Abstract
N-linked glycans are structurally diverse polysaccharides that represent significant biological relevance due to their involvement in disease progression and cancer. Due to their complex nature, N-linked glycans pose many analytical challenges requiring the continued development of analytical technologies. Infrared matrix-assisted laser desorption electrospray ionization (IR-MALDESI) is a hybrid ionization technique commonly used for mass spectrometry imaging (MSI) applications. Previous work demonstrated IR-MALDESI to significantly preserve sialic acid containing N-linked glycans that otherwise require chemical derivatization prior to detection. Here we demonstrate the first analysis of N-linked glycans in situ by IR-MALDESI MSI. Formalin-fixed paraffin-embedded (FFPE) human prostate tissue was analyzed in negative ionization mode after tissue washing, antigen retrieval, and pneumatic application of PNGase F for enzymatic digestion of N-linked glycans. 53 N-linked glycans were confidently identified in the prostate sample where more than 60% contained sialic acid residues. This work demonstrates the first steps in N-linked glycan imaging of biological tissues by IR-MALDESI MSI. Raw data files are available in MassIVE (identifier: MSV000088414).
Keywords: IR-MALDESI, N-Linked Glycans, Mass Spectrometry Imaging, Prostate Cancer
Graphical Abstract

INTRODUCTION
Glycosylation represents one of the most common post-translational modifications in humans, wherein a glycan moiety is enzymatically added to a protein and mediates its function in tumor progress and signal transduction.1 N-linked glycans are polysaccharides covalently bound to asparagine residues on proteins containing an N-X-T/S motif where X is any amino acid except proline. The structural diversity of N-linked glycans (isomeric monosaccharides, ring conformations, linkages, and protein localization) contribute to the analytical challenges to investigate the biological importance of N-linked glycans. Additionally, N-linked glycans containing N-acetylneuraminic acid (sialic acid) face additional challenges due to the lability and ionization preference of the monosaccharide. These challenges demand for improved analytical techniques addressing these unique difficulties to confidently investigate the biological role of N-linked glycans in various disease states.
Common workflows for analysis of N-linked glycans utilize liquid chromatography-mass spectrometry where sample preparation requires homogenization of tissue samples resulting in the loss of any molecular spatial specificity.2,3 Mass spectrometry imaging (MSI) methodologies allow the visualization of molecular spatial distributions across tissue types which is important in understanding their biological importance and biological role in tumor regions within a tissue sample. Thus far, successful MSI of N-linked glycans has primarily used matrix-assisted laser desorption ionization (MALDI).4–6 While significant progress has been made for N-linked glycan imaging, previous research indicates that MALDI results in fewer sialic acid species compared to electrospray ionization (ESI) due to internal energy deposited by MALDI matrices during proton transfer.7 Chemical derivatization methods have been developed to overcome this challenge, requiring more time and resources involved in sample preperation.8 Additionally, sialic acid contains a negative charge due to the presence of a carboxylic acid indicating a preference for negative ionization mode. Negative mode analyses are less sensitive in MALDI measurements and thus, glycan imaging has primarily operated in positive ionization mode.9,10 Therefore, an ESI-like ionization method could be useful for N-linked glycan imaging without the need of additional chemical derivatization methods during sample preparation.
Infrared matrix-assisted laser desorption electrospray ionization (IR-MALDESI), commonly used for MSI applications, utilizes a mid-IR laser to resonantly excite water found in biological samples and desorbs neutral species into the gas-phase. Neutral species are then post-ionized by an orthogonal electrospray plume in an ESI-like fashion. By combination of both MALDI and ESI characteristics, IR-MALDESI achieves advantages from both soft ionization sources such as spatial correlation from MALDI and ionization characteristics from ESI. IR-MALDESI interfaced with Orbitrap technology yields high mass measurement accuracy (MMA) and high resolving power critical for confident detection of biomolecules with untargeted MSI datasets. IR-MALDESI has established its utility for sampling a variety of complex sample types such as bones and zebrafish for analysis of metabolites and lipids.11–14 Recently, we demonstrated the use of IR-MALDESI in the direct analysis of native N-linked glycans enzymatically cleaved from glycoproteins without any use of chemical derivatization.15
In this work, we demonstrate the first IR-MALDESI MSI analysis of N-linked glycans. Formalin-fixed paraffin-embedded (FFPE) human prostate was chosen for analysis due to its high abundance of N-linked glycans in the tissue as well as their biological importance in prostate cancer.16–18 Previously established sample preparation methods were utilized to prepare prostate samples for the enzymatic digestion of N-linked glycans prior to analysis by IR-MALDESI.19 This proof-of-concept study demonstrates the utility of IR-MALDESI for N-linked glycan imaging, laying the ground work for future studies.
METHODS
FFPE human prostate cancer tissue (Gleason Score 6) was obtained from MUSC Hollings Cancer Center Biorepository, under a MUSC Institutional Review Board for Human Research approved protocol (Pro 17669). The FFPE human prostate sample was prepared for analysis using previously published methods which are summarized below.19 All solvents and materials were purchased from Fisher Scientific (Hampton, NH, USA) unless otherwise specified.
Dewaxing/Delipidation
The FFPE human prostate tissue section was heated at 60°C for 1 hour to melt the paraffin residue around the tissue section. After heating, the prostate section was cooled to room temperature on the benchtop for approximately 5 minutes. The prostate tissue was rinsed with 8 washes in Coplin jars to remove salts and lipids from the prostate tissue.19,20 Tissue washes were composed of histology-grade xylenes (×2, 3 min each), 200 proof ethanol (×2, 1 minute each), 95% ethanol (1 minute), 70% ethanol (1 minute), and LC-MS grade water (×2, 3 minutes each). Following tissue washes, the prostate tissue was vacuum desiccated for 5 minutes.
Antigen Retrieval
A vegetable steamer (Rival, Kansas City, Missouri, USA) was filled to water line and preheated to produce steam for antigen retrieval. The prostate section was steamed for 30 minutes in a side opening five-slide mailer filled with 5.6 mM citraconic acid buffer (pH~3, Sigma-Aldrich, St. Louis, MO, USA). After steaming, the slide-mailer was removed from steamer and placed into cool tap water for 5 minutes to slowly cool down the prostate sample. The tissue section was slowly cooled down by removing half the buffer, replenishing with LC-MS grade water and incubating for 5 minutes for a total of 3 times. The prostate tissue was then rinsed with LC-MS grade water and vacuum desiccated for 5 minutes.
Enzymatic Digestion of N-Linked Glycans
Exactly 100 μg/mL of PNGase F PRIME-LY (Bulldog Bio, Portsmouth, NH, USA) was evenly sprayed onto prostate tissue using the TM-Sprayer (HT-X Technologies, Carrboro, NC, USA) at 25 μL/min, 1200 mm/min velocity, 3 mm spacing, 5 mm overspray margins, and 15 passes in a crisscross pattern. Nitrogen gas (Arc3 Gases, Raleigh, NC, USA) was set to 10 psi and the nozzle was heated to 45°C. N-linked glycans were then enzymatically cleaved during incubation at 37°C for 2 hours in a humidity chamber where the relative humidity was greater than 80%.
IR-MALDESI MSI Analysis
The digested human prostate tissue was placed directly on the Peltier-cooled translational stage inside the sample enclosure purged with nitrogen gas (Arc3 Gases, Raleigh, NC, USA) and a thin ice layer was formed by controlled exposure to humidity. An electrospray ionization source was achieved with 60% acetonitrile and 1 mM acetic acid solution at a flow rate of 2 μL/min and spray voltage of 3600 V for negative ionization mode.21 A mid-IR laser (JGM Associates, Burlington, MA, USA) operating at 2.97 μm was used for laser ablation at 1.2 mJ per voxel with a spatial resolution of 150 μm. IR-MALDESI was coupled to an Orbitrap Exploris 240 mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) for high resolution accurate mass spectrometry at a resolving power of 240,000FWHM at 200 m/z. Automatic gain control (AGC) was disabled and a fixed injection time of 90 ms was set to synchronize the laser ablation event with collection of ionized glycans in the ion routing multipole. The prostate sample was analyzed in two separate regions of interest to evaluate ESI stability halfway through. The instrument was mass calibrated the morning of analysis to achieve high mass measurement accuracy (<2.5 ppm).
A small portion of the tissue was left unanalyzed so that multiply-charged peaks could be fragmented, and putative structures could be further identified. Three ions were individually isolated with a window of 2 Da and the normalized collision energy (NCE) was ramped up every 5 scans to get a range of fragmentation patterns for each precursor ion.
Data Analysis
Thermo .RAW data files were converted to mzML files using MSConvert22 and then to imzML files using imzMLconverter23. All ion images were generated and analyzed using MSiReader, an open-source MATLAB software for imaging analyses.24,25 The two imzML files are separated with a black border in each ion image. The MSiImage tool in MSiReader was used to overlay the optical image onto ion images to compare morphological regions. GlyConnect, an experimentally curated glycomic database, was used to search previously identified N-linked glycans found in human samples.26
GlycoHunter was used to quickly search for multiply-charged peaks detected by IR-MALDESI.27 Peak pairs with a 0.5017 (z=2) and 0.3345 Da (z=3) mass offset were searched in the data with a 10 ppm mass offset m/z tolerance and a minimum abundance threshold of 1000 ions/s. These multiply-charged ions were searched in GlycoMod, a theoretical glycan database, where each identification must be within 2.5 ppm MMA, contain the core N-linked glycan structure (Hex3HexNAc2), and not include pentose, KDN, or HexA monosaccharides.28
For further identification of putative structures, the predicted structures were drawn in GlycoWorkbench and the glycosidic cleavages were computed and searched within each MS/MS data file.29
RESULTS
IR-MALDESI was used to spatially investigate the distribution of N-linked glycans in the human prostate. The human prostate tissue served as a model system for the first demonstration of glycan imaging by IR-MALDESI due to the biological involvement of N-linked glycans in prostate cancer.16–18 Figure 1 shows the optical image of the FFPE human prostate tissue section taken prior to IR-MALDESI MSI analysis, depicting large and diverse morphological regions. Sample preparation procedures not typically used prior to IR-MALDESI analyses were required to enzymatically cleave and detect N-linked glycans in the prostate sample (Figure 2). Tissue washes in xylenes, ethanol, and water were required to remove paraffin residue, salts, metabolites, and lipids that would otherwise suppress and dominate the recorded mass spectra. Protein crosslinking was reduced by antigen retrieval allowing enzyme access to the glycosylation sites. Finally, PNGase F was pneumatically sprayed over the human prostate before digestion at 37°C in a humidity chamber. Enzymatically cleaved N-linked glycans were then analyzed by IR-MALDESI in negative ionization mode due to previous work indicating negative mode as a more sensitive method for negatively charged sialylated species with reduced ambient background ions.15
Figure 1.

Optical image of FFPE human prostate tissue showing multiple large morphological features. The tumorous region as determined by H&E staining is roughly outlined. A few examples of glandular and stromal regions are highlighted and will be discussed later with ion image distributions.
Figure 2.

Experimental workflow implemented in this study for the analysis of N-linked glycans. Tissue washing was utilized for dewaxing and delipidating the tissue. Antigen retrieval was required to reduce protein crosslinking prior to enzymatic digestion by pneumatically applied PNGase F. IR-MALDESI MSI was used to spatially detect N-Linked glycans in negative ionization mode. Data analysis included spatial interpretation in MSiReader and peak picking of multiply-charged species via GlycoHunter.
Native N-linked glycans were detected as multiply-charged species in negative ionization mode by IR-MALDESI MSI. Due to the significant amount of N-linked glycans observed in each individual spectrum, GlyConnect was used to specifically search for previously identified N-linked glycans specific to the human species. N-linked glycans reported in GlyConnect were exported and filtered to structures that contained at minimum 3 hexose and 2 N-acetylglucosamines with the monoisotopic peaks of the second or third charge-state overlapping the experimental mass range used in this study. This filtering criteria resulted in 580 previously identified N-linked glycans reported in GlyConnect that were searched within this dataset. The ion images for the multiply-charged monoisotopic peaks were exported and filtered down to tissue-specific ions with spatially informative ion images (i.e. ions with sporadic signal were filtered out). Isotopic distributions for tissue-specific ions were then investigated to confirm the presence of a multiply-charged distribution. A total of 53 multiply-charged deprotonated N-linked glycans were detected by IR-MALDESI with high mass measurement accuracy (MMA, <2.5 ppm) in the human prostate sample (Table 1).
Table 1.
53 multiply-charged deprotonated N-linked glycans detected in human FFPE prostate tissue with high mass measurement accuracy (MMA). These identifications were found by searching for the human N-linked glycans reported in GlyConnect. Each identification is represented in short-hand notation where H=Hexose, N=N-Acetylglucosamine, F=Fucose, S=N-Acetyl-Neuraminic Acid, G=N-Glycolyl-Neuraminic Acid, and U=Sulphate Modification.
| # | Charge | Experimental m/z | Database ID | MMA (ppm) |
|---|---|---|---|---|
| 1 | 2 | 616.2089 | H5N2 | 0.9 |
| 2 | 2 | 697.2353 | H6N2 | 0.8 |
| 3 | 2 | 709.7512 | H4N3F1 | 0.7 |
| 4 | 3 | 739.9203 | H5N4S2 | 0.1 |
| 5 | 2 | 778.2623 | H7N2 | 0.0 |
| 6 | 2 | 782.2695 | H4N3S1 | 1.2 |
| 7 | 3 | 788.6054 | H5N4F1S2 | 1.2 |
| 8 | 3 | 789.2782 | H7N6 | 0.0 |
| 9 | 2 | 798.7750 | H6N3 | 0.7 |
| 10 | 2 | 811.2899 | H4N4F1 | 1.8 |
| 11 | 3 | 813.2844 | H6N5F1S1 | 1.1 |
| 12 | 3 | 815.2565 | H5N4F1S2U1 | 2.6 |
| 13 | 2 | 819.2882 | H5N4 | 0.7 |
| 14 | 2 | 855.2989 | H4N3F1S1 | 0.5 |
| 15 | 2 | 859.2877 | H8N2 | 1.1 |
| 16 | 3 | 861.6301 | H6N5S2 | 1.2 |
| 17 | 3 | 866.5885 | H6N5F1S1U2 | 1.5 |
| 18 | 3 | 886.3090 | H7N6S1 | 1.2 |
| 19 | 2 | 892.3173 | H5N4F1 | 0.5 |
| 20 | 3 | 910.3166 | H6N5F1S2 | 0.6 |
| 21 | 2 | 932.2953 | H5N4F1U1 | 0.9 |
| 22 | 3 | 934.9963 | H7N6F1S1 | −0.3 |
| 23 | 3 | 936.9697 | H6N5F1S2U1 | −0.4 |
| 24 | 2 | 940.3138 | H9N2 | 1.4 |
| 25 | 2 | 944.3216 | H6N3S1 | 1.7 |
| 26 | 2 | 956.8365 | H4N4F1S1 | 2.7 |
| 27 | 3 | 958.6623 | H6N5S3 | 0.7 |
| 28 | 2 | 964.8356 | H5N4S1 | 0.9 |
| 29 | 2 | 972.2729 | H5N4F1U2 | 1.7 |
| 30 | 2 | 1001.8549 | H6N5 | 0.0 |
| 31 | 3 | 1007.3479 | H6N5F1S3 | 0.9 |
| 32 | 3 | 1032.0267 | H7N6F1S2 | 1.1 |
| 33 | 2 | 1037.8648 | H5N4S1F1 | 0.6 |
| 34 | 2 | 1058.3771 | H4N5F1S1 | 1.6 |
| 35 | 2 | 1074.8822 | H6N5F1 | 1.5 |
| 36 | 2 | 1077.8422 | H5N4F1S1U1 | 1.6 |
| 37 | 3 | 1080.3739 | H7N6S3 | −0.2 |
| 38 | 2 | 1098.3553 | H4N5F1S1U1 | 1.7 |
| 39 | 2 | 1110.3831 | H5N4S2 | 1.0 |
| 40 | 3 | 1129.0582 | H7N6F1S3 | 1.3 |
| 41 | 2 | 1147.4005 | H6N5S1 | 1.9 |
| 42 | 2 | 1147.9128 | H6N5F2 | 0.0 |
| 43 | 2 | 1183.4121 | H5N4F1S2 | 1.0 |
| 44 | 2 | 1184.4210 | H7N6 | 0.0 |
| 45 | 2 | 1203.9254 | H4N5F1S2 | 0.9 |
| 46 | 2 | 1220.4306 | H6N5F1S1 | 0.8 |
| 47 | 2 | 1257.4486 | H7N6F1 | 1.1 |
| 48 | 2 | 1292.9481 | H6N5S2 | 1.7 |
| 49 | 2 | 1329.9673 | H7N6S1 | 1.1 |
| 50 | 2 | 1365.9774 | H6N5F1S2 | 1.4 |
| 51 | 3 | 1372.4791 | H9N8F1S3 | 1.5 |
| 52 | 2 | 1402.9950 | H7N6F1S1 | 1.9 |
| 53 | 2 | 1475.5152 | H7N6S2 | 0.8 |
More than 60% of the N-linked glycans detected by IR-MALDESI (Table 1) contain at least one sialic acid residue which is a considerable amount given that no chemical derivatization steps were used to stabilize the labile monosaccharide. This observation is supported by previous investigations into the internal energy deposited into a molecule during ionization (using thermometer ions) demonstrating that IR-MALDESI is a soft ionization method that produces ions with comparable internal energy to conventional ESI measurements.30 The mechanism in which IR-MALDESI produces soft ions begins with IR absorption of endogenous and exogenous water/ice matrix. Neutral species can be vibrationally activated in a multi-photon process as a result of IR irradiation, but these neutral species quickly enter the charged ESI droplets and are cooled by via vibrational relaxation giving up their internal energy by raising the temperature of the solvent. Thus, labile species, even when sufficient internal energy is deposited in a molecule when irradiated, are not given the time to dissociate prior to the internal energy being removed.
In order to demonstrate the critical importance of IR-MALDESI for glycan imaging methodologies, it is important that the N-linked glycans detected in this study also have spatially informative ion images and show close alignment with the morphological features of the prostate sample. Figure 3 demonstrates the colocalization of the ion images with the morphological features of three spatially distinct N-linked glycans within the prostate sample. The optical image taken prior to IR-MALDESI analysis (Figure 1) was overlayed in MSiReader to correlate these regions together. A zoomed-in region of each unique spatial distribution shows alignment with the morphological regions. For example, Figure 3A shows an ion particularly distributed in a small glandular region while the ion shown in Figure 3B is distributed across the stromal tissue of the prostate. The ion represented in Figure 3C is well distributed across the entire prostate sample, but of importance is the decrease in abundance inside the large glandular region highlighted in the zoomed-in image. This ion is observed to have a decrease in abundance inside the glandular tissue of this region; however, the abundance of this ion closely aligns with the connective tissue between each of the individual glands. The spatial distribution of all N-linked glycans detected by IR-MALDESI are shown in Figure S1.
Figure 3.

Colocalization with spatial features of human prostate sample. Ion images of three putatively identified N-linked glycans are shown for reference (left). The optical image of the prostate sample is overlayed on top of each ion image (middle) to show colocalization with morphological features of the tissue with smaller regions of interest zoomed-in to exhibit close alignment (right). The black line seen halfway through the ion images was added by MSiReader to separate two regions of interest collected for this prostate sample.
After identification of the 53 N-linked glycans, it became clear that there was a significant number of unidentified multiply-charged species in the prostate sample. For example, Figure 4A shows the mass spectrum #7719 that shows more unidentified multiply-charged peaks than putatively identified N-linked glycans from GlyConnect which are highlighted green. Considering that multiply-charged peaks are not present in the IR-MALDESI background, these peaks represent tissue-specific ions and are most likely N-linked glycans that were enzymatically cleaved by PNGase F. These multiply-charged peaks were first extracted using GlycoHunter and then searched in GlycoMod for theoretical identifications. This resulted in a total of 604 peak pairs in spectrum #7719 alone, which was further reduced down to 123 unique peak pairs by deisotoping and verifying the detection of the isotopic distribution (Figure S2).
Figure 4.
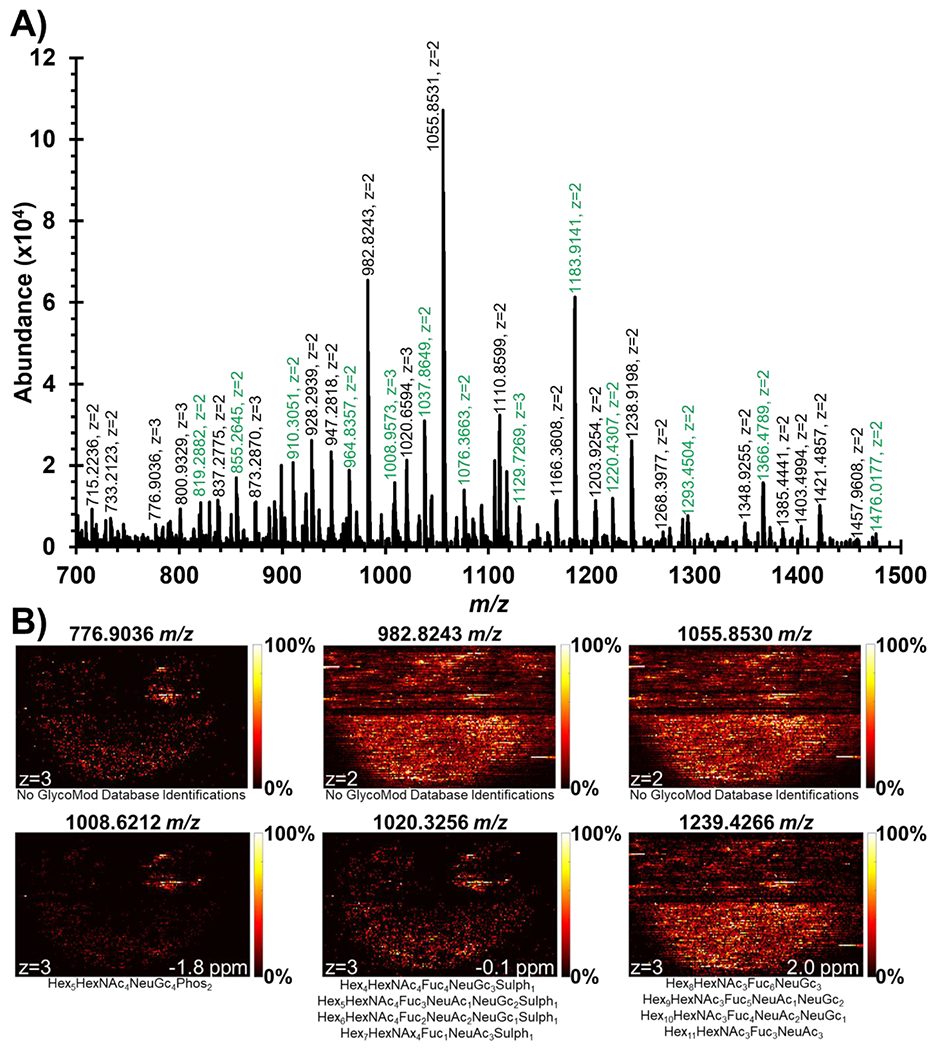
A) Mass spectrum #7719 showing significant number of multiply-charged peaks. Labels highlighted in green were already annotated by GlyConnect database searching (Table 1). This scan was searched for peak pairs via GlycoHunter with a 0.5017 Da or 0.3345 Da m/z offset to find multiply-charged monoisotopic peaks to search within GlycoMod database. B) Ion images of multiply-charged peaks with no GlycoMod database identifications and potential sulfate/phosphate modifications represent significant spatially informative distributions that could be of biological importance to the human prostate sample. The black line seen halfway through the ion images was added by MSiReader to separate two regions of interest collected for this prostate sample.
A majority of the monoisotopic peaks found by GlycoHunter did not have any theoretical matches to GlycoMod (Figure S3) and thus were left unidentified by database searching. 43 multiply-charged GlycoHunter peaks, having tissue-specific spatial distributions and isotopic distributions, matched theoretical structures reported in GlycoMod, many of which were already annotated by searching for GlyConnect N-linked glycans (Table 1). 23 multiply-charged peaks not previously identified by GlyConnect were identified by GlycoMod (Figure S4), in which case most contained sulfate or phosphate modifications that are under-reported and thus not present in GlyConnect. Figure 4B shows six selected ion images demonstrating that these ions found in spectrum #7719 by GlycoHunter had similar spatial distributions to GlyConnect ions and could represent biologically relevant N-linked glycans.
Tandem mass spectrometry was performed on three N-linked glycans in order to confirm the presence of putatively identified structures as well as one of the unidentified multiply-charged peaks. The putative structures provided by GlyConnect were drawn in GlycoWorkbench and its glycosidic cleavages were predicted.29 For example, the predicted fragments for 1037.8650 m/z that were detected in the MS/MS file are shown in Figure 5A. The combination of the biantennary branches, as well as the loss of the reducing end all confirm and lead to Hex5HexNAc4Fuc1NeuAc1 being the most likely structure. Additionally, each fragment that resulted in the loss of the reducing end was not detected with a fucose, confirming the presence of a core fucose. The unidentified multiply-charged precursor could not be drawn and have computed fragments in GlycoWorkbench. However, the unknown multiply-charged precursor was observed to fragment in extremely similar patterns to the 1037.8650 m/z ion (Figure 5B). Additionally, the 1st charge state of the 1037.8650 m/z ion was detected as a fragment in the unknown MS/MS spectrum. This indicates that Hex5HexNAc4Fuc1NeuAc1 is likely the core structure of the unidentified ion with an additional modification. These ions have a neutral mass difference of 35.9763 Da which corresponds by accurate mass to a chlorine adduct and loss of proton ([M-H++Cl]2−).
Figure 5.

Fragmentation spectra for a putatively identified N-linked glycan and an unidentified multiply-charged peak. A) The glycosidic cleavages shown for the 1037 m/z peak confirm the putative identification of Hex5HexNAc4Fuc1NeuAc1. B) The unknown peak at 1055 m/z showed extremely similar fragmentation peaks to the 1037 m/z peak. Additionally, the singly charged form of Hex5HexNAc4Fuc1NeuAc1 was also detected indicating that this is likely the core structure of the 1055 m/z peak with an additional unknown modification.
After discovering the mass offset likely represented a mixed-mode ionization with chlorine adducts, we searched the data for other possible chlorinated N-linked glycans. This led to the annotation of 36 N-linked glycan peaks that were previously extracted by GlycoHunter but left unidentified by database searching (Table S1). Although chlorines have distinctive isotopic patterns, the 37Cl isotopologue could not be resolved due to the resolving power at the detected m/z values as well as the m/z spacing of multiply-charged peaks. However, the isotopic distributions of the detected N-linked glycans very closely align with the theoretical distribution of the glycans with chlorinated adducts. Figure 6A shows Hex5HexNAc4Fuc1NeuAc1 is likely adducted with one chlorine and the loss of a proton. The presence of one chlorine adduct causes a 40% increase in the abundance of the A+2 isotopic peak. The observed isotopic distribution closely aligns with the increase in abundance as demonstrated by the chi-squared value which tests for goodness of fit between two distributions. Similarly, Figure 6B represents an N-linked glycan detected with 2 chlorine adducts which causes a 200% increase in the A+2 peak which is clearly observed in the experimental data.
Figure 6.

Isotopic distributions of two detected N-linked glycans with theoretical distributions of chlorine and deprotonated adducts overlayed. The [M-2H+]2+ isotopic distribution would be observed at a lower m/z value but has been shifted to compare probabilities of abundance. Chi-squared values testing a goodness of fit indicate that the N-linked glycans were detected with chlorine adducts.
CONCLUSIONS
A new methodology to spatially detect N-linked glycans in a biological tissue was created using IR-MALDESI MSI. A total of 53 N-linked glycans were confidently identified based on previous identifications found in literature. More than 60% of these identifications included sialic acid residues indicating the softness of IR-MALDESI to preserve these labile species. A significant amount of multiply-charged peaks were discovered via GlycoHunter in a single scan which led to potential sulfate/phosphate modifications as well as many chlorine adducted structures. This proof-of-concept effort demonstrates that N-linked glycan imaging by IR-MALDESI can be carried out without the need for chemical derivatization.
Supplementary Material
ACKNOWLEDGEMENTS
IR-MALDESI-MSI measurements were carried out in the Molecular Education, Technology, and Research Innovation Center (METRIC) at North Carolina State University which is supported by the State of North Carolina. The authors gratefully acknowledge the financial support received from the National Institutes of Health (R01GM087964 and R56AG063885) and North Carolina State University.
Footnotes
SUPPORTING INFORMATION
Ion images of 53 multiply-charged N-linked glycans detected in human prostate (Figure S1); Examples of isotopic distributions investigated after peak pair extraction via GlycoHunter (Figure S2); Ion images of multiply-charged peaks found by GlycoHunter that have no database identifications in GlycoMod or GlyConnect (Figure S3); Ion images of N-linked glycans found by GlycoHunter and annotated by GlycoMod (Figure S4); List of N-linked glycans detected with chlorinated adducts with high mass measurement accuracy (Table S1)
REFERENCES
- (1).Hart GW; Copeland RJ Glycomics Hits the Big Time. Cell 2010, 143 (5), 672–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Abdul Rahman S; Bergström E; Watson CJ; Wilson KM; Ashford DA; Thomas JR; Ungar D; Thomas-Oates JE Filter-Aided N-Glycan Separation (FANGS): A Convenient Sample Preparation Method for Mass Spectrometric N-Glycan Profiling. J. Proteome Res 2014, 13 (3), 1167–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Hecht ES; McCord JP; Muddiman DC A Quantitative Glycomics and Proteomics Combined Purification Strategy. J. Vis. Exp 2016, 2016 (109), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Powers TW; Jones EE; Betesh LR; Romano PR; Gao P; Copland JA; Mehta AS; Drake RR Matrix Assisted Laser Desorption Ionization Imaging Mass Spectrometry Workflow for Spatial Profiling Analysis of N-Linked Glycan Expression in Tissues. Anal. Chem 2013, 85 (20), 9799–9806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Eshghi ST; Yang S; Wang X; Shah P; Li X; Zhang H Imaging of N-Linked Glycans from Formalin-Fixed Paraffin-Embedded Tissue Sections Using MALDI Mass Spectrometry. ACS Chem. Biol 2014, 9 (9), 2149–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Shi Y; Li Z; Felder MA; Yu Q; Shi X; Peng Y; Cao Q; Wang B; Puglielli L; Patankar MS; Li L Mass Spectrometry Imaging of N-Glycans from Formalin-Fixed Paraffin-Embedded Tissue Sections Using a Novel Subatmospheric Pressure Ionization Source. Anal. Chem 2019, 91 (20), 12942–12947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Harvey DJ Structural Determination of N-Linked Glycans by Matrix-Assisted Laser Desorption/Ionization and Electrospray Ionization Mass Spectrometry. Proteomics 2005, 5 (7), 1774–1786. [DOI] [PubMed] [Google Scholar]
- (8).Holst S; Heijs B; De Haan N; Van Zeijl RJM; Briaire-De Bruijn IH; Van Pelt GW; Mehta AS; Angel PM; Mesker WE; Tollenaar RA; Drake RR; Bovée JVMG; McDonnell LA; Wuhrer M Linkage-Specific in Situ Sialic Acid Derivatization for N-Glycan Mass Spectrometry Imaging of Formalin-Fixed Paraffin-Embedded Tissues. Anal. Chem 2016, 88 (11), 5904–5913. [DOI] [PubMed] [Google Scholar]
- (9).Powell AK; Harvey DJ Stabilization of Sialic Acids InN-Linked Oligosaccharides and Gangliosides for Analysis by Positive Ion Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry. Rapid Commun. Mass Spectrom 1996, 10 (9), 1027–1032. [DOI] [PubMed] [Google Scholar]
- (10).Everest-Dass AV; Briggs MT; Kaur G; Oehler MK; Hoffmann P; Packer NH N-Glycan MALDI Imaging Mass Spectrometry on Formalin-Fixed Paraffin-Embedded Tissue Enables the Delineation of Ovarian Cancer Tissues. Mol. Cell. Proteomics 2016, 15 (9), 3003–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Khodjaniyazova S; Hanne NJ; Cole JH; Muddiman DC Mass Spectrometry Imaging (MSI) of Fresh Bones Using Infrared Matrix-Assisted Laser Desorption Electrospray Ionization (IR-MALDESI). Anal. Methods 2019, 11 (46), 5929–5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Stutts WL; Knuth MM; Ekelöf M; Mahapatra D; Kullman SW; Muddiman DC Methods for Cryosectioning and Mass Spectrometry Imaging of Whole-Body Zebrafish. J. Am. Soc. Mass Spectrom 2020, 31 (4), 768–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Pace CL; Horman B; Patisaul H; Muddiman DC Analysis of Neurotransmitters in Rat Placenta Exposed to Flame Retardants Using IR-MALDESI Mass Spectrometry Imaging. Anal. Bioanal. Chem 2020, 412, 3745–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Xi Y; Tu A; Muddiman DC Lipidomic Profiling of Single Mammalian Cells by Infrared Matrix-Assisted Laser Desorption Electrospray Ionization (IR-MALDESI). Anal. Bioanal. Chem 2020, 412 (29), 8211–8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Pace CL; Muddiman DC Direct Analysis of Native N -Linked Glycans by IR-MALDESI. J. Am. Soc. Mass Spectrom 2020, 31 (8), 1759–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Scott E; Munkley J Glycans as Biomarkers in Prostate Cancer. Int. J. Mol. Sci 2019, 20 (6), 1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Taniguchi N; Kizuka Y Glycans and Cancer. In Advances in Cancer Research; Elsevier Inc., 2015; Vol. 126, pp 11–51. [DOI] [PubMed] [Google Scholar]
- (18).Pinho SS; Reis CA Glycosylation in Cancer: Mechanisms and Clinical Implications. Nat. Rev. Cancer 2015, 15 (9), 540–555. [DOI] [PubMed] [Google Scholar]
- (19).Drake RR; Powers TW; Norris-Caneda K; Mehta AS; Angel PM In Situ Imaging of N-Glycans by MALDI Imaging Mass Spectrometry of Fresh or Formalin-Fixed Paraffin-Embedded Tissue. Curr. Protoc. Protein Sci 2018, 94 (1), 1–21. [DOI] [PubMed] [Google Scholar]
- (20).Powers TW; Neely BA; Shao Y; Tang H; Troyer DA; Mehta AS; Haab BB; Drake RR MALDI Imaging Mass Spectrometry Profiling of N-Glycans in Formalin-Fixed Paraffin Embedded Clinical Tissue Blocks and Tissue Microarrays. PLoS One 2014, 9 (9), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Bagley MC; Ekelöf M; Muddiman DC Determination of Optimal Electrospray Parameters for Lipidomics in Infrared-Matrix-Assisted Laser Desorption Electrospray Ionization Mass Spectrometry Imaging. J. Am. Soc. Mass Spectrom 2020, 31 (2), 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Kessner D; Chambers M; Burke R; Agus D; Mallick P ProteoWizard: Open Source Software for Rapid Proteomics Tools Development. Bioinformatics 2008, 24 (21), 2534–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Race AM; Styles IB; Bunch J Inclusive Sharing of Mass Spectrometry Imaging Data Requires a Converter for All. J. Proteomics 2012, 75 (16), 5111–5112. [DOI] [PubMed] [Google Scholar]
- (24).Robichaud G; Garrard KP; Barry JA; Muddiman DC MSiReader: An Open-Source Interface to View and Analyze High Resolving Power MS Imaging Files on Matlab Platform. J. Am. Soc. Mass Spectrom 2013, 24 (5), 718–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Bokhart MT; Nazari M; Garrard KP; Muddiman DC MSiReader v1.0: Evolving Open-Source Mass Spectrometry Imaging Software for Targeted and Untargeted Analyses. J. Am. Soc. Mass Spectrom 2018, 29 (1), 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Mariethoz J; Alocci D; Gastaldello A; Horlacher O; Gasteiger E; Rojas-Macias M; Karlsson NG; Packer NH; Lisacek F Glycomics@ExPASy: Bridging the Gap. Mol. Cell. Proteomics 2018, 17 (11), 2164–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Kalmar JG; Garrard KP; Muddiman DC GlycoHunter: An Open-Source Software for the Detection and Relative Quantification of INLIGHT-Labeled N-Linked Glycans. J. Proteome Res 2021, 20 (4), 1855–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Cooper CA; Gasteiger E; Packer NH GlycoMod - A Software Tool for Determining Glycosylation Compositions from Mass Spectrometric Data. Proteomics 2001, 1 (2), 340–349. [DOI] [PubMed] [Google Scholar]
- (29).Ceroni A; Maass K; Geyer H; Geyer R; Dell A; Haslam SM GlycoWorkbench: A Tool for the Computer-Assisted Annotation of Mass Spectra of Glycans. J. Proteome Res 2008, 7 (4), 1650–1659. [DOI] [PubMed] [Google Scholar]
- (30).Tu A; Muddiman DC Internal Energy Deposition in Infrared Matrix-Assisted Laser Desorption Electrospray Ionization With and Without the Use of Ice as a Matrix. J. Am. Soc. Mass Spectrom 2019, 30 (11), 2380–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


