Highlights
-
•
MGO induced structural changes of tropomyosin (TM) during thermal processing.
-
•
Lys, Arg, Asp, and Gln residues of TM were modified by MGO treatment.
-
•
TM-MGO can reduce the mediator and cytokine releases in RBL-2H3 cells.
-
•
MGO decreased TM-specific IgE/IgG1, histamine and mMCP-1 levels in vivo.
-
•
MGO caused changes in TM allergic epitopes resulting in lower allergenicity.
Keywords: Shrimp, Tropomyosin, Methylglyoxal, Allergenicity
Abstract
This study aimed to analyze the effect of methylglyoxal (MGO) on the structure and allergenicity of shrimp tropomyosin (TM) during thermal processing. The structural changes were determined by SDS-PAGE, intrinsic fluorescence, circular dichroism, and HPLC-MS/MS. The allergenicity was evaluated by in vitro and in vivo experiments. MGO could cause conformational structural changes in TM during thermal processing. Moreover, the Lys, Arg, Asp, and Gln residues of TM were modified by MGO, which could destroy and/or mask TM epitopes. In addition, TM-MGO samples could lead to lower mediators and cytokines released from RBL-2H3 cells. In vivo, TM-MGO caused a significant reduction in antibodies, histamine, and mast cell protease 1 levels in sera. These results indicate that MGO can modify the allergic epitopes and reduce the allergenicity of shrimp TM during thermal processing. The study will help to understand the changes in the allergenic properties of shrimp products during thermal processing.
Introduction
Shrimp is favored by consumers for its rich nutrition and delicious taste. It is widely used as a functional ingredient in food processing. However, shrimp also is one of the common “big-8” food allergens. Shrimp allergy is an important food safety issue affecting allergic patients’ health (Li et al., 2020, Ruethers et al., 2018, Shek et al., 2010). Shrimp allergy generally caused immunoglobulin E (IgE)-mediated allergic symptoms, including asthma, urticaria, and even shock and death (Renz et al., 2018, Volpicella et al., 2019).
Tropomyosin (TM) was considered a major allergenic protein in shrimp, which can cause up to 80 % of allergic reactions (Fan et al., 2022). TM is a heat-stable protein with 32–38 kDa molecular weight and 4.2 isoelectric points. More than 90 % of shrimp-allergic people contain TM-specific antibodies (Tsedendorj et al., 2018, Zhang et al., 2021, Zhang et al., 2019). Therefore, the allergenicity of shrimp should be considered when it is used as a food ingredient.
Many studies have reported that the allergenicity of shrimp TM could be influenced by different processing methods, such as thermal processing, high pressure, irradiation, and so on. Among them, thermal processing is widely considered utilizing during food processing (Xu et al., 2022, Zhang et al., 2021). However, thermal processing could cause complex reactions within food ingredients including protein oxidation and Maillard reaction (Misnan et al., 2016, Zhao et al., 2022, Zhao et al., 2022). Maillard reaction is induced by reducing sugars, which could alter the structures and physicochemical properties of dietary proteins due to sugar degradation products. a-Dicarbonylic compounds (a-DCs) like glyoxal (GO) and methylglyoxal (MGO) are important intermediates of the Maillard reaction that exist during thermal processing (Dasanayaka et al., 2022, Gupta et al., 2018, Zhang et al., 2022). In addition, the a-DCs also inevitably react with the shrimp protein as the shrimp reheats.
a-DCs could interact with the side-chain groups of arginine (Arg) and lysine (Lys) residues. Among all of the a-DCs, MGO exists the most widely in heat-processed foods (Kim et al., 2021, Wang et al., 2020, Zheng et al., 2022, Zhuang et al., 2020). MGO in fats and oils is also formed by lipid degradation during processing. The amount of MGO formed in fish oil heated at 60℃ for 7 days was 2.03–2.89 mg/kg (Fujioka et al., 2004). The previous study reported that MGO could modify the amino acid residues (Arg 184, 185) of glutathione peroxidase (Hirahara et al., 2020, Schmitz et al., 2017). The sugar degradation of basic amino acids (such as Lys and Arg) could change the functional properties of protein and decrease its nutritional value. However, the effects of MGO on the allergenicity of shrimp TM haven’t been investigated during thermal processing.
The study aimed to analyze the influences of MGO on shrimp TM structure and allergenicity during thermal processing. The structures of the treated samples were measured by SDS-PAGE, circular dichroism (CD), intrinsic fluorescence (IF), ultraviolet (UV), and high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS), and the allergenicity was evaluated in vitro and in vivo experiments. The study will provide basic data for the safety evaluation of shrimp products.
Materials and methods
Chemical
The ice fresh shrimp was purchased at the local supermarket (Qingdao, China). MGO, bovine serum albumin (BSA), and Al(OH)3 were acquired from Sigma Chemical Co. (St. Louis, MO, USA). HRP-labeled goat anti-human IgE antibody was acquired from Zhongshan jinqiao Co. (Beijing, China). ELISA kits for histamine, mouse mast cell protease 1 (mMCP-1), interleukin 4 (IL-4), and interleukin 13 (IL-13) were acquired from R&D Systems (Minneapolis, MN, USA), respectively.
Human sera
The allergic sera to TM were collected from allergic patients for shrimp. All the patient records showed anaphylaxis with different allergic symptoms. Moreover, special IgE levels above 5 kU/L for allergic sera were acquired. The selected sera were stored at −20 ∼ -70 °C before the experiment (Table S1 of Support Information).
Purification of shrimp TM
The samples were prepared as described previously (Wang et al. 2018). The fresh shrimp muscle was extracted with two volumes of a solution containing 1 mol L−1 KHCO3, 20 mmol L−1 KCl, and 0.1 mol L−1 dithiothreitol. The resultant homogenate was centrifuged at 4000 × g for 10 min five times. The extract was then discarded, and the shrimp homogenate was extracted in 1 L of 95 % ethanol for 10 min and extracted in 1 L of diethyl ether for 10 min in triplicate, respectively. Later, the shrimp homogenate was dried for 2 h and extracted by incubating overnight with 5 volumes of a solution containing 0.01 mol L−1 NaN3 (pH 7.4) and 1 mol L−1 KCl at 4℃. The protein was then salted out with precisely 30 % (NH4)2SO4. Finally, the solution was heated in a boiling water bath for 8 min and then was immediately cooled on ice, followed by a centrifugation at 8000 × g. The supernatant containing the purified TM was collected and stored at −20 ℃ until use.
Samples preparation
The purified shrimp TM was diluted to 2 mg mL−1 with phosphate buffer solution (PBS, 0.01 mol/L, pH 7.4). MGO was added to the purified shrimp TM solution to keep a mass ratio of TM to MGO with 1:4, 1:8, and 1:16, respectively. The final samples were then heated at 60 °C, 80 °C, and 100 °C for 15 min, respectively. After the reaction, all the samples were cooled to below 4 °C. The unreacted MGO was dialyzed to remove by deionized water at 4 °C, and finally, all the samples were freeze-dried and kept at −20 ∼ -80 °C.
SDS-PAGE analysis
SDS-PAGE was carried out by the previous study (Lv et al., 2017). Each protein sample was mixed 4:1 with loading buffer (containing 5 % 2-mercaptoethanol), and then the sample mixtures were heated in boiling water for 6 min. After heating, the samples were loaded on prefab gel. After electrophoresis, the prefab gels were dyed using Coomassie Brilliant Blue R-250G overnight and were subsequently destained with deionized water to visualize proteins. The result was acquired using Tanon-4200SF (Tanon Science and Technology Co., Ltd, Shanghai, China).
Structural analysis
UV absorption
The method of UV absorption spectra was referenced to the report of Yuan et al (Yuan et al., 2017). All the samples were adjusted to 0.5 mg mL−1 using PBS (0.01 mol/L, pH 7.4) and scanned at 200 nm- 350 nm with a 400 nm min−1 scanning speed. PBS solution (pH 7.4) was considered as one blank.
Intrinsic fluorescence
The intrinsic fluorescence was determined referenced to the report of Wu et al. (Wu et al., 2011). The samples were diluted to 1 mg mL−1 in PBS and then measured by a Hitachi F2700 fluorescence spectrometer (Shimadzu, Kyoto, Japan). The parameters were set as follows: 300 nm min−1 scanning speed, 280 nm excitation wavelength, 300–400 nm emission wavelength, and 2.5 nm slit width.
Circular dichroism
CD spectroscopy was carried out to determine the secondary structure of MGO treated TM (TM-MGO) during thermal processing. The detailed experiment was measured by a JASCO J-815CD spectropolarimeter (Japan Spectroscopic Co. Inc., Japan) based on the previous method (Lv, Lin, et al., 2019). The samples were diluted to 1 mg ml−1. The detailed parameters were set as follows: 1 nm bandwidth, 100 nm min−1 scan speed,190 to 260 nm wavelength ranges, and 0.25 s response time.
ELISA assay
ELISA assay was determined by our previous study (Lv et al., 2019). The TM samples (100 μL) in 50 mmol/L sodium carbonate buffer (pH 9.6) were added to a 96-wells plate and then kept overnight at 4 °C. 100 μL pooled human sera (diluted 1:60) was used as a primary antibody. Moreover, 100 μL HRP-labelled goat antihuman IgE antibody (diluted 1:2000) was used as the secondary antibody. After incubation and washing, TMB (100 μL) was added to react for 20 min at 37 °C. And then the reaction was terminated by sulfuric acid (2 mol/L). Finally, the absorbance was determined on an ELISA plate reader.
RBL-2H3 cells assays
RBL-2H3 cells were cultured by the study of Zhang et al. with some modifications (Zhang et al., 2019). RBL-2H3 cells (1x107 cells/mL) were pre-incubated for 20 h with 100 μL allergic patients’ sera (v/v = 4:1) and further washed three times with Tyrode’s buffer. And then 10 μL TM-MGO samples (5 mg/mL) in Tyrode's buffer were added to incubate for 4 h at 37 °C, respectively. Finally, the supernatant was collected from the cells to measure β-hexosaminidase, histamine, IL-4 and IL-13 contents using ELISA kits.
Mice experiment for sensitization and challenge
According to the results of structures and RBL-2H3 cells, TM and TM-MGO samples at 100℃ were selected for the mice experiment. Forty female BALB/c mice (Six-week-old) were acquired by SPF Biotechnology (Qingdao, China). All experiments involving animals were performed following established guidelines and the experimental protocols were in accordance with the Ethical Committee of Experimental Animal Care of the Qingdao Agricultural University. All the mice were acclimated for at least 1 week before sensitization and then divided into five groups: control (no stimulation), TM, and TM-MGO (1:4; 1:8; 1:16) groups. The sensitization experiment was performed by the method of Samadi et al (Samadi et al., 2020). As shown in Fig. 5A, mice were immunized with 5 mg of native TM or TM-MGO samples with 15 μg alum adjuvant on days 0, 7, 14, and 21. The control groups were sensitized with 500 μL PBS containing 15 μg alum adjuvant. On day 28, all the mice were challenged with 500 μL PBS containing 20 mg of TM, and TM-MGO samples, respectively. The control group was challenged with 500 μL PBS. After 1 h, anaphylactic symptoms were assayed by an established scoring system (Table S2 of Support Information)). All mice were then euthanized on day 30, and the sera were acquired to determine IgE, IgG, IgG1, mMCP-1, and histamine levels by ELISA kits.
Fig. 5.
(A) Sensitization experiments. (B) Hypersensitivity symptoms were scored on a scale from 0 (no symptoms) to 5 (death), as shown in Table S1 of the Support Information. Levels of (C) IgE, (D) IgG, (E) IgG1, (F) plasma histamine, and (G) mMCP-1 in serum from mice. Levels of (H) IL-4; (I) IL-5; (J) IL-13 and (K) IFN-γ on the secretions of cytokines from splenic lymphocytes, respectively. Results are expressed as the mean ± SD. (∗∗) p < 0.01, and (∗) p < 0.05 represent significant differences compared to the TM group.
Cytokine analysis for spleen cell
The spleen cells were acquired from mice spleens. Subsequently, the spleen cells were cultured in RPMI-1640 medium containing 1 % penicillin–streptomycin and 10 % FBS solution. The spleen cells (2*105 cells/mL) were re-stimulated with the different samples for 3 days. Finally, the cells were centrifuged to collect the supernatant, which was acquired to determine interferon γ (IFN-γ), IL-4, and IL-13 contents by ELISA kits.
Statistical analysis
The obtained data were processed by GraphPad Prism 9.0.2 software. Moreover, the results are expressed as means ± SD. The differences were analyzed by ANOVA with SPSS Statistics (Chicago, USA). Statistical significance was considered at P < 0.05.
Results
SDS-PAGE analysis
The SDS-PAGE results are shown in Fig. 1A. The purified TM has a clear band with ∼ 36 kDa molecular weight under the reaction conditions of 60℃, 80℃, and 100℃, respectively (Fig. 1A, Lane 0). The molecular weight for all treated bands of TM-MGO samples was about 36 kDa at 60℃. Interestingly, the protein bands slightly migrate after MGO treatment at 80℃, and the bands with increasing MGO concentration migrate more obviously. And the molecular weight of different TM-MGO samples is about 42–45 kDa. In addition, compared with the protein bands at 80℃, the protein bands of TM-MGO samples migrate and formed the aggregation at 100℃. And the band formed the largest aggregation with a ratio of TM and MGO (nTM: nMGO = 1:16).
Fig. 1.
(A) SDS-PAGE of shrimp TM modified by MGO during thermal processing (a: Incubated at 60, b: Incubated at 80℃, c: Incubated at 100℃, lane M: protein marker; lane 0: control; lane 1: nTM: nMGO = 1:4; lane 2: nTM: nMGO = 1:8; lane 3: nTM: nMGO = 1:16). (B) The IgE binding capacity of shrimp TM modified by MGO (a: control; b: nTM: nMGO = 1:4; c: nTM: nMGO = 1:8; d: nTM: nMGO = 1:16. The data represent the mean ± SD of triplicate measurements. ∗, p < 0.05; ∗∗, p < 0.01.).
The IgE binding capacity
To determine whether TM-MGO samples influence the immunoreactivity, the IgE binding capacity of TM-MGO was assayed by ELISA (Fig. 1B). The IgE binding capacity of TM-MGO (nTM: nMGO = 1:4; nTM: nMGO = 1:8; nTM: nMGO = 1:16) samples decreased by 6.3 ± 0.5 %, 10.5 ± 0.7 % and 14.7 ± 0.8 % at 60 ℃, respectively. while the IgE-binding capacity decreased by 9.7 ± 0.4 %, 13.9 ± 0.6 % and 22.6 ± 0.7 % at 80 ℃, respectively. In addition, the IgE-binding capacity decreased by 16.3 ± 0.6 %, 20.9 ± 0.9 % and 33.7 % ± 1.3 % at 100 ℃, respectively. Thus, the IgE binding of TM was reduced with increasing MGO concentration.
Intrinsic fluorescence analysis
Intrinsic fluorescence spectrum could measure the conformational structure of the protein. The samples of TM-MGO with different MGO concentrations were subjected to the intrinsic fluorescence spectrum at 60℃,80℃,and 100℃, respectively (Fig. 2 A). The intrinsic fluorescence absorption peak of shrimp TM was ∼ 315 nm, and the intensity of the intrinsic fluorescence spectrum gradually decreased with the increasing MGO concentration. Compared with the maximum fluorescence absorption peak of TM, the peak of TM-MGO (nTM: nMGO = 1:4; nTM: nMGO = 1:8; nTM: nMGO = 1:16) samples decreased by 20.8 %, 35 % and 50 % at 60℃, respectively (Fig. 2 A-a). The maximum fluorescence absorption peak decreased by 7.69 %, 38.46 %, and 84.62 % at 80℃, respectively (Fig. 2 A-b). In addition, the maximum fluorescence absorption peaks decreased by 40.9 %, 45.45 %, and 63.63 % at 100℃, respectively (Fig. 2 A-c). Moreover, the maximum fluorescence absorption appeared blue shifting from 320 nm to 313 nm.
Fig. 2.
(A) UV absorption spectra, (B) Intrinsic fluorescence spectra, and (C) Circular dichroism of shrimp TM modified by MGO during thermal processing (a: Incubated at 60, b: Incubated at 80℃, c: Incubated at 100℃).
CD analysis
CD spectrum can be utilized to analyze the secondary structure of the protein. The results of the TM-MGO samples were shown by CD spectrum in Fig. 2 B. The purified TM showed a positive peak near 198 nm, a negative peak at 212 nm and 223 nm, and a negative groove between 212 nm and 223 nm at 60℃, 80℃, and 100℃, respectively. The peak of the CD spectrum gradually decreased with increasing MGO concentration.
HPLC-MS/MS analysis
The binding sites of TM-MGO samples were determined by the proteomics approach. According to the SDS-PAGE results of TM-MGO samples (Fig. 1A), the target bands of TM-MGO samples were sliced and further analyzed by HPLC-MS/MS. The result showed that the amino acid residues of TM were modified by MGO, which mainly occurred in the amino acids residues of lysine (Lys), Arginine (Arg), Aspartic acid (Asp), and Glutamine (Gln). The molecular weight increased by 54 Da based on a Mascot search. Moreover, a total of 27 peptides were modified with MGO treatment by HPLC-MS/MS analysis (Table 1).
Table 1.
List of methylglyoxal-modified peptides of shrimp tropomyosin identified after LC-MS/MS.
| Methylglyoxal-modified peptide | site | z | Observed mass | Mr (expt) | Mr (calc) | |
|---|---|---|---|---|---|---|
|
K |
KANIQLVEKDKA | 74 | 2 | 615.3449 | 1228.6753 | 1228.6663 |
| KANIQLVEKDKA | 76 | 2 | 410.5660 | 1228.6761 | 1228.6663 | |
| RKLAMVEADLERA | 168 | 2 | 681.8550 | 1361.6954 | 1361.6860 | |
| KSLEVSEEKANQRE | 213 | 2 | 731.3674 | 1460.7203 | 1460.7107 | |
| RMDALENQLKEARF | 149 | 2 | 745.3755 | 1488.7365 | 1488.7242 | |
| KANQREEAYKEQIKT | 212 | 2 | 839.9273 | 1677.8401 | 1677.8322 | |
| RVVGNNLKSLEVSEEKA | 205 | 2 | 858.9616 | 1715.9087 | 1715.8941 | |
| REEAYKEQIKTLTNKL | 236 | 3 | 589.6492 | 1765.9257 | 1765.9097 | |
| RAEKSEEEVHNLQKRM | 48 | 3 | 590.3021 | 1767.8845 | 1767.8751 | |
| RFLAEEADRKYDEVARK | 161 | 3 | 628.6471 | 1882.9194 | 1882.9061 | |
| RAETGESKIVELEEELRV | 189 | 2 | 952.4842 | 1902.9538 | 1902.9422 | |
| KEVDRLEDELVNEKEKY | 264 | 2 | 958.9807 | 1915.9469 | 1915.9374 | |
| RLNTATTKLAEASQAADESERM | 112 | 2 | 1089.5365 | 2177.0584 | 2177.0447 | |
| KEANNRAEKSEEEVHNLQKR | 37 | 3 | 733.0247 | 2196.0522 | 2196.0406 | |
| KDNAMDRADTLEQQNKEANNRA | 30 | 4 | 807.6975 | 2420.0707 | 2420.0622 | |
|
R |
RAEFAERSVQKL | 244 | 2 | 618.8176 | 1235.6206 | 1235.6146 |
| KAAEARAEFAERS | 238 | 2 | 646.8184 | 1291.6222 | 1291.6156 | |
| KALSNAEGEVAALNRRI | 90 | 2 | 821.9349 | 1641.8553 | 1641.8434 | |
| KANQREEAYKEQIKT | 217 | 3 | 560.2907 | 1677.8501 | 1677.8322 | |
| RKLAMVEADLERAEERA | 178 | 2 | 924.4686 | 1846.9226 | 1846.9094 | |
| RFLAEEADRKYDEVARK | 160 | 3 | 628.6487 | 1882.9244 | 1882.9061 | |
| KDKALSNAEGEVAALNRRI | 91 | 3 | 629.3351 | 1884.9834 | 1884.9653 | |
|
D |
RFLAEEADRKYDEVARK | 163 | 2 | 942.4667 | 1882.9189 | 1882.9061 |
| KEVDRLEDELVNEKEKY | 258 | 3 | 639.6573 | 1915.9502 | 1915.9374 | |
| RLNTATTKLAEASQAADESERM | 121 | 3 | 726.6818 | 2177.0236 | 2177.0447 | |
|
Q |
RRIQLLEEDLERS | 93 | 3 | 495.9382 | 1484.7928 | 1484.7834 |
| RLNTATTKLAEASQAADESERM | 118 | 3 | 726.6949 | 2177.0630 | 2177.0447 |
As shown in Fig. 3, the TM-MGO adducts involved two or more nucleophilic sites. Fig. 3-A shows the MGO adduct formation on the Gln94 residue in the peptide 90Arg-Arg-Ile-Gln-Leu-Leu-Glu-Glu-Asp-Leu-Glu-Arg-Ser102. Fig. 3-B shows MGO adduction in the amino acid residues of Asp32 in the peptide 21Arg-Phe-Leu-Ala-Glu-Glu-Ala-Asp-Arg-Lys-Tyr-Asp-Glu-Val-Ala-Arg-Lys37. Moreover, Arg29 also was modified with the same peptides by MGO (Fig. 3-C). The MGO adduct on the Lys112 residue was found in the peptide 105Arg-Leu-Asn-Thr-Ala-Thr-Thr-Lys-Leu-Ala-Glu-Ala-Ser-Gln-Ala-Ala-Asp-Glu-Ser-Glu-Arg-Met126 (Fig. 3-D).
Fig. 3.
(A) HPLC-MS/MS spectrum for a peptide containing Gln from TM-MGO samples. MGO adduction (*, +54) was observed on the lysine side-chain group in Arg-Arg-Ile-Gln-Leu-Leu-Glu-Glu-Asp-Leu-Glu-Arg-Ser. (B) HPLC-MS/MS spectrum for a peptide containing Asp from TM-MGO samples. MGO adduction (*, +54) was observed on the lysine side-chain group in Arg-Phe-Leu-Ala-Glu-Glu-Ala-Asp-Arg-Lys-Tyr-Asp-Glu-Val-Ala-Arg-Lys. (C) HPLC-MS/MS spectrum for a peptide containing Arg from TM-MGO samples. MGO adduction (*, +54) was observed on the lysine side-chain group in Arg-Phe-Leu-Ala-Glu-Glu-Ala-Asp-Arg-Lys-Tyr-Asp-Glu-Val-Ala-Arg-Lys. (D) HPLC-MS/MS spectrum for a peptide containing Lys from TM-MGO samples. MGO adduction (*, +54) was observed on the lysine side-chain group in Arg-Leu-Asn-Thr-Ala-Thr-Thr-Lys-Leu-Ala-Glu-Ala-Ser-Gln-Ala-Ala-Asp-Glu-Ser-Glu-Arg-Met.
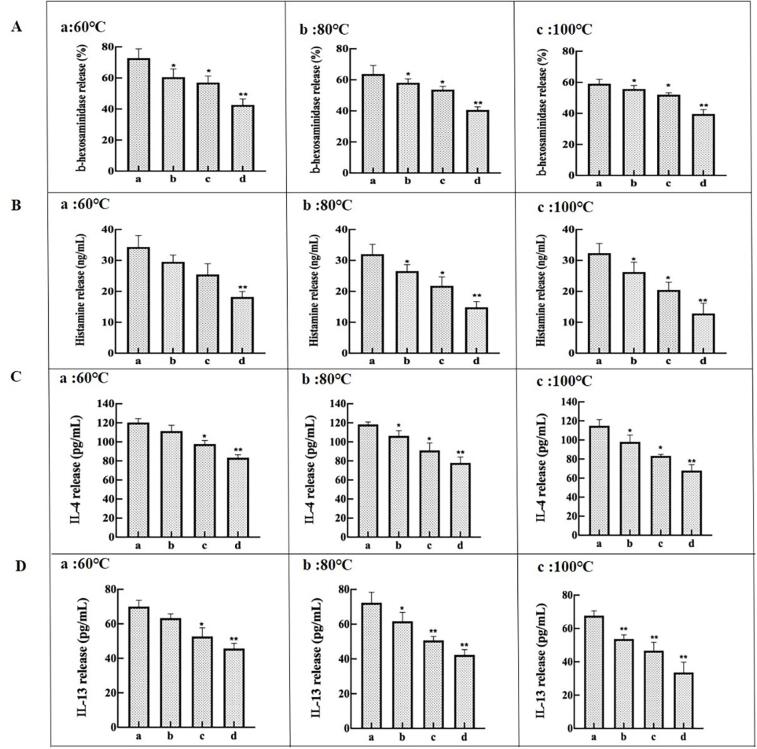
RBL-2H3 cells assay
The potential allergenicity of TM-MGO samples could be assayed by RBL-2H3 cells (Figure 4). RBL-2H3 cells can lead to mediator release (β-hexosaminidase and histamine release) and cytokines release (IL-4 and IL-13). The release of β-hexosaminidase decreased (22.37 %, 26.32 %, and 43.42 %) in cells exposed to TM-MGO (nTM: nMGO = 1:4; nTM: nMGO = 1:8; nTM: nMGO = 1:16) compared with TM alone at 60℃ (Figure 4A-a). In addition, TM-MGO also caused β-hexosaminidase release to decline (6.45 %, 11.11 %, and 31.75 %) at 80℃ and (3.39 %, 6.78 %, and 30.5 %) at 100℃ (Figure 4A-b, c), respectively. Histamine release of TM-MGO (nTM: nMGO = 1:4; nTM: nMGO = 1:8; nTM: nMGO = 1:16) samples decreased by 17.65 %, 23.53 %, and 47.06 % at 60 ℃ (Figure 4B-a). Histamine release of TM-MGO showed a similar tendency at 80℃ and 100 ℃ (Figure 4B-b, c). Interestingly, mediator release decreased more pronounced with the higher temperature. Moreover, RBL-2H3 cells could also release cytokines such as IL-4 and IL-13. TM-MGO (nTM: nMGO = 1:4; nTM: nMGO = 1:8; nTM: nMGO = 1:16) samples lead to a significant decrease for IL-4 release (2.52 %, 17.65 %, and 26.05 %) at 60℃, (13.45 %, 19.33 %, and 32.77 %) at 80℃, and (15.52 %, 28.45 %, and 41.38 %) at 100℃, respectively (Fig. 4 C). IL-13 release of RBL-2H3 cells decreased by 8.82 %, 17.65 %, and 35.29 % with TM-MGO (nTM: nMGO = 1:4; nTM: nMGO = 1:8; nTM: nMGO = 1:16) treatment at 60 ℃, respectively (Fig. 4D-a). IL-13 release was also reduced with increasing MGO concentration at 80℃ and 100℃, respectively. Similarly, the cytokines releases occurred with a decreased tendency with increasing temperature from 60℃ to 100℃.
Fig. 4.
Impact of TM-MGO on mediators release (A. β-hexosaminidase; B histamine) and cytokines release (C. IL-4; D. IL-13) from RBL-2H3 cells sensitized with anti-TM sera. a: control; b: nTM: nMGO = 1:4; c: nTM: nMGO = 1:8; d: nTM: nMGO = 1:16. The data represent the mean ± SD of triplicate measurements. ∗, p < 0.05; ∗∗, p < 0.01.
In vivo assay
A BALB/c mice model was considered to evaluate the allergenicity of TM-MGO samples as shown in Fig. 5 A. The anaphylactic symptoms were evaluated following the challenge with TM and TM-MGO samples by an established scoring system. The result showed that the anaphylactic symptoms following the challenge with TM-MGO samples were less severe (Fig. 5B). Exposure of mice to TM-MGO (nTM: nMGO = 1:16) result in a significant (p < 0.01) decline of TM-specific antibodies levels in sera (Fig. 5 C-E). The TM-specific IgE/IgG/IgG1 level of the TM-MGO (nTM: nMGO = 1:16) group was ∼2-3 fold lower than that in the purified TM group. Moreover, compared with the TM group, serum histamine level was significantly (p < 0.01) decreased in the TM-MGO group (Fig. 5F). The mMCP-1 level decreased from 122.39 ng/ml to 93.25 ng/ml for the TM-MGO (nTM: nMGO = 1:16) group (Fig. 5G). In addition, Th2-related cytokines including IL-13 and IL-4 from spleen cells were significantly lower after stimulation with TM-MGO samples (Fig. 4 H and I). Interestingly, after re-stimulation with TM-MGO samples, Th1-related cytokine including IFN-γ from spleen cells was increased (Fig. 5 J).
Discussion
This study indicated that the allergenicity of TM-MGO samples is reduced during thermal processing. SDS-PAGE results showed that the bands of shrimp TM essentially unchanged during different thermal processing (Fig. 1A), which illustrated that shrimp TM is a thermally stable protein. Moreover, the molecular weight of TM-MGO samples increased from 60℃ to 100℃. TM-MGO (nTM: nMGO = 1:16) sample formed the aggregation at 100℃. Interestingly, the IgE binding ability of TM-MGO (nTM: nMGO = 1:16) also significantly dropped at 100℃. The reduced IgE binding ability might be attributed to the changes of TM allergic epitopes after MGO treatment during thermal processing. The conformational and linear structural alteration of TM could destroy and/or mask a few allergic epitopes, which could subsequently cause the reduction of IgE binding ability (Song et al., 2015, Lv et al., 2016). The present study demonstrated that MGO could result in changes in the molecular properties of TM. Our findings support existing studies, which confirmed that food allergens aggregations via different processing methods could influence their allergenicity (Shao et al., 2021, Liu et al., 2021).
The aggregation of TM after MGO treatment could cause the change of TM structure. Intrinsic fluorescence of protein is one of the structural characteristics used to evaluate the conformational changes. The intrinsic fluorescence intensity of proteins depends on the changes in the side chain groups of tryptophan and tyrosine residues. Fig. 2A shows the intrinsic fluorescence absorption of TM-MGO samples at 60℃, 80℃, and 100℃. It has been reported above that shrimp TM does not contain tryptophan (Zheng et al., 2011), so tyrosine was considered to analyze the changes of fluorescence intensity for TM-MGO samples. According to the result of their fluorescence absorption peak, the change of endogenous fluorescence peak was not obvious at different temperatures.However, the fluorescence absorption peak decreased more pronounced with the increasing MGO concentration. It is speculated that the lowering of fluorescence intensity might be attributed to the changes in the microenvironment around specific amino acid residues. In addition, this result might also be caused by protein aggregation, which could result in the lower exposure of amino acids in an aqueous environment.
Circular dichroism spectroscopy was used to characterize the secondary structure of protein (Huang et al., 2021). The secondary structure of TM-MGO samples was altered in response to MGO modification during thermal processing. The presence of two peaks at 212 nm and 223 nm indicates an α-helix in TM, while the negative groove at 212 nm-223 nm and a positive peak near 198 nm indicated the β-sheet structure in TM. With increasing the concentration of MGO, the positive peak at 198 nm, the negative peak at 220 nm, and the groove between 210 nm and 225 nm all show a decreasing trend at 60℃, 80℃, and 100℃, respectively. The result indicates that the α-helix and β-sheet contents of TM-MGO samples decreased compared to the purified TM. With increasing the temperature, the contents of α-helix and β-sheet structures didn’t change pronounced, indicating that temperature only has a slight effect on the secondary structure of TM and TM-MGO samples.
The proteomics technology was utilized to analyze the sites of TM modified with MGO treatment. According to the above results, the modified amino acid residues of TM after MGO treatment can alter the conformational (secondary or tertiary) and linear structure, which could influence the functional properties of TM, including allergenicity. Interestingly, the binding sites of amino acids in the side-chains of Lys, Arg, Asp, and Gln were also the sites of shrimp TM allergic IgE epitopes (Lv et al., 2016, Yuan et al., 2017). Therefore, MGO could influence the IgE binding capacity of TM during thermal processing. Further research needs to analyze and evaluate the allergenicity of TM through in vitro and in vivo experiments.
The allergenicity of TM-MGO samples was further assayed by the RBL-2H3 cell model. The IgE-mediated RBL-2H3 cells are reliable models that were considered to evaluate type I allergic responses. The degree of allergic symptoms is generally dependent upon the cellular degranulation components (such as mediators and cytokines releases), which are important indicators to examine body condition. The present study indicated that TM-MGO samples significantly inhibited the mediators and cytokines releases from RBL-2H3 cells at different temperatures, respectively. Zhang et al. (2019) reported that peanut allergens were less likely to trigger RBL-2H3 cell degranulation including mediators and cytokines releases during thermal processing. The possible reason was that modification of shrimp TM via MGO could alter allergic epitopes by Intra/intermolecular interactions. Further, it was speculated that MGO might mask the IgE allergic epitopes, which subsequently hindered basophil and mast cell mediator and cytokine releases for RBL-2H3 cells. The decrease in mast cell degranulation could correlate with the suppression of allergic reactions better.
The results of the in vivo experiment demonstrated that shrimp TM triggered allergic symptoms. Compared to shrimp TM, TM-MGO samples could produce lower allergenicity. The anaphylactic symptoms of mice were relieved more significantly after the TM-MGO samples groups. Moreover, the specific IgE, IgG, and IgG1 levels from mice sera were substantially reduced in TM-MGO group. In addition, the mMCP-1 and histamine levels were also reduced. The results sufficiently illustrated that TM-MGO samples altered the conformational and linear structures of TM, masking the IgE allergic epitopes, thus influencing the potential allergenicity of TM. Interestingly, the decrease of mMCP-1 level for TM-MGO groups furtherly explained the reduced mast cell degranulation reactions. Furthermore, the cytokine production from mice splenocytes were also determined to analyze the immune responses. Th1-related cytokine (IFN-γ) could stimulate Th1 cells to inhibit IgE production, whereas Th2-related cytokines (IL-13 and IL-4) could induce IgG to IgG1 and produce IgE. Compared with TM group, TM-MGO groups upregulated IFN-γ level while downregulated IL-13 and IL-4 levels, thereby leading to a reduction in IgG1 and IgE in mice. Therefore, the results of cytokines from mice splenocytes indicated that TM-MGO samples could promote Th1 and suppress Th2 immune response, inhibiting shrimp TM allergic reaction. The mitigating allergenicity further suggested the reduction in shrimp TM allergic epitopes.
Conclusions
MGO could change the conformational and linear structure, and decrease the potential allergenicity of shrimp TM during thermal processing. The aggregation formation of TM-MGO samples at high temperatures leads to the decrease of intrinsic fluorescence. Consequently, the secondary structure of shrimp TM became considerably disordered. The side-chain amino acid residues including Lys, Arg, Asp, and Gln was modified, which could destroy and/or shield the allergic epitopes. In addition, the allergenicity of TM-MGO samples was reduced by in vitro and in vivo experiments. TM-MGO samples reduced the IgE-binding ability and inhibited the release of mediators and cytokines in vitro. TM-MGO samples reduced their allergenicity during thermal processing by In vivo experiment. This current study was expected to understand the changes in the allergenic properties of shrimp products during thermal processing, and to provide valuable insights for further research into different allergens in various other types of allergenic foods.
CRediT authorship contribution statement
Qingli Yang: Conceptualization, Data curation, Methodology, Writing – original draft. Xin Qu: Writing – review & editing, Supervision. Xiudan Wang: Formal analysis, Investigation. Hongxia Che: Formal analysis, Data curation. Ziqian Huang: Formal analysis, Validation. Xinyu Ge: Resources, Investigation. Liangtao Lv: Resources, Writing – review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to express their thanks to Natural Science Foundation of Shandong Province (ZR2022QC125) and the High-level Talent Start-up Fund from Qingdao Agricultural University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100532.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- Dasanayaka B.P., Li Z., Pramod S.N., Chen Y., Khan M.U., Lin H. A review on food processing and preparation methods for altering fish allergenicity. Critical Reviews in Food Science and Nutrition. 2022;62(7):1951–1970. doi: 10.1080/10408398.2020.1848791. [DOI] [PubMed] [Google Scholar]
- Fan S., Ma J., Li C., Wang Y., Zeng W., Li Q., Zhou J., Wang L., Wang Y., Zhang Y. Determination of Tropomyosin in Shrimp and Crab by Liquid Chromatography-Tandem Mass Spectrometry Based on Immunoaffinity Purification. Frontiers in Nutrition. 2022;9 doi: 10.3389/fnut.2022.848294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka K., Shibamoto T. Formation of genotoxic dicarbonyl compounds in dietary oils upon oxidation. Lipids. 2004;39(5):481–486. doi: 10.1007/s11745-004-1254-y. [DOI] [PubMed] [Google Scholar]
- Gupta R.K., Gupta K., Sharma A., Das M., Ansari I.A., Dwivedi P.D. Maillard reaction in food allergy: Pros and cons. Critical Reviews in Food Science and Nutrition. 2018;58(2):208–226. doi: 10.1080/10408398.2016.1152949. [DOI] [PubMed] [Google Scholar]
- Hirahara I., Kusano E., Jin D., Takai S. Hypermetabolism of glutathione, glutamate and ornithine via redox imbalance in methylglyoxal-induced peritoneal injury rats. Journal of Biochemistry. 2020;167(2):185–194. doi: 10.1093/jb/mvz077. [DOI] [PubMed] [Google Scholar]
- Huang M., Mao Y., Li H., Yang H. Kappa-carrageenan enhances the gelation and structural changes of egg yolk via electrostatic interactions with yolk protein. Food chemistry. 2021;360 doi: 10.1016/j.foodchem.2021.129972. [DOI] [PubMed] [Google Scholar]
- Kim Y., Ahn H., Lee K.G. Analysis of glyoxal, methylglyoxal and diacetyl in soy sauce. Food Science and Biotechnology. 2021;30(11):1403–1408. doi: 10.1007/s10068-021-00918-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Ogorodova L.M., Mahesh P.A., Wang M.H., Fedorova O.S., Leung T.F.…Wong G.W.K. Comparative Study of Food Allergies in Children from China, India, and Russia: The EuroPrevall-INCO Surveys. The Journal of Allergy and Clinical Immunology. Practice. 2020;8(4):1349–1358 e1316. doi: 10.1016/j.jaip.2019.11.042. [DOI] [PubMed] [Google Scholar]
- Liu M., Huan F., Han T.J., Liu S.H., Li M.S., Yang Y., Wu Y.H., Chen G.X., Cao M.J., Liu G.M. Combination Processing Method Reduced IgE-Binding Activity of Litopenaeus vannamei by Modifying Lysine, Arginine, and Cysteine on Multiple Allergen Epitopes. Journal of agricultural and food chemistry. 2021;69(16):4865–4873. doi: 10.1021/acs.jafc.1c00718. [DOI] [PubMed] [Google Scholar]
- Lv L., Lin H., Li Z., Ahmed I., Chen G. Determining the effect of malondialdehyde on the IgE-binding capacity of shrimp tropomyosin upon in vitro digestion. Journal of the Science of Food and Agriculture. 2017;97:4588–4594. doi: 10.1002/jsfa.8328. [DOI] [PubMed] [Google Scholar]
- Lv L., Lin H., Li Z., Nayak B., Ahmed I., Tian S.…Zhao J. Structural changes of 2,2'-azobis(2-amidinopropane) dihydrochloride (AAPH) treated shrimp tropomyosin decrease allergenicity. Food Chemistry. 2019;274:547–557. doi: 10.1016/j.foodchem.2018.09.030. [DOI] [PubMed] [Google Scholar]
- Lv L., Lin H., Li Z., Yuan F., Gao Q., Ma J. Effect of 4-hydroxy-2-nonenal treatment on the IgE binding capacity and structure of shrimp (Metapenaeus ensis) tropomyosin. Food Chemistry. 2016;212:313–322. doi: 10.1016/j.foodchem.2016.05.152. [DOI] [PubMed] [Google Scholar]
- Lv L., Tian S., Ahmed I., Ramesh Pavase T., Lin H., Xu L.…Liu F. Effect of laccase-catalyzed cross-linking on the structure and allergenicity of Paralichthys olivaceus parvalbumin mediated by propyl gallate. Food Chemistry. 2019;297 doi: 10.1016/j.foodchem.2019.124972. [DOI] [PubMed] [Google Scholar]
- Misnan, R., Salahudin Abd Aziz, N., Mohamad Yadzir, Z. H., Bakhtiar, F., Abdullah, N., & Murad, S. (2016). Impacts of Thermal Treatments on Major and Minor Allergens of Sea Snail, Cerithidea obtusa (Obtuse Horn Shell). Iranian Journal of Allergy, Asthma, and Immunology, 15(4), 309-316. https://www.ncbi.nlm.nih.gov/pubmed/27921412. [PubMed]
- Renz H., Allen K.J., Sicherer S.H., Sampson H.A., Lack G., Beyer K., Oettgen H.C. Food allergy. Nature Review. Disease Primers. 2018;4:17098. doi: 10.1038/nrdp.2017.98. [DOI] [PubMed] [Google Scholar]
- Ruethers T., Taki A.C., Johnston E.B., Nugraha R., Le T.T.K., Kalic T.…Lopata A.L. Seafood allergy: A comprehensive review of fish and shellfish allergens. Molecular Immunology. 2018;100:28–57. doi: 10.1016/j.molimm.2018.04.008. [DOI] [PubMed] [Google Scholar]
- Samadi N., Klems M., Heiden D., Bauer R., Kitzmuller C., Weidmann E.…Untersmayr E. Nitrated food proteins induce a regulatory immune response associated with allergy prevention after oral exposure in a Balb/c mouse food allergy model. Allergy. 2020;75(2):412–422. doi: 10.1111/all.14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A.E., de Souza L.F., Dos Santos B., Maher P., Lopes F.M., Londero G.F.…Dafre A.L. Methylglyoxal-Induced Protection Response and Toxicity: Role of Glutathione Reductase and Thioredoxin Systems. Neurotoxicity Research. 2017;32(3):340–350. doi: 10.1007/s12640-017-9738-5. [DOI] [PubMed] [Google Scholar]
- Shao Y.H., Zhang Y., Zhang L., Liu J., Tu Z.C. Mechanism of Reduction in Allergenicity and Altered Human Intestinal Microbiota of Digested β-Lactoglobulin Modified by Ultrasonic Pretreatment Combined with Glycation. Journal of agricultural and food chemistry. 2021;69(46):14004–14012. doi: 10.1021/acs.jafc.1c03501. [DOI] [PubMed] [Google Scholar]
- Shek, L. P., Cabrera-Morales, E. A., Soh, S. E., Gerez, I., Ng, P. Z., Yi, F. C., . . . Lee, B. W. (2010). A population-based questionnaire survey on the prevalence of peanut, tree nut, and shellfish allergy in 2 Asian populations. Journal of Allergy and Clinical Immunology, 126(2), 324-331, 331 e321-327. 10.1016/j.jaci.2010.06.003. [DOI] [PubMed]
- Song Y., Li Z., Lin H., Du S., Hao Z., Lin H., Zhu Z. Effect of malondialdehyde treatment on the IgE binding capacity and conformational structure of shrimp tropomyosin. Food chemistry. 2015;175:374–380. doi: 10.1016/j.foodchem.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Tsedendorj O., Chinuki Y., Ueda K., Kohno K., Adachi A., Morita E. Tropomyosin is a minor but distinct allergen in patients with shrimp allergies in Japan. Journal of Cutaneous Immunology and Allergy. 2018;1:100–108. doi: 10.1002/cia2.12019. [DOI] [Google Scholar]
- Volpicella M., Leoni C., Dileo M.C.G., Ceci L.R. Progress in the Analysis of Food Allergens through Molecular Biology Approaches. Cells. 2019;8(9) doi: 10.3390/cells8091073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zou L., Yuan F., Lv L., Tian S., Li Z., Lin H. Inhibition of advanced glycation endproducts during fish sausage preparation by transglutaminase and chitosan oligosaccharides induced enzymatic glycosylation. Food Function. 2018;9(1):253–262. doi: 10.1039/c7fo01092c. [DOI] [PubMed] [Google Scholar]
- Wang Y., Li X., Wu S., Dong L., Hu Y., Wang J.…Wang S. Methylglyoxal Decoration of Glutenin during Heat Processing Could Alleviate the Resulting Allergic Reaction in Mice. Nutrients. 2020;12(9) doi: 10.3390/nu12092844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Wu H., Liu M., Liu Z., Xu H., Lai F. Analysis of binding interaction between (−)-epigallocatechin (EGC) and β-lactoglobulin by multi-spectroscopic method. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2011;82(1):164–168. doi: 10.1016/j.saa.2011.07.028. [DOI] [PubMed] [Google Scholar]
- Xu L.L., Gao H.Y., Yang F., Wen Y.Q., Zhang H.W., Lin H.…Gasset M. Major shrimp allergen peptidomics signatures and potential biomarkers of heat processing. Food Chemistry. 2022;382 doi: 10.1016/j.foodchem.2022.132567. [DOI] [PubMed] [Google Scholar]
- Yuan F., Lv L., Li Z., Mi N., Chen H., Lin H. Effect of transglutaminase-catalyzed glycosylation on the allergenicity and conformational structure of shrimp (Metapenaeus ensis) tropomyosin. Food Chemistry. 2017;219:215–222. doi: 10.1016/j.foodchem.2016.09.139. [DOI] [PubMed] [Google Scholar]
- Zhang P., Gao J., Che H., Xue W., Yang D. Molecular Basis of IgE-Mediated Shrimp Allergy and Heat Desensitization. Nutrients. 2021;13(10) doi: 10.3390/nu13103397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Huang Z., Li H., Cen C., Zheng R., Lili C.…Fu L. Deciphering Changes in the Structure and IgE-Binding Ability of Ovalbumin Glycated by α-Dicarbonyl Compounds under Simulated Heating. Journal of Agricultural and Food Chemistry. 2022;70(6):1984–1995. doi: 10.1021/acs.jafc.1c06939. [DOI] [PubMed] [Google Scholar]
- Zhang T., Shi Y., Zhao Y., Wang J., Wang M., Niu B., Chen Q. Different thermal processing effects on peanut allergenicity. Journal of the Science of Food and Agriculture. 2019;99(5):2321–2328. doi: 10.1002/jsfa.9430. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Li X.M., Li Z., Lin H. Investigation of glycated shrimp tropomyosin as a hypoallergen for potential immunotherapy. Food & Function. 2021;12(6):2750–2759. doi: 10.1039/d0fo03039b. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Xiao H., Zhang X., Zhou P. Insight into the effects of deglycosylation and glycation of shrimp tropomyosin on in vivo allergenicity and mast cell function. Food & Function. 2019;10(7):3934–3941. doi: 10.1039/C9FO00699K. [DOI] [PubMed] [Google Scholar]
- Zhao J., Li Y., Xu L., Ji Y., Zeng J., Timira V.…Li Z. Insight into IgG/IgE binding ability, in vitro digestibility and structural changes of shrimp (Litopenaeus vannamei) soluble extracts with thermal processing. Food Chemistry. 2022;381 doi: 10.1016/j.foodchem.2022.132177. [DOI] [PubMed] [Google Scholar]
- Zhao J., Li Y., Xu L., Zeng J., Liu Y., Timira V.…Li Z. Thermal induced the structural alterations, increased IgG/IgE binding capacity and reduced immunodetection recovery of tropomyosin from shrimp (Litopenaeus vannamei) Food Chemistry. 2022;391 doi: 10.1016/j.foodchem.2022.133215. [DOI] [PubMed] [Google Scholar]
- Zheng L.N., Lin H., Pawar R., Li Z.X., Li M.H. Mapping IgE binding epitopes of major shrimp (Penaeus monodon) allergen with immunoinformatics tools. Food and chemical toxicology. 2011;49(11):2954–2960. doi: 10.1016/j.fct.2011.07.043. [DOI] [PubMed] [Google Scholar]
- Zheng L., van Dongen K.C.W., Bakker W., Miro Estruch I., Rietjens I. The Influence of Intracellular Glutathione Levels on the Induction of Nrf2-Mediated Gene Expression by alpha-Dicarbonyl Precursors of Advanced Glycation End Products. Nutrients. 2022;14(7):1364. doi: 10.3390/nu14071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Y., Dong L., Wang J.P., Wang S.J., Wang S. Formation and migration of alpha-dicarbonyl compounds during storage and reheating of a sugary food simulation system. Journal of the Science of Food and Agriculture. 2020;100(5):2296–2304. doi: 10.1002/jsfa.10263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.