Abstract
Background:
Chronic liver disease has emerged as a leading cause of non-acquired immune deficiency syndrome (AIDS)-related mortality in hepatitis C virus (HCV)/human immunodeficiency virus (HIV)-coinfected patients. The relationship between CD4 cell count and HIV-related opportunistic infections and tumors has been well characterized; however, it is unclear whether CD4 cell count is associated with HCV-related hepatic events.
Methods:
This observational cohort study enrolled HCV/HIV-coinfected patients from the National Free Antiretroviral Treatment Program of China from 2004 to 2019 in Guangzhou. The primary outcome was a composite of hepatic events, including cirrhosis complications, hepatocellular carcinoma (HCC), and liver-related mortality. Kaplan–Meier survival and multivariate logistic regression analyses were performed.
Results:
Among the 793 patients, 43 developed hepatic events during a median follow-up of 6.7 years, including 35 cirrhosis complications, 13 HCC cases, and 14 cases of liver-related mortality. The 5-year and 10-year cumulative incidences of hepatic events were 4.2% and 9.3%, respectively. Patients who developed hepatic events had a less satisfactory increase in CD4 cell count, lower peak CD4 (354.5 cells/μL vs. 560.0 cells/μL, P < 0.001), and lower percentage of peak CD4 > 500 cells/μL (30.2% vs. 60.7%, P < 0.001) after the initiation of antiretroviral therapy (ART) than those who did not. The cumulative incidences of hepatic events were higher in patients with lower peak CD4 levels with adjusted odds ratios of 3.96 (95% confidence interval [CI]: 1.51–10.40), 2.25 (95% CI: 0.87–5.86), and 0.98 (95% CI: 0.35–2.74) for patients with peak CD4 at <200 cells/μL, 200–350 cells/μL, and 351 to 500 cells/μL, respectively, relative to those with peak CD4 > 500 cells/μL. Peak CD4 was negatively associated with the risk of hepatic events in a dose–response manner (P-value for trend = 0.004).
Conclusion:
Persistently low CD4 cell counts after ART are independently associated with a high risk of hepatic events in HCV/HIV-coinfected patients, highlighting the important role of immune reconstitution in improving liver outcomes.
Keywords: CD4 cell count, Co-infection, Hepatic events, Hepatitis C virus, Human immunodeficiency virus
Introduction
Hepatitis C virus (HCV) and human immunodeficiency virus (HIV) infection are major global public health concerns, with overlapping transmission modes and affected populations. Globally, the prevalence of HCV infection has been estimated to be approximately 6.2% in people living with HIV.[1] In China, the estimated prevalence of HCV infection was as high as 18.2% in people living with HIV, especially in some populations, such as injection drug users and men who have sex with men. In addition, HCV/HIV-coinfected patients were reported to have a higher risk of all-cause mortality, virological failure to antiretroviral therapy (ART), and loss to follow-up compared to HIV monoinfected patients.[2]
It is well established that the course of HCV infection is accelerated in HIV-coinfected individuals, with faster progression of liver fibrosis leading to a high risk of hepatic events, including liver cirrhosis, end-stage liver disease, and hepatocellular carcinoma (HCC).[3–6] With the development of potent and safe antiretroviral regimens in the past two decades, HIV replication was effectively suppressed, leading to immune reconstitution (reflected by CD4 cell count recovery to >500 cells/μL) and a substantial decrease in acquired immune deficiency syndrome (AIDS)-related morbidities.[7,8] Consequently, the relative importance of other traditionally non-AIDS-related morbidities has increased. Chronic liver disease has emerged as a leading cause of non-AIDS-related mortality in HCV/HIV-coinfected patients.[9–11] The relationship between CD4 cell count and HIV-positive opportunistic infections and tumors has been well characterized in previous studies.[12] However, it is unclear whether the CD4 cell count is associated with the risk of hepatic events among HCV/HIV-coinfected patients because previous studies reported inconsistent results.[13–15]
The National Free Antiretroviral Treatment Program was a prospective cohort established and managed by the National Center for AIDS/sexually transmitted disease (STD) Control and Prevention, China Centers for Disease Control and Prevention (China CDC).[16,17] The cohort provides a unique opportunity to monitor and follow up on HCV/HIV-coinfected patients receiving ART and to assess clinical characteristics and outcomes in this population. In this study, we enrolled and followed HCV/HIV-coinfected patients from the National Free Antiretroviral Treatment Program, aiming to provide robust clinical evidence on the association between CD4 cell count and the occurrence of hepatic events.
Methods
Ethical approval
This study was approved by the Ethics Committee of Guangzhou Eighth People's Hospital (No.202033166) and was conducted in accordance with the guidelines of the Declaration of Helsinki and the principles of good clinical practice. All patients provided written informed consent to have their data used (anonymously) for research purposes.
Study design and participants
This was an observational cohort study with patients from the National Free Antiretroviral Treatment Program of China, which is a prospectively enrolled, observational national cohort and has been previously described.[2] Briefly, HIV-positive patients in China were eligible to receive ART recommended in the guidelines when they met the national treatment criteria. Since 2002, HIV-infected patients in China with a CD4 cell count of 200 cells/μL or less, total lymphocyte count of <1200 cells/μL, or World Health Organization (WHO) disease stage 3 or 4 have been eligible for free combination ART. However, since 2008, the CD4 cell count threshold for treatment has increased to 350 cells/μL. Between 2003 and 2005, the first-line HIV treatment regimen recommended in China was zidovudine or stavudine plus didanosine and nevirapine or efavirenz in accordance with WHO recommendations. Since 2005, these regimens have been gradually replaced by zidovudine or stavudine plus lamivudine and nevirapine. After 2010, as recommended by the WHO, stavudine was phased out, and tenofovir was introduced in the first-line regimen. Standardized reporting forms at baseline (initiation of ART) and follow-up were completed by trained local health workers.
In this study, consecutive HIV-positive patients visiting the Infectious Disease Center at Guangzhou Eighth People's Hospital from October 2004 to December 2019 were screened. Patients who met the following criteria were included: ART-naïve patients aged 18 years or older, confirmed HIV infection by HIV antibody or HIV-1 ribonucleic acid (RNA), and positive HCV antibody. Patients who did not have detection of HCV RNA, had a history of HCC before enrollment, or were followed for <3 months were excluded. During the study period, interferon- or direct-acting antiviral drug (DAA)-based anti-HCV treatment could be initiated per the standard of care. Standard-of-care anti-HCV treatment was defined as a prescription of >8 weeks of a DAA-containing regimen or >24 weeks of a pegylated interferon/ribavirin regimen. Sustained virologic response (SVR) was defined as undetectable HCV RNA ≥12 weeks after completion of treatment.
Data collection
Data collected included demographic characteristics, route of HIV infection, date of diagnosis, laboratory test results, treatment regimen start and stop dates and reasons for change, and reasons for treatment termination. Recommended laboratory tests of the National Free Antiretroviral Treatment Program included complete blood count, CD4 cell count, CD8 cell count, liver panel, liver biochemistry, blood lipid levels, fasting glucose levels, hepatitis B virus (HBV) surface antigen, and HCV antibody. HIV-1 RNA testing, measurement of HCV RNA levels and HCV genotypes, and liver imaging were performed per standard of care. HIV-1 RNA was detected by a COBASTM automatic virus load analysis system (COBAS TapMan48, Roche, Switzerland), and the detection limit was 20 copies/mL. HCV genotyping was performed using a detection kit (PCR-fluorescent probe method, Triplex International Biosciences Co., LTD, Xiamen, Fujian, China). HCV RNA was detected by a COBASTM automatic virus load analysis system (COBAS TapMan48, Roche), and the lower detection limit was 15 IU/mL. Relevant clinical, laboratory, radiological, and histological data were extracted from past medical records. The aspartate aminotransferase-to-platelet ratio index (APRI) and fibrosis 4 marker (FIB-4) score were calculated to estimate liver fibrosis.
Outcomes
The primary outcome was a composite endpoint of hepatic events after ART, including cirrhosis complications, HCC, and liver-related mortality. The secondary outcomes were the cumulative probability of HCC, all-cause mortality, and liver-related mortality. Among patients without baseline cirrhosis, incident (new diagnosis) cirrhosis was also determined. Hepatic events were defined as any cirrhosis complications, HCC, and/or liver-related mortality.[18] Cirrhosis complications included ascites, spontaneous bacterial peritonitis, variceal bleeding, hepatic encephalopathy, and hepatorenal syndrome. The diagnoses were based on pathology reports, hospital discharge summaries, or consultation notes. Liver-related mortality was defined as death related to cirrhosis complications and/or HCC. Patients were censored at dropout, loss to follow-up, death, or on December 31, 2020, whichever came first. The diagnosis of cirrhosis was based on (1) liver histology or radiological findings of coarse liver echotexture with nodularity and small liver size or (2) the presence of features of portal hypertension (including ascites, splenomegaly, and varices) noted on liver imaging or (3) APRI >2.0 or FIB-4 >3.25.
Statistical analysis
Data are expressed as counts and percentages for categorical variables and as medians and interquartile ranges (IQRs) for continuous variables. Qualitative and quantitative differences between subgroups were analyzed using the chi-squared test or Fisher's exact test for categorical parameters and Student's t-test or the Mann–Whitney test for continuous parameters, as appropriate. The Kaplan–Meier method was used to estimate the cumulative probability of hepatic events over time. Univariate and multivariate logistic regression analyses were performed to evaluate the odds ratios (ORs) with 95% confidence intervals (CIs) across longitudinal peak CD4 cell count categories using >500 cells/μL as the reference group. Subgroup analyses were performed according to age (<40 years vs. ≥40 years), baseline CD4 cell count (<200 cells/μL vs. ≥200 cells/μL), cirrhosis (yes vs. no), and anti-HCV treatment (yes vs. no). All statistical tests were two-sided. Statistical significance was set at P < 0.05. All analyses were performed with SPSS software, version 26.0 (IBM, Armonk, NY, USA).
Results
A total of 1217 consecutive adult HCV/HIV-coinfected patients were included; of these, 201 were excluded for lack of detection of HCV RNA, 223 for follow-up <3 months, and no patients had HCC before study enrollment; thus, 793 were finally enrolled in this study.
Patient characteristics
Of the 793 studied patients, the median age was 38.0 years (IQR: 33.0–44.0), 81.7% (640/793) were males, and 62.3% (494/793) had a history of intravenous drug abuse [Table 1]. The overall baseline CD4 cell count was quite low, with a median of 119.0 cells/μL (IQR: 32.0–236.0 cells/μL); 67.0% (523/793) of patients had CD4 <200 cells/μL, 24.3% (190/793) had CD4 between 200 cells/μL and 350 cells/μL, 6.3% (49/793) had CD4 between 351 cells/μL and 500 cells/μL, and only 2.4% (19/793) had CD4 >500 cells/μL. Over 80% of patients received nucleoside reverse transcriptase inhibitors (NRTIs) plus non-nucleoside reverse transcriptase inhibitors (NNRTIs) as an initial antiretroviral regimen. Among the 157 patients with available data, the median HIV RNA before ART was 4.7 log10 copies/mL (IQR: 3.9–5.1 log10 copies/mL). Nearly one-quarter (23.7%) of patients had predefined liver cirrhosis, and 16.9% (130/770) of patients were coinfected with HBV. The median HCV RNA was 6.0 log10 IU/mL (IQR: 3.7–6.8 log10 IU/mL), with 17.8% having undetectable HCV RNA at study enrollment. Of the 244 patients who underwent genotype testing, genotype 6 (53.3%) was the most common, followed by genotype 3 (27.9%), genotype 1 (16.4%), and genotype 2 (2.5%).
Table 1.
Baseline characteristics of studied HCV/HIV-coinfected patients.
| Characteristics | Patients (N = 793) |
| Age at enrollment (years) | 38.0 (33.0–44.0) |
| Male | 640 (81.7) |
| BMI (kg/m2) | 19.9 (18.4–21.9) |
| HIV transmission | 793 |
| IDU | 494 (62.3) |
| Sexual | 245 (30.9) |
| Other | 54 (6.8) |
| CD4 count (cells/μL) | 119.0 (32.0–236.0) |
| CD4 count categories | 781 |
| <200 (cells/μL) | 523 (67.0) |
| 200–350 (cells/μL) | 190 (24.3) |
| 351–500 (cells/μL) | 49 (6.3) |
| >500 (cells/μL) | 19 (2.4) |
| CD4/CD8 ratio | 0.158 (0.058–0.289) |
| HIV RNA (log10 copies/mL)∗ | 4.7 (3.9–5.1) |
| Initial antiretroviral regimen | 793 |
| NRTIs + NNRTIs | 641 (80.8) |
| NRTIs + PIs | 93 (11.7) |
| Other regimens/unknown | 59 (7.4) |
| Liver cirrhosis | 188 (23.7) |
| Platelet (×109/L) | 163.0 (114.0–207.0) |
| Hemoglobin (g/L) | 123.0 (105.0–139.0) |
| ALT (U/L) | 37.0 (26.0–56.8) |
| Albumin (g/L) | 38.0 (31.0–42.1) |
| Total bilirubin (μmol/L) | 9.1 (6.8–12.5) |
| HBsAg positive | 130/770 (16.9) |
| HCV RNA (log10 IU/mL) | 6.0 (3.7–6.8) |
| Undetectable HCV RNA | 141 (17.8) |
| HCV genotype | 244 |
| 1 | 40 (16.4) |
| 2 | 6 (2.5) |
| 3 | 68 (27.9) |
| 6 | 130 (53.3) |
Data are presented as n, n/N (%), n (%) or median (interquartile range). ∗Data were available for 157 patients.
ALT: Alanine aminotransferase; BMI: Body mass index; HBsAg: Hepatitis B surface antigen; HBV: Hepatitis B virus; HCV: Hepatitis C virus; HIV: Human immunodeficiency virus; IDU: Intravenous drug-using; NNRTIs: Non-nucleoside reverse transcriptase inhibitors; NRTIs: Nucleoside reverse transcriptase inhibitors; PIs: Protease inhibitors.
Incidence and features of clinical outcomes
During the study period, 386/793 (48.7%) patients had initiated anti-HCV treatment, among whom 91 (23.6%) patients received interferon, 247 (64.0%) received DAAs, and 48 (12.4%) switched from interferon to DAAs because there was no SVR after interferon treatment. The overall SVR rate was 96.9% (347/358), specifically 57.7% (75/130) in patients with interferon-based treatment and 98.9% (272/275) in those with DAA-based treatment [Supplementary Table 1].
During a median follow-up of 6.7 years (IQR: 3.2–9.7 years), 43 patients developed hepatic events, including 35 cirrhosis complications, 13 HCC cases, and 14 cases of liver-related mortality. In addition, of the 605 patients without baseline cirrhosis, 84 had incident cirrhosis. A total of 64 patients were known to have died during the study, 14 were adjudicated to be HCV related, 35 HIV related, and 15 other causes related. The incidence rate per 1000 person-years and cumulative percentages at years 5 and 10 for each individual are shown in Table 2. The overall 5-year and 10-year cumulative incidences of hepatic events were 4.2% and 9.3%, respectively. Supplementary Table 2 shows the baseline characteristics of patients with and without hepatic events. Compared to patients without hepatic events, those with hepatic events were older and less likely to receive NRTIs plus NNRTIs, whereas they were more likely to be triple infected, to have liver cirrhosis corroborated by lower platelet and hemoglobin levels, and to have poor liver function corroborated by higher alanine aminotransferase and bilirubin levels and lower albumin levels. However, sex, HIV transmission route, HCV RNA load, and HCV genotypes were comparable between patients with and without hepatic events.
Table 2.
Clinical outcomes among HCV/HIV-coinfected patients with and without baseline cirrhosis.
| Clinical outcomes | Patients | Person-years | Incidence per 1000 person-years (95% CI) | 5-year cumulative incidence (%) | 10-year cumulative incidence (%) |
| Hepatic events | 43 | 5073 | 8.5 (6.0–11.0) | 4.2 | 9.3 |
| Cirrhosis decompensation | 35 | 5065 | 6.9 (4.6–9.2) | 3.5 | 6.6 |
| SBP | 26 | – | – | – | – |
| Variceal bleeding | 16 | – | – | – | – |
| Hepatic encephalopathy | 8 | – | – | – | – |
| Hepatorenal syndrome | 4 | – | – | – | – |
| HCC | 13 | 5098 | 2.6 (1.2–3.9) | 1.2 | 3.5 |
| Liver-related mortality | 14 | 5112 | 2.7 (1.3–4.2) | 1.1 | 3.1 |
| Incident cirrhosis | 84/605∗ | 3932 | 21.4 (16.8–25.9) | 7.9 | 18.0 |
| All-cause mortality | 64 | 5123 | 12.5 (9.5–15.5) | 6.5 | 10.8 |
The CI is determined by assuming that the ratio conforms to the normal distribution.
The 605 patients without baseline cirrhosis.
CI: Confidence interval; HCC: Hepatocellular carcinoma; HCV: Hepatitis C virus; HIV: Human immunodeficiency virus; SBP: Spontaneous bacterial peritonitis.
Association between CD4 cell count and hepatic events
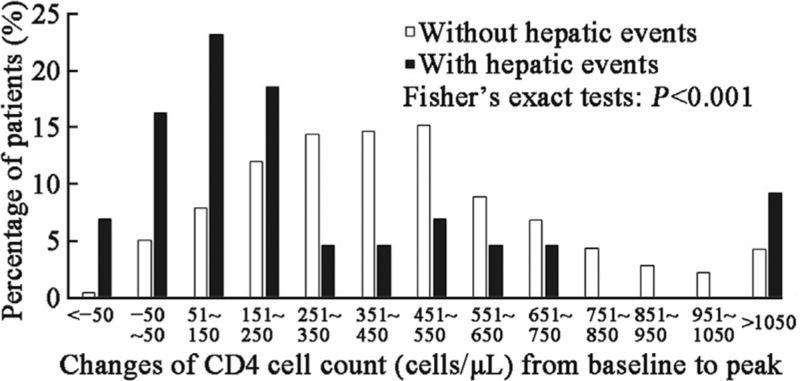
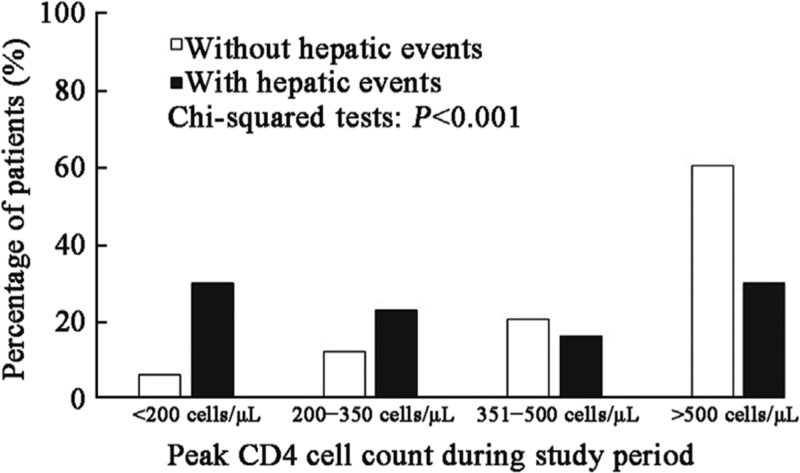
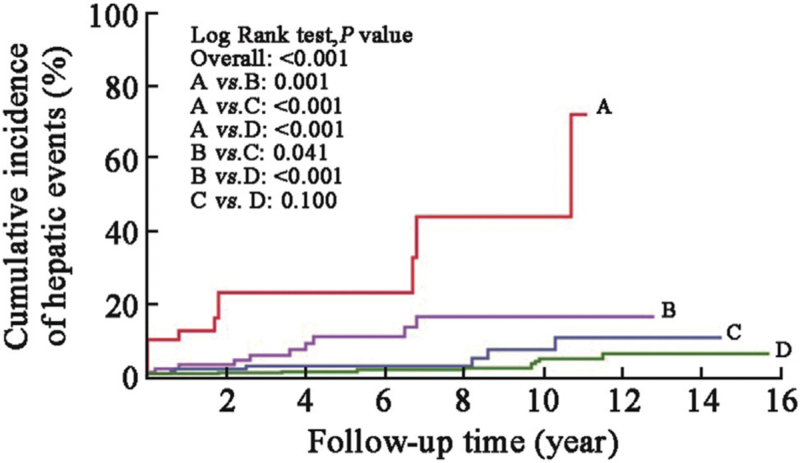
The baseline CD4 cell count (median: 146.0 cells/μL vs. 118.0 cells/μL, t = −0.369, P = 0.692) and categories between patients with and without hepatic events were comparable [Supplementary Table 2]. All patients received ART per standard of care, and most of them experienced varying degrees of CD4 cell count elevation after the initiation of ART; however, the increase in CD4 cell count in patients with hepatic events was less robust than that in patients without hepatic events [Figure 1]. The median time for CD4 cell count to peak in the entire cohort was 4.3 years (IQR: 1.8–7.1 years), with 3.2 years in patients with hepatic events and 4.8 years in those without hepatic events. The peak CD4 cell count was significantly lower among patients with hepatic events than among those without (median: 354.5 cells/μL vs. 560.0 cells/μL, t = −3.356, P = 0.001). Overall, 7.6%, 12.9%, 20.6%, and 59.0% of the patients reached peak CD4 cell counts at <200 cells/μL, 200 to 350 cells/μL, 351 to 500 cells/μL, and >500 cells/μL, respectively. The percentage of peak CD4 cell counts >500 cells/μL in patients with hepatic events was significantly lower than that in patients without hepatic events (30.2% vs. 60.7%, χ2 = 41.477, P < 0.001, Figure 2). The 5-year and 10-year cumulative incidences of hepatic events were 22.9% and 43.8% among patients with peak CD4 cell counts <200 cells/μL, 10.8% and 16.3% among those with peak CD4 cell counts between 200 and 350 cells/μL, 2.7% and 7.2% among those with peak CD4 cell counts between 351 and 500 cells/μL, and 1.1% and 4.7% among those with peak CD4 cell counts >500 cells/μL, respectively (log-rank P < 0.001, Figure 3).
Figure 1.
Changes in CD4 cell count between patients with and without hepatic events. Columns indicate the percentage of patients categorized according to the changes in CD4 cell count from baseline to peak. White column indicates the percentage of patients without hepatic events, and black column indicates patients with hepatic events.
Figure 2.
Peak CD4 cell count between patients with and without hepatic events. Columns indicate the percentage of patients categorized according to peak CD4 cell count during the study period. White column indicates patients without hepatic events, and black column indicates patients with hepatic events.
Figure 3.
Cumulative incidence of hepatic events in subgroups stratified by peak CD4 cell count during the study period. A: Peak CD4 <200 cells/μL; B: Peak CD4 at 200–350 cells/μL; C: Peak CD4 at 351–500 cells/μL; D: Peak CD4 >500 cells/μL.
After adjusting for potential confounders, including age, sex, baseline CD4 cell count, antiretroviral regimen, liver cirrhosis, liver function, HBsAg status, HCV RNA, and anti-HCV treatment, the adjusted odds ratios (aORs) were 3.96 (95% CI: 1.51–10.40), 2.25 (95% CI: 0.87–5.86), and 0.98 (95% CI: 0.35–2.74) for patients with peak CD4 cell counts <200 cells/μL, 200 to 350 cells/μL, and 351 to 500 cells/μL, respectively, relative to those with peak CD4 cell counts >500 cells/μL [Table 3]. Further analysis showed that peak CD4 cell count after ART was negatively associated with the risk of hepatic events in a dose–response manner (P value for trend = 0.04). Similar results were found in subgroup analyses according to age, baseline CD4 cell count, cirrhosis, and anti-HCV treatment [Supplementary Table 3]. We further evaluated the relationship between CD4 cell count and HCC, liver-related death, and all-cause death and found that persistently low CD4 cell count was a risk factor for these individual events [Supplementary Table 4].
Table 3.
Risk of hepatic events according to peak CD4 count among HCV/HIV-coinfected patients.
| Peak CD4 cell count | |||||
| Characteristics | >500 cells/μL | 351–500 cells/μL | 200–350 cells/μL | <200 cells/μL | P value for trend |
| Patients | 468 | 163 | 102 | 60 | – |
| Hepatic events | 13 | 7 | 10 | 13 | – |
| Person-years | 3529 | 910 | 492 | 142 | – |
| Incidence per 1000 PYs (95% CI) | 3.7 (1.7–5.7) | 7.7 (2.0–13.4) | 20.3 (7.9–32.8) | 91.5 (44.1–139.0) | |
| Multivariable aOR (95% CI), model 1 | 1.00 (Ref) | 1.57 (0.62–4.01) | 3.80 (1.62–8.94) | 9.68 (4.24–22.10) | <0.001 |
| P-value of model 1 | 0.345 | 0.002 | <0.001 | ||
| Multivariable aOR (95% CI), Model 2 | 1.00 (Ref) | 1.54 (0.60–3.95) | 2.92 (1.19–7.13) | 7.36 (3.09–17.52) | <0.001 |
| P-value of model 2 | 0.368 | 0.019 | <0.001 | ||
| Multivariable aOR (95% CI), Model 3 | 1.00 (Ref) | 1.28 (0.49–3.33) | 2.54 (1.01–6.38) | 5.50 (2.23–13.60) | <0.001 |
| P-value of model 3 | 0.619 | 0.048 | <0.001 | ||
| Multivariable aOR (95% CI), Model 4 | 1.00 (Ref) | 0.98 (0.35–2.74) | 2.25 (0.87–5.86) | 3.96 (1.51–10.40) | 0.004 |
| P-value of model 4 | 0.974 | 0.095 | 0.005 | ||
Multivariate logistics regression models, backward Wald method was used. Model 1, Adjusted for age at enrollment and gender (male and female); Model 2, Model 1 plus adjustment for the baseline CD4 cell count and initial antiretroviral regimen (NRTIs plus NNRTIs, NRTIs plus PIs, other regimens/unknown); Model 3, Model 2 plus adjustment for the liver cirrhosis (with and without), platelet, hemoglobin, ALT, albumin, total bilirubin; Model 4, Model 3 plus adjustment for the HBsAg status (positive and negative), HCV RNA, and anti-HCV treatment (with and without).
The CI is determined by assuming that the ratio conforms to the normal distribution.
ALT: Alanine aminotransferase; CI: Confidence interval; HBsAg: Hepatitis B surface antigen; HCV: Hepatitis C virus; HIV: Human immunodeficiency virus; NRTIs: Nucleoside reverse transcriptase inhibitors; NNRTIs: Non-nucleoside reverse transcriptase inhibitors; PIs: Protease inhibitors; PYs: Person years; RNA: Ribonucleic acid; –: Not applicable.
Discussion
In this long-term observational cohort study comprising HCV/HIV-coinfected patients with good adherence to ART in China, we found that persistently low CD4 cell counts were independently associated with a high risk of hepatic events and that the association may have a dose–response effect.
The relationship between CD4 cell count and the risk of hepatic events among HCV/HIV-coinfected patients remains controversial. In a multicohort collaboration of HCV/HIV-coinfected individuals in Europe and Canada, Gjaerde et al[14] showed that a low current CD4 cell count is associated with a higher incidence of both HCC and other liver events, including liver-related deaths or decompensations. Kramer et al[13] also found that a recent low CD4 cell count is associated with an increased risk for HCC among veterans with HCV/HIV-coinfection in the United States and that the association is even stronger in the cirrhosis-only subgroup. However, in a French cohort of HCV/HIV-coinfected patients with liver cirrhosis, Salmon et al[15] did not find a significant association between CD4 cell count and risk of HCC; they believed that any association is likely confounded by cirrhosis, as concomitant splenic sequestration of lymphocytes artificially lowers the CD4 cell count.[19]
In our study, neither the CD4 cell count nor categories at baseline were significantly associated with hepatic events. However, when considering the longitudinal change in CD4 cell count after ART and adjusting for liver cirrhosis, anti-HCV treatment, and other potential confounders, we found that patients with a peak CD4 count of ≤200 cells/μL had a nearly four-fold increased risk of hepatic events compared to those with a peak CD4 cell count >500 cells/μL. This observation is consistent with reports from Gjaerde et al and Kramer et al[20–22] and other previous studies on HIV individuals. More importantly, by stratifying the peak CD4 cell count, we demonstrated that the above association had a dose–response effect; more specifically, the lower the peak CD4 cell count, the higher the risk of hepatic events. This association was supported by subgroup analyses.
Previous studies have reported that effective ART could lower the progression of hepatic fibrosis and liver disease.[23,24] On the other hand, CD4 cells are a key component of the adaptive immune response against tumors, and increasing evidence suggests that CD4 cells can be highly potent antitumor effectors.[25] Our study not only supports recommendations that antiretroviral treatment should aim at reaching and maintaining a high CD4 cell count to prevent malignancy,[20] but also highlights the important role of immune reconstitution in improving long-term liver outcomes in HCV/HIV-coinfected patients. Our results emphasize the need for early initiation of antiviral therapy in HCV/HIV-coinfected patients in clinical practice to improve immune reconstitution and reduce the incidence of end-stage liver disease. In patients with poor immune reconstitution, closer monitoring is needed for the early detection of adverse outcomes and timely intervention, thus improving the long-term prognosis of patients.
As expected, we found that liver cirrhosis (aOR: 4.72, 95% CI: 2.30–9.71) and the absence of anti-HCV treatment (aOR: 2.37, 95% CI: 1.03–5.43) were risk factors for hepatic events. In addition, coinfection with HBV (triple infection), a well-known risk factor for hepatic events, was verified in the present study (aOR: 2.18, 95% CI: 0.99–4.78). In the sensitivity analysis, we tried to exclude patients with triple infection and found that the relationship between the peak CD4 cell count and hepatic events was still consistent (data not shown). Interestingly, we found that the proportion of patients receiving NRTIs plus NNRTIs as an initial antiretroviral regimen was lower in patients with hepatic events than in those without. NRTIs plus NNRTIs as an initial antiretroviral regimen were negatively associated with the occurrence of hepatic events (aOR: 0.33, 95% CI: 0.13–0.82). This could be explained by the fact that most patients with hepatic events had baseline cirrhosis; in this case, physicians are less likely to prescribe NNRTIs because of their potential hepatotoxicity.[26] Nonetheless, the impact of the antiretroviral regimen on long-term liver outcomes needs further investigation. In this study, only 157 patients had HIV-1 RNA testing before ART, with a median of 4.7 log10 copies/mL. HIV RNA is an expensive test that is usually not affordable for some AIDS patients because of their poor financial situation. On the other hand, the early clinical practice did not emphasize the need for HIV RNA testing before ART, whereas the required tests were HIV antibody and CD4 cell count, so only a small percentage of patients had HIV RNA test results. Based on the available data, although we did not find a correlation between HIV RNA load and hepatic events, their relationship needs to be further clarified by more well-designed studies.
Our study had a few limitations. First, compared with previous multicenter and multicohort studies, the present study was a single-center study with a relatively small sample size. However, most of the previous studies were conducted in European and North American populations with predominantly HCV genotype 1, whereas this study included Asian populations, and most of the patients were genotype 6, thus providing novel evidence to fill the gap of previous ones. Second, the enrollment period of this study spanned a long period of time, and the patients had fairly low baseline CD4 cell counts. Some of them may have been lost to follow-up or died due to AIDS-related opportunistic infections and tumors; therefore, the incidence of hepatic events would be underestimated. However, sensitivity analyses stratified by baseline CD4 cell count (<200 cells/μL vs. ≥200 cells/μL) showed that the primary results remained consistent. Third, this study is a real-world observational study. Unlike strictly interventional studies, clinical information and test results are based on patient self-report and historical medical record information, and there are inevitably missing data, including lifestyle details such as alcohol consumption, smoking, and comorbidities such as fatty liver diseases and diabetes. However, the indicators closely related to hepatic events collected in this study are relatively complete, which maximizes the reliability of the conclusions. In addition, a relatively large number of patients in this study were excluded due to the absence of HCV RNA testing and short follow-up time (N = 424), but we compared the baseline characteristics between included and excluded patients. We found that they were comparable in gender, ART regimens, and coinfection with HBV, although the included patients were slightly younger, with a slightly higher CD4 cell count and a lower proportion of liver cirrhosis. In short, our study population is in line with the current characteristics of patients with HIV infection; thus, it has a good representativeness.
In conclusion, persistently low CD4 cell counts after the initiation of ART are independently associated with a high risk of hepatic events in HCV/HIV-coinfected patients, highlighting the important role of immune reconstitution in improving liver outcomes. Future intervention studies are needed to analyze the impact of promoting immune reconstitution on the long-term outcomes in this population.
Acknowledgments
We thank all the patients who took part in this study and provided samples to support the scientific research.
Funding
This study was supported by grants from the Guangzhou Basic Research Program on People's Livelihood Science and Technology (No. 202002020005), the National Natural Science Foundation of China (No. 82072265), and the Chinese 13th Five-Year National Science and Technology Major Project (No. 2017ZX10202101-003-001).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Lin W, Zhong H, Wen C, He Y, Zheng X, Li H, Chen X, He H, Chen J, Chen L, Liu C, Tang X, Cai W, Li L. Persistently low CD4 cell counts are associated with hepatic events in HCV/HIV coinfected patients: data from the national free antiretroviral treatment program of China. Chin Med J 2022;135:2699–2705. doi: 10.1097/CM9.0000000000002502
Weiyin Lin, Huolin Zhong, and Chunyan Wen contributed equally to this study.
Supplemental digital content is available for this article.
References
- 1.Platt L, Easterbrook P, Gower E, McDonald B, Sabin K, McGowan C, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis 2016; 16:797–808. doi: 10.1016/S1473-3099(15)00485-5. [DOI] [PubMed] [Google Scholar]
- 2.Zhang F, Zhu H, Wu Y, Dou Z, Zhang Y, Kleinman N, et al. HIV, hepatitis B virus, and hepatitis C virus co-infection in patients in the China National Free Antiretroviral Treatment Program, 2010–12: a retrospective observational cohort study. Lancet Infect Dis 2014; 14:1065–1072. doi: 10.1016/S1473-3099(14)70946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology 1999; 30:1054–1058. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 4.Graham CS, Baden LR, Yu E, Mrus JM, Carnie J, Heeren T, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis 2001; 33:562–569. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 5.Anderson KB, Guest JL, Rimland D. Hepatitis C virus coinfection increases mortality in HIV-infected patients in the highly active antiretroviral therapy era: data from the HIV Atlanta VA Cohort Study. Clin Infect Dis 2004; 39:1507–1513. doi: 10.1086/425360. [DOI] [PubMed] [Google Scholar]
- 6.Macías J, Berenguer J, Japón MA, Girón JA, Rivero A, López-Cortés LF, et al. Fast fibrosis progression between repeated liver biopsies in patients coinfected with human immunodeficiency virus/hepatitis C virus. Hepatology 2009; 50:1056–1063. doi: 10.1002/hep.23136. [DOI] [PubMed] [Google Scholar]
- 7.Yang X, Su B, Zhang X, Liu Y, Wu H, Zhang T. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: challenges of immunological non-responders. J Leukoc Biol 2020; 107:597–612. doi: 10.1002/JLB.4MR1019-189R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS 2017; 12:6–11. doi: 10.1097/COH.0000000000000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber R, Sabin CA, Friis-Møller N, Reiss P, El-Sadr WM, Kirk O. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006; 166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 10.Smith CJ, Ryom L, Weber R, Morlat P, Pradier C, Reiss P, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 2014; 384:241–248. doi: 10.1016/S0140-6736(14)60604-8. [DOI] [PubMed] [Google Scholar]
- 11.Lu Y, Robinson M, Zhang FJ. Human immunodeficiency virus and hepatitis C virus co-infection: epidemiology, natural history and the situation in China. Chin Med J (Engl) 2009; 122:93–97. doi: 10.3760/cma.j.issn.0366-6999.2009.01.017. [PubMed] [Google Scholar]
- 12.Saharia KK, Koup RA. T cell susceptibility to HIV influences outcome of opportunistic infections. Cell 2013; 155:505–514. doi: 10.1016/j.cell.2013.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramer JR, Kowalkowski MA, Duan Z, Chiao EY. The effect of HIV viral control on the incidence of hepatocellular carcinoma in veterans with hepatitis C and HIV coinfection. J Acquir Immune Defic Syndr 2015; 68:456–462. doi: 10.1097/QAI.0000000000000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gjaerde LI, Shepherd L, Jablonowska E, Lazzarin A, Rougemont M, Darling K, et al. Trends in incidences and risk factors for hepatocellular carcinoma and other liver events in HIV and hepatitis C virus-coinfected individuals from 2001 to 2014: a multicohort study. Clin Infect Dis 2016; 63:821–829. doi: 10.1093/cid/ciw380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salmon D, Bani-Sadr F, Loko M, Stitou H, Gervais A, Durant J, et al. Insulin resistance is associated with a higher risk of hepatocellular carcinoma in cirrhotic HIV/HCV-co-infected patients: results from ANRS CO13 HEPAVIH. J Hepatol 2012; 56:862–868. doi: 10.1016/j.jhep.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Zhang FJ, Pan J, Yu L, Wen Y, Zhao Y. Current progress of China's free ART program. Cell Res 2005; 15:877–882. doi: 10.1038/sj.cr.7290362. [DOI] [PubMed] [Google Scholar]
- 17.Ma Y, Zhang F, Zhao Y, Zang C, Zhao D, Dou Z, et al. Cohort profile: the Chinese national free antiretroviral treatment cohort. Int J Epidemiol 2010; 39:973–979. doi: 10.1093/ije/dyp233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong GL, Chan HL, Mak CW, Lee SK, Ip ZM, Lam AT, et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology 2013; 58:1537–1547. doi: 10.1002/hep.26301. [DOI] [PubMed] [Google Scholar]
- 19.McGovern BH, Golan Y, Lopez M, Pratt D, Lawton A, Moore G, et al. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis 2007; 44:431–437. doi: 10.1086/509580. [DOI] [PubMed] [Google Scholar]
- 20.Clifford GM, Rickenbach M, Polesel J, Dal Maso L, Steffen I, Ledergerber B, et al. Influence of HIV-related immunodeficiency on the risk of hepatocellular carcinoma. AIDS 2008; 22:2135–2141. doi: 10.1097/QAD.0b013e32831103ad. [DOI] [PubMed] [Google Scholar]
- 21.Bruyand M, Dabis F, Vandenhende M, Lazaro E, Neau D, Leleux O, et al. HIV-induced immune deficiency is associated with a higher risk of hepatocarcinoma, ANRS CO3 Aquitaine Cohort, France, 1998–2008. J Hepatol 2011; 55:1058–1062. doi: 10.1016/j.jhep.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 22.Ioannou GN, Bryson CL, Weiss NS, Miller R, Scott JD, Boyko EJ. The prevalence of cirrhosis and hepatocellular carcinoma in patients with human immunodeficiency virus infection. Hepatology 2013; 57:249–257. doi: 10.1002/hep.25800. [DOI] [PubMed] [Google Scholar]
- 23.Bräu N, Salvatore M, Ríos-Bedoya CF, Fernández-Carbia A, Paronetto F, Rodríguez-Orengo JF, et al. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol 2006; 44:47–55. doi: 10.1016/j.jhep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Anderson JP, Tchetgen Tchetgen EJ, Lo Re V, Tate JP, Williams PL, Seage GR, et al. Antiretroviral therapy reduces the rate of hepatic decompensation among HIV- and hepatitis C virus-coinfected veterans. Clin Infect Dis 2014; 58:719–727. doi: 10.1093/cid/cit779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernard D, Ventresca MS, Marshall LA, Evelegh C, Wan Y, Bramson JL. Processing of tumor antigen differentially impacts the development of helper and effector CD4+ T-cell responses. Mol Ther 2010; 18:1224–1232. doi: 10.1038/mt.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovari H, Weber R. Influence of antiretroviral therapy on liver disease. Curr Opin HIV AIDS 2011; 6:272–277. doi: 10.1097/COH.0b013e3283473405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.