b* value, which was lower than that in the control fruits by 15.99 %. Compared with the changes in L*, a*, and b* values, the h° values decreased with storage period (Fig. 2D). Statistical analysis revealed that the chromaticity h° value of the SAEW-treated fruits was significantly higher than that of the control fruits during 6–24 d (P < 0.05). This suggests that SAEW can intensify the brightness of carambola fruit, preserve its bright green color, and delay fruit yellowing (Fig. 6E). In the control and SAEW-treated fruits, the chromaticity a* value presented a positive correlation with fruit yellowing index (P < 0.05), while the reverse correlation (P < 0.05) was found between chromaticity h° value and fruit yellowing index (Supplementary Fig. S1). In addition, the chromaticity b* values of the two treatments were positively correlated with the peel browning index (P < 0.05). These indicate that yellowing and browning of carambola fruit was characterized by changes in chromaticity.
Fig. 2.
SAEW induced variations in the apparent color characteristics of postharvest carambola fruit. (A) value of chromaticity L*; (B) value of chromaticity a*; (C) value of chromaticity b*; and (D) value of chromaticity h°. Values are expressed as the mean ± standard error (n = 3). Differences in samples were based on one-way ANOVA with t-test. * and ** represents statistical significance at P < 0.05 and P < 0.01, respectively.
A previous report confirmed that SAEW (ACC = 21 mg/L) treatment for 5 min can inhibit the color change in the flesh of Nanhui peaches during storage (Zhou et al. 2012). SAEW combined with AEW treatment played an important role in delaying the color change of lettuce (Han, Liao, Ai, Ding & Wang 2021). In addition, 2,4-epibrassinolide can delay the color change in harvested carambola fruit by enhancing L* and C* values and inhibiting the decrease in h° value (Zhu et al. 2021). Brassinolide treatment can delay the process of carambola fruit color change from green to yellow by increasing L* value and inhibiting the increase in a* and b* values (Duan et al., 2022). Consistently, our results show that SAEW treatment is effective in inhibiting the apparent color change and improving the quality of carambola fruit.
SAEW treatment delays yellowing of carambola fruit by inhibiting chlorophyll degradation and carotenoid accumulation
Apart from L*, a*, b*, and h° values, chlorophyll and carotenoids are crucial components that affect the apparent fruit quality (Chen et al., 2020a). Chlorophyll and carotenoids are common pigments that play pivotal roles in the transformation and formation of fruit color (Ahmad and Ali, 2019, Duan et al., 2022). The chlorophyll content in the control fruits rapidly decreased during storage, whereas that in SAEW-treated fruits initially increased within 0–6 d but then gradually decreased (Fig. 3A). Compared with the control, SAEW treatment significantly delayed the decline in chlorophyll content. No significant difference in carotenoid content was observed between SAEW-treated and control fruits during 0–12 d. However, the carotenoid content in the SAEW-treated fruits was 81.5 % and 79.5 % that of the control fruits on days 18 and 24, respectively (Fig. 3B). The yellowing index of carambola fruits increased with storage period (Fig. 3C). During 6–24 d, the yellowing index of the control fruits was significantly higher than that of SAEW-treated fruits (P < 0.01). The fruit yellowing index (Fig. 3C) was significantly negatively correlated with chlorophyll content (Fig. 3A) in the control carambola fruits throughout the storage period (P < 0.05). These results indicate that the yellowing index of carambola was strongly correlated with chlorophyll and carotenoid contents. In addition, SAEW treatment could delay the yellowing of carambola by inhibiting the degradation of chlorophyll and accumulation of carotenoids, which further demonstrate that SAEW is vital for maintaining the apparent quality of carambola fruit. Data analysis (Supplementary Fig. S1) showed a positive correlation (P < 0.05) between fruit yellowing index and peel browning index in control and SAEW-treated fruits. Also, the fruit yellowing index of control fruits was negatively related to the commercial acceptability rate (P < 0.05). Thus, the decrease in chlorophyll content as well as the increase in carotenoids and fruit yellowing index may be responsible for the decrease in fruit quality of popcorn. Thus, the delayed decrease in chlorophyll content as well as the increase in carotenoids and fruit yellowing index may be responsible for the delayed deterioration in fruit quality of SAEW-treated carambola fruits.
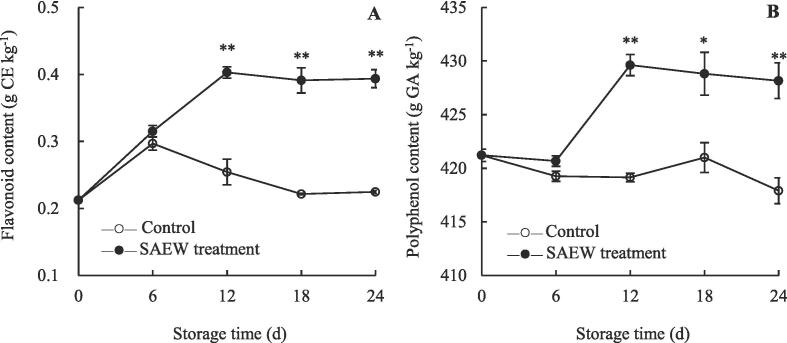
SAEW induces the accumulation of flavonoids and polyphenols in carambola pulp
Flavonoids and polyphenols are important bioactive components of postharvest fruits that contribute to their nutritional quality (Chen et al., 2020a). Moreover, they act as antioxidant agents in the antioxidant defense system and are involved in fruit senescence regulation (Chen et al., 2015, Duan et al., 2022, Zhao et al., 2021). The content of flavonoids in the control fruits displayed a marked increase within 0–6 d, and then gradually decreased until storage day 24 (Fig. 4A). During storage, the content of flavonoids in SAEW-treated fruits exhibited a different trend to that in the control fruits, showing a rapid increase at 0–12 d and reaching the highest level on 12 d, which was maintained until the end of the storage period. Moreover, the flavonoid content of SAEW-treated fruits was 1.41-, 1.63-, and 1.64-fold higher than that of the control fruits on days 12, 18, and 24, respectively. This may be attributed to SAEW-induced the maintenance of cell membrane structural integrity and SAEW-altered activity of some enzymes, which resulted in the accumulation of flavonoids throughout the storage period (Li et al., 2020, Zhao et al., 2021). Similarly, the polyphenol content of SAEW-treated fruits displayed a slight reduction at 0–6 d, a rapid increase during 6–12 d, and a stable level after 12 d of storage (Fig. 4B). Compared with the SAEW-treated group, the polyphenol content of the control group slightly decreased and remained at a lower level throughout the storage period. During 12–24 d, SAEW-treated fruits showed significantly higher polyphenol contents than the control fruits (P < 0.05).
Fig. 4.
Changes in the pulp flavonoid (A) and total polyphenol (B) contents of postharvest carambola fruits. Values are expressed as the mean ± standard error (n = 3). Differences in samples were based on one-way ANOVA with t-test. * and ** represents statistical significance at P < 0.05 and P < 0.01, respectively.
Zhao et al. (2021) previously demonstrated that SAEW treatment can improve the antioxidant capacity of fresh-cut kiwifruit by increasing the contents of flavonoids and total phenols. SAEW treatment delayed the reduction of total phenolics content in eggplant (Li et al. 2020). Brassinolide treatment was also shown to delay the quality deterioration of carambola fruit by promoting the accumulation of total phenols and flavonoids (Duan et al. 2022). Altogether, we can speculate that SAEW treatment maintains the quality and delays the senescence of carambola fruit by inducing the biosynthesis of flavonoids and polyphenols.
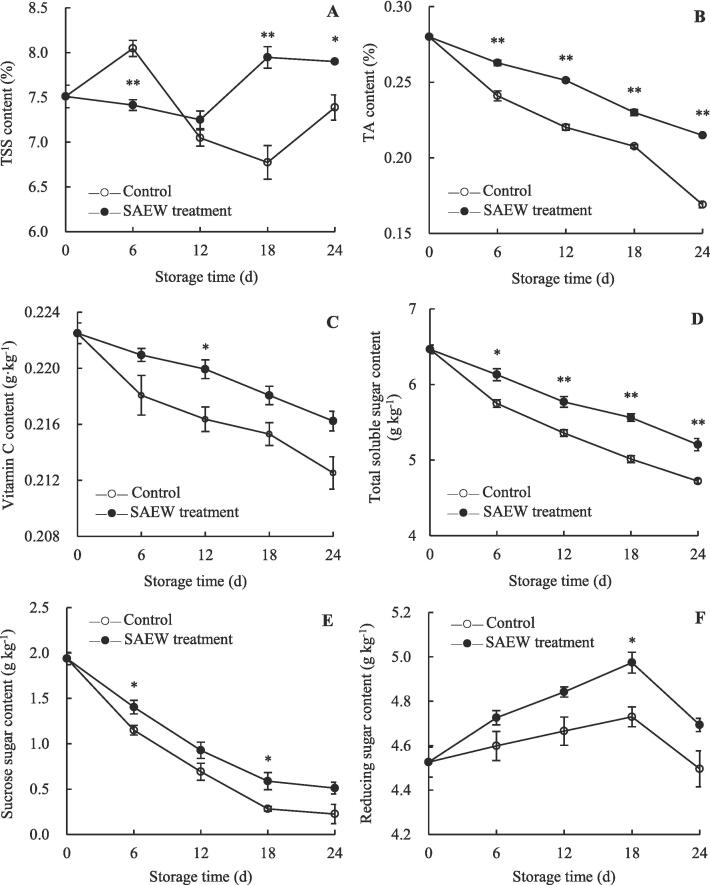
Effects of SAEW on the contents of reducing sugar, sucrose, vitamin C, total soluble sugar, TSS, and TA in carambola pulp
The pulp of carambola fruit contains numerous nutrients and flavor substances, such as reducing sugar, sucrose, vitamin C, total soluble sugar, and TSS, and exhibits high TA, which are commonly used as fruit nutritional indicators (Chen et al., 2017, Duan et al., 2022, Gol et al., 2013, Mekontso et al., 2021). The TSS content of the control fruits was remarkably lower than that of SAEW-treated fruits at day 6 (Fig. 5A). The contents of TA in the control and SAEW-treated groups decreased with storage period (Fig. 5B). Statistical analysis revealed that the content of TA in the control fruits was significantly lower than that in SAEW-treated fruits throughout the storage period (P < 0.01). Similarly, as the storage period increased, the level of vitamin C in carambola pulp decreased remarkably, whereas SAEW treatment delayed this decrease (Fig. 5C).
Fig. 5.
The role of SAEW in the regulation of pulp TSS (A), TA (B), vitamin C (C), total soluble sugar (D), sucrose (E), and reducing sugar (F) contents of postharvest carambola fruits. Values are expressed as the mean ± standard error (n = 3). Differences in samples were based on one-way ANOVA with t-test. * and ** represents statistical significance at P < 0.05 and P < 0.01, respectively.
During storage 0–24 d, the contents of sucrose and total soluble sugar rapidly decreased in SAEW-treated and control fruits (Fig. 5D and E). However, over the same storage period, higher contents of sucrose and total soluble sugar were observed in SAEW-treated fruits. The changes in the contents of reducing sugar differed compared with those in the contents of sucrose and total soluble sugar. In the control fruits, the content of reducing sugar was enhanced during 0–18 d and then shown a decreasing trend (Fig. 5F). Similar trend was observed in SAEW-treated fruits; however, the content of reducing sugar in these fruits was considerably higher than that in the control fruits throughout the storage period. For both the treatments, the contents of vitamin C, sucrose, total soluble sugar, and reducing sugar showed a negative correlation (P < 0.05) with the fruit yellowing index (Supplementary Fig. S1). The contents of TA, vitamin C, sucrose, and total soluble sugar were positively (P < 0.05) correlated with commercial acceptability rate in the control and SAEW-treated fruits, but negatively (P < 0.05) correlated with peel browning index (except for the correlation between sucrose content and commercial acceptability rate in SAEW-treated fruits) (Supplementary Fig. S1).
A recent study demonstrated that SAEW treatment can improve the nutritional indicators of pea sprouts, such as vitamin C, total protein, and soluble sugar (Zhang et al., 2019). Similarly, SAEW treatment improved the nutritional indicators of fresh-cut kiwifruit by inhibiting the conversion of starch into sugars and decreasing the content of TA (Zhao et al. 2021). In addition, Chen et al. (2020a) reported that acidic electrolyzed water treatment can maintain the nutritional quality of longan fruit by inhibiting the rate of fruit respiration. Our results also showed that SAEW treatment can retain higher contents of reducing sugar, sucrose sugar, vitamin C, total soluble sugar, TSS, and TA, which may be attributed to the inhibition of respiration rate and reduced nutrient consumption of the harvested carambola fruit. Overall, these results indicate that SAEW treatment improves the quality of carambola fruit.
Effects of SAEW on the commercial acceptability rate, percentage weight loss, firmness, and peel browning index of carambola fruit
The commercial acceptability rate, percentage weight loss, firmness, and peel browning index of carambola fruit are crucial indices that reflect its storage properties during storage. The commercial acceptability rate of SAEW-treated and control fruits did not change at day 0–6, and then rapidly declined (Fig. 6A). In addition, the commercial acceptability rate of the control fruits was significantly lower than that of SAEW-treated fruits at 12–24 d (P < 0.05). In contrast, the commercial acceptability rate of SAEW-treated fruits was higher than that of the control fruits by 42.22 %, 32.22 %, and 33.33 % on days 12, 18, and 24, respectively, indicating that SAEW treatment maintains a high commercial acceptability rate of fruits. The percentage weight loss in the control carambola fruits steadily increased with storage period (Fig. 6B). A similar trend was noted in SAEW-treated fruits, but with relatively lower percentage weight loss than the control fruits throughout the storage period. In contrast, the firmness of carambola fruit decreased during storage (Fig. 6C), but the loss was delayed by SAEW treatment. Statistical analysis revealed that SAEW treatment significantly inhibited the decrease in fruit firmness during 6–18 d (P < 0.05). The correlation analysis (Supplementary Fig. S1) confirms that percentage weight loss in the control and SAEW treatments was negatively (P < 0.01) associated with commercial acceptability rate and positively (P < 0.05) correlated with peel browning index. For firmness in both treatments, it presented a positive relationship (P < 0.05) with commercial acceptability rate and a negative relationship (P < 0.01) with peel browning index.
The development of carambola peel browning is strongly correlated with pericarp tissue structure, mechanical and chilling injury, and pathogen infection, which along with phenolic compounds can cause the loss of cell membrane integrity and enzymatic browning catalyzed by phenol oxidase (Chen et al., 2020a, Lin et al., 2020, Zhu et al., 2021). As the duration of storage progressed, the browning index of carambola peel exhibited an increasing trend (Fig. 6D). Statistical analysis demonstrated that during 12–24 d, the extent of browning of SAEW-treated peels was significantly lower than that of the control peels (P < 0.01). The peel cell membrane permeability (Fig. 1B) was positively correlated with peel browning index (Fig. 6D) in the SAEW-treated group throughout the storage period (r = 0.9407, P < 0.01). These results confirm that SAEW treatment inhibits the browning of carambola peels by maintaining the cell membrane integrity.
Treatment with 1-methylcyclopropene was previously shown to maintain the storage quality of carambola fruit by providing a high commercial acceptability rate and increased firmness, suppressing its percentage weight loss, and delaying its decay (Chen, Zhang, Lin, Lin, & Lin, 2013). Similarly, carambola fruit treated with brassinolide inhibited the occurrence of chilling injury and extended the shelf life by increasing flesh firmness and lowering its percentage weight loss compared with those in the control fruit (Duan et al. 2022). Additionally, SAEW treatment maintained the high storage quality of fresh-cut kiwifruit by reducing percentage weight loss and retaining fruit firmness (Zhao et al., 2021). Consistently, our results indicated that SAEW treatment inhibited the increase in percentage weight loss and peel browning and maintained higher commercial acceptability rate and increased fruit firmness, resulting in better storage properties of harvested carambola.
Conclusions
In summary, this study confirmed that SAEW treatment can significantly hinder fruit senescence and retain the quality and storage properties of carambola fruit. In particular, our results indicate that SAEW treatment inhibits cell membrane permeability, lowers respiration rate, decreases percentage weight loss as well as peel browning index, maintains fruit firmness, and enhances its commercial acceptability rate and apparent quality. In addition, SAEW treatment improved the nutritional properties of carambola fruit by inducing the accumulation of higher contents of flavonoids, polyphenols, reducing sugar, sucrose, vitamin C, total soluble sugar, TSS, and TA. These results suggest that SAEW treatment is a promising strategy for improving the quality and storage properties of harvested carambola fruit. These results indicated that SAEW treatment has great potential as a viable treatment to improve the quality and storage properties in carambola fruit throughout storage and could be used as a preservation technology for other fruits in the future.
CRediT authorship contribution statement
Jing Zhang: Investigation, Data curation, Formal analysis, Writing – original draft. Qingqing Liu: Investigation, Data curation, Formal analysis, Writing – original draft. Xuezhen Chen: Investigation, Data curation, Formal analysis. Meiling Li: Investigation. Mingyu Lin: Investigation. Yihui Chen: Supervision, Resources, Conceptualization, Project administration, Writing – review & editing. Hetong Lin: Supervision, Project administration, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Science and Technology Innovation Foundation at Fujian Agriculture and Forestry University of China (Grant numbers CXZX2020121A, KFb22079XA and CXZX2020115A).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100555.
Contributor Information
Yihui Chen, Email: harris2197395@163.com.
Hetong Lin, Email: hetonglin@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The authors do not have permission to share data.
References
- Ahmad A., Ali A. Improvement of postharvest quality, regulation of antioxidants capacity and softening enzymes activity of cold-stored carambola in response to polyamines application. Postharvest Biology and Technology. 2019;148:208–217. doi: 10.1016/j.postharvbio.2018.10.017. [DOI] [Google Scholar]
- Ali Z.M., Chin L., Marimuthu M., Lazan H. Low temperature storage and modified atmosphere packaging of carambola fruit and their effects on ripening related texture changes, wall modification and chilling injury symptoms. Postharvest Biology and Technology. 2004;33:181–192. doi: 10.1016/j.postharvbio.2004.02.007. [DOI] [Google Scholar]
- Chen M.Y., Lin H.T., Zhang S., Lin Y.F., Chen Y.H., Lin Y.X. Effects of adenosine triphosphate (ATP) treatment on postharvest physiology, quality and storage behavior of longan fruit. Food and Bioprocess Technology. 2015;8:971–982. doi: 10.1007/s11947-014-1462-z. [DOI] [Google Scholar]
- Chen S.W., Hsu M.C., Fang H.H., Tsai S.H., Liang Y.S. Effect of harvest season, maturity and storage temperature on storability of carambola 'Honglong' fruit. Scientia Horticulturae. 2017;220:42–51. doi: 10.1016/j.scienta.2017.03.047. [DOI] [Google Scholar]
- Chen Y.H., Sun J.Z., Lin H.T., Lin M.S., Lin Y.F., Wang H.…Hung Y.C. Salicylic acid reduces the incidence of Phomopsis longanae Chi infection in harvested longan fruit by affecting the energy status and respiratory metabolism. Postharvest Biology and Technology. 2020;160 doi: 10.1016/j.postharvbio.2019.111035. [DOI] [Google Scholar]
- Chen Y.H., Xie H.L., Tang J.Y., Lin M.S., Hung Y.C., Lin H.T. Effects of acidic electrolyzed water treatment on storability, quality attributes and nutritive properties of longan fruit during storage. Food Chemistry. 2020;320 doi: 10.1016/j.foodchem.2020.126641. [DOI] [PubMed] [Google Scholar]
- Chen Y.H., Zhang H., Lin H.T., Lin Y.F., Lin Y. Effects of different concentrations of 1-methylcyclopropene (1-MCP) treatments on physiology and fresh-keeping of harvested carambola fruits. Chinese Journal of Tropical Crops. 2013;34:2283–2288. doi: 10.3969/j.issn.1000-2561.2013.11.036. [DOI] [Google Scholar]
- Chen Y.H., Zhang H., Lin H.T., Lin Y.F., Lin Y. Effects of 1-methylcyclopropene (1-MCP) treatment on postharvest physiology and storage quality of carambola fruits. Modern Food Science and Technology. 2014;30:16–21. doi: 10.13982/j.mfst.1673-9078.2014.01.022. [DOI] [Google Scholar]
- Ding T., Ge Z., Shi J., Xu Y.T., Jones C.L., Liu D.H. Impact of slightly acidic electrolyzed water (SAEW) and ultrasound on microbial loads and quality of fresh fruits. LWT-Food Science and Technology. 2015;60:1195–1199. doi: 10.1016/j.lwt.2014.09.012. [DOI] [Google Scholar]
- Duan W.H., Mekontso F.N., Li W., Tian J.X., Li J.K., Wang Q. Alleviation of postharvest rib-edge darkening and chilling injury of carambola fruit by brassinolide under low temperature storage. Scientia Horticulturae. 2022;299 doi: 10.1016/j.scienta.2022.111015. [DOI] [Google Scholar]
- Gol N.B., Chaudhari M.L., Ramana Rao T.V. Effect of edible coating on quality and shelf life of carambola (Averrhoa Carambola L.) fruit during storage. Journal of Food Science and Technology. 2013;52:78–91. doi: 10.1007/s13197-013-0988-9. [DOI] [Google Scholar]
- Han R.W., Liao X.Y., Ai C.M., Ding T., Wang J. Sequential treatment with slightly acidic electrolyzed water (SAEW) and UVC light-emitting diodes (UVC-LEDs) for decontamination of Salmonella Typhimurium on lettuce. Food Control. 2021;123 doi: 10.1016/j.foodcont.2020.107738. [DOI] [Google Scholar]
- Huang Y.R., Hung Y.C., Hsu S.Y., Huang Y.W., Hwang D.F. Application of electrolyzed water in the food industry. Food Control. 2008;19:329–345. doi: 10.1016/j.foodcont.2007.08.012. [DOI] [Google Scholar]
- Imahori Y., Bai J.H., Ford B.L., Baldwin E.A. Effect of storage temperature on chilling injury and activity of antioxidant enzymes in carambola ‘Arkin’ fruit. Journal of Food Processing Preservation. 2021;45:15178. doi: 10.1111/jfpp.15178. [DOI] [Google Scholar]
- Jiang X.J., Lin H.T., Shi J., Neethirajan S., Lin Y.F., Chen Y.H.…Lin Y.X. Effects of a novel chitosan formulation treatment on quality attributes and storage behavior of harvested litchi fruit. Food Chemistry. 2018;252:134–141. doi: 10.1016/j.foodchem.2018.01.095. [DOI] [PubMed] [Google Scholar]
- Li X.H., Yue H., Xu S.S., Tian J.Y., Zhao Y., Xu J.F. The effect of electrolyzed water on fresh-cut eggplant in storage period. LWT-Food Science and Technology. 2020;123 doi: 10.1016/j.lwt.2020.109080. [DOI] [Google Scholar]
- Lin Y.X., Lin Y.F., Chen Y.H., Wang H., Shi J., Lin H.T. Hydrogen peroxide induced changes in energy status and respiration metabolism of harvested longan fruit in relation to pericarp browning. Journal of Agricultural and Food Chemistry. 2016;64:4627–4632. doi: 10.1021/acs.jafc.6b01430. [DOI] [PubMed] [Google Scholar]
- Lin Y.Z., Li N., Lin H.T., Lin M.S., Chen Y.H., Wang H.…Lin Y.F. Effects of chitosan treatment on the storability and quality properties of longan fruit during storage. Food Chemistry. 2020;306 doi: 10.1016/j.foodchem.2019.125627. [DOI] [PubMed] [Google Scholar]
- Mekontso F.N., Duan W.H., Cisse E.H.M., Chen T.Y., Xu X.B. Alleviation of postharvest chilling injury of carambola fruit by γ-aminobutyric acid: Physiological, biochemical, and structural characterization. Frontiers in Nutrition. 2021;8 doi: 10.3389/fnut.2021.752583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa M.A., Ali A., Seymour G., Tucker G. Enhancing the antioxidant content of carambola (Averrhoa carambola) during cold storage and methyl jasmonate treatments. Postharvest Biology and Technology. 2016;118:79–86. doi: 10.1016/j.postharvbio.2016.03.021. [DOI] [Google Scholar]
- Nimitkeatkai H., Techavuthiporn C., Boonyaritthongchai P., Supapvanich S. Commercial active packaging maintaining physicochemical qualities of carambola fruit during cold storage. Food Packaging and Shelf Life. 2022;32 doi: 10.1016/j.fpsl.2022.100834. [DOI] [Google Scholar]
- Omar A.F., Atan H., Matjafri M.Z. Visible spectral linearisation, gradient shift and normalisation in quantifying carambola acidity. Food Biophysics. 2012;7:289–295. doi: 10.1007/s11483-012-9267-y. [DOI] [Google Scholar]
- Rahman S., Khan I., Oh D.H. Electrolyzed water as a novel sanitizer in the food industry: Current trends and future perspectives. Comprehensive Reviews in Food Science and Food Safety. 2016;15:471–490. doi: 10.1111/1541-4337.12200. [DOI] [PubMed] [Google Scholar]
- Roy P.K., Rahaman M.F.D., Iqbal Hossain M., Han N., Nahar S., Ashrafudoulla M., Ha S.D. Elimination of Vibrio parahaemolyticus biofilms on crab and shrimp surfaces using ultraviolet C irradiation coupled with sodium hypochlorite and slightly acidic electrolyzed water. Food Control. 2021;128 doi: 10.1016/j.foodcont.2021.108179. [DOI] [Google Scholar]
- Saravanakumar K., Sathiyaseelan A., Mariadoss A.V.A., Chelliah R., Shin S., Park S.…Wang M.H. Slightly acidic electrolyzed water combination with antioxidants and fumaric acid treatment to maintain the quality of fresh-cut bell peppers. LWT-Food Science and Technology. 2021;147 doi: 10.1016/j.lwt.2021.111565. [DOI] [Google Scholar]
- Shui, G. H., & Leong, L. P. (2006). Residue from star fruit as valuable source for functional food ingredients and antioxidant nutraceuticals. Food Chemistry, 97, 277–284. http://doi.org./10.1016/j.foodchem.2005.03.048.
- Song H., Lee J.Y., Lee H.W., Ha J.H. Inactivation of bacteria causing soft rot disease in fresh cut cabbage using slightly acidic electrolyzed water. Food Control. 2021;128 doi: 10.1016/j.foodcont.2021.108217. [DOI] [Google Scholar]
- Sun J.Z., Jiang X.J., Chen Y.H., Lin M.S., Tang J.Y., Lin Q.…Lin H.T. Recent trends and applications of electrolyzed oxidizing water in fresh foodstuff preservation and safety control. Food Chemistry. 2022;369 doi: 10.1016/j.foodcont.2021.108217. [DOI] [PubMed] [Google Scholar]
- Tango C.N., Imran K., Ngnitcho Kounkeu P.F., Momna R., Hussain M.S., Oh D.H. Slightly acidic electrolyzed water combined with chemical and physical treatments to decontaminate bacteria on fresh fruits. Food Microbiology. 2017;67:97–105. doi: 10.1016/j.fm.2017.06.007. [DOI] [PubMed] [Google Scholar]
- Wang H.X., Zhang Y.Q., Jiang H.T., Cao J.K., Jiang W.B. A comprehensive review of effects of electrolyzed water and plasma-activated water on growth, chemical compositions, microbiological safety and postharvest quality of sprouts. Trends in Food Science & Technology. 2022;129:449–462. doi: 10.1016/j.tifs.2022.10.017. [DOI] [Google Scholar]
- Wrolstad R.E., Durst R.W., Lee J. Tracking color and pigment changes in anthocyanin products. Trends in Food Science & Technology. 2005;16:423–428. https://doi:10.1016/j.tifs.2005.03.019 [Google Scholar]
- Yoon S.R., Lee J.Y., Yang J.S., Ha J.H. Bactericidal effects of diluted slightly acidic electrolyzed water in quantitative suspension and cabbage tests. LWT-Food Science and Technology. 2021;152 doi: 10.1016/j.lwt.2021.112291. [DOI] [Google Scholar]
- Zainudin M.A.M., Hamid A.A., Anwar F., Osman A., Saari N. Variation of bioactive compounds and antioxidant activity of carambola (Averrhoa carambola L.) fruit at different ripening stages. Scientia Horticulturae. 2014;172:325–331. doi: 10.1016/j.scienta.2014.04.007. [DOI] [Google Scholar]
- Zhang C.L., Cao W., Hung Y.C., Li B.M. Disinfection effect of slightly acidic electrolyzed water on celery and cilantro. Food Control. 2016;69:147–151. doi: 10.1016/j.foodcont.2016.04.039. [DOI] [Google Scholar]
- Zhang C.L., Zhang Y.Y., Zhao Z.Y., Liu W.F., Chen Y.Q., Yang G.J.…Gao Y.F. The application of slightly acidic electrolyzed water in pea sprout production to ensure food safety, biological and nutritional quality of the sprout. Food Control. 2019;104:83–90. doi: 10.1016/j.foodcont.2019.04.029. [DOI] [Google Scholar]
- Zhang W.L., Cao J.K., Jiang W.B. Application of electrolyzed water in postharvest fruits and vegetables storage: A review. Trends in Food Science and Technology. 2021;114:599–607. doi: 10.1016/j.tifs.2021.06.005. [DOI] [Google Scholar]
- Zhao L., Li S., Yang H. Recent advances on research of electrolyzed water and its applications. Current Opinion in Food Science. 2021;41:180–188. doi: 10.1016/j.cofs.2021.03.004. [DOI] [Google Scholar]
- Zhao X.M., Meng X.M., Li W.X., Cheng R.Y., Liu P., Ma M. Effect of hydrogen-rich water and slightly acidic electrolyzed water treatments on storage and preservation of fresh-cut kiwifruit. Journal of Food Measurement and Characterization. 2021;15:5203–5210. doi: 10.1007/s11694-021-01000-x. [DOI] [Google Scholar]
- Zhou R., Zhang G.X., Hu Y.S., Wu H., Xie J., Luo Y.D. Reductions in flesh discolouration and internal morphological changes in Nanhui peaches (Prunus persica (L.) Batsch, cv. Nanhui) by electrolysed water and 1-methylcyclopropene treatment during refrigerated storage. Food Chemistry. 2012;135:985–992. doi: 10.1016/j.foodchem.2012.05.040. [DOI] [PubMed] [Google Scholar]
- Zhu X.Y., Chen Y.X., Li J.Y., Ding X.C., Xiao S.L., Fan S.L.…Li X.P. Exogenous 2,4-epibrassinolide treatment maintains the quality of carambola fruit associated with enhanced antioxidant capacity and alternative respiratory metabolism. Frontiers in Plant Science. 2021;12 doi: 10.3389/fpls.2021.678295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.