Abstract
Background
Referral to palliative medicine (PM) has been shown to improve quality of life, reduce hospitalizations, and improve survival. Limited data exist about PM utilization among racial minorities with gynecologic malignancies. Our objective was to assess differences in palliative medicine referrals and end of life interventions (within the last 30 days of life) by race and ethnicity in a diverse population of gynecologic oncology patients.
Methods
A retrospective cohort study of patients receiving gynecologic oncologic care at a tertiary referral center between 2017 – 2019 was conducted. Patients had either metastatic disease at the time of diagnosis or recurrence. Demographic and clinical data were abstracted. Exploratory analyses were done using chi-square and rank sum tests. Tests were two-sided with significance set at P < .05.
Results
A total of 186 patients were included. Of those, 82 (44.1%) were referred to palliative medicine. Underrepresented minorities accounted for 47.3% of patients. English was identified as the primary language for 69.9% of the patients and Spanish in 24.2%. Over 90% of patients had insurance coverage. Ovarian cancer (37.6%) and uterine cancer (32.8%) were the most common sites of origin. Most patients (75%) had advanced stage at the time of diagnosis. Race and language spoken were not associated with referral to PM. Black patients were more likely to have been prescribed appetite stimulants compared to White patients (41% vs 24%, P = .038). Black patients also had a higher number of emergency department visits compared to White patients during the study timeframe. Chemotherapy in the last 30 days of life was also more likely to be given to Black patients compared to White (P = .019).
Conclusions
Race was associated with variation in interventions and healthcare utilization near end-of-life. Understanding the etiologies of these differences is crucial to inform interventions for care optimization as it relates specifically to the health of minority patients.
Keywords: cancer, palliative care, health care, health care disparities, end-of-life, Gynecologic Oncology
Background
Gynecologic malignancies will account for 115,130 of the new cancer cases diagnosed in the United States in 2022.1 Most patients diagnosed with an advanced gynecologic malignancy have an incurable chronic condition that carries a large symptom burden, either directly related to the primary malignancy or due to complications related to treatment. In addition to the standard of care treatment for gynecologic malignancies, evidence supports simultaneous palliative medicine involvement in patient care to improve overall patient outcomes.2-5
Fauci et al reported that 58% of patients with gynecologic cancer received chemotherapy within the final 6 months of life, 7% received radiotherapy, 59% underwent at least one procedure for curative therapy, and 85% required one or multiple inpatient admissions.6 In the study by Soares et al patients who received chemotherapy in the last 30 days of life were more likely to visit the emergency department, undergo medical imaging, and die in the hospital compared to those who did not use chemotherapy,7 raising concerns about the futility of such interventions.
Despite the recognized importance of palliative medicine in cancer patients, studies on implementation and outcomes have minimal representation from minority groups. In a meta-analysis by Pirl et al examining racial and ethnic representation in studies of early palliative care involvement for patients with advanced cancer, only 75% reported ethnicity data, and less than half (38%) included African American (AA)/Black or Hispanic patients. Of those studies that included minority patients, the total number of patients included represented less than 25% of the cohort, and few focused specifically on gynecologic cancers.8 Cancer health disparities in women diagnosed with gynecologic cancers in minoritized populations have been well documented.9-11 Given the paucity of data in the context of palliative care, it is important that further work is done to understand potential disparities among these populations. Our objectives were to evaluate patterns of referral to palliative medicine and to assess differences in end-of-life interventions among women with gynecologic cancers by race in a diverse patient population. If such differences exist, they may inform interventions for care optimization and elimination of structural barriers.
Methods
Ethics Statement
This study received ethics approval from the University of Miami (Human Subject Research Office (M809), 1400 NW 10th Avenue, Suite 1200A Miami, FL 33136), Protocol Approval Number #20200275. Given the design of the study, approval was obtained for both waiver of consent and full waiver of authorization.
Design, Setting and Participants
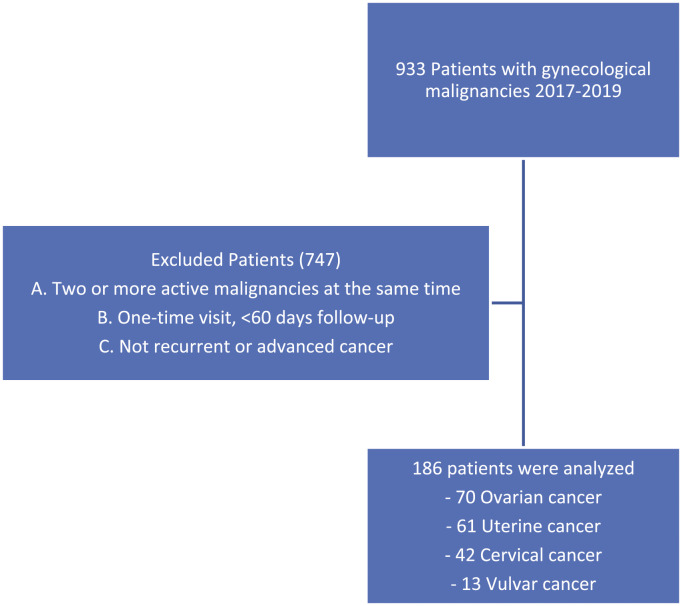
A retrospective review was conducted of patients treated for a gynecologic oncology (GYO) malignancy at Sylvester Comprehensive Cancer Center (SCCC) during the years 2017-2019 with the following diagnoses: platinum resistant/refractory or recurrent ovarian cancer; advanced endometrial cancer (Stage III-IV) or recurrent disease; advanced cervical cancer (Stage III-IV) or recurrent disease; and advanced vulvar or vaginal cancer (Stage III-IV) or recurrent disease. The study timeframe of 2017 to 2019 was selected based on known tumor registry data from our clinic. This timeframe would allow for evaluation of 700 to 800 subjects with approximately 25% to 30% meeting inclusion criteria outlined above. Patients were excluded if they had multiple (two or more) active cancers, patients who did not meet the cancer stage criteria, and those were seen only once by the Gynecologic Oncology service (Figure 1).
Figure 1.
Inclusion and exclusion criteria.
Patient-specific information was obtained from the electronic medical record for all patients who met inclusion criteria, regardless of palliative medicine referral. Variables abstracted included: demographic information (age, race/ethnicity, personal country of origin, zip code, insurance type, familial country of origin, BMI, smoking status, illicit drug use), cancer related data (cancer type, stage, evidence of metastasis, emergency visits and hospitalizations), and palliative medicine related data. Specific data collected regarding palliative medicine for all subjects included: referral to palliative medicine (defined as an order placed into the electronic medical record in the outpatient or hospital-based setting), Eastern Cooperative Oncology Group (ECOG) performance score, referral indication (symptom management, goals of care, or both), and end of life (EOL) interventions such as chemotherapy and surgical interventions within the last 30 days of life. For evaluation of high-intensity care received during the end-of-life period, chemotherapy use within the last 30 days of life, surgical interventions within the last 30 days of life, hospital admissions, emergency department visits, as well as hospice referral were used. These factors were identified and selected based on published studies evaluating patients with cancer, including gynecologic malignancies.12-14
Data Analysis
The sociodemographic and clinical data were summarized using descriptive statistics with total count and corresponding percentage. Chi-square analysis (or Fisher’s exact, when appropriate), and Wilcoxon’s Rank Sum Test were performed to assess study variables and referral practices to palliative medicine. All statistical analysis tests were two-sided with statistical significance set at P < .05. Statistical analysis was completed using IBM SPSS Statistics for Windows, version 28 (IBM Corp., Armonk, N.Y., USA).
Results
A total of 186 patients met inclusion criteria. Demographic data are shown in (Table 1). Most of the patients included in this study spoke English as their primary language (69.9%) with approximately 25% speaking Spanish. The mean age at diagnosis was 58 years. About half of the patients were White (49.5%), 24.7% were Black, and 22.6% were Hispanic. Ovarian cancer (37.6%) and uterine cancer (32.8%) were the most common diagnoses. Over 75% of patients had Stage III or IV at the time of diagnosis with abdominal or lymph node involvement being the most common sites of tumor spread (86% and 67.7%). Despite an advanced stage at diagnosis, over 50% of our patients had an ECOG score of 0-1 at the time of first contact.
Table 1.
Patient Demographics.
| Variables | N (%) | |
|---|---|---|
| Language spoken | English | 130 (69.9%) |
| Spanish | 45 (24.2%) | |
| Creole | 6 (3.2%) | |
| Other | 5 (2.6%) | |
| Age at diagnosis (Mean, SD) | 58.35 (±12.971) [min 21 – max 90] | |
| Race/Ethnicity | Asian | 6 (3.2%) |
| Black or AA | 46 (24.7%) | |
| Hispanic or Latina | 42 (22.6%) | |
| Unknown | 1 (.5%) | |
| White | 90 (49.5%) | |
| BMI at diagnosis (Mean, SD) | 26.7 (±6.9) [min 14.12 – max 61.00] | |
| Last Available BMI | 25.7 (±6.8) [min 13.50 – max 63.60] | |
| Country of birth | USA | 38 (20.4%) |
| Foreign | 41 (22%) | |
| Unknown | 107 (57.5%) | |
| Tobacco use | Yes | 26 (14%) |
| No | 160 (86%) | |
| Illicit drugs | Yes | 5 (2.7%) |
| No | 181 (97.3%) | |
| Insurance | Medicaid | 16 (8.6%) |
| Medicare | 43 (23.1%) | |
| Private insurance | 124 (66.7%) | |
| Other* | 3 (1.6%) | |
| Organ site | Cervix | 42 (22.6%) |
| Ovary | 70 (37.6%) | |
| Uterus | 61 (32.8%) | |
| Vulva | 13 (7.0%) | |
| Stage at diagnosis | I | 17 (9.1%) |
| II | 23 (12.4%) | |
| III | 66 (35.5%) | |
| IV | 80 (43.0%) | |
| Metastatic disease | Yes | 176 (94.6%) |
| No | 7 (3.8%) | |
| Palliative referral | Yes | 82 (44.1%) |
| No | 104 (55.9%) | |
| Referred and seen | Yes | 67 (82%) |
| No | 15 (18%) | |
| Reasons for referral | Goals of care | 9 (10.9%) |
| Symptom Management | 60 (73.1%) | |
| Both | 13 (15.8%) | |
| ECOG 1st | 0 | 62 (33.3%) |
| 1 | 48 (25.8) | |
| 2 | 5 (2.7%) | |
| 3 | 2 (1.1%) | |
| Unknown | 69 (37.1%) | |
*Other (two with no insurance, one with Veteran Affairs insurance coverage). BMI kg/m2 (Body Mass Index). ECOG (Eastern Cooperative Oncology Group).
Palliative medicine referral was provided to 82 (44.2%) patients with 67 (37%) ultimately seeing a palliative medicine provider. The most common indication for palliative medicine referral was symptom management (73%). Symptom management included any evaluation or visit for recommendations of care for malignancy related symptoms (pain, nausea, insomnia, etc.) (Table 1).
The use of analgesics, antiemetics and bowel regimens (cathartics) was high, and was reported in 84.4%, 76.3%, and 60.2% respectively. Prescriptions for agitation (41.4%) and appetite stimulation (28.5%) were less commonly provided. Sixty-seven percent (67%) of the cohort had at least 1-3 visits to the emergency department (ED) or hospitalizations, while more than 7 visits or hospitalizations were documented in 14.5% of the cohort. Chemotherapy and procedures in the last 30 days of life was noted in 5.9% of the cohort for both interventions. A total of 68 (36.6%) patients expired during the study interval (Table 2).
Table 2.
Pharmacologic Classification of Drugs Used by Study Patients, Emergency Department and Hospitalization, and Interventions in Last 30 days of Life.
| Variables | N (%) | |
|---|---|---|
| Analgesic use | No | 29 (15.6%) |
| Yes | 157 (84.4%) | |
| Antiemetic use | No | 44 (23.7%) |
| Yes | 142 (76.3%) | |
| Agitation Medication use | No | 109 (58.6%) |
| Yes | 77 (41.4%) | |
| Appetite stimulant use | No | 133 (71.5%) |
| Yes | 53 (28.5%) | |
| Bowel regimen use | No | 74 (39.8%) |
| Yes | 112 (60.2%) | |
| Admissions to emergency department prior to palliative referral | 0-3 | 125 (67.2%) |
| 4-7 | 41 (22%) | |
| 8 or more | 20 (10.8%) | |
| Admissions After palliative visit | 0-3 | 55 (29.7%) |
| 4-7 | 11 (5.9%) | |
| 8 or more | 4 (2.1%) | |
| Not referred or referred to hospice after or unknown | 116 (63.4%) | |
| Chemotherapy in last 30 days | Yes | 13 (19%) |
| No | 55 (81%) | |
| Procedure in last 30 days | Yes | 11 (16%) |
| No | 57 (84%) | |
| Patient expired | Yes | 68 (36.6%) |
| No | 118 (64.3%) | |
No significant associations were identified among various socio-demographic factors and a referral to palliative medicine among our cohort (Table 3). Patients who were referred to palliative medicine were more likely to be prescribed analgesics, antiemetics, medications for agitation, bowel regimen, and appetite stimulants than patients who were not referred (Table 4). A higher proportion of patients who were referred to PM were seen in the ED or admitted to the hospital across the treatment continuum. Palliative referral was also more common among patients who expired, with 69% (47/61) of them meeting with a palliative medicine provider at least once prior to expiration. Within the referral group, the mean time from referral to palliative medicine to time of death was 82 days (1-519). Over 31% of patients were referred to palliative medicine <30 days prior to end-of-life, with only 8.5% referred in the last 7 days of life (Table 5).
Table 3.
Socio-Demographic and Clinical Factors Associated With Palliative Medicine Referral.
| Variable | No PM referral (N = 104) | PM referral (N = 82) | P value | |
|---|---|---|---|---|
| Language spoken | English | 69 (66.3%) | 61 (74.4%) | .262 |
| Other | 35 (33.7%) | 21 (25.6%) | ||
| Age at diagnosis | — | 59 (±12.6) | 57 (±13.3) | .378 |
| Race | White | 83 (79.8%) | 57 (69.5%) | .125 |
| Black | 21 (20.2%) | 25 (30.5%) | ||
| Country of birth | US born | 23 (22.1%) | 15 (18.3%) | .520 |
| Foreign | 25 (24%) | 16 (19.5%) | ||
| Unknown | 56 (53.8%) | 51 (62.2%) | ||
| Type of insurance | Private | 70 (67.3%) | 54 (65.9%) | .876 |
| Other | 34 (32.7%) | 28 (34.1%) | ||
| Organ site | Cervix/Vulva | 26 (25.0%) | 29 (35.4%) | .196 |
| Ovary | 39 (37.5%) | 31 (37.8%) | ||
| Uterus | 39 (37.5%) | 22 (26.8%) | ||
| Stage at diagnosis | I | 10 (9.6%) | 7 (8.5%) | .068 |
| II | 10 (9.6%) | 13 (15.9%) | ||
| III | 31 (29.8%) | 35 (42.7%) | ||
| IV | 53 (51.0%) | 27 (32.9%) | ||
| First ECOG score | 0 | 36 (34.6%) | 26 (31.7%) | .584 |
| 1 | 22 (21.2%) | 26 (31.7%) | ||
| 2 | 3 (2.9%) | 2 (2.4%) | ||
| 3 | 1 (1.0%) | 1 (1.2%) | ||
| Unknown | 42 (40.4%) | 27 (32.9%) | ||
ECOG: Eastern Cooperative Oncology Group.
Table 4.
Clinical Factors Associated With Palliative Medicine Referral.
| Variable | No PM referral (N = 104) | PM referral (N = 82) | P value | |
|---|---|---|---|---|
| Analgesic use | Yes | 77 (74.0%) | 80 (97.6%) | <.001 |
| No | 27 (26.0%) | 2 (2.4%) | ||
| Antiemetic use | Yes | 71 (68.3%) | 71 (86.6%) | .005 |
| No | 33 (31.7%) | 11 (13.4%) | ||
| Agitation Rx use | Yes | 33 (31.7%) | 44 (53.7%) | .003 |
| No | 71 (68.3%) | 38 (46.3%) | ||
| Appetite stimulant use | Yes | 18 (17.3%) | 35 (42.7%) | <.001 |
| No | 86 (82.7%) | 47 (57.3%) | ||
| Bowel regimen use | Yes | 48 (46.2%) | 64 (78.0%) | <.001 |
| No | 56 (53.8%) | 18 (22.0%) | ||
| Emergency department visits since diagnosis | 0-5 | 97 (93%) | 55 (67%) | <.001 |
| 6+ | 7 (7%) | 27 (33%) | ||
| Patient expired | Yes | 21 (20.2%) | 47 (57.3%) | <.001 |
| No | 83 (79.8%) | 35 (42.7%) | ||
| Chemo in last 30 days | Yes | 4 (19%) | 9 (19%) | 1.00 |
| No | 17 (81%) | 38 (81%%) | ||
| Procedure in last 30 days | Yes | 4 (19%) | 7 (15%) | .727 |
| No | 17 (81%) | 40 (85%) | ||
| Hospice referral | Yes | 14 (13%) | 53 (65%) | <.001 |
| No | 90 (87%) | 29 (35%) | ||
Table 5.
Time From Palliative Medicine Referral to Patient Demise.
| Time from Referral to Patient Demise | N (%) |
|---|---|
| <7 days | 4 (8.5) |
| 7 to 14 days | 2 (4.3%) |
| 15 to 30 days | 9 (19.1%) |
| <30 days | 32 (68.1%) |
Differences in palliative medicine referral, resource utilization, and EOL interventions by race and language spoken are shown in Tables 6 and 7. A higher proportion of Black patients were prescribed appetite stimulants compared to White patients (41% vs 24%, P = .038). Black patients also had a higher number of emergency department visits/hospitalization (P = .035). Chemotherapy in the last 30 days of life was higher among Black patients compared to White (38.8% vs 12%, P = .019). Language spoken was not a significant factor associated with medication use or interventions in the last 30 days of life.
Table 6.
Sub-group analysis by patient race.
| Variable | White | Black | P value | |
|---|---|---|---|---|
| Analgesic use | Yes | 120 (86%) | 37 (80%) | .482 |
| No | 20 (14%) | 9 (20%) | ||
| Antiemetic use | Yes | 107 (76%) | 35 (76%) | 1.00 |
| No | 33 (24%) | 11 (24%) | ||
| Agitation Medication use | Yes | 77 (55%) | 14 (30%) | .088 |
| No | 63 (45%) | 32 (70%) | ||
| Appetite stimulant use | Yes | 34 (24%) | 19 (41%) | .038 |
| No | 106 (76%) | 27 (59%) | ||
| Bowel regimen use | Yes | 82 (59%) | 30 (65%) | .489 |
| No | 58 (41%) | 16 (35%) | ||
| Admission or emergency department visit since diagnosis | 0-5 | 119 (85%) | 33 (71%) | .035 |
| 6+ | 21 (15%) | 13 (28%) | ||
| Chemo in last 30 days | Yes | 6 (12%) | 7 (38.8%) | .019 |
| No | 44 (88%) | 11 (61.1%) | ||
| Surgery in last 30 days | Yes | 7 (14%) | 4 (8.7%) | .469 |
| No | 43 (86%) | 14 (30.4%) | ||
| Hospice referral | Yes | 45 (32.1%) | 22 (47.8%) | .076 |
| No | 95 (67.9%) | 24 (52.5%) | ||
| Hospice enrollment | Yes | 43 (30.7%) | 19 (41.3%) | .209 |
| No | 97 (69.3%) | 27 (58.7%) | ||
Table 7.
Sub-group analysis by patient spoken language.
| Variable | English | Non-English | P value | |
|---|---|---|---|---|
| Analgesic use | Yes | 110 (84.6%) | 47 (83.9%) | 1.00 |
| No | 20 (15.4%) | 9 (16.1%) | ||
| Antiemetic use | Yes | 100 (76.9%) | 42 (75%) | .851 |
| No | 30 (23.1%) | 14 (25%) | ||
| Agitation Medication use | Yes | 58 (44.6%) | 19 (33.9%) | .197 |
| No | 72 (55.4%) | 37 (66.1%) | ||
| Appetite stimulant use | Yes | 37 (28.5%) | 16 (28.6%) | 1.00 |
| No | 93 (71.5%) | 40 (71.4%) | ||
| Bowel regimen use | Yes | 76 (58.5%) | 36 (64.3%) | .515 |
| No | 54 (41.5%) | 20 (35.7%) | ||
| Admission or emergency department visit since diagnosis | 0-5 | 105 (81%) | 47 (84%) | .120 |
| 5+ | 25 (19%) | 9 (16%) | ||
| Chemo in last 30 days | Yes | 9 (18%) | 4 (22%) | 1.00 |
| No | 41 (82%) | 14 (78%) | ||
| Surgery in last 30 days | Yes | 7 (14%) | 4 (22%) | .736 |
| No | 43 (86%) | 14 (78%) | ||
| Hospice referral | Yes | 50 (38.5%) | 17 (30.4%) | .321 |
| No | 80 (61.5%) | 39 (69.6%) | ||
| Hospice enrollment | Yes | 45 (34.6%) | 17 (30.4%) | .614 |
| No | 85 (65.4%) | 39 (69.6%) | ||
On review of patients who were referred to palliative medicine but did not complete a consultation (Supplementary Table), organ site was noted to be the only significant variable, with patients who had ovarian cancer being less likely to complete a consultation compared to those with uterine, cervical, or vulvar malignancies. Given the small sample size of patients who didn’t complete consultation despite a referral placed (15 subjects), no clear conclusions can be made regarding specific patient populations who might be more at risk for non-completion of palliative medicine consultation.
Discussion
In this investigation we demonstrated that there were no differences in palliative care referral by race, but that palliative care referral was associated with greater utilization of resources for symptom control. Variation in specific tools for alleviation of symptoms, such as appetite stimulants, and differences in futile measures near end of life did, however, vary by race. Our unique population, inclusive of more than 20% confirmed immigrants and >50% racial/ethnic minorities provides a unique lens to the needs of these women in the setting of advanced or recurrent cancer.
Studies evaluating end-of-life interventions among minority patients undergoing care for advanced or metastatic gynecologic malignancies are limited, given that data on racial disparities among these patients is inconsistently included. Barbera et al reported on health services received near end-of-life among 2,040 gynecologic cancer patients, but no racial or ethnic data were provided.15 A recent study by Islam et al published in 2021, in which patients with advanced gynecologic cancer were studied to evaluate racial or ethnic disparities in palliative medicine utilization, did include data about race/ethnicity. However, over 70% of the cohort was comprised of non-Hispanic White patients (73.5%) with approximately 17% of patients identified as non-Hispanic Black, or Hispanic.16 In contrast, our study cohort consisted of 49.5% non-Hispanic White, with non-Hispanic Black and Hispanic patients representing 24.7% and 22.6% respectively.
In our cohort, Black patients had a higher number of emergency department visits and/or hospitalizations compared to White patients (Hispanic and Non-Hispanic combined) during the study timeframe. Black patients were also noted to have a higher use of chemotherapy in the last 30 days of life compared to White patients (38.8% vs 11.7%). This is consistent with findings from Yang et al reporting a significantly higher percentage of aggressive end-of-life interventions in Non-Hispanic Black patients, including higher ICU admission in the last 30 days of life as well as chemotherapy in the last 14 days of life.17,18 The increased use of emergency department visits or hospitalizations by Black patients has also been seen in other studies.19,20 Among patients with ovarian cancer, Taylor et al similarly found differences in end-of-life care among minority patients, with Black patients being more likely to visit the emergency department within the last 30 days of life compared to White patients.21 The reason for this observation is not definitively known and is likely multifactorial, with complex social aspects such as cultural preferences, religious beliefs, overall trust in the medical profession, lack of advanced care planning, as well as patients’ and caregivers’ emergency care preferences.22,23
Smith et al, using data from the Coping with Cancer study, demonstrated that Black and Hispanic patients were less likely to have an advanced care plan, and more likely to want life-prolonging care, even with only a few days left to live.24 These patients were less likely to acknowledge their terminally ill status, citing religion as a very important factor. In a study by LoPresti et al, African American patients perceived a greater need for hospice, but more frequently had inadequate knowledge of hospice care.22 Additionally, there was less documentation of advanced care plans among Hispanics and African Americans, with some of the reasons provided being religious or cultural differences, caregiver respect for autonomy, access barriers, and acknowledgment of end-of-life care options. Mack et al noted that end-of-life discussions in white patients translated to less aggressive interventions, which was not the case in black or Hispanics.25 In Black and Hispanic patients, end-of-life discussions resulted in more DNR orders being placed but didn’t translate into less aggressive interventions during end-of-life care, raising question about the quality and impact of that counseling.22,24-27
Use of appetite stimulants among Black patients was higher compared to that of White patients. Retrospective studies in patients with pancreatic cancer have shown that cachexia disproportionately affects Black patients more than White, with no well-established explanation.28 In another publication by Lambda et al, the use of various types of medications for symptom control were lower among African Americans and Hispanics patients compared to White.29 This study included both male and female patients as well as various cancer types such as lung, breast, esophageal, testicular, and ovarian, among many other, factors which can obscure the utilization pattern among gynecologic malignancies which could explain the different observed outcomes.
When assessing the timeliness of palliative medicine referral for patients with advanced or recurrent gynecologic malignancies, a single institution retrospective analysis by Nitecki et al showed that among patients with metastatic or advanced ovarian cancer, 38% of them were referred to palliative medicine within 30 days from death, and 17% within one week.30 Nevadunsky et al evaluated 100 racially diverse patients who died from primary gynecologic malignancies at a single institution, with 49% being referred to palliative medicine and only 18% being referred more than 30 days prior to their death.31 In our cohort, 31% of patients were referred within 30 days of death, with 8.5% referred within the last week of life, suggesting that our cohort may have been referred earlier in the disease course. Unlike the population studied by Nitecki et al which included 85% of White patients, our patient population is more racially diverse with Black and Hispanic patients comprising over 47% of the cohort, similar distribution to those patients evaluated and reported by Nevadunsky et al in their study.
The proportion of patients who had a referral to hospice, while not statistically significant, was higher among Black patients relative to White patients (47.8% vs 32.1%, P = .076). This finding is consistent with a retrospective, cross-sectional study by Johnson et al, which included 35% (644) of patients with neoplasm related admissions with over 50% of the population being African American or Hispanic didn’t show any statistical association between inpatient Palliative Medicine consultation and discharge to hospice in regard to race or ethnicity.32 Similar to their reported findings, we identified a significant association between palliative medicine consultation and hospice referral (<.001), an expected finding given that over 50% of our cohort was referred to palliative medicine within the last 30 days of life, reflective of the advanced state of disease and clinical condition. Notably, the use of chemotherapy was higher in Black patients within the last 30 days of life, despite the relatively high proportion of referrals to hospice. While the reasons for this are likely multifactorial, it suggests that futile treatments may be more utilized in this population or that hospice referrals are later when death may be imminent.
Strengths and Limitations
Our study was limited by its retrospective nature, with the associated risk of information bias, especially because data abstraction was completed by review of an electronic medical record with multiple users. This was a particular limitation when attempting to identify reasons for patients not being seen by palliative medicine providers after a referral had been placed, which was the case among 15 patients within the study population. Additionally, it was done at a single institution, which may limit generalizability. Despite these limitations, however, we present data on a diverse group of gynecologic cancer patients with a sizable number of minorities included. During the study interval, the gynecologic oncology or palliative medicine faculty did not change, so there is no anticipated temporal bias which may distort referral patterns.
Conclusion
Despite similar trends in referral to palliative medicine across racial groups, the use of symptom-alleviating medications and utilization of aggressive interventions near the end-of-life significantly varies. Improve counseling practices regarding EOL care such as advanced care planning as well as qualitative research to better understand EOL perceptions and values among Black and other minorities should be considered to improve clinical understanding of care preferences, and the numerous sociocultural influences which inform these preferences.
Supplemental Material
Supplemental Material for Palliative Medicine Referral and End-of-Life Interventions Among Racial and Ethnic Minority Patients With Advanced or Recurrent Gynecologic Cancer by Angel Tabuyo-Martin, Angelica Torres-Morales, Marie J. Pitteloud, Alisha Kshetry, Carina Oltmann, J. Matt Pearson, Mariana Khawand, Matthew P. Schlumbrecht and Julia C. Sanchez in Cancer Control
Acknowledgments
The authors would like to thank the Sylvester Comprehensive Cancer Center for its generous support of this project.
Author Contributions: Angel Tabuyo-Martin: Conceptualization, Methodology, Formal Analysis, Investigation, Writing-Original Draft, Visualization, Project administration. Angelica Torres-Morales: Investigation, Conceptualization, Writing - Original Draft. Marie J. Pitteloud: Investigation, Conceptualization, Writing - Original Draft. Alisha Kshetry: Investigation. Carina Oltmann: Writing - Review & Editing. Joseph M. Pearson: Writing - Review & Editing. Mariana Khawand: Writing - Review & Editing. Sophia HL George: Writing - Review & Editing. Matthew P. Schlumbrecht: Conceptualization, Methodology, Writing - Review & Editing, Supervision, Project administration. Julia C. Sanchez: Conceptualization, Methodology, Writing - Review & Editing, Supervision, Project administration.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by the University of Miami Institutional Review Board (IRB Protocol #20200275).
Informed Consent: Informed consent for patient information to be published in this article was not obtained because approval was received for both waiver of consent and full waiver of authorization based on the study design.
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the University of Miami Institutional Review Board (IRB Protocol #20200275).
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Angel Tabuyo-Martin https://orcid.org/0000-0001-8624-2630
Carina Oltmann https://orcid.org/0000-0001-5517-0080
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics. CA Cancer J Clin. 2022;72(1):7-33. [DOI] [PubMed] [Google Scholar]
- 2.Haun MW, Estel S, Rucker G, et al. Early palliative care for adults with advanced cancer. Cochrane Database Syst Rev. 2017;6:CD011129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med. 2010;363(8):733-742. [DOI] [PubMed] [Google Scholar]
- 4.Fulton JJ, LeBlanc TW, Cutson TM, et al. Integrated outpatient palliative care for patients with advanced cancer: A systematic review and meta-analysis. Palliat Med. 2019;33(2):123-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112. [DOI] [PubMed] [Google Scholar]
- 6.Fauci J, Schneider K, Walters C, et al. The utilization of palliative care in gynecologic oncology patients near the end of life. Gynecol Oncol. 2012;127(1):175-179. [DOI] [PubMed] [Google Scholar]
- 7.Soares LGL, Gomes RV, Palma A, Japiassu AM. Quality indicators of end-of-life care among privately insured people with cancer in Brazil. Am J Hosp Palliat Care. 2020;37(8):594-599. [DOI] [PubMed] [Google Scholar]
- 8.Pirl WF, Saez-Flores E, Schlumbrecht M, Nipp R, Traeger LN, Kobetz E. Race and ethnicity in the evidence for integrating palliative care into oncology. J Oncol Pract. 2018;14(6):e346-e356. [DOI] [PubMed] [Google Scholar]
- 9.Collins Y, Holcomb K, Chapman-Davis E, Khabele D, Farley JH. Gynecologic cancer disparities: A report from the health disparities taskforce of the society of gynecologic oncology. Gynecol Oncol. 2014;133(2):353-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clair K, Bristow RE. Looking at cancer health disparities in gynecologic oncology in 2020. Curr Opin Obstet Gynecol. 2021;33(4):355-359. [DOI] [PubMed] [Google Scholar]
- 11.Bristow RE, Powell MA, Al-Hammadi N, et al. Disparities in ovarian cancer care quality and survival according to race and socioeconomic status. J Natl Cancer Inst. 2013;105(11):823-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneiter MK, Karlekar MB, Crispens MA, Prescott LS, Brown AJ. The earlier the better: The role of palliative care consultation on aggressive end of life care, hospice utilization, and advance care planning documentation among gynecologic oncology patients. Support Care Cancer. 2019;27(5):1927-1934. [DOI] [PubMed] [Google Scholar]
- 13.Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22(2):315-321. [DOI] [PubMed] [Google Scholar]
- 14.Hicks-Courant K, Kanter GP, Schapira MM, Brensinger CM, Liu Q, Ko EM. Intensity of end-of-life care for gynecologic cancer patients by primary oncologist specialty. Int J Gynecol Cancer. 2022;32(6):695-703. [DOI] [PubMed] [Google Scholar]
- 15.Barbera L, ELit L, Krzyzanowska M, Saskin R, Bierman AS. End of life care for women with gynecologic cancers. Gynecol Oncol. 2010;118(2):196-201. [DOI] [PubMed] [Google Scholar]
- 16.Islam JY, Deveaux A, Previs RA, Akinyemiju T. Racial disparities in palliative care utilization among metastatic gynecological cancer patients living at last follow-up: An analysis of the National Cancer Data Base. Data Brief. 2021;34:106705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang A, Goldin D, Nova J, Malhotra J, Cantor JC, Tsui J. Racial disparities in health care utilization at the end of life among New Jersey medicaid beneficiaries with advanced cancer. JCO Oncol Pract. 2020;16(6):e538-e548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnato AE, Anthony DL, Skinner J, Gallagher PM, Fisher ES. Racial and ethnic differences in preferences for end-of-life treatment. J Gen Intern Med. 2009;24(6):695-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown LE, Burton R, Hixon B, et al. Factors influencing emergency department preference for access to healthcare. West J Emerg Med. 2012;13(5):410-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henson LA, Gao W, Higginson IJ, et al. Emergency department attendance by patients with cancer in their last month of life: A systematic review and meta-analysis. J Clin Oncol. 2015;33(4):370-376. [DOI] [PubMed] [Google Scholar]
- 21.Taylor JS, Rajan SS, Zhang N, et al. End-of-Life racial and ethnic disparities among patients with ovarian cancer. J Clin Oncol. 2017;35(16):1829-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LoPresti MA, Dement F, Gold HT. End-of-Life care for people with cancer from ethnic minority groups: A systematic review. Am J Hosp Palliat Care. 2016;33(3):291-305. [DOI] [PubMed] [Google Scholar]
- 23.Jones T, Luth EA, Lin SY, Brody AA. Advance care planning, palliative care, and end-of-life care interventions for racial and ethnic underrepresented groups: A systematic review. J Pain Symptom Manage. 2021;62(3):e248-e260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith AK, McCarthy EP, Paulk E, et al. Racial and ethnic differences in advance care planning among patients with cancer: Impact of terminal illness acknowledgment, religiousness, and treatment preferences. J Clin Oncol. 2008;26(25):4131-4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mack JW, Paulk ME, Viswanath K, Prigerson HG. Racial disparities in the outcomes of communication on medical care received near death. Arch Intern Med. 2010;170(17):1533-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bullock K. The influence of culture on end-of-life decision making. J Soc Work End Life Palliat Care. 2011;7(1):83-98. [DOI] [PubMed] [Google Scholar]
- 27.Rabow MW, Dibble SL, Pantilat SZ, McPhee SJ. The comprehensive care team: A controlled trial of outpatient palliative medicine consultation. Arch Intern Med. 2004;164(1):83-91. [DOI] [PubMed] [Google Scholar]
- 28.Permuth JB, Clark Daly A, Jeong D, et al. Racial and ethnic disparities in a state-wide registry of patients with pancreatic cancer and an exploratory investigation of cancer cachexia as a contributor to observed inequities. Cancer Med. 2019;8(6):3314-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamba N, Mehanna E, Kearney RB, et al. Racial disparities in supportive medication use among older patients with brain metastases: A population-based analysis. Neuro Oncol. 2020;22(9):1339-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nitecki R, Diver EJ, Kamdar MM, et al. Patterns of palliative care referral in ovarian cancer: A single institution 5 year retrospective analysis. Gynecol Oncol. 2018;148(3):521-526. [DOI] [PubMed] [Google Scholar]
- 31.Nevadunsky NS, Gordon S, Spoozak L, et al. The role and timing of palliative medicine consultation for women with gynecologic malignancies: Association with end of life interventions and direct hospital costs. Gynecol Oncol. 2014;132(1):3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson T, Walton S, Levine S, Fister E, Baron A, O’Mahony S. Racial and ethnic disparity in palliative care and hospice use. Am J Manag Care. 2020;26(2):e36-e40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Palliative Medicine Referral and End-of-Life Interventions Among Racial and Ethnic Minority Patients With Advanced or Recurrent Gynecologic Cancer by Angel Tabuyo-Martin, Angelica Torres-Morales, Marie J. Pitteloud, Alisha Kshetry, Carina Oltmann, J. Matt Pearson, Mariana Khawand, Matthew P. Schlumbrecht and Julia C. Sanchez in Cancer Control