Graphical abstract

Keywords: Intelligent packaging film, Anthocyanins, Freshness monitoring, Antioxidant, Edible film
Highlights
-
•
An intelligent food packaging was prepared from black rice anthocyanin, chitosan and pectin.
-
•
This intelligent packaging can monitor the freshness of meat in real time.
-
•
With natural active substances, the membrane has high safety.
Abstract
With the improvement of consumer awareness of food safety and the increasing concern about plastic pollution, the development of novel intelligent packaging film is imminent. This project aims to develop an environmentally friendly pH-sensitive intelligent food packaging film for meat freshness monitoring. In this study, anthocyanin-rich extract from black rice (AEBR) was added to composite film formed by the co-polymerisation of pectin and chitosan. AEBR showed strong antioxidant activity, and different colour responses to different conditions. The mechanical properties of the composite film remarkably improved when AEBR was incorporated into. Besides, the introduction of anthocyanins enables the colour of composite film to change from red to blue with the degree of meat spoilage increased which shows the indicative effect of composite films on meat putrification. Therefore, the AEBR-loaded pectin/chitosan film could be used as an indicator to monitor meat freshness in real-time.
Introduction
Meat is rich in fat and protein, which is prone to oxidation and microbial contamination during storage (Anas et al., 2019). Therefore, every year, an enormous amount of meat is discarded and wasted because of spoilage in the meat supply chain, which caused huge economic losses. In addition, conventional physiochemical detection, microbial properties, and sensory evaluation of food freshness have disadvantages such as cumbersome operation, long detection period and inaccurate evaluation (Alkhaled et al., 2021), which make it difficult to obtain accurate real-time information on meat freshness for producers, sellers and consumers and further lead to the waste of meat in the food supply chain. Therefore, the development of intelligent active food packaging that can detect the freshness of meat in real-time and extend the shelf life of meat is conducive to avoiding the waste of meat in the food supply chain and enabling consumers to accurately grasp the quality information of meat, which is of great significance for standardizing the order of food market and safeguarding the rights and interests of consumers.
The development of intelligent food packaging is extremely rapid. In 1962, Lawdermilt (Lawdermilt, 1962) proposed the use of indicator labels based on pH indicators (such as magenta acid) and CO2 absorbers (KOH) to indicate the change in acidity during dairy product spoilage. However, single-dye solutions feature some problems, such as the small colour range of pH and non-obvious colour change (Valuk et al., 2005). Continuous research revealed that the pH indicator label produced by mixing various dyes can achieve good indication results (Kuswandi & Nurfawaidi, 2017). Unfortunately, if chemical dyes are used for food safety testing, then food may be contaminated by the volatilisation of toxic substances, which may harm people’s health (Latos-Brozio & Masek, 2020). Therefore, chemical dyes are not the most suitable materials for the production of intelligent food packaging (Shao et al., 2021, Vu and Won, 2013). Recent theoretical developments have revealed that anthocyanins are a class of natural pH indicators that have antioxidant and bacteriostatic activities (Moradi et al., 2019). Anthocyanins can react with the gas generated during food spoilage and have a wide range of colour development and reversibility (Zhang J. et al., 2019). Therefore, anthocyanins are ideal materials for the addition of intelligent food packaging films as pH indicators and preservative ingredients (Dainelli et al., 2008). Currently, one of the major topics to be investigated in intelligent packaging is the use of anthocyanins to prepare pH-sensitive composite films. In 2017, Choi (Choi et al., 2017) developed an intelligent packaging film with purple potato anthocyanins as a pH indicator to detect the freshness of pork.
Rice is a vital crop for human beings and about half the world population, including almost all of East and Southeast Asia, is completely dependent on rice as staple food. There are many varieties of rice, and the content of anthocyanin in different varieties is different. Among them, the content of anthocyanin in black rice (Oryza sativa L) is particularly prominent (Shao et al., 2018). Black rice has many physiological activities, such as anti-oxidation, lipid-lowering, anticancer, antibacterial and anti-allergy functions (Ito & Lacerda, 2019). Studies have shown that the physiological activity of black rice is mainly attributed to the abundant anthocyanins in the seed coat (Wu et al., 2019). Moreover, black rice shows excellent antioxidant activity due to its high content of phenols, especially anthocyanins, and has the potential to be used in the preparation of active packaging to delay meat spoilage (Fang et al., 2020). However, the application of black rice anthocyanins in intelligent packaging film is rarely studied (Shao et al., 2021, Wu et al., 2019).
In this experiment, we tested the detailed performance of anthocyanins in preservation and intelligent indication. Anthocyanins were used as a pH indicator to prepare the intelligent active packaging film based on chitosan and pectin. The effects of different doses of AEBR on the physical properties of composite film were tested. In addition, we characterized the changes of AEBR and AEBR-loaded pectin/chitosan film under volatile ammonia and meat preservation models by UV–vis chromatography to explore the feasibility and discolouration mechanism of anthocyanin film in practical application.
Experiment
Materials
Black rice (Oryza sativa L.), pork and beef were bought from Xin Hua Capital Supermarket in Zhang Zhou. Pectin (d-galacturonic acid content: ≥74 %, mean molecular weight: 109625, esterification degree: 55 %) and chitosan (deacetylation degree: 95.6 %, viscosity: 100–200 mPa·s) were provided by Shanghai Macklin Biochemical Technology Co., ltd. (Shanghai, China). Ascorbic acid (Vc) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) were provided by Shanghai Yuanye Bio-Technology Co., ltd. Absolute ethanol, acetic acid, potassium chloride, hydrochloric acid (37 %), ammonia (28 %) and sodium acetate were obtained from Xilong Scientific Co., ltd. All reagents were of analytical grade except as otherwise noted.
Extraction of anthocyanins from black rice
Firstly, black rice was crushed using a grinder (TAISITE Instrument, China) and sifted through a 40 mesh sieve to obtain a dry powder material, which was stored in a brown reagent bottle in the dark. Subsequently, 2 % acetic acid–water solution and absolute ethyl alcohol (1:1) were mixed as the extractant. The black rice flour and extractant were mixed at the solid–liquid ratio of 1:20 (g: mL). After 30 min of ultrasonic treatment, the mixture was heated in a water bath at 50 ℃ for 90 min. The supernatant was centrifuged at 4 ℃ and 4000 rpm, and the impurities were precipitated again. Lastly, the solvent was removed by freeze-drying, and the solid powder, the anthocyanin-rich extract from black rice (AEBR), was collected (Pang et al., 2018).
Determination of the total anthocyanins content of the AEBR
The total anthocyanin content (TAC) of the AEBR was determined by differential pH method with cyanidin-3-glucoside as the reference material by referring to the previous study (Wang P. et al., 2022). The anthocyanins were added to buffer solutions with pH 1.0 (0.5 mol/L citric acid/HCl) and pH 4.5 (0.5 mol/L citric acid/dipotassium phosphate) and dissolved completely. The absorbances of the two solutions at 513 and 700 nm were measured by MultiSkan Go (Thermo Scientific, U.S.A) after the solutions was left in the dark for 60 min. The TAC of the AEBR was calculated as follows:
| (1) |
where A (absorbance) = ( − )pH1.0 − ( − )pH4.5; MW is the relative molecular weight for cyanidin-3-O-glucoside (449.2 g/mol); DF is the dilution factor (V/Wt); ε is the molar absorptivity for cyanidin-3-O-glucoside (26900 L/mol·cm−1); and L is the optical path (cm).
Intelligent indication of the AEBR
Colour responses of the AEBR to pH values
Buffer solutions with pH values from 1 to 13 were prepared, and 5 mg AEBR was dissolved in 100 mL of the buffer solutions. The absorption characteristics and pH response of the AEBR were analysed by a MultiSkan Go microplate reader (Thermo Fisher Co., ltd., USA).
Colour response of the AEBR to volatile ammonia
AEBR solution (25 mL, 50 mg/L) and ammonia solution (5 mL, 0.6 wt%) were added to the inner and outer chambers of the Conway diffusion dish, respectively. The UV–Vis spectra of the AEBR solution were obtained every 10 min by a microplate reader.
Colour response of the AEBR to the odour of spoiled meat
AEBR solution (25 mL, 50 mg/L) and 25 g meat (pork and beef were used separately) were added to the inner and outer chambers of the Conway diffusion dish, respectively. The UV–Vis spectra of the AEBR solution were obtained every 24 h by a microplate reader.
Antioxidant activity of the AEBR
DPPH solution (72 mL) was placed into 18 centrifuge tubes (10 mL). Exactly 0.1–0.8 mL of AEBR solution (20 mg/L) and 0.1–0.8 mL of ascorbic acid solution (50 mg/L) were placed into the centrifuge tubes. All samples were placed in the dark for 30 min and scanned at full wavelength by a microplate reader. The DPPH radical scavenging rate was calculated according to Formula (2) by adding 4 mL of DPPH into the centrifuge tube as the blank control (Zhang W. et al., 2019):
| (2) |
where A0 is the absorption of the blank control group and An is the absorption of the sample.
Preparation of anthocyanin/pectin/chitosan (APC) composite films
Firstly, 8 g chitosan was added to 200 mL of deionised water and stirred magnetically for 30 min until evenly dispersed. Then, 2 mL of glacial acetic acid was slowly added to the solution, and chitosan was fully dissolved by magnetic stirring for 12 h. Afterwards, 4 g pectin, 2 mL of glycerol and a certain amount of AEBR were dissolved in 100 mL of deionised water. Then, the two solutions were mixed and stirred magnetically for 4 h until fully mixed. Finally, 20 mL of the membrane solution was added to a Petri dish with 9 cm diameter and placed in a drying oven at 40 ℃ to remove moisture to obtain the composite film (Ye et al., 2020).
The composite films with AEBR contents of 0, 0.1, 0.2, 0.3 and 0.4 g were coded as APC0, APC1, APC2, APC3 and APC4, respectively.
Measurement of the mechanical properties of the APC composite films
The APC composite films were cut into 4 mm × 50 mm dumbbell-shaped strips, which were customised according to GB/T-258 III. Both ends of the prepared samples were clamped with a TA-RT-LIT probe, and the mechanical properties of the APC composite films were analysed by a BROOKFIELD (USA) CT3-10 K texturiser.
Intelligent indication of the APC composite films
Colour response of the APC composite films to pH values
The composite films were cut into squares with 4 cm edge and soaked in buffer solution (20 mL) with pH 1.0–13.0 on a glass dish for 5 min. The colour change of the composite film was recorded using a colourimeter. The result was expressed as Lab colour space (L*: lightness, a*: red to green; b*: yellow to blue).
Response of the APC composite films to the odour of spoiled meat
A 4 cm × 4 cm composite film and 25 g meat (pork and beef are used separately) were added to the inner and outer chambers of the Conway diffusion dish, respectively. The UV–Vis spectra of the composite film were determined every 24 h by a microplate reader.
Characterization
The surface molecular groups of the AEBR and APC composite films were monitored using a Nicolet IS 10 Fourier transform infrared (FTIR) spectrometer (Thermo Fisher Co., ltd., USA) at the scanning resolution of 4 cm−1 in the frequency region from 4000 cm−1 to 400 cm−1, and the number of scans was 64. A JSM-6010LA scanning electron microscope (Electronics Co., ltd., Japan) was used to observe the micro-images of AEBR in an appropriate field of vision.
Statistical analysis
All tests were repeated thrice. Statistical analyses were performed using SPSS 16.0. One-way ANOVA and independent sample t-test were conducted to compare differences, and p < 0.05 indicated a significant difference.
Results and discussion
Typical characteristics of the AEBR
Fig. 1(a) shows the FTIR spectrum of the AEBR. A wide and strong absorption peak could be observed at 3340 cm−1, which was ascribed to the presence of the phenolic and alcoholic hydroxyl groups of the AEBR (de Oliveira et al., 2018). The peak at 2979 cm−1 was caused by the methylene stretching vibration on the glycosyl group (Fug et al., 2016). Besides, acyl groups were observed in the AEBR. The characteristic peak of the stretching vibration of the conjugated carbonyl group was observed at ∼ 1644 cm−1 (Huang et al., 2017), which corresponded to the structure of the α-carbonyl group on the benzene ring. The absorption peaks at ∼ 1455 and ∼ 1415 cm−1 were assigned to the skeleton vibrations of aromatic and heterocyclic rings of benzopyran in the anthocyanins. A series of absorption peaks at ∼ 1383, ∼1327 and ∼ 1273 cm−1 corresponded to the stretching vibration of carbon–oxygen bonds in the glucosyl rings of the AEBR (Liu et al., 2019). Therefore, black rice anthocyanins are a kind of partially acylated compound consisting of a six-membered heterocyclic ring with one oxygen heteroatom and –OH and –OCH3 groups in the benzene ring structure.
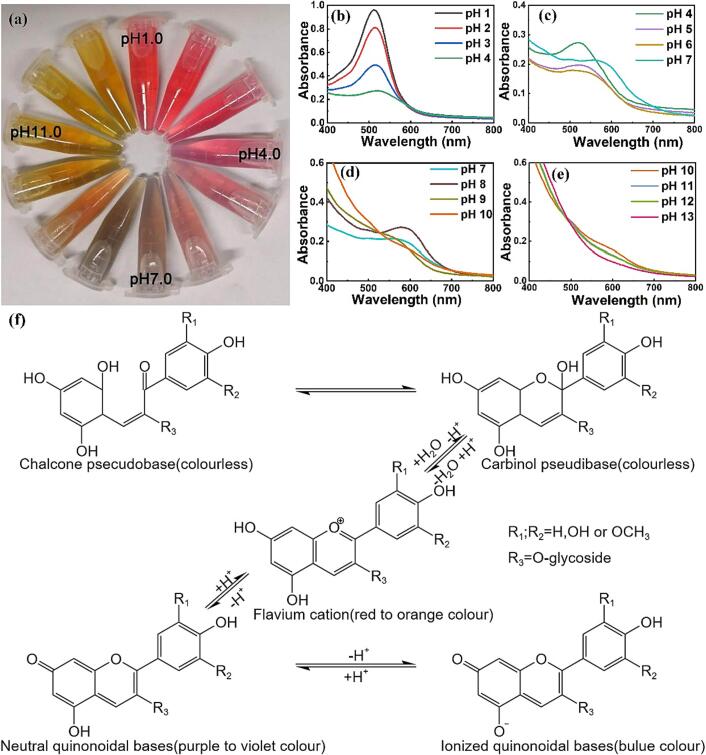
Fig. 1.
FTIR spectrum (a), apparent morphology (b), UV–Vis spectra (c) and DPPH radical scavenging (d) of the AEBR.
Fig. 1(b) presents the apparent morphology of the AEBR. The surface of the anthocyanins had many folds and wrinkles, which might be caused by the structure formed by the crosslinking of several polysaccharides in the anthocyanins. This structure greatly increased the surface area of the AEBR (Zhu et al., 2019). Considering the special structure of the AEBR, it can react sensitively with spoilage gases and demonstrate good ability for intelligent indication (Li et al., 2019).
The TAC value of the AEBR was determined based on the UV–Vis spectra at pH 1.0 and 4.5. The TAC value of the AEBR determined by pH differential calculation was 468.37 mg/g as shown in Fig. 1(c). The antioxidant activity of the AEBR was characterised by DPPH radical scavenging, and the result is presented in Fig. 1(d). In this part of the experiment, the AEBR and ascorbic acid had the same concentration of 50 mg/L. As shown in the figure, the DPPH radical scavenging of the two free radical scavengers increased with the increase in dose. The IC50 values of DPPH radical scavenging by the AEBR and ascorbic acid were 4.46 and 34.56 µg/L, respectively, which indicated that the scavenging capacity of the AEBR was much higher than that of ascorbic acid. Free radicals are atoms or groups with unpaired electrons, which are formed by the homogeneous splitting of the covalent bonds of compounds under external conditions, such as light and heat. In food, the number of free radicals increases with time (Liang et al., 2019). Free radicals weaken the resistance of cells and produce chemicals that destroy cells, making food susceptible to microbial infection and oxidative deterioration. The AEBR had a strong antioxidant activity; thus, it can scavenge free radicals and inhibit food spoilage. Therefore, the AEBR can help preserve food effectively (Ahmad et al., 2018, Pang et al., 2018).
Intelligent indication of the AEBR
Colour response of the AEBR to pH
Anthocyanins are plant polyphenols that are widely distributed in nature. They exist in most fruit seed coats to varying degrees. During the ripening of black rice, a large amount of anthocyanins accumulate in the seed coat, making brown rice appear red, yellow and even black (Ito & Lacerda, 2019). The colour response of the AEBR to pH is shown in Fig. 2(a), and the corresponding UV–Vis spectra are presented in Fig. 2(b–e), corresponding to the four structural changes in anthocyanins at different pH values. From pH 1.0 to 4.0, the absorption peak appeared at 520 nm because of the existence of anthocyanins in the form of red flavylium cation. The absorption peak strength at 520 nm decreased gradually with the increase in pH value because of the conversion of flavylium cations into colourless methanol and chalcone pseudo-bases (Ebrahimi Tirtashi et al., 2019). When the pH value increased to 7.0, the flavylium cation almost disappeared, and the maximum absorption wavelength of the anthocyanins red-shifted (Fig. 2c). When the pH value reached 8.0, the solution was yellow-green, and the maximum absorption wavelength of anthocyanins was 600 nm. These changes are due to the transformation of anthocyanins into the blue quinone structure and the methylation reaction on its aromatic ring (Sinopoli et al., 2019). As the pH value continued to rise, the colour of anthocyanins gradually changed to yellow, and the maximum absorbance increased accordingly (Fig. 2d). When the pH further increased to 9.0–13.0, the solution turned yellow, and the absorption peak at 600 nm decreased with the increase in pH. Therefore, the quinone structure of the anthocyanins was unstable under strong alkali environment, and the resonant-stable quinone anion formed. Therefore, AEBR was sensitive to pH and could change from amaranth to red, colourless, green and yellow-green with different pH values, which allows the AEBR to act efficiently in the real-time monitoring of food freshness.
Fig. 2.
Colour (a), UV–Vis spectra (b, c, d, e) and structural changes (f) of the AEBR at different pH values Significance in difference indicated by different capital letter, p ≤ 0.05.
Colour response of the AEBR to volatile ammonia and meat spoilage odour
Amines produced by amino acid decarboxylation are important indicators of food spoilage and microbial growth. These amines comprise basic gases, such as ammonia, hydrogen sulphide and dimethylamine. They can alter the pH of the surrounding environment, resulting in different colour changes in anthocyanins (Mansur et al., 2019). We aimed to test the colour response of the AEBR to ammonia and meat samples, evaluate the sensitivity of the AEBR to spoilage gases and evaluate the feasibility of the use of the AEBR to monitor food spoilage. We used ammonia as the characteristic gas of spoilage to simulate the volatilisation of nitrogen-containing compounds in meat products during spoilage. We used pork and beef as the meat samples and carried out experiments under daily living conditions.
Fig. 3 (a) and (b) shows the visible spectra of the AEBR exposed to ammonia for 80 min at intervals of 10 min. During the first 10 min, the absorbance of the anthocyanin solution decreased sharply. This is due to the rise in the pH of the solution in the presence of ammonia gas, which causes the anthocyanins to change from the red configuration to the colourless configuration. With the extension of exposure time, the absorbance of the solution began to rise and red shift occurred. This is obviously because the pH of the solution continues to rise, and the molecular configuration of the anthocyanins gradually changes to quinone base.
Fig. 3.
UV–Vis spectra of the AEBR under volatilisation of ammonia (a, b) and spoilage odour of pork (c) and beef (d).
As shown in Fig. 3 (c) and (d), the absorption values of the AEBR on pork and beef were the same. The absorption peak of anthocyanins at 520 nm decreased gradually in the first 2 days, accompanied with colour fading. This result indicated that the red flavylium cation structure of anthocyanins gradually decreased and converted into colourless substances, such as methanol and chalcone pseudo-bases. The flavylium cation structure of the AEBR almost disappeared, its maximum absorption wavelength red-shifted and the absorption peak further decreased with the increase in pork and beef spoilage. The anthocyanins turned chartreuse at this time. A blue quinone structure formed, and the methylation of aromatic rings in the structure made anthocyanins turn yellow (Tang et al., 2019). Hence, the AEBR showed different colour changes depending on the degree of food spoilage.
FTIR spectra, mechanical properties and dissolution characteristics of APC composite films
The composite films were prepared by mixing chitosan and pectin, during which different amounts of anthocyanins were added. The apparent groups of pectin, chitosan and composite film were characterised by FTIR, and the results are presented in Fig. 4 (a) and (b). The broad band at ∼ 3442 cm−1 was assigned to –OH, and the peak at ∼ 2924 cm−1 was caused by the stretching vibration of –CH2 and –CH3. Besides, in Fig. 5(b), the peak at ∼ 1635 cm−1 in the spectrum of pectin was attributed to the associated –COOH, and that in the spectrum of chitosan was assigned to the flexural vibration of –NH2(Xiong et al., 2016). In addition, the peaks at 1120–965 cm−1 corresponded to the C—O—C of pectin and chitosan. Moreover, in the spectrum of the APC2 composite film, two new peaks appeared at ∼ 1547 and ∼ 1395 cm−1, which might be caused by the stretching vibration of bands Ⅱ and III of amide. This condition could be deduced from the fact that the –COOH of pectin bonded to the –NH2 of chitosan and formed an amide bond (Wang S. et al., 2022).
Fig. 4.
FTIR spectra (a, b), tensile strength (c), tensile modulus (d), UV–Vis spectra (e) and dissolution characteristic (f) of the APC composite films Significance in difference indicated by different capital letter, p ≤ 0.05.
Fig. 5.
Response of the APC2 composite film to meat spoilage odour. (a, b): FTIR spectra of the response of the composite film during pork spoilage; (c, d) FTIR spectra of the response of the composite film during beef spoilage; (e): UV–Vis spectra of the response of the composite film during pork spoilage; (f): UV–Vis spectra of the response of the composite film during beef spoilage.
Mechanical properties are one of the most important properties of food packaging film. Tensile strength and tensile modulus were measured and are presented in Fig. 4(c) and (d). The tensile strength of the composite films increased with the additive dosage of the AEBR. A possible explanation for this phenomenon was that the AEBR containing sugar moiety filled the gel network formed by pectin and chitosan and therefore enhanced the tensile strength of the composite film. Besides, the addition of the AEBR also had a positive effect on the tensile modulus of the composite films, and APC2 obtained the highest value. However, the tensile modulus of the composite film began to decrease with the further increase in AEBR content. This outcome might be due to excessive anthocyanins entering the gel network formed by pectin and chitosan, making the structure more loose. In addition, the modulus reflects the properties of the material, and the change in tensile modulus can predict the interaction between molecules. It could be seen that anthocyanins not only bound to the deprotonated carboxyl groups of the pectin side chains through electrostatic interaction due to the structure of the flavylium cation, but also formed hydrogen bonds to chitosan and pectin, which enhanced the density of the chitosan/pectin cross-linked network, resulting in enhancement of mechanical properties of the composite film (Xiong et al., 2017). However, when the amount of anthocyanin exceeds the optimal ratio, the balance of electrostatic interaction between pectin and chitosan with opposite charge was broken, resulting in the loose structure of the network and the reduction of the tensile modulus (Xiong & Ma, 2017). Furthermore, the tensile modulus of APC4 was still higher than that of APC0, indicating that the addition of anthocyanins is positive for the mechanical properties of the composite film due to the presence of hydrogen bonds.
The UV–Vis spectra of the composite films are presented in Fig. 4(e). The maximum absorption wavelength of the composite film was 575 nm, and a high AEBR dosage results in a higher absorbance of the composite film. After the composite film was soaked in deionised water for 30 min, the UV spectrum of the aqueous solution was characterised. The result is shown in Fig. 4(f), which reflects the dissolution characteristic of the AEBR in the composite film. When the anthocyanin content reached 0.3 g, the anthocyanins were dissolved obviously in the composite film and could be distinguished by naked eyes.
Intelligent indicator of the APC composite films
Colour response of the APC composite films
The AEBR showed different colours in different pH environments, which endows it a potential application in food intelligent indicator packaging film. Then, the composite film containing anthocyanins were added into buffer solutions with different pH values, and their colour changes were recorded as shown in Table 1 to determine whether AEBR could maintain an intelligent indicator function in composite films. As mentioned in Section 3.3, excessive anthocyanins would lead to their dissolution from the composite film; therefore, the APC2 composite film was used to measure the intelligent indicator characteristics of the composite films.
Table 1.
Colour response of the APC2 composite film at different pH values.
| L | a* | b* | ||
|---|---|---|---|---|
| pH 1 |  |
49.5 ± 2.4 | 36.7 ± 2.8 | 5.1 ± 1.1 |
| pH 2 |  |
52.9 ± 5.3 | 26.2 ± 3.4 | 6.3 ± 0.9 |
| pH 3 |  |
48.5 ± 4.7 | 21 ± 2.4 | 6.3 ± 1.2 |
| pH 4 |  |
45.7 ± 2.9 | 14.6 ± 1.6 | 2.9 ± 0.7 |
| pH 5 |  |
41.1 ± 3.4 | 8.6 ± 0.8 | 0.4 ± 0.7 |
| pH 6 |  |
41.3 ± 5.9 | 4.4 ± 1.0 | −1.4 ± 0.7 |
| pH 7 |  |
33.1 ± 3.4 | 0.6 ± 0.5 | −2.3 ± 0.8 |
| pH 8 |  |
41.8 ± 3.2 | −0.2 ± 0.4 | −3.1 ± 0.7 |
| pH 9 |  |
43.5 ± 2.6 | 1.6 ± 1.2 | −3.3 ± 0.8 |
| pH 10 |  |
46.9 ± 4.0 | −2.2 ± 0.4 | −3.2 ± 0.9 |
| pH 11 |  |
52.7 ± 4.3 | −2.7 ± 0.7 | −4.0 ± 1.8 |
| pH 12 |  |
43.3 ± 2.1 | −0.4 ± 0.5 | −6.1 ± 0.9 |
| pH 13 |  |
53.3 ± 1.3 | 0 ± 0.5 | 7.1 ± 0.3 |
As shown in Table 1, the composite film showed a magenta colour at pH 1.0–4.0. a* and b* values decreased gradually with the gradual increase in pH. This finding indicated that the magenta colour of the film became lighter, and blue became the dominant colour. The structure of the AEBR in the composite film still changed from red flavylium cation to colourless methanol pseudo-base and chalcone (Ebrahimi Tirtashi et al., 2019). Beyond pH 5.0, the values of a* and b* continued to decrease, and the blue colour continued to deepen. However, at pH 13.0, the composite film suddenly turned chartreuse, which was due to the instability of blue ionised quinone alkali in the anthocyanin structure of the film under strong alkali conditions and the formation of resonantly stable quinone anions (Sinopoli et al., 2019). Hence, the AEBR could maintain its original pH response after being added to the composite film, and the change in colour caused by the change in its own structure reflected the pH environment of the AEBR. Given the sensitivity of anthocyanins to pH, the composite film with anthocyanins can be used to indicate the freshness of food.
Response of the APC composite film to meat spoilage odour
The experimental results showed that the composite film could inherit the properties of anthocyanins well after the addition of the AEBR into the composite film. Therefore, the AEBR composite film was used to wrap fresh pork and beef, and its infrared and visible absorption spectra were analysed to further explore the discolouration effect and practicability of the AEBR composite film.
The FTIR spectra of the APC2 composite films were measured and are shown in Fig. 5(a–d). Similar to the FTIR spectra of anthocyanins, a strong and wide absorption peak was observed at 3402 cm−1 because of the existence of hydroxyl groups and polysaccharides on the benzene ring and the hydrogen bond association between hydroxyl groups. The peaks at 2924 cm−1 were attributed to the stretching vibration of methylene and methine, respectively. The peak at 1642 cm−1 was the stretching vibration characteristic peak of the conjugated carbonyl group, which corresponded to the structure of α-carbonyl group in the benzene ring of the AEBR. The characteristic peak at 1548 cm−1 was caused by the stretching vibration of the nitro group in the anthocyanins’ carbon chain. A new peak appeared at 1535 cm−1 on the second day, which was assigned to the stretching vibration of nitro groups on aromatic rings (Stoyanov et al., 2023). The pork and beef deteriorated with the increase in the preservation time, resulting in the increase in volatile basic nitrogen content in the system. The elevated volatile basic nitrogen content destroyed the skeleton structure of the AEBR and changed the structure and colour of the anthocyanins (Zeng et al., 2019). As depicted in Fig. 5(e) and (f), the colour of the AEBR in the composite films changed remarkably with the increase in meat spoilage and the release of volatile basic nitrogen. This result was due to the alteration in the anthocyanin structure under alkaline conditions (Jiang et al., 2020). Therefore, the AEBR can be added to the composite film to indicate the freshness of food.
Conclusion
As natural pigments, black rice anthocyanins have abundant sources, high safety coefficient and good antioxidant capacity. They can prevent food spoilage and prolong food shelf life. The AEBR showed different colour changes depending on the changes in food freshness. Furthermore, when the AEBR was added into the pectin–chitosan composite film, the AEBR maintained its original properties and changed in structure in accordance with the changes in the environment. Therefore, the AEBR is an ideal composite material for intelligent indicator packaging film (Kalpana et al., 2019). It may be used in the preparation of pH-sensitive intelligent food packaging film, which can be used as an intelligent indicator of food freshness. It is convenient for consumers to identify food freshness. This study provides a reference for the development and application of anthocyanins from black rice.
At present, the research of intelligent packaging is deepening. However, the application of anthocyanin intelligent film are still facing a significant challenge. Future research on the establishment of accurate indication relationship between indicators and food freshness and the enhancement of hydrophobicity of composite membranes might expand the application prospect of anthocyanin intelligent composite films.
CRediT authorship contribution statement
Fansen Zeng: Investigation, Data curation, Formal analysis, Writing – review & editing. Yanqi Ye: Data curation, Investigation. Jingna Liu: Writing – original draft, Formal analysis, Funding acquisition. Peng Fei: Supervision, Project administration, Funding acquisition, Formal analysis, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors gratefully acknowledge the Natural Science Foundation of Fujian Province of China (2021J01999 and 2022J01905). In addition, the authors would like to thank Shuting Ren from Shiyanjia Lab (www.shiyanjia.com) for the FTIR analysis.
Contributor Information
Jingna Liu, Email: liujingnaa@163.com.
Peng Fei, Email: fp@bio.mnnu.edu.cn, fp@bio.mnnu.edu.com.
Data availability
Data will be made available on request.
References
- Ahmad M., Ashraf B., Gani A., Gani A. Microencapsulation of saffron anthocyanins using β glucan and β cyclodextrin: Microcapsule characterization, release behaviour & antioxidant potential during in-vitro digestion. International Journal of Biological Macromolecules. 2018;109:435–442. doi: 10.1016/j.ijbiomac.2017.11.122. [DOI] [PubMed] [Google Scholar]
- Alkhaled A., Parrish C., Adedeji A. Comprehensive Reviews in Food Science and Food Safety. 2021. Emerging non-destructive approaches for meat quality and safety evaluation A review BD 03272021; p. 20. [DOI] [PubMed] [Google Scholar]
- Anas, M., Ahmad, S., & Malik, A. (2019). Microbial Escalation in Meat and Meat Products and Its Consequences. In (pp. 29-49).
- Choi I., Lee J.Y., Lacroix M., Han J. Intelligent pH indicator film composed of agar/potato starch and anthocyanin extracts from purple sweet potato. Food Chemistry. 2017;218:122–128. doi: 10.1016/j.foodchem.2016.09.050. [DOI] [PubMed] [Google Scholar]
- Dainelli D., Gontard N., Spyropoulos D., Zondervan-van den Beuken E., Tobback P. Active and intelligent food packaging: Legal aspects and safety concerns. Trends in Food Science & Technology. 2008;19 S103–S112. [Google Scholar]
- de Oliveira I.R.N., Roque J.V., Maia M.P., Stringheta P.C., Teófilo R.F. New strategy for determination of anthocyanins, polyphenols and antioxidant capacity of Brassica oleracea liquid extract using infrared spectroscopies and multivariate regression. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2018;194:172–180. doi: 10.1016/j.saa.2018.01.006. [DOI] [PubMed] [Google Scholar]
- Ebrahimi Tirtashi F., Moradi M., Tajik H., Forough M., Ezati P., Kuswandi B. Cellulose/chitosan pH-responsive indicator incorporated with carrot anthocyanins for intelligent food packaging. International Journal of Biological Macromolecules. 2019;136:920–926. doi: 10.1016/j.ijbiomac.2019.06.148. [DOI] [PubMed] [Google Scholar]
- Fang J., Luo Y., Yuan K., Guo Y., Jin S. Preparation and evaluation of an encapsulated anthocyanin complex for enhancing the stability of anthocyanin. LWT-Food Science and Technology. 2020;117 [Google Scholar]
- Fug F., Rohe K., Vargas J., Nies C., Springborg M., Possart W. 4,4′-methylene diphenyl diisocyanate–Conformational space, normal vibrations and infrared spectra. Polymer. 2016;99:671–683. [Google Scholar]
- Huang L., Zhou Y., Meng L., Wu D., He Y. Comparison of different CCD detectors and chemometrics for predicting total anthocyanin content and antioxidant activity of mulberry fruit using visible and near infrared hyperspectral imaging technique. Food Chemistry. 2017;224:1–10. doi: 10.1016/j.foodchem.2016.12.037. [DOI] [PubMed] [Google Scholar]
- Ito V.C., Lacerda L.G. Black rice (Oryza sativa L.): A review of its historical aspects, chemical composition, nutritional and functional properties, and applications and processing technologies. Food Chemistry. 2019;301 doi: 10.1016/j.foodchem.2019.125304. [DOI] [PubMed] [Google Scholar]
- Jiang G., Hou X., Zeng X., Zhang C., Wu H., Shen G.…Zhang Z. Preparation and characterization of indicator films from carboxymethyl-cellulose/starch and purple sweet potato (Ipomoea batatas (L.) lam) anthocyanins for monitoring fish freshness. International Journal of Biological Macromolecules. 2020;143:359–372. doi: 10.1016/j.ijbiomac.2019.12.024. [DOI] [PubMed] [Google Scholar]
- Kalpana S., Priyadarshini S.R., Maria Leena M., Moses J.A., Anandharamakrishnan C. Intelligent packaging: Trends and applications in food systems. Trends in Food Science & Technology. 2019;93:145–157. [Google Scholar]
- Kuswandi B., Nurfawaidi A. On-package dual sensors label based on pH indicators for real-time monitoring of beef freshness. Food Control. 2017;82:91–100. [Google Scholar]
- Latos-Brozio M., Masek A. The application of natural food colorants as indicator substances in intelligent biodegradable packaging materials. Food and Chemical Toxicology. 2020;135 doi: 10.1016/j.fct.2019.110975. [DOI] [PubMed] [Google Scholar]
- Lawdermilt, R. F. (1962) Spoilage indicator for food containers. US-3067015-A.Washington, DC: U.S. Patent and Trademark Office.
- Li Y., Ying Y., Zhou Y., Ge Y., Yuan C., Wu C., Hu Y. A pH-indicating intelligent packaging composed of chitosan-purple potato extractions strength by surface-deacetylated chitin nanofibers. International Journal of Biological Macromolecules. 2019;127:376–384. doi: 10.1016/j.ijbiomac.2019.01.060. [DOI] [PubMed] [Google Scholar]
- Liang T., Sun G., Cao L., Li J., Wang L. A pH and NH3 sensing intelligent film based on Artemisia sphaerocephala Krasch. gum and red cabbage anthocyanins anchored by carboxymethyl cellulose sodium added as a host complex. Food Hydrocolloids. 2019;87:858–868. [Google Scholar]
- Liu Y., Qin Y., Bai R., Zhang X., Yuan L., Liu J. Preparation of pH-sensitive and antioxidant packaging films based on κ-carrageenan and mulberry polyphenolic extract. International Journal of Biological Macromolecules. 2019;134:993–1001. doi: 10.1016/j.ijbiomac.2019.05.175. [DOI] [PubMed] [Google Scholar]
- Mansur A.R., Seo D.-H., Song E.-J., Song N.-E., Hwang S.H., Yoo M., Nam T.G. Identifying potential spoilage markers in beef stored in chilled air or vacuum packaging by HS-SPME-GC-TOF/MS coupled with multivariate analysis. LWT-Food Science and Technology. 2019;112 [Google Scholar]
- Moradi M., Tajik H., Almasi H., Forough M., Ezati P. A novel pH-sensing indicator based on bacterial cellulose nanofibers and black carrot anthocyanins for monitoring fish freshness. Carbohydrate Polymers. 2019;222 doi: 10.1016/j.carbpol.2019.115030. [DOI] [PubMed] [Google Scholar]
- Pang Y., Ahmed S., Xu Y., Beta T., Zhu Z., Shao Y., Bao J. Bound phenolic compounds and antioxidant properties of whole grain and bran of white, red and black rice. Food Chem. 2018;240:212–221. doi: 10.1016/j.foodchem.2017.07.095. [DOI] [PubMed] [Google Scholar]
- Shao P., Liu L., Yu J., Lin Y., Gao H., Chen H., Sun P. An overview of intelligent freshness indicator packaging for food quality and safety monitoring. Trends in Food Science & Technology. 2021;118:285–296. [Google Scholar]
- Shao Y., Hu Z., Yu Y., Mou R., Zhu Z., Beta T. Phenolic acids, anthocyanins, proanthocyanidins, antioxidant activity, minerals and their correlations in non-pigmented, red, and black rice. Food Chemistry. 2018;239:733–741. doi: 10.1016/j.foodchem.2017.07.009. [DOI] [PubMed] [Google Scholar]
- Sinopoli A., Calogero G., Bartolotta A. Computational aspects of anthocyanidins and anthocyanins: A review. Food Chemistry. 2019;297 doi: 10.1016/j.foodchem.2019.05.172. [DOI] [PubMed] [Google Scholar]
- Stoyanov S., Yancheva D., Velcheva E., Stamboliyska B. Anion and radical anion products of flutamide studied by IR spectra and density functional calculations. Journal of Molecular Structure. 2023;1271 [Google Scholar]
- Tang B., He Y., Liu J., Zhang J., Li J., Zhou J.…Wang X. Kinetic investigation into pH-dependent color of anthocyanin and its sensing performance. Dyes and Pigments. 2019;170 [Google Scholar]
- Valuk V.F., Duportail G., Pivovarenko V.G. A wide-range fluorescent pH-indicator based on 3-hydroxyflavone structure. Journal of Photochemistry and Photobiology A: Chemistry. 2005;175(2):226–231. [Google Scholar]
- Vu C.H.T., Won K. Novel water-resistant UV-activated oxygen indicator for intelligent food packaging. Food Chemistry. 2013;140(1):52–56. doi: 10.1016/j.foodchem.2013.02.056. [DOI] [PubMed] [Google Scholar]
- Wang P., Liu J., Zhuang Y., Fei P. Acylating blueberry anthocyanins with fatty acids: Improvement of their lipid solubility and antioxidant activities. Food Chemistry: X. 2022;15 doi: 10.1016/j.fochx.2022.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Gao Z., Liu L., Li M., Zuo A., Guo J. Preparation, in vitro and in vivo evaluation of chitosan-sodium alginate-ethyl cellulose polyelectrolyte film as a novel buccal mucosal delivery vehicle. European Journal of Pharmaceutical Sciences. 2022;168 doi: 10.1016/j.ejps.2021.106085. [DOI] [PubMed] [Google Scholar]
- Wu C., Sun J., Zheng P., Kang X., Chen M., Li Y.…Pang J. Preparation of an intelligent film based on chitosan/oxidized chitin nanocrystals incorporating black rice bran anthocyanins for seafood spoilage monitoring. Carbohydrate Polymers. 2019;222 doi: 10.1016/j.carbpol.2019.115006. [DOI] [PubMed] [Google Scholar]
- Xiong Z., Ma M. Enhanced ovalbumin stability at oil-water interface by phosphorylation and identification of phosphorylation site using MALDI-TOF mass spectrometry. Colloids and Surfaces B: Biointerfaces. 2017;153:253–262. doi: 10.1016/j.colsurfb.2017.02.027. [DOI] [PubMed] [Google Scholar]
- Xiong Z., Ma M., Jin G., Xu Q. Effects of site-specific phosphorylation on the mechanical properties of ovalbumin-based hydrogels. International Journal of Biological Macromolecules. 2017;102:1286–1296. doi: 10.1016/j.ijbiomac.2017.05.028. [DOI] [PubMed] [Google Scholar]
- Xiong Z., Zhang M., Ma M. Emulsifying properties of ovalbumin: Improvement and mechanism by phosphorylation in the presence of sodium tripolyphosphate. Food Hydrocolloids. 2016;60:29–37. [Google Scholar]
- Ye Y., Zeng F., Zhang M., Zheng S., Li J., Fei P. Hydrophobic edible composite packaging membrane based on low-methoxyl pectin/chitosan: Effects of lotus leaf cutin. Food Packaging and Shelf Life. 2020;26 [Google Scholar]
- Zeng P., Chen X., Qin Y., Zhang Y., Wang X., Wang J.…Zhang Y. Preparation and characterization of a novel colorimetric indicator film based on gelatin/polyvinyl alcohol incorporating mulberry anthocyanin extracts for monitoring fish freshness. Food Research International. 2019;126 doi: 10.1016/j.foodres.2019.108604. [DOI] [PubMed] [Google Scholar]
- Zhang J., Zou X., Zhai X., Huang X., Jiang C., Holmes M. Preparation of an intelligent pH film based on biodegradable polymers and roselle anthocyanins for monitoring pork freshness. Food Chemistry. 2019;272:306–312. doi: 10.1016/j.foodchem.2018.08.041. [DOI] [PubMed] [Google Scholar]
- Zhang W., Jiang H., Yang J., Jin M., Du Y., Sun Q.…Xu H. Safety assessment and antioxidant evaluation of betulin by LC-MS combined with free radical assays. Analytical Biochemistry. 2019;587 doi: 10.1016/j.ab.2019.113460. [DOI] [PubMed] [Google Scholar]
- Zhu K., Yu D., Chen X., Song G. Preparation, characterization and controlled-release property of Fe3+ cross-linked hydrogels based on peach gum polysaccharide. Food Hydrocolloids. 2019;87:260–269. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.