Abstract
Approximately half of breast cancers (BCs), historically categorized as human epidermal growth factor receptor 2 (HER2)-negative, have low expression of HER2 defined as an immunohistochemical (IHC) score of 1+ or 2+ with negative in situ hybridization. Retrospective evidence suggest that HER2-low BC does not represent a distinct subtype from a biological and prognostic perspective. Nonetheless, it currently constitutes an essential biomarker to guide treatment selection and its introduction has led to reconsidering the binary classification of HER2 status according to which only patients with HER2-positive BC were thought to derive benefit from anti-HER2 therapies. Trastuzumab deruxtecan has recently been approved by the U.S. Food and Drug Administration for the treatment of patients with HER2-low metastatic BC based on the results of the DESTINY-Breast04 phase III trial, and other antibody–drug conjugates (ADCs) targeting HER2 are showing promising results. Treatment paradigms for both triple-negative and hormone receptor-positive BCs exhibiting HER2-low expression are thus rapidly evolving. Given its therapeutic implications, it is essential to accurately recognize the level of HER2 expression, and the development of more sensitive and reliable methods for HER2 testing and scoring is warranted, especially since the minimum threshold of HER2 expression required for T-DXd efficacy is currently under investigation. Given the signs of activity of T-DXd even in patients with HER2-0 (IHC 0) disease, an evolution in the way we define HER2-low is anticipated. Considering the expansion of the therapeutic armamentarium for BC patients, with several ADCs approaching the clinic, research efforts are needed to clarify whether the expression level of targets can enrich for responders to a given ADC as well as to understand mechanisms of resistance with the goal of optimizing the sequencing of ADCs.
Keywords: antibody–drug conjugates, breast cancer, HER2-low, human epidermal growth factor receptor 2, sacituzumab govitecan, sequencing, trastuzumab deruxtecan
Introduction
The development of anti-human epidermal growth factor receptor 2 (HER2) drugs has significantly improved the prognosis of patients with HER2-positive breast cancer (BC).1 This subtype of BC is defined according to the 2018 American Society of Clinical Oncology and College of American Pathology HER2 testing guidelines by HER2 overexpression on an immunohistochemical (IHC) assay (score 3+) and/or gene amplification on an in situ hybridization (ISH) assay.2 The remaining majority of BCs (80–85%) that do not overexpress HER2 are currently defined as HER2-negative since traditional HER2-targeting therapies are not effective in such cases.3,4 Nevertheless, the availability of novel anti-HER2 agents is gradually transforming the way we define HER2, moving away from the binary classification with the introduction of the new entity of ‘HER2-low’ BC. This represents approximately half of all BCs and is defined as a HER2 IHC score of 1+ or 2+ with negative ISH assay.5 The DESTINY-Breast04 trial has recently demonstrated the benefit of treating patients with HER2-low metastatic BC (mBC) with the anti-HER2 antibody–drug conjugate (ADC) trastuzumab deruxtecan (T-DXd).6 This represents a major therapeutic step forward for those patients with BC historically classified as hormone receptor (HR)-positive/HER2-negative or triple-negative BC (TNBC) and initially treated according to their HR status, but with very limited treatment options once chemo-refractory.7 However, along with the therapeutic opportunities offered by the emergence of this new subgroup of HER2-low BC, several challenges emerged related to the understanding of its biological role, pathological diagnosis, and the definition itself of HER2-low.
The aim of this review is to provide an updated overview of the knowledge about HER2-low BC, explore how its introduction is changing our clinical practice with novel pitfalls to address, and how its definition may evolve in the coming years.
Identification of HER2-low BC
The current clinical definition of HER2-low BC used in clinical practice and ongoing clinical trials relies on the standard IHC and ISH approach; thus, tumors with low level of HER2 expression (defined as a HER2 IHC score of 1+ or 2+) and no detectable ERBB2 amplification fall into this category.5 It should be noted that this testing method was originally developed to identify patients who could benefit from trastuzumab, thus aimed at identifying tumors with HER2 overexpression and not to differentiate low levels of HER2 expression from lack of HER2. Consequently, it is unclear whether IHC and ISH assays are adequate for the detection of low HER2 expression.
HER2 ISH testing allows to detect HER2 gene amplification that is directly related to protein overexpression, and no protein overexpression is observed in the absence of gene amplification. However, for novel ADCs, antitumoral effects have been observed in BC regardless of gene amplification, underscoring the inadequacy of ISH assay to identify all potential responders.6,8
HER2 IHC testing allows semiquantitative assessment of membrane protein expression, with scores ranging from 0 to 3+. However, several preanalytical and analytical issues may impact on evaluation of low range of HER2 expression, ultimately leading to high discordance of HER2 IHC testing when evaluated by different pathologists. Different studies reported discrepancy of HER2 status on IHC observed in local and central laboratories. Lambein et al. found that 85% of BC specimens (n = 102) locally scored as HER2-0 were HER2-low at centralized reanalysis.9 Another recently published study aimed at evaluating the accuracy of HER2 IHC in the low range (0 and 1+) reported only 26% concordance among pathologists compared with 58% concordance between scores 2+ and 3+.10 Notably, in DESTINY-Breast04, tumor responses were similar in both HER2 IHC score 1+ and 2+ subgroups, suggesting that the entity of T-DXd antitumor activity is not predictable using IHC.6
Since inaccuracy of HER2 evaluation could lead to the misassignment of many patients for treatment with T-DXd, further efforts will be required to develop more sensitive and reproducible HER2 testing methods. In this framework, novel quantitative assays are under development with the goal of improving our ability to identify patients with HER2-low BC including the HERmark Breast Cancer assay,11,12 immunofluorescence-based automated quantitative analysis methods,13,14 and quantitative IHC method.15 Moreover, the evaluation of HER2 mRNA expression levels may represent a potential complement or alternative method to IHC/ISH in the assessment of BC samples to estimate which patients may benefit from ADCs.16 Before entering clinical practice, all these new methods for HER2 detection will need extensive analytic and clinical validation, and their value in the identification of threshold levels of HER2 for defining HER2-low BC will be investigated.
Biology of HER2-low BC
Following the recognition of HER2-low as a fundamental biomarker for the treatment of patients with BC, research efforts have been directed toward the definition of the biological significance of HER2-low expression, exploring its differences from HER2 IHC score 0 (HER2-0) BC. Analyses of large retrospective cohorts of patients with BC often reported inconsistent findings and there is still no agreement in the field.17–35 Most studies have identified no major difference in clinicopathologic characteristics nor prognosis for HER2-low expression,24,30,31,36 few studies have instead suggested a better29,32 or worse prognosis25 associated with HER2-low BC (Table 1). Of note, a positive correlation between estrogen receptor (ER) level and HER2-low expression has been reported, with approximately 60% of HR-positive/HER2-negative BC being HER2-low, compared with up to 40% of TNBC.24,28,29,37,38 This association could represent a possible confounding factor for studies aimed at defining the clinicopathologic features and prognostic role of HER2-low expression. Indeed, any feature found enriched among HER2-low BC compared with HER2-0 may be related to the higher HR expression, which remains the major determinant of tumor behavior and prognosis in the absence of HER2 overexpression. Similarly, only minor differences in PAM50 subtypes distribution were observed between HER2-low and HER2-0 tumors when the confounding factor of HR expression was considered, suggesting the absence of gene expression correlates for HER2-low BC. Indeed, at PAM50, TNBC were mostly basal-like (85%) and HR-positive were mostly luminal (90%), regardless of HER2-low expression.24
Table 1.
Studies conducted to evaluate the prognostic role of HER2-low expression in early and advanced BC.
| Study | Study population | HER2-low (n) | HER2-0 (n) |
Endpoints explored | Prognostic impact of HER2-low expression (significant findings highlighted) |
|---|---|---|---|---|---|
| Early setting | |||||
| Won et al.17 | 30491 HR+ and TNBC | 9056 | 20985 | OS; BCSS | No OS difference in both HR+ (p = 0.086) and
TNBC (p = 0.170) HER2-low
versus HER2-0 Better BCSS in HR+ (99.4% versus 99.1%, p = 0.003) and TNBC (97.2% versus 95.9%; p = 0.023) HER2-low |
| Tan et al.18 | 28280 HR+ and TNBC | 12,260 | 16020 | RFS; OS |
Better RFS (HR: 0.92, 95% CI: 0.86–0.99,
p = 0.022) and OS (HR: 0.89, 95% CI:
0.81–0.97; p = 0.012) in HR+
HER2-low.
No RFS difference (HR: 0.92, 95% CI: 0.81–1.05, p = 0.226) and longer OS (HR: 0.82, 95% CI: 0.69–0.96, p = 0.017) in HER2-low TNBC |
| Tarantino et al.28 | 5235 HR+ and TNBC | 2917 | 2318 | pCR; DFS, DDFS, OS | No pCR differences when stratifying by HR status No DFS, DDFS and OS differences in HR+ and TNBC HER2-low versus HER2-0 |
| Horisawa et al.20 | 4918 HR+ and TNBC | 3169 | 2860 | 5y-DFS; 5y-OS | No 5y-DFS (91.6% versus 90.1%;
p = 0.151) and 5y-OS (96.7%
versus 94.9%; p = 0.215)
differences in HR+ HER2-low versus
HER2-0 No 5y-DFS (74% versus 78.7% p = 0.306) and 5y-OS (86.5% versus 79.3%; p = 0.152) difference in HER2-low versus HER2-0 TNBC |
| Denkert et al.29 | 2310 HR+ and TNBC | 1098 | 1212 | pCR; 3y-DFS; 3y-OS |
pCR lower in HER2-low
versus
HER2-0 HR+ (17.5%
versus
23.6%; p = 0.024); no pCR
differences in HER2-low versus HER2-0 TNBC
(50.1% versus 48.0%;
p = 0.21) Higher 3y-DFS (84.5% versus 74.4% p = 0.0076) and 3y-OS (90.2% versus 84.3% p = 0.016) in HER2-low versus HER2-0 HR+ No 3y-DFS (82.8% versus 79.3%; p = 0.39) and 3y-OS (92.3% versus 88.4%; p = 0.13) differences in HER2-low versus HER2-0 TNBC |
| De Moura Leite et al.30 | 855 HR+ and TNBC | 285 | 570 | pCR; 5y-RFS | No pCR differences in HR+ (13% versus 9.5%;
p = 0.27) and TNBC (51%
versus 47%; p = 0.64)
HER2-low versus HER2-0 No 5y-RFS differences in HR+ (72.1% versus 71.7%; p = 0.47) and TNBC (75.6% versus 70.8%; p = 0.23) HER2-low versus HER2-0 |
| Agostinetto et al.31 | 804 HR+ and TNBC | 410 | 394 | PFI, DFI, OS | No differences in DFI, PFI and OS |
| Mutai et al.32 | 608 HR+ | 304 | 304 | DDFS, DFS, OS stratified by OncotypeDx score |
In high-genomic risk (RS > 25), HER2-low associated
with superior OS (HR: 0.31, 95% CI: 0.11–0.78,
p = 0.01), DFS (HR: 0.40, 95% CI:
0.20–0.82, p = 0.01) and DDFS (HR: 0.26,
95% CI: 0.11–0.63, p = 0.002)
No differences in low genomic risk (RS ⩽ 25) |
| Jacot et al.33 | 296 TNBC | 48 | 248 | RFS; OS | No differences in RFS and OS HER2 2+ (compared with HER2 0/1+ tumors) associated with worse RFS (HR = 3.16, 95% CI: 1.27–7.85, p = 0.034) in univariate analysis |
| Almstedt et al.34 | 351 HR+ and TNBC | 198 | 153 | 15y-DFS; 15y-OS |
Higher 15y-DFS (67.5%, 95% CI: 61.0–74.7
versus
47.3%, 95% CI: 39.9–56.1, p < 0.001)
and 15y-OS (75.4%, 95% CI: 69.4–81.9
versus
66.8%, 95% CI: 59.5–74.9, p = 0.009) in
HER2-low
versus
HER2-0
OS difference in HR+ (p = 0.039) but not in TNBC (p = 0.086) |
| Tarantino et al.35 | 276 HR+ and TNBC inflammatory BC | 140 | 136 | iDFS | No differences in 2y-iDFS in HR+ (63% versus
63%, HR: 1.10, 95% CI: 0.57–2.13) and TNBC (28%
versus 25%, HR: 1.19, 95% CI:
0.69–2.04) No differences in 2y-OS in HR+ (80% versus 81%, HR: 0.82, 95% CI: 0.39–1.73) and TNBC (34% versus 47%, HR: 1.34, 95% CI: 0.74–2.41) |
| Domergue et al.19 | 437 TNBC | 121 | 316 | pCR | No pCR (35.7% versus 41.8%, p = 0.284), iDFS (p = 0.487) and OS (p = 0.329) differences in HER2-low versus HER2-0 |
| Chen et al.21 | 2099 HR+ BC | 1732 | 367 | 5y-DFS | No 5y-DFS differences in HER2-0 versus HER2-low (92.3% versus 93.3%, p = 0.83) |
| Advanced setting | |||||
| Gampenrieder et al.22 | 1378 HR+ and TNBC | 608 | 770 | OS | No OS differences in HR+ (HR: 0.89; 95% CI: 0.74–1.05; p = 0.171) and in TNBC (HR: 0.92; 95% CI: 0.68–1.25; p = 0.585) HER2-low versus HER2-0 |
| Tarantino et al.23 | 232 HR+ and TNBC | NR | NR | OS | No OS differences in HR+ and TNBC HER2-low versus HER2-0 |
| Schettini et al.24 | 1304 HR+ and TNBC | 795 | 501 | OS | No statistically significantly differences in OS between HER2-low and HER2-0 (p = 0.787) |
| Bao et al.25 | 106 HR+/HER2- | 82 | 24 | PFS on CDK4/6i | Shorter mPFS (8.9 months, 95% CI: 6.49–11.30 versus 18.8 months, 95% CI: 9.44–28.16; p = 0.01) in HER2-low versus HER2-0 |
| De Calbiac et al.26 | 15054 HR+ and TNBC | 4671 | 10383 | PFS1; OS | No PFS1 difference (HR: 0.99, 95% CI: 0.95–1.02; p = 0.45) and slightly better OS (HR: 0.95, 95% CI: 0.91–0.99; p = 0.02) in HER2-low versus HER2-0 |
| Li et al.27 | 1433 HR+ and TNBC | 618 | 815 | OS |
OS higher in HR+ HER2-low
versus
HER2-0 (54.9
versus
48.1 months, p = 0.011).
No OS difference in TNBC HER2-low versus HER2-0 |
BC, breast cancer; BCSS, breast cancer-specific survival; CDK4/6i, cyclin-dependent kinase 4/6 inhibitors; CI, confidence interval; DDFS, distant disease-free survival; DFI, disease-free interval; DFS, disease-free survival; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; iDFS, invasive disease-free survival; NR, not reported; OS, overall survival; pCR, pathological complete response; PFI, progression-free interval; PFS, progression-free survival; RFS, relapse-free survival; RS, recurrence score; TNBC, triple-negative breast cancer; 5y-DFS, 5-year disease-free survival; 5y-OS, 5-year overall survival. Statistically significant p values are highlighted in bold.
The HER2-low expression has been shown to be highly unstable during time with a relevant portion of HER2-low tumors turning HER2-0, and vice versa, either on residual disease after neoadjuvant therapy,39 or after tumor relapse.23,37 This dynamism is likely due to multiple factors, as it is known that HER2 expression can be modulated by various stimuli from the tumor microenvironment as well as by effect of therapies.5,40 Other possible confounders are pre-analytical and analytical issues of HER2 testing methods that lead to high discordance in pathology testing.9,10 Moreover, heterogeneous HER2 expression can be found across a single tumor location or different metastatic sites.41 Regardless of the specific reason, this observation highlights the need to reassess HER2 status during the course of a patient’s disease, even when the tumor was HER2-0 on prior biopsy in order to potentially allow access to treatment with T-DXd in case of change to HER2-low expression. Of note, in the DESTINY-Breast04 trial, both archived or fresh tumor-biopsy specimens were acceptable for enrollment and the presence of prior HER2-0 samples was not an exclusion criterion.6 Which is the right time point to define HER2-low remains to be clarified; however, even patients whose last biopsy tested HER2-0 could be considered for treatment with T-DXd if they had HER2-low score on any prior biopsies. To better capture the spatial and temporal evolution of HER2-low expression, a useful tool could be liquid biopsy. Identification of HER2-low expressing circulating tumor cells (CTCs) could provide additional information to tissue biopsies to map tumor heterogeneity and evolution, better reflecting the current status of the disease. Indeed, it has been shown that HER2-negative CTCs can spontaneously convert into HER2-expressing CTCs and vice versa.42 Moreover, different studies revealed a significant discordance in HER2 status between the primary tumor and corresponding CTCs.43–45 The DETECT III trial showed preliminary positive results of adding lapatinib to standard chemotherapy in patients with HER2-negative mBC and HER2-positive CTCs.46 Larger studies are needed to validate this hypothesis, and the possibility of targeting CTCs with low HER2 expression with novel ADCs could be explored in the future.
ADCs in HER2-low BC
Until recently, HER2-low expression has never influenced clinical practice, as these tumors were considered ineligible for anti-HER2 therapies. Indeed, the blockade of HER2 with trastuzumab failed to prove any beneficial effect when added to standard adjuvant chemotherapy in HER2-low early-stage BC patients within a large phase III trial.3 Trastuzumab emtansine (T-DM1), the first anti-HER2 ADC to be approved for patients with BC, demonstrated poor activity among patients with heterogeneous and/or low HER2 expression.4,47,48 This reinforced the notion that only addiction to the HER2 pathway associated with HER2 overexpression warranted treatment with HER2-targeting drugs. Nevertheless, the development of a new generation of anti-HER2 ADCs reshaped this scenario leading to the recognition of HER2-low as a new targetable subgroup of BCs. To date, four anti-HER2 ADCs have proven activity in HER2-low BC: T-DXd, trastuzumab duocarmazine, disitamab vedotin, and MRG002. This novel generation of ADCs harbors one or more of the distinctive features that may explain activity in HER2-low BCs such as more potent cytotoxic payload, higher drug-to-antibody ratio (DAR), or the ability to elicit the so-called bystander effect. Indeed, the features of the linker that allow interstitial cleavage of these novel ADCs along with the release of membrane-permeable free payload from target cells enable the killing of neighboring cells regardless of their HER2 expression, ultimately resulting in clinical activity in case of low or heterogeneous antigen expression.49
Trastuzumab deruxtecan
T-DXd is an ADC in which a HER2-directed antibody is conjugated through a tetrapeptide-based cleavable linker with the topoisomerase I (TOP1) inhibitor deruxtecan (DXd), an exatecan derivative. It has a high DAR of 8:1. Results of the phase III DESTINY-Breast04 trial led T-DXd to become a new standard of care for patients with pretreated HER2-low mBC.6 This study randomized 557 patients with HER2-low mBC (494 HR-positive, 58 triple-negative), pretreated with endocrine therapy (if HR-positive) and up to two lines of chemotherapy, in a 2:1 ratio to receive T-DXd or physician’s choice chemotherapy (capecitabine, eribulin, gemcitabine, eribulin, paclitaxel, or nab-paclitaxel). Treatment with T-DXd resulted in improved progression-free survival (PFS) compared with chemotherapy among HR-positive patients [primary endpoint, 10.1 versus 5.4 months, hazard ratio (HR) 0.51, 95% confidence interval (CI): 0.40–0.64, p < 0.001], as well as in the overall study population (9.9 versus 5.1 months, HR: 0.50, 95% CI: 0.40–0.63, p < 0.001). Overall survival (OS) was also improved among HR-positive patients (23.9 versus 17.5 months, HR: 0.64, 95% CI: 0.48–0.86, p = 0.003) and in the overall population (23.4 versus 16.8 months, HR: 0.64, 95% CI: 0.49–0.84, p = 0.001). Among HR-positive patients, the objective response rate (ORR) was increased in T-DXd arm (52.6%) compared with control arm (16.3%) with a median duration of response of 10.7 and 6.8 months, respectively. A consistent benefit was observed for T-DXd across all prespecified subgroups. As for HER2 IHC status, patients benefited similarly from T-DXd regardless of HER2 IHC score 1+ or 2+ with a PFS of 10.3 versus 10.1 months, respectively, and a paradoxically greater reduction in risk of progression (52% versus 45%) observed in the 1+ subgroup compared with tumors with a 2+ score. An exploratory analysis restricted to TNBC patients showed improved PFS (8.5 versus 2.9 months, HR: 0.46, 95% CI: 0.24–0.89), OS (18.2 versus 8.3 months, HR: 0.48, 95% CI: 0.24–0.95), and ORR (50% versus 16.7%) with T-DXd compared with chemotherapy. In the experimental arm, the most common treatment-related adverse events (TRAEs) of any grade were nausea (73.0%), fatigue (47.7%), and alopecia (37.7%). The most common adverse events (AEs) of grade ⩾3 were neutropenia (13.7%), anemia (8.1%), and fatigue (7.5%). Drug-related interstitial lung disease (ILD) or pneumonitis occurred in 45 patients (12.1%) receiving T-DXd, mostly grade 1 and 2 events, with three (0.8%) fatal events. The median time to onset of ILD was 129 days (range, 26–710). Additionally, 17 patients (4.6%) had left ventricular dysfunction with 5 patients (1.5%) experiencing a decline >20% from baseline of the left ventricular ejection function.
To further enhance the benefit observed with T-DXd in HER2-low BC, combination strategies are currently being explored. Given the strong biological rationale, one of the most interesting is the combination with immunotherapy.50 T-DXd has shown to exert immunomodulating activity, enhancing antitumor immunity and ultimately synergizing with anti-programmed death-ligand 1 (PD-L1).51 In the BEGONIA trial, T-DXd was evaluated as first-line treatment in combination with durvalumab for patients with HR-negative/HER2-low mBC, demonstrating a promising response rate (8/12, all partial responses, 87.5% ongoing at data cutoff), with responses observed regardless of PD-L1 expression.52 A 38.1% rate of grade 3 AEs was reported, mainly hematologic toxicities, and two cases of pneumonitis (grades 2 and 3) were observed. The latter is a major concern when combining immunotherapy and T-DXd since ILD represents an overlapping toxicity with potential higher risk for combination. Another early-phase Ib trial is evaluating T-DXd combined with nivolumab in patients with pretreated HER2-expressing mBC.53 Among the 16 HER2-low patients treated, the combination with nivolumab did not seem superior to what observed with T-DXd alone in the DESTINY-Breast04 trial, reporting an ORR of 50% and a mPFS of 7 months. This observation suggests that to maximize the synergism between T-DXd and immunotherapy the combination should be incorporated in earlier lines when the host is less immunosuppressed and the tumor less heterogeneous, factors that could impair the efficacy of immunotherapy.54 Other combinations of T-DXd with immune checkpoint inhibitors, chemotherapy, as well as targeted therapies in HER2-low BC are currently being investigated in the DESTINY-Breast08 platform trial (NCT04556773).
Trastuzumab duocarmazine
Trastuzumab duocarmazine (SYD985) consists of an anti-HER2 antibody bound to a DNA-alkylating duocarmycin payload through a cleavable linker. Despite its low DAR of 2.8:1, SYD985 showed to be more potent than T-DM1 in HER2-low tumors in preclinical models.55 In a phase I trial conducted in patients with treatment-refractory locally advanced or metastatic solid tumors and variable HER2 status, 49 HER2-low mBC patients were enrolled in the BC dose expansion cohort.8 The observed ORR was 28% in the HR-positive/HER2-low (n = 32) and 40% in the HR-negative/HER2-low (n = 17), and mPFS was similar in the two subgroups (4.1 and 4.9 months, respectively). Ocular toxicity dominated the toxicity profile of SYD985 with 71% of patients experiencing ocular AEs of any grade, mainly conjunctivitis, dry eye, lacrimation increase, and keratitis. Most of these events were of grade ⩽2 and though dose reduction, dose delay, or the use of prophylactic eye drops did not substantially change the rate of ocular toxicities, most patients were able to continue treatment. Myelosuppression was lower with SYD985 than with other anti-HER2 ADCs (grade ⩾3 thrombocytopenia < 1% and neutropenia 6%), a relevant finding when considering combination strategies. Pneumonitis was reported as a dose-limiting toxicity at the highest administered dose of 2.4 mg/kg in the dose-escalation phase (one death), but the risk was diminished at the recommended phase II dose of 1.2 mg/kg with one grade 4 pneumonitis observed (1%).
Disitamab vedotin
Disitamab vedotin (RC48-ADC) is an ADC composed of a novel anti-HER2 humanized antibody, hertuzumab, conjugated to a microtubule inhibitor monomethyl auristatin E (MMAE) payload through a cleavable linker. This compound showed relevant activity in a cohort of 48 HER2-low mBC patients, reporting an ORR of 39.6% and a mPFS of 5.7 months, slightly higher in HER2 IHC score 2+/ISH-negative subgroup compared to HER2 IHC 1+.56 The most common TRAEs were transaminase increase, hypoesthesia, and myelosuppression, mostly grade 1–2. A phase III trial is ongoing in China to compare the efficacy and safety of RC48-ADC with physician’s choice chemotherapy (paclitaxel, docetaxel, vinorelbine, or capecitabine) in HER2-low mBC patients previously treated with maximum of two lines of chemotherapy, including anthracyclines (NCT04400695). Of note, the low expression of HER2 in this trial is defined as IHC 2+ and ISH-negative, thus excluding IHC 1+ BC.
MRG002
MRG002 is a novel HER2-targeted ADC composed of modified trastuzumab conjugated through a cleavable linker to a MMAE payload. Considering the efficacy demonstrated in preclinical studies, it has been tested in a phase II trial including patients with pretreated metastatic HER2-low BC.57 At primary analysis, 52 patients were evaluable for response reporting an ORR of 33%, similar in both the HER2 IHC 1+ and IHC 2+/ISH-negative subgroups, a disease control rate of 75%, and a mPFS of 5.6 months. Responses were similar in the HR-negative/HER2-low subgroup, although only eight TNBC patients were enrolled. The toxicity profile was dominated by hematological toxicity, with decreased neutrophil count being the most common grade ⩾3 TRAE (14.3%), liver and gastrointestinal toxicities mainly of grade 1 or 2. No treatment-related death was reported.
How to treat HER2-low mBC
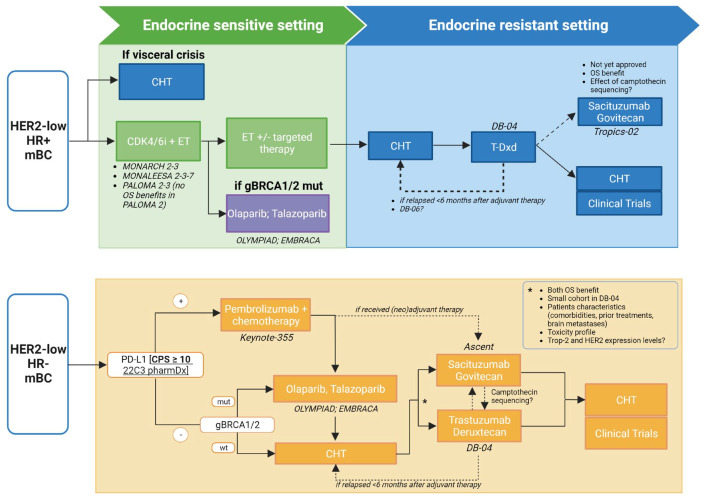
Based on the practice-changing results of the DESTINY-Breast04 trial, T-DXd has been approved by the U.S. Food and Drug Administration in August 2022 and has also been recommended in National Comprehensive Cancer Network guidelines for patients with previously treated HER2-low mBC. The statistical design of this trial has allowed to confirm a benefit of T-DXd over chemotherapy both in HR-positive patients and the overall study population, including HR-negative patients (i.e. TNBC).6 Nevertheless, while the outcome in HR-positive was a primary endpoint and this represented a large cohort, the study was not powered to assess outcomes in the TNBC subgroup, which was relatively small. Consequently, distinct considerations are required to define the optimal treatment sequencing for the two HR-expressing subgroups (Figure 1).
Figure 1.
Proposed treatment algorithm for patients with HR-positive/HER2-low and HR-negative/HER2-low mBC.
CDK4/6i, cyclin-dependent kinase 4/6 inhibitors; CHT, chemotherapy; CPS, combined positive score; DB-04, DESTINY-Breast04; DB-06, DESTINY-Breast06; ET, endocrine therapy; gBRCA1/2, germinal BRCA1/2; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; mBC, metastatic breast cancer; mut, mutated; OS, overall survival; PD-L1, programmed death-ligand 1; T-Dxd, trastuzumab deruxtecan; Trop-2, trophoblast cell surface antigen 2; wt, wild type. Created with BioRender.com.
HR-positive/HER2-low mBC
For patients with HR-positive/HER2-low mBC, the standard first-line treatment in the absence of visceral crisis remains the combination of endocrine therapy plus cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6i), namely abemaciclib, palbociclib, or ribociclib.7,58 As endocrine resistance may develop through the bidirectional crosstalk between ER and HER2 pathways,40 the association between low levels of HER2 expression and clinical outcome of patients with HR-positive/HER2-negative BC treated with CDK4/6i has been investigated. Small retrospective analyses reported conflicting evidence on the prognostic value of HER2-low expression during CDK4/6i therapy,25,59,60 highlighting the necessity of more data to understand the real implication of this biomarker in patients with HR-positive/HER2-negative tumors.
As subsequent lines, further endocrine therapy possibly associated with targeted therapy (e.g. mTOR or PI3K inhibitors) could be considered and, after exhaustion of endocrine-based treatment, subsequent lines of single-agent chemotherapy are recommended.7,58 DESTINY-Breast04 allowed enrollment of patients with HR-positive BC that had received prior endocrine treatment and at least one but no more than two prior lines of chemotherapy for metastatic disease, which represent the ideal setting for the use of T-DXd in clinical practice. However, with the limits of cross-trial comparison, the robust and sustained activities observed with T-DXd compare favorably to that obtained with first-line chemotherapy. The ongoing randomized phase III DESTINY-Breast06 trial will elucidate if T-DXd is superior to standard chemotherapy in patients with HR-positive/HER2-low BC who progressed on prior endocrine therapy and are naïve to chemotherapy, possibly positioning T-DXd as first non-endocrine-based treatment for this population (NCT04494425). Based on the inclusion criteria of DESTINY-Breast04, T-DXd can be evaluated immediately after exhaustion of endocrine-based therapies also for patients experiencing metastatic recurrence during or within 6 months after completing adjuvant chemotherapy. Of note, the PFS benefit with T-DXd was obtained regardless of previous CDK4/6i treatment.6
Other treatment options available for HR-positive/HER2-low BC patients having a germline BRCA mutation are poly (ADP-ribose) polymerase (PARP) inhibitors (PARPi), namely olaparib and talazoparib, given the PFS advantage demonstrated over single-agent chemotherapy, although differently from T-DXd none of the approved PARPi has yet demonstrated an OS advantage in this setting.61,62 Additionally, the phase III TROPiCS-02 trial has recently demonstrated a significant PFS (5.5 versus 4.0 months; HR: 0.66; 95% CI: 0.53–0.83; p = 0.0003) and OS improvement (14.4 versus 11.2 months; HR: 0.79; p = 0.020) with the anti-Trop2 ADC sacituzumab govitecan (SG) compared with chemotherapy, regardless of HER2 expression.63 However, the more heavily pretreated population enrolled in TROPiCS-02 (at least one endocrine-based therapy, CDK4/6i, and two to four lines of chemotherapy) than those enrolled DESTINY-Breast04 could position SG in later lines of treatment, after T-DXd. In DESTINY-Breast04, prior treatment with ADC that consists of an exatecan derivative (TOP1 inhibitor) was an exclusion criterion, while others were allowed. Understanding mechanisms governing resistance will help to understand how to sequence ADCs. Regarding sequence, the percentage of patients previously treated with alpelisib, everolimus, PARPi, or other biologic treatments that received T-DXd in the DESTINY-Breast04 is unknown so, although acceptable, sequencing in clinical practice requires caution.
HR-negative/HER2-low mBC
For patients with HR-negative/HER2-low BC treatment commonly involves first-line chemotherapy, plus immunotherapy if PD-L1 positive, followed by sequential lines of single-agent chemotherapy.7,58 As for HR-positive BC given the PFS advantage over chemotherapy, PARPi are considered for patients with germline BRCA mutation.61,62
For patients with HR-negative/HER2-low BC, the results of DESTINY-Breast04 position T-DXd after first-line chemotherapy or as front-line therapy for those patients with early recurrence after (neo)adjuvant chemotherapy. Despite this study was not powered to assess outcomes in the TNBC subgroup and the small number of TNBC included (n = 58), the clear benefit observed in this subset of patients along with the poor prognosis of TNBC warrants the use of T-DXd in TNBC with low HER2 expression. However, its role should be considered in light of the approval of SG for TNBC patients who have received at least two prior systemic therapies (⩾1 in metastatic setting) based on the results of ASCENT trial.64 The latter was a study restricted to TNBC patients (n = 529) and powered for survival outcomes in this cohort: SG demonstrated a relevant PFS (HR: 0.41; 95% CI: 0.32–0.52; p < 0.001) and OS (HR: 0.48; 95% CI: 0.38–0.59; p < 0.001) benefit over chemotherapy. A post hoc analysis of ASCENT trial evaluated the efficacy of SG by HER2 status, reporting a clinical benefit with SG in HER2-0 and HER2-low mTNBC consistent with the overall population.65 In the HER2-low subgroup treated with SG the mPFS was 6.2 months and mOS was 14 months. Since no direct comparison exists between T-DXd and SG, the choice of which ADC prioritize requires a careful assessment of the available literature and a shared decision-making process with the patient on the basis of the different efficacy and toxicity profile of these two agents.
Brain metastases and other patients’ characteristics
The mainstay of treatment for patients with brain metastases remains local approach, with radiotherapy or surgery, and systemic therapy.58 However, systemic treatment could represent an appealing alternative approach to whole-brain radiotherapy (WBRT) when more limited approach such as stereotactic radiotherapy is not indicated, aiming at preventing or delaying neurocognitive decline associated with WBRT. Both ASCENT64 and DESTINY-Breast046 allowed inclusion of patients with treated and stable brain metastases and the different intracranial activity of SG and T-DXd may guide patient’s treatment selection. As for the former, 61 patients enrolled in the ASCENT had stable brain metastases at screening and a sub-analysis restricted to this population revealed that SG was numerically better than chemotherapy for tumor response (3% versus 0%) and mPFS (2.8 versus 1.6 months), but not OS.66 Moreover, preclinical evidence and mass spectroscopy studies in patients that underwent craniotomy for BC brain metastases or recurrent glioblastoma during SG treatment support intracranial activity of SG.67 Further studies are ongoing to better evaluate the role of SG in patients with brain metastases (SWOG-S2007, NCT04647916).
Regarding T-DXd, no data have been presented regarding its activity restricted to HER2-low BC patients with stable brain metastases treated in DESTINY-Breast04 (n = 24). In the DAISY trial, the efficacy of T-DXd in patients with treated and asymptomatic brain metastases according to HER2 status has been recently reported: T-DXd showed meaningful antitumor activity in this population and efficacy in patients with HER2-low BC was promising (ORR of 30% and clinical benefit rate of 50%).68 More data on T-DXd intracranial activity come from HER2-positive disease; indeed, outcomes were comparable in patients with stable brain metastases at baseline (HR = 0.25) treated in DESTINY-Breast03 study to those without brain metastases, with an objective intracranial response rate of 63.9%.69 The phase II DEBBRAH trial showed high intracranial activity in patients with HER2-positive BC with stable or active brain metastases.70 Similarly, the phase II TUXEDO-1 trial showed an intracranial response rate of 73.3% in patients with active brain metastases from HER2-positive BC71. Altogether, these results suggest that T-DXd could be administered as treatment for patients with active brain metastases from HER2-positive BC, in case immediate local intervention is not indicated. Interestingly, an additional cohort of DEBBRAH trial is currently evaluating patients with HER2-low BC whose results could inform on T-DXd activity in HER2-low BC patients with brain metastases and/or leptomeningeal carcinomatosis.
Furthermore, other patients’ characteristics must be taken into account in selecting a given ADC. Indeed, besides some common side effects such as hematological toxicities, nausea, and vomiting, others are more typical for a given ADC. For instance, patients treated with T-DXd may be at increased risk of developing left ventricular dysfunction and treatment with T-DXd has not been studied in patients with a history of clinically significant cardiac disease or left ventricular ejection fraction less than 50% prior to initiation of treatment.6 Moreover, additional risk factors which may increase the possibility of cardiac events such as previous treatment with anthracycline agents might be considered in the choice of treatment. ILD is another relevant AE observed with T-DXd,72 thus patients with a history of noninfectious ILD treated with glucocorticoids or suspected ILD on imaging at screening were not eligible for inclusion in DESTINY-Breast04. On the other hand, SG can cause severe diarrhea; thus, it should be considered with caution for patients with gastrointestinal disorders.64 Additionally, for SG, a role for pharmacogenomic testing is emerging since patients who are homozygous for the uridine diphosphate-glucuronosyl transferase 1A1 (UGT1A1)*28 allele are at increased risk for neutropenia, febrile neutropenia, and anemia.73
Challenges for the future: patient selection and ADCs sequencing
Given the increasing number of ADCs approaching the clinic, even for HER2-low BC patients, and the practical impossibility of a head-to-head comparison for all of them, prediction of their activity through biomarker selection may be critical for a more accurate choice of treatment sequencing. The semiquantitative IHC assay currently used to define HER2-low BC does not allow to predict the activity of T-DXd. In DESTINY-Breast04, patients derived similar benefit from T-DXd regardless of the HER2 IHC score with a slightly longer PFS (10.3 versus 10.1 months) and higher reduction in risk of progression (52% versus 45%) in the HER2 1+ subgroup compared with tumors HER2 2+/ISH-negative6. Differently, the phase II DAISY trial found a slightly different activity depending on the level of HER2 expression: indeed, response rate was higher in HER2-positive subgroup (ORR 71%) with slight differences in the activity of T-DXd noted among HER2-low and HER2-0 pretreated BC patients (ORR of 37.5% and 30%, mPFS of 6.7 and 4.2 months, respectively), although the small number of patients included in this study limits to derive definitive conclusion.74 Notably, translational analysis from this study reported that beyond the magnitude of HER2 expression also its spatial distribution matters, identifying a cluster of response.75 This could be related to the bystander effect, so large areas of target-negative cells distant to HER2 expressing tumor cells could impair the killing of the first. This adds an additional layer of complexity, but it opens the possibility to dissect HER2 intratumoral heterogeneity and identify clusters that are predictive of response that could be identified through artificial intelligence algorithms.
In the ASCENT trial, although even patients with Trop-2-low BC benefited from SG, the magnitude of response seems to be related to the level of target expression.76 Although Trop-2 testing is nowadays not recommended since SG outperformed chemotherapy in all expression subgroups, its expression level could be crucial in selecting which ADC to prioritize. The same may happen with other ADCs directed against novel targets being investigated for the treatment of this disease that could be prioritized through biomarker selection. Besides the identification of reliable biomarkers, this approach will require the development of validated assays and cutoffs to define antigen positivity as well as to better understand the implication of dynamic expression of several targets.
Another upcoming challenge will be to unravel the mechanism through which resistance develops. Indeed, some ADCs target the same antigen or have a similar payload that acts through a shared mechanism and the question will be whether these could be used sequentially. As for traditional chemotherapies, sequential ADC administration seems feasible and effective. Initial evidence comes from HER2-positive BC in which both T-DXd77 and trastuzumab duocarmazine8 have demonstrated activity in patients previously treated with T-DM1, likely due to the different payload of these novel ADCs. Moreover, in the TROPION-PanTumor01 trial testing datopotamab deruxtecan (Dato-DXd) 30% of patients previously received a TOP1 inhibitor-based ADC.78 Although the ORR increased when removing patients pretreated with these ADCs, several responses were observed in this subset, suggesting that sequencing may still be effective. Regarding SG, RNA and whole-exome sequencing of pre- and post-progression specimens from three patients revealed that Trop-2 expression is required for response to SG and that resistance to this agent can emerge from genomic alterations in the antibody or payload targets, respectively Trop-2 and TOP1.79 Similarly, in the DAISY study, in 13 out of 20 patients (65%) treated with T-DXd was observed a decrease in HER2 expression at progression.75 These findings could inform therapeutic strategies to overcome resistance. For example, for some HER2-low BC patients, both SG and T-DXd are available treatment options, and both have a TOP1 inhibitor payload. Therefore, resistance determined by alterations in TOP1 would likely confer cross-resistance while target alteration would not affect the efficacy of the sequence.
HER2-ultralow: a return to the dichotomy?
The recently presented data of the DAISY trial suggested meaningful activity of T-DXd even in patients with HER2-0 mBC (ORR 30%, PFS 4.2 months).74 The role of T-DXd in this subgroup of patients is being investigated also in the ongoing DESTINY-Breast06 (NCT04494425) that includes patients with HER2 IHC 0 but minimal expression (IHC score > 0 but < 1+), today defined as HER2-ultralow. Several hypotheses have been put forward to explain the activity of T-DXd in HER2-0 BC. Primarily, the subgroup of HER2 IHC 0 includes not only the absence of membrane staining, but also the incomplete and faint or barely perceptible membrane staining in ⩽ 10% of tumor cells.2 This minimal expression could be exploited by novel ADCs such as T-DXd to enable cytotoxic drug delivery. Another explanation of activity could be related to the limitations of pathology testing. Indeed, as most normal and BC cells express some degree of HER280 it is possible that the lack of IHC membrane staining may represent a false negative due to artifact of formalin fixation process or insufficient sensitivity of the IHC assay in the low ranges of HER2 expression. Thus, misdiagnosis and low concordance among pathologists on scores 0 and 1+ should be taken into account.9,10 Moreover, the spatial and temporal intratumor heterogeneity of HER2 may hamper the correct identification of true HER2 expression level. Finally, the presence of unconjugated payload in the bloodstream owing to incomplete conjugation during production and/or linker lability could contribute to ADCs’ activity in tumors with low or minimal HER2 expression.81
Regardless of the mechanism, the activity of T-DXd in HER2-0 suggests the inadequacy of the current HER2-low definition and testing assay. Emerging quantitative HER2 assays may improve our classification of HER2 expression and prediction of ADCs’ activity. Whether future studies will confirm the activity of next-generation ADCs like T-DXd in HER2-0 BC, clinical practice will likely return to the dichotomous HER2 classification based on which for HER2-positive BC there is an arsenal of anti-HER2 agents (monoclonal antibodies, tyrosine kinase inhibitors, and ADCs) while for the whole group of HER2-negative BC T-DXd, and possibly other ADCs in the future, will be available. Emerging quantitative HER2 assays may improve our classification and prediction of ADCs activity possibly leading to consider HER2 expression as a continuous spectrum, just as for ER and PgR.
Conclusions
The introduction of the novel targetable subset of HER2-low BC has led to a remarkable change in the treatment algorithms for patients with BC. HER2-low expression identifies patients with mBC who can derive benefit from T-DXd. However, along with this meaningful advancement, several challenges have recently emerged in the field. We will need to optimize strategies for the identification of HER2-low BC through the development of more sensitive and reproducible HER2 assays. These assays, together with the results from ongoing trials, may lead to an evolution in the way we define HER2-low in the future. Moreover, with the availability of other ADCs for the treatment of BC, further investigations could provide optimized strategies for the treatment of HER2-low BC, such as by prioritizing a given ADC based on its target expression level, as well as by anticipating potential cross-resistance through the unraveling of mechanisms of resistance to these agents. Ultimately, a refined understanding of how ADCs work and stop working will allow us to fully exploit their therapeutic potential and hopefully, extend the benefit of targeting HER2 to a larger population of patients with mBC.
Acknowledgments
Not applicable.
Footnotes
ORCID iD: Paolo Tarantino  https://orcid.org/0000-0001-8686-0228
https://orcid.org/0000-0001-8686-0228
Contributor Information
Eleonora Nicolò, Division of New Drugs and Early Drug Development, European Institute of Oncology IRCCS, Milan, Italy; Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy.
Luca Boscolo Bielo, Division of New Drugs and Early Drug Development, European Institute of Oncology IRCCS, Milan, Italy; Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy.
Giuseppe Curigliano, Division of New Drugs and Early Drug Development, European Institute of Oncology IRCCS, Milan, Italy; Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy.
Paolo Tarantino, Division of New Drugs and Early Drug Development, European Institute of Oncology IRCCS, Milan, Italy; Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy; Breast Oncology Center, Dana-Farber Cancer Institute, 450 Brookline Avenue, Boston, MA 02215, USA; Harvard Medical School, Boston, USA.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution(s): Eleonora Nicolò: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Luca Boscolo Bielo: Conceptualization; Data curation; Formal analysis; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Giuseppe Curigliano: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing – original draft; Writing – review & editing.
Paolo Tarantino: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Eleonora Nicolò was supported by an American-Italian Cancer Foundation Post-Doctoral Research Fellowship.
GC served as consultant or advisor for Roche, Lilly and Bristol-Myers Squibb, served on the speaker’s bureau for Roche, Pfizer and Lilly, received travel funding from Pfizer and Roche and received honoraria from Roche, Pfizer, Lilly, Novartis, and SEAGEN, all outside the submitted work. PT has served as consultant/advisor for AstraZeneca, Daiichi Sankyo and Lilly outside the submitted work. EN and LBB do not report potential conflicts of interest
Availability of data and materials: Not applicable.
References
- 1. Martínez-Sáez O, Prat A. Current and future management of HER2-positive metastatic breast cancer. JCO Oncol Pract 2021; 17: 594–604. [DOI] [PubMed] [Google Scholar]
- 2. Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. J Clin Oncol 2018; 36: 2105–2122. [DOI] [PubMed] [Google Scholar]
- 3. Fehrenbacher L, Cecchini RS, Geyer CE, Jr, et al. NSABP B-47/NRG oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1+ or 2+. J Clin Oncol 2020; 38: 444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burris HA, Rugo HS, Vukelja SJ, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2) –positive breast cancer after prior HER2-directed therapy. J Clin Oncol 2011; 29: 398–405. [DOI] [PubMed] [Google Scholar]
- 5. Tarantino P, Hamilton E, Tolaney SM, et al. HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol 2020; 38: 1951–1962. [DOI] [PubMed] [Google Scholar]
- 6. Modi S, Jacot W, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med 2022; 387: 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Loibl S, Poortmans P, Morrow M, et al. Breast cancer. Lancet 2021; 397: 1750–1769. [DOI] [PubMed] [Google Scholar]
- 8. Banerji U, van Herpen CML, Saura C, et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol 2019; 20: 1124–1135. [DOI] [PubMed] [Google Scholar]
- 9. Lambein K, Van Bockstal M, Vandemaele L, et al. Distinguishing score 0 from score 1+ in HER2 immunohistochemistry-negative breast cancer. Am J Clin Pathol 2013; 140: 561–566. [DOI] [PubMed] [Google Scholar]
- 10. Fernandez AI, Liu M, Bellizzi A, et al. examination of low erbb2 protein expression in breast cancer tissue. JAMA Oncol 2022; 8: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Larson JS, Goodman LJ, Tan Y, et al. Analytical validation of a highly quantitative, sensitive, accurate, and reproducible assay (HERmark®) for the measurement of HER2 total protein and HER2 homodimers in FFPE breast cancer tumor specimens. Patholog Res Int 2010; 2010: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yardley DA, Kaufman PA, Huang W, et al. Quantitative measurement of HER2 expression in breast cancers: comparison with ‘real-world’ routine HER2 testing in a multicenter collaborative biomarker study and correlation with overall survival. Breast Cancer Res 2015; 17: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harigopal M, Barlow WE, Tedeschi G, et al. Multiplexed assessment of the southwest oncology group-directed intergroup breast cancer trial S9313 by AQUA shows that both high and low levels of HER2 are associated with poor outcome. Am J Pathol 2010; 176: 1639–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moutafi M, Robbins CJ, Yaghoobi V, et al. Quantitative measurement of HER2 expression to subclassify ERBB2 unamplified breast cancer. Lab Invest 2022; 102: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 15. Jensen K, Krusenstjerna-Hafstrøm R, Lohse J, et al. A novel quantitative immunohistochemistry method for precise protein measurements directly in formalin-fixed, paraffin-embedded specimens: analytical performance measuring HER2. Mod Pathol 2017; 30: 180–193. [DOI] [PubMed] [Google Scholar]
- 16. Griguolo G, Brasó-Maristany F, González-Farré B, et al. ERBB2 mRNA expression and response to ado-trastuzumab emtansine (T-DM1) in HER2-positive breast cancer. Cancers (Basel) 2020; 12: 1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Won HS, Ahn J, Kim Y, et al. Clinical significance of HER2-low expression in early breast cancer: a nationwide study from the Korean Breast Cancer Society. Breast Cancer Res 2022; 24: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tan RSYC, Ong WS, Lee K-H, et al. HER2 expression, copy number variation and survival outcomes in HER2-low non-metastatic breast cancer: an international multicentre cohort study and TCGA-METABRIC analysis. BMC Med 2022; 20: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Domergue C, Martin E, Lemarié C, et al. Impact of HER2 status on pathological response after neoadjuvant chemotherapy in early triple-negative breast cancer. Cancers (Basel) 2022; 14: 2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Horisawa N, Adachi Y, Takatsuka D, et al. The frequency of low HER2 expression in breast cancer and a comparison of prognosis between patients with HER2-low and HER2-negative breast cancer by HR status. Breast Cancer 2022; 29: 234–241. [DOI] [PubMed] [Google Scholar]
- 21. Chen M, Chen W, Liu D, et al. Prognostic values of clinical and molecular features in HER2 low-breast cancer with hormonal receptor overexpression: features of HER2-low breast cancer. Breast Cancer 2022; 29: 844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gampenrieder SP, Rinnerthaler G, Tinchon C, et al. Landscape of HER2-low metastatic breast cancer (MBC): results from the Austrian AGMT_MBC-Registry. Breast Cancer Res 2021; 23: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tarantino P, Gandini S, Nicolò E, et al. Evolution of low HER2 expression between early and advanced-stage breast cancer. Eur J Cancer 2022; 163: 35–43. [DOI] [PubMed] [Google Scholar]
- 24. Schettini F, Chic N, Brasó-Maristany F, et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer 2021; 7: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bao KKH, Sutanto L, Tse SSW, et al. The association of ERBB2 -low expression with the efficacy of cyclin-dependent kinase 4/6 inhibitor in hormone receptor–positive, ERBB2 -negative metastatic breast cancer. JAMA Netw Open 2021; 4: e2133132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Calbiac O, Lusque A, Mailliez A, et al. Comparison of management and outcomes in ERBB2 -low vs ERBB2 -zero metastatic breast cancer in France. JAMA Netw Open 2022; 5: e2231170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Y, Abudureheiyimu N, Mo H, et al. In real life, low-level HER2 expression may be associated with better outcome in HER2-negative breast cancer: a study of the national cancer center, China. Front Oncol 2022; 11: 774577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tarantino P, Jin Q, Tayob N, et al. Prognostic and biologic significance of ERBB2-low expression in early-stage breast cancer. JAMA Oncol 2022; 8: 1177–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Denkert C, Seither F, Schneeweiss A, et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol 2021; 22: 1151–1161. [DOI] [PubMed] [Google Scholar]
- 30. de Moura Leite L, Cesca MG, Tavares MC, et al. HER2-low status and response to neoadjuvant chemotherapy in HER2 negative early breast cancer. Breast Cancer Res Treat 2021; 190: 155–163. [DOI] [PubMed] [Google Scholar]
- 31. Agostinetto E, Rediti M, Fimereli D, et al. HER2-low breast cancer: molecular characteristics and prognosis. Cancers (Basel) 2021; 13: 2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mutai R, Barkan T, Moore A, et al. Prognostic impact of HER2-low expression in hormone receptor positive early breast cancer. The Breast 2021; 60: 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jacot W, Maran-Gonzalez A, Massol O, et al. Prognostic value of HER2-low expression in non-metastatic triple-negative breast cancer and correlation with other biomarkers. Cancers (Basel) 2021; 13: 6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Almstedt K, Heimes A-S, Kappenberg F, et al. Long-term prognostic significance of HER2-low and HER2-zero in node-negative breast cancer. Eur J Cancer 2022; 173: 10–19. [DOI] [PubMed] [Google Scholar]
- 35. Tarantino P, Niman SM, Erick TK, et al. HER2-low inflammatory breast cancer: clinicopathologic features and prognostic implications. Eur J Cancer 2022; 174: 277–286. [DOI] [PubMed] [Google Scholar]
- 36. Viale G, Niikura N, Tokunaga E, et al. Retrospective study to estimate the prevalence of HER2-low breast cancer (BC) and describe its clinicopathological characteristics. J Clin Oncol 2022; 40: 1087–1087. [Google Scholar]
- 37. Miglietta F, Griguolo G, Bottosso M, et al. Evolution of HER2-low expression from primary to recurrent breast cancer. NPJ Breast Cancer 2021; 7: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Scott M, Vandenberghe ME, Scorer P, et al. Prevalence of HER2 low in breast cancer subtypes using the VENTANA anti-HER2/neu (4B5) assay. J Clin Oncol 2021; 39: 1021–1021. [Google Scholar]
- 39. Miglietta F, Griguolo G, Bottosso M, et al. HER2-low-positive breast cancer: evolution from primary tumor to residual disease after neoadjuvant treatment. NPJ Breast Cancer 2022; 8: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Osborne CK, Shou J, Massarweh S, et al. Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin Cancer Res 2005; 11: 865s–870s. [PubMed] [Google Scholar]
- 41. Hamilton E, Shastry M, Shiller SM, et al. Targeting HER2 heterogeneity in breast cancer. Cancer Treat Rev 2021; 100: 102286. [DOI] [PubMed] [Google Scholar]
- 42. Jordan NV, Bardia A, Wittner BS, et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature 2016; 537: 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ignatiadis M, Rothé F, Chaboteaux C, et al. HER2-positive circulating tumor cells in breast cancer. PLoS One 2011; 6: e15624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pestrin M, Bessi S, Galardi F, et al. Correlation of HER2 status between primary tumors and corresponding circulating tumor cells in advanced breast cancer patients. Breast Cancer Res Treat 2009; 118: 523–530. [DOI] [PubMed] [Google Scholar]
- 45. Fehm T, Müller V, Aktas B, et al. HER2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast Cancer Res Treat 2010; 124: 403–412. [DOI] [PubMed] [Google Scholar]
- 46. Fehm T, Mueller V, Banys-Paluchowski M, et al. Abstract PD3-12: efficacy of the tyrosine kinase inhibitor lapatinib in the treatment of patients with HER2-negative metastatic breast cancer and HER2-positive circulating tumor cells-results from the randomized phase III DETECT III trial. Cancer Res 2021; 81: PD3-12. [Google Scholar]
- 47. Krop IE, LoRusso P, Miller KD, et al. A phase II study of trastuzumab emtansine in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer who were previously treated with trastuzumab, lapatinib, an anthracycline, a taxane, and capecitabine. J Clin Oncol 2012; 30: 3234–3241. [DOI] [PubMed] [Google Scholar]
- 48. Filho OM, Viale G, Stein S, et al. Impact of HER2 heterogeneity on treatment response of early-stage HER2-positive breast cancer: phase II neoadjuvant clinical trial of T-DM1 combined with pertuzumab. Cancer Discov 2021; 11: 2474–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Giugliano F, Corti C, Tarantino P, et al. Bystander effect of antibody–drug conjugates: fact or fiction? Curr Oncol Rep 2022; 24: 809–817. [DOI] [PubMed] [Google Scholar]
- 50. Nicolò E, Giugliano F, Ascione L, et al. Combining antibody-drug conjugates with immunotherapy in solid tumors: current landscape and future perspectives. Cancer Treat Rev 2022; 106: 102395. [DOI] [PubMed] [Google Scholar]
- 51. Iwata TN, Ishii C, Ishida S, et al. A HER2-targeting antibody–drug conjugate, trastuzumab deruxtecan (DS-8201a), enhances antitumor immunity in a mouse model. Mol Cancer Ther 2018; 17: 1494–1503. [DOI] [PubMed] [Google Scholar]
- 52. Schmid P, Im S-A, Armstrong A, et al. BEGONIA: Phase 1b/2 study of durvalumab (D) combinations in locally advanced/metastatic triple-negative breast cancer (TNBC)—Initial results from arm 1, d+paclitaxel (P), and arm 6, d+trastuzumab deruxtecan (T-DXd). J Clin Oncol 2021; 39: 1023–1023. [Google Scholar]
- 53. Hamilton EP, Shapiro CL, Boni V, et al. 162O primary analysis from DS8201-A-U105: A 2-part, open label, phase Ib trial assessing trastuzumab deruxtecan (T-DXd) with nivolumab (nivo) in patients (pts) with HER2-expressing advanced breast cancer. Ann Oncol 2022; 33: S196. [Google Scholar]
- 54. Vasan N, Baselga J, Hyman DM. A view on drug resistance in cancer. Nature 2019; 575: 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van der Lee MMC, Groothuis PG, Ubink R, et al. The preclinical profile of the duocarmycin-based HER2-targeting ADC SYD985 predicts for clinical benefit in low HER2-expressing breast cancers. Mol Cancer Ther 2015; 14: 692–703. [DOI] [PubMed] [Google Scholar]
- 56. Wang J, Liu Y, Zhang Q, et al. RC48-ADC, a HER2-targeting antibody-drug conjugate, in patients with HER2-positive and HER2-low expressing advanced or metastatic breast cancer: a pooled analysis of two studies. J Clin Oncol 2021; 39: 1022–1022. [Google Scholar]
- 57. Jiang Z, Sun T, Wang X, et al. A multiple center, open-label, single-arm, phase II clinical trial of MRG002, an HER2-targeted antibody-drug conjugate, in patients with HER2-low expressing advanced or metastatic breast cancer. J Clin Oncol 2022; 40: 1102–1102.35015587 [Google Scholar]
- 58. Gennari A, André F, Barrios CH, et al. ESMO clinical practice guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol 2021; 32: 1475–1495. [DOI] [PubMed] [Google Scholar]
- 59. Bortot L, Basile D, Targato G, et al. 295P Clinical characterization and outcome of a HER2-low metastatic breast cancer (mBC) cohort receiving first-line treatment (1L) with ET +/- CDK 4/6 inhibitor (CDKi). Ann Oncol 2021; 32: S493. [Google Scholar]
- 60. Lapuchesky LS, Bortz M, Waisberg F, et al. CDK4/6 inhibitors outcomes in patients with advanced breast cancer based on HER2-low expression. J Clin Oncol 2022; 40: 1056. [Google Scholar]
- 61. Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med 2018; 379: 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Robson M, Im S-A, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 2017; 377: 523–533. [DOI] [PubMed] [Google Scholar]
- 63. Rugo HS, Bardia A, Marmé F, et al. LBA76-overall survival (OS) results from the phase III TROPiCS-02 study of sacituzumab govitecan (SG) vs treatment of physician’s choice (TPC) in patients (pts) with HR+/HER2- metastatic breast cancer (mBC). Ann Oncol 2022; 33: S808–S869. [Google Scholar]
- 64. Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med 2021; 384: 1529–1541. [DOI] [PubMed] [Google Scholar]
- 65. Hurvitz SA, Bardia A, Punie K, et al. 168P Sacituzumab govitecan (SG) efficacy in patients with metastatic triple-negative breast cancer (mTNBC) by HER2 immunohistochemistry (IHC) status: findings from the phase III ASCENT study. Ann Oncol 2022; 33: S200–S201. [Google Scholar]
- 66. Diéras V, Weaver R, Tolaney SM, et al. Abstract PD13-07: subgroup analysis of patients with brain metastases from the phase 3 ASCENT study of sacituzumab govitecan versus chemotherapy in metastatic triple-negative breast cancer. Cancer Res 2021; 81: PD13-07. [Google Scholar]
- 67. Brenner AJ, Pandey R, Chiou J, et al. Abstract PD13-05: delivery and activity of SN-38 by sacituzumab govitecan in breast cancer brain metastases. Cancer Res 2021; 81: PD13-05. [Google Scholar]
- 68. Epaillard N, Lusque A, Pistilli B, et al. 260P–Antitumor activity of trastuzumab deruxtecan (T-DXd) in patients with metastatic breast cancer (mBC) and brain metastases (BMs) from DAISY trial. Ann Oncol 2022; 33: S88–S121. [Google Scholar]
- 69. Hurvitz S, Kim S-B, Chung W-P, et al. Abstract GS3-01: Trastuzumab deruxtecan (T-DXd; DS-8201a) vs. trastuzumab emtansine (T-DM1) in patients (pts) with HER2+ metastatic breast cancer (mBC): subgroup analyses from the randomized phase 3 study DESTINY-Breast03. Cancer Res 2022; 82: GS3-01. [Google Scholar]
- 70. Pérez-García JM, Vaz Batista M, Cortez P, et al. Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: The DEBBRAH trial. Neuro Oncol 2023; 25: 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bartsch R, Berghoff AS, Furtner J, et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat Med 2022; 28: 1840–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tarantino P, Modi S, Tolaney SM, et al. Interstitial lung disease induced by anti-ERBB2 antibody-drug conjugates. JAMA Oncol 2021; 7: 1873. [DOI] [PubMed] [Google Scholar]
- 73. Rugo HS, Tolaney SM, Loirat D, et al. Abstract PS11-09: impact of UGT1A1 status on the safety profile of sacituzumab govitecan in the phase 3 ASCENT study in patients with metastatic triple-negative breast cancer. Cancer Res 2021; 81: PS11-09. [Google Scholar]
- 74. Diéras V, Deluche E, Lusque A, et al. Abstract PD8-02: Trastuzumab deruxtecan (T-DXd) for advanced breast cancer patients (ABC), regardless HER2 status: a phase II study with biomarkers analysis (DAISY). Cancer Res 2022; 82: PD8-02. [Google Scholar]
- 75. Mosele MF, Lusque A, Dieras V, et al. LBA1 Unraveling the mechanism of action and resistance to trastuzumab deruxtecan (T-DXd): biomarker analyses from patients from DAISY trial. Ann Oncol 2022; 33: S123. [Google Scholar]
- 76. Bardia A, Tolaney SM, Punie K, et al. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann Oncol 2021; 32: 1148–1156. [DOI] [PubMed] [Google Scholar]
- 77. Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med 2020; 382: 610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Krop I, Juric D, Shimizu T, et al. Abstract GS1-05: Datopotamab deruxtecan in advanced/metastatic HER2- breast cancer: results from the phase 1 TROPION-PanTumor01 study. Cancer Res 2022; 82: GS1-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Coates JT, Sun S, Leshchiner I, et al. Parallel genomic alterations of antigen and payload targets mediate polyclonal acquired clinical resistance to sacituzumab govitecan in triple-negative breast cancer. Cancer Discov 2021; 11: 2436–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/ neu proto-oncogene in human breast and ovarian cancer. Science 1989; 244: 707–712. [DOI] [PubMed] [Google Scholar]
- 81. Drago JZ, Modi S, Chandarlapaty S. Unlocking the potential of antibody–drug conjugates for cancer therapy. Nat Rev Clin Oncol 2021; 18: 327–344. [DOI] [PMC free article] [PubMed] [Google Scholar]