Abstract
Antiretroviral therapy against human immunodeficiency virus (HIV) is effective in controlling viral replication but cannot completely eliminate HIV due to the persistence of the HIV reservoir. Innate and adaptive immune responses have been proposed to contribute to preventing HIV acquisition, controlling HIV replication and eliminating HIV-infected cells. However, the immune responses naturally induced in HIV-infected individuals rarely eradicate HIV infection, which may be caused by immune escape, an inadequate magnitude and breadth of immune responses, and immune exhaustion. Optimizing these immune responses may solve the problems of epitope escape and insufficient sustained memory responses. Moreover, immune interventions aimed at improving host immune response can reduce HIV reservoirs, which have become one focus in the development of innovative strategies to eliminate HIV reservoirs. In this review, we focus on the immune response against HIV and how antiviral immune responses affect HIV reservoirs. We also discuss the development of innovative strategies aiming to eliminate HIV reservoirs and promoting functional cure of HIV infection.
Keywords: Antiviral immune response, Functional HIV cure, HIV reservoir, Human immunodeficiency virus, Immune interventions
Introduction
Although effective antiretroviral therapy (ART) can reduce HIV replication, viremia rebounds within weeks of ART interruption. This viral rebound might be fueled by reservoirs that are established early in HIV infection and influenced by multiple virological and immunological factors. A potential source of viremia might be the reactivation of transcriptionally silent but replication-competent viruses harbored in latently infected cells.[1] In addition, previous studies have shown that HIV reservoirs decay very slowly, with a half-life of approximately 44 months.[2] Therefore, people living with HIV (PLWH) cannot be cured by ART on its own, and must receive lifelong ART.
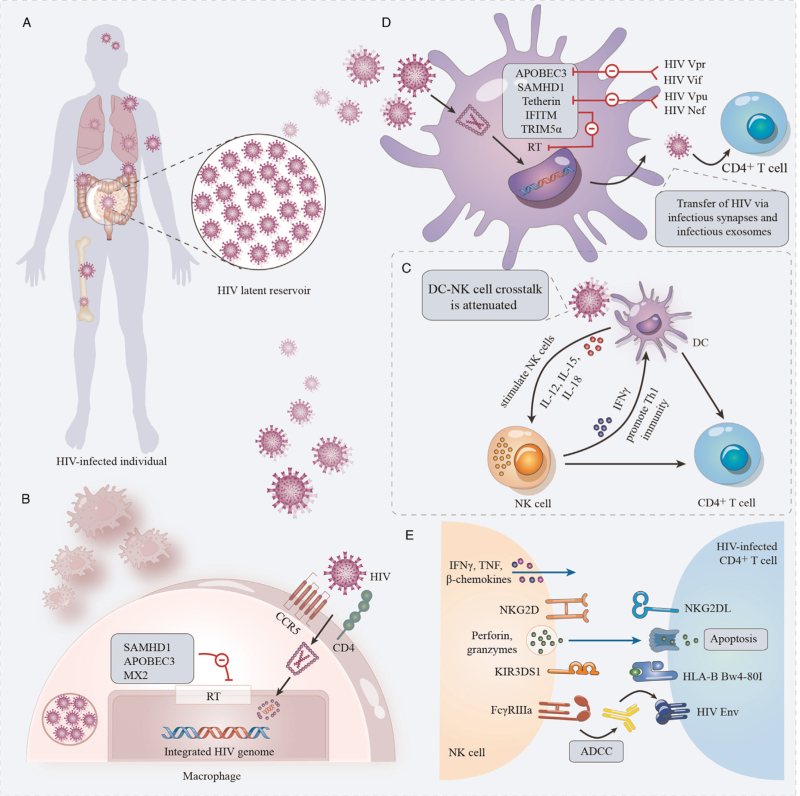
A replication-competent form of HIV persists in a specific cell type or anatomical site for a longer time than in the main pool of actively replicating viruses, forming HIV reservoirs [Figure 1A].[1] Although there are multiple reservoirs, the persistence of replication-competent HIV in resting memory CD4+ T cells represents a major obstacle to HIV eradication.[3] Activated CD4+ T cells integrate the HIV provirus and survive viral cytopathic effects and cytolytic host effector mechanisms to revert to a resting memory state.[4] Moreover, HIV latency can also be established directly in resting CD4+ T cells.[5] In addition to establishing reservoirs in all subsets of CD4+ T cells, HIV persists in macrophages, dendritic cells (DCs) and astrocytes, which can also form HIV reservoirs.[6,7] The elimination of these latently infected cells is critical to curing HIV infection. However, biomarkers of the HIV reservoir that facilitate the elimination of these latently infected cells are challenging to identify. Studies have reported that the immune checkpoint molecule programmed death-1 might be potential biomarkers expressed on latently infected cells, also not exclusive.[6] Additional markers will need to be identified for a more improved targeting of HIV reservoirs.
Figure 1.
Innate immune response and HIV reservoir. (A) HIV reservoirs. HIV reservoirs are established early in HIV infection and hide in immune-privileged anatomic sites, including the brain, bone marrow, lungs, lymph nodes, and GALT. (B) Macrophages and HIV reservoirs. Host restriction factors, including SAMHD1, APOBEC3, and MX2, can influence HIV latency in macrophages by inhibiting RT. (C) DC–NK cell crosstalk. DCs are activated by HIV; secrete pro-inflammatory cytokines, including IL-12, IL-15, and IFNs; and stimulate NK cells. Activated NK cells secrete IFN-γ to promote DC maturation and Th1 immunity. DC–NK cell crosstalk is attenuated in HIV infection. (D) DCs and HIV reservoirs. Viral restriction factors play an important role in HIV reservoirs in DCs, including SAMHD1, IFITM proteins, TRIM5α, Tetherin, and APOBEC3. HIV can counteract the immune response induced by these host restriction factors by encoding viral accessory proteins, including Vpr and Vif, which can antagonize APOBEC3, and Vpu and nef, which can antagonize Tetherin. (E) NK cells and HIV reservoirs. NK cells can recognize and eliminate HIV-infected cells through many different mechanisms, mainly cytokine secretion, ADCC, cytotoxic granule exocytosis, and death receptor pathway activity, which may be effective for reducing the size of the latent HIV reservoir and become a promising strategy for achieving a functional HIV cure. ADCC: Antibody-dependent cellular cytotoxicity; APOBEC3: Apolipoprotein B Editing Complex3; CCR5: C-C chemokine receptor 5; CD4:cluster of differentiation 4; DCs: Dendritic cells; GALT: Gut-associated lymphoid tissue; HIV: Human immunodeficiency virus; HLA-B: Hhuman leukocyte antigen-B; IFITM: Interferon-induced transmembrane; IFN-γ: Interferon γ; IL-12: Interleukin-12; KIR: Killer immunoglobulin-like receptors; MX2: Myxovirus-resistance protein 2; NK: Natural killer; NKG2DL: Natural killer group 2 member D ligand; NKG2D: Natural killer group 2D; RT: Reverse transcription; SAMHD1: Sterile α-motif/histidine-aspartate domain-containing protein 1; Tetherin: Bone marrow stromal antigen 2; Th1 immunity: T helper 1 immunity; TNF: Tumor necrosis factor; TRIM5α: Tripartite motif containing 5 alpha.
Host antiviral immune responses, including innate and adaptive immune responses, might impact and shape the HIV reservoir, playing an essential role in eliminating HIV.[8] Determining the roles of the innate and adaptive immune response, HIV-specific antibodies (Abs) and B cells in viral control and elimination are the main research goals for the next 5 years.[9] Recently, the “Esperanza patient” has received great attention for achieving a sterilizing cure of HIV infection. In the Esperanza patient, no intact HIV proviruses or replication-competent HIV viral particles were detected in large numbers of cells (>1.5 billion in total).[10] The mechanisms leading to the abortive HIV infection in this patient might be attributed to innate immune cells, HIV-specific B cell and T cell responses, and cell-intrinsic restriction of viral replication steps. However, it is clear that immune-mediated mechanisms have difficulties in controlling HIV infection and eliminating HIV in most HIV-infected individuals naturally. Therefore, a combination of interventions aiming to induce an HIV-specific immune response and to prevent HIV infection and spread might be a useful strategy for controlling viral reservoirs and achieving a functional HIV cure. Here, we discuss how antiviral immune responses affect the HIV reservoir, as well as their implications for immune interventions that aim to cure HIV.
Innate Immune Response and HIV Reservoir
Innate immunity is the first line of defense against viral infection, and innate immune cells play an essential role in the onset of HIV infection. During acute HIV infection, viral pathogen-associated molecular patterns are recognized by pattern recognition receptors expressed by infected cells, which triggers intracellular innate immune responses that provide antiviral defenses and aim to achieve viral restriction.[11] These responses also induce the production of cytokines and chemokines, which can recruit and activate innate immune cells such as macrophages, natural killer (NK) cells and DCs, leading to the control of viral spread and activation of the adaptive immune response.[11] This innate immunity might be used to improve HIV cure strategies by enhancing the clearance of early infected cells by cytokines or immune cells.
Macrophages
Evidence has shown that HIV infects and maintains infection in macrophages.[12] However, the detection of HIV ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) in macrophages does not indicate that this cell type has the ability to produce replication-component virus.[13] The mechanism of HIV persistence in macrophages is not completely understood.
A large amount of unintegrated HIV DNA has been found in macrophages, which can persist for a long time and is conducive to viral persistence.[14] Interferon (IFN)/IFN-stimulated gene (ISG) signaling in HIV-infected macrophages may play important roles in HIV latency. Host restriction factors, such as sterile α-motif/histidine-aspartate domain-containing protein 1 (SAMHD1), apolipoprotein B editing complex (APOBEC) 3 and myxovirus-resistance protein 2 (MX2), have been shown to have a large impact on HIV latency in macrophages [Figure 1B]. SAMHD1 can inhibit HIV reverse transcription (RT) through the depletion of cellular deoxynucleoside triphosphates (dNTPs).[15,16] The low intracellular dNTP concentration in macrophages caused by SAMHD1 may affect RT of viral complementary DNA (cDNA), restricting proviral DNA synthesis.[17] APOBEC3 proteins, a family of cytidine deaminases, play critical roles in intrinsic responses to HIV infection and the development of the HIV reservoir. APOBEC3 proteins inhibit HIV infection by deaminating deoxycytidine on single-stranded viral cDNA produced by RT, inducing G-to-A mutations in newly synthesized HIV DNA.[18] The hypermutation of HIV genomes leads to the accumulation of defective proviruses in the HIV reservoir, which cannot produce functional viruses after reactivation. Other host restriction factors that might restrict HIV RT and integration include MX2, which works by hindering nuclear accumulation and inhibiting the integration of proviral DNA into the host genome.[19] In addition to host restriction factors, cellular transcription factors, such as chicken ovalbumin upstream promoter transcription factor (COUP-TF)-interacting protein 2 (CTIP2), viral transactivator of transcription (Tat) protein and microRNAs, influence HIV latency in macrophages.[17] Marban et al[20] showed that CTIP2 can recruit histone deacetylase (HDAC)1 and HDAC2 and establish a heterochromatic environment at the HIV promoter, leading to HIV silencing.
During HIV infection, although macrophages can produce cytokines and chemokines to recruit innate and adaptive immune cells and thus induce an efficient antiviral response against HIV, HIV can overcome these antiviral immune responses through several mechanisms in macrophages. HIV-infected macrophages can resist the cytopathic effects of HIV infection, which might promote the development of the HIV reservoir in macrophages.[21] The expression of triggering receptor expressed on myeloid cells 1 (TREM1) is critical to protect HIV-infected macrophages from HIV-induced apoptosis. Campbell et al[22] identified a mechanism by which HIV can promote macrophage survival through TREM1-dependent upregulation of B-cell lymphoma-2 (Bcl-2) family proteins and mitofusins, which can inhibit Bcl-2-interacting mediator of cell death (Bim)-mediated disruption of the mitochondrial membrane potential and subsequent apoptosis. In addition, the killing of macrophages by CD8+ cytotoxic T lymphocytes (CTLs) is mediated by caspase-3 and granzyme B, whereas the CTL-mediated killing of CD4+ T cells by caspase and granzyme B is independent.[23] Therefore, this inefficient CTL-mediated killing of macrophages may contribute to the setup of HIV reservoir in macrophages.
Macrophages are heterogeneous and non-dividing cells. Tissue-resident macrophages are difficult to obtain, which makes it challenging to investigate their role. Although animal models have produced robust evidence for the persistence of viral DNA in macrophages,[24] the complexity of the contribution of macrophage heterogeneity to HIV persistence cannot be completely explained. Therefore, whether macrophages can serve as a viable reservoir for latent HIV infection remains debatable.
NK cells
NK cells play an essential role in innate immune responses to HIV infection.[25] A recent study showed that NK cells migrated into lymph node follicles and controlled simian immunodeficiency virus (SIV) replication in African green monkeys.[26] In addition, changes in the receptor repertoire and function of NK cells were also observed during HIV infection.[27] A recent study has also demonstrated the associations between the receptor-ligand repertoire of NK cell and markers of HIV persistence, which suggests that NK cells might influence the level of HIV reservoir.[28]
NK cells can recognize and eliminate virus-infected cells through many different mechanisms, mainly cytokine secretion, antibody-dependent cellular cytotoxicity (ADCC), cytotoxic granule exocytosis and death receptor pathway activity [Figure 1E]. The secretion of cytokines, such as IFN-γ, tumor necrosis factor (TNF) and β-chemokines, has been observed in chronic HIV infection, and these cytokines influence antiviral immunity and restrict HIV spread.[27] The β-chemokines produced by NK cells, such as CC-chemokine ligand (CCL) 3, CCL4 and CCL5, function as natural ligands of C-C chemokine receptor 5 (CCR5), which can inhibit HIV infection of target cells.[29] Moreover, killer immunoglobulin-like receptors (KIRs) expressed on NK cells can interact with human leukocyte antigen (HLA) molecules to regulate the activity of NK cells against HIV-infected cells and control the progression of HIV infection. The presence of KIR3DS1 and HLA-B Bw4-80I is associated with improved HIV control, further supporting the role of NK cells in mediating the protective effect against HIV infection progression.[30] In addition, in the context of the anti-HIV immune response, an upregulated inhibitory receptor T cell immunoreceptor with immunoglobulin (Ig) and immunoreceptor tyrosine-based inhibition motif (ITIM) domain (TIGIT) expression on NK cells correlates inversely with CD4-T cell counts and positively with plasma HIV viral loads by reducing the NK production of IFN-γ.[31] Zhang et al[32] recently found that TIGIT expression on NK cells impaired the ability of NK cells in inhibiting HIV-1 replication, which provides an insight to the role of NK cells in the anti-HIV immune response in relation to TIGIT expression.
HIV Nef has been shown to downregulate the expression of major histocompatibility complex (MHC) class I molecules on the surface of infected cells to escape recognition and lysis by CD8+ T cells.[33] However, reduced MHC class I expression might avoid CTL recognition but increase the lysis of HIV-infected cells by NK cells. Interestingly, HIV Nef can downregulate HLA-A and HLA-B but not HLA-C or HLA-E.[34] The presence of HLA-C and HLA-E may play an important role in avoiding the mass activation of NK cells and protect HIV-infected cells against NK cell lysis. The ability of HIV to balance escape from CTLs and protection from NK cell-mediated attack is important for HIV immune evasion and HIV persistence.[35] In addition, HIV infection results in the upregulation of ligands for natural killer group 2D (NKG2D), which can induce the activation of NK cells.[36] However, HIV can also limit and regulate the expression of some NKG2D ligands by expressing viral accessory proteins.[37]
Furthermore, ADCC mediated by NK cells may be associated with control of HIV infection. However, ADCC is also restricted by HIV immune evasion.[38] In addition to exerting antiviral activity, NK cells also play important roles in immune regulation and adaptive immune response development. Several studies have demonstrated that NK cells can regulate the functions of DCs and T cells.[39] However, HIV infection can interfere with the crosstalk between NK cells and DCs [Figure 1C].[39] Although there are currently no data to identify a direct effect of NK cells on the HIV reservoir, the recognition of general stress signals induced early in HIV infection by NK cells may have a crucial role in the development of the reservoir.
Dendritic cells
The populations of DCs mainly include plasmacytoid DCs (pDCs), myeloid DCs and monocyte-derived DCs, which are susceptible to infection with HIV and can transfer HIV to CD4+ T cells to enhance HIV transmission. DCs can mediate HIV trans-infection through infectious synapses and infectious exosomes,[40] allowing efficient virus dissemination. Follicular DCs can capture and maintain large amounts of HIV, acting as a persistent HIV reservoir.[41] In addition, Langerhans cells may be a potential HIV reservoir.[42]
However, DCs are the main antigen-presenting cells that link innate and adaptive immune responses and play a critical role in the induction of protective immune responses against HIV. DCs can activate immune responses against HIV by mediating the differentiation, activation and proliferation of T and B cells.[43] During HIV infection, DCs can produce type I IFNs by sensing viral products, which can induce ISGs and upregulate class II HLA and costimulatory molecules, leading to restricted viral replication and increased expression of molecules involved in antigen (Ag) presentation and costimulation.[43] As for macrophages, the viral restriction factors in DCs induced by type I IFNs also play an important role in inhibiting HIV replication, including SAMHD1, interferon-induced transmembrane proteins, tripartite motif containing 5 alpha, bone marrow stromal antigen 2 (BST2 or Tetherin) and APOBEC3.[44] However, HIV can counteract the immune response induced by these host restriction factors by encoding viral accessory proteins, including Vpr and Vif, which can antagonize APOBEC3, and Vpu and Nef, which can antagonize Tetherin [Figure 1D].[44] Therefore, DCs may contribute to HIV immune control by numerous mechanisms, and further studies will need to be performed to take advantage of these DC functions for HIV control.
Adaptive Immune Response and HIV Reservoir
Few individuals can naturally control HIV infection by immune-mediated mechanisms. This HIV control might be attributed to a broad and fine regulation of the adaptive immune responses that participate to limit the size of the HIV reservoirs,[45] suggesting that immunotherapies consisting of enhanced T cell and B cell responses might contribute to achieving a functional HIV cure.
Antibody responses
Accumulating evidence has demonstrated that HIV-specific Abs play an important role in HIV control. These Abs have the capability to bind with HIV Ags and prevent the virus from invading host target cells. They can also meditate a wide range of functions by direct neutralization of viral infectivity or through interactions between the Fc domain of the antibody and Fc receptors, complement proteins, or lectins to promote HIV elimination. These later functions mainly include ADCC, antibody-dependent cellular phagocytosis, complement-dependent cytotoxicity and anti-inflammatory activities.[46]
Broadly neutralizing Abs (bNAbs) with breadth and potency can neutralize a wide spectrum of HIV variants, exhibiting potential for effectively decreasing HIV reservoirs. Early first-generation bNAbs displayed a good neutralizing breadth but had limited potency, resulting in little clinical benefit. With the development of single-cell antibody cloning methods, second-generation bNAbs have shown the potential for a role in achieving a functional HIV cure.[47] bNAbs mainly target five epitopes on HIV envelope (Env) including the CD4-binding site, the V3- and V1/V2-glycans on gp120, the gp120-gp41 interface and the gp41 membrane-proximal region (MPER).[48] A study showed that bNAbs could reduce the HIV reservoir and interfere with its establishment in humanized mice.[49] Another study evaluated the presence of plasma bNAbs in HIV-infected infants. It demonstrated that plasma bNAbs could effectively target diverse autologous circulating viruses in multivariant HIV infection.[50] However, HIV can avoid Ab control by changing the variable epitopes, leading to neutralization escape. Changes in the HIV envelope conformation and glycan abundance may also affect the exposure of relatively conserved epitopes and induce HIV immune escape.[51]
In addition, HIV infection can occur via genital mucosal tissues, which comprise a multicellular layer of stratified squamous epithelial cells. Both cell-free and cell-associated infectious HIV can infect host cells.[46] Previous studies suggest that cell-to-cell HIV transmission makes a large contribution to its rapid dissemination throughout the mucosal site of the human body. Indeed, bNAbs could contribute to protection against HIV transmission through cell-free or cell-to-cell HIV transmission pathways,[40] which would lead to new therapeutic and next-generation therapeutic approaches for reducing the HIV reservoirs and inhibiting HIV transmission.
CD8+ T cell responses
CD8+ T cells play a critical role in the antiviral immune response against HIV. Evidence has shown that CD8+ T cells might mediate the control of HIV replication and the prevention of HIV infection progression. The common features from elite controllers were described previously: HIV-specific CD8+ T cells mediate durable HIV control.[52] During acute HIV infection, CD8+ T cells are involved in the post-peak decline in HIV viremia. CD8+ T cells can recognize HLA class I molecules on the surface of HIV-infected cells and lyse those cells through the secretion of perforin and granzymes. The secretion of cytokines, including IFN-γ, TNF-α, CCL5 and macrophage inflammatory proteins 1α and 1β, also has antiviral effects. In addition, CTLs can eliminate infected cells via Fas/Fas-ligand interactions and the TNF-related apoptosis-inducing ligand (TRAIL)/TRAIL receptor (TRAIL-R) pathways.[53] The ability of CTLs to effectively recognize and eliminate HIV-infected cells is essential for reducing HIV reservoirs. Although CTLs can inhibit defective proviruses, their ability to control intact proviruses is limited.[54] The effectiveness of CD8+ T cells in restraining HIV reservoirs remains unclear. In addition, CTL-mediated HIV eradication faces some obstacles, such as HIV immune escape, CD8+ T cell exhaustion, CD8+ T cell compartmentalization and HIV latency.[55] A better understanding of the roles of CD8+ T cells related to HIV reservoirs could provide promising immune strategies for the prevention and cure of HIV/AIDS.
In addition to the CTL response, the CD8+ T cell non-cytotoxic antiviral response (CNAR) can suppress HIV replication. A recent study showed that non-cytolytic CD8+ T cells could mediate HIV suppression by silencing long terminal repeat (LTR)-dependent HIV transcription and that this activity was an MHC-independent immunoregulatory mechanism that regulated the proliferation and activation of CD4+ T cells.[56] The CNAR is also associated with the secretion of a soluble CD8+ T cell antiviral factor (CAF) that contributes to blocking HIV transcription and affects all HIV isolates. The CNAR and CAF might have characteristics of both innate and adaptive responses and play a role by inhibiting HIV transcription.[53]
Immune Interventions for HIV Cure
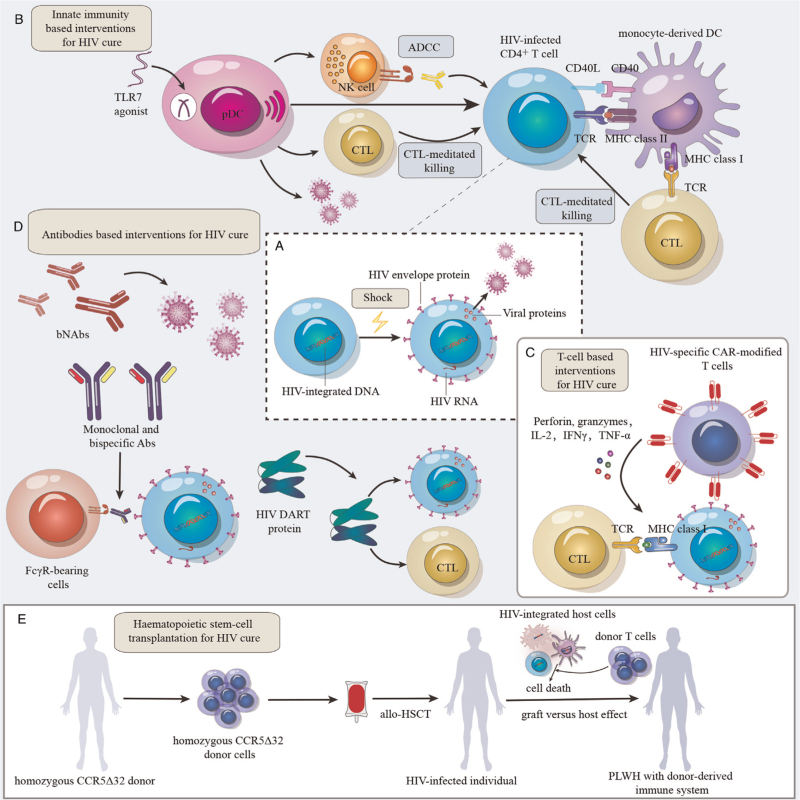
A sustained HIV-specific immune response is essential for the elimination of HIV-infected cells during ART administration. Many studies have focused on “Shock and Kill” strategies to eliminate HIV reservoirs. “Shock and Kill” aims to reverse HIV latency and increase viral gene expression [Figure 2A], followed by clearance of infected cells via immune-mediated killing by NK cells, CTLs and bNAbs.[57] However, “Shock and Kill” has been challenging to achieve. The localization of reactivated CD4+ T cells in protected sites, insufficient expression of HIV Ags by reactivated CD4+ T cells, low levels and impaired function of HIV-specific CD8+ T cells, and presence of CTL escape mutants might affect the effectiveness of “Shock and Kill” strategies.[58] Therefore, insufficient antigen induction by latency-reversing agents (LRAs) and the lack of effective clearance by immunotherapies are the main concerns. A deep understanding of antiviral immune responses and immune escape strategies could contribute to the development of immune approaches targeting the HIV reservoir, which has implications for generating a functional HIV cure.
Figure 2.
Immune interventions for HIV cure. (A) “Shock” HIV out of hiding. HIV latency in reservoirs is reversed, leading to increases in viral gene expression and viral protein production. (B) Innate immunity-based interventions for HIV cure. TLR7 agonists and pDCs reactivate HIV-infected cells. HIV-infected cells can be recognized and killed by CTLs and NK cells. MoDC induce CTL and CD4+ T cell responses through Ag presentation. DC-based immunotherapeutic vaccines also involve CD40L, which can enhance DC maturation, interleukin(IL)-12p70 (IL-12p70) production and Ag presentation. (C) T cell-based interventions for HIV cure. CTL-mediated immunotherapy plays a critical role in eliminating the HIV reservoir. CAR-T cells target HIV binding sites on the surface of reactivated reservoir cells and secrete granzymes and cytokines to kill HIV-infected cells. (D) Antibody-based interventions for HIV cure. HIV-specific bNAbs can bind with different epitopes of HIV Env and promote the elimination of HIV reservoirs. Bispecific Abs can simultaneously bind with two different Ag binding sites or epitopes with different inhibitory effects on HIV Env to produce immune responses. DART proteins can improve the recognition of HIV Env on the surface of infected cells and recruit effector cells to eliminate infected cells. (E) Hematopoietic stem cell transplantation for HIV cure. Allo-HSCT with homozygous CCR5Δ32 donor cells may achieve HIV remission, further supporting the development of functional HIV cures. The graft-versus-host effect may be a key factor in achieving a sterilizing cure of HIV infection after allo-HSCT. Abs: Antibodies; ADCC: Antibody-dependent cellular cytotoxicity; Ag: Antigen; allo-HSCT: Allogeneic hematopoietic stem cell transplantation; bNAbs: Broadly neutralizing Abs; CAR-T: Chimeric antigen receptor T; CCR5: C-C chemokine receptor 5; CD40: Cluster of differentiation 40; CD40L: CD40 ligand; CTLs: Cytotoxic T lymphocytes; DART: Dual-affinity retargeting; DC: Dendritic cell; DNA: Deoxyribonucleic acid; FcγR: Fc gamma receptor; HIV:Human immunodeficiency virus; HIV Env: HIV envelope; IFNγ: Interferon γ; IL-2: Interleukin-2; MHC: Major histocompatibility complex; MoDC: Monocyte-derived DCs; NK: Natural killer; pDCs: Plasmacytoid DCs; PLWH: People living with HIV; RNA: Ribonucleic acid; TCR: T-cell receptor; TLR7: Toll-like receptor 7; TNF-α: Tumor necrosis factor-α.
Innate immunity-based interventions for HIV cure
Innate immunity might play critical roles in reversing HIV latency and eliminating HIV-infected cells. Studies have shown that innate immunity, currently mainly considered to be mediated by DCs and NK cells, can improve the effectiveness of HIV cure strategies.[59] DCs have been reported to reactivate latently infected cells in “Shock and Kill” strategies through their antigen presentation function. Tsai et al[60] showed that the selective Toll-like receptor 7 (TLR7) agonist GS-9620 could induce HIV reactivation and improve immune effector functions specifically targeting HIV-infected cells. They suggested that the induction of latency reversal by GS-9620 requires the presence of pDCs and production of IFN-α. A recent phase 1b clinical trial also showed that GS-9620 was associated with an induction of immune cell activation, a reduction in intact proviral DNA during ART, and an increase in the rebound time after ART interruption.[61] Therefore, TLR7 agonists can be combined with therapeutic vaccines and bNAbs to enhance the HIV-specific T cell response and ADCC, thereby improving the elimination of HIV-infected cells [Figure 2B]. In addition, DC-based immunotherapeutic vaccines have been developed to promote the recovery of the host immune response by DC-specific antigen presentation. AGS-004 is a DC-based immunotherapy that consists of mature autologous DCs coelectroporated with transcribed RNA encoding autologous HIV Ags (Gag, Vpr, Rev, and Nef) and synthetically derived CD40L [Figure 2B]. A recent study investigated the impact of the HDAC inhibitor vorinostat combined with AGS-004 on the HIV reservoir. However, the interventions had no effect on the HIV reservoir, as measured by a quantitative viral outgrowth assay.[62] This result might be attributable to the small sample size of the study, uncertain efficacy of vorinostat in vivo, and absence of HIV-specific CD8+ T cell responses. In addition, NK cells have been shown to play an essential role in immune control of HIV infection. Immunotherapies aimed at restoring the functionality of NK cells in HIV infection and improving the NK cell survival capacity and the ability to recognize latently HIV-infected cells might be effective in reducing the size of the latent HIV reservoir. These strategies might become promising for functional cure of HIV.
T cell-based interventions for HIV cure
Previous studies showed that stimulation of HIV-specific CTLs might facilitate the elimination of the HIV reservoir after viral reactivation. Increasing evidence supports that focusing on CTL responses targeting specific regions of the viral proteome is beneficial for HIV cure.
Increasing preclinical evidence suggests that vaccine-induced CD8+ T cell-mediated immunity can provide protection against retroviral infection and establish durable remission after therapeutic vaccination.[63] HIV-specific CD8+ T cells have also been shown to provide durable control following HIV infection, which can enhance vaccine-induced humoral immunity offering incomplete protection. Fan et al[64] evaluated the effect of adoptively transferred vaccine-induced HIV subtype C Env-specific CTLs on plasma viremia after ART interruption in a macaque subtype B simian-human immunodeficiency virus (SHIV) model. They demonstrated that autologous Env-specific T cells enhanced by therapeutic vaccination could inhibit SHIV rebound in ART-free macaques. Another study evaluated the relationship between Gag-specific CD8+ T cell responses and the anti-SIV efficacy of CD8+ T cells after vaccination. It indicated that the induction of Gag-specific CD8+ T cells through therapeutic vaccination could enhance the antiviral efficacy of CD8+ T cells.[65] These data suggest that CTL-mediated immunotherapy plays a critical role in suppressing HIV rebound and eliminating the HIV reservoir.
The effective and sustained T cell response that chimeric antigen receptor (CAR)-T cells can afford might be critical in the development of effective strategies for HIV cure [Figure 2C]. Studies have demonstrated that HIV-specific CAR-modified CD4+ T cells directly suppress HIV replication in vitro and eliminate virus-infected cells.[66] Maldini et al[67] developed dual CD4-based CAR-T cells with distinct costimulatory domains to optimize HIV-specific CAR-T cell therapy. This study showed that these cells could promote HIV suppression and reduce HIV viremia and the tissue viral burden in a bone marrow, liver, thymus (BLT) humanized mouse model. However, some potential limitations exist for CAR-T cell therapy. The target epitopes must be expressed on the cell surface at a high density, and all current CARs target highly variable envelope glycoproteins. The other important obstacle to overcome before testing CAR-T cells in PLWH is the lack of safe and effective LRAs.
Antibody-based interventions for HIV cure
As with T cell responses, an antibody-mediated immune response can be evoked to promote a functional HIV cure. In recent years, HIV bNAbs, which are being explored as potential therapeutic and preventative agents, have been identified to bind with different epitopes of HIV Env [Figure 2D]. The protective effect of bNAbs has been shown in humanized mouse and non-human primate animal models. Furthermore, the effectiveness of bNAbs in HIV-infected individuals is being evaluated in clinical trials.
Clinically advanced bNAbs, including the CD4 binding site-targeting bNAbs 3BNC117 and VRC01, have been evaluated in HIV-infected individuals in clinical trials. A clinical trial showed that the combination of 3BNC117 and 10–1074 could maintain long-term viral suppression in individuals with antibody-sensitive HIV reservoirs following ART interruption.[68] A phase 1b clinical trial also concluded that the combination of 3BNC117 and 10–1074 more effectively suppressed HIV viremia than either antibody alone.[69] In addition, Astronomo et al[70] showed that intravenous VRC01 was distributed in rectal and vaginal tissues and could protect against ex vivo HIV challenge. Other clinical trials also showed that VRC01 could promote HIV remission by preventing viral replication and eliminating infected cells.[71] However, recently, two randomized clinical trials found that VRC01 could not prevent HIV acquisition, which suggests that a broader and more potent combination of Abs should be used. In addition to CD4 binding site-specific Abs, the bNAbs currently in advanced clinical development include agents targeting the V3-glycan site (PGT121 and 10–1074), MPER epitope (10E8) and V2-glycan site (PGDM1400 and CAP256).[47] Together these studies emphasize that Abs can prevent HIV infection if fully sensitive to the virus.[72] The bNAb monotherapy trials showed that a single bNAb therapy might select for HIV-resistant variants; therefore, bNAb combinations might be more effective against HIV than monotherapies, which should be recommended.
To confront the emergence of viral escape mutants, the antiviral activity of bispecific and multispecific Abs against HIV has been explored. Bispecific Abs can simultaneously bind with two distinct Ag binding sites or epitopes with different inhibitory effects on the pathogen or can connect to cells to produce immune responses. The dual-affinity retargeting (DART) scaffold is a bNAb-based modality that provides relatively good stability, manufacturability, and potency [Figure 2D]. [73] Previous studies have focused on DART proteins that can recognize both the HIV envelope and CD3 molecules. This combination should induce the clearance of HIV-infected cells by increasing cytotoxic T cell recruitment to Env-expressing infected cells.[74] However, these molecules mainly target HIV-infected cells and do not play a role in viral neutralization. Other studies have reported the development of bispecific anti-Env Abs with an enhanced neutralization breadth and potency, which play a critical role in the control of HIV infection.[75] In addition, a trispecific antibody that combines three HIV-specific bNAbs demonstrated outstanding HIV coverage with remarkable potency in neutralization assays, which showed the effectiveness of trispecific Abs for HIV prevention and functional cure.[76]
Hematopoietic stem cell transplantation for HIV cure
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) might dramatically reduce the HIV reservoir when complete donor chimerism is achieved, which might be sufficient to achieve HIV remission [Figure 2E]. The “Berlin patient” received two allo-HSCTs using cells from a donor with a homozygous mutation in CCR5 (CCR5Δ32/Δ32) and achieved sustained HIV remission.[77] The “London patient”, who underwent allo-HSCT, has been in HIV remission for 30 months with no detectable replication-competent virus in diverse HIV reservoir sites.[78] Another study also suggested that allo-HSCT with homozygous CCR5Δ32 donor cells might achieve HIV remission, further supporting the development of strategies for functional HIV cure based on preventing CCR5 expression.[79] A multicenter randomized controlled trial demonstrated that human umbilical cord mesenchymal stem cell infusion in chronic immune non-responder HIV-infected patients was safe and well tolerated.[80] In addition, HLA-mismatched allogeneic adoptive immunotherapy has been shown to be safe and potentially contribute to achieving better immune restoration in immunosuppressed AIDS patients.[81]
The mechanisms of HIV eradication associated with allo-HSCT have been investigated. Salgado et al[82] found that 5 of 6 participants who received allo-HSCT with CCR5-wild-type donor cells eliminated all measurable HIV reservoirs. They also demonstrated that the graft-versus-HIV-reservoir effect might be a key factor in achieving a sterilizing cure of HIV infection after allo-HSCT. However, studies have indicated that the SHIV DNA reservoir cannot be eradicated after allo-HSCT in a non-human primate model, despite high levels of donor chimerism and graft-versus-host disease.[83] Allo-HSCT may result in a reduction in the HIV reservoir, but viral rebound can still occur. Eberhard et al[84] described the immunological reconstitution and HIV-specific T cell response breadth and functionality of 16 HIV-infected individuals who received allo-HSCT. High T cell activation was found after early allo-HSCT, which might increase the risk of engrafted cell infection in deep tissues and reseeding of HIV reservoirs, suggesting the importance of maintaining ART after allo-HSCT. In addition, Xu et al[85] recently reported the use of clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR associated protein 9 (Cas9) gene editing in a patient with acute lymphoblastic leukemia who was infected with HIV. Authors selected an HLA-compatible person as the donor for stem-cell transplantation in this patient, and the donor stem cells were subjected to genome editing with the use of CRISPR–Cas9 technology to knock out CCR5 before infusion into the HIV-infected recipient. The edited stem cells were engrafted, and at 19 months after transplantation, the leukemia was in remission, and the patient continues to have HIV infection and to receive ART. Although we achieved successful transplantation and long-term engraftment of CRISPR-edited hematopoietic stem and progenitor cells, the percentage of CCR5 disruption in lymphocytes was only approximately 5%, which indicates the need for further research into this approach.[85] Further studies on CCR5-defective cells, especially cells used for cord blood transplantation, engineered CCR5-knockout cells for autologous and allogeneic transplantation, and allogeneic transplantation with ART might be needed to develop an effective cure strategy and achieve HIV remission.
Conclusions
The HIV reservoir represents a major barrier to HIV eradication. Effective innate and adaptive immune responses are essential for elimination of the HIV reservoir. However, host immune responses might occur too late or be insufficient, resulting in an inability to effectively eliminate the HIV reservoir. Therefore, the development of effective immunological approaches will most likely be needed. In addition, future work should specifically focus on combining immunotherapeutic strategies linking of innate and adaptive immune responses and antiretroviral drugs to eradicate HIV reservoirs. Complex combination of synergistic strategies may represent a promising way forward for HIV cure.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Nos. NSFC, 81974303 to BS, and 82072271 to TZ), the High-Level Public Health Specialized Talents Project of Beijing Municipal Health Commission (Nos. 2022-1-007 to TZ and 2022-2-018 to BS), the “Climbing the peak (Dengfeng)” Talent Training Program of Beijing Hospitals Authority (No. DFL20191701 to TZ), the Beijing Health Technologies Promotion Program (No. BHTPP2020 to TZ), the Beijing Key Laboratory for HIV/AIDS Research (No. BZ0089), the ANRS (Agence Nationale de Recherches sur le SIDA et les hépatites virales), the Investissements d’Avenir program managed by the ANR under reference ANR-10-LABX-77 and EHVA (No. 681032, Horizon 2020). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest
None.
Footnotes
How to cite this article: Li S, Moog C, Zhang T, Su B. HIV reservoir: antiviral immune responses and immune interventions for curing HIV infection. Chin Med J 2022;135:2667–2676. doi: 10.1097/CM9.0000000000002479
References
- 1.Van Lint C, Bouchat S, Marcello A. HIV-1 transcription and latency: an update. Retrovirology 2013; 10:67.doi:10.1186/1742-4690-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med 2003; 9:727–728. doi:10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 3.Wei M, Shao YM. Transient CD4-cell-depletion therapy for HIV/AIDS cure. Chin Med J 2021; 134:1930–1932. doi:10.1097/CM9.0000000000001654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chun TW, Finzi D, Margolick J, Chadwick K, Schwartz D, Siliciano RF. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med 1995; 1:1284–1290. doi:10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- 5.Chavez L, Calvanese V, Verdin E. HIV latency is established directly and early in both resting and activated primary CD4T cells. PLoS Pathog 2015; 11:e1004955.doi:10.1371/journal.ppat.1004955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sengupta S, Siliciano RF. Targeting the latent reservoir for HIV-1. Immunity 2018; 48:872–895. doi:10.1016/j.immuni.2018.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao JC, Deng K. Heterogeneity of HIV-1 latent reservoirs. Chin Med J 2020; 133:2867–2873. doi:10.1097/CM9.0000000000001085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang ND, Li TS. Factors associated with the size of HIV DNA reservoir. Chin Med J 2017; 130:224–230. doi:10.4103/0366-6999.198009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deeks SG, Archin N, Cannon P, Collins S, Jones RB, de Jong M, et al. Research priorities for an HIV cure: international AIDS society global scientific strategy 2021. Nat Med 2021; 27:2085–2098. doi:10.1038/s41591-021-01590-5. [DOI] [PubMed] [Google Scholar]
- 10.Turk G, Seiger K, Lian X, Sun W, Parsons EM, Gao C, et al. A possible sterilizing cure of HIV-1 infection without stem cell transplantation. Ann Intern Med 2022; 175:95–100. doi:10.7326/L21-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altfeld M, Gale M, Jr. Innate immunity against HIV-1 infection. Nat Immunol 2015; 16:554–562. doi:10.1038/ni.3157. [DOI] [PubMed] [Google Scholar]
- 12.Andrade VM, Mavian C, Babic D, Cordeiro T, Sharkey M, Barrios L, et al. A minor population of macrophage-tropic HIV-1 variants is identified in recrudescing viremia following analytic treatment interruption. Proc Natl Acad Sci U S A 2020; 117:9981–9990. doi:10.1073/pnas.1917034117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burdo TH. Editor's commentary for special issue: “the role of macrophages in HIV persistence”. J Neuroimmune Pharmacol 2019; 14:2–5. doi:10.1007/s11481-019-09836-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rensen E, Mueller F, Scoca V, Parmar JJ, Souque P, Zimmer C, et al. Clustering and reverse transcription of HIV-1 genomes in nuclear niches of macrophages. EMBO J 2021; 40:e105247.doi:10.15252/embj.2020105247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lahouassa H, Daddacha W, Hofmann H, Ayinde D, Logue EC, Dragin L, et al. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat Immunol 2012; 13:223–228. doi:10.1038/ni.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su B, Biedma ME, Lederle A, Peressin M, Lambotin M, Proust A, et al. Dendritic cell-lymphocyte cross talk downregulates host restriction factor SAMHD1 and stimulates HIV-1 replication in dendritic cells. J Virol 2014; 88:5109–5121. doi:10.1128/JVI.03057-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veenhuis RT, Abreu CM, Shirk EN, Gama L, Clements JE. HIV replication and latency in monocytes and macrophages. Semin Immunol 2021; 51:101472.doi:10.1016/j.smim.2021.101472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stavrou S, Ross SR. APOBEC3 proteins in viral immunity. J Immunol 2015; 195:4565–4570. doi:10.4049/jimmunol.1501504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goujon C, Moncorge O, Bauby H, Doyle T, Ward CC, Schaller T, et al. Human MX2 is an interferon-induced post-entry inhibitor of HIV-1 infection. Nature 2013; 502:559–562. doi:10.1038/nature12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marban C, Suzanne S, Dequiedt F, de Walque S, Redel L, Van Lint C, et al. Recruitment of chromatin-modifying enzymes by CTIP2 promotes HIV-1 transcriptional silencing. EMBO J 2007; 26:412–423. doi:10.1038/sj.emboj.7601516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hendricks CM, Cordeiro T, Gomes AP, Stevenson M. The interplay of HIV-1 and macrophages in viral persistence. Front Microbiol 2021; 12:646447.doi:10.3389/fmicb.2021.646447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell GR, To RK, Spector SA, TREM-1. Protects HIV-1-infected macrophages from apoptosis through maintenance of mitochondrial function. mBio 2019; 10:e02638-19.doi:10.1128/mBio.02638-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clayton KL, Collins DR, Lengieza J, Ghebremichael M, Dotiwala F, Lieberman J, et al. Resistance of HIV-infected macrophages to CD8(+) T lymphocyte-mediated killing drives activation of the immune system. Nat Immunol 2018; 19:475–486. doi:10.1038/s41590-018-0085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avalos CR, Abreu CM, Queen SE, Li M, Price S, Shirk EN, et al. Brain macrophages in simian immunodeficiency virus-infected, antiretroviral-suppressed macaques: a functional latent reservoir. mBio 2017; 8:e01186-17.doi:10.1128/mBio.01186-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang QY, Zhang X, Su B, Liu LF, Yang XD, Tang B, et al. Increased early activation of CD56dimCD16dim/- natural killer cells in immunological non-responders correlates with CD4+ T-cell recovery. Chin Med J 2020; 133:2928–2939. doi:10.1097/CM9.0000000000001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huot N, Jacquelin B, Garcia-Tellez T, Rascle P, Ploquin MJ, Madec Y, et al. Natural killer cells migrate into and control simian immunodeficiency virus replication in lymph node follicles in African green monkeys. Nat Med 2017; 23:1277–1286. doi:10.1038/nm.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bjorkstrom NK, Strunz B, Ljunggren HG. Natural killer cells in antiviral immunity. Nat Rev Immunol 2022; 22:112–123. doi:10.1038/s41577-021-00558-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivison GT, Vendrame E, Martinez-Colon GJ, Ranganath T, Vergara R, Zhao NQ, et al. Natural killer cell receptors and ligands are associated with markers of HIV-1 persistence in chronically infected ART suppressed patients. Front Cell Infect Microbiol 2022; 12:757846.doi:10.3389/fcimb.2022.757846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott-Algara D, Truong LX, Versmisse P, David A, Luong TT, Nguyen NV, et al. Cutting edge: increased NK cell activity in HIV-1-exposed but uninfected Vietnamese intravascular drug users. J Immunol 2003; 171:5663–5667. doi:10.4049/jimmunol.171.11.5663. [DOI] [PubMed] [Google Scholar]
- 30.Song R, Lisovsky I, Lebouche B, Routy JP, Bruneau J, Bernard NF. HIV protective KIR3DL1/S1-HLA-B genotypes influence NK cell-mediated inhibition of HIV replication in autologous CD4 targets. PLoS Pathog 2014; 10:e1003867.doi:10.1371/journal.ppat.1003867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin X, Liu T, Wang Z, Ma M, Lei J, Zhang Z, et al. Expression of the inhibitory receptor TIGIT is up-regulated specifically on NK cells with CD226 activating receptor from HIV-infected individuals. Front Immunol 2018; 9:2341.doi:10.3389/fimmu.2018.02341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Lu X, Cheung AKL, Zhang Q, Liu Z, Li Z, et al. Analysis of the characteristics of TIGIT-expressing CD3(-)CD56(+)NK cells in controlling different stages of HIV-1 infection. Front Immunol 2021; 12:602492.doi:10.3389/fimmu.2021.602492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 1998; 391:397–401. doi:10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 34.Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, et al. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 1999; 10:661–671. doi:10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 35.Specht A, DeGottardi MQ, Schindler M, Hahn B, Evans DT, Kirchhoff F. Selective downmodulation of HLA-A and -B by Nef alleles from different groups of primate lentiviruses. Virology 2008; 373:229–237. doi:10.1016/j.virol.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 36.Desimio MG, Covino DA, Doria M. Potential of the NKG2D/NKG2DL axis in NK cell-mediated clearance of the HIV-1 reservoir. Int J Mol Sci 2019; 20:4490.doi:10.3390/ijms20184490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norman JM, Mashiba M, McNamara LA, Onafuwa-Nuga A, Chiari-Fort E, Shen W, et al. The antiviral factor APOBEC3G enhances the recognition of HIV-infected primary T cells by natural killer cells. Nat Immunol 2011; 12:975–983. doi:10.1038/ni.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richard J, Prevost J, Alsahafi N, Ding S, Finzi A. Impact of HIV-1 envelope conformation on ADCC responses. Trends Microbiol 2018; 26:253–265. doi:10.1016/j.tim.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 39.Altfeld M, Fadda L, Frleta D, Bhardwaj N. DCs and NK cells: critical effectors in the immune response to HIV-1. Nat Rev Immunol 2011; 11:176–186. doi:10.1038/nri2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su B, Peressin M, Ducloy C, Penichon J, Mayr LM, Laumond G, et al. Short communication: exploring antibody potential as prophylactic/therapeutic strategies for prevention of early mucosal HIV-1 infection. AIDS Res Hum Retroviruses 2015; 31:1187–1191. doi:10.1089/AID.2015.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ollerton MT, Berger EA, Connick E, Burton GF. HIV-1-specific chimeric antigen receptor T cells fail to recognize and eliminate the follicular dendritic cell HIV reservoir in vitro. J Virol 2020; 94:e00190-20.doi:10.1128/JVI.00190-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mayr L, Su B, Moog C. Langerhans cells: the ‘Yin and Yang’ of HIV restriction and transmission. Trends Microbiol 2017; 25:170–172. doi:10.1016/j.tim.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 43.Martin-Gayo E, Yu XG. Role of dendritic cells in natural immune control of HIV-1 infection. Front Immunol 2019; 10:1306.doi:10.3389/fimmu.2019.01306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nijmeijer BM, Langedijk CJM, Geijtenbeek TBH, Mucosal Dendritic Cell Subsets. Control HIV-1's viral fitness. Annu Rev Virol 2020; 7:385–402. doi:10.1146/annurev-virology-020520-025625. [DOI] [PubMed] [Google Scholar]
- 45.Hartana CA, Yu XG. Immunological effector mechanisms in HIV-1 elite controllers. Curr Opin HIV AIDS 2021; 16:243–248. doi:10.1097/COH.0000000000000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su B, Dispinseri S, Iannone V, Zhang T, Wu H, Carapito R, et al. Update on Fc-mediated antibody functions against HIV-1 beyond neutralization. Front Immunol 2019; 10:2968.doi:10.3389/fimmu.2019.02968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caskey M, Klein F, Nussenzweig MC. Broadly neutralizing anti-HIV-1 monoclonal antibodies in the clinic. Nat Med 2019; 25:547–553. doi:10.1038/s41591-019-0412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haynes BF, Shaw GM, Korber B, Kelsoe G, Sodroski J, Hahn BH, et al. HIV-Host interactions: implications for vaccine design. Cell Host Microbe 2016; 19:292–303. doi:10.1016/j.chom.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halper-Stromberg A, Lu CL, Klein F, Horwitz JA, Bournazos S, Nogueira L, et al. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell 2014; 158:989–999. doi:10.1016/j.cell.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mishra N, Sharma S, Dobhal A, Kumar S, Chawla H, Singh R, et al. Broadly neutralizing plasma antibodies effective against autologous circulating viruses in infants with multivariant HIV-1 infection. Nat Commun 2020; 11:4409.doi:10.1038/s41467-020-18225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Behrens AJ, Vasiljevic S, Pritchard LK, Harvey DJ, Andev RS, Krumm SA, et al. Composition and antigenic effects of individual glycan sites of a trimeric HIV-1 envelope glycoprotein. Cell Rep 2016; 14:2695–2706. doi:10.1016/j.celrep.2016.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shasha D, Karel D, Angiuli O, Greenblatt A, Ghebremichael M, Yu X, et al. Elite controller CD8+ T cells exhibit comparable viral inhibition capacity, but better sustained effector properties compared to chronic progressors. J Leukoc Biol 2016; 100:1425–1433. doi:10.1189/jlb.4A0915-422R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morvan MG, Teque FC, Locher CP, Levy JA. The CD8(+) T cell noncytotoxic antiviral responses. Microbiol Mol Biol Rev 2021; 85:e00155-20.doi:10.1128/MMBR.00155-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang C, Hu W, Jin JH, Zhou MJ, Song JW, Deng JN, et al. The role of CD8T cells in controlling HIV beyond the antigen-specific face. HIV Med 2020; 21:692–700. doi:10.1111/hiv.13021. [DOI] [PubMed] [Google Scholar]
- 55.Jones RB, Walker BD. HIV-specific CD8(+) T cells and HIV eradication. J Clin Invest 2016; 126:455–463. doi:10.1172/JCI80566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zanoni M, Palesch D, Pinacchio C, Statzu M, Tharp GK, Paiardini M, et al. Innate, non-cytolytic CD8+ T cell-mediated suppression of HIV replication by MHC-independent inhibition of virus transcription. PLoS Pathog 2020; 16:e1008821.doi:10.1371/journal.ppat.1008821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zaongo SD, Wang Y, Ma P, Song FZ, Chen YK. Selective elimination of host cells harboring replication-competent human immunodeficiency virus reservoirs: a promising therapeutic strategy for HIV cure. Chin Med J 2021; 134:2776–2787. doi:10.1097/CM9.0000000000001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferrari G, Pollara J, Tomaras GD, Haynes BF. Humoral and innate antiviral immunity as tools to clear persistent HIV infection. J Infect Dis 2017; 215: (suppl_3): S152–S159. doi:10.1093/infdis/jiw555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Board NL, Moskovljevic M, Wu F, Siliciano RF, Siliciano JD. Engaging innate immunity in HIV-1 cure strategies. Nat Rev Immunol 2022; 22:499–512. doi:10.1038/s41577-021-00649-1. [DOI] [PubMed] [Google Scholar]
- 60.Tsai A, Irrinki A, Kaur J, Cihlar T, Kukolj G, Sloan DD, et al. Toll-Like receptor 7 agonist GS-9620 induces HIV expression and HIV-specific immunity in cells from HIV-infected individuals on suppressive antiretroviral therapy. J Virol 2017; 91:e02166-16.doi:10.1128/JVI.02166-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.SenGupta D, Brinson C, DeJesus E, Mills A, Shalit P, Guo S, et al. The TLR7 agonist vesatolimod induced a modest delay in viral rebound in HIV controllers after cessation of antiretroviral therapy. Sci Transl Med 2021; 13:eabg3071.doi:10.1126/scitranslmed.abg3071. [DOI] [PubMed] [Google Scholar]
- 62.Gay CL, Kuruc JD, Falcinelli SD, Warren JA, Reifeis SA, Kirchherr JL, et al. Assessing the impact of AGS-004, a dendritic cell-based immunotherapy, and vorinostat on persistent HIV-1 Infection. Sci Rep 2020; 10:5134.doi:10.1038/s41598-020-61878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Collins DR, Gaiha GD, Walker BD. CD8(+) T cells in HIV control, cure and prevention. Nat Rev Immunol 2020; 20:471–482. doi:10.1038/s41577-020-0274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fan J, Liang H, Ji X, Wang S, Xue J, Li D, et al. CTL-mediated immunotherapy can suppress SHIV rebound in ART-free macaques. Nat Commun 2019; 10:2257.doi:10.1038/s41467-019-09725-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakamura-Hoshi M, Takahara Y, Matsuoka S, Ishii H, Seki S, Nomura T, et al. Therapeutic vaccine-mediated Gag-specific CD8(+) T-cell induction under anti-retroviral therapy augments anti-virus efficacy of CD8(+) cells in simian immunodeficiency virus-infected macaques. Sci Rep 2020; 10:11394.doi:10.1038/s41598-020-68267-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maldini CR, Gayout K, Leibman RS, Dopkin DL, Mills JP, Shan X, et al. HIV-resistant and HIV-specific CAR-modified CD4(+) T cells mitigate HIV disease progression and confer CD4(+) T cell help in vivo. Mol Ther 2020; 28:1585–1599. doi:10.1016/j.ymthe.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maldini CR, Claiborne DT, Okawa K, Chen T, Dopkin DL, Shan X, et al. Dual CD4-based CAR T cells with distinct costimulatory domains mitigate HIV pathogenesis in vivo. Nat Med 2020; 26:1776–1787. doi:10.1038/s41591-020-1039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mendoza P, Gruell H, Nogueira L, Pai JA, Butler AL, Millard K, et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature 2018; 561:479–484. doi:10.1038/s41586-018-0531-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bar-On Y, Gruell H, Schoofs T, Pai JA, Nogueira L, Butler AL, et al. Safety and antiviral activity of combination HIV-1 broadly neutralizing antibodies in viremic individuals. Nat Med 2018; 24:1701–1707. doi:10.1038/s41591-018-0186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Astronomo RD, Lemos MP, Narpala SR, Czartoski J, Fleming LB, Seaton KE, et al. Rectal tissue and vaginal tissue from intravenous VRC01 recipients show protection against ex vivo HIV-1 challenge. J Clin Invest 2021; 131:e146975.doi:10.1172/JCI146975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crowell TA, Colby DJ, Pinyakorn S, Sacdalan C, Pagliuzza A, Intasan J, et al. Safety and efficacy of VRC01 broadly neutralising antibodies in adults with acutely treated HIV (RV397): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet HIV 2019; 6:e297–e306. doi:10.1016/S2352-3018(19)30053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Corey L, Gilbert PB, Juraska M, Montefiori DC, Morris L, Karuna ST, et al. Two randomized trials of neutralizing antibodies to prevent HIV-1 acquisition. N Engl J Med 2021; 384:1003–1014. doi:10.1056/NEJMoa2031738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferrari G, Haynes BF, Koenig S, Nordstrom JL, Margolis DM, Tomaras GD. Envelope-specific antibodies and antibody-derived molecules for treating and curing HIV infection. Nat Rev Drug Discov 2016; 15:823–834. doi:10.1038/nrd.2016.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pegu A, Asokan M, Wu L, Wang K, Hataye J, Casazza JP, et al. Activation and lysis of human CD4 cells latently infected with HIV-1. Nat Commun 2015; 6:8447.doi:10.1038/ncomms9447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bournazos S, Gazumyan A, Seaman MS, Nussenzweig MC, Ravetch JV. Bispecific anti-HIV-1 antibodies with enhanced breadth and potency. Cell 2016; 165:1609–1620. doi:10.1016/j.cell.2016.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Steinhardt JJ, Guenaga J, Turner HL, McKee K, Louder MK, O’Dell S, et al. Rational design of a trispecific antibody targeting the HIV-1 Env with elevated anti-viral activity. Nat Commun 2018; 9:877.doi:10.1038/s41467-018-03335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Allers K, Hutter G, Hofmann J, Loddenkemper C, Rieger K, Thiel E, et al. Evidence for the cure of HIV infection by CCR5Delta32/Delta32 stem cell transplantation. Blood 2011; 117:2791–2799. doi:10.1182/blood-2010-09-309591. [DOI] [PubMed] [Google Scholar]
- 78.Gupta RK, Peppa D, Hill AL, Galvez C, Salgado M, Pace M, et al. Evidence for HIV-1 cure after CCR5Delta32/Delta32 allogeneic haemopoietic stem-cell transplantation 30 months post analytical treatment interruption: a case report. Lancet HIV 2020; 7:e340–e347. doi:10.1016/S2352-3018(20)30069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gupta RK, Abdul-Jawad S, McCoy LE, Mok HP, Peppa D, Salgado M, et al. HIV-1 remission following CCR5Delta32/Delta32 haematopoietic stem-cell transplantation. Nature 2019; 568:244–248. doi:10.1038/s41586-019-1027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang L, Zhang Z, Xu R, Wang X, Shu Z, Chen X, et al. Human umbilical cord mesenchymal stem cell transfusion in immune non-responders with AIDS: a multicenter randomized controlled trial. Signal Transduct Target Ther 2021; 6:217.doi:10.1038/s41392-021-00607-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu R, Zhang JY, Tu B, Xu Z, Huang HH, Huang L, et al. HLA-mismatched allogeneic adoptive immune therapy in severely immunosuppressed AIDS patients. Signal Transduct Target Ther 2021; 6:174.doi:10.1038/s41392-021-00550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salgado M, Kwon M, Galvez C, Badiola J, Nijhuis M, Bandera A, et al. Mechanisms that contribute to a profound reduction of the HIV-1 reservoir after allogeneic stem cell transplant. Ann Intern Med 2018; 169:674–683. doi:10.7326/M18-0759. [DOI] [PubMed] [Google Scholar]
- 83.Colonna L, Peterson CW, Schell JB, Carlson JM, Tkachev V, Brown M, et al. Evidence for persistence of the SHIV reservoir early after MHC haploidentical hematopoietic stem cell transplantation. Nat Commun 2018; 9:4438.doi:10.1038/s41467-018-06736-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eberhard JM, Angin M, Passaes C, Salgado M, Monceaux V, Knops E, et al. Vulnerability to reservoir reseeding due to high immune activation after allogeneic hematopoietic stem cell transplantation in individuals with HIV-1. Sci Transl Med 2020; 12:eaay9355.doi:10.1126/scitranslmed.aay9355. [DOI] [PubMed] [Google Scholar]
- 85.Xu L, Wang J, Liu Y, Xie L, Su B, Mou D, et al. CRISPR-edited stem cells in a patient with HIV and acute lymphocytic leukemia. N Engl J Med 2019; 381:1240–1247. doi:10.1056/NEJMoa1817426. [DOI] [PubMed] [Google Scholar]