Abstract
Our purpose was to report clinical features in bilateral white dot syndrome in a 47-year-old female patient who was tested positive for the SARS-CoV-2. A 47-year-old female visited our department with complaints of bilateral photophobia and blurred vision in both her eyes. She visited our department during the pandemic period after her PCR-proven SARS-CoV-2 positivity. Her symptoms were chills and fever with a temperature of 40.0°C, associated with fatigue, sweat, and complete loss of taste. Besides basic ophthalmological examinations, ocular diagnostic testing were made to differentiate between specific white dot syndromes with suggestive features of fluorescein angiography, optical coherence tomography, and fundus autofluorescence. Laboratory tests were ordered, including immunserological and haematological ones. Eye examination revealed mild bilateral vitritis and white dots in the fundus of both eyes, including the macula explaining the blurred vision. Herpes simplex virus reactivation was proved, after the SARS-CoV-2 infection. Local corticosteroids were given according to the European Reference Network's recommendations for patients with uveitis during the COVID-19 pandemic. Our report demonstrates that white dot syndrome with blurred vision could be associated with SARS-CoV-2 infection, being potentially sight-threatening because of macular involvement. Ophthalmological examinations found posterior uveitis white dot syndrome, and this should call attention to the risk of acute 2019-CoV infection or occurred 2019-CoV infection. Immunodeficiency favours the occurrence of other viral infections, such as herpes virus infections. Everybody should be aware of the risk of 2019-CoV infection, especially professionals, social workers, and those who work or live with elder people and people with immunodeficiency.
Keywords: SARS-CoV-2 uveitis, COVID-19 white dot, Herpes simplex, Multiple evanescent white dot syndrome, Viral prodrome
Introduction
The SARS-CoV-2 virus is known as the severe acute respiratory syndrome coronavirus. In March 2020, the World Health Organization (WHO) declared the COVID-19 outbreak as pandemic. The cause is the coronavirus disease 2019 (COVID-19) [1]. Comparing with other viral outbreaks, COVID-19 infection has a relatively high morbidity and mortality rate, and the treatment options for COVID-19 infection are currently limited.
The mean incubation time from COVID-19 infection until the appearance of symptoms ranges from 4 to 7 days, while some of the patients have no or just a few symptoms. The majority of the patients with severe disease develop acute respiratory distress syndrome [2]. Coronaviruses (CoVs) are in a family of single-stranded, positive RNA (ribonucleic acid) viruses, characterized by spike proteins projecting from their envelopes [1, 2]. Current guidelines based on the experience from previous epidemics recommend the protection of the nose, the mouth, and the eyes because they contain susceptible mucous membranes.
The COVID-19 is also known to affect visual system, causing ocular manifestation. The ocular system may play a role in viral transmission. The virus may shed from ocular surface secretions and tears which facilitates viral spreading. The initial viral infection may also occur on ocular surface tissues. Otherwise, the virus may spread to the respiratory system of the same individual with the ocular system acting as a conduit [1, 2, 3].
The SARS-CoV-2 may also constitute the risk factor for reactivation of the herpes family viruses. Herpesviridae family uses latency as an escape or evasion mechanism for the host's immune system. The most common gate for human herpes viruses is the pharynx. After getting inside the human body, they use various mechanisms to spread. After the initial infection, herpes viruses remain in a latent state in different cells. They can be later reactivated in cases of immunodeficiency. This can occur by many reasons, like stress, malnutrition, immunosupressive drugs, and infections by other pathological agents like viruses [3, 4, 5].
Case Report
A 47-year-old female patient came to our department because of bilateral photophobia and blurred vision in her eyes and decreased vision in her left eye. She visited our department in November 2020. She tested negative for the SARS-CoV-2 infection a week before admission to our department. She was positive for SARS-CoV-2 21 days before the ocular signs appeared, and then she presented chills and fever with a temperature of 40.0°C, associated with complete loss of taste. The ocular symptoms are displayed in Table 1.
Table 1.
Ocular signs
| Blurred vision-both sides |
| Decreased vision-left side |
| Photophobia-both sides |
| Mild vitritis-both sides |
| Multifocal, greyish-white placoid lesions at the level of RPE-both sides |
| Macular involvement-left side |
The ocular anamnesis excluded previous episodes of uveitis, ocular, and systemic infections or autoimmune diseases. Ophthalmological history proved that she has had annual check-ups for hypermetropy of +3.5 dioptres (D) in her right and +4.5 dioptres in her left eye, without any remarkable ocular pathology. At present, her vision decreased in her left eye. According to the patient, her vision got worse gradually and progressively. Best corrected visual acuity (BCVA) of the right eye was 1.0 with +3.5 D and blurred vision; BCVA of the left eye was 0.2 with +4.5 D correction. Intraocular pressure was in normal range and slit lamp examination did not show any alterations of the anterior segments of both sides. We did not see any cells in the anterior chamber.
With dilated funduscopic examinations, there was mild vitritis on both sides probably because of the “spilled over” cells from the anterior chamber. The optic disc was normal on both sides, and no swelling was detected. Multifocal, flat, and grayish-white placoid lesions in the retinal pigment epithelium (RPE) level of the retina on both sides were revealed. On the left side, the macular region was involved.
Multiple evanescent white dot syndrome (MEWDS) is one of the diagnoses within the family of white dot syndromes. The white dot syndromes produce yellow-white retinal lesions classically located at the retinal pigment epithelium or outer retina. Symptoms of MEWDS include unilateral/bilateral blurred vision, visual field loss, photopsias, and floaters.
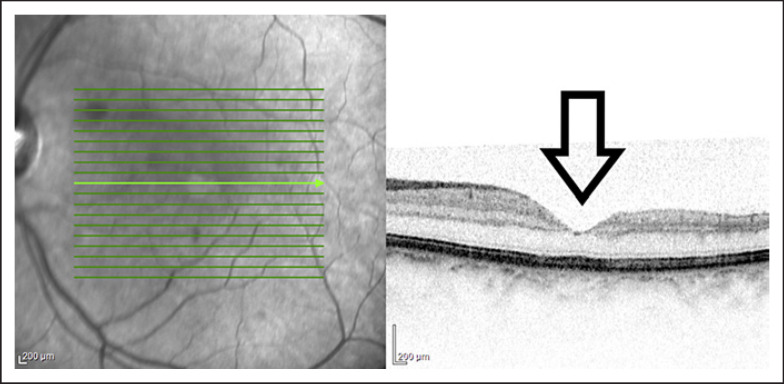
MEWDS is rarely bilateral, and in these cases, the ocular involvement is usually asymmetric. In our case, only the left eye was symptomatic due to the macular involvement [6]. Optical coherence tomography (Heidelberg Spectralis) showed inflammatory lesions in the level of the outer retina. The disruption of the ellipsoid zone could be seen and that could make the foveal area granular. Furthermore, optical coherence tomography showed swelling of the outer retinal layers and granules at the level of RPE [7].
Figure 1. Discontinuities in inner segment-outer segment junction and mild attenuation of external limiting membrane have been reported in acute phase. Recurrent episodes may result in the thinning of the outer nuclear layer.
Fig. 1.
OCT, Heidelberg. Left eye with macular involvement. Pigment epithelial detachment at first visit. OCT shows small projections in the outer retina corresponding to dots. Near infrared reflectance imaging highlight the granular fovea. OCT, optical coherence tomography.
Wide field fluorescein angiography (FLAG; Optos, California) was performed, showing early and late hyperfluorescence of the white spots. Diffuse and patchy late staining was detected at the level of RPE and retina. The wreath-like hyperfluorescence corresponded to the dots and could be seen clinically. After resolutions of the acute lesions, window defects could be noted, corresponding to the clinical granularity seen. No vasculitis could be detected. We performed Optos FLAG to get to know more information about the periphery of the eyes.
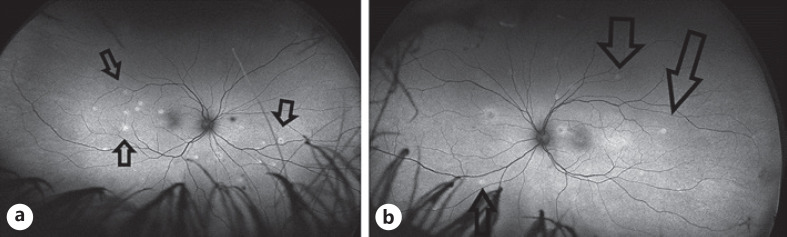
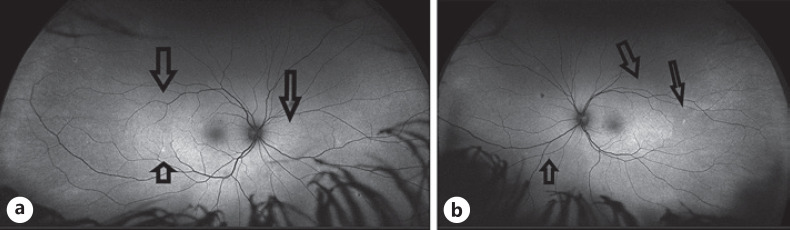
The initial autofluorescence images (fundus autofluorescence, (Optos, California)) showed hyperautofluorescence corresponding to the white dots. Figure 2. In the recovery phase, the areas of hyperautofluorescence became less and smaller [8, 9] Figure 3.
Fig. 2.
Optos, California − autofluorescent pictures: before treatment. aInflammatory whitish lesions on the right fundus before the therapy. bInflammatory whitish lesions on the left fundus.
Fig. 3.
aOptos, California − autofluorescent pictures after treatment the white spots are getting disappeared on the right eye. bThe left fundus after therapy.
We suspected infectious (viral) and immune-related origin, complicated by macular involvement so the patient underwent haematological and serological examinations for uveitis. According to the European Reference Network's recommendations for patients with uveitis during the COVID-19 pandemic, we started local therapy. The European Reference Networks are virtual networks connecting healthcare professionals around Europe with expertise in rare diseases, which allows them to discuss patient's diagnosis and care. This network helps to make any decision in treating uveitis during COVID-19 pandemic. This included corticosteroid injection (1 mg, dexamethasone, ratiopharm) into the orbital floor of both sides, every other day, all together five times. We gave corticosteroid drops because we were positive that there was an anterior uveitis. Beside corticosteroid eye drops (5 times a day), dilatation of the pupil was initiated (cycloplegicedol eye drops 5 times a day) in order to prevent severe ciliary spasm and synechiae formation. Additionally, we gave acyclovir orally 5 × 800 mg/day.
The clinical symptoms regressed completely in 4 weeks and at the 1-month follow-up visit, the whitish inflammatory dots regressed but did not disappear totally. The macular involvement on the left eye resolved. The patient's visual acuity returned to 1.0 with hyperopic spherical correction.
The laboratory data, as reported in Table 2, excluded antinuclear antigen-associated uveitis, HLA-B27 positivity, lupus anti-coagulant (LAC), toxoplasma, cytomegalovirus, Borellia, Toxocara, and Epstein-Barr antibodies-associated uveitis. We examined the patient after COVID infection, the lab values were taken at her first visit at the Department of Ophthalmology.
Table 2.
Laboratory findings
| Laboratory tests | Value |
|---|---|
| SARS-CoV-2 IgM | Negative |
| SARS-CoV-2 IgG | Positive |
| Borellia IgM Western blot | Negative |
| Borellia IgG Western blot | Negative |
| Anti-CMV IgM | Negative |
| Anti-CMV IgG | Negative |
| Anti-EBV IgM | Negative |
| Anti-EBV IgG | Positive |
| Anti-HSV-1/2 IgM | Positive |
| Anti-HSV-1/2 IgG | Positive |
| Anti-HCV IgM | Negative |
| Anti-HCV IgG | Negative |
| Toxocara canis antibody (IgM, IgG) | Negative |
| Toxoplasma gondii (IgM, IgG) | Negative |
| HLA-B27 | Negative |
| ANA | Negative |
| LCA | Negative |
ANA, antinuclear antigen.
The Herpes simplex IgM and IgG levels were elevated. Table 2 shows the laboratory findings.
The patient had elevated IgG for SARS-CoV-2 from the nasopharyngeal swab using RT-PCR (reverse transcription polymerase chain reaction) and confirmed the past infection of the SARS-CoV-2 virus. Reverse transcription polymerase chain reaction is used to prove that the patient had a past infection of SARS-CoV-2. The test is based on the extraction of SARS-CoV-2 virus nucleic acid from blood specimen, followed by combined reverse transcription of viral RNA and PCR amplification using real-time reverse transcriptase PCR (RT-PCR) methods.
Discussion
CoVs can produce many types of ocular manifestations from anterior segment pathologies like conjunctivitis and anterior uveitis to sight-threatening conditions like retinitis and optic neuritis [3, 4]. CoV creates two different phases: the first is represented by the primary infection which induces a trigger of the immune system, while the second phase is probably an autoimmune disease like reaction-based pathology [10]. The lesions presented by our patient support the hypothesis that a herpes infection can manifest after SARS-CoV-2 infection.
Multiple evanescent white dot syndrome is an acute, multifocal, and rarely bilateral retinopathy. The multiple white infiltrations or foci can be seen at the level of the outer retina. There is a strong female predominance. Recent reports revealed female predominance in MEWDS ranging from 50 to 91%; the reason is unknown.
We do not know the definite origin of MEWDS, but infectious and/or immune origin is suspected. The occurrence of MEWDS following hepatitis B, varicella, meningococcus infection, or vaccination suggests environmental triggers [4, 11, 12].
In our case, SARS-CoV-2 could trigger the inactive herpes simplex infection that caused MEWDS. Recovery of vison in a few weeks was coincident with the return of the serum IgM values to normal [5, 12].
The HLA locus maybe important; a preliminary study found the frequency of HLA-B51 haplotype to be 3.5 times more common in patients with MEWDS than in normal group. Our patient was negative for HLA-B27.
The natural course of MEWDS is excellent, and no intervention is required, but in the time of SARS-CoV-2, local steroid therapy is recommended to keep the best visual acuity [13]. Periocular and intraocular corticosteroids are not suitable for recommendation in management of MEWDS, but in time of SARS-CoV-2 pandemic, it is recommended although there is no abundant evidence to prove whether the resolution of MEWDS is self-limiting or relays on corticosteroid.
Statement of Ethics
The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. We state that written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images. Information revealing the subject's identity is avoided. All patients can be identified by numbers or aliases and not by their real names. Human subjects have been performed with the approval of Regional and Institutional Review Board of Human Investigations in University of Szeged and with appropriate participant's informed consent in compliance with the Helsinki Declaration. This study protocol was reviewed and approved by Regional and Institutional Review Board of Human Investigations in University of Szeged, approval number 5053.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding was received for this study.
Author Contributions
Lilla Smeller: patient examination, photodocumentation, therapy, and writing the publication. Edit Toth-Molnar: following the publication. Nicolette Sohar: patient examination and following the therapy.
Data Availability Statement
Data are available and they can be found in our department. All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Funding Statement
No funding was received for this study.
References
- 1.Emrani J, Ahmed M, Jeffers-Francis L, Teleha JC, Mowa N, Newman RH, et al. SARS-COV-2, infection, transmission, transcription, translation, proteins, and treatment: a review. Int J Biol Macromol. 2021 Dec 15;193((Pt B)):1249–1273. doi: 10.1016/j.ijbiomac.2021.10.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ocansey S, Abu EK, Abraham CH, Owusu-Ansah A, Boadi-Kusi SB, Ilechie AA, et al. Ocular symptoms of SARS-CoV-2: indication of possible ocular transmission or viral shedding. Ocul Immunol Inflamm. 2020 Nov 16;28((8)):1269–1279. doi: 10.1080/09273948.2020.1799035. [DOI] [PubMed] [Google Scholar]
- 3.Sen M, Honavar SG, Sharma N, Sachdev MS. COVID-19 and eye: a review of ophthalmic manifestations of COVID-19. Indian J Ophthalmol. 2021 Mar;69((3)):488–509. doi: 10.4103/ijo.IJO_297_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Balc'h P, Pinceaux K, Pronier C, Seguin P, Tadié JM, Reizine F. Herpes simplex virus and cytomegalovirus reactivations among severe COVID-19 patients. Crit Care. 2020;24:530. doi: 10.1186/s13054-020-03252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maldonado MD, Romero-Aibar J, Pérez-San-Gregorio MA. COVID-19 pandemic as a risk factor for the reactivation of herpes viruses. Epidemiol Infect. 2021;149:e145. doi: 10.1017/S0950268821001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veronese C, Morara CMM, Armstrong GW, Ciardella AP. Bilateral multiple evanescent white dot syndrome. Int Ophthalmol. 2018 Oct;38((5)):2153–2158. doi: 10.1007/s10792-017-0673-5. [DOI] [PubMed] [Google Scholar]
- 7.Essilfie J, Bacci T, Abdelhakim AH, Ramtohul P, Turchi F, Freund KB, et al. Are there two forms of multiple evanescent white dot syndrome? Retina. 2022 Feb;42((2)):227–235. doi: 10.1097/IAE.0000000000003288. [DOI] [PubMed] [Google Scholar]
- 8.Furino C, Boscia F, Cardascia N, Alessio G, Sborgia C. Fundus autofluorescence and multiple evanescent white dot syndrome. Retina. 2009 Jan;29((1)):60–3. doi: 10.1097/IAE.0b013e31818c5e04. [DOI] [PubMed] [Google Scholar]
- 9.Seah I, Agrawal R. Can the Coronavirus Disease 2019 (COVID-19) affect the eyes? A review of coronaviruses and ocular implications in humans and animals. Ocul Immunol Inflamm. 2020;28((3)):391–395. doi: 10.1080/09273948.2020.1738501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maddur MS, Vani J, Lacroix-Desmazes S, Kaveri S, Bayry J. Autoimmunity as a predisposition for infectious diseases. PLoS Pathog. 2010 Nov 4;6((11)):e1001077. doi: 10.1371/journal.ppat.1001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tartari F, Spadotto A, Zengarini C, Zanoni R, Guglielmo A, Adorno A, et al. Herpes zoster in COVID-19-positive patients. Int J Dermatol. 2020;59((8)):1028–1029. doi: 10.1111/ijd.15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beltrán-García J, Osca-Verdegal R, Pallardó FV, Ferreres J, Rodríguez M, Mulet S, et al. Sepsis and coronavirus disease 2019: common features and anti-inflammatory therapeutic approaches. Crit Care Med. 2020;48((12)):1841–1844. doi: 10.1097/CCM.0000000000004625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith JR, Lai TYY. Managing uveitis during the COVID-19 pandemic. Ophthalmology. 2020;127((9)):e65–7. doi: 10.1016/j.ophtha.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available and they can be found in our department. All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.