Abstract
Background:
Abnormal lipids are strong predictors of cardiovascular disease in type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM). However, the potential associations of insulin resistance (IR) and beta-cell function (BCF) with abnormal lipids in newly diagnosed T1DM or T2DM patients are not fully understood.
Methods:
A cross-sectional survey of 15,928 participants was conducted. Homeostasis model assessment and postprandial C-peptide levels were used to estimate IR and BCF. A restricted cubic spline (RCS) nested in binary logistic regression was used to examine the associations of IR and BCF with abnormal lipids.
Results:
High triglyceride (TG), low high-density lipoprotein cholesterol, and high low-density lipoprotein cholesterol (LDL-C) accounted for 49.7%, 47.8%, and 59.2% of the participants, respectively. In multivariable analysis, high IR was associated with an increased risk of high TGs (P for trend <0.001) in T1DM and is associated with an elevated risk of high TG and low HDL-C (all P for trend <0.01) in T2DM. Low BCF was not associated with risks of dyslipidemia in patients with T1DM or T2DM after adjustment for potential confounders.
Conclusion:
High IR had different associations with the risk of dyslipidemia in newly diagnosed T1DM and T2DM patients, suggesting that early treatment that improves IR may benefit abnormal lipid metabolism.
Keywords: Beta-cell function, Dyslipidemia, Insulin resistance, Type 1 diabetes, Type 2 diabetes
Introduction
Cardiovascular disease (CVD) is one of the most severe complications, and the leading cause of death in both type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM).[1,2] It has been established that some lipid components, such as high triglyceride (TG) and low high-density lipoprotein cholesterol (HDL-C), are risk factors for diabetes. Conversely, abnormal lipids are highly predictive of CVD in patients with diabetes.[3,4] Disorders in carbohydrate metabolism in diabetes can cause or worsen abnormal lipid metabolism in various ways. In both T1DM and T2DM, poor glycemic control can increase serum levels of TG and low-density lipoprotein cholesterol (LDL-C) and decrease levels of HDL-C.[5] Indeed, it is essential to understand the biological links between diabetes and lipid abnormalities to reduce the increasing burden of CVD in patients with diabetes.
Pathophysiologically, insulin resistance (IR) and decreased beta-cell function (BCF) are two major contributors to diabetes. Interactions between IR and pancreatic BCF play a key role in the pathogenesis of T1DM and T2DM.[6] T1DM primarily arises from BCF impairment, while T2DM results from IR along with inadequate BCF.[7] The decreased BCF and IR in diabetes may also play a key role in worsening lipid metabolism. Furthermore, insulin deficiency or IR can increase TG by reducing the suppression of TG lipolysis, thus, increasing fatty acids in the liver and decreasing HDL-C by reducing the inhibition of ApoA-I expression needed for HDL synthe-sis.[8] However, it remains unknown whether decreased BCF or increased IR contributes most to abnormal metabolism in different lipid components, i.e., high TG, low HDL-C, and high LDL-C.
Statin treatment was associated with a 37% reduction in major CVD events in individuals with T2DM,[9] but the residual risk of CVD remains substantially high. It is necessary to understand the associations of the two fundamental features of diabetes, decreased BCF and increased IR, for different abnormal lipid components to better control CVD risk factors. Therefore, we conducted a cross-sectional study in China and aimed to explore whether decreased BCF and increased IR in newly diagnosed T1DM or T2DM are associated with abnormal lipids, i.e., high TG, low HDL-C, and high LDL-C, with the use of restricted cubic spline (RCS) to detect these potential non-linear associations.
Methods
Ethical approval
This study was approved by the Ethics Committee of the Second Xiangya Hospital, Central South University in China (No. 2014032), and written consent was obtained from all participants.
Study design and population
From April 2015 to October 2017, we conducted a nationwide, multicenter, cross-sectional survey of 18,976 participants with newly diagnosed diabetes in China. In this survey, we invited 46 tertiary care hospitals across all seven geographic regions of China from 20 provinces and four municipalities to participate in this cross-sectional survey.
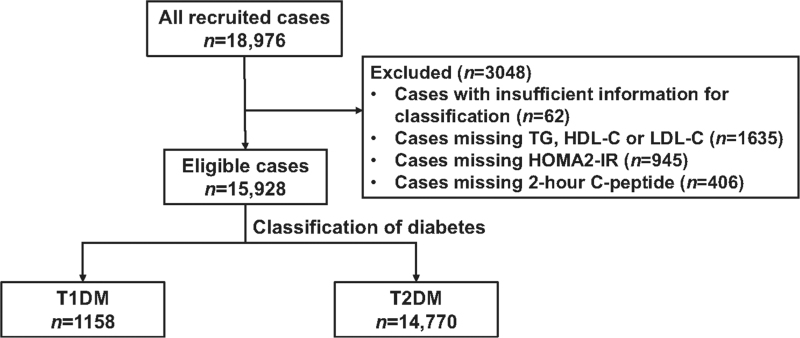
The inclusion criteria were set as: (1) diagnosis of diabetes based on the World Health Organization 1999 criteria; (2) age 18 years and older; (3) diabetes duration <1 year; (4) outpatients attending clinics in the Department of Endocrinology. Individuals were excluded if pregnant at the time of diabetes diagnosis, if they had gestational diabetes mellitus, or if they had co-existing acute diseases such as infection or acute myocardial infarction that could affect glucose metabolism. Specific types of diabetes due to other causes (e.g., monogenic diabetes), diseases of the exocrine pancreas (e.g., cystic fibrosis), and drug- or chemical-induced diabetes (e.g., in the treatment of human immunodeficiency virus/acquired immunodeficiency syndrome or after organ transplantation) were excluded as well. In addition, we excluded 62 cases with insufficient data for disease classification, 1635 cases missing lipid data, 945 cases missing homeostasis model assessment of insulin resistance (HOMA2-IR) data, and 406 cases missing 2-h prandial C-peptide data. The remaining 15,928 patients were included in this analysis [Figure 1].
Figure 1.
Flow diagram of the study patients. HDL-C: High-density lipoprotein cholesterol; HOMA2-B: Homeostasis model assessment of beta-cell function; HOMA2-IR: Homeostasis model assessment of insulin resistance; LDL-C: Low-density lipoprotein cholesterol; T1DM: Type 1 diabetes mellitus; T2DM: Type 2 diabetes mellitus; TG: Triglyceride.
Data collection procedures
Demographic characteristics (i.e., age and sex), clinical features, and lifestyle risk factors (i.e., exercise habits, diet, smoking, and alcohol consumption) were collected using a standard questionnaire administered by research nurses at each of the 46 participating hospitals. Physical activities were defined as engaging in exercise more than three times a week for at least 30 min a session. A diet habit was defined as engaging a healthy-eating plan which is naturally rich in nutrients and low in fat and calories. Current smoking was defined as either daily or occasional (less than daily) smoking. Alcohol consumption was defined as either daily or occasional (less than daily) drinking. The nurses used standardized procedures to measure height, weight, waist circumference, hip circumference, systolic blood pressure (SBP), and diastolic blood pressure. Drug use information was retrieved from case notes.
Laboratory assays
Fasting plasma glucose (FPG), total cholesterol, TGs, HDL-C, LDL-C, plasma hemoglobin A1c (HbA1c), and fasting C-peptide were directly assayed using standard methods at the study sites at the time of the patients’ visits. Postprandial blood samples were tested for 2-h postprandial plasma glucose (PPG) and C-peptide after a mixed meal. The core laboratory performed serum glutamic acid decarboxylase antibodies (GADA) assays via a standardized radioligand assay.[10] Serum samples for GADA assays from other hospitals were shipped on ice within 1 day and stored at –80°C in the core laboratory. The assay was assessed in the 2016 islet autoantibody standardization program (IASP 2016).
Classification of diabetes
The classification of T1DM and T2DM was based on clinical characteristics and diabetes-related biochemical measurement results, including fasting and 2-h PPG and C-peptide, lipids levels, HbA1c, and GADA serum levels. T1DM was diagnosed based on insulin-dependent diabetes, prone to ketoacidosis, or presence of GADA positivity. T1DM was further divided into classic T1DM and latent autoimmune diabetes in adults (LADA). Classic T1DM was defined according to the classification of diabetes by the American Diabetes Association (ADA) and World Health Organization and was diagnosed based on insulin-dependent diabetes, prone to ketoacidosis, or presence of GADA positivity. LADA was defined as GADA positivity in patients with non-insulin requiring diabetes for at least the first 6 months. T2DM was diagnosed as GADA-negative and insulin-independent patients.
Evaluation of IR and BCF
HOMA2-IR was estimated based on C peptide levels and plasma glucose using the HOMA calculator (https://www.dtu.ox.ac.uk/homacalculator/). BCF was based on 2-h postprandial C peptide levels.
Definition of dyslipidemia
As recommended by the ADA,[11] high TG was defined as TG >1.7 mmol/L, low HDL-C was defined as HDL-C <1.0 mmol/L (males) and <1.3 mmol/L (females), and high LDL-C was defined as LDL-C >2.6 mmol/L.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation or median (interquartile range) based on the evaluation of a normal distribution; categorical variables were given as a number (percent). For analysis of continuous variables, the Student t test or Mann–Whitney test was performed to compare differences between groups where appropriate. Frequency differences were compared using the chi-square test. R (version 4.0.2) and SPSS (version 26, IBM Corp, Armonk, NY, USA) were used to perform all the analyses. P < 0.05 was considered statistically significant.
Because there are no data to suggest that HOMA-IR and postprandial C-peptide were linearly associated with abnormal lipids in diabetes, RCS analyses nested in the multivariable logistic regression analyses were used to check full-range associations of HOMA2-IR and postprandial C-peptide with different dyslipidemia in T1DM and T2DM, respectively. We first performed univariable analysis and then multivariable analyses with adjustment for age, sex, current smoking, current drinking, body mass index (BMI, calculated as kg/m2), HbA1c, SBP, and current use of drugs including lipid-lowering treatment, oral anti-diabetic treatment, and insulin treatment to obtain full-range associations of HOMA2-IR and postprandial C-peptide with different dyslipidemia in T1DM and T2DM. In the RCS analysis, four knots were chosen because four knots were able to offer an adequate fit of the model and represent a good compromise between flexibility and the loss of precision caused by overfitting a small sample.[12] We identified threshold points of HOMA2-IR and postprandial C-peptide at the points, if any, where dyslipidemia risk started to rise or fall sharply, as in the previous investigations.[13]We further stratified HOMA2-IR and postprandial C-peptide at the identified cutoff points and repeated the univariable and multivariable analyses to obtain odds ratios (ORs) and 95% confidence intervals (CIs) of HOMA2-IR and postprandial C-peptide in categorical variables as stratified at these threshold points for high TG, low HDL-C, and high LDL-C among patients with T1DM and T2DM, respectively.
Results
Characteristics of the study participants
The mean age of the patients was 50.3 ± 13.3 years. Patients with T1DM were significantly younger, leaner, and had lower blood pressure, better lipid metabolic parameters; however, they had higher FPG and HbA1c levels than those with T2DM. Patients with T1DM were less insulin-resistant, less likely to undergo diet modification, be engaged in exercise, or need a lipid-lowering therapy than those with T2DM [Table 1].
Table 1.
Characteristics of the study participants with newly diagnosed diabetes.
| Characteristics | T1DM | T2DM | Statistics | P values |
| n | 1158 | 14,770 | ||
| Age (years) | 43.1 ± 14.8 | 50.8 ± 13.0 | –17.347∗ | <0.001 |
| Male, n (%) | 674 (58.2) | 8868 (60.0) | 1.508‡ | 0.231 |
| BMI (kg/m2) | 21.8 ± 3.7 | 24.8 ± 3.6 | –26.437∗ | <0.001 |
| FPG (mmol/L) | 9.4 ± 4.2 | 9.1 ± 3.5 | 2.591∗ | 0.002 |
| HbA1c (%) | 10.7 ± 3.2 | 9.4 ± 2.7 | 13.858∗ | <0.001 |
| SBP (mmHg) | 121.0 ± 15.8 | 127.8 ± 16.3 | –13.462∗ | <0.001 |
| DBP (mmHg) | 76.5 ± 10.7 | 80.2 ± 10.5 | –11.017∗ | <0.001 |
| Waist circumference (cm) | 81.3 ± 10.5 | 88.4 ± 10.6 | –21.278∗ | <0.001 |
| PPG (mmol/L) | 16.7 ± 6.6 | 15.3 ± 5.7 | 6.651∗ | <0.001 |
| TG (mmol/L) | 1.2 (0.8–1.8) | 1.7 (1.2–2.7) | 19.617† | <0.001 |
| TG >1.7 mmol/L, n (%) | 336 (29.0) | 7587 (51.3) | 214.603‡ | <0.001 |
| Total cholesterol (mmol/L) | 4.5 ± 1.3 | 4.8 ± 1.3 | –8.050∗ | <0.001 |
| LDL-C (mmol/L) | 2.7±1.0 | 2.9 ± 1.0 | –5.230∗ | <0.001 |
| LDL-C >2.6 mmol/L, n (%) | 582 (50.3) | 8846 (59.9) | 41.248‡ | <0.001 |
| HDL-C (mmol/L) | 1.2 (1.0–1.5) | 1.1 (0.9–1.3) | –6.699† | <0.001 |
| HDL-C <1.0 mmol/L, male, <1.3 mmol/L female, n (%) | 459 (39.6) | 7153 (48.4) | 32.266† | <0.001 |
| Family history of diabetes, n (%) | 275 (24.1) | 4233 (29.3) | 13.906‡ | <0.001 |
| Fasting C-peptide (nmol/L) | 200.0 (81.3–409.0) | 566.2 (363.3–803.0) | 33.587† | <0.001 |
| Postprandial C-peptide (nmol/L) | 423.5 (160.0–964.5) | 1435.2 (879.9–2243.1) | 34.357† | <0.001 |
| HOMA2-IR | 0.6 (0.2–1.2) | 1.5 (1.0–2.2) | 32.241† | <0.001 |
| Current smoking, n (%) | 350 (30.6) | 4382 (30.0) | 0.137‡ | 0.737 |
| Current drinking, n (%) | 159 (14.0) | 2594 (17.9) | 11.265‡ | <0.001 |
| Diet treatment, n (%) | 547 (54.3) | 6836 (62.3) | 24.829‡ | <0.001 |
| Physical activity, n (%) | 451 (44.8) | 5835 (53.2) | 26.031‡ | <0.001 |
| Lipid lowering drugs, n (%) | 66 (5.7) | 1611 (12.4) | 30.944‡ | <0.001 |
| Insulin treatment, n (%) | 291 (25.5) | 1159 (8.0) | 385.717‡ | <0.001 |
Data are presented as mean ± SD, median (interquartile range), or n (%). Comparisons were performed with Mann-Whitney test or t test for continuous variables depending on the normal distribution and the chi-square test for categorical variables. P value <0.05 was considered significant.
t values.
Z values.
χ2 values.
BMI: Body mass index; DBP: Diastolic blood pressure; FPG: Fasting plasma glucose; HbA1c: Hemoglobin A1c; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; PPG: Postprandial plasma glucose; SBP: Systolic blood pressure; SD: Standard deviation; T1DM: Type 1 diabetes mellitus; T2DM: Type 2 diabetes mellitus; TG: Triglyceride.
S- or U-shaped associations between HOMA2-IR or postprandial C-peptide and the risk of dyslipidemia in T1DM and T2DM
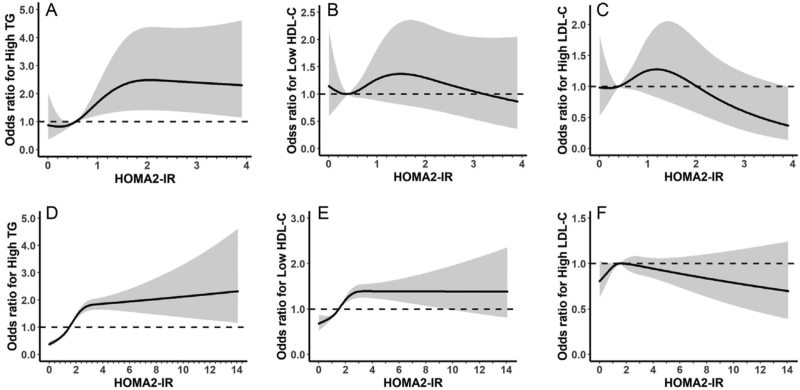
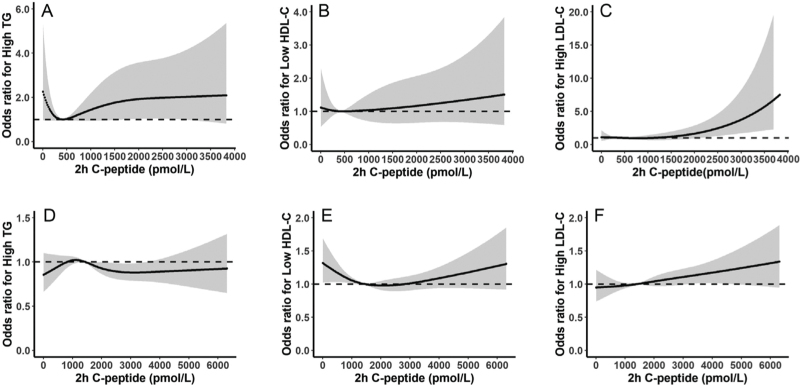
We modeled the associations of HOMA2-IR and postprandial C-peptide with the risk of dyslipidemia using RCS models in T1DM and T2DM after adjustment for age, sex, HOMA2-IR (or postprandial C-peptide where appropriate), current smoking, current drinking, BMI, HbA1c, SBP, lipid-lowering treatment, oral anti-diabetic treatment, and insulin treatment [Figures 2 and 3].
Figure 2.
Associations of HOMA2-IR with risk of dyslipidemia in T1DM (A-C) and T2DM (D-F). The curves and the gray region stand for the spline lines and 95% CIs for high TG (A, D), low HDL-C (B, E), and high LDL-C (C, F). High TG was defined as TG >1.7 mmol/L, low HDL-C was defined as HDL-C <1.0 mmol/L in males and 1.3 mmol/L in females, high LDL-C was defined as LDL-C >2.6 mmol/L. CIs: Confidence intervals; HOMA2-IR: Homeostasis model assessment of insulin resistance; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; T1DM: Type 1 diabetes mellitus; T2DM: Type 2 diabetes mellitus; TG: Triglyceride.
Figure 3.
Associations of prandial C-peptide with risk of dyslipidemia in T1DM (A–C) and T2DM (D–F). The curves and the gray region stand for the spline lines and 95% CIs for high TG (A, D), low HDL (B, E), and high LDL (C, F). High TG was defined as TG >1.7 mmol/L, low HDL-C was defined as HDL-C <1.0 mmol/L in males and 1.3 mmol/L in females, high LDL-C was defined as LDL-C >2.6 mmol/L. CIs: Confidence intervals; HDL-C: High-density lipoprotein cholesterol; HOMA2-B: Homeostasis model assessment of beta-cell function; LDL-C: Low-density lipoprotein cholesterol; T1DM: Type 1 diabetes mellitus; T2DM: Type 2 diabetes mellitus; TG: Triglyceride.
The associations of HOMA2-IR and the risks of dyslipidemia (high TG, low HDL-C, and high LDL-C) were S-shaped in T1DM with a positively linear association when HOMA2-IR was between 0.5 and 1.5 to 2.0 [Figure 2A–C]. However, in patients with T2DM, HOMA2-IR was positively associated with the risk of dyslipidemia when HOMA2-IR <2.0 and leveled off for the risk of high TG and low HDL-C and showed a negative association with the risk of high LDL-C when HOMA2-IR >2.0 [Figure 2D–F].
The associations of BCF (estimated by postprandial C-peptide) and the risk of dyslipidemia were different in T1DM compared with those in T2DM. In patients with T1DM, there was a U-shaped association between postprandial C-peptide and the risk of dyslipidemia with the highest/lowest risk related to postprandial C-peptide of about 500 to 1000 pmol/L [Figure 3]. In patients with T2DM, however, postprandial C-peptide was positively associated with the risk of high TG when <1000 pmol/L and then started to fall until about 2500 pmol/L [Figure 3]. There was a U-shaped association between postprandial C-peptide and the risk of low HDL-C with the lowest risk related to postprandial C-peptide of 2000 pmol/L. Postprandial C-peptide was positively associated with the risk of high LDL-C in patients with T2DM [Figure 3].
From above, the risk of abnormal lipid metabolism started to change steeply mainly from 1 to 2 of HOMA2-IR and at 1000 pmol/L of postprandial C-peptide, therefore, we selected these values as threshold points for further logistic regression analysis.
Associations between HOMA2-IR and the risk of dyslipidemia in T1DM and T2DM
The risks of dyslipidemia (high TG, low HDL-C, and high LDL-C) associated with HOMA2-IR were estimated by both univariate logistic regression and multivariable logistic regression with adjustments for age, sex, BCF categories, current smoking, current drinking, BMI, HbA1c, SBP, lipid-lowering treatment, oral anti-diabetic treatment, and insulin treatment in T1DM and T2DM [Table 2]. In patients with T1DM, high HOMA2-IR was associated with an elevated risk of high TG (P for trend <0.001), and low HDL-C (P for trend = 0.005) in univariable regression models; however, it was only associated with an increased risk of high TG (ORs of HOMA2-IR ≥2, ≥1–<2 vs. <1: 4.20, 95% CI 2.26–7.90; 2.05, 95% CI 1.33–3.13, P for trend <0.001) after adjustment for potential confounders [Table 2]. When T1DM was further divided into classic T1DM and LADA, high HOMA2-IR was only associated with an elevated risk of high TG in patients with classic T1DM (P for trend <0.05) and LADA (P for trend <0.001) after adjustment for potential confounders [Supplementary Table 1]. In patients with T2DM, high HOMA2-IR was associated with an elevated risk of high TG, low HDL-C, and high LDL-C (all P for trend ≤0.001) in univariable regression models but was only associated with an increased risk of high TG (ORs of HOMA2-IR ≥2, ≥1−<2 vs. <1: 2.56, 95% CI: 2.26–2.91; 1.61, 95% CI 1.45–1.79, P for trend <0.001) and low HDL-C (ORs of HOMA2-IR ≥2, ≥1−<2 vs. <1: 1.64, 95% CI 1.45–1.86; 1.26, 95% CI 1.13–1.40, P for trend <0.001) after adjustment for potential confounders. HOMA-IR was not associated with high LDL-C in patients with T1DM or T2DM after adjustment for potential confounders [Table 2].
Table 2.
OR of IR for abnormal lipid profile in T1DM and T2DM.
| High TG | Low HDL-C | High LDL-C | |||||||
| IR | n (%) | OR (95% CI) | P values | n (%) | OR (95% CI) | P values | n (%) | OR (95% CI) | P values |
| Univariable | |||||||||
| T1DM | <0.001∗ | 0.005∗ | 0.166∗ | ||||||
| High (≥2) | 67 (62.6) | 5.98 (3.61–10.02) | <0.001 | 55 (51.4) | 1.89 (1.17–3.07) | 0.010 | 66 (61.7) | 1.41 (0.87–2.30) | 0.168 |
| Median (≥1−<2) | 93 (38.0) | 2.19 (1.54–3.11) | <0.001 | 108 (44.1) | 1.39 (1.00–1.93) | 0.052 | 133 (54.3) | 1.14 (0.82–1.58) | 0.444 |
| Low (<1) | 176 (21.8) | Ref | 296 (36.7) | Ref | 383 (47.5) | Ref | |||
| T2DM | <0.001∗ | <0.001∗ | 0.001∗ | ||||||
| High (≥2) | 2913 (65.6) | 3.32 (3.01–3.67) | <0.001 | 2509 (56.5) | 1.84 (1.67–2.02) | <0.001 | 2735 (61.6) | 1.22 (1.11–1.35) | <0.001 |
| Median (≥1−<2) | 3233 (50.0) | 1.73 (1.59–1.89) | <0.001 | 3017 (46.7) | 1.23 (1.13–1.34) | <0.001 | 3858 (59.7) | 1.11 (1.02–1.21) | 0.016 |
| Low (<1) | 1441 (37.3) | Ref | 1627 (42.1) | Ref | 2253 (58.3) | Ref | |||
| Multivariable | |||||||||
| T1DM | <0.001∗ | 0.238∗ | 0.987∗ | ||||||
| High (≥2) | 67 (62.6) | 4.20 (2.26–7.90) | <0.001 | 55 (51.4) | 1.29 (0.71 –2.36) | 0.401 | 66 (61.7) | 0.92 (0.51–1.67) | 0.790 |
| Median (≥1−<2) | 93 (38.0) | 2.05 (1.33–3.13) | <0.001 | 108 (44.1) | 1.32 (0.88 –1.97) | 0.176 | 133 (54.3) | 1.09 (0.74–1.62) | 0.649 |
| Low (<1) | 176 (21.8) | Ref | 296 (36.7) | Ref | 383 (47.5) | Ref | |||
| T2DM | <0.001∗ | <0.001∗ | 0.275∗ | ||||||
| High (≥2) | 2913 (65.6) | 2.56 (2.26–2.91) | <0.001 | 2509 (56.5) | 1.64 (1.45–1.86) | <0.001 | 2735 (61.6) | 1.07 (0.95–1.21) | 0.277 |
| Median (>1−<2) | 3233 (50.0) | 1.61 (1.45–1.79) | <0.001 | 3017 (46.7) | 1.26 (1.13–1.40) | <0.001 | 3858 (59.7) | 1.03 (0.93–1.15) | 0.558 |
| Low (<1) | 1441 (37.3) | Ref | 1627 (42.1) | Ref | 2253 (58.3) | Ref | |||
The univariate model was adjusted for BCF categories; the multivariate model was adjusted for age, sex, BCF categories, current smoking status, current drinking status, BMI, HbA1c, SBP, use of lipid lower drugs, use of oral antidiabetic drugs, and insulin treatment. P value <0.05 was considered significant.
P for trend.
BCF: Beta-cell function; BMI: Body mass index; CI: Confidence interval; HbA1c: Hemoglobin A1c; HDL-C: High-density lipoprotein cholesterol; IR: Insulin resistance; LDL-C: Low-density lipoprotein cholesterol; n: study number; OR: Odds ratio; SBP: Systolic blood pressure; T1DM: Type 1 diabetes mellitus; T2DM: Type 2 diabetes mellitus; TG: Triglyceride.
Associations between postprandial C-peptide and risk of dyslipidemia in T1DM and T2DM
The risks of dyslipidemia (high TG, low HDL-C, and high LDL-C) associated with postprandial C-peptide were estimated by both univariate logistic regression and multivariable logistic regression with adjustments for age, sex, IR categories, current smoking, current drinking, BMI, HbA1c, SBP, lipid-lowering treatment, oral anti-diabetic treatment, and insulin treatment in T1DM and T2DM [Table 3]. In patients with T1DM, low postprandial C-peptide was not associated with the risks of dyslipidemia in both the univariable analysis and the multivariable analysis (P > 0.05). When T1DM was further divided into classic T1DM and LADA, low postprandial C-peptide was not associated with the risks of dyslipidemia in patients with classic T1DM or LADA after adjustment for potential confounders [Supplementary Table 2]. While in patients with T2DM, low postprandial C-peptide, i.e., <1000 pmol/L vs. ≥1000 pmol/L, was associated with an increased risk of high LDL-C in univariable analysis (OR: 1.14, 95% CI 1.05–1.23, P = 0.001) but not in multivariable analysis. Postprandial C-peptide was not associated with high TG and low HDL-C in patients with T2DM after adjustment for potential confounders [Table 3].
Table 3.
OR of postprandial C-peptide for abnormal lipid profile in T1DM and T2DM.
| High TG | Low HDL-C | High LDL-C | |||||||
| Postprandial C-peptide (pmol/L) | n (%) | OR (95% CI) | P values | n (%) | OR (95% CI) | P values | n (%) | OR (95% CI) | P values |
| Univariable | |||||||||
| T1DM | |||||||||
| High (≥1000) | 116 (42.5) | Ref | 121 (44.3) | Ref | 161 (59.0) | Ref | |||
| Low (<1000) | 220 (24.9) | 1.00 (0.69–1.46) | 0.983 | 338 (38.2) | 1.05 (0.74–1.49) | 0.787 | 421 (47.6) | 0.73 (0.51–1.03) | 0.072 |
| T2DM | |||||||||
| High (≥1000) | 5506 (53.8) | Ref | 5064 (49.4) | Ref | 6088 (59.4) | Ref | |||
| Low (<1000) | 2081 (46.0) | 1.07 (0.99–1.16) | 0.074 | 2089 (46.1) | 1.06 (0.98–1.15) | 0.146 | 2758 (60.9) | 1.14 (1.05–1.23) | 0.001 |
| Multivariable | |||||||||
| T1DM | |||||||||
| High (≥1000) | 116 (42.5) | Ref | 121 (44.3) | Ref | 161 (59.0) | Ref | |||
| Low (<1000) | 220 (24.9) | 0.90 (0.56–1.46) | 0.676 | 338 (38.2) | 1.01 (0.65–1.57) | 0.974 | 421 (47.6) | 0.73 (0.47–1.11) | 0.141 |
| T2DM | |||||||||
| High (≥1000) | 5506 (53.8) | Ref | 5064 (49.4) | Ref | 6088 (59.4) | Ref | |||
| Low (<1000) | 2081 (46.0) | 0.91 (0.81–1.01) | 0.069 | 2089 (46.1) | 1.09 (0.98–1.21) | 0.118 | 2758 (60.9) | 0.91 (0.82–1.01) | 0.078 |
The univariate model was adjusted for IR categories; the multivariate model was adjusted for age, sex, IR categories, current smoking status, current drinking status, BMI, HbA1c, SBP, use of lipid lower drugs, use of oral antidiabetic drugs, and insulin treatment. BMI: Body mass index; CI: Confidence interval; HbA1c: Hemoglobin A1c; HDL-C: High-density lipoprotein cholesterol; IR: Insulin resistance; LDL-C: Low-density lipoprotein cholesterol; OR: Odds ratio; SBP: Systolic blood pressure; T1DM: Type 1 diabetes mellitus; T2DM: Type 2 diabetes mellitus; TG: Triglyceride.
Discussion
In this study, we found that high IR as estimated by HOMA-IR had different associations with the risk of dyslipidemia in patients with newly-onset T1DM and T2DM. In T1DM, high IR was associated with the risk of high TG while in T2DM, high IR was associated with increased risks of high TG and low HDL-C in multivari-able analysis.
Studies in different populations reported consistent findings regarding the associations between IR and dyslipidemia. High TG and low HDL-C were associated with an increased IR in Chinese elderly patients with newly-onset diabetes[14] and Japanese non-obese patients with T2DM.[15] The Coronary Artery Calcification in the Type 1 Diabetes Study found that high TG but not HDL-C or LDL-C was inversely associated with glucose infusion in American adult patients with T1DM.[16] The first study only included elderly patients and the latter two cohorts had an 8-year and 23-year duration of diabetes. Our study confirmed the above findings using a large representative sample of patients with newly diagnosed diabetes.
The associations of IR with high TG and low HDL-C are biologically plausible. Lipid abnormalities associated with IR are very likely to be initiated by the resistance of adipocytes to insulin. Insulin-resistant fat cells lead to increased hydrolysis of TGs and release of fatty acids to the liver.[17] This can increase TG synthesis in the liver and stimulate the assembly and secretion of very-low-density lipoprotein (VLDL), the main transporter of fasting TG and is a major contributor to hypertriglyceridemia.[18] Decreased degradation of apolipoprotein B, the predominant surface protein of VLDL, was seen within the IR states resulting from increased free fatty acids, thus causing an overproduction of VLDL.[19] An increase in TG-rich lipoproteins is often associated with an increase in small dense LDL and a decrease in HDL levels. Hypertriglycer-idemia stimulates the transfer of TG-rich lipoproteins to HDL and LDL in exchange for cholesteryl esters,[20] leading to an increased HDL and LDL TG content. Furthermore, the TG content is then converted to small dense LDL and small HDL. The expression of Apo-I, which can dissociate from TG-rich HDL, is decreased in patients with diabetes or IR states, leading to a reduction in HDL levels.[17]
In this study, we did not detect the associations between BCF and the risks of dyslipidemia in patients with T1DM or T2DM. A previous study has shown that log (TG)/HDL-C was associated with BCF in patients with T2DM, but these patients had a long disease duration of 14(9) years.[21] Moreover, Dullaart et al[22] found that bCf was not significantly correlated with HDL-C in patients with well-controlled T2DM but was significantly correlated with HDL functional biomarkers.
A biological link between low BCF and abnormal lipid metabolism is also plausible. Excess exposure of beta-cells to free fatty acids can decrease beta-cell secretory function and cause cellular death.[23] Moreover, insulin plays a central role in the regulation of lipid metabolism. Insulin inhibits lipolysis in the adipose tissue resulting in reduced circulating free fatty acids. Insulin also inhibits VLDL production and promotes the catabolism of TG-rich lipoproteins by activating lipoprotein lipase.[24] Thus, a relative insulin deficiency could increase VLDL production resulting in hypertriglyceridemia. Insulin also stimulates the clearance of LDL by increasing LDL receptor expression and activity.[25] However, we did not observe the significant associations between BCF and risk of dyslipidemia in those with T1DM or T2DM. Possible explanations could be: (1) T2DM is primarily characterized by IR instead of BCF, the prevalence of BCF in T2DM is low. (2) The prevalence of dyslipidemia in T1DM is relatively low. Lipid abnormalities in T1DM are more frequent in those with poor glycemic control,[26] which is observed in several studies.[27–29] The lipid profile is similar in T1DM patients with good glycemic control and within the general population.[3] (3) An earlier study showed that insulin therapy might resolve lipid abnormalities in 24 h in
T1DM patients with diabetic ketoacidosis, by increasing TG-rich lipoprotein catabolism.[30] This finding may suggest that dyslipidemia affected by insulin insufficiency can be rapidly resolved by insulin treatment in T1DM.
Our study has clinical significance. We found that high IR is associated with an increased risk of high TG in T1DM. Evidence also showed that anti-diabetic drugs like glucagon-like peptide-1 receptor agonists (exenatide), sodium-glucose cotransporter 2 inhibitors, and metformin combined with insulin treatment have some beneficial effects in T1DM, such as contributing to weight loss or reducing insulin requirements.[31–34] These findings suggest that such anti-diabetic drugs combined with insulin therapy may potentially benefit lipid metabolism by increasing insulin sensitivity and improving CVD outcomes in patients with T1DM, especially for those with obesity and IR.
Our study has some limitations. First, our study was a cross-sectional survey, and causality cannot be established. It is also possible that some of the associations between high IR with high TG and low HDL-C had reverse causal relationships. Second, the use of drugs, especially, lipid-lowering drugs may have major confounding effects on our conclusions. Although information regarding the use of these drugs was documented and we made careful adjustments for use of those drugs. The adjustment cannot completely remove all of their confounding effects and residual confounding effects may be significant. Third, newly-onset diagnosed patients may have BCF inhibition due to high glucose levels, resulting in lower serum C-peptide and HOMA2-IR in those with poor glycemic control; thus, leading to inaccurate estimations of these associations in the study.
To conclude, in patients with newly diagnosed diabetes, IR had different associations with risk of dyslipidemia in T1DM and T2DM, supporting early use of anti-diabetic therapies that improve IR because it may have beneficial effects for lipid metabolism and therefore, reduced risk of CVD in the future.
Acknowledgements
Author would like to thank all of the patients and investigators involved at the 46 participating centers of the National Clinical Research Center for Metabolic Diseases. The members of National Clinical Research Center for Metabolic Diseases (investigators and hospitals); Linong Ji, Xueyao Han, Ling Chen, Xiaoling Cai, Peking University People Hospital; Lixin Guo, Xiaofan Jia, Shan Ding, Beijing Hospital; Xinhua Xiao, Cuijuan Qi, Xiaojing Wang, Peking Union Medical College Hospital; Zhongyan Shan, Yaxin Lai, Zhuo Zhang, The First Hospital of China Medical University; Yu Liu, Yan Cheng, Hanqing Cai, The Second Hospital of Jilin University; Yadong Sun, Yan Ma, Haiying Wang, People Hospital of Jilin Province; Yiming Li, Chaoyun Zhang, Shuo Zhang, Hua Shan Hospital, Fudan University; Tao Yang, Hao Dai, Mei Zhang, The First Affiliated Hospital with Nanjing Medical University; Liyong Yang, Peiwen Wu, Xiaofang Yan, The First Affiliated Hospital of Fujian Medical University; Yangang Wang, Fang Wang, Hong Chen, The Affiliated Hospital of Qingdao University; Qifu Li, Rong Li, The First Affiliated Hospital of Chongqing Medical University; Qiuhe Ji, Li Wang, Xiangyang Liu, Xijing Hospital, Fourth Military Medical University; Jing Liu, Suhong Wei, Gansu Provincial Hospital; Yun Zhu, Rui Ma, The First Affiliated Hospital of Xinjiang Medical University; Gebo Wen, Xinhua Xiao, Jianping Qin, The First Affiliated Hospital of University of South China; Jian Kuang, Yan Lin, Guangdong General Hospital; Shaoda Lin, Kun Lin, the First Affiliated Hospital of Shantou University Medical College; Li Li, Heji Hospital Affiliated to Changzhi Medical College; Gan Huang, Shuoming Luo, The Second Xiangya Hospital of Central South University; Huibiao Quan, Leweihua Lin, Hainan General Hospital; Hongyu Kuang, Weihua Wu, The First Affiliated Hospital of Harbin Medical University; Yuling He, The First Affiliated Hospital of Guangxi Medical University; Xiaoyan Chen, Yuyu Tan, The First Affiliated Hospital of Guangzhou Medical University; Ling He, Guangzhou First People Hospital; Chao Zheng, The Second Affiliated Hospital of Wenzhou Medical University; Jianying Liu, Zhifang Yang, The First Affiliated Hospital of Nanchang University; Xiaoyang Lai, The Second Affiliated Hospital of Nan-chang University; Ling Hu, Yan Zhu, Ying Hu, The Third Affiliated Hospital of Nanchang University; Xuqing Li, Henan Provincial People Hospital; Hong Li, Yushan Xu, The First Affiliated Hospital of Kunming Medical University; Yang Ou, The First People Hospital of Yunnan Province; Jianping Wang, The Second Hospital University of South China; Changqing Luo, Xiaoyue Wang, The First People Hospital of YueYang; Zhiming Deng, Shenglian Gan, The First People Hospital of Changde City; Zhaohui Mo, Ping Jin, Honghui He, The Third Xiangya Hospital of Central South University; Qiuxia Huang, Dongguan People Hospital; Fang Wang, Heping Hospital Affiliated to Changzhi Medical College; Yi Zhang, Zhenzhen Hong, First Hospital of Quanzhou Affiliated to Fujian Medical University; Yuezhong Ren, Pengfei Shan, The Second Affiliated Hospital of Zhejiang University School of Medicine; Caifeng Yan, Hui Zhang, Northern Jiangsu People Hospital; Zhiwen Liu, Shanghai Xuhui District Central Hospital; Meibiao Zhang, The First People Hospital of Huaihua; Ming Liu, Heting Wang, Tianjin Medical University General Hospital; Liujun Fu, The First Affiliated Hospital of the Henan University of Science and Technology; Hui Fang, Tangshan Gongren Hospital; Hui Sun, The Affiliated Hospital of Inner Mongolia Medical University.
Funding
This work was supported by grants from the National Science and Technology Infrastructure Program (Nos. 2013BAI09B12, 2015BAI12B13) and the National Key R&D Program of China (Nos. 2016YFC1305000, 2017YFC1309604).
Supplementary Material
Footnotes
How to cite this article: Tang X, Yan X, Zhou H, Huang G, Niu X, Jiang H, Su H, Yang X, Li X, Zhou Z. Associations of insulin resistance and beta-cell function with abnormal lipid profile in newly diagnosed diabetes. Chin Med J 2022;135:2554–2562. doi: 10.1097/CM9.0000000000002075
Supplemental digital content is available for this article.
References
- 1.Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia 2001; 44: (Suppl 2): S14–S21. doi: 10.1007/pl00002934. [DOI] [PubMed] [Google Scholar]
- 2.Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawren-son RA, Colhoun HM. All-cause mortality rates in patients with type 1 diabetes mellitus compared with a non-diabetic population from the UK general practice research database, 1992–1999. Diabetologia 2006; 49:660–666. doi: 10.1007/s00125-005-0120-4. [DOI] [PubMed] [Google Scholar]
- 3.de Ferranti SD, de Boer IH, Fonseca V, Fox CS, Golden SH, Lavie CJ, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Circulation 2014; 130:1110–1130. doi: 10.1161/CIR.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 4.Martin-Timon I, Sevillano-Collantes C, Segura-Galindo A, Del Canizo-Gomez FJ. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes 2014; 5:444–470. doi: 10.4239/wjd.v5.i4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahn SE. Clinical review 135: the importance of beta-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab 2001; 86:4047–4058. doi: 10.1210/jcem.86.9.7713. [DOI] [PubMed] [Google Scholar]
- 6.Vladu M, Clenciu D, Efrem IC, Fortofoiu MC, Amzolini A, Micu ST, et al. Insulin resistance and chronic kidney disease in patients with type 1 diabetes mellitus. J Nutr Metab 2017; 2017:6425359.doi: 10.1155/2017/6425359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein BJ. Insulin resistance as the core defect in type 2 diabetes mellitus. Am J Cardiol 2002; 90:3G–10G. doi: 10.1016/s0002-9149 (02)02553-5. [DOI] [PubMed] [Google Scholar]
- 8.Feingold KR. Dyslipidemia in diabetes. In: Endotext. South Dartmouth MDText.com, Inc, 2000. doi: 10.1016/s0002-9149(02) 02553-5. [Google Scholar]
- 9.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696. doi: 10.1016/S0140-6736 (04)16895-5. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Z, Xiang Y, Ji L, Jia W, Ning G, Huang G, et al. Frequency, immunogenetics, and clinical characteristics of latent autoimmune diabetes in China (LADA China study): a nationwide, multicenter, clinic-based cross-sectional study. Diabetes 2013; 62:543–550. doi: 10.2337/db12-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998; 21:2191–2192. doi: 10.2337/diacare.21.12. 2191. [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association. Standards of medical care in diabetes - 2013. Diabetes Care 2013; 36: (Suppl 1): S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Huo X, Cao YF, Li SN, Du Z, Shao P, et al. Bile acid metabolites in early pregnancy and risk of gestational diabetes in Chinese women: a nested case-control study. EBioMedicine 2018; 35:317–324. doi: 10.1016/j.ebiom.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pang SJ, Man QQ, Song S, Song PK, Liu Z, Li YQ, et al. Association of lipid parameters with insulin resistance in different glycemic among the elderly population (in Chinese). Chin J Prevent Med 2018; 52:629–635. doi:10.3760/cma.j.issn.0253-9624.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Taniguchi A, Nakai Y, Sakai M, Yoshii S, Hamanaka D, Hatae Y, et al. Relationship of regional adiposity to insulin resistance and serum triglyceride levels in nonobese Japanese type 2 diabetic patients. Metabolism 2002; 51:544–548. doi: 10.1053/meta.2002. 31984. [DOI] [PubMed] [Google Scholar]
- 16.Maahs DM, Nadeau K, Snell-Bergeon JK, Schauer I, Bergman B, West NA, et al. Association of insulin sensitivity to lipids across the lifespan in people with Type 1 diabetes. Diabet Med 2011; 28:148–155. doi: 10.1111/j.1464-5491.2010.03143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest 2000; 106:453–458. doi: 10.1172/JCI10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adiels M, Olofsson SO, Taskinen MR, Boren J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol 2008; 28:1225–1236. doi: 10.1161/ATVBAHA.107.160192. [DOI] [PubMed] [Google Scholar]
- 19.Arca M, Pigna G, Favoccia C. Mechanisms of diabetic dyslipidemia: relevance for atherogenesis. Curr Vasc Pharmacol 2012; 10:684–686. doi: 10.2174/157016112803520864. [DOI] [PubMed] [Google Scholar]
- 20.Guerin M, Le Goff W, Lassel TS, Van Tol A, Steiner G, Chapman MJ. Atherogenic role of elevated CE transfer from HDL to VLDL(1) and dense LDL in type 2 diabetes: impact of the degree of triglyceridemia. Arterioscler Thromb Vasc Biol 2001; 21:282–288. doi: 10.1161/01. atv.21.2.282. [DOI] [PubMed] [Google Scholar]
- 21.Hermans MP, Ahn SA, Rousseau MF. log(TG)/HDL-C is related to both residual cardiometabolic risk and beta-cell function loss in type 2 diabetes males. Cardiovasc Diabetol 2010; 9:88.doi: 10.1186/1475-2840-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dullaart RP, Annema W, de Boer JF, Tietge UJ. Pancreatic beta-cell function relates positively to HDL functionality in well-controlled type 2 diabetes mellitus. Atherosclerosis 2012; 222:567–573. doi: 10.1016/j.atherosclerosis.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 23.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev 2008; 29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verges B. New insight into the pathophysiology of lipid abnormalities in type 2 diabetes. Diabetes Metab 2005; 31:429–439. doi: 10.1016/s1262-3636(07)70213-6. [DOI] [PubMed] [Google Scholar]
- 25.Chait A, Bierman EL, Albers JJ. Low-density lipoprotein receptor activity in cultured human skin fibroblasts. Mechanism of insulin-induced stimulation. J Clin Invest 1979; 64:1309–1319. doi: 10.1172/JCI109587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dullaart RP. Plasma lipoprotein abnormalities in type 1 (insulin-dependent) diabetes mellitus. Neth J Med 1995; 46:44–54. doi: 10.1016/0300-2977(94)00048-e. [DOI] [PubMed] [Google Scholar]
- 27.The DCCT Research Group. Lipid and lipoprotein levels in patients with IDDM diabetes control and complication trial experience. Diabetes Care 1992; 15:886–894. doi: 10.2337/diac-are.15.7.886. [DOI] [PubMed] [Google Scholar]
- 28.Marcovecchio ML, Dalton RN, Prevost AT, Acerini CL, Barrett TG, Cooper JD, et al. Prevalence of abnormal lipid profiles and the relationship with the development of microalbuminuria in adolescents with type 1 diabetes. Diabetes Care 2009; 32:658–663. doi: 10.2337/dc08-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guy J, Ogden L, Wadwa RP, Hamman RF, Mayer-Davis EJ, Liese AD, et al. Lipid and lipoprotein profiles in youth with and without type 1 diabetes: the SEARCH for Diabetes in Youth case-control study. Diabetes Care 2009; 32:416–420. doi: 10.2337/dc08-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weidman SW, Ragland JB, Fisher JN, Jr, Kitabchi AE, Sabesin SM. Effects of insulin on plasma lipoproteins in diabetic ketoacidosis: evidence for a change in high density lipoprotein composition during treatment. J Lipid Res 1982; 23:171–182. [PubMed] [Google Scholar]
- 31.Guyton J, Jeon M, Brooks A. Glucagon-like peptide 1 receptor agonists in type 1 diabetes mellitus. Am J Health Syst Pharm 2019; 76:1739–1748. doi: 10.1093/ajhp/zxz179. [DOI] [PubMed] [Google Scholar]
- 32.Buse JB, Garg SK, Rosenstock J, Bailey TS, Banks P, Bode BW, et al. Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes: the North American inTandem1 Study. Diabetes Care 2018; 41:1970–1980. doi: 10.2337/dc18-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Danne T, Cariou B, Banks P, Brandle M, Brath H, Franek E, et al. HbA1c and hypoglycemia reductions at 24 and 52 weeks with sotagliflozin in combination with insulin in adults with type 1 diabetes: the European inTandem2 Study. Diabetes Care 2018; 41:1981–1990. doi: 10.2337/dc18-0342. [DOI] [PubMed] [Google Scholar]
- 34.Petrie JR, Chaturvedi N, Ford I, Brouwers M, Greenlaw N, Tillin T, et al. Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 2017; 5:597–609. doi: 10.1016/S2213-8587(17)30194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.