Abstract
Keeping up with the global scenario, diabetes prevalence is on rise in India. Inadequate glycemic control is a major cause of diabetes-related morbidity and mortality. The conventional standards of care (SOC) in diabetes, including self-monitoring of blood glucose and measurement of glycated hemoglobin, have supported achievement of glycemic control, yet there are a few limitations. With the use of current technologies and metrics, such as continuous glucose monitoring (CGM) and standardized CGM data reporting, the continuous real-time glucose levels can be measured, and importantly, the percentage of time above, below, and within the target glucose range can be calculated, which facilitates patient-centric care, a current goal in diabetes management. International consensus recommendations endorse the incorporation of CGM and CGM data reporting in SOC for diabetes management. The guidelines provide time in range (TIR) thresholds for different patient populations and different types of diabetes. However, extrapolation of these global guidelines does not aptly cover the Indian population, which has diverse diet, culture, and religious practices. In this context, a consensus meeting was held in India in 2021 with experts in the field of diabetes care. The purpose of the meeting was to develop consensus recommendations for TIR thresholds for different patient profiles in India. Those expert recommendations, together with an evidence-based review, are reported here. The aim of this agreement is to aid clinicians across India to routinely use CGM and CGM data reports for optimizing individualized diabetes care, by implementing clinical targets for TIR.
Keywords: Consensus recommendations, Continuous glucose monitoring, Elderly, Gestational diabetes mellitus, Pregnancy, Time in range(s), Type 1 diabetes mellitus, Type 2 diabetes mellitus
Key Summary Points
| Guidelines and consensus recommendations on the use of CGM-based metrics in routine diabetes care have been developed. |
| When compared with traditional glucose metrics such as HbA1c, CGM data reports can give additional glucose management measures such as TIR and glycemic variability. |
| Time in range (above, below, or within the glucose target) is a powerful metric for capturing dynamic fluctuations in blood glucose levels. |
| When compared with their Western counterparts, optimizing diabetes management in the Indian population necessitates tailored CGM and TIR as new metrics. |
Introduction
Diabetes is a growing health epidemic worldwide [1]. Recent statistics indicate that 9.3% of the global population had diabetes in 2019, affecting 463 million people [2]. In 2019, there were 77 million people with diabetes in India, the second-highest number of people with diabetes in the world [2]. These statistics highlight the need for urgent nationwide efforts to more effectively manage this rapidly growing health challenge [3]. This will necessitate the adoption and effective implementation of newer strategies for glucose management. Continuous glucose monitoring (CGM) utilizes glucose metrics such as time in range (TIR), and has successfully empowered healthcare providers (HCPs), patients, and caregivers to effectively manage diabetes [4].
Compared with standard glucose metrics, such as glycated hemoglobin (HbA1c), CGM data reports can provide additional glucose management metrics including TIRs and glycemic variability (GV) [5]. The ambulatory glucose profile (AGP) report displays consolidated CGM data, which enable clinicians to evaluate overall glycemia and assess the patterns of concern, which facilitates informed decision-making on therapy [6].
Guidelines and consensus recommendations have been developed on the use of CGM-based metrics in routine diabetes care [4, 7]. A recent South Asian expert census highlighted the TIR recommendations for South Asian population [8]. It has been shown that a TIR of > 70% corresponds to an HbA1c level of < 7.5% for the Indian population [8]. The authors highlighted the lower utilization of CGM in the Indian population. In developing countries such as India, patients are not properly educated, thus, it is cumbersome to educate the patients on the understanding and interpretation of interstitial glucose values and graphs. Furthermore, the recommendations were limited to TIR limits and CGM frequency [8]. However, while applying and agreeing to TIR targets for the Indian population, special considerations should be made for the population diversity in terms of geographic region, cultural differences, dietary variations, and religious practices such as fasting [4]. Thus, it can be postulated that there is a dearth of appropriate clinical practice-based guidelines and consensus recommendations tailored to the diverse population in India. Therefore, a consensus meeting was held in India in 2021, where an expert group met and discussed the current challenges of diabetes management based on their clinical experience. They also discussed the current recommendations for clinical targets for CGM data interpretation in clinical practice and finally made recommendations to develop a streamlined approach for appropriate clinical CGM metric targets tailored to the needs of the Indian population.
Achieving Glycemic Control with the “Gold-Standard” Metrics: Why the Need to “Go Beyond”?
India has the second largest population of people with diabetes, and the numbers are expected to surpass China by 2035. The Indian population has a high burden of diabetes, owing to distinctive biochemical and clinical characteristics (Asian Indian phenotype) that includes higher prevalence of insulin resistance, abdominal fat despite low body mass index, lower adiponectin levels, and so on [9]. Early onset of diabetes, as reported by Nagarathna et al. [9], and poor glycemic control in India has been documented by two large studies: the DiabCare India study and the ICMR– INDIAB study, and contribute to diabetes comorbidities and mortality [10, 11]. Current accepted metrics involve glycated hemoglobin and plasma glucose levels. It is well established that diabetes control is suboptimal in India despite the availability of these metrics.
Glycated Hemoglobin
Glycated hemoglobin (HbA1c) is a universally accepted tool for the periodic monitoring of glycemic control, along with self-monitoring of blood glucose (SMBG) [7, 12, 13]. Although HbA1c is considered the gold standard for monitoring glucose control, it has many disadvantages, such as an inability to show any dynamic fluctuations in blood glucose levels and the limitation in detecting time spent in hypoglycemia or hyperglycemia each day [7, 12–15]. Furthermore, HbA1c is an unreliable measure in patients with anemia, hemoglobinopathies, iron deficiency, and during pregnancy [7, 11, 12, 15]. One of the biggest limitations of this metric in an Indian-specific context is the lack of availability of standardized HbA1C testing. Moreover, India is a developing economy and much of the healthcare costs are borne by patients themselves, thus, the additional cost of HbA1C assay may prove to be a limiting factor in many cases, particularly in rural areas [16, 17].
Self-Monitoring of Glucose
This monitoring method informs patients about their current glucose level to support dietary, exercise, insulin, antidiabetic medications, or other treatment parameter-related decisions [18]. However, a few disadvantages of SMBG include inconvenient and painful data collection from finger pricking, difficulty in maintaining adherence to testing and a testing plan, user errors in glucose measurement, anxiety regarding test results, and patient’s inertia [7, 19–21]. Most importantly, even when performed frequently, blood glucose level measurements can only provide sporadic glucose data and do not fully capture dynamic glycemic changes [7].
With these limitations of traditional glucose metrics, sustained efforts have been made by diabetes experts to discover more reliable parameters to assess glycemic control, “moving beyond HbA1c.” This has culminated in the emergence of the concept of TIRs [22].
Apart from conventional limitations, a recent Research Society for the Study of Diabetes (RSSDI) consensus highlighted the limited and unstructured manner of SMBG implementation in the Indian population. Only 0.2% of the total Indian population who has diabetes uses SMBG for diabetes management [20].
Continuous Glucose Monitoring: A Direct and Integrated Measure of Glycemic Monitoring
Continuous glucose monitoring is a diabetes monitoring technology that provides more detailed, actionable information, and has distinct advantages over SMBG [22]. It aids critical decision-making regarding the initiation and optimization of therapeutic agents, and is highly suitable for the measurement of TIR [7, 23, 24].
Saboo et al. highlighted the importance of CGM as an alternative to HbA1C testing, which follows a one-size fits all approach. Moreover, a strong association between TIR and vascular complications was identified and the need to implement this tool in diabetes management was put forward [22].
Time in Range as a Powerful Metric for Glycemic Control
Time in range (above, below, or within the glucose target) is a powerful metric, which is very effective in capturing dynamic variations in blood glucose levels [7, 24–26]. Recent studies on TIR have demonstrated its versatility in ensuring glycemic control [27–32]. The descriptions on TIR and the use of standardized CGM report have been officially included into the American Diabetes Association (ADA) standard of care (SOC) since 2020, and have been recommended for assessment of glycemic control [26]. Furthermore, as per ADA SOC 2022, a single-page standardized CGM report such as ambulatory glucose profile needs to be considered as a standard format for all CGM devices. Also, recommendations regarding frequency and interpretations of CGM data were included [30]. Multiple randomized trials such as DIAMOND and IMPACT have demonstrated the superiority of CGM over SMBG in achieving glycemic control, while the REPLACE study suggested that CGM could safely and effectively replace SMBG [33–36].
The current guidelines recommend a range of 70–180 mg/dL (3.9–10 mmol/L) for people with type 1 or type 2 diabetes mellitus (T1DM or T2DM). However, for pregnant women with T1DM, T2DM, or gestational diabetes mellitus (GDM), a target range of 63–140 mg/dL (3.5–7.8 mmol/L) is recommended [7].
Correlation between HbA1c and TIR
There is a good correlation between HbA1c and TIR in T1DM or T2DM [7, 27, 36, 37], which may enable TIR to become the preferred metric for predicting the risk of diabetes complications, determining the outcomes of clinical studies, and assessing patients’ glycemic control [38].
A study evaluated the relationship between these two metrics, and reported a correlation of 84%. The study revealed an inverse relationship between these two parameters and showed that a 10% change in TIR corresponds to a 0.8% (9 mmol/mol) change in HbA1c [37].
Another study revealed that although a TIR 70–180 of 50% is associated with an average HbA1c level of about 8%, the range of this HbA1c could oscillate between 6.6% and 9.2% [39]. This study found that an increase in TIR 70–180 of 10% is associated with a decrease of 0.6% in HbA1c [39]. While in the Caucasian population, every 10% increase in TIR corresponds to a 0.8% reduction in HbA1c, an Indian study showed that a TIR value > 70% corresponded to an HbA1c level < 7.5% in the Asian Indian population [40].
Time in Range Measurement and the Ambulatory Glucose Report
The AGP is a report generated using data from a CGM [6]. Initially developed by Mazze et al., the AGP report covers a 14-day composite profile of glucose variations [7, 41].
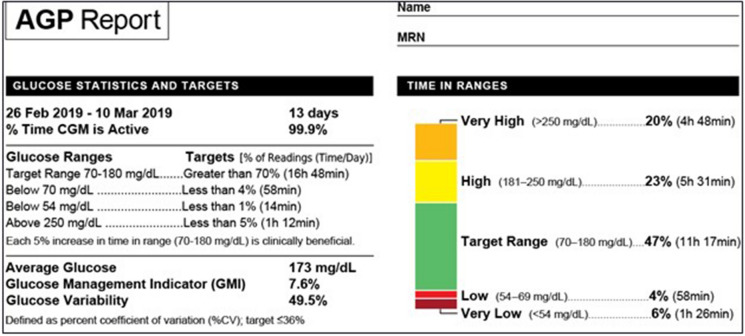
The AGP report provides graphical information on ten CGM metrics and glycemic graphs, time in various glycemic ranges, glucose variability, and glycemic exposure. Three broad categories in the AGP report include (1) Glucose statistics and TIR, (2) AGP, and (3) daily glucose profiles (Fig. 1) [7].
Fig. 1.
Glucose statistics and TIRs section of the AGP report
(Adapted from Battelino 2019) [7]
In the 2022 SOC ADA recommendations, AGP has been accepted as standardized format for CGM reporting [30].
Glucose Statistics and TIR
This includes the number and percentage of days the device has been active, average glucose, glucose management indicator, GV [reported as a % coefficient of variation (CV)], and TIRs. TIR information is color coded for the percentage of time and number of hours spent in the following ranges: 70–180 mg/dL (time in range, green); 54–69 mg/dL (time below range, level 1, red); < 54 mg/dL (time below range, level 2, maroon); 181–250 mg/dL (time above range, level 1, yellow); and > 250 mg/dL (time above range, level 2, mustard) (Fig. 1) [7].
International Consensus on TIR and Implications in an Indian Setting
In February 2019, the Advanced Technologies and Treatments for Diabetes (ATTD) Congress formalized guidelines for CGM and TIR goals, which have been endorsed by the ADA and the American Association of Clinical Endocrinologists/American College of Endocrinology (AACE/ACE) [7, 25, 42].
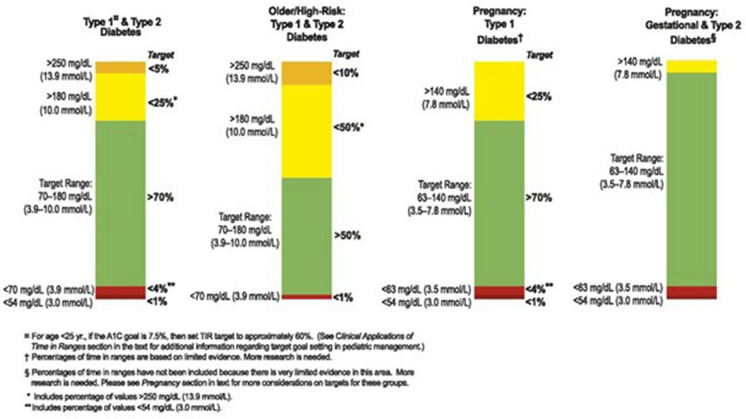
A summary of TIR goals for different diabetic subpopulations is shown in Fig. 2.
Fig. 2.
Summary of TIR recommendations
(Adapted from Battelino et al. 2019) [7]
The Research Society for the Study of Diabetes in India—Endocrine Society of India (RSSDI-ESI)—recommends the use of CGP (including AGP) in association with HbA1c and SMBG in people with T2DM on intensive insulin therapy who fail to achieve glycemic targets. Its use has been recommended in pregnant women with frequent hypoglycemia. For maximum benefits, CGMs are recommended to be used daily [43]. However, diverse patient populations with diabetes have not been aptly covered in the existing guidelines, especially in the Indian context.
Time in Range: A Need for Customized Guidelines for India?
In the Indian context, optimizing diabetes care with TIR would also require attention to additional considerations as follows:
High-Carbohydrate Diet
In the Indian population, the daily carbohydrate consumption is very high, which is associated with higher postprandial excursions [44, 45]. Therefore, the achievable percentage of TIR for the Indian population could significantly deviate from those of the global average. Hence, setting personalized achievable TIR should be considered in clinical setting.
Ethnic Differences
The genetic, racial, and ethnic differences impact glucose metabolism and insulin resistance, limiting the applicability of Western guidelines to the Indian population [43, 46]. Ethnic differences also account for the high glycemic response of the Indian population to calorie intake, as compared with the Western population, which possibly indicates that the percentage TIR target should be ≥ 70% for the Indian population [44].
Cultural and Religious Practices
India has various religions and cultures, and the Indian population follow several types of rituals and festivals. The combination of fasting and feasting lifestyle pattern is observed by the majority of the people during religious occasions [47, 48]. As the quantity of fluid and food intake is markedly altered during such occasions, the TIR should be used and carefully monitored during such phases [47].
Geographic Variation
It is well known that, compared with other ethnicities, South Asians have an increased risk of diabetes [49]. Even across different geographic locations within India, the prevalence and susceptibility of diabetes are markedly different [50, 51]. The prevalence and susceptibility of diabetes in North India are lower than those of South India [51]. Therefore, routine glucose monitoring involving the use of TIR should encompass such geographic variations in the country.
Psychosocial Behavior
When it comes to altering dietary habits, it is very challenging for the Indian diabetes population because of their nonchalant attitude [4]. Hence, the use of CGM can be recommended to guide the change of dietary habits.
Unique Clusters of T2DM
Recently, based on metabolic traits, subtypes or clusters of T2DM have been identified, which behave differently with regard to risk of complications, phenotypes, and clinical presentation [52, 53]. These clusters include severe autoimmune diabetes (SAID), severe insulin-deficient diabetes (SIDD), severe insulin-resistant diabetes (SIRD), mild obesity-related diabetes (MOD), and mild age-related diabetes (MARD) [52]. Anjana et al. identified four replicable clusters [combined insulin resistant and deficient diabetes (CIRDD), insulin-resistant obese diabetes (IROD), SIDD, and MARD], in 19,084 people with T2DM, of which two (CIRDD and IROD) were unique to the Indian population [53]. Therefore, unique clusters/subtypes of T2DM further necessitate the customization of TIR goals for each of these T2DM subtypes.
All these factors indicate why the Indian population needs specific personalized CGM and TIR as additional new metrics, as compared with their Western counterparts [4].
To address these challenges, Abbott Diabetes Care India, held a virtual Advisory Board Meeting in May 2020, where leading diabetologists and endocrinologists participated to discuss the current challenges in diabetes awareness and management and to discuss the possible scenarios where CGM could be implemented.
Consensus Statements on Using Time in Range for Different Patient Profiles in India
For Adult and Pediatric Population with T1DM on Insulin Therapy
Continuous glucose monitoring and TIR should be continuously used in patients who can afford it.
Continuous glucose monitoring and TIR are recommended for all pediatric patients with T1DM to minimize time below range (TBR) and reduce instances of hospitalization.
The use of TIR is helpful in people with T1DM (including the pediatric population) in adjusting diet and lifestyle changes for better clinical outcomes.
Number of Sensors to be Used for Glucose Monitoring
When cost is not a factor, it is recommended to use continuously
When on insulin, at least 2–4 sensors per year are recommended.
For basal-bolus therapy, more frequent use of sensors is recommended, if affordable.
For premix insulin (with slightly more hypoglycemia) at least 2–4 sensors should be used in a year.
For Patients with Oral Antidiabetic Drug (OAD) Inadequacy Requiring Initiation of Insulin Therapy
The use of CGM with TIR metric included for 2 weeks will help in readjustment or modification of treatment in these patients and waivers the need to wait for the 3-month duration to check the HbA1c status and efficacy of the treatment.
Routine use of TIR is recommended for patients on basal insulin.
TIR as a Glucose Metric for Diet and Lifestyle Compliance
Use of CGM helps in adjusting diet and physical activity levels by minimizing hypoglycemic events and hyperglycemia.
For newly diagnosed cases, patients are often reluctant to start medications, wherein the use of CGM helps to identify glucose patterns and to achieve glycemic targets with lifestyle changes.
Patients on polypills are afraid to initiate insulin, and CGM may help them to alter their lifestyle or to initiate insulin if required.
TIR for Hospitalized Patients
Continuous glucose monitoring can aid the clinicians in effective clinical decision-making by monitoring real-time glycemic status. Therapy can be initiated and/or modified at the clinician’s discretion.
Some medications and medical conditions such as inflammation and cancer can affect interstitial glucose levels, thus, precautions must be taken by clinician while interpreting TIR values. In these cases, both TIR and SMBG tools need to be used for best decision-making practices.
It is strongly recommended to utilize TIR in case of hypoglycemia.
Post-hospitalization diabetes management including insulin titration can be performed based on TIR readings.
TIR for Pregnant Women with T1DM/T2DM or GDM
For a woman with T1DM planning to conceive, having ≥ 70% TIR within 70–140 mg/dL should be considered.
For a woman with T2DM, or first-time detected hyperglycemic woman during pregnancy, or for GDM, having a TIR ≥ 90% within the 70–140 mg/dL range should be considered.
TIR for Elderly Population with T1DM or T2DM
In elderly patients, those with a high risk of hypoglycemia, unawareness of hypoglycemia, or established cardiovascular disease (CVD), ≥ 50% TIR is suggested to be the goal, and with < 1% for time below 54 mg/dL [7].
For Pandemic Situation
Since patients are more dependent on partial self-care in hospitals during the pandemic, the use of TIR is crucial in therapy-related decision-making among hospitalized non-intensive care unit (non-ICU) patients.
In the context of the pandemic, CGM is cost effective. Therefore, it is strongly recommended
The use of TIR needs further validation in the intensive care unit (ICU) setting.
TIR is very useful for at-home patients affected by the pandemic for self-care.
Overall Recommendations
For the management of glucose for patients living with diabetes, the use of TIR provided by CGM with the use of HbA1c can be regarded as the new SOC, other glucose metrics including fasting plasma glucose (FPG) and postprandial glucose (PPG) can also be considered, and one must be mindful regarding the few limitations of CGM as it is based on interstitial fluid measurements.
Awareness of TIR should be increased both among physicians and patients.
Overall, apart from the ICU setting, in every pandemic situation, including homecare, post hospitalization, quarantine, and in-hospital, the use of TIR is strongly recommended.
There is often discordance between CGM and glucometer readings of glucose levels, which should also be kept in mind. However, with the advent of recent CGM devices, such as the FreeStyle Libre system, such discordance has become less frequent.
Utility of Time in Range in the Management of Diabetes Complications
Several studies have correlated TIR with diabetes complications and risk factors, which support the role of TIR as an important outcome variable to assess glycemic control in clinical studies and practice [22].
Diabetic Retinopathy
A study showed that the severity of this complication is inversely correlated with TIR, and the prevalence of diabetic retinopathy decreased with increasing TIR [31]. A 10% reduction in TIR increases the risk of retinopathy by 64%, and mean TIR was lower in patients who developed retinopathy versus those who did not develop this complication [31].
Microalbuminuria
A reanalysis of the Diabetes Control and Complications Trial (DCCT) revealed that the mean TIR was 32% in participants who developed microalbuminuria versus 42% in those who did not develop this complication [31]. Further, a 10% reduction in TIR increased the risk of microalbuminuria by 40% [31].
An Indian study revealed that patients who came for regular follow-ups spent a significantly lower amount of time in the abnormal HbA1c range compared with patients who were irregular for follow-ups; besides, the former had a lower risk of diabetic nephropathy and retinopathy [54].
Outcomes following Surgery
It has been suggested that people with diabetes having TIR > 80% have a significantly lower risk of wound infection and spend less time in ICUs [37]. Similarly, patients without diabetes with TIR over 80% have been shown to have better surgical outcomes compared with those with TIR < 80% [37].
Benefits of Time in Range in Special Populations
Pregnancy
During pregnancy, in the first to third trimesters, hyperglycemia or time above range (TAR) is known to reduce from 40% (10 h) to 33% (8 h) [55]. During the first, second, and third trimesters, pregnant women with T1DM spend 50% (12 h), 55% (13 h), and 60% (14 h) in the target range of 63–140 mg/dL (3.5–7.8 mmol/L), respectively [55]. Clinicians and the prospective mother should strive to increase TIR while reducing TAR and TBR [7, 55]. Ideally, for the best neonatal outcomes, women with T1DM should have TIR > 70% (16 h, 48 min) and TAR < 25% (6 h) from the inception of pregnancy [7, 55]. Data from the CONCEPTT study report that prospective mothers could reduce the risk of large for gestational age (LGA) babies, neonatal hypoglycemia, and neonatal ICU admission by employing CGM-based methods [29].
A study revealed that 16.7% of women in South India with GDM were not diagnosed, suggesting a need for supplementary methods for capturing pregnancy-related diabetic complications [56].
Pediatrics, Adolescents, and Young Adults
The SELFY study involving children and adolescents aged 4–17 years with T1DM demonstrated the noninferiority and superiority of CGM over SMBG in achieving improved glycemic control [57].
An epidemiologic study utilizing the Indian Council of Medical Research (ICMR) Registry of Young-Onset Diabetes (YDR) indicated that the high burden of hospitalization in this subpopulation is primarily due to uncontrolled hyperglycemia [58].
Older Individuals and High-Risk Individuals
A recent study showed that the clinical profiles of Asian Indians with T2DM who lived beyond 90 years were significantly different from patients aged 50–60 years [59]. Therefore, in older individuals there should be a focus on minimizing the TBR of < 70 mg/dL (< 3.9 mmol/L) and avoiding excessive hyperglycemia [7].
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Conclusions
Several consensus documents and guidelines recommend the incorporation of TIR in routine clinical practice. However, there is a scarcity of consensus recommendations for TIR in different Indian patient profiles. A review of TIR consensus guidelines for the population in India may support HCPs across the country to optimize diabetes management in different patient profiles, potentially improving glycemic control and quality of life for patients. Diabetes management requires varying approaches in different parts of the Western world as it does in India. India is large and diverse and the approach to DM in India needs to suit each unique region.
Acknowledgements
We would like to thank BioQuest Solutions Pvt Ltd for editorial support.
Author Contributions
All authors VM, SJ, AM, JK, AGU, BS, PK, MC, AB, RK, have contributed equally to the conceptualization, drafting, review and approval of this paper.
Authorship
All the named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
Funding for the manuscript and medical writing services was from Abbott Diabetes Care along with the journal fees (as applicable).
Disclosures
Viswanathan Mohan, Shashank Joshi, Ambrish Mithal, Jothydev Kesavadev, Ambika G Unnikrishnan, Banshi Saboo, Prasanna Kumar, Manoj Chawla, Abhijit Bhograj and Rajiv Kovil.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Papatheodorou K, Banach M, Bekiari E, Rizzo M, Edmonds M. Complications of diabetes 2017. J Diabetes Res. 2018;2018:3086167. doi: 10.1155/2018/3086167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Diabetes Federation. Diabetes Atlas 9th Edition. 2019. https://www.diabetesatlas.org/en/. Accessed 04 May 2021.
- 3.Anjana RM, Deepa M, Pradeepa R, et al. Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR–INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017;5(8):585–596. doi: 10.1016/S2213-8587(17)30174-2. [DOI] [PubMed] [Google Scholar]
- 4.Chawla M, Saboo B, Jha S, et al. Consensus and recommendations on continuous glucose monitoring. J Diabetol. 2019;10:4–14. doi: 10.4103/jod.jod_45_18. [DOI] [Google Scholar]
- 5.Dovc K, Battelino T. Time in range centered diabetes care. Clin Pediatr Endocrinol. 2021;30(1):1–10. doi: 10.1297/cpe.30.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unnikrishnan AG, Saboo B, Joshi S, et al. Consensus statement on use of ambulatory glucose profile in patients with type 2 diabetes mellitus receiving oral antidiabetic drugs. J Assoc Physicians India. 2019;67(11):76–83. [PubMed] [Google Scholar]
- 7.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabe Care. 2019;42(8):1593–1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kesavadev J, Misra A, Saboo B, et al. Time-in-range and frequency of continuous glucose monitoring: recommendations for South Asia. Diabetes Metab Syndr. 2022;16(1):102345. doi: 10.1016/j.dsx.2021.102345. [DOI] [PubMed] [Google Scholar]
- 9.Nagarathna R, Bali P, Anand A, et al. Prevalence of diabetes and its determinants in the young adults Indian population-call for yoga intervention. Front Endocrinol (Lausanne) 2020;11:507064. doi: 10.3389/fendo.2020.507064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohan V, Shah SN, Joshi SR, et al. Current status of management, control, complications and psychosocial aspects of people with diabetes in India: results from the DiabCare India 2011 Study. Indian J Endocrinol Metab. 2014;18(3):370–378. doi: 10.4103/2230-8210.129715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unnikrishnan R, Anjana RM, Deepa M, et al. Glycemic control among individuals with self–reported diabetes in India—the ICMR–INDIAB Study. Diabetes Technol Ther. 2014;16(9):596–603. doi: 10.1089/dia.2014.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonora E, Tuomilehto J. The pros and cons of diagnosing diabetes with A1C. Diabetes Care. 2011;34(Suppl 2):S184–S190. doi: 10.2337/dc11-s216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright LA, Hirsch IB. Metrics beyond hemoglobin A1C in diabetes management: time in range, hypoglycemia, and other parameters. Diabetes Technol Ther. 2017;19(S2):S16–S26. doi: 10.1089/dia.2017.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovatchev B, Cobelli C. Glucose variability: timing, risk analysis, and relationship to hypoglycemia in diabetes. Diabetes Care. 2016;39(4):502–510. doi: 10.2337/dc15-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The fallacy of average: How using HbA1c alone to assess glycemic control can be misleading. Diabetes Care. 2017;40(8):994–999. doi: 10.2337/dc17-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sriram S, Khan MM. Effect of health insurance program for the poor on out-of-pocket inpatient care cost in India: evidence from a nationally representative cross-sectional survey. BMC Health Serv Res. 2020;20(1):1–21. doi: 10.1186/s12913-020-05692-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unnikrishnan R, Mohan V. Challenges in estimation of glycated hemoglobin in India. Diabetes Technol Ther. 2013;15(10):897–899. doi: 10.1089/dia.2013.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergenstal RM, Gavin JR., 3rd The role of self–monitoring of blood glucose in the care of people with diabetes: report of a global consensus conference. Am J Med. 2005;118(Suppl 9A):1s–6s. doi: 10.1016/j.amjmed.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 19.Erbach M, Freckmann G, Hinzmann R, et al. Interferences and limitations in blood glucose self–testing: an overview of the current knowledge. J Diabetes Sci Technol. 2016;10(5):1161–1168. doi: 10.1177/1932296816641433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao PV, Makkar BM, Kumar A, et al. RSSDI consensus on self-monitoring of blood glucose in types 1 and 2 diabetes mellitus in India. Int J Diabetes Dev Ctries. 2018;38(3):260–279. doi: 10.1007/s13410-018-0677-3. [DOI] [Google Scholar]
- 21.Patton SR, Clements MA. Continuous glucose monitoring versus self–monitoring of blood glucose in children with type 1 diabetes– are there pros and cons for both? US Endocrinol. 2012;8(1):27–29. doi: 10.17925/USE.2012.08.01.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saboo B, Kesavadev J, Shankar A, et al. Time-in-range as a target in type 2 diabetes: an urgent need. Heliyon. 2021;7(1):e05967. doi: 10.1016/j.heliyon.2021.e05967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reddy N, Verma N, Dungan K, et al. Monitoring technologies – continuous glucose monitoring, mobile technology, biomarkers of glycemic control. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext. South Dartmouth: MDText.com, Inc; 2022. pp. 2000–2020. [Google Scholar]
- 24.Ajjan R, Slattery D, Wright E. Continuous glucose monitoring: a brief review for primary care practitioners. Adv Ther. 2019;36(3):579–596. doi: 10.1007/s12325-019-0870-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–1640. doi: 10.2337/dc17-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Diabetes Association 6. Glycemic targets: standards of medical care in diabetes 2020. Diabetes Care. 2020;43(Suppl 1):S66–S76. doi: 10.2337/dc20-S006. [DOI] [PubMed] [Google Scholar]
- 27.Advani A. Positioning time in range in diabetes management. Diabetologia. 2020;63(2):242–252. doi: 10.1007/s00125-019-05027-0. [DOI] [PubMed] [Google Scholar]
- 28.Murphy HR, Rayman G, Duffield K, et al. Changes in the glycemic profiles of women with type 1 and type 2 diabetes during pregnancy. Diabetes Care. 2007;30(11):2785–2791. doi: 10.2337/dc07-0500. [DOI] [PubMed] [Google Scholar]
- 29.Feig DS, Donovan LE, Corcoy R, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet. 2017;390(10110):2347–2359. doi: 10.1016/S0140-6736(17)32400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Diabetes Association Standards of medical care in diabetes-2022 abridged for primary care providers. Clin Diabetes. 2022;40(1):10–38. doi: 10.2337/cd22-as01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42(3):400–405. doi: 10.2337/dc18-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu J, Ma X, Zhou J, et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care. 2018;41(11):2370–2376. doi: 10.2337/dc18-1131. [DOI] [PubMed] [Google Scholar]
- 33.Irace C, Cutruzzola A, Nuzzi A, et al. Clinical use of a 180–day implantable glucose sensor improves glycated hemoglobin and time in range in patients with type 1 diabetes. Diabetes Obes Metab. 2020;22(7):1056–1061. doi: 10.1111/dom.13993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317(4):371–378. doi: 10.1001/jama.2016.19975. [DOI] [PubMed] [Google Scholar]
- 35.Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016;388(10057):2254–2263. doi: 10.1016/S0140-6736(16)31535-5. [DOI] [PubMed] [Google Scholar]
- 36.Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline J-P, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8(1):55–73. doi: 10.1007/s13300-016-0223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vigersky RA, McMahon C. The relationship of hemoglobin A1C to time–in–range in people with diabetes. Diabetes Technol Ther. 2019;21(2):81–85. doi: 10.1089/dia.2018.0310. [DOI] [PubMed] [Google Scholar]
- 38.Hirsch IB, Sherr JL, Hood KK. Connecting the dots: validation of time in range metrics with microvascular outcomes. Diabetes Care. 2019;42(3):345–348. doi: 10.2337/dci18-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beck RW, Bergenstal RM, Cheng P, et al. The relationships between time in range, hyperglycemia metrics, and HbA1c. J Diabetes Sci Technol. 2019;13(4):614–626. doi: 10.1177/1932296818822496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kesavadev J, Shankar A, Krishnan G, et al. Is Time-in-range independent of A1C? A study in Asian Indian population. Diabetes. 2020;69(Suppl 1):880. doi: 10.2337/db20-880-P. [DOI] [Google Scholar]
- 41.Mazze RS, Lucido D, Langer O, Hartmann K, Rodbard D. Ambulatory glucose profile: Representation of verified self-monitored blood glucose data. Diabetes Care. 1987;10(1):111–117. doi: 10.2337/diacare.10.1.111. [DOI] [PubMed] [Google Scholar]
- 42.Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2020 executive summary. Endocr Pract. 2020;26(1):107–139. doi: 10.4158/CS-2019-0472. [DOI] [PubMed] [Google Scholar]
- 43.Chawla R, Madhu SV, Makkar BM, et al. RSSDI-ESI Clinical Practice Recommendations for the management of type 2 diabetes mellitus 2020. Indian J Endocrinol Metab. 2020;24(1):1–122. doi: 10.4103/ijem.IJEM_225_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Modi KD, Jha S, Banzal S, et al. Adoption of Gla-100 in India and its impact on insulin usage patterns. J Assoc Physicians India. 2020;68(12):25–30. [PubMed] [Google Scholar]
- 45.Mohan V, Radhika G, Sathya RM, Tamil SR, Ganesan A, Sudha V. Dietary carbohydrates, glycaemic load, food groups and newly detected type 2 diabetes among urban Asian Indian population in Chennai, India (Chennai Urban Rural Epidemiology Study 59) Br J Nutr. 2009;102(10):1498–1506. doi: 10.1017/S0007114509990468. [DOI] [PubMed] [Google Scholar]
- 46.Unnikrishnan R, Anjana RM, Mohan V. Diabetes mellitus and its complications in India. Nat Rev Endocrinol. 2016;12(6):357–370. doi: 10.1038/nrendo.2016.53. [DOI] [PubMed] [Google Scholar]
- 47.Gupta L, Khandelwal D, Singla R, Gupta P, Kalra S. Pragmatic dietary advice for diabetes during Navratris. Indian J Endocrinol Metab. 2017;21(1):231–237. doi: 10.4103/2230-8210.196009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saboo B, Joshi S, Shah SN, et al. Management of diabetes during fasting and feasting in India. J Assoc Physicians India. 2019;67(9):70–77. [PubMed] [Google Scholar]
- 49.Narayan KMV, Kanaya AM. Why are South Asians prone to type 2 diabetes? A hypothesis based on underexplored pathways. Diabetologia. 2020;63(6):1103–1109. doi: 10.1007/s00125-020-05132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Unnikrishnan R, Anjana RM, Mohan V. Diabetes in South Asians: is the phenotype different? Diabetes. 2014;63(1):53–55. doi: 10.2337/db13-1592. [DOI] [PubMed] [Google Scholar]
- 51.Hernandez AM, Jia P, Kim HY, Cuadros DF. Geographic variation and associated covariates of diabetes prevalence in India. JAMA Netw Open. 2020;3(5):e203865. doi: 10.1001/jamanetworkopen.2020.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahlqvist E, Prasad RB, Groop L. Subtypes of type 2 diabetes determined from clinical parameters. Diabetes. 2020;69(10):2086–2093. doi: 10.2337/dbi20-0001. [DOI] [PubMed] [Google Scholar]
- 53.Anjana RM, Pradeepa R, Unnikrishnan R, et al. New and unique clusters of type 2 diabetes identified in indians. J Assoc Physicians India. 2021;69(2):58–61. [PubMed] [Google Scholar]
- 54.Anjana RM, Shanthirani CS, Unnikrishnan R, et al. Regularity of follow–up, glycemic burden, and risk of microvascular complications in patients with type 2 diabetes: a 9–year follow–up study. Acta Diabetol. 2015;52(3):601–609. doi: 10.1007/s00592-014-0701-0. [DOI] [PubMed] [Google Scholar]
- 55.Murphy HR. Continuous glucose monitoring targets in type 1 diabetes pregnancy: every 5%time in range matters. Diabetologia. 2019;62(7):1123–1128. doi: 10.1007/s00125-019-4904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nallaperumal S, Bhavadharini B, Mahalakshmi MM, et al. Comparison of the world health organization and the International association of diabetes and pregnancy study groups criteria in diagnosing gestational diabetes mellitus in South Indians. Indian J Endocrinol Metab. 2013;17(5):906–909. doi: 10.4103/2230-8210.117241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campbell FM, Murphy NP, Stewart C, Biester T, Kordonouri O. Outcomes of using flash glucose monitoring technology by children and young people with type 1 diabetes in a single arm study. Pediatr Diabetes. 2018;19(7):1294–1301. doi: 10.1111/pedi.12735. [DOI] [PubMed] [Google Scholar]
- 58.Praveen PA, Madhu SV, Viswanathan M, et al. Demographic and clinical profile of youth onset diabetes patients in India-Results from the baseline data of a clinic based registry of people with diabetes in India with young age at onset-[YDR-02] Pediatr Diabetes. 2021;22(1):15–21. doi: 10.1111/pedi.12973. [DOI] [PubMed] [Google Scholar]
- 59.Viswanathan M, Ranjit Mohan A, Ranjit U, et al. Clinical Profile of elderly patients (over 90 years) with type 2 diabetes seen at a diabetes center in south India. Diabetes Technol Ther. 2020;22(2):79. doi: 10.1089/dia.2019.0219. [DOI] [PubMed] [Google Scholar]